2071:
1927:
38:
400:
1985:
2012:
1327:
1888:
1871:
1854:
1802:
1782:
2156:
1076:
2214:
2077:
2002:. These reagents activate the acyl chloride via a nucleophilic catalysis mechanism. The amine attacks the carbonyl bond and presumably first forms a transient tetrahedral intermediate, then forms a quaternary acylammonium salt by the displacement of the leaving group. This quaternary acylammonium salt is more susceptible to attack by alcohols or other nucleophiles.
1901:
are the most reactive acyl derivatives, and can easily be converted into any of the others. Acid halides will react with carboxylic acids to form anhydrides. If the structure of the acid and the acid chloride are different, the product is a mixed anhydride. First, the carboxylic acid attacks the acid
2070:
1948:. Acid halides hydrolyze in the presence of water to produce carboxylic acids, but this type of reaction is rarely useful, since carboxylic acids are typically used to synthesize acid halides. Most reactions with acid halides are carried out in the presence of a non-nucleophilic base, such as
2567:
2046:, although mixtures of products can result. While a carbon nucleophile will react with the acid halide first to produce a ketone, the ketone is also susceptible to nucleophilic attack, and can be converted to a tertiary alcohol. For example, when
2088:– can add to acid halides just once to give ketones. The reaction between an acid halide and a Gilman reagent is not a nucleophilic acyl substitution reaction, however, and is thought to proceed via a radical pathway. The
2401:
1320:
with the liberated chloride, forming the acid anhydride and releasing regenerated molecule of DMF. Relative to thionyl chloride, oxalyl chloride is more expensive but also a milder reagent and therefore more selective.
1047:
1612:
687:
2092:
can also be used to convert acid halides to ketones. In this reaction, the acid halide is first converted to an N–methoxy–N–methylamide, known as a
Weinreb amide. When a carbon nucleophile – such as a Grignard or
1299:
1491:
1750:
830:
539:
1194:
394:
1926:
1820:
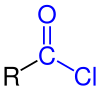
Acid chlorides are useful for the preparation of amides, esters, anhydrides. These reactions generate chloride, which can be undesirable.Acyl chlorides are used to prepare
2222:
Because of the harsh conditions and the reactivity of the intermediates, this otherwise quite useful reaction tends to be messy, as well as environmentally unfriendly.
333:
The simplest stable acyl chloride is acetyl chloride; formyl chloride is not stable at room temperature, although it can be prepared at –60 °C or below.
2896:
K. Venkataraman; D. R. Wagle (1979). "Cyanuric chloride : a useful reagent for converting carboxylic acids into chlorides, esters, amides and peptides".
1317:
2205:
3042:"Computational Studies of Nucleophilic Substitution at Carbonyl Carbon: the S N 2 Mechanism versus the Tetrahedral Intermediate in Organic Synthesis"
2618:
2583:
3030:
C. H. Bamford and C. F. H. Tipper, Comprehensive
Chemical Kinetics: Ester Formation and Hydrolysis and Related Reactions, Elsevier, Amsterdam, 1972.
2066:) is an intermediate in this reaction, it is impossible to isolate because it reacts with a second equivalent of MeMgBr rapidly after being formed.
2252:
849:
3194:
1506:
561:
1760:
Acyl chloride are reactive, versatile reagents. Acyl chlorides have a greater reactivity than other carboxylic acid derivatives like acid
1214:
1352:
3216:
David A. Shirley (2011). "The
Synthesis of Ketones from Acid Halides and Organometallic Compounds of Magnesium, Zinc, and Cadmium".
1627:
702:
431:
3250:
1120:
835:
Similarly, benzotrichlorides react with carboxylic acids to the acid chloride. This conversion is practiced for the reaction of
3301:
2489:
1980:
2 reaction involves a concerted reaction, the tetrahedral addition-elimination pathway involves a discernible intermediate).
346:
2173:
2054:) is treated with two equivalents of a Grignard reagent, such as methyl magnesium bromide (MeMgBr), 2-phenyl-2-propanol (
3233:
2635:
2600:
2836:
2738:
2700:
2463:
2430:
1089:) are gases and residual thionyl chloride can be easily removed as a result of its low boiling point (76 °C).
276:
The names pivalyl chloride and acrylyl chloride are less commonly used, although they are arguably more logical.)
2231:
836:
2581:
Samel, Ulf-Rainer; Kohler, Walter; Gamer, Armin Otto; Keuser, Ullrich (2005). "Propionic acid and derivatives".
403:
Structure of 3,5-dinitrobenzoyl chloride with selected bond distances (picometers) and bond angles shown in red.
2968:
2204:) – coordinates to the halogen on the acid halide, activating the compound towards nucleophilic attack by an
2155:
17:
2208:
aromatic ring. For especially electron-rich aromatic rings, the reaction will proceed without a Lewis acid.
112:
takes priority, acyl chlorides are named by taking the name of the parent carboxylic acid, and substituting
2169:
2879:
1338:
Acid chlorides can be used as a chloride source. Thus acetyl chloride can be distilled from a mixture of
37:
2137:
1316:
that which reacts with the carboxylic acid to form a mixed imino-anhydride. This structure undergoes an
322:. For example, acetic acid boils at 118 °C, whereas acetyl chloride boils at 51 °C. Like most
3327:
2565:, Phillip R. DeVrou, W. Bryan Waites, Robert E. Young, "Process for preparing acetyl chloride"
2562:
3258:
2141:
2133:
2023:
1945:
2124:
also afford ketones, reflecting the low nucleophilicity of these lithium diorganocopper compounds.
