124:
27:
251:
Katoa, Y.; K. Suwaa; S. Yokoyamab; T. Yabeb; H. Ikutaa; Y. Uchimotoa; M. Wakihara (December 2002). "Thermally stable solid polymer electrolyte containing borate ester groups for lithium secondary battery".
140:
that flows under low stress, but breaks under higher stresses and pressures. This combination of fluid-like and solid-like properties makes it a
276:
192:
306:
216:
Cassassa, E. Z.; A. M. Sarquis; C. H. Van Dyke (January 1986). "The
Gelation of Polyvinyl Alcohol with Borax".
311:
39:
116:
is weak because of the ease with which the slime flows and pulls apart. However, even though this
316:
8:
137:
233:
261:
97:
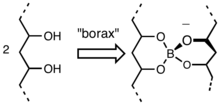
that cross links the chains of the PVA. Borate esters form readily by condensation of
109:
70:
62:
237:
257:
225:
141:
30:
Flubber polymer with green food coloring added. The polymer is normally colorless.
145:
20:
300:
105:
94:
112:
held together side-by-side by the borate molecules. It is evident that this
171:
161:
52:
277:"Polyvinyl Alcohol Slime - Gelfand Center - Carnegie Mellon University"
193:"Polyvinyl Alcohol Slime - Gelfand Center - Carnegie Mellon University"
117:
113:
58:
229:
123:
26:
215:
98:
74:
127:
Structure for borate ester that comprises crosslinking in "slime".
250:
149:
120:
is weak, it does alter the properties of the resulting polymer.
66:
166:
78:
108:. The resulting polymer network is composed of strands of
90:
104:
The individual polymer chains are bound together by
298:
144:. Its behavior can also be described as being
244:
69:compound. Slime can be made by combining
122:
25:
299:
274:
209:
190:
13:
14:
328:
93:process entails formation of a
57:is a rubbery polymer formed by
268:
184:
1:
275:University, Carnegie Mellon.
262:10.1016/s0167-2738(02)00370-3
218:Journal of Chemical Education
191:University, Carnegie Mellon.
177:
131:
7:
155:
84:
40:The Absent-Minded Professor
10:
333:
18:
19:For the 1997 film, see
128:
101:and the B-OH groups.
31:
307:Chemistry experiments
126:
37:(named from the film
29:
312:Non-Newtonian fluids
256:. 152–153: 155–159.
138:non-Newtonian fluid
106:weak hydrogen bonds
254:Solid State Ionics
129:
32:
110:polyvinyl alcohol
71:polyvinyl-acetate
63:polyvinyl alcohol
324:
291:
290:
288:
287:
272:
266:
265:
248:
242:
241:
230:10.1021/ed063p57
213:
207:
206:
204:
203:
188:
332:
331:
327:
326:
325:
323:
322:
321:
297:
296:
295:
294:
285:
283:
273:
269:
249:
245:
214:
210:
201:
199:
189:
185:
180:
158:
134:
99:hydroxyl groups
87:
24:
17:
16:Type of gelatin
12:
11:
5:
330:
320:
319:
314:
309:
293:
292:
267:
243:
208:
182:
181:
179:
176:
175:
174:
169:
164:
157:
154:
133:
130:
86:
83:
21:Flubber (film)
15:
9:
6:
4:
3:
2:
329:
318:
315:
313:
310:
308:
305:
304:
302:
282:
278:
271:
263:
259:
255:
247:
239:
235:
231:
227:
223:
219:
212:
198:
194:
187:
183:
173:
170:
168:
165:
163:
160:
159:
153:
151:
147:
143:
142:Maxwell fluid
139:
136:Flubber is a
125:
121:
119:
118:cross linking
115:
114:cross linking
111:
107:
102:
100:
96:
92:
82:
80:
76:
72:
68:
65:(PVA) with a
64:
60:
59:cross-linking
56:
55:
50:
46:
42:
41:
36:
28:
22:
317:Sensory toys
284:. Retrieved
280:
270:
253:
246:
221:
217:
211:
200:. Retrieved
196:
186:
146:viscoplastic
135:
103:
95:borate ester
88:
53:
48:
44:
38:
34:
33:
281:www.cmu.edu
197:www.cmu.edu
172:Slime (toy)
162:Silly Putty
301:Categories
286:2022-11-01
202:2022-11-01
178:References
150:gelatinous
132:Properties
224:(1): 57.
75:adhesives
238:94114885
156:See also
91:gelation
85:Reaction
73:-based
35:Flubber
236:
67:borate
49:Glurch
234:S2CID
167:Gunge
79:borax
77:with
54:Slime
51:, or
45:Glorp
89:The
258:doi
226:doi
148:or
81:.
61:of
43:),
303::
279:.
232:.
222:63
220:.
195:.
152:.
47:,
289:.
264:.
260::
240:.
228::
205:.
23:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.