151:
103:
210:
Aldonic acids are typically used in industrial applications for their ability to degrade naturally in the environment. This can be attributed to their affinity with water, as the polar bonds within the carboxylic acid group of aldonic acids allow them to interact with aquatic systems.
202:. The incorporation of these organic sugars into synthetic materials allow for a more renewable alternative to oil-based polymer synthesis, and increased structural durability within polymer chains.
167:
Anaerobic bacteria can also perform dehydrogenation to produce aldonic acids. This is done by synthesizing enzymes that are able to selectively oxidize aldoses to their corresponding aldonic acid.
17:
214:
The structural diversity of aldonic acids also allow for various properties. Their ring formation creates an added layer of rigidity when integrated with other materials.
123:. Cyanide in ammonia reacts with an aldose to produce an intermediate, which is then reacted with a hydronium ion to form an aldonic acid.
70:
The nomenclature of aldonic acids and their lactones is based on replacing the suffix "-ose" with "onic acid" or "onolactone". Hence, D-
191:, and other chemicals. As such, the use of aldonic acids for chemical applications is of growing interest to various industries.
321:
187:
are commonly oxidized to obtain aldonic acids. These products can then be used as the building blocks for preservatives,
641:"Polymers from sugars: cyclic monomer synthesis, ring-opening polymerisation, material properties and applications"
67:
carbon at the terminal end bearing the aldehyde, and they cannot form R−O−R′ linkages between different molecules.
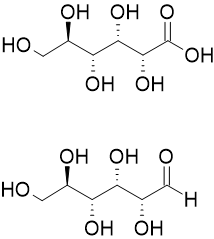
426:"Benedict's Solution, a Reagent for Measuring Reducing Sugars: the Clinical Chemistry of Stanley R. Benedict"
527:
Mehtiö, Tuomas; Toivari, Mervi; Wiebe, Marilyn G.; Harlin, Ali; Penttilä, Merja; Koivula, Anu (2016-09-02).
63:(-COOH group). Aldonic acids are generally found in their ring form. However, these rings do not have a
274:
528:
254:(4 ed.). Research Triangle Park, NC: International Union of Pure and Applied Chemistry (IUPAC).
640:
688:"Structures and vibrational spectra of indole carboxylic acids. Part I. Indole-2-carboxylic acid"
529:"Production and applications of carbohydrate-derived sugar acids as generic biobased chemicals"
579:
Galbis, Juan A.; García-Martín, M. de Gracia; de Paz, M. Violante; Galbis, Elsa (2016-02-10).
155:
140:
136:
64:
194:
Aldonic acids can be used as the natural starting materials to synthetic products including
739:
699:
470:
Wieschalka, Stefan; Blombach, Bastian; Bott, Michael; Eikmanns, Bernhard J. (March 2013).
8:
275:"Lactones: Classification, synthesis, biological activities, and industrial applications"
703:
580:
504:
471:
371:
442:
425:
273:
Sartori, Suélen Karine; Diaz, Marisa Alves
Nogueira; Diaz-Muñoz, Gaspar (2021-03-26).
715:
668:
660:
618:
610:
556:
548:
509:
491:
447:
406:
358:
317:
294:
120:
687:
711:
707:
652:
600:
592:
540:
499:
483:
437:
398:
366:
350:
286:
255:
188:
544:
60:
596:
79:
290:
733:
719:
664:
614:
552:
495:
451:
410:
402:
362:
298:
75:
21:
686:
Morzyk-Ociepa, Barbara; Michalska, Danuta; Pietraszko, Adam (January 2004).
487:
259:
95:
Aldonic acids are most commonly prepared by the oxidation of the sugar with
672:
622:
560:
513:
386:
228:
223:
199:
639:
Gregory, Georgina L.; López-Vidal, Eva M.; Buchard, Antoine (2017-02-14).
