3610:, its volume would be the sum of the unmixed components. The volume of 0.2 kg pure ethanol is 0.2 kg x 1.27 L/kg = 0.254 L, and the volume of 0.8 kg pure water is 0.8 kg x 1.0018 L/kg = 0.80144 L, so the ideal solution volume would be 0.254 L + 0.80144 L = 1.055 L. The nonideality of the solution is reflected by a slight decrease (roughly 2.2%, 1.0326 rather than 1.055 L/kg) in the volume of the combined system upon mixing. As the percent ethanol goes up toward 100%, the apparent molar volume rises to the molar volume of pure ethanol.
3530:
3583:
1207:
855:
1202:{\displaystyle {}^{\phi }{\tilde {V}}_{1}={\frac {V-V_{0}}{n_{1}}}=\left({\frac {m}{\rho }}-{\frac {m_{0}}{\rho _{0}^{0}}}\right){\frac {1}{n_{1}}}=\left({\frac {m_{1}+m_{0}}{\rho }}-{\frac {m_{0}}{\rho _{0}^{0}}}\right){\frac {1}{n_{1}}}=\left({\frac {m_{0}}{\rho }}-{\frac {m_{0}}{\rho _{0}^{0}}}\right){\frac {1}{n_{1}}}+{\frac {m_{1}}{\rho n_{1}}}}
3511:
2335:
4534:
1753:
5156:
There are situations when there is no rigorous way to define which is solvent and which is solute like in the case of liquid mixtures (say water and ethanol) that can dissolve or not a solid like sugar or salt. In these cases apparent molar properties can and must be ascribed to all components of the
3602:
of 1.0326 liters per kg at 20 °C, while pure water is 1.0018 L/kg (1.0018 cc/g). The apparent volume of the added ethanol is 1.0326 L – 0.8 kg x 1.0018 L/kg = 0.2317 L. The number of moles of ethanol is 0.2 kg / (0.04607 kg/mol) = 4.341 mol, so that the apparent molar volume
55:, provided that the properties of the other solution components are assumed to remain constant during the addition. However this assumption is often not justified, since the values of apparent molar properties of a component may be quite different from its molar properties in the pure state.
3574:−6.7 cm/mole. This means that their solutions in a given amount of water have a smaller volume than the same amount of pure water. (The effect is small, however.) The physical reason is that nearby water molecules are strongly attracted to the ions so that they occupy less space.
2068:
1466:
1333:
4292:
2673:
for comparison. In other words, we assume that the volume of the solvent does not change, and we use the partial molar volume where the number of moles of the solute is exactly zero ("the molar volume"). Thus, in the defining expression for apparent molar volume
3280:
3219:
5255:
This labelling is arbitrary. For mixtures of two liquids either may be described as solvent. For mixtures of a liquid and a solid, the liquid is usually identified as the solvent and the solid as the solute, but the theory is still valid if the labels are
4043:
3776:
2142:
2854:
2667:
1899:
197:
4305:
1524:
4298:
The sum of products molalities – apparent molar volumes of solutes in their binary solutions equals the product between the sum of molalities of solutes and apparent molar volume in ternary of multicomponent solution mentioned above.
1517:
The sum of products molalities – apparent molar volumes of solutes in their binary solutions equals the product between the sum of molalities of solutes and apparent molar volume in ternary of multicomponent solution mentioned above.
401:
is the number of moles of the solute in the solution. By dividing this relation to the molar amount of one component a relation between the apparent molar property of a component and the mixing ratio of components can be obtained.