2089:
3322:
1910:. The tetrahedral intermediate collapses, ejecting chloride ion as the leaving group and forming
2730:
2723:
1761:
1097:
840:
2479:
1313:
327:
1984:
3147:
2518:
2434:
1057:
In the laboratory, acyl chlorides are generally prepared by treating carboxylic acids with
399:
3262:
1960:
The alcoholysis of acyl halides (the alkoxy-dehalogenation) is believed to proceed via an
336:
Acyl chlorides hydrolyze (react with water) to form the corresponding carboxylic acid and
8:
2145:
2113:
2112:, which in turn are susceptible to the attack by second equivalent to yield the tertiary
2011:
1841:
1326:
3151:
2522:
2539:
2506:
2243:
2235:
2189:
1887:
1870:
1853:
1801:
544:
109:
2910:
1781:
3297:
3229:
3163:
3116:
3108:
3069:
3061:
3013:
3005:
2964:
2941:
2898:
2832:
2734:
2696:
2631:
2596:
2544:
2485:
2459:
2197:
1618:
1309:
1305:
1069:
422:
337:
323:
279:
When other functional groups take priority, acyl chlorides are considered prefixes —
46:
3221:
3155:
3100:
3053:
2997:
2933:
2906:
2861:
2802:
2775:
2688:
2660:
2623:
2588:
2534:
2526:
2451:
2446:
Sih, John C. (2001-04-15), "Formyl
Chloride", in John Wiley & Sons, Ltd (ed.),
2105:
2047:
2039:
2022:
The use of two phases (aqueous for amine, organic for acyl chloride) is called the
1339:
1058:
693:
418:
224:
181:
62:
58:
2101:
by the carbonyl and N–methoxy oxygens, preventing further nucleophilic additions.
3135:
2924:
Sonntag, Norman O. V. (1953-04-01). "The
Reactions of Aliphatic Acid Chlorides".
2239:
1833:
1205:
319:
141:
85:
77:
31:
3225:
3089:"Mechanism of Amine-Catalyzed Ester Formation from an Acid Chloride and Alcohol"
3040:
Fox, Joseph M.; Dmitrenko, Olga; Liao, Lian-an; Bach, Robert D. (October 2004).
2121:
2085:
1821:
1497:
1107:) is popular, although excess reagent is required. Phosphorus pentachloride (
548:
2986:"SN2 Mechanism for Alcoholysis, Aminolysis, and Hydrolysis of Acetyl Chloride"
2984:
Bentley, T. William; Llewellyn, Gareth; McAlister, J. Anthony (January 1996).
2692:
2530:
2084:
Unlike most other carbon nucleophiles, lithium dialkylcuprates – often called
3316:
3167:
3112:
3088:
3065:
3041:
3009:
2985:
2945:
2865:
2806:
2779:
2664:
2627:
2592:
2505:
Wang, Hong-Yong; Xie, Min-Hao; Luo, Shi-Neng; Zou, Pei; Liu, Ya-Ling (2009).
2455:
2396:{\displaystyle {\ce {IrCl(CO)(PPh3)2 + CH3COCl -> CH3COIrCl2(CO)(PPh3)2}}}
2181:
2117:
2094:
1075:
1042:{\displaystyle {\ce {C6H4(CCl3)2 + C6H4(CO2H)2 -> 2 C6H4(COCl)2 + 2 HCl}}}
315:
3120:
3073:
3017:
2548:
2149:
2059:
1969:
1961:
1898:
204:
161:
30:
This article is about the functional group. For the chemical compound, see
2683:
Martin Ansell (1972). "Preparation of acyl halides". In Saul Patai (ed.).
2213:
2144:, a bulky hydride donor, reduces acyl chlorides to aldehydes, as does the
2412:
1911:
1343:
125:
97:
2937:
2753:
J. S. Pizey, Synthetic
Reagents, Vol. 1, Halsted Press, New York, 1974.
2177:
1607:{\displaystyle {\ce {RCO2H + Ph3P + CCl4 -> RCOCl + Ph3PO + HCCl3}}}
682:{\displaystyle {\ce {CH3CH2CO2H + COCl2 -> CH3CH2COCl + HCl + CO2}}}
73:
3159:
3104:
3057:
3001:
2026:. This approach is used in the preparation of nylon via the so-called
2098:
1999:
1294:{\displaystyle {\ce {RCO2H + ClCOCOCl -> RCOCl + CO + CO2 + HCl}}}
3136:"The nylon rope trick: Demonstration of condensation polymerization"
1246:
1082:
Thionyl chloride is a well-suited reagent as the by-products (HCl,
318:, acyl chlorides have lower boiling and melting points than similar
2616:
Maki, Takao; Takeda, Kazuo (2002). "Benzoic acid and derivatives".
1991:
1952:, to neutralize the hydrohalic acid that is formed as a byproduct.
1949:
1486:{\displaystyle {\ce {CH3CO2H + C6H5COCl -> CH3COCl + C6H5CO2H}}}
552:
2477:
2043:
1815:
417:
The industrial route to acetyl chloride involves the reaction of
2076:
1968:(Scheme 10). However, the mechanism can also be tetrahedral or
1812:
This hydrolysis is usually a nuisance rather than intentional.
2826:
2109:
1745:{\displaystyle {\ce {RCO2H + C3N3Cl3 -> RCOCl + C3N3Cl2OH}}}
825:{\displaystyle {\ce {C6H5CCl3 + H2O -> C6H5C(O)Cl + 2 HCl}}}
534:{\displaystyle {\ce {(CH3CO)2O + HCl -> CH3COCl + CH3CO2H}}}
2961:
Strategic
Applications of Named Reactions in Organic Synthesis
1189:{\displaystyle {\ce {RCO2H + PCl5 -> RCOCl + POCl3 + HCl}}}
2415:, and they react violently with water, alcohols, and amines.
1941:
1937:
1933:
1837:
1829:
1825:
1769:
1765:
1114:) is also effective, but only one chloride is transferred:
3294:
Organotransition Metal
Chemistry: From Bonding to Catalysis
2230:
Acyl chlorides react with low-valent metal centers to give
1092:
2895:
2831:. Englewood Cliffs, N.J: Prentice Hall. pp. 666–762.
2687:. PATAI'S Chemistry of Functional Groups. pp. 35–68.
692:
Benzoyl chloride is produced by the partial hydrolysis of
3087:
Hubbard, Patricia; Brittain, William J. (February 1998).
2983:
2651:
Helferich, B.; Schaefer, W. (1929). "n-Butyrl chloride".
2478:
Richard O.C. Norman; James M. Coxon (16 September 1993).
