48:
820:
substrates cause an opposite change in spectral properties, a "reverse type I" spectrum, by processes that are as yet unclear. Inhibitors and certain substrates that bind directly to the heme iron give rise to the type II difference spectrum, with a maximum at 430 nm and a minimum at 390 nm (see inset graph in figure). If no reducing equivalents are available, this complex may remain stable, allowing the degree of binding to be determined from absorbance measurements
837:
672:
791:
785:
Depending on the substrate and enzyme involved, P450 enzymes can catalyze any of a wide variety of reactions. A hypothetical hydroxylation is illustrated. After the hydroxylated product has been released from the active site, the enzyme returns to its original state, with a water molecule returning
824:
C: If carbon monoxide (CO) binds to reduced P450, the catalytic cycle is interrupted. This reaction yields the classic CO difference spectrum with a maximum at 450 nm. However, the interruptive and inhibitory effects of CO varies upon different CYPs such that the CYP3A family is relatively less
819:
Binding of substrate is reflected in the spectral properties of the enzyme, with an increase in absorbance at 390 nm and a decrease at 420 nm. This can be measured by difference spectroscopies and is referred to as the "type I" difference spectrum (see inset graph in figure). Some
719:, on the side opposite to the axial thiolate. Substrate binding induces a change in the conformation of the active site, often displacing a water molecule from the distal axial coordination position of the heme iron, and changing the state of the heme iron from low-spin to high-spin.
802:
An alternative route for mono-oxygenation is via the "peroxide shunt" (path "S" in figure). This pathway entails oxidation of the ferric-substrate complex with oxygen-atom donors such as peroxides and hypochlorites. A hypothetical peroxide "XOOH" is shown in the
398:
is used synonymously. These names should never be used as according to the nomenclature convention (as they denote a P450 in family number 450). However, some gene or enzyme names for P450s are also referred to by historical names (e.g.
627:
The most common reaction catalyzed by cytochromes P450 is a monooxygenase reaction, e.g., insertion of one atom of oxygen into the aliphatic position of an organic substrate (RH), while the other oxygen atom is
1481:
Smith AT, Pazicni S, Marvin KA, Stevens DJ, Paulsen KM, Burstyn JN (April 2015). "Functional divergence of heme-thiolate proteins: a classification based on spectroscopic attributes".
211:
620:
2885:
1175:
663:
also rely on an Fe=O intermediate but lack hemes. Methane monooxygenase, which converts methane to methanol, are non-heme iron-and iron-copper-based enzymes.
292:, and participate in many biosyntheses. By hydroxylation, CYP450 enzymes convert xenobiotics into hydrophilic derivatives, which are more readily excreted.
797:
utilized by cytochrome P450 for conversion of hydrocarbons to alcohols via the action of "compound I", an iron(IV) oxide bound to a heme radical cation.
2215:
762:
The peroxo group formed in step 4 is rapidly protonated twice, releasing one molecule of water and forming the highly reactive species referred to as
3350:
468:
identity, while members of subfamilies must share at least 55% amino-acid identity. Nomenclature committees assign and track both base gene names (
2794:
143:
131:
2934:
509:
3106:
660:
3088:
3084:
2424:
2247:
850:
403:
for CYP102A1) or functional names, denoting the catalytic activity and the name of the compound used as substrate. Examples include
2773:
2293:
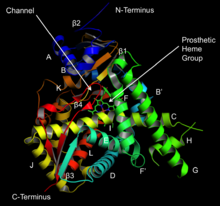
1276:
Sligar SG, Cinti DL, Gibson GG, Schenkman JB (October 1979). "Spin state control of the hepatic cytochrome P450 redox potential".
17:
3357:
3066:
2590:
1534:
1314:
Rittle J, Green MT (November 2010). "Cytochrome P450 Compound I: Capture, Characterization, and C-H Bond
Activation Kinetics".
1377:
1109:
1082:
111:
1442:
Hopper CP, Zambrana PN, Goebel U, Wollborn J (June 2021). "A brief history of carbon monoxide and its therapeutic origins".
1198:
Meunier B, de Visser SP, Shaik S (September 2004). "Mechanism of oxidation reactions catalyzed by cytochrome p450 enzymes".
231:
3453:
3010:
2926:
472:
31:
733:
Molecular oxygen binds to the resulting ferrous heme center at the distal axial coordination position, initially giving a
3818:
3251:
2950:
940:
Danielson PB (December 2002). "The cytochrome P450 superfamily: biochemistry, evolution and drug metabolism in humans".
868:
Estabrook RW (December 2003). "A passion for P450s (Remembrances of the early history of research on cytochrome P450)".
766:(or just Compound I). This highly reactive intermediate was isolated in 2010, P450 Compound 1 is an iron(IV) oxo (or
3519:
2990:
734:
2225:
3329:
3127:
2968:
2847:
2546:
3313:
3120:
365:, a capital letter indicating the subfamily, and another numeral for the individual gene. The convention is to
219:
3675:
3024:
2994:
2954:
2942:
2240:
975:
3178:
3092:
3080:
688:
The active site of cytochrome P450 contains a heme-iron center. The iron is tethered to the protein via a
3230:
2930:
2598:
855:
1241:
Poulos TL, Finzel BC, Howard AJ (June 1987). "High-resolution crystal structure of cytochrome P450cam".
47:
3113:
3052:
3048:
3036:
3032:
3028:
1527:
698:. This cysteine and several flanking residues are highly conserved in known P450s, and have the formal
215:
3808:
3776:
3763:
3750:
3737:
3724:
3711:
3698:
3660:
3416:
3301:
3070:
741:
723:
513:
449:, lanosterol 14-α-demethylase, sometimes unofficially abbreviated to LDM according to its substrate (
351:
155:
124:
501:
Based on the nature of the electron transfer proteins, P450s can be classified into several groups:
160:
3798:
3670:
3624:
3567:
3485:
3223:
3170:
3165:
3020:
2871:
2268:
2233:
1602:
808:
794:
3572:
3272:
3145:
1680:
1624:
528:
496:
312:
300:
267:
1661:
464:
The current nomenclature guidelines suggest that members of new CYP families share at least 40%
3099:
3044:
3040:
3006:
2938:
2520:
416:
3593:
3512:
3336:
2842:
1520:
616:
598:
546:
1512:
3665:
3294:
3191:
3062:
2961:
2602:
1805:
1791:
1777:
1763:
1323:
1046:
Nelson DR (January 2011). "Progress in tracing the evolutionary paths of cytochrome P450".