4936:
5069:
Of course the complement volume of a component in respect to other components of the mixture can be defined as a difference between the volume of the mixture and the volume of a binary submixture of a given composition like:
4800:
4673:
738:
3889:
4544:
and define the apparent molar volume of each solute with reference to a binary system containing both other components: water and the other solute. The apparent molar volumes of each of the two solutes are then
1910:
1348:
844:
3537:
of the mixture is smaller than the sum of the individual volumes, as the water can lodge in the spaces between the sand grains. A similar situation with a different mechanism occurs when ethanol is mixed with
1215:
5151:
3092:
2432:
4102:
2516:
3025:
3506:{\displaystyle \ln \gamma _{s}={\frac {h-\nu }{\nu }}\ln(1+{\frac {br_{a}}{55.5}})-{\frac {h}{\nu }}\ln(1-{\frac {br_{a}}{55.5}})+{\frac {br_{a}(r_{a}+h-\nu )}{55.5(1+{\frac {br_{a}}{55.5}})}}}
2134:
3103:
612:
555:
501:
451:
362:
5035:
4987:
2717:
3236:
between the apparent molar volume of a dissolved electrolyte in a concentrated solution and the molar volume of the solvent (water) can be linked to the statistical component of the
2967:
5423:
Apparent molar volumes and apparent molar heat capacities of Pr(NO3)3(aq), Gd(NO3)3(aq), Ho(NO3)3(aq), and Y(NO3)3(aq) at T = (288.15, 298.15, 313.15, and 328.15) K and p = 0.1 MPa
3900:
3097:
Quantitatively, the relation between partial molar properties and the apparent ones can be derived from the definition of the apparent quantities and of the molality. For volume,
509:
that the molar volume of the solvent is unchanged by the addition of solute. However this assumption must often be considered unrealistic as shown in the examples below, so that
1512:
3641:
2552:
2330:{\displaystyle {\bar {V_{0}}}={\Big (}{\frac {\partial V}{\partial n_{0}}}{\Big )}_{T,p,n_{1}},{\bar {V_{1}}}={\Big (}{\frac {\partial V}{\partial n_{1}}}{\Big )}_{T,p,n_{0}}}
273:
2921:
616:. Some authors have reported apparent molar volumes of both (liquid) components of the same solution. This procedure can be extended to ternary and multicomponent mixtures.
619:
Apparent quantities can also be expressed using mass instead of number of moles. This expression produces apparent specific quantities, like the apparent specific volume.
3264:
4529:{\displaystyle {}^{\phi }{\tilde {V}}_{123..}(b_{1}+b_{2}+b_{3}+...)=b_{1}{}^{\phi }{\tilde {V}}_{1}+b_{2}{}^{\phi }{\tilde {V}}_{2}+b_{3}{}^{\phi }{\tilde {V}}_{3}+...}
2725:
2560:
1770:
1748:{\displaystyle {}^{\phi }{\tilde {V}}_{123..}(b_{1}+b_{2}+b_{3}+...)=b_{1}{}^{\phi }{\tilde {V}}_{1}+b_{2}{}^{\phi }{\tilde {V}}_{2}+b_{3}{}^{\phi }{\tilde {V}}_{3}+...}
68:
5353:
4094:
2885:
397:
308:
229:
3603:
is 0.2317 L / 4.341 mol = 0.0532 L / mol = 53.2 cc/mole (1.16 cc/g). However pure ethanol has a molar volume at this temperature of 58.4 cc/mole (1.27 cc/g).
4067:
455:. The first term is equal to the volume of the same quantity of solvent with no solute, and the second term is the change of volume on addition of the solute.
5752:
4811:
4678:
4551:
5286:
DENSITIES, EXCESS MOLAR AND PARTIAL MOLAR VOLUMES FOR DIETHYLSULFOXIDE WITH METHANOL OR ETHANOL BINARY SYSTEMS AT TEMPERATURE RANGE 298.15 – 323.15 K
625:
5324:
3793:
5413:
3786:
of the materials and therefore unambiguously defined in multicomponent systems. For example, partial molar volume is defined for each component
5381:
2063:{\displaystyle {}^{\phi }{\tilde {V}}_{1}={\frac {V}{n_{1}}}-{\tilde {V}}_{0}{\frac {n_{0}}{n_{1}}}={\frac {V}{n_{1}}}-{\tilde {V}}_{0}r_{01}}
5328:
1764:
A relation between the apparent molar of a component of a mixture and molar mixing ratio can be obtained by dividing the definition relation
1461:{\displaystyle {}^{\phi }{\tilde {V}}_{12..}={\frac {1}{b_{T}}}\left({\frac {1}{\rho }}-{\frac {1}{\rho _{0}^{0}}}\right)+{\frac {M}{\rho }}}
5454:
1328:{\displaystyle {}^{\phi }{\tilde {V}}_{1}={\frac {1}{b}}\left({\frac {1}{\rho }}-{\frac {1}{\rho _{0}^{0}}}\right)+{\frac {M_{1}}{\rho }}}
786:
1338:
For more solutes the above equality is modified with the mean molar mass of the solutes as if they were a single solute with molality b
5076:
3590:
Another example of the apparent molar volume of the second component is less than its molar volume as a pure substance is the case of
3030:
3551:
has a volume of 27 cm per mole, but the apparent molar volume at low concentrations is only 16.6 cc/mole. In fact, some aqueous
4287:{\displaystyle {}^{\phi }{\tilde {V}}(n_{1},n_{2},n_{3},..)={}^{\phi }{\tilde {V}}_{123..}={\frac {V-V_{0}}{n_{1}+n_{2}+n_{3}+...}}}
5285:
2344:
2078:
Note the contrasting definitions between partial molar quantity and apparent molar quantity: in the case of partial molar volumes
3599:
3894:
One description of ternary aqueous solutions considers only the weighted mean apparent molar volume of the solutes, defined as
2437:
746:
Apparent (molar) properties are not constants (even at a given temperature), but are functions of the composition. At infinite
5361:
2972:
5298:
Glueckauf, E. (1955). "The
Influence of Ionic Hydration on Activity Coefficients in Concentrated Electrolyte Solutions".