2389:
2376:
2347:
2334:
2315:
2299:
2286:
1735:
1722:
1709:
1687:
1674:
1661:
1642:
1600:
1581:
1559:
1540:
1521:
1476:
1463:
1450:
1431:
1412:
1399:
1380:
1367:
1281:
1229:
1176:
1154:
1135:
1025:
1004:
991:
971:
955:
937:
924:
908:
895:
877:
864:
791:
778:
759:
743:
730:
717:
675:
650:
637:
621:
602:
589:
576:
524:
511:
492:
467:
451:
367:
2766:
Allen, C. F. H.; Barker, W. E. (1932). "Desoxybenzoin".
2246:, converting square planar Ir(I) to octahedral Ir(III):
1792:
Acyl chlorides hydrolyze, yielding the carboxylic acid:
2120:
reagents stops at the ketone stage. The reaction with
1052:
2884:
e-EROS: Encyclopedia of
Reagents for Organic Synthesis
2580:
1199:
2255:
2038:
Acid halides react with carbon nucleophiles, such as
1630:
1509:
1355:
1308:(DMF), which reacts with oxalyl chloride to give the
1217:
1123:
852:
705:
564:
434:
389:{\displaystyle {\ce {RCOCl + H2O -> RCOOH + HCl}}}
349:
3296:. New York: University Science Books. p. 1160.
3134:
Morgan, Paul W.; Kwolek, Stephanie L. (April 1959).
3039:
1944:, respectively, in a reaction formally known as the
2958:
2722:
2395:
1744:
1606:
1485:
1293:
1188:
1041:
824:
681:
533:
388:
96:. Acyl chlorides are the most important subset of
2720:
2650:
3314:
3215:
2963:. London: Elsevier Academic Press. p. 398.
2793:Adams, Roger (1923). "p-Nitrobenzoyl Chloride".
2097:reagent – adds to a Weinreb amide, the metal is
3086:
1832:, by reacting acid chlorides with: a salt of a
1496:Other methods that do not form HCl include the
2619:Ullmann's Encyclopedia of Industrial Chemistry
2584:Ullmann's Encyclopedia of Industrial Chemistry
2471:
2448:Encyclopedia of Reagents for Organic Synthesis
2411:Low molecular weight acyl chlorides are often
1816:Alcoholysis, aminolysis, and related reactions
84:). A specific example of an acyl chloride is
41:General chemical structure of an acyl chloride
3291:
2851:
2682:
2504:
3133:
2729:. Oxford: Oxford University Press. pp.
2481:Principles of Organic Synthesis, 3rd Edition
2212:
2116:. The reaction of acyl halides with certain
2033:
240:(Idiosyncratically, for some trivial names,
2822:
2820:
2818:
2816:
2765:
2058:) is obtained in excellent yield. Although
1918:. Deprotonation gives the mixed anhydride,
1333:
3248:
2852:L. P. Kyrides (1940). "Fumaryl Chloride".
2827:Boyd, Robert W.; Morrison, Robert (1992).
2761:
2759:
2716:
2714:
2712:
2615:
2010:
1983:
1886:
1869:
1852:
1800:
1780:
2538:
1034:
980:
817:
2813:
2172:, acid halides act as electrophiles for
2142:Lithium tri-tert-butoxyaluminium hydride
2075:
1093:Laboratory methods: phosphorus chlorides
398:
36:
3193:was invoked but never defined (see the
3183:
2923:
2880:Triphenylphosphine-carbon tetrachloride
2756:
2709:
2678:
2676:
2674:
14:
3315:
3276:
2952:
2163:
1976:in highly polar solvents (while the S
27:Organic compound with a –C(=O)Cl group
2959:Kürti, László; Barbara Czakó (2005).
2792:
2225:
2671:
2152:over a poisoned palladium catalyst.
1053:Laboratory methods: thionyl chloride
412:
76:. They are reactive derivatives of
3188:
2445:
2174:electrophilic aromatic substitution
1936:react with acid halides to produce
1906:) to give tetrahedral intermediate
1204:Another method involves the use of
1200:Laboratory methods: oxalyl chloride
68:. Their formula is usually written
24:
2154:
1074:
330:reveals a band near 1750 cm.
25:
3339:
2431:Nomenclature of Organic Chemistry
3255:VirtualText of Organic Chemistry
3189:Cite error: The named reference
3093:The Journal of Organic Chemistry
3046:The Journal of Organic Chemistry
2990:The Journal of Organic Chemistry
2511:Acta Crystallographica Section E
2069:
1925:
1325:
1068:). The reaction is catalyzed by
3285:
3242:
3209:
3200:
3174:
3127:
3080:
3033:
3024:
2977:
2917:
2889:
2872:
2845:
2786:
2747:
2232:transition metal acyl complexes
837:1,4-bis(trichloromethyl)benzene
547:is produced by chlorination of
103:
2644:
2609:
2574:
2555:
2498:
2450:, John Wiley & Sons, Ltd,
2439:
2424:
2379:
2363:
2357:
2351:
2321:
2289:
2273:
2267:
2261:
2132:Acyl chlorides are reduced by
1690:
1562:
1418:
1157:
1015:
1009:
974:
961:
942:
898:
882:
804:
798:
765:
624:
479:
457:
438:
373:
13:
1:
3282:Kürti and Czakó 2005, p. 176.
3251:"Carboxylic Acid Derivatives"
3206:Kürti and Czakó 2005, p. 478.
3140:Journal of Chemical Education
2911:10.1016/S0040-4039(00)71006-9
2507:"3,5-Dinitrobenzoyl chloride"
2418:
1998:-dimethylformamide, catalyze
1304:The reaction is catalysed by
309:
2127:
2108:, convert acyl chlorides to
2104:Carbon nucleophiles such as
1955:
1922:, and an equivalent of HCl.
1755:
407:
314:Lacking the ability to form
7:
3226:10.1002/0471264180.or008.02
2138:diisobutylaluminium hydride
10:
3344:
3180:McMurry 1996, pp. 826–827.
2721:Clayden, Jonathan (2001).