612:
560:
198:
811:, have been investigated with synthetic analogues, consisting of iron oxo heme complexes.
8:
3813:
3629:
3446:
3152:
2980:
2071:
2041:
1965:
1607:
358:
255:
167:
1327:
1151:
1126:
675:
The "Fe(V) intermediate" at the bottom left is a simplification: it is an Fe(IV) with a
3562:
2946:
2055:
1737:
1463:
1419:
1394:
1347:
1223:
1023:
996:
893:
676:
1395:"Oxygen Activation and Radical Transformations in Heme Proteins and Metalloporphyrins"
1101:
136:
2255:
2036:
1931:
1498:
1467:
1455:
1424:
1373:
1351:
1339:
1293:
1289:
1258:
1254:
1215:
1173:
1156:
1105:
1063:
1028:
957:
885:
304:
296:
206:
116:
1227:
897:
80:
3803:
3608:
3603:
3577:
3505:
1941:
1900:
1830:
1490:
1447:
1414:
1406:
1331:
1285:
1250:
1207:
1146:
1138:
1097:
1055:
1018:
1008:
949:
877:
779:
276:
194:
172:
148:
3655:
3639:
3552:
3395:
2050:
1179:
1059:
476:
324:
92:
1410:
1142:
922:
469:
3693:
3634:
2703:
2097:
2092:
1451:
1127:"Direct Methane Oxidation by Copper- and Iron-Dependent Methane Monooxygenases"
842:
274:. However, they are not omnipresent; for example, they have not been found in
3792:
3598:
3557:
3478:
3382:
3343:
3265:
3258:
3244:
3204:
3185:
3159:
2202:
2161:
2076:
1883:
1665:
1648:
1587:
1013:
953:
749:
652:
517:
271:
1335:
3547:
3001:
2975:
2879:
2865:
2858:
2764:
2744:
2737:
2720:
2662:
2645:
2638:
2631:
2624:
2617:
2585:
2578:
2190:
2131:
2007:
1993:
1979:
1869:
1844:
1502:
1459:
1428:
1343:
1219:
1160:
1067:
1032:
961:
889:
881:
527:) can also contribute reducing power to this system after being reduced by
350:
encoding P450 enzymes, and the enzymes themselves, are designated with the
1262:
120:
3771:
3706:
3542:
2571:
2527:
2138:
1551:
1297:
771:
593:
550:
408:
380:
367:
362:
2256:
2185:
2143:
2046:
1569:
745:
716:
564:
465:
308:
289:
285:
104:
1494:
1211:
387:
nomenclature is the official naming convention, although occasionally
3745:
3719:
3471:
2672:
2260:
2126:
775:
727:
602:
316:
3428:
3388:
3375:
3320:
3287:
3237:
3215:
3210:
3196:
836:
692:
689:
656:
611:
which do not require external reducing power. Notable ones include
328:
87:
706:- - . In general, the P450 catalytic cycle proceeds as follows:
581:
in which both electrons required by the CYP come from cytochrome b
2897:
2824:
2814:
2804:
2787:
2777:
2769:
2757:
2753:
2749:
2730:
2682:
2655:
2610:
2606:
2594:
2485:
2480:
2475:
2450:
2445:
2429:
2349:
2344:
2324:
1543:
1182:
699:
446:
281:
99:
3758:
3528:
2564:
2560:
2550:
2536:
2532:
2500:
2495:
2490:
2470:
2465:
2460:
2455:
2419:
2414:
2409:
2389:
2384:
2379:
2374:
2369:
2364:
2359:
2354:
2339:
2334:
2329:
2319:
2314:
2298:
2288:
2283:
1560:
786:
to occupy the distal coordination position of the iron nucleus.
767:
695:
480:
404:
376:
336:
259:
226:
327:. Most P450s require a protein partner to deliver one or more
3732:
2851:
2713:
2400:
1612:
1542:
1372:(3rd ed.). New York: Kluwer Academic/Plenum Publishers.
1048:
Biochimica et
Biophysica Acta (BBA) - Proteins and Proteomics
778:
and thiolate ligands. Evidence for the alternative perferryl
722:
Substrate binding induces electron transfer from NAD(P)H via
659:
groups) use CYP enzymes, but many other hydroxylases exist.
629:
484:
320:
53:
1441:
671:
2905:
1721:
1709:
1704:
1636:
1547:
347:
332:
263:
188:
75:
3497:
1275:
790:
1951:
1692:
1480:
702:
signature consensus pattern - - x - - {F} - - {P} -
1370:
Cytochrome P450: structure, mechanism, and biochemistry
1083:"Electron transfer proteins of cytochrome P450 systems"
295:
P450s are, in general, the terminal oxidase enzymes in
1197:
832:
1367:
1278:
1240:
770:) species with an additional oxidizing equivalent
371:the name when referring to the gene. For example,
3790:
646:
1193:
1191:
1124:
740:A second electron is transferred, from either
270:that mostly, but not exclusively, function as
3513:
2241:
1528:
1074:
1363:
1361:
1188:
1392:
1313:
933:
3520:
3506:
2248:
2234:
1535:
1521:
1092:. Advances in Molecular and Cell Biology.
759:adduct to give a short-lived peroxo state.
661:Alpha-ketoglutarate-dependent hydroxylases
46:
2216:disorders of globin and globulin proteins
1418:
1358:
1309:
1307:
1150:
1125:Tucci FJ, Rosenzweig AC (February 2024).
1080:
1022:
1012:
939:
867:
851:Cytochrome P450 oxidoreductase deficiency
553:to transfer electrons from NADPH to P450.
52:Structure of lanosterol 14α-demethylase (
1234:
789:
670:
508:in which electrons are transferred from
1269:
14:
3791:
1304:
1045:
994:
361:, followed by a number indicating the
303:. The term "P450" is derived from the
3501:
2229:
1516:
917:
915:
715:Substrate binds in proximity to the
375:is the gene that encodes the enzyme
280:. In mammals, these enzymes oxidize
32:Cytochrome P450 (individual enzymes)
807:Mechanistic details, including the
24:
861:
709:
25:
3830:
1393:Huang X, Groves JT (March 2018).
912:
490:
485:CYP Allele Nomenclature Committee
835:
516:(variously CPR, POR, or CYPOR).
383:(acetaminophen) metabolism. The
379:—one of the enzymes involved in
1474:
1435:
1386:
1368:Ortiz de Montellano PR (2005).