3783:
5818:
5499:
3618:
Apparent quantities can underline interactions in electrolyte – non-electrolyte systems which show interactions like
3214:{\displaystyle {\bar {V_{1}}}={}^{\phi }{\tilde {V}}_{1}+b{\frac {\partial {}^{\phi }{\tilde {V}}_{1}}{\partial b}}.}
5447:
2081:
571:
514:
460:
410:
321:
4992:
4947:
2677:
780:
of that solute (and of the densities of the solution and solvent). The volume of solution per mole of solute is
5589:
3547:
The apparent molar volume of salt is usually less than the molar volume of the solid salt. For instance, solid
4038:{\displaystyle {}^{\phi }{\tilde {V}}(n_{1},n_{2})={}^{\phi }{\tilde {V}}_{12}={\frac {V-V_{0}}{n_{1}+n_{2}}}}
3638:(3-component) solution with one solvent and two solutes as an example, there would still be only one equation
2518:
always holds. In contrast, in the definition of apparent molar volume, the molar volume of the pure solvent,
2926:
3634:
For multicomponent solutions, apparent molar properties can be defined in several ways. For the volume of a
5742:
35:
or solution is a quantity defined with the purpose of isolating the contribution of each component to the
5813:
5672:
5440:
5181:
3771:{\displaystyle (V={\tilde {V}}_{0}n_{0}+{}^{\phi }{\tilde {V}}_{1}n_{1}+{}^{\phi }{\tilde {V}}_{2}n_{2})}
3224:
5727:
1471:
5410:
5405:
2521:
242:
5823:
5619:
2890:
5604:
5539:
2849:{\displaystyle V=V_{0}+{}^{\phi }{V}_{1}\ ={\tilde {V}}_{0}n_{0}+{}^{\phi }{\tilde {V}}_{1}n_{1}\,}
2662:{\displaystyle {\tilde {V_{0}}}={\Big (}{\frac {\partial V}{\partial n_{0}}}{\Big )}_{T,p,n_{1}=0}}
1894:{\displaystyle V=V_{0}+{}^{\phi }{V}_{1}\ ={\tilde {V}}_{0}n_{0}+{}^{\phi }{\tilde {V}}_{1}n_{1}\,}
192:{\displaystyle V=V_{0}+{}^{\phi }{V}_{1}\ ={\tilde {V}}_{0}n_{0}+{}^{\phi }{\tilde {V}}_{1}n_{1}\,}
3626:, but also give insights in ion-ion interactions, especially by their dependence on temperature.
3242:
47:
of that component added, when all of that component is added to the solution. It is described as
4096:
the volume of pure water. This method can be extended for mixtures with more than 3 components.
5657:
5652:
5509:
5236:
5216:
3779:
751:
5275:
Rock, Peter A., Chemical
Thermodynamics, MacMillan 1969, p.227-230 for water-ethanol mixtures.