2484:. CRC Press. p. 371.
2406:
2140:to give primary alcohols.
29:
3259:Michigan State University
2693:10.1002/9780470771273.ch2
2561:
2531:10.1107/S1600536809036228
2134:lithium aluminium hydride
2034:Reactions with carbanions
2024:Schotten-Baumann reaction
1946:Schotten-Baumann reaction
2866:10.15227/orgsyn.020.0051
2807:10.15227/orgsyn.003.0075
2780:10.15227/orgsyn.012.0016
2665:10.15227/orgsyn.009.0032
2628:10.1002/14356007.a03_555
2593:10.1002/14356007.a22_223
2456:10.1002/047084289x.rf026
2170:Friedel–Crafts acylation
2090:Weinreb ketone synthesis
1334:Other laboratory methods
108:Where the acyl chloride
2622:. Weinheim: Wiley-VCH.
2587:. Weinheim: Wiley-VCH.
2234:. Illustrative is the
2159:The Rosenmund reduction
3292:Hartwig, John (2010).
2882:Taschner, Michael J.
2397:
2217:
2160:
2081:
1746:
1617:Another is the use of
1608:
1487:
1295:
1190:
1098:Phosphorus trichloride
1079:
1043:
841:terephthaloyl chloride
826:
683:
535:
404:
390:
42:
2563:US patent 5672749
2398:
2216:
2158:
2079:
1747:
1609:
1488:
1296:
1191:
1078:
1072:and other additives.
1044:
827:
684:
536:
402:
391:
328:infrared spectroscopy
40:
2435:R-5.7.6 Acid halides
2253:
1628:
1507:
1353:
1215:
1121:
850:
703:
562:
432:
347:
3152:1959JChEd..36..182M
2938:10.1021/cr60162a001
2523:2009AcCrE..65o2460W
2391:
2378:
2349:
2336:
2317:
2301:
2288:
2164:Acylation of arenes
2146:Rosenmund reduction
1737:
1724:
1711:
1689:
1676:
1663:
1644:
1602:
1583:
1561:
1542:
1523:
1478:
1465:
1452:
1433:
1414:
1401:
1382:
1369:
1283:
1256:
1231:
1178:
1156:
1137:
1027:
1006:
993:
973:
957:
939:
926:
910:
897:
879:
866:
793:
780:
761:
745:
732:
719:
677:
652:
639:
623:
604:
591:
578:
526:
513:
494:
469:
453:
369:
2393:
2366:
2361:
2337:
2324:
2305:
2276:
2271:
2236:oxidative addition
2226:Oxidative addition
2218:
2190:iron(III) chloride
2161:
2082:
1742:
1725:
1712:
1699:
1677:
1664:
1651:
1632:
1604:
1590:
1571:
1549:
1530:
1511:
1483:
1466:
1453:
1440:
1421:
1402:
1389:
1370:
1357:
1291:
1271:
1219:
1186:
1166:
1144:
1125:
1080:
1039:
1007:
994:
981:
945:
940:
927:
914:
885:
880:
867:
854:
822:
781:
768:
749:
733:
720:
707:
679:
665:
640:
627:
611:
592:
579:
566:
545:Propionyl chloride
531:
514:
501:
482:
441:
436:
405:
386:
357:
324:carbonyl compounds
251:For example, pival
43:
3328:Functional groups
3303:978-1-938787-15-7
3160:10.1021/ed036p182
3105:10.1021/jo9716643
3058:10.1021/jo049494z
3052:(21): 7317–7328.
3002:10.1021/jo9609844
2996:(22): 7927–7932.
2905:(32): 3037–3040.
2899:Tetrahedron Lett.
2854:Organic Syntheses
2829:Organic Chemistry
2795:Organic Syntheses
2768:Organic Syntheses
2725:Organic chemistry
2653:Organic Syntheses
2491:978-0-7487-6162-3
2369:
2356:
2340:
2327:
2320:
2308:
2279:
2266:
2259:
2198:aluminum chloride
2106:Grignard reagents
1740:
1728:
1715:
1702:
1695:
1680:
1667:
1654:
1647:
1635:
1619:cyanuric chloride
1593:
1586:
1574:
1567:
1552:
1545:
1533:
1526:
1514:
1481:
1469:
1456:
1443:
1436:
1424:
1417:
1405:
1392:
1385:
1373:
1360:
1318:acyl substitution
1310:Vilsmeier reagent
1306:dimethylformamide
1289:
1274:
1267:
1261:
1257:
1240:
1234:
1222:
1184:
1169:
1162:
1147:
1140:
1128:
1070:dimethylformamide
1037:
1014:
997:
984:
960:
948:
930:
917:
888:
870:
857:
820:
810:
803:
796:
784:
771:
764:
752:
736:
723:
710:
668:
661:
655:
643:
630:
614:
607:
595:
582:
569:
529:
517:
504:
497:
485:
478:
472:
456:
444:
423:hydrogen chloride
413:Industrial routes
384:
378:
372:
360:
353:
338:hydrochloric acid
47:organic chemistry
16:(Redirected from
3335:
3308:
3307:
3289:
3283:
3280:
3274:
3273:
3271:
3270:
3261:. Archived from
3249:William Reusch.
3246:
3240:
3239:
3213:
3207:
3204:
3198:
3192:
3187:
3181:
3178:
3172:
3171:
3131:
3125:
3124:
3084:
3078:
3077:
3037:
3031:
3028:
3022:
3021:
2981:
2975:
2974:
2956:
2950:
2949:
2926:Chemical Reviews
2921:
2915:
2914:
2893:
2887:
2876:
2870:
2869:
2849:
2843:
2842:
2824:
2811:
2810:
2790:
2784:
2783:
2763:
2754:
2751:
2745:
2744:
2728:
2718:
2707:
2706:
2680:
2669:
2668:
2648:
2642:
2641:
2613:
2607:
2606:
2578:
2572:
2571:
2570:
2566:
2559:
2553:
2552:
2542:
2502:
2496:
2495:
2475:
2469:
2468:
2443:
2437:
2428:
2402:
2400:
2399:
2394:
2392:
2390:
2387:
2382:
2377:
2374:
2367:
2360:
2354:
2348:
2345:
2338:
2335:
2332:
2325:
2318:
2316:
2313:
2306:
2300:
2297:
2292:
2287:
2284:
2277:
2270:
2264:
2257:
2080:A Weinreb amide.