354:
605:
472:"Bio-based production of organic acids with C orynebacterium glutamicum"
338:
154:
The reaction scheme of an aldose being oxidized by the copper ions in a
143:
reagents. Copper ions react with an aldose to form a red precipitate, Cu
656:
36:
150:
106:
The reaction mechanism of bromine and water being used to oxidize the
195:
184:
180:
107:
102:
52:
424:
Simoni, Robert D.; Hill, Robert L.; Vaughan, Martha (April 2002).
343:
Journal of
Research of the National Bureau of Standards, Section A
176:
158:
solution. The R group provided is an example of a sugar backbone.
126:
119:
Alternatively, they arise by homologation of an aldose using the
96:
71:
25:
16:
685:
578:
132:
56:
469:
249:
316:. Springer advanced texts in chemistry. New York: Springer.
251:
The IUPAC Compendium of
Chemical Terminology: The Gold Book
638:
526:
272:
131:Aldonic acids are the products of the oxidation of
423:
90:
731:
581:"Synthetic Polymers from Sugar-Based Monomers"
127:Oxidation by Benedict's and Fehling's reagents
20:The skeletal structure of an aldonic acid,
604:
503:
441:
370:
149:
101:
15:
51:OH. They are obtained by oxidizing the
732:
336:
634:
632:
465:
463:
461:
311:
39:with the general chemical formula, HO
574:
572:
570:
314:Essentials of carbohydrate chemistry
247:
162:
114:
339:"Oxidation of aldoses with bromine"
13:
629:
458:
14:
751:
567:
533:Critical Reviews in Biotechnology
337:Isbell, Horace S. (March 1962).
679:
430:Journal of Biological Chemistry
170:
712:10.1016/j.molstruc.2003.09.027
692:Journal of Molecular Structure
520:
417:
379:
330:
305:
266:
241:
91:Oxidation by bromine and water
1:
545:10.3109/07388551.2015.1060189
443:10.1016/s0021-9258(19)61050-1
234:
205:
99:and water under neutral pH.
85:
7:
597:10.1021/acs.chemrev.5b00242
217:
10:
756:
248:Gold, Victor, ed. (2019).
291:10.1016/j.tet.2021.132001
403:10.15227/orgsyn.036.0038
175:In commercial settings,
645:Chemical Communications
488:10.1111/1751-7915.12013
476:Microbial Biotechnology
312:Robyt, John F. (1998).
260:10.1351/goldbook.a00212
24:(top), and its aldose,
159:
111:
29:
387:"d-gulonic-y-lactone"
355:10.6028/jres.066a.023
153:
105:
19:
704:2004JMoSt.688...79M
55:(-CHO group) of an
657:10.1039/C6CC09578J
160:
156:Benedict's reagent
112:
30:
651:(14): 2198–2217.
391:Organic Syntheses
323:978-0-387-94951-2
163:Natural synthesis
121:Strecker reaction
115:Strecker reaction
74:is oxidized to D-
747:
724:
723:
683:
677:
676:
636:
627:
626:
608:
591:(3): 1600–1636.
585:Chemical Reviews
576:
565:
564:
524:
518:
517:
507:
467:
456:
455:
445:
421:
415:
414:
383:
377:
376:
374:
334:
328:
327:
309:
303:
302:
270:
264:
263:
245:
189:buffering agents
755:
754:
750:
749:
748:
746:
745:
744:
730:
729:
728:
727:
684:
680:
637:
630:
577:
568:
525:
521:
468:
459:
422:
418:
385:
384:
380:
335:
331:
324:
310:
306:
271:
267:
246:
242:
237:
220:
208:
173:
165:
146:
129:
117:
93:
88:
61:carboxylic acid
50:
46:
42:
12:
11:
5:
753:
743:
742:
726:
725:
698:(1–3): 79–86.
678:
628:
566:
539:(5): 904–916.
519:
457:
416:
378:
349:(3): 233–239.