870:Drug Metabolism and Disposition
814:
730:, converting Fe(III) to Fe(II).
342:
299:chains, broadly categorized as
1167:
1118:
1039:
997:"The cytochrome p450 homepage"
988:
968:
567:to transfer electrons to P450.
13:
1:
1102:10.1016/S1569-2558(08)60339-2
905:
647:Related hydroxylation enzymes
183:Available protein structures:
1290:10.1016/0006-291X(79)91916-8
1255:10.1016/0022-2836(87)90190-2
1243:Journal of Molecular Biology
1060:10.1016/j.bbapap.2010.08.008
683:
666:
7:
3527:
1411:10.1021/acs.chemrev.7b00373
1143:10.1021/acs.chemrev.3c00727
856:Cytochrome P450 engineering
828:
10:
3835:
3819:Integral membrane proteins
1452:10.1016/j.niox.2021.04.001
1185:consensus pattern for P450
995:Nelson DR (October 2009).
542:Mitochondrial P450 systems
494:
335:(and eventually molecular
29:
3684:
3676:Michaelis–Menten kinetics
3648:
3617:
3586:
3535:
3463:
3438:
3367:
3137:
2915:
2834:
2692:
2509:
2438:
2398:
2307:
2276:
2211:
2178:
2152:
2117:
2110:
2085:
2064:
2029:
1922:
1862:
1823:
1756:
1745:
1736:
1659:
1584:
1577:
1568:
1559:
742:cytochrome P450 reductase
737:similar to oxy-myoglobin.
724:cytochrome P450 reductase
514:cytochrome P450 reductase
453:anosterol) and activity (
415:synthase, abbreviated to
323:state and complexed with
225:
205:
187:
182:
178:
166:
154:
142:
130:
110:
98:
86:
74:
66:
61:
45:
40:
3568:Diffusion-limited enzyme
1014:10.1186/1479-7364-4-1-59
954:10.2174/1389200023337054
809:oxygen rebound mechanism
795:Oxygen rebound mechanism
655:reactions (insertion of
470:Cytochrome P450 Homepage
315:of the enzyme (450
1336:10.1126/science.1193478
942:Current Drug Metabolism
505:Microsomal P450 systems
497:P450-containing systems
301:P450-containing systems
18:Cytochrome P450 oxidase
976:"NCBI sequence viewer"
882:10.1124/dmd.31.12.1461
798:
726:or another associated
680:
556:Bacterial P450 systems
3661:Eadie–Hofstee diagram
3594:Allosteric regulation
793:
674:
639:+ NADPH + H → ROH + H
621:allene oxide synthase
617:prostacyclin synthase
547:adrenodoxin reductase
30:Further information:
3671:Lineweaver–Burk plot
1081:Hanukoglu I (1996).
619:(CYP8), and CYP74A (
613:thromboxane synthase
605:is fused to the CYP.
597:species, in which a
591:originally found in
561:ferredoxin reductase
319:) when it is in the
2072:Glycated hemoglobin
2042:Carbaminohemoglobin
1328:2010Sci...330..933R
1090:Adv. Mol. Cell Biol
755:, reducing the Fe-O
677:radical heme ligand
601:-domain-containing
588:FMN/Fd/P450 systems
3630:Enzyme superfamily
3563:Enzyme promiscuity
1446:. 111–112: 45–63.
1178:2019-10-18 at the
799:
681:
475:2010-06-27 at the
313:absorption maximum
305:spectrophotometric
3786:
3785:
3495:
3494:
2223:
2222:
2174:
2173:
2170:
2169:
2106:
2105:
2037:Carboxyhemoglobin
2025:
2024:
1918:
1917:
1732:
1731:
1495:10.1021/cr500056m
1379:978-0-306-48324-0
1322:(6006): 933–937.
1212:10.1021/cr020443g
1111:978-0-7623-0113-3
923:"Cytochrome P450"
876:(12): 1461–1473.
608:P450 only systems
297:electron transfer
241:
240:
237:
236:
232:structure summary
16:(Redirected from
3826:
3809:Pharmacokinetics
3666:Hanes–Woolf plot
3609:Enzyme activator
3604:Enzyme inhibitor
3578:Enzyme catalysis
3522:
3515:
3508:
3499:
3498:
2267:(Most belong to
2250:
2243:
2236:
2227:
2226:
2115:
2114:
1754:
1753:
1743:
1742:
1582:
1581:
1575:
1574:
1566:
1565:
1537:
1530:
1523:
1514:
1513:
1507:
1506:
1489:(7): 2532–2558.
1483:Chemical Reviews
1478:
1472:
1471:
1439:
1433:
1432:
1422:
1405:(5): 2491–2553.
1399:Chemical Reviews
1390:
1384:
1383:
1365:
1356:
1355:
1311:
1302:
1301:
1273:
1267:
1266:
1238:
1232:
1231:
1206:(9): 3947–3980.
1200:Chemical Reviews
1195:
1186:
1171:
1165:
1164:
1154:
1137:(3): 1288–1320.