2073:
2048:benzoyl chloride
2028:nylon rope trick
2014:
1987:
1929:
1890:
1873:
1856:
1844:, respectively.
1804:
1784:
1751:
1749:
1748:
1743:
1741:
1738:
1736:
1733:
1726:
1723:
1720:
1713:
1710:
1707:
1700:
1693:
1688:
1685:
1678:
1675:
1672:
1665:
1662:
1659:
1652:
1645:
1643:
1640:
1633:
1613:
1611:
1610:
1605:
1603:
1601:
1598:
1591:
1584:
1582:
1579:
1572:
1565:
1560:
1557:
1550:
1543:
1541:
1538:
1531:
1524:
1522:
1519:
1512:
1492:
1490:
1489:
1484:
1482:
1479:
1477:
1474:
1467:
1464:
1461:
1454:
1451:
1448:
1441:
1434:
1432:
1429:
1422:
1415:
1413:
1410:
1403:
1400:
1397:
1390:
1383:
1381:
1378:
1371:
1368:
1365:
1358:
1340:benzoyl chloride
1329:
1300:
1298:
1297:
1292:
1290:
1287:
1282:
1279:
1272:
1265:
1259:
1258:
1242:
1238:
1232:
1230:
1227:
1220:
1195:
1193:
1192:
1187:
1185:
1182:
1177:
1174:
1167:
1160:
1155:
1152:
1145:
1138:
1136:
1133:
1126:
1113:
1106:
1088:
1067:
1059:thionyl chloride
1048:
1046:
1045:
1040:
1038:
1035:
1026:
1023:
1018:
1012:
1005:
1002:
995:
992:
989:
982:
972:
969:
964:
958:
956:
953:
946:
938:
935:
928:
925:
922:
915:
909:
906:
901:
896:
893:
886:
878:
875:
868:
865:
862:
855:
831:
829:
828:
823:
821:
818:
808:
807:
801:
794:
792:
789:
782:
779:
776:
769:
762:
760:
757:
750:
744:
741:
734:
731:
728:
721:
718:
715:
708:
694:benzotrichloride
688:
686:
685:
680:
678:
676:
673:
666:
659:
653:
651:
648:
641:
638:
635:
628:
622:
619:
612:
605:
603:
600:
593:
590:
587:
580:
577:
574:
567:
540:
538:
537:
532:
530:
527:
525:
522:
515:
512:
509:
502:
495:
493:
490:
483:
476:
470:
468:
465:
460:
454:
452:
449:
442:
419:acetic anhydride
395:
393:
392:
387:
385:
382:
376:
370:
368:
365:
358:
351:
320:carboxylic acids
305:
201:
157:
95:
83:
78:carboxylic acids
71:
67:
63:functional group
59:organic compound
21:
3343:
3342:
3338:
3337:
3336:
3334:
3333:
3332:
3313:
3312:
3311:
3304:
3290:
3286:
3281:
3277:
3268:
3266:
3247:
3243:
3236:
3214:
3210:
3205:
3201:
3190:
3184:
3179:
3175:
3132:
3128:
3085:
3081:
3038:
3034:
3029:
3025:
2982:
2978:
2971:
2957:
2953:
2922:
2918:
2894:
2890:
2877:
2873:
2850:
2846:
2839:
2825:
2814:
2791:
2787:
2764:
2757:
2752:
2748:
2741:
2719:
2710:
2703:
2681:
2672:
2649:
2645:
2638:
2614:
2610:
2603:
2579:
2575:
2568:
2560:
2556:
2503:
2499:
2492:
2476:
2472:
2466:
2444:
2440:
2429:
2425:
2421:
2409:
2388:
2383:
2375:
2370:
2362:
2350:
2346:
2341:
2333:
2328:
2314:
2309:
2298:
2293:
2285:
2280:
2272:
2260:
2256:
2254:
2251:
2250:
2244:Vaska's complex
2240:acetyl chloride
2228:
2203:
2195:
2187:
2166:
2130:
2122:Gilman reagents
2086:Gilman reagents
2036:
1990:Bases, such as
1979:
1973:
1965:
1958:
1834:carboxylic acid
1822:acid anhydrides
1818:
1758:
1734:
1729:
1721:
1716:
1708:
1703:
1686:
1681:
1673:
1668:
1660:
1655:
1641:
1636:
1631:
1629:
1626:
1625:
1599:
1594:
1580:
1575:
1558:
1553:
1539:
1534:
1520:
1515:
1510:
1508:
1505:
1504:
1475:
1470:
1462:
1457:
1449:
1444:
1430:
1425:
1411:
1406:
1398:
1393:
1379:
1374:
1366:
1361:
1356:
1354:
1351:
1350:
1336:
1280:
1275:
1241:
1228:
1223:
1218:
1216:
1213:
1212:
1206:oxalyl chloride
1202:
1175:
1170:
1153:
1148:
1134:
1129:
1124:
1122:
1119:
1118:
1112:
1108:
1105:
1101:
1095:
1087:
1083:
1066:
1062:
1055:
1024:
1019:
1008:
1003:
998:
990:
985:
970:
965:
954:
949:
941:
936:
931:
923:
918:
907:
902:
894:
889:
881:
876:
871:
863:
858:
853:
851:
848:
847:
797:
790:
785:
777:
772:
758:
753:
742:
737:
729:
724:
716:
711:
706:
704:
701:
700:
674:
669:
649:
644:
636:
631:
620:
615:
601:
596:
588:
583:
575:
570:
565:
563:
560:
559:
523:
518:
510:
505:
491:
486:
466:
461:
450:
445:
437:
435:
433:
430:
429:
415:
410:
366:
361:
350:
348:
345:
344:
312:
303:
291:
288:acetic acid (CH
287:
281:chlorocarbonyl-
219:
215:
196:
192:
176:
172:
160:
152:
136:
124:
106:
93:
89:
86:acetyl chloride
81:
72:, where R is a
69:
65:
35:
32:Acetyl chloride
28:
23:
22:
15:
12:
11:
5:
3341:
3331:
3330:
3325:
3323:Acyl chlorides
3310:
3309:
3302:
3284:
3275:
3241:
3235:978-0471264187
3234:
3218:Org. Reactions
3208:
3199:
3182:
3173:
3126:
3099:(3): 677–683.