329:
322:
304:
265:
239:
238:
236:
233:
232:
231:
226:
219:
216:
207:
204:
172:
169:
164:
161:
144:
128:
125:
116:
113:
108:aldehyde group
92:
89:
87:
84:
80:gluconolactone
48:
44:
40:
9:
6:
4:
3:
2:
752:
741:
738:
737:
735:
721:
717:
713:
709:
705:
701:
697:
693:
689:
682:
674:
670:
666:
662:
658:
654:
650:
646:
642:
635:
633:
624:
620:
616:
612:
607:
602:
598:
594:
590:
586:
582:
575:
573:
571:
562:
558:
554:
550:
546:
542:
538:
534:
530:
523:
515:
511:
506:
501:
497:
493:
489:
485:
482:(2): 87–102.
481:
477:
473:
466:
464:
462:
453:
449:
444:
439:
436:(16): e5–e6.
435:
431:
427:
420:
412:
408:
404:
400:
396:
392:
388:
382:
373:
368:
364:
360:
356:
352:
348:
344:
340:
333:
325:
319:
315:
308:
300:
296:
292:
288:
284:
280:
276:
269:
261:
257:
253:
252:
244:
240:
230:
227:
225:
224:Aldaric acids
222:
221:
215:
212:
203:
201:
197:
192:
190:
186:
182:
178:
168:
157:
152:
148:
142:
138:
134:
124:
122:
110:of an aldose.
109:
104:
100:
98:
83:
81:
77:
76:gluconic acid
73:
68:
66:
62:
58:
54:
38:
34:
33:Aldonic acids
27:
23:
22:gluconic acid
18:
695:
691:
681:
648:
644:
606:11441/154263
588:
584:
536:
532:
522:
479:
475:
433:
429:
419:
397:: 38. 1956.
394:
390:
381:
346:
342:
332:
313:
307:
282:
278:
268:
250:
243:
229:Uronic acids
213:
209:
200:polyurethane
193:
174:
171:Applications
166:
130:
118:
94:
69:
32:
31:
740:Sugar acids
279:Tetrahedron
37:sugar acids
285:: 132001.
235:References
206:Properties
196:polyesters
147:O.
137:Benedict's
59:to form a
720:0022-2860
665:1364-548X
615:0009-2665
553:0738-8551
496:1751-7915
452:0021-9258
411:0078-6209
363:0022-4332
299:0040-4020
185:arabinose
181:galactose
141:Fehling's
86:Synthesis
28:(bottom).
734:Category
673:28127607
623:26291239
561:26177333
514:23199277
218:See also
53:aldehyde
700:Bibcode
505:3917452
372:5310681
177:glucose
133:aldoses
97:bromine
72:glucose
43:C(CHOH)
26:glucose
718:
671:
663:
621:
613:
559:
551:
512:
502:
494:
450:
409:
369:
361:
320:
297:
78:and D-
65:chiral
57:aldose
183:, or
716:ISSN
669:PMID
661:ISSN
619:PMID
611:ISSN
557:PMID
549:ISSN
510:PMID
492:ISSN
448:ISSN
407:ISSN
359:ISSN
318:ISBN
295:ISSN
198:and
35:are
708:doi
696:688
653:doi
601:hdl
593:doi
589:116
541:doi
500:PMC
484:doi
438:doi
434:277
399:doi
367:PMC
351:doi
347:66A
287:doi
256:doi
139:or
135:by
736::
714:.
706:.
694:.
690:.
667:.
659:.
649:53
647:.
643:.
631:^
617:.
609:.
599:.
587:.
583:.
569:^
555:.
547:.
537:36
535:.
531:.
508:.
498:.
490:.
478:.
474:.
460:^
446:.
432:.
428:.
405:.
395:36
393:.
389:.
365:.
357:.
345:.
341:.
293:.
283:84
281:.
277:.
179:,
82:.
47:CH
722:.
710::
702::
675:.
655::
625:.
603::
595::
563:.
543::
516:.
486::
480:6
454:.
440::
413:.
401::
375:.
353::
326:.
301:.
289::
262:.
258::
145:2
49:2
45:n
41:2
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.