5757:
5737:
5221:
3582:
566:
An apparent molar quantity can be similarly defined for the component identified as solvent
5471:
5463:
5206:
4072:
3237:
2863:
849:
Subtracting the volume of pure solvent per mole of solute gives the apparent molal volume:
375:
286:
207:
28:
8:
5584:
5554:
5037:
is not to be confused with volumes of partial binary mixtures with one common component
4944:
The apparent molar volume of two components or solutes considered as one pseudocomponent
3027:, and so the apparent molar volume and partial molar volume of the solute also converge:
5186:
4052:
3778:, which is insufficient to determine the two apparent volumes. (This is in contrast to
5411:
The (p,ρ,T) Properties and
Apparent Molar Volumes of ethanol solutions of LiI or ZnCl2
3607:
5762:
5721:
5624:
5357:
3225:
Relation to the activity coefficient of an electrolyte and its solvation shell number
4931:{\displaystyle {}^{\phi }{\tilde {V}}_{0}={\frac {V-V(solute\ 1+solute\ 2)}{n_{0}}}}
16:
Difference in properties of one mole of substance in a mixture vs. an ideal solution
5697:
5692:
5524:
5484:
5307:
5201:
5196:
5176:
3564:
3529:
3517:
where ν is the number of ions due to dissociation of the electrolyte, and b is the
4795:{\displaystyle {}^{\phi }{\tilde {V}}_{2}={\frac {V-V(solvent+solute\ 1)}{n_{2}}}}
4668:{\displaystyle {}^{\phi }{\tilde {V}}_{1}={\frac {V-V(solvent+solute\ 2)}{n_{1}}}}
5682:
5667:
5599:
5564:
5549:
5544:
5529:
5494:
5417:
5211:
5166:
3267:
5427:
5422:
5344:
Calculated from data in the CRC Handbook of
Chemistry and Physics, 49th edition.
5732:
5687:
5677:
5594:
5489:
5479:
5171:
733:{\displaystyle V=V_{0}+{}^{\phi }{V}_{1}\ =v_{0}m_{0}+{}^{\phi }{v}_{1}m_{1}\,}
58:
For instance, the volume of a solution containing two components identified as
36:
20:
773:
The apparent (molal) volume of a solute can be expressed as a function of the
5807:
5777:
5614:
5574:
5519:
762:
3884:{\displaystyle {\bar {V_{i}}}=(\partial V/\partial n_{i})_{T,p,n_{j\neq i}}}
1904:
to the number of moles of one component. This gives the following relation:
747:
5634:
5629:
5231:
5191:
5052:
3595:
278:
39:. It shows the change in the corresponding solution property (for example,
5787:
5702:
5311:
4941:
However, this is an unsatisfactory description of volumetric properties.
3623:
3552:
3534:
757:
Some apparent molar properties that are commonly used are apparent molar
313:
44:
5782:
5772:
5767:
5644:
5288:
PROCEEDINGS OF THE YEREVAN STATE UNIVERSITY no.2, p.17-25. See Table 4.
5226:
3619:
2887:
is attributed to the pure solvent, while the "leftover" excess volume,
5792:
5747:
5662:
5432:
2923:, is considered to originate from the solute. At high dilution with
51:
because it appears to represent the molar property of that component
839:{\displaystyle {\frac {1}{\rho }}\left({\frac {1}{b}}+M_{1}\right).}
5609:
3518:
774:
758:
5712:
5514:
5504:
3591:
59:
32:
5146:{\displaystyle {}^{c}{\tilde {V}}_{2}={\frac {V-V_{01}}{n_{2}}}}
3087:{\displaystyle {}^{\phi }{\tilde {V}}_{1}\approx {\bar {V}}_{1}}
367:
234:
40:
743:
where the specific quantities are denoted with small letters.
