70:
588:
574:
98:
89:
661:
1174:
61:
1121:
878:
This equilibrium does not involve a change in hydrogen ion concentration, which would predict that the equilibrium is independent of pH. The red line on the predominance diagram is not quite horizontal due to the simultaneous equilibrium with the chromate ion. The hydrogen chromate ion may be
1263:
Subsequent leaching of this material at higher temperatures dissolves the chromates, leaving a residue of insoluble iron oxide. Normally the chromate solution is further processed to make chromium metal, but a chromate salt may be obtained directly from the liquor.
1511:
Brito, F.; Ascanioa, J.; Mateoa, S.; Hernándeza, C.; Araujoa, L.; Gili, P.; Martín-Zarzab, P.; Domínguez, S.; Mederos, A. (1997). "Equilibria of chromate(VI) species in acid medium and ab initio studies of these species".
1723:
of chromium (VI) compounds. Chromium (VI) compounds cause cancer of the lung. Also positive associations have been observed between exposure to chromium (VI) compounds and cancer of the nose and nasal sinuses. There is
1422:"Effect of solution acidity on the crystallization of polychromates in uranyl-bearing systems: synthesis and crystal structures of Rb2[(UO2)(Cr2O7)(NO3)2] and two new polymorphs of Rb2Cr3O10"
1681:
719:
and the analytical concentration of chromium. The chromate ion is the predominant species in alkaline solutions, but dichromate can become the predominant ion in acidic solutions.
1597:
Anger, Gerd; Halstenberg, Jost; Hochgeschwender, Klaus; Scherhag, Christoph; Korallus, Ulrich; Knopf, Herbert; Schmidt, Peter; Ohlinger, Manfred (2005). "Chromium
Compounds".
1275:, which can occur as spectacular long red crystals, is the most commonly found chromate mineral. Rare potassium chromate minerals and related compounds are found in the
1332:. The use of chromate compounds in manufactured goods is restricted in the EU (and by market commonality the rest of the world) by EU Parliament directive on the
186:
2627:
2535:
1700:
1333:
1778:
1165:, chromates and dichromates convert into trivalent chromium, Cr, salts of which typically have a distinctively different blue-green color.
386:
1677:
1887:
1158:
was used for a very long time before environmental regulations discouraged its use. When used as oxidizing agents or titrants in a
2620:
178:
1694:
1643:
1578:
1483:
1404:
3149:
2613:
1548:
351:
2403:
1771:
487:
1208:
in the presence of air. The chromium is oxidized to the hexavalent form, while the iron forms iron(III) oxide, Fe
1614:
1420:
Nazarchuk, Evgeny V.; Siidra, Oleg I.; Charkin, Dmitry O.; Kalmykov, Stepan N.; Kotova, Elena L. (2021-02-01).
1839:
116:
587:
281:
3113:
2454:
1764:
1345:
621:
311:
1728:
in experimental animals for the carcinogenicity of chromium (VI) compounds. Chromium (VI) compounds are
892:
573:
3144:
2868:
2549:
2511:
2292:
1501:. A comprehensive database of published data on equilibrium constants of metal complexes and ligands.
1421:
20:
2857:
1154:
are only very slightly soluble in water and are thus used as pigments. The lead-containing pigment
337:
329:
1292:
2258:
2216:
2192:
1200:, found as brittle metallic black crystals or granules. Chromite ore is heated with a mixture of
1142:
to protect metals from corrosion and to improve paint adhesion. Chromate and dichromate salts of
3103:
2900:
2421:
2282:
2272:
1631:
1543:, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic Press/De Gruyter,
2569:
2362:
2352:
2337:
2306:
2240:
2226:
2134:
2030:
2020:
2015:
1985:
1801:
1568:
1475:
1396:
1051:
shows that chromates are weaker oxidizing agent in alkaline solution than in acid solution.
1002:
2977:
2372:
2316:
2129:
2040:
2005:
2000:
1967:
1952:
1938:
1928:
1821:
1151:
722:
Further condensation reactions can occur in strongly acidic solution with the formation of
712:
672:
664:
593:
559:
299:
291:
153:
145:
1746:
8:
3013:
2760:
2436:
2382:
2202:
2050:
1871:
1365:
1297:
1138:, mainly sodium dichromate, were produced in 1985. Chromates and dichromates are used in
1135:
134:
27:
215:
207:
3139:
2742:
2722:
2702:
2692:
2664:
2342:
1995:
1787:
1449:
1368:
of chromium. Thus, when pCr = 2, the chromium concentration is 10 mol/L.
620:, replaces one or more oxygen atoms. In acid solution the unstable blue peroxo complex
579:
1525:
3035:
2995:
2959:
2922:
2828:
2804:
2444:
1690:
1639:
1610:
1574:
1544:
1479:
1453:
1441:
1400:
1201:
1162:
637:
601:
2794:
2712:
2678:
2521:
2497:
2178:
2173:
2104:
1602:
1570:
Toxic
Substances Controls Guide: Federal Regulation of Chemicals in the Environment
1521:
1467:
1433:
1388:
1205:
414:
1596:
270:
262:
2770:
2732:
2650:
1720:
1048:
998:
552:
548:
1498:
2892:
2586:
1276:
1139:
481:
467:
19:
This article is about the salts of the chromium(VI) anion. For other uses, see
1751:
3133:
2814:
2784:
1606:
1445:
1329:
1317:
1155:
1128:
986:
value for this reaction shows that it can be ignored at pH > 4.