3079:
3032:
3023:
2976:
2969:
2951:
2932:(2): 237–416.
2916:
2888:
2871:
2844:
2837:
2812:
2785:
2755:
2746:
2739:
2708:
2701:
2670:
2643:
2637:978-3527306732
2636:
2608:
2602:978-3527306732
2601:
2573:
2554:
2497:
2490:
2470:
2464:
2438:
2422:
2420:
2417:
2408:
2405:
2404:
2403:
2386:
2381:
2373:
2365:
2359:
2353:
2344:
2331:
2323:
2312:
2304:
2296:
2291:
2283:
2275:
2269:
2263:
2227:
2224:
2220:
2219:
2201:
2193:
2185:
2165:
2162:
2129:
2126:
2035:
2032:
2020:
2019:
2018:
2017:
2016:
2015:
1977:
1971:
1963:
1957:
1954:
1896:
1895:
1894:
1893:
1892:
1891:
1879:
1878:
1877:
1876:
1875:
1874:
1862:
1861:
1860:
1859:
1858:
1857:
1817:
1814:
1810:
1809:
1808:
1807:
1806:
1805:
1790:
1789:
1788:
1787:
1786:
1785:
1757:
1754:
1753:
1752:
1732:
1719:
1706:
1698:
1692:
1684:
1671:
1658:
1650:
1639:
1615:
1614:
1597:
1589:
1578:
1570:
1564:
1556:
1548:
1537:
1529:
1518:
1498:Appel reaction
1494:
1493:
1473:
1460:
1447:
1439:
1428:
1420:
1409:
1396:
1388:
1377:
1364:
1335:
1332:
1331:
1330:
1302:
1301:
1286:
1278:
1270:
1264:
1255:
1252:
1249:
1245:
1237:
1226:
1201:
1198:
1197:
1196:
1181:
1173:
1165:
1159:
1151:
1143:
1132:
1110:
1103:
1094:
1091:
1085:
1064:
1054:
1051:
1050:
1049:
1033:
1030:
1022:
1017:
1011:
1001:
988:
979:
976:
968:
963:
952:
944:
934:
921:
913:
905:
900:
892:
884:
874:
861:
833:
832:
816:
813:
806:
800:
788:
775:
767:
756:
748:
740:
727:
714:
690:
689:
672:
664:
658:
647:
634:
626:
618:
610:
599:
586:
573:
549:propionic acid
542:
541:
521:
508:
500:
489:
481:
475:
464:
459:
448:
440:
414:
411:
409:
406:
397:
396:
381:
375:
364:
356:
316:hydrogen bonds
311:
308:
307:
306:
301:
296:)acetic acid (
294:chlorocarbonyl
289:
238:
237:
217:
213:
202:
194:
190:
174:
170:
158:
150:
134:
105:
102:
91:
26:
9:
6:
4:
3:
2:
3340:
3329:
3326:
3324:
3321:
3320:
3318:
3305:
3299:
3295:
3288:
3279:
3265:on 2016-05-16
3264:
3260:
3256:
3252:
3245:
3237:
3231:
3227:
3223:
3219:
3212:
3203:
3196:
3186:
3177:
3169:
3165:
3161:
3157:
3153:
3149:
3145:
3141:
3137:
3130:
3122:
3118:
3114:
3110:
3106:
3102:
3098:
3094:
3090:
3083:
3075:
3071:
3067:
3063:
3059:
3055:
3051:
3047:
3043:
3036:
3027:
3019:
3015:
3011:
3007:
3003:
2999:
2995:
2991:
2987:
2980:
2972:
2966:
2962:
2955:
2947:
2943:
2939:
2935:
2931:
2927:
2920:
2912:
2908:
2904:
2901:
2900:
2892:
2885:
2881:
2875:
2867:
2863:
2859:
2855:
2848:
2840:
2838:0-13-643669-2
2834:
2830:
2823:
2821:
2819:
2817:
2808:
2804:
2800:
2796:
2789:
2781:
2777:
2773:
2769:
2762:
2760:
2750:
2742:
2740:0-19-850346-6
2736:
2732:
2727:
2726:
2717:
2715:
2713:
2704:
2702:9780470771273
2698:
2694:
2690:
2686:
2679:
2677:
2675:
2666:
2662:
2658:
2654:
2647:
2639:
2633:
2629:
2625:
2621:
2620:
2612:
2604:
2598:
2594:
2590:
2586:
2585:
2577:
2564:
2558:
2550:
2546:
2541:
2536:
2532:
2528:
2524:
2520:
2517:(10): o2460.