5534:
2427:{\displaystyle dV={\bar {V_{0}}}dn_{0}+{\bar {V_{1}}}dn_{1}}
3560:
3556:
3548:
2511:{\displaystyle V={\bar {V_{0}}}n_{0}+{\bar {V_{1}}}n_{1}}
2073:
505:
may then be considered as the molar volume of the solute
3613:
281:(at the same temperature and pressure as the solution),
3629:
3020:{\displaystyle {\tilde {V_{0}}}\approx {\bar {V_{0}}}}
5428:
Isotopic effects for electrolytes apparent properties
5372:
Harned, Owen, op. cit. third edition 1958, p. 398-399
5079:
4995:
4950:
4814:
4681:
4554:
4308:
4105:
4075:
4055:
3903:
3796:
3644:
3533:
Everyday example: when sand is mixed with water, the
3283:
3245:
3106:
3033:
2975:
2929:
2893:
2866:
2728:
2680:
2563:
2524:
2440:
2347:
2145:
2084:
1913:
1773:
1527:
1474:
1351:
1218:
858:
789:
628:
574:
517:
463:
413:
378:
324:
289:
245:
210:
71:
5753:
List of boiling and freezing information of solvents
750:, an apparent molar property and the corresponding
5145:
5029:
4981:
4930:
4794:
4667:
4528:
4286:
4088:
4061:
4037:
3883:
3770:
3505:
3258:
3213:
3086:
3019:
2961:
2915:
2879:
2848:
2711:
2661:
2546:
2510:
2426:
2329:
2128:
2062:
1893:
1747:
1506:
1460:
1327:
1201:
838:
732:
606:
549:
495:
445:
391:
356:
302:
267:
223:
191:
4540:Another method is to treat the ternary system as
2623:
2588:
2297:
2262:
2205:
2170:
237:of the pure solvent before adding the solute and
5805:
5406:Apparent Molar Properties: Solutions: Background
5333:The Physical Chemistry of Electrolytic Solutions
3586:Excess volume of a mixture of ethanol and water
5448:
4805:The apparent molar volume of the solvent is:
2129:{\displaystyle {\bar {V_{0}}},{\bar {V_{1}}}}
607:{\displaystyle {}^{\phi }{\tilde {V}}_{0}\,}
550:{\displaystyle {}^{\phi }{\tilde {V}}_{1}\,}
496:{\displaystyle {}^{\phi }{\tilde {V}}_{1}\,}
446:{\displaystyle {}^{\phi }{\tilde {V}}_{1}\,}
357:{\displaystyle {}^{\phi }{\tilde {V}}_{1}\,}
5284:H. H. Ghazoyan and Sh. A. Markarian (2014)
5030:{\displaystyle {}^{\phi }{\tilde {V}}_{ij}}
4982:{\displaystyle {}^{\phi }{\tilde {V}}_{12}}
2554:, is used instead, which can be written as
1759:
5455:
5441:
2712:{\displaystyle {}^{\phi }{\tilde {V}}_{1}}
405:This equation serves as the definition of
5356:Apelblat, Alexander (Springer 2014) p.50
5297:
2845:
1890:
729:
603:
546:
492:
442:
353:
188:
3581:
3528:
2962:{\displaystyle n_{0}\gg n_{1}\approx 0}
768:
5806:
5462:
3555:have negative apparent molar volumes:
2074:Relation to partial (molar) quantities
5436:
3614:Electrolyte – non-electrolyte systems
3630:Multicomponent mixtures or solutions
366:is the apparent molar volume of the
5300:Transactions of the Faraday Society
13:
3833:
3822:
3199:
3166:
2604:
2596:
2278:
2270:
2186:
2178:
14:
5835:
5399:
2136:, defined by partial derivatives
1507:{\displaystyle M=\sum y_{i}M_{i}}
2547:{\displaystyle {\tilde {V}}_{0}}
268:{\displaystyle {\tilde {V}}_{0}}
5387:
5055:form a certain ternary mixture
3542:
2916:{\displaystyle {}^{\phi }V_{1}}
5375:
5366:
5347:
5338:
5318:
5291:
5278:
5269:
5249:
5096:
5012:
4967:
4912:
4858:
4831:
4776:
4725:
4698:
4649:
4598:
4571:
4502:
4461:
4420:
4388:
4337:
4325:
4197:
4175:
4127:
4121:
3973:
3951:
3925:
3919:
3847:
3819:
3810:
3765:
3743:
3702:
3661:
3645:
3497:
3466:
3458:
3433:
3411:
3380:
3358:
3327:
3185:
3145:
3120:
3072:
3050:
3011:
2989:
2823:
2782:
2697:
2577:
2532:
2492:
2460:
2405:
2370:
2251:
2159:
2120:
2098:
2038:
1972:
1930:
1868:
1827:
1721:
1680:
1639:
1607:
1556:
1544:
1368:
1235:
875:
591:
534:
480:
430:
341:
253:
166:
125:
1:
5263:
3594:in water. For example, at 20
765:, and apparent molar volume.