1131:Chemical Reviews
1122:
1116:
1115:
1087:
1078:
1072:
1071:
1043:
1037:
1036:
1026:
1016:
992:
986:
985:
983:
982:
972:
966:
965:
937:
931:
930:
919:
901:
845:
840:
839:
277:Escherichia coli
244:Cytochromes P450
180:
179:
50:
38:
37:
27:Class of enzymes
21:
3834:
3833:
3829:
3828:
3827:
3825:
3824:
3823:
3799:Cytochrome P450
3789:
3788:
3787:
3782:
3694:Oxidoreductases
3680:
3656:Enzyme kinetics
3644:
3640:List of enzymes
3613:
3582:
3553:Catalytic triad
3531:
3526:
3496:
3491:
3459:
3434:
3396:Halloween genes
3363:
3133:
2911:
2830:
2688:
2505:
2434:
2394:
2303:
2272:
2265:cytochrome P450
2254:
2224:
2219:
2207:
2196:Cytochrome P450
2166:
2148:
2102:
2081:
2060:
2051:Deoxyhemoglobin
2021:
2017:
2013:
2003:
1999:
1989:
1985:
1975:
1971:
1961:
1957:
1947:
1937:
1914:
1910:
1906:
1896:
1892:
1887:
1879:
1875:
1858:
1854:
1850:
1840:
1836:
1819:
1815:
1811:
1806:HbE Portland II
1801:
1797:
1787:
1783:
1773:
1769:
1748:
1728:
1655:
1586:Alpha locus on
1555:
1541:
1511:
1510:
1479:
1475:
1440:
1436:
1391:
1387:
1380:
1366:
1359:
1312:
1305:
1274:
1270:
1239:
1235:
1196:
1189:
1180:Wayback Machine
1172:
1168:
1123:
1119:
1112:
1085:
1079:
1075:
1044:
1040:
993:
989:
980:
978:
974:
973:
969:
938:
934:
921:
920:
913:
908:
864:
862:Further reading
841:
834:
831:
817:
764:P450 Compound 1
758:
753:
735:dioxygen adduct
712:
710:Catalytic cycle
686:
669:
649:
644:
642:
638:
584:
577:
573:
559:which employ a
538:
532:
526:
521:
499:
493:
477:Wayback Machine
437:
414:
402:
396:
345:
325:carbon monoxide
132:OPM superfamily
57:
41:Cytochrome P450
34:
28:
23:
22:
15:
12:
11:
5:
3832:
3822:
3821:
3816:
3811:
3806:
3801:
3784:
3783:
3781:
3780:
3767:
3754:
3741:
3728:
3715:
3702:
3688:
3686:
3682:
3681:
3679:
3678:
3673:
3668:
3663:
3658:
3652:
3650:
3646:
3645:
3643:
3642:
3637:
3632:
3627:
3621:
3619:
3618:Classification
3615:
3614:
3612:
3611:
3606:
3601:
3596:
3590:
3588:
3584:
3583:
3581:
3580:
3575:
3570:
3565:
3560:
3555:
3550:
3545:
3539:
3537:
3533:
3532:
3525:
3524:
3517:
3510:
3502:
3493:
3492:
3490:
3489:
3482:
3475:
3467:
3465:
3461:
3460:
3458:
3457:
3450:
3442:
3440:
3436:
3435:
3433:
3432:
3425:
3393:
3392:
3391:
3379:
3371:
3369:
3365:
3364:
3362:
3361:
3354:
3347:
3340:
3333:
3326:
3325:
3324:
3317:
3305:
3298:
3291:
3284:
3283:
3282:
3279:
3269:
3262:
3255:
3248:
3241:
3234:
3227:
3220:
3219:
3218:
3213:
3201:
3200:
3199:
3194:
3182:
3175:
3174:
3173:
3168:
3156:
3149:
3141:
3139:
3135:
3134:
3132:
3131:
3124:
3117:
3110:
3103:
3096:
3074:
3056:
3014:
2998:
2984:
2972:
2965:
2958:
2919:
2917:
2913:
2912:
2910:
2909:
2902:
2901:
2900:
2890:
2889:
2888:
2876:
2875:
2874:
2862:
2855:
2838:
2836:
2832:
2831:
2829:
2828:
2818:
2808:
2798:
2791:
2781:
2761:
2741:
2734:
2724:
2717:
2707:
2696:
2694:
2690:
2689:
2687:
2686:
2676:
2666:
2659:
2649:
2642:
2635:
2628:
2621:
2614:
2582:
2575:
2568:
2554:
2540:
2524:
2513:
2511:
2507:
2506:
2504:
2503:
2498:
2493:
2488:
2483:
2478:
2473:
2468:
2463:
2458:
2453:
2448:
2442:
2440:
2436:
2435:
2433:
2432:
2427:
2422:
2417:
2412:
2406:
2404:
2396:
2395:
2393:
2392:
2387:
2382:
2377:
2372:
2367:
2362:
2357:
2352:
2347:
2342:
2337:
2332:
2327:
2322:
2317:
2311:
2309:
2305:
2304:
2302:
2301:
2296:
2291:
2286:
2280:
2278:
2274:
2273:
2253:
2252:
2245:
2238:
2230:
2221:
2220:
2212:
2209:
2208:
2206:
2205:
2200:
2199:
2198:
2193:
2182:
2180:
2176:
2175:
2172:
2171:
2168:
2167:
2165:
2164:
2158:
2156:
2150:
2149:
2147:
2146:
2141:
2136:
2135:
2134:
2123:
2121:
2112:
2108:
2107:
2104:
2103:
2101:
2100:
2098:Erythrocruorin
2095:
2089:
2087:
2083:
2082:
2080:
2079:
2074:
2068:
2066:
2062:
2061:
2059:
2058:
2056:Sulfhemoglobin
2053:
2044:
2039:
2033:
2031:
2027:
2026:
2023:
2022:
2020:
2019:
2015:
2011:
2005:
2001:
1997:
1991:
1987:
1983:
1977:
1973:
1969:
1963:
1959:
1955:
1949:
1945:
1939:
1935:
1928:
1926:
1920:
1919:
1916:
1915:
1913:
1912:
1908:
1904:
1898:
1894:
1890:
1885:
1881:
1877:
1873:
1866:
1864:
1860:
1859:
1857:
1856:
1852:
1848:
1842:
1838:
1834:
1827:
1825:
1821:
1820:
1818:
1817:
1813:
1809:
1803:
1799:
1795:
1792:HbE Portland I
1789:
1785:
1781:
1775:
1771:
1767:
1760:
1758:
1751:
1740:
1734:
1733:
1730:
1729:
1727:
1726:
1725:
1724:
1714:
1713:
1712:
1707:
1697:
1696:
1695:
1685:
1684:
1683:
1672:
1670:
1657:
1656:
1654:
1653:
1652:
1651:
1641:
1640:
1639:
1629:
1628:
1627:
1617:
1616:
1615:
1610:
1605:
1594:
1592:
1579:
1572:
1563:
1557:
1556:
1540:
1539:
1532:
1525:
1517:
1509:
1508:
1473:
1434:
1385:
1378:
1357:
1303:
1284:(3): 925–932.
1268:
1249:(3): 687–700.
1233:
1187:
1166:
1117:
1110:
1073:
1038:
1001:Human Genomics
987:
967:
948:(6): 561–597.