2605:
928:
is not well characterized. Reported values vary between about −0.8 and 1.6.
3074:
2468:
1437:
1309:
1305:
1143:
1005:
it to oxidation state +3. In acid solution the aquated Cr ion is produced.
880:
472:
69:
43:
660:
1510:
1313:
1173:
60:
31:
1280:
236:
2071:
1147:
1124:
457:
39:
97:
1756:
1182:
1120:
788:
773:
35:
1747:
National
Pollutant Inventory - Chromium(VI) and compounds fact sheet
1539:
Holleman, Arnold
Frederik; Wiberg, Egon (2001), Wiberg, Nils (ed.),
1316:. Also positive associations have been observed between exposure to
931:
The dichromate ion is a somewhat weaker base than the chromate ion:
480:
Except where otherwise noted, data are given for materials in their
88:
1268:
1189:
1178:
641:
633:
605:
544:
540:
226:
185:
177:
1334:
Restriction of
Hazardous Substances (RoHS) Directive (2002/95/EC)
1312:). Inhaling particles of hexavalent chromium compounds can cause
556:
248:
1636:
Industrial
Minerals & Rocks: Commodities, Markets, and Uses
1321:
671:
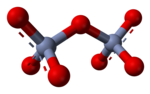
In aqueous solution, chromate and dichromate anions exist in a
1419:
1047:
In alkaline solution chromium(III) hydroxide is produced. The
1364:
pCr is equal to the negative of the decimal logarithm of the
1301:
1159:
197:
166:
316:
2064:
1325:
715:
shows that the position of the equilibrium depends on both
1880:
1815:
776:
of chromium(VI) have structures made up of tetrahedral CrO
1001:. Commonly three electrons are added to a chromium atom,
644:
results in the formation of the more stable complex CrO(O
1659:
1426:
562:, chromate and dichromate ions can be interconvertible.
716:
1689:. Lyon: International Agency for Research on Cancer.
1566:
1168:
997:The chromate and dichromate ions are fairly strong
833:It is also in equilibrium with the dichromate ion:
1629:
1188:The primary chromium ore is the mixed metal oxide
1466:
1387:
992:
3131:
1752:Demonstration of chromate-dichromate equilibrium
269:
261:
1683:Volume 100C: Arsenic, Metals, Fibres, and Dusts
1134:Approximately 136,000 tonnes (150,000 tons) of
152:
144:
23:. See also for disambiguation of derived terms.
1599:Ullmann's Encyclopedia of Industrial Chemistry
1538:
2635:
2621:
1772:
1029:+ 14 H + 6 e → 2 Cr + 7 H
462:115.994 g mol and 215.988 g mol
370:dichromate: InChI=1S/2Cr.7O/q;;;;;;;2*-1
1567:Worobec, Mary Devine; Hogue, Cheryl (1992).
879:protonated, with the formation of molecular
2628:
2614:
1779:
1765:
1177:Crocoite specimen from the Red Lead Mine,
1592:
1590:
1532:
298:
290:
1670:
1562:
1560:
1172:
1119:
659:
1267:Chromate containing minerals are rare.
655:
336:
328:
235:
3132:
1786:
1630:Papp, John F.; Lipin Bruce R. (2006).
1623:
1587:
636:molecule, which may be extracted into
565:
360:chromate: InChI=1S/Cr.4O/q;;;2*-1
2609:
1760:
1557:
1283:– the only known dichromate mineral.
373:Key: SOCTUWSJJQCPFX-UHFFFAOYSA-N
363:Key: ZCDOYSPFYFSLEW-UHFFFAOYSA-N
214:
206:
1676:
518:salts contain the dichromate anion,
1293:Hexavalent chromium § Toxicity
251:
13:
502:salts contain the chromate anion,
399:dichromate: O=(=O)()O(=O)(=O)
96:
87:
68:
59:
14:
3161:
1740:
1169:Natural occurrence and production
1730:carcinogenic to humans (Group 1)
632:, is formed; it is an uncharged
586:
572:
1652:
1115:
783:The hydrogen chromate ion, HCrO
484:(at 25 °C , 100 kPa).
1504:
1492:
1460:
1413:
1381:
1358:
1103:
1035:
993:Oxidation–reduction properties
966:
820:
1:
1526:10.1016/S0277-5387(97)00128-9
1375:
2065:Organochromium(II) compounds
7:
1881:Organochromium(I) compounds
1816:Organochromium(0) compounds
1346:Chromate conversion coating
1339:
1286:
622:Chromium(VI) oxide peroxide
604:, giving products in which
83:
55:
10:
3166:
3150:Transition metal oxyanions
1660:"Mines, Minerals and More"
1290:
551:and are moderately strong
25:
18:
3065:
2952:
2891:
2850:
2643:
2637:Chromates and dichromates
2585:
2435:
2396:
2330:
2122:
2097:
1921:
1864:
1794:
1573:. BNA Books. p. 13.