2516:
2512:
2508:
2501:
2493:
2487:
2483:
2482:
2474:
2467:
2465:9780471936237
2461:
2457:
2453:
2449:
2442:
2436:
2432:
2427:
2423:
2416:
2414:
2384:
2371:
2342:
2329:
2310:
2302:
2294:
2281:
2249:
2248:
2247:
2245:
2241:
2237:
2233:
2223:
2215:
2211:
2210:
2209:
2207:
2199:
2191:
2183:
2182:zinc chloride
2179:
2175:
2171:
2157:
2153:
2151:
2147:
2143:
2139:
2135:
2125:
2123:
2119:
2118:organocadmium
2115:
2111:
2107:
2102:
2100:
2096:
2095:organolithium
2091:
2087:
2078:
2074:
2072:
2067:
2065:
2061:
2057:
2053:
2049:
2045:
2041:
2031:
2029:
2025:
2013:
2009:
2008:
2007:
2006:
2005:
2004:
2003:
2001:
1997:
1993:
1988:
1986:
1981:
1975:
1967:
1953:
1951:
1947:
1943:
1939:
1935:
1932:Alcohols and
1930:
1928:
1923:
1921:
1917:
1913:
1909:
1905:
1900:
1889:
1885:
1884:
1883:
1882:
1881:
1880:
1872:
1868:
1867:
1866:
1865:
1864:
1863:
1855:
1851:
1850:
1849:
1848:
1847:
1846:
1845:
1843:
1839:
1835:
1831:
1827:
1823:
1813:
1803:
1799:
1798:
1797:
1796:
1795:
1794:
1793:
1783:
1779:
1778:
1777:
1776:
1775:
1774:
1773:
1771:
1767:
1763:
1730:
1717:
1704:
1696:
1682:
1669:
1656:
1648:
1637:
1624:
1623:
1622:
1620:
1595:
1587:
1576:
1568:
1554:
1546:
1535:
1527:
1516:
1503:
1502:
1501:
1499:
1471:
1458:
1445:
1437:
1426:
1407:
1394:
1386:
1375:
1362:
1349:
1348:
1347:
1345:
1341:
1328:
1324:
1323:
1322:
1319:
1315:
1312:, an iminium
1311:
1307:
1284:
1276:
1268:
1262:
1253:
1250:
1247:
1243:
1235:
1224:
1211:
1210:
1209:
1207:
1179:
1171:
1163:
1149:
1141:
1130:
1117:
1116:
1115:
1099:
1090:
1077:
1073:
1071:
1060:
1031:
1028:
1020:
999:
986:
977:
966:
950:
932:
919:
911:
903:
890:
872:
859:
846:
845:
844:
842:
838:
814:
811:
786:
773:
754:
746:
738:
725:
712:
699:
698:
697:
695:
670:
662:
656:
645:
632:
616:
608:
597:
584:
571:
558:
557:
556:
554:
550:
546:
519:
506:
498:
487:
473:
462:
446:
428:
427:
426:
424:
420:
401:
379:
362:
354:
343:
342:
341:
339:
334:
331:
329:
325:
321:
317:
299:
295:
286:
285:
284:
282:
277:
275:
273:
269:becomes acryl
268:
267:
262:
261:
257:becomes pival
256:
255:
250:
247:
243:
242:-oyl chloride
235:
231:
230:
229:
222:
211:
210:
209:
203:
199:
188:
187:
186:
179:
168:
167:
166:
159:
155:
148:
147:
146:
139:
132:
131:
130:
123:
122:
121:
119:
115:
111:
101:
99:
87:
79:
75:
64:
60:
56:
55:acid chloride
52:
51:acyl chloride
48:
39:
33:
19:
18:Acid chloride
3293:
3287:
3278:
3267:. Retrieved
3263:the original
3254:
3244:
3217:
3211:
3202:
3185:
3176:
3143:
3139:
3129:
3096:
3092:
3082:
3049:
3045:
3035:
3026:
2993:
2989:
2979:
2960:
2954:
2929:
2925:
2919:
2902:
2897:
2891:
2883:
2874:
2857:
2853:
2847:
2828:
2798:
2794:
2788:
2771:
2767:
2749:
2724:
2685:Acyl Halides
2684:
2656:
2652:
2646:
2617:
2611:
2582:
2576:
2557:
2514:
2510:
2500:
2480:
2473:
2447:
2441:
2426:
2413:lachrymators
2410:
2229:
2221:
2167:
2150:hydrogen gas
2131:
2103:
2083:
2068:
2063:
2060:acetophenone
2055:
2051:
2037:
2027:
2021:
1995:
1989:
1982:
1959:
1931:
1924:
1919:
1915:
1907:
1903:
1899:Acid halides
1897:
1819:
1811:
1791:
1759:
1616:
1495:
1337:
1314:intermediate
1303:
1203:
1096:
1081:
1056:
834:
691:
543:
416:
335:
332:
313:
297:
293:
280:
278:
272:oyl chloride
271:
270:
265:
264:
260:oyl chloride
259:
258:
253:
252:
248:
245:
244:substitutes
241:
239:
233:
227:
226:
220:
207:
206:
197:
184:
183:
177:
164:
163:
153:
144:
143:
137:
128:
127:
117:
114:-yl chloride
113:
107:
104:Nomenclature
98:acyl halides
54:
50:
44:
1966:2 mechanism
1344:acetic acid
292:COOH) → (
228:yl chloride
185:yl chloride
145:yl chloride
3317:Categories
3269:2009-02-19
3146:(4): 182.
2970:0124297854
2419:References
2180:– such as
2178:Lewis acid
2000:acylations
1902:chloride (
1762:anhydrides
310:Properties
74:side chain
3220:: 28–58.
3195:help page
3168:0021-9584
3113:0022-3263
3066:0022-3263
3010:0022-3263
2946:0009-2665
2322:⟶
2206:activated
2128:Reduction
2040:Grignards
1956:Mechanism
1756:Reactions
1691:⟶
1563:⟶
1419:⟶
1158:⟶
975:⟶
766:⟶
625:⟶
480:⟶
408:Synthesis
374:⟶
263:and acryl
82:R−C(=O)OH
61:with the
3121:11672060
3074:15471486
3018:11667754
2549:21577915
2099:chelated
2044:enolates
1992:pyridine
1950:pyridine
1914:species
1840:, or an
1244:→
1239:ClCOCOCl
839:to give
553:phosgene
246:-ic acid
120:. Thus:
118:-ic acid
66:−C(=O)Cl
57:) is an
3191:mcmurry
3148:Bibcode
2731:276–296
2540:2970283
2519:Bibcode
2407:Hazards
2168:In the
2114:alcohol
2110:ketones
1912:oxonium
1842:alcohol
266:ic acid
254:ic acid
208:ic acid
165:ic acid
129:ic acid
3300:
3232:
3166:
3119:
3111:
3072:
3064:
3016:
3008:
2967:
2944:
2886:, 2001
2860:: 51.
2835:
2801:: 75.
2774:: 16.
2737:
2699:
2659:: 32.