932:
910:
909:
907:
904:
903:
902:
863:
860:
859:
858:
853:
847:
846:
843:Biology portal
830:
827:
816:
813:
805:
804:
788:
787:
783:
760:
756:
751:
738:
731:
720:
711:
708:
685:
682:
668:
665:
648:
645:
640:
636:
634:
625:
624:
609:
606:
589:
586:
582:
579:
575:
571:
568:
557:
554:
543:
540:
536:
530:
524:
519:
506:
495:Main article:
492:
491:Classification
489:
435:
412:
400:
394:
344:
341:
331:to reduce the
272:monooxygenases
239:
238:
235:
234:
229:
223:
222:
209:
203:
202:
192:
185:
184:
176:
175:
170:
164:
163:
158:
152:
151:
146:
140:
139:
134:
128:
127:
114:
108:
107:
102:
96:
95:
90:
84:
83:
78:
72:
71:
68:
64:
63:
59:
58:
51:
43:
42:
26:
9:
6:
4:
3:
2:
3831:
3820:
3817:
3815:
3812:
3810:
3807:
3805:
3802:
3800:
3797:
3796:
3794:
3778:
3774:
3773:
3768:
3765:
3761:
3760:
3755:
3752:
3748:
3747:
3742:
3739:
3735:
3734:
3729:
3726:
3722:
3721:
3716:
3713:
3709:
3708:
3703:
3700:
3696:
3695:
3690:
3689:
3687:
3683:
3677:
3674:
3672:
3669:
3667:
3664:
3662:
3659:
3657:
3654:
3653:
3651:
3647:
3641:
3638:
3636:
3635:Enzyme family
3633:
3631:
3628:
3626:
3623:
3622:
3620:
3616:
3610:
3607:
3605:
3602:
3600:
3599:Cooperativity
3597:
3595:
3592:
3591:
3589:
3585:
3579:
3576:
3574:
3571:
3569:
3566:
3564:
3561:
3559:
3558:Oxyanion hole
3556:
3554:
3551:
3549:
3546:
3544:
3541:
3540:
3538:
3534:
3530:
3523:
3518:
3516:
3511:
3509:
3504:
3503:
3500:
3488:
3487:
3483:
3481:
3480:
3476:
3474:
3473:
3469:
3468:
3466:
3462:
3456:
3455:
3451:
3449:
3448:
3444:
3443:
3441:
3437:
3431:
3430:
3426:
3423:
3419:
3418:
3413:
3409:
3405:
3401:
3397:
3394:
3390:
3387:
3386:
3385:
3384:
3380:
3378:
3377:
3373:
3372:
3370:
3366:
3360:
3359:
3355:
3353:
3352:
3348:
3346:
3345:
3341:
3339:
3338:
3334:
3332:
3331:
3327:
3323:
3322:
3318:
3316:
3315:
3311:
3310:
3309:
3306:
3304:
3303:
3299:
3297:
3296:
3292:
3290:
3289:
3285:
3280:
3277:
3276:
3275:
3274:
3270:
3268:
3267:
3263:
3261:
3260:
3256:
3254:
3253:
3249:
3247:
3246:
3242:
3240:
3239:
3235:
3233:
3232:
3228:
3226:
3225:
3221:
3217:
3214:
3212:
3209:
3208:
3207:
3206:
3202:
3198:
3195:
3193:
3190:
3189:
3188:
3187:
3183:
3181:
3180:
3176:
3172:
3169:
3167:
3164:
3163:
3162:
3161:
3157:
3155:
3154:
3150:
3148:
3147:
3143:
3142:
3140:
3136:
3130:
3129:
3125:
3123:
3122:
3118:
3116:
3115:
3111:
3109:
3108:
3104:
3102:
3101:
3097:
3094:
3090:
3086:
3082:
3078:
3075:
3072:
3068:
3064:
3060:
3057:
3054:
3050:
3046:
3042:
3038:
3034:
3030:
3026:
3022:
3018:
3015:
3012:
3008:
3004:
3003:
2999:
2996:
2992:
2988:
2985:
2982:
2978:
2977:
2973:
2971:
2970:
2966:
2964:
2963:
2959:
2956:
2952:
2948:
2944:
2940:
2936:
2932:
2928:
2924:
2921:
2920:
2918:
2914:
2908:
2907:
2903:
2899:
2896:
2895:
2894:
2891:
2887:
2884:
2883:
2882:
2881:
2877:
2873:
2870:
2869:
2868:
2867:
2863:
2861:
2860:
2856:
2853:
2849:
2845:
2844:
2840:
2839:
2837:
2833:
2826:
2822:
2819:
2816:
2812:
2809:
2806:
2802:
2799:
2797:
2796:
2792:
2789:
2785:
2782:
2779:
2775:
2771:
2767:
2766:
2762:
2759:
2755:
2751:
2747:
2746:
2742:
2740:
2739:
2735:
2732:
2728:
2725:
2723:
2722:
2718:
2715:
2711:
2708:
2705:
2701:
2698:
2697:
2695:
2691:
2684:
2680:
2677:
2674:
2670:
2667:
2665:
2664:
2660:
2657:
2653:
2650:
2648:
2647:
2643:
2641:
2640:
2636:
2634:
2633:
2629:
2627:
2626:
2622:
2620:
2619:
2615:
2612:
2608:
2604:
2600:
2596:
2592:
2588:
2587:
2583:
2581:
2580:
2576:
2574:
2573:
2569:
2566:
2562:
2558:
2555:
2552:
2548:
2544:
2541:
2538:
2534:
2530:
2529:
2525:
2522:
2518:
2515:
2514:
2512:
2508:
2502:
2499:
2497:
2494:
2492:
2489:
2487:
2484:
2482:
2479:
2477:
2474:
2472:
2469:
2467:
2464:
2462:
2459:
2457:
2454:
2452:
2449:
2447:
2444:
2443:
2441:
2437:
2431:
2428:
2426:
2423:
2421:
2418:
2416:
2413:
2411:
2408:
2407:
2405:
2402:
2397:
2391:
2388:
2386:
2383:
2381:
2378:
2376:
2373:
2371:
2368:
2366:
2363:
2361:
2358:
2356:
2353:
2351:
2348:
2346:
2343:
2341:
2338:
2336:
2333:
2331:
2328:
2326:
2323:
2321:
2318:
2316:
2313:
2312:
2310:
2306:
2300:
2297:
2295:
2292:
2290:
2287:
2285:
2282:
2281:
2279:
2275:
2270:
2266:
2262:
2258:
2251:
2246:
2244:
2239:
2237:
2232:
2231:
2228:
2218:
2217:
2210:
2204:
2203:Methemalbumin
2201:
2197:
2194:
2192:
2189:
2188:
2187:
2184:
2183:
2181:
2177:
2163:
2162:Leghemoglobin
2160:
2159:
2157:
2155:
2151:
2145:
2142:
2140:
2137:
2133:
2130:
2129:
2128:
2125:
2124:
2122:
2120:
2116:
2113:
2109:
2099:
2096:
2094:
2093:Chlorocruorin
2091:
2090:
2088:
2084:
2078:
2077:Methemoglobin
2075:
2073:
2070:
2069:
2067:
2063:
2057:
2054:
2052:
2048:
2047:Oxyhemoglobin
2045:
2043:
2040:
2038:
2035:
2034:
2032:
2028:
2009:
2006:
1995:
1992:
1981:
1978:
1967:
1964:
1953:
1950:
1943:
1940:
1933:
1930:
1929:
1927:
1925:
1921:
1902:
1899:
1888:
1882:
1871:
1868:
1867:
1865:
1861:
1846:
1843:
1832:
1829:
1828:
1826:
1822:
1807:
1804:
1793:
1790:
1779:
1776:
1765:
1762:
1761:
1759:
1755:
1752:
1750:
1744:
1741:
1739:
1735:
1723:
1720:
1719:
1718:
1715:
1711:
1708:
1706:
1703:
1702:
1701:
1698:
1694:
1691:
1690:
1689:
1686:
1682:
1679:
1678:
1677:
1674:
1673:
1671:
1669:
1667:
1663:
1658:
1650:
1647:
1646:
1645:
1642:
1638:
1635:
1634:
1633:
1630:
1626:
1623:
1622:
1621:
1618:
1614:
1611:
1609:
1606:
1604:
1601:
1600:
1599:
1596:
1595:
1593:
1591:
1589:
1583:
1580:
1576:
1573:
1571:
1567:
1564:
1562:
1558:
1553:
1549:
1546:that contain
1545:
1538:
1533:
1531:
1526:
1524:
1519:
1518:
1515:
1504:
1500:
1496:
1492:
1488:
1484:
1477:
1469:
1465:
1461:
1457:
1453:
1449:
1445:
1438:
1430:
1426:
1421:
1416:
1412:
1408:
1404:
1400:
1396:
1389:
1381:
1375:
1371:
1364:
1362:
1353:
1349:
1345:
1341:
1337:
1333:
1329:
1325:
1321:
1317:
1310:
1308:
1299:
1295:
1291:
1287:
1283:
1279:
1272:
1264:
1260:
1256:
1252:
1248:
1244:
1237:
1229:
1225:
1221:
1217:
1213:
1209:
1205:
1201:
1194:
1192:
1184:
1181:
1177:
1174:
1170:
1162:
1158:
1153:
1148:
1144:
1140:
1136:
1132:
1128:
1121:
1113:
1107:
1103:
1099:
1095:
1091:
1084:
1077:
1069:
1065:
1061:
1057:
1053:
1049:
1042:
1034:
1030:
1025:
1020:
1015:
1010:
1006:
1002:
998:
991:
977:
971:
963:
959:
955:
951:
947:
943:
936:
928:
924:
918:
916:
911:
899:
895:
891:
887:
883:
879:
875:
871:
866:
865:
857:
854:
852:
849:
848:
844:
838:
833:
826:
823:
812:
810:
801:
800:
796:
792:
784:
781:
777:
773:
769:
765:
761:
754:
747:
743:
739:
736:
732:
729:
725:
721:
718:
714:
713:
707:
705:
701:
697:
694:
691:
678:
673:
664:
662:
658:
654:
653:hydroxylation
633:
631:
622:
618:
614:
610:
607:
604:
600:
596:
595:
590:
587:
580:
578:/P450 systems
569:
566:
562:
558:
555:
552:
548:
545:which employ
544:
541:
534:
522:
515:
511:
507:
504:
503:
502:
498:
488:
486:
482:
478:
474:
471:
467:
462:
461:ethylation).
460:
456:
452:
448:
444:
440:
434:
430:
426:
422:
418:
410:
406:
397:
390:
386:
382:
378:
374:
370:
369:
364:
360:
356:
353:
349:
340:
338:
334:
330:
326:
322:
318:
314:
310:
306:
302:
298:
293:
291:
287:
283:
279:
278:
273:
269:
265:
261:
257:
253:
249:
245:
233:
230:
228:
224:
221:
217:
213:
210:
208:
204:
200:
196:
193:
190:
186:
181:
177:
174:
171:
169:
165:
162:
159:
157:
153:
150:
147:
145:
141:
138:
135:
133:
129:
126:
122:
118:
115:
113:
109:
106:
103:
101:
97:
94:
91:
89:
85:
82:
79:
77:
73:
69:
65:
60:
55:
49:
44:
39:
36:
33:
19:
3772:Translocases
3769:
3756:
3743:
3730:
3717:
3707:Transferases
3704:
3691:
3548:Binding site
3484:
3477:
3470:
3452:
3445:
3427:
3421:
3415:
3411:
3407:
3403:
3399:
3381:
3374:
3356:
3349:
3342:
3335:
3328:
3319:
3312:
3307:
3300:
3293:
3286:
3271:
3264:
3257:
3250:
3243:
3236:
3229:
3222:
3203:
3184:
3177:
3158:
3151:
3144:
3126:
3119:
3112:
3105:
3098:
3076:
3058:
3016:
3000:
2986:
2974:
2967:
2960:
2922:
2904:
2892:
2878:
2864:
2857:
2841:
2820:
2810:
2800:
2793:
2783:
2763:
2743:
2736:
2726:
2719:
2709:
2699:
2678:
2668:
2661:
2651:
2644:
2637:
2630:
2623:
2616:
2584:
2577:
2570:
2556:
2542:
2526:
2516:
2264:
2213:
2195:
2191:Cytochrome b
2153:
2132:Metmyoglobin
2118:
1923:
1749:development:
1746:
1716:
1699:
1687:
1675:
1660:
1643:
1631:
1619:
1597:
1585:
1552:hemoproteins
1486:
1482:
1476:
1444:Nitric Oxide
1443:
1437:
1402:
1398:
1388:
1369:
1319:
1315:
1281:
1277:
1271:
1246:
1242:
1236:
1203:
1199:
1169:
1134:
1130:
1120:
1093:
1089:
1076:
1054:(1): 14–18.
1051:
1047:
1041:
1007:(1): 59–65.
1004:
1000:
990:
979:. Retrieved
970:
945:
941:
935:
926:
873:
869:
821:
818:
815:Spectroscopy
806:
763:
750:cytochrome b
703:
687:
650:
626:
592:
529:cytochrome b
518:Cytochrome b
500:
463:
458:
454:
450:
442:
438:
432:
428:
424:
420:
392:
388:
384:
372:
366:
354:
346:
343:Nomenclature
307:peak at the
294:
275:
251:
247:
243:
242:
35:
3543:Active site
2257:Cytochromes
2139:Neuroglobin
2065:Other human
1778:HbE Gower 2
1764:HbE Gower 1
782:is lacking.
780:iron(V)-oxo
772:delocalized
746:ferredoxins
594:Rhodococcus
551:adrenodoxin
409:thromboxane
381:paracetamol
363:gene family
359:superfamily
352:root symbol
290:xenobiotics
286:fatty acids
262:containing
256:superfamily
144:OPM protein
62:Identifiers
3814:Metabolism
3793:Categories
3746:Isomerases
3720:Hydrolases
3587:Regulation
3464:CYP701-999
3439:CYP501-699
3368:CYP301-499
3138:CYP101-281
2261:oxygenases
2186:Cytochrome
2144:Cytoglobin
1924:pathology:
1747:stages of
1662:Beta locus
1570:Hemoglobin
981:2007-11-19
906:References
825:affected.
717:heme group
632:to water:
565:ferredoxin
466:amino-acid
309:wavelength
195:structures
168:Membranome
3625:EC number
3472:CYP704B22
2214:see also
2127:Myoglobin
2030:Compounds
1901:HbF/Fetal
1831:HbF/Fetal
1757:Embryonic
1738:Tetramers
1468:233205099
1352:206528205
1096:: 29–55.
776:porphyrin
774:over the
728:reductase
684:Structure
667:Mechanism
603:reductase
533:reductase
368:italicize
329:electrons
105:PDOC00081
93:IPR001128
3649:Kinetics
3573:Cofactor
3536:Activity
3486:CYP720A1
3454:CYP504B1
3429:CYP318A1
3422:CYP315A1
3417:CYP314A1
3412:CYP307A2
3408:CYP307A1
3404:CYP306A1
3400:CYP302A1
3376:CYP303A1
3351:CYP199A2
3330:CYP176A1
3288:CYP154C3
3252:CYP125A1
3238:CYP119A1
3231:CYP113A1
3179:CYP106A2
3153:CYP102A1
3146:CYP101A1
2916:CYP71-99
2835:CYP51-69
2693:CYP21-49
2086:Nonhuman
1578:Subunits
1544:Proteins
1503:25763468
1460:33838343
1429:29286645
1344:21071661
1228:33927145
1220:15352783
1176:Archived
1161:38305159
1152:10923174
1068:20736090
1033:19951895
962:12369887
927:InterPro
898:43655270
890:14625342
829:See also
822:in vitro
803:diagram.
693:thiolate
690:cysteine
657:hydroxyl
643:O + NADP
615:(CYP5),
473:Archived
441:ynthase
357:for the
282:steroids
268:cofactor
254:) are a
212:RCSB PDB
88:InterPro
3804:EC 1.14
3759:Ligases
3529:Enzymes
3358:CYP255A
3337:CYP183A
3302:CYP161C
3295:CYP158A
3128:CYP99A3
3121:CYP97C1
3114:CYP93E1
3107:CYP90C1
3100:CYP88D6
2969:CYP73A1
2962:CYP72A1
2510:CYP5-20
1561:Globins
1420:5855008
1324:Bibcode
1316:Science
1263:3656428
1183:PROSITE
1024:3500189
700:PROSITE
630:reduced
483:names (
447:CYP51A1
445:), and
321:reduced
311:of the
260:enzymes
161:cd00302
100:PROSITE
81:PF00067
3733:Lyases
3479:CYP710
3447:CYP503
3383:CYP305
3344:CYP197
3308:CYP170
3273:CYP152
3266:CYP147
3259:CYP139
3245:CYP123
3224:CYP111
3205:CYP109
3186:CYP107
3160:CYP105
2399:CYP3 (
2154:plant:
2119:human:
1613:pseudo
1501:
1466:
1458:
1427:
1417:
1376:
1350:
1342:
1298:228675
1296:
1261:
1226:
1218:
1159:
1149:
1108:
1066:
1031:
1021:
960:
896:
888:
768:ferryl
696:ligand
635:RH + O
563:and a
481:allele
479:) and
417:TBXAS1
405:CYP5A1
389:CYP450
377:CYP2E1
373:CYP2E1
337:oxygen
227:PDBsum
201:
191:
125:SUPFAM
67:Symbol
3685:Types
3077:CYP81
3059:CYP80
3017:CYP79
3002:CYP76
2987:CYP75
2976:CYP74
2923:CYP71
2906:CYP61
2893:CYP56
2880:CYP55
2866:CYP53
2859:CYP52
2843:CYP51
2821:CYP46
2811:CYP39
2801:CYP35
2795:CYP29
2784:CYP28
2765:CYP27
2745:CYP26
2738:CYP25
2727:CYP24
2721:CYP23
2710:CYP22
2700:CYP21
2679:CYP20
2669:CYP19
2663:CYP18
2652:CYP17
2646:CYP16
2639:CYP15
2632:CYP14
2625:CYP13
2618:CYP12
2586:CYP11
2579:CYP10
2401:CYP3A
2271:1.14)
2179:Other
2111:Other
1942:Barts
1863:Adult
1824:Fetal
1464:S2CID
1348:S2CID
1224:S2CID
1086:(PDF)
894:S2CID
748:, or
651:Many
574:R/cyb
510:NADPH
348:Genes
266:as a
248:P450s
121:SCOPe
112:SCOP2
54:CYP51
3777:list
3770:EC7
3764:list
3757:EC6
3751:list
3744:EC5
3738:list
3731:EC4
3725:list
3718:EC3
3712:list
3705:EC2
3699:list
3692:EC1
2572:CYP9
2557:CYP8
2543:CYP7
2528:CYP6
2517:CYP5
2439:CYP4
2308:CYP2
2277:CYP1
1722:HBE1
1710:HBG2
1705:HBG1
1637:HBQ1
1608:HBA2
1603:HBA1
1548:heme
1499:PMID
1456:PMID
1425:PMID
1374:ISBN
1340:PMID
1294:PMID
1259:PMID
1216:PMID
1157:PMID
1106:ISBN
1064:PMID
1052:1814
1029:PMID
958:PMID
886:PMID
549:and
535:(CYB
523:(cyb
512:via
431:ane
423:hrom
399:P450
333:iron
264:heme
252:CYPs
220:PDBj
216:PDBe
199:ECOD
189:Pfam
149:2bdm
117:2cpp
76:Pfam
70:p450
2955:BA1
2951:AV1
2947:AJ4
2486:F22
2481:F12
2476:F11
2451:A22
2446:A11
2430:A43
2425:A37
2350:C19
2345:C18
2325:A13
2008:HbO
1994:HbE
1980:HbC
1966:HbS
1952:HbD
1932:HbH
1884:HbA
1870:HbA
1845:HbA
1693:HBD
1681:HBB
1664:on
1649:HBM
1625:HBZ
1491:doi
1487:115
1448:doi
1415:PMC
1407:doi
1403:118
1332:doi
1320:330
1286:doi
1251:doi
1247:195
1208:doi
1204:104
1147:PMC
1139:doi
1135:124
1098:doi
1056:doi
1019:PMC
1009:doi
950:doi
878:doi
599:FMN
570:CYB
539:R).
487:).
401:BM3
395:450
393:CYP
391:or
385:CYP
355:CYP
339:).
288:,
258:of
250:or
207:PDB
173:265
156:CDD
3795::
3420:,
3414:,
3410:,
3406:,
3402:,
3398:(
3389:M2
3321:B1
3314:A1
3281:B1
3278:A1
3216:E1
3211:B1
3197:G1
3192:A1
3171:D7
3166:A1
3093:E9
3091:,
3089:E7
3087:,
3085:E3
3083:,
3081:E1
3071:G2
3069:,
3067:B1
3065:,
3063:A1
3053:D4
3051:,
3049:D3
3047:,
3045:D2
3043:,
3041:D1
3039:,
3037:B3
3035:,
3033:B2
3031:,
3029:B1
3027:,
3025:A2
3023:,
3021:A1
3011:M7
3009:,
3007:B6
2995:B1
2993:,
2991:A1
2981:D1
2953:,
2949:,
2945:,
2943:Z6
2941:,
2939:C4
2937:,
2935:C3
2933:,
2931:C2
2929:,
2927:C1
2898:A1
2886:A1
2872:A1
2852:F1
2850:,
2848:A1
2825:A1
2815:A1
2805:B1
2788:A1
2778:C1
2776:,
2774:B1
2772:,
2770:A1
2758:C1
2756:,
2754:B1
2752:,
2750:A1
2731:A1
2714:A1
2704:A2
2683:A1
2673:A1
2656:A1
2611:C1
2609:,
2607:B3
2605:,
2603:B2
2601:,
2599:B1
2597:,
2595:A2
2593:,
2591:A1
2565:B1
2563:,
2561:A1
2551:B1
2549:,
2547:A1
2537:M2
2535:,
2533:G1
2521:A1
2501:Z1
2496:X1
2491:V2
2471:F8
2466:F3
2461:F2
2456:B1
2420:A7
2415:A5
2410:A4
2390:W1
2385:U1
2380:S1
2375:R1
2370:J2
2365:F1
2360:E1
2355:D6
2340:C9
2335:C8
2330:B6
2320:A7
2315:A6
2299:B1
2294:A5
2289:A2
2284:A1
2269:EC
2263::
2259:,
2010:(α
1996:(α
1982:(α
1968:(α
1954:(α
1944:(γ
1934:(β
1903:(α
1889:(α
1872:(α
1847:(α
1833:(α
1808:(ζ
1794:(ζ
1780:(α
1766:(ζ
1666:11
1588:16
1497:.
1485:.
1462:.
1454:.
1423:.
1413:.
1401:.
1397:.
1360:^
1346:.
1338:.
1330:.
1318:.
1306:^
1292:.
1282:90
1280:.
1257:.
1245:.
1222:.
1214:.
1202:.
1190:^
1155:.
1145:.
1133:.
1129:.
1104:.
1094:14
1088:.
1062:.
1050:.
1027:.
1017:.
1003:.
999:.
956:.
944:.
925:.
914:^
892:.
884:.
874:31
872:.
744:,
623:).
407:,
317:nm
284:,
218:;
214:;
197:/
137:39
123:/
119:/
3779:)
3775:(
3766:)
3762:(
3753:)
3749:(
3740:)
3736:(
3727:)
3723:(
3714:)
3710:(
3701:)
3697:(
3521:e
3514:t
3507:v
3424:)
3095:)
3079:(
3073:)
3061:(
3055:)
3019:(
3013:)
3005:(
2997:)
2989:(
2983:)
2979:(
2957:)
2925:(
2854:)
2846:(
2827:)
2823:(
2817:)
2813:(
2807:)
2803:(
2790:)
2786:(
2780:)
2768:(
2760:)
2748:(
2733:)
2729:(
2716:)
2712:(
2706:)
2702:(
2685:)
2681:(
2675:)
2671:(
2658:)
2654:(
2613:)
2589:(
2567:)
2559:(
2553:)
2545:(
2539:)
2531:(
2523:)
2519:(
2403:)
2249:e
2242:t
2235:v
2049:/
2018:)
2016:2
2014:β
2012:2
2004:)
2002:2
2000:β
1998:2
1990:)
1988:2
1986:β
1984:2
1976:)
1974:2
1972:β
1970:2
1962:)
1960:2
1958:β
1956:2
1948:)
1946:4
1938:)
1936:4
1911:)
1909:2
1907:γ
1905:2
1897:)
1895:2
1893:δ
1891:2
1886:2
1880:)
1878:2
1876:β
1874:2
1855:)
1853:2
1851:β
1849:2
1841:)
1839:2
1837:γ
1835:2
1816:)
1814:2
1812:β
1810:2
1802:)
1800:2
1798:γ
1796:2
1788:)
1786:2
1784:ε
1782:2
1774:)
1772:2
1770:ε
1768:2
1717:ε
1700:γ
1688:δ
1676:β
1668::
1644:μ
1632:θ
1620:ζ
1598:α
1590::
1554:)
1550:(
1536:e
1529:t
1522:v
1505:.
1493::
1470:.
1450::
1431:.
1409::
1382:.
1354:.
1334::
1326::
1300:.
1288::
1265:.
1253::
1230:.
1210::
1163:.
1141::
1114:.
1100::
1070:.
1058::
1035:.
1011::
1005:4
984:.
964:.
952::
946:3
929:.
900:.
880::
757:2
752:5
704:C
679:.
641:2
637:2
585:.
583:5
576:5
572:5
537:5
531:5
525:5
520:5
459:M
457:e
455:D
451:L
443:1
439:S
436:2
433:A
429:X
427:o
425:B
421:T
419:(
413:2
411:A
246:(
56:)
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.