1472:Chemistry of the Elements
1470:; Earnshaw, Alan (1997).
1393:Chemistry of the Elements
1391:; Earnshaw, Alan (1997).
1111: = −0.13 V
478:
407:
382:
347:
127:
115:
110:
82:
54:
1607:10.1002/14356007.a07_067
1351:
1043: = 1.33 V
50:Chromate and dichromate
26:Not to be confused with
2595:Chromate and dichromate
1601:. Weinheim: Wiley-VCH.
780:units sharing corners.
394:chromate: (=O)(=O)
121:Chromate and dichromate
1438:10.1515/zkri-2020-0078
1185:
1131:
668:
101:
92:
73:
64:
1638:(7th ed.). SME.
1476:Butterworth-Heinemann
1468:Greenwood, Norman N.
1397:Butterworth-Heinemann
1389:Greenwood, Norman N.
1291:Further information:
1176:
1152:alkaline earth metals
1123:
901:for the equilibrium
663:
600:Chromates react with
117:Systematic IUPAC name
100:
91:
72:
63:
713:predominance diagram
673:chemical equilibrium
665:Predominance diagram
656:Acid–base properties
594:potassium dichromate
1726:sufficient evidence
1717:sufficient evidence
1541:Inorganic Chemistry
1366:molar concentration
1298:Hexavalent chromium
1136:hexavalent chromium
566:Chemical properties
51:
28:chromite (compound)
16:Chromium(VI) anions
1788:Chromium compounds
1719:in humans for the
1186:
1132:
669:
580:potassium chromate
488:Infobox references
102:
93:
74:
65:
49:
3127:
3126:
2948:
2947:
2603:
2602:
2098:Chromium(II, III)
2093:
2092:
1917:
1916:
1860:
1859:
1696:978-92-832-0135-9
1645:978-0-87335-233-8
1580:978-0-87179-752-0
1520:(21): 3835–3846.
1499:IUPAC SC-Database
1485:978-0-08-037941-8
1406:978-0-08-037941-8
1300:compounds can be
1202:calcium carbonate
1163:chemical reaction
975: = 1.18
602:hydrogen peroxide
496:Chemical compound
494:
493:
335:dichromate:
312:CompTox Dashboard
297:dichromate:
268:dichromate:
234:dichromate:
213:dichromate:
187:Interactive image
184:dichromate:
179:Interactive image
151:dichromate:
106:
105:
78:
77:
3157:
3145:Oxidizing agents
3119:
3109:
3099:
3084:
3057:
3031:
3009:
2991:
2973:
2940:
2918:
2883:
2864:
2848:
2847:
2842:
2824:
2810:
2800:
2790:
2780:
2766:
2756:
2738:
2728:
2718:
2708:
2698:
2688:
2674:
2660:
2630:
2623:
2616:
2607:
2606:
2575:
2564:
2545:
2531:
2517:
2507:
2493:
2478:
2464:
2450:
2427:
2417:
2388:
2378:
2368:
2358:
2348:
2322:
2312:
2302:
2288:
2278:
2268:
2254:
2236:
2222:
2212:
2198:
2188:
2169:
2114:
2085:
2062:
2061:
2056:
2046:
2036:
2026:
2011:
1991:
1981:
1963:
1948:
1934:
1909:
1878:
1877:
1853:
1835:
1813:
1812:
1807:
1781:
1774:
1767:
1758:
1757:
1735:
1734:
1712:
1711:
1705:
1699:. Archived from
1688:
1674:
1668:
1667:
1656:
1650:
1649:
1627:
1621:
1620:
1594:
1585:
1584:
1564:
1555:
1553:
1536:
1530:
1529:
1508:
1502:
1496:
1490:
1489:
1478:. p. 1009.
1474:(2nd ed.).
1464:
1458:
1457:
1417:
1411:
1410:
1395:(2nd ed.).
1385:
1369:
1362:
1279:. Among them is
1206:sodium carbonate
1104:
1101:
1100:
1099:
1090:
1089:
1088:
1078:
1076:
1075:
1065:
1064:
1063:
1036:
1028:
1027:
1026:
1018:
1017:
999:oxidizing agents
967:
963:
961:
960:
957:
948:
947:
944:
924:
922:
921:
918:
869:
868:
867:
859:
858:
848:
847:
846:
829: ≈ 5.9
821:
817:
816:
815:
805:
804:
803:
771:
770:
769:
761:
760:
746:
745:
744:
736:
735:
707:
701:
700:
697:
688:
687:
684:
619:
618:
617:
590:
576:
553:oxidizing agents
538:
537:
536:
528:
527:
513:
512:
511:
451:
450:
449:
441:
440:
430:
429:
428:
415:Chemical formula
340:
332:
320:
318:
302:
294:
273:
265:
253:
239:
218:
210:
189:
181:
156:
148:
84:
56:
52:
48:
3165:
3164:
3160:
3159:
3158:
3156:
3155:
3154:
3130:
3129:
3128:
3123:
3118:
3114:
3108:
3104:
3098:
3094:
3090:
3086:
3083:
3079:
3075:
3061:
3056:
3052:
3048:
3044:
3040:
3036:
3030:
3026:
3022:
3018:
3014:
3008:
3004:
3000:
2996:
2990:
2986:
2982:
2978:
2972:
2968:
2964:
2960:
2944:
2939:
2935:
2931:
2927:
2923:
2917:
2913:
2909:
2905:
2901:
2893:Chromate esters
2887:
2881:
2877:
2873:
2869:
2862:
2858:
2851:Chlorochromates
2846:
2841:
2837:
2833:
2829:
2823:
2819:
2815:
2809:
2805:
2799:
2795:
2789:
2785:
2779:
2775:
2771:
2765:
2761:
2755:
2751:
2747:
2743:
2737:
2733:
2727:
2723:
2717:
2713:
2707:
2703:
2697:
2693:
2687:
2683:
2679:
2673:
2669:
2665:
2659:
2655:
2651:
2639:
2634:
2604:
2599:
2581:
2574:
2570:
2562:
2558:
2554:
2550:
2544:
2540:
2536:
2530:
2526:
2522:
2516:
2512:
2506:
2502:
2498:
2492:
2488:
2484:
2480:
2477:
2473:
2469:
2463:
2459:
2455:
2449:
2445:
2431:
2426:
2422:
2416:
2412:
2408:
2404:
2392:
2387:
2383:
2377:
2373:
2367:
2363:
2357:
2353:
2347:
2343:
2326:
2321:
2317:
2311:
2307:
2301:
2297:
2293:
2287:
2283:
2277:
2273:
2267:
2263:
2259:
2253:
2249:
2245:
2241:
2235:
2231:
2227:
2221:
2217:
2211:
2207:
2203:
2197:
2193:
2187:
2183:
2179:
2167:
2163:
2159:
2155:
2151:
2147:
2143:
2139:
2135:
2118:
2113:
2109:
2105:
2089:
2084:
2080:
2076:
2072:
2060:
2055:
2051:
2045:
2041:
2035:
2031:
2025:
2021:
2010:
2006:
1990:
1986:
1980:
1976:
1972:
1968:
1961:
1957:
1953:
1947:
1943:
1939:
1933:
1929:
1913:
1908:
1904:
1900:
1896:
1892:
1888:
1876:
1856:
1852:
1848:
1844:
1840:
1834:
1830:
1826:
1822:
1811:
1806:
1802:
1790:
1785:
1743:
1738:
1721:carcinogenicity
1709:
1707:
1703:
1697:
1686:
1675:
1671:
1658:
1657:
1653:
1646:
1628:
1624:
1617:
1595:
1588:
1581:
1565:
1558:
1551:
1537:
1533:
1509:
1505:
1497:
1493:
1486:
1465:
1461:
1418:
1414:
1407:
1399:. p. 637.
1386:
1382:
1378:
1373:
1372:
1363:
1359:
1354:
1342:
1295:
1289:
1274:
1259:
1255:
1251:
1247:
1243:
1239:
1235:
1231:
1227:
1223:
1215:
1211:
1199:
1195:
1171:
1118:
1110:
1102:
1098:
1096:
1095:
1094:
1092:
1087:
1084:
1083:
1082:
1080:
1074:
1071:
1070:
1069:
1067:
1062:
1059:
1058:
1057:
1055:
1049:redox potential
1042:
1034:
1032:
1025:
1022:
1021:
1020:
1016:
1013:
1012:
1011:
1009:
995:
989:
985:
974:
965:
958:
955:
954:
952:
945:
942:
941:
939:
935:
919:
916:
915:
913:
909:
905:
899:
890:
886:
873:
866:
863:
862:
861:
857:
854:
853:
852:
850:
845:
842:
841:
840:
838:
828:
819:
814:
811:
810:
809:
807:
802:
799:
798:
797:
795:
786:
779:
768:
765:
764:
763:
759:
756:
755:
754:
752:
743:
740:
739:
738:
734:
731:
730:
729:
727:
705:
698:
695:
694:
692:
685:
682:
681:
679:
658:
651:
647:
631:
627:
616:
613:
612:
611:
609:
596:
591:
582:
577:
568:
549:oxidation state
535:
532:
531:
530:
526:
523:
522:
521:
519:
510:
507:
506:
505:
503:
497:
490:
485:
448:
445:
444:
443:
439:
436:
435:
434:
432:
427:
424:
423:
422:
420:
417:
403:
400:
395:
390:
389:
378:
375:
374:
371:
365:
364:
361:
355:
354:
343:
327:chromate:
321:
314:
305:
289:chromate:
276:
260:chromate:
254:
242:
221:
205:chromate:
192:
176:chromate:
170:
159:
143:chromate:
137:
123:
122:
47:
24:
17:
12:
11:
5:
3163:
3153:
3152:
3147:
3142:
3125:
3124:
3122:
3121:
3116:
3111:
3106:
3101:
3096:
3092:
3088:
3081:
3077:
3071:
3069:
3063:
3062:
3060:
3059:
3054:
3050:
3046:
3042:
3038:
3033:
3028:
3024:
3020:
3016:
3011:
3006:
3002:
2998:
2993:
2988:
2984:
2980:
2975:
2970:
2966:
2962:
2956:
2954:
2950:
2949:
2946:
2945:
2943:
2942:
2937:
2933:
2929:
2925:
2920:
2915:
2911:
2907:
2903:
2897:
2895:
2889:
2888:
2886:
2885:
2879:
2875:
2871:
2866:
2860:
2854:
2852:
2845:
2844:
2839:
2835:
2831:
2826:
2821:
2817:
2812:
2807:
2802:
2797:
2792:
2787:
2782:
2777:
2773:
2768:
2763:
2758:
2753:
2749:
2745:
2740:
2735:
2730:
2725:
2720:
2715:
2710:
2705:
2700:
2695:
2690:
2685:
2681:
2676:
2671:
2667:
2662:
2657:
2653:
2647:
2645:
2641:
2640:
2633:
2632:
2625:
2618:
2610:
2601:
2600:
2598:
2597:
2591:
2589:
2587:Polyatomic ion
2583:
2582:
2580:
2579:
2577:(hypothetical)
2572:
2566:
2560:
2556:
2552:
2547:
2542:
2538:
2533:
2528:
2524:
2519:
2514:
2509:
2504:
2500:
2495:
2490:
2486:
2482:
2475:
2471:
2466:
2461:
2457:
2452:
2447:
2441:
2439:
2433:
2432:
2430:
2429:
2424:
2419:
2414:
2410:
2406:
2400:
2398:
2394:
2393:
2391:
2390:
2385:
2380:
2375:
2370:
2365:
2360:
2355:
2350:
2345:
2340:
2334:
2332:
2328:
2327:
2325:
2324:
2319:
2314:
2309:
2304:
2299:
2295:
2290:
2285:
2280:
2275:
2270:
2265:
2261:
2256:
2251:
2247:
2243:
2238:
2233:
2229:
2224:
2219:
2214:
2209:
2205:
2200:
2195:
2190:
2185:
2181:
2176:
2171:
2165:
2161:
2157:
2153:
2149:
2145:
2141:
2137:
2132:
2126:
2124:
2120:
2119:
2117:
2116:
2111:
2107:
2101:
2099:
2095:
2094:
2091:
2090:
2088:
2087:
2082:
2078:
2074:
2068:
2066:
2059:
2058:
2053:
2048:
2043:
2038:
2033:
2028:
2023:
2018:
2013:
2008:
2003:
1998:
1993:
1988:
1983:
1978:
1974:
1970:
1965:
1959:
1955:
1950:
1945:
1941:
1936:
1931:
1925:
1923:
1919:
1918:
1915:
1914:
1912:
1911:
1906:
1902:
1898:
1894:
1890:
1884:
1882:
1875:
1874:
1868:
1866:
1862:
1861:
1858:
1857:
1855:
1850:
1846:
1842:
1837:
1832:
1828:
1824:
1819:
1817:
1810:
1809:
1804:
1798:
1796:
1792:
1791:
1784:
1783:
1776:
1769:
1761:
1755:
1754:
1749:
1742:
1741:External links
1739:
1737:
1736:
1695:
1669:
1664:www.mindat.org
1651:
1644:
1622:
1615:
1586:
1579:
1556:
1549:
1531:
1503:
1491:
1484:
1459:
1432:(1–2): 11–21.
1412:
1405:
1379:
1377:
1374:
1371:
1370:
1356:
1355:
1353:
1350:
1349:
1348:
1341:
1338:
1320:compounds and
1288:
1285:
1277:Atacama desert
1272:
1261:
1260:
1257:
1253:
1249:
1245:
1241:
1237:
1233:
1229:
1225:
1221:
1213:
1209:
1197:
1193:
1170:
1167:
1140:chrome plating
1117:
1114:
1113:
1112:
1108:
1097:
1085:
1072:
1060:
1045:
1044:
1040:
1030:
1023:
1014:
994:
991:
983:
977:
976:
972:
950:
937:
926:
925:
911:
907:
897:
888:
884:
876:
875:
871:
864:
855:
843:
831:
830:
826:
812:
800:
784:
777:
766:
757:
749:tetrachromates
741:
732:
709:
708:
703:
690:
657:
654:
649:
645:
640:. Addition of
629:
625:
614:
598:
597:
592:
585:
583:
578:
571:
567:
564:
533:
524:
508:
495:
492:
491:
486:
482:standard state
479:
476:
475:
470:
468:Conjugate acid
464:
463:
460:
454:
453:
446:
437:
425:
418:
413:
410:
409:
405:
404:
402:
401:
398:
396:
393:
385:
384:
383:
380:
379:
377:
376:
372:
369:
368:
366:
362:
359:
358:
350:
349:
348:
345:
344:
342:
341:
333:
324:
322:
310:
307:
306:
304:
303:
295:
286:
284:
278:
277:
275:
274:
266:
257:
255:
247:
244:
243:
241:
240:
231:
229:
223:
222:
220:
219:
211:
202:
200:
194:
193:
191:
190:
182:
173:
171:
164:
161:
160:
158:
157:
149:
140:
138:
133:
130:
129:
125:
124:
120:
119:
113:
112:
108:
107:
104:
103:
94:
80:
79:
76:
75:
66:
15:
9:
6:
4:
3:
2:
3162:
3151:
3148:
3146:
3143:
3141:
3138:
3137:
3135:
3120:
3112:
3110:
3102:
3100:
3073:
3072:
3070:
3068:
3064:
3058:
3034:
3032:
3012:
3010:
2994:
2992:
2976:
2974:
2958:
2957:
2955:
2951:
2941:
2921:
2919:
2899:
2898:
2896:
2894:
2890:
2884:
2867:
2865:
2856:
2855:
2853:
2849:
2843:
2827:
2825:
2813:
2811:
2803:
2801:
2793:
2791:
2783:
2781:
2769:
2767:
2759:
2757:
2741:
2739:
2731:
2729:
2721:
2719:
2711:
2709:
2701:
2699:
2691:
2689:
2677:
2675:
2663:
2661:
2649:
2648:
2646:
2642:
2638:
2631:
2626:
2624:
2619:
2617:
2612:
2611:
2608:
2596:
2593:
2592:
2590:
2588:
2584:
2578:
2576:
2567:
2565:
2548:
2546:
2534:
2532:
2520:
2518:
2510:
2508:
2496:
2494:
2467:
2465:
2453:
2451:
2443:
2442:
2440:
2438:
2434:
2428:
2420:
2418:
2402:
2401:
2399:
2395:
2389:
2381:
2379:
2371:
2369:
2361:
2359:
2351:
2349:
2341:
2339:
2336:
2335:
2333:
2329:
2323:
2315:
2313:
2305:
2303:
2291:
2289:
2281:
2279:
2271:
2269:
2257:
2255:
2239:
2237:
2225:
2223:
2215:
2213:
2201:
2199:
2191:
2189:
2177:
2175:
2172:
2170:
2133:
2131:
2128:
2127:
2125:
2123:Chromium(III)
2121:
2115:
2103:
2102:
2100:
2096:
2086:
2070:
2069:
2067:
2063:
2057:
2049:
2047:
2039:
2037:
2029:
2027:
2019:
2017:
2014:
2012:
2004:
2002:
1999:
1997:
1994:
1992:
1984:
1982:
1966:
1964:
1951:
1949:
1937:
1935:
1927:
1926:
1924:
1920:
1910:
1886:
1885:
1883:
1879:
1873:
1870:
1869:
1867:
1863:
1854:
1838:
1836:
1820:
1818:
1814:
1808:
1800:
1799:
1797:
1793:
1789:
1782:
1777:
1775:
1770:
1768:
1763:
1762:
1759:
1753:
1750:
1748:
1745:
1744:
1733:
1731:
1727:
1722:
1718:
1706:on 2020-03-17
1702:
1698:
1692:
1685:
1684:
1679:
1673:
1665:
1661:
1655:
1647:
1641:
1637:
1633:
1626:
1618:
1612:
1608:
1604:
1600:
1593:
1591:
1582:
1576:
1572:
1571:
1563:
1561:
1552:
1550:0-12-352651-5
1546:
1542:
1535:
1527:
1523:
1519:
1515:
1507:
1500:
1495:
1487:
1481:
1477:
1473:
1469:
1463:
1455:
1451:
1447:
1443:
1439:
1435:
1431:
1427:
1423:
1416:
1408:
1402:
1398:
1394:
1390:
1384:
1380:
1367:
1361:
1357:
1347:
1344:
1343:
1337:
1335:
1331:
1330:nasal sinuses
1327:
1323:
1319:
1318:chromium (VI)
1315:
1311:
1307:
1303:
1299:
1294:
1284:
1282:
1278:
1270:
1265:
1219:
1218:
1217:
1207:
1203:
1191:
1184:
1180:
1175:
1166:
1164:
1161:
1157:
1156:chrome yellow
1153:
1149:
1145:
1141:
1137:
1130:
1129:Chrome yellow
1126:
1122:
1107:
1054:
1053:
1052:
1050:
1039:
1008:
1007:
1006:
1004:
1000:
990:
987:
982:
971:
934:
933:
932:
929:
904:
903:
902:
900:
896:
882:
836:
835:
834:
825:
794:
793:
792:
790:
781:
775:
750:
725:
720:
718:
714:
678:
677:
676:
674:
666:
662:
653:
643:
639:
635:
623:
607:
603:
595:
589:
584:
581:
575:
570:
569:
563:
561:
558:
554:
550:
546:
542:
517:
501:
489:
483:
477:
474:
471:
469:
466:
465:
461:
459:
456:
455:
419:
416:
412:
411:
406:
397:
392:
391:
388:
381:
367:
357:
356:
353:
346:
339:
338:DTXSID5074004
334:
331:
330:DTXSID7065675
326:
325:
323:
313:
309:
308:
301:
296:
293:
288:
287:
285:
283:
280:
279:
272:
267:
264:
259:
258:
256:
250:
246:
245:
238:
233:
232:
230:
228:
225:
224:
217:
212:
209:
204:
203:
201:
199:
196:
195:
188:
183:
180:
175:
174:
172:
168:
163:
162:
155:
150:
147:
142:
141:
139:
136:
132:
131:
126:
118:
114:
109:
99:
95:
90:
86:
85:
81:
71:
67:
62:
58:
57:
53:
45:
41:
37:
33:
29:
22:
3066:
2636:
2594:
2568:
2437:Chromium(VI)
2331:Chromium(IV)
1922:Chromium(II)
1729:
1725:
1716:
1714:
1708:. Retrieved
1701:the original
1682:
1672:
1663:
1654:
1635:
1625:
1598:
1569:
1540:
1534:
1517:
1513:
1506:
1494:
1471:
1462:
1429:
1425:
1415:
1392:
1383:
1360:
1310:IARC Group 1
1306:carcinogenic
1296:
1266:
1262:
1187:
1144:heavy metals
1133:
1116:Applications
1105:
1046:
1037:
996:
988:
980:
978:
969:
930:
927:
894:
881:chromic acid
877:
832:
823:
782:
748:
724:trichromates
723:
721:
710:
689:+ 2 H ⇌ Cr
670:
667:for chromate
599:
515:
499:
498:
473:Chromic acid
128:Identifiers
44:tetrachromat
2953:Dichromates
2397:Chromium(V)
1865:Chromium(I)
1795:Chromium(0)
1314:lung cancer
1256:+ 8 CO
1248:+ 2 Fe
1240:→ 8 Na
1228:+ 8 Na
1220:4 FeCr
1148:lanthanides
1127:painted in
539:. They are
408:Properties
216:CHEBI:33141
208:CHEBI:35404
32:monochromat
3134:Categories
1710:2020-01-05
1632:"Chromite"
1616:3527306730
1514:Polyhedron
1376:References
1236:+ 7 O
1125:School bus
891:, but the
772:. All poly
547:in the +6
516:Dichromate
458:Molar mass
300:9LKY4BFN2V
292:9S2Y101D6M
165:3D model (
154:13907-47-6
146:13907-45-4
135:CAS Number
40:trichromat
3140:Chromates
2644:Chromates
1715:There is
1680:(2012) .
1454:231808339
1446:2196-7105
1183:Australia
789:weak acid
774:oxyanions
541:oxyanions
36:dichromat
2928:(OSi(OCH
2160:][CH
1340:See also
1287:Toxicity
1281:lópezite
1269:Crocoite
1190:chromite
1179:Tasmania
1079:+ 3 e →
1003:reducing
642:pyridine
634:covalent
606:peroxide
560:solution
555:. In an
545:chromium
500:Chromate
227:DrugBank
21:Chromate
3067:Related
3015:[NH
2830:[NH
2136:[Cr
1324:of the
1271:, PbCrO
914:⇌ HCrO
837:2
787:, is a
624:, CrO(O
557:aqueous
452:
249:PubChem
237:DB14182
3037:[C
2906:(OC(CH
2878:NH]CrO
2870:[C
2559:NH]CrO
2551:[C
2294:Cr(ClO
2218:Cr(OH)
1803:Cr(CO)
1693:
1642:
1613:
1577:
1547:
1482:
1452:
1444:
1403:
1322:cancer
1192:, FeCr
1081:Cr(OH)
747:, and
387:SMILES
111:Names
3105:EuCrO
2806:PbCrO
2796:CdCrO
2786:ZnCrO
2762:NiCrO
2734:BaCrO
2724:SrCrO
2714:CaCrO
2704:MgCrO
2694:BeCrO
2456:CrO(O
2180:Cr(NO
1969:Cr(CH
1704:(PDF)
1687:(PDF)
1450:S2CID
1352:Notes
1302:toxic
1160:redox
979:The p
949:⇌ Cr
680:2 CrO
638:ether
352:InChI
271:24503
263:24461
198:ChEBI
167:JSmol
42:, or
2859:KCrO
2748:(CrO
2513:CrOF
2409:Cr(O
2374:CrBr
2364:CrCl
2338:CrSi
2308:CrBr
2284:CrCl
2194:CrPO
2140:O(CH
2073:Cr(C
2042:CrBr
2032:CrCl
2016:CrSe
2007:CrSO
1987:CrSi
1954:Cr(C
1905:(CO)
1849:(CO)
1823:Cr(C
1691:ISBN
1678:IARC
1640:ISBN
1611:ISBN
1575:ISBN
1545:ISBN
1480:ISBN
1442:ISSN
1401:ISBN
1328:and
1326:nose
1304:and
1204:and
1150:and
1091:+ 5
1066:+ 4
839:HCrO
818:+ H;
796:HCrO
711:The
652:py.
431:and
282:UNII
3115:CrO
3080:CrO
3045:NH]
2924:CrO
2902:CrO
2838:CrO
2820:CrO
2776:CrO
2684:CrO
2670:CrO
2656:CrO
2571:CrF
2537:CrO
2523:CrO
2499:CrO
2474:CrO
2446:CrO
2423:CrF
2384:CrI
2354:CrF
2344:CrO
2318:CrI
2274:CrF
2246:(SO
2174:CrN
2130:CrB
2052:CrI
2022:CrF
2001:CrS
1996:CrO
1930:CrH
1872:CrH
1841:CrC
1603:doi
1522:doi
1434:doi
1430:236
1244:CrO
1056:CrO
962:+ H
936:HCr
923:+ H
910:CrO
887:CrO
883:, H
870:+ H
808:CrO
702:+ H
543:of
504:CrO
421:CrO
317:EPA
252:CID
3136::
3091:Cr
3049:Cr
3023:Cr
3001:Cr
2997:Ag
2983:Cr
2965:Cr
2961:Na
2882:Cl
2863:Cl
2816:Pb
2772:Ag
2744:Fe
2680:Cs
2652:Na
2563:Cl
2541:Br
2527:Cl
2485:Cr
2264:Te
2260:Cr
2242:Cr
2228:Cr
2204:Cr
2164:CO
2156:O)
2152:(H
2144:CO
2106:Cr
1973:CO
1940:Cr
1893:(C
1889:Cr
1713:.
1662:.
1634:.
1609:.
1589:^
1559:^
1518:16
1516:.
1448:.
1440:.
1428:.
1424:.
1336:.
1232:CO
1216::
1181:,
1146:,
1093:OH
1010:Cr
956:2−
851:Cr
849:⇌
806:⇌
791::
767:13
753:Cr
751:,
742:10
728:Cr
726:,
717:pH
696:2−
683:2−
675:.
608:,
520:Cr
514:.
433:Cr
38:,
34:,
30:,
3117:5
3107:4
3097:7
3095:O
3093:2
3089:2
3087:H
3085:/
3082:4
3078:2
3076:H
3055:7
3053:O
3051:2
3047:2
3043:5
3041:H
3039:5
3029:7
3027:O
3025:2
3021:2
3019:]
3017:4
3007:7
3005:O
3003:2
2999:2
2989:7
2987:O
2985:2
2981:2
2979:K
2971:7
2969:O
2967:2
2963:2
2938:2
2936:)
2934:3
2932:)
2930:3
2926:2
2916:2
2914:)
2912:3
2910:)
2908:3
2904:2
2880:3
2876:5
2874:H
2872:5
2861:3
2840:4
2836:2
2834:]
2832:4
2822:5
2818:2
2808:4
2798:4
2788:4
2778:4
2774:2
2764:4
2754:3
2752:)
2750:4
2746:2
2736:4
2726:4
2716:4
2706:4
2696:4
2686:4
2682:2
2672:4
2668:2
2666:K
2658:4
2654:2
2629:e
2622:t
2615:v
2573:6
2561:3
2557:5
2555:H
2553:5
2543:2
2539:2
2529:2
2525:2
2515:4
2505:2
2503:F
2501:2
2491:7
2489:O
2487:2
2483:2
2481:H
2479:/
2476:4
2472:2
2470:H
2462:2
2460:)
2458:2
2448:3
2425:5
2415:4
2413:)
2411:2
2407:3
2405:K
2386:4
2376:4
2366:4
2356:4
2346:2
2320:3
2310:3
2300:3
2298:)
2296:4
2286:3
2276:3
2266:3
2262:2
2252:3
2250:)
2248:4
2244:2
2234:3
2232:S
2230:2
2220:3
2210:3
2208:O
2206:2
2196:4
2186:3
2184:)
2182:3
2168:]
2166:2
2162:3
2158:3
2154:2
2150:6
2148:)
2146:2
2142:3
2138:3
2112:2
2110:C
2108:3
2083:2
2081:)
2079:5
2077:H
2075:5
2054:2
2044:2
2034:2
2024:2
2009:4
1989:2
1979:2
1977:)
1975:2
1971:3
1962:)
1960:4
1958:O
1956:2
1946:2
1944:C
1942:3
1932:2
1907:6
1903:2
1901:)
1899:5
1897:H
1895:5
1891:2
1851:3
1847:6
1845:H
1843:6
1833:2
1831:)
1829:6
1827:H
1825:6
1805:6
1780:e
1773:t
1766:v
1732:.
1666:.
1648:.
1619:.
1605::
1583:.
1554:.
1528:.
1524::
1488:.
1456:.
1436::
1409:.
1308:(
1273:4
1258:2
1254:3
1252:O
1250:2
1246:4
1242:2
1238:2
1234:3
1230:2
1226:4
1224:O
1222:2
1214:3
1212:O
1210:2
1198:4
1196:O
1194:2
1109:0
1106:ε
1086:3
1077:O
1073:2
1068:H
1061:4
1041:0
1038:ε
1033:O
1031:2
1024:7
1019:O
1015:2
984:a
981:K
973:a
970:K
968:p
964:,
959:7
953:O
951:2
946:7
943:−
940:O
938:2
920:4
917:−
912:4
908:2
906:H
898:a
895:K
893:p
889:4
885:2
874:O
872:2
865:7
860:O
856:2
844:4
827:a
824:K
822:p
813:4
801:4
785:4
778:4
762:O
758:4
737:O
733:3
706:O
704:2
699:7
693:O
691:2
686:4
650:2
648:)
646:2
630:2
628:)
626:2
615:2
610:O
534:7
529:O
525:2
509:4
447:7
442:O
438:2
426:4
319:)
315:(
169:)
46:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.