2634:
2599:
2569:
2547:
2537:
2488:
2462:
2339:COIrCl
2196:), or
2148:using
1942:amides
1938:esters
1934:amines
1830:esters
1826:amides
1770:amides
1766:esters
223:) →
180:) →
140:) →
110:moiety
70:R−COCl
2200:(AlCl
2192:(FeCl
2184:(ZnCl
1838:amine
1836:, an
1694:RCOCl
1566:RCOCl
1260:RCOCl
1161:RCOCl
551:with
421:with
377:RCOOH
352:RCOCl
304:COOH)
232:(C3H7
225:butyr
205:butyr
182:benzo
162:benzo
49:, an
3298:ISBN
3230:ISBN
3164:ISSN
3117:PMID
3109:ISSN
3070:PMID
3062:ISSN
3014:PMID
3006:ISSN
2965:ISBN
2942:ISSN
2833:ISBN
2735:ISBN
2697:ISBN
2632:ISBN
2597:ISBN
2545:PMID
2486:ISBN
2460:ISBN
2319:COCl
2258:IrCl
2176:. A
2136:and
2042:and
1940:and
1828:and
1592:HCCl
1435:COCl
1416:COCl
1342:and
1168:POCl
1063:SOCl
1013:COCl
654:COCl
613:COCl
496:COCl
298:ClOC
234:COCl
221:COOH
198:COCl
178:COOH
154:COCl
142:acet
138:COOH
126:acet
116:for
94:COCl
53:(or
3222:doi
3156:doi
3101:doi
3054:doi
2998:doi
2934:doi
2907:doi
2862:doi
2803:doi
2776:doi
2689:doi
2661:doi
2624:doi
2589:doi
2535:PMC
2527:doi
2452:doi
2368:PPh
2278:PPh
2242:to
2238:of
2188:),
2030:.
1996:N,N
1994:or
1768:or
1634:RCO
1551:CCl
1513:RCO
1288:HCl
1221:RCO
1183:HCl
1146:PCl
1127:RCO
1109:PCl
1102:PCl
1036:HCl
887:CCl
819:HCl
735:CCl
660:HCl
477:HCl
425::
383:HCl
149:(CH
133:(CH
45:In
3319::
3257:.
3253:.
3228:.
3197:).
3162:.
3154:.
3144:36
3142:.
3138:.
3115:.
3107:.
3097:63
3095:.
3091:.
3068:.
3060:.
3050:69
3048:.
3044:.
3012:.
3004:.
2994:61
2992:.
2988:.
2940:.
2930:52
2928:.
2903:20
2858:20
2856:.
2815:^
2797:.
2772:12
2770:.
2758:^
2733:.
2711:^
2695:.
2673:^
2655:.
2630:.
2595:.
2543:.
2533:.
2525:.
2515:65
2513:.
2509:.
2458:,
2433:,
2355:CO
2326:CH
2307:CH
2265:CO
1824:,
1772::
1764:,
1739:OH
1727:Cl
1679:Cl
1621::
1585:PO
1573:Ph
1532:Ph
1500::
1468:CO
1423:CH
1372:CO
1359:CH
1346::
1273:CO
1266:CO
1208::
1084:SO
947:CO
843::
809:Cl
696::
667:CO
642:CH
629:CH
594:CO
581:CH
568:CH
555::
516:CO
503:CH
484:CH
455:CO
443:CH
340::
326:,
300:CH
283::
212:(C
189:(C
169:(C
100:.
90:CH
88:,
3306:.
3272:.
3238:.
3224::
3170:.
3158::
3150::
3123:.
3103::
3076:.
3056::
3020:.
3000::
2973:.
2948:.
2936::
2913:.
2909::
2878:"
2868:.
2864::
2841:.
2809:.
2805::
2799:3
2782:.
2778::
2743:.
2705:.
2691::
2667:.
2663::
2657:9
2640:.
2626::
2605:.
2591::
2551:.
2529::
2521::
2494:.
2454::
2385:2
2380:)
2372:3
2364:(
2358:)
2352:(
2343:2
2330:3
2311:3
2303:+
2295:2
2290:)
2282:3
2274:(
2268:)
2262:(
2202:3
2194:3
2186:2
2064:2
2062:(
2056:3
2052:1
2050:(
1978:N
1974:1
1972:N
1970:S
1964:N
1962:S
1920:4
1916:3
1908:2
1904:1
1731:2
1718:3
1714:N
1705:3
1701:C
1697:+
1683:3
1670:3
1666:N
1657:3
1653:C
1649:+
1646:H
1638:2
1596:3
1588:+
1577:3
1569:+
1555:4
1547:+
1544:P
1536:3
1528:+
1525:H
1517:2
1480:H
1472:2
1459:5
1455:H
1446:6
1442:C
1438:+
1427:3
1408:5
1404:H
1395:6
1391:C
1387:+
1384:H
1376:2
1363:3
1285:+
1277:2
1269:+
1263:+
1254:F
1251:M
1248:D
1236:+
1233:H
1225:2
1180:+
1172:3
1164:+
1150:5
1142:+
1139:H
1131:2
1111:5
1104:3
1100:(
1086:2
1065:2
1061:(
1032:2
1029:+
1021:2
1016:)
1010:(
1000:4
996:H
987:6
983:C
978:2
967:2
962:)
959:H
951:2
943:(
933:4
929:H
920:6
916:C
912:+
904:2
899:)
891:3
883:(
873:4
869:H
860:6
856:C
815:2
812:+
805:)
802:O
799:(
795:C
787:5
783:H
774:6
770:C
763:O
755:2
751:H
747:+
739:3
726:5
722:H
713:6
709:C
671:2
663:+
657:+
646:2
633:3
617:2
609:+
606:H
598:2
585:2
572:3
528:H
520:2
507:3
499:+
488:3
474:+
471:O
463:2
458:)
447:3
439:(
380:+
371:O
363:2
359:H
355:+
302:2
290:3
274:.
249:.
236:)
218:7
216:H
214:3
200:)
195:5
193:H
191:6
175:5
173:H
171:6
156:)
151:3
135:3
92:3
80:(
34:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.