5743:Inorganic nonaqueous solvent
7:
5182:Enthalpy change of solution
5160:
4069:is the solution volume and
3524:
3266:of the electrolyte and its
3259:{\displaystyle \gamma _{s}}
37:non-ideality of the mixture
10:
5840:
5728:Acid dissociation constant
3577:
5711:
5643:
5573:
5470:
5051:which mixed in a certain
3782:, which are well-defined
3600:the solution has a volume
5819:Thermodynamic properties
5242:
3780:partial molar properties
1760:Relation to mixing ratio
559:is described only as an
5693:Solubility table (data)
5560:Apparent molar property
62:and solute is given by
25:apparent molar property
5658:Total dissolved solids
5653:Solubility equilibrium
5578:and related quantities
5237:Thermodynamic activity
5217:Partial molar property
5147:
5031:
4983:
4932:
4796:
4669:
4530:
4288:
4090:
4063:
4039:
3885:
3772:
3587:
3539:
3507:
3260:
3215:
3088:
3021:
2963:
2917:
2881:
2850:
2713:
2663:
2548:
2512:
2428:
2331:
2130:
2064:
1895:
1749:
1508:
1462:
1329:
1203:
840:
752:partial molar property
734:
608:
551:
497:
447:
393:
358:
304:
269:
225:
193:
5758:Partition coefficient
5738:Polar aprotic solvent
5222:Excess molar quantity
5148:
5032:
4984:
4933:
4797:
4670:
4531:
4289:
4091:
4089:{\displaystyle V_{0}}
4064:
4040:
3886:
3773:
3606:If the solution were
3585:
3532:
3508:
3261:
3216:
3089:
3022:
2964:
2918:
2882:
2880:{\displaystyle V_{0}}
2851:
2714:
2664:
2549:
2513:
2429:
2332:
2131:
2065:
1896:
1750:
1509:
1463:
1330:
1204:
841:
735:
609:
552:
498:
448:
394:
392:{\displaystyle n_{1}}
359:
305:
303:{\displaystyle n_{0}}
270:
226:
224:{\displaystyle V_{0}}
194:
5673:Enthalpy of solution
5600:Volume concentration
5595:Number concentration
5312:10.1039/TF9555101235
5207:Ion transport number
5077:
4993:
4948:
4812:
4679:
4552:
4306:
4103:
4073:
4053:
3901:
3794:
3784:intensive properties
3642:
3281:
3243:
3238:activity coefficient
3104:
3031:
2973:
2927:
2891:
2864:
2726:
2678:
2561:
2522:
2438:
2345:
2143:
2082:
1911:
1771:
1525:
1472:
1349:
1216:
856:
787:
769:Relation to molality
626:
572:
515:
461:
411:
376:
322:
287:
243:
208:
69:
5585:Molar concentration
5555:Dilution (equation)
1437:
1297:
1142:
1063:
969:
5814:Physical chemistry
5625:Isotopic abundance
5590:Mass concentration
5464:Chemical solutions
5416:2016-03-04 at the
5187:Enthalpy of mixing
5143:
5027:
4979:
4928:
4792:
4665:
4526:
4284:
4086:
4059:
4035:
3881:
3768:
3588:
3540:
3503:
3256:
3211:
3084:
3017:
2959:
2913:
2877:
2846:
2709:
2659:
2544:
2508:
2424:
2327:
2126:
2060:
1891:
1745:
1504:
1458:
1423:
1325:
1283:
1199:
1128:
1049:
955:
836:
730:
604:
547:
493:
443:
389:
354:
300:
265:
221:
189:
5801:
5800:
5362:978-3-319-11233-6
5141:
5099:
5015:
4970:
4926:
4908:
4881:
4834:
4790:
4772:
4701:
4663:
4645:
4574:
4505:
4464:
4423:
4328:
4282:
4200:
4124:
4062:{\displaystyle V}
4033:
3976:
3922:
3813:
3746:
3705:
3664:
3501:
3495:
3409:
3372:
3356:
3319:
3206:
3188:
3148:
3123:
3075:
3053:
3014:
2992:
2826:
2785:
2771:
2700:
2618:
2580:
2535:
2495:
2463:
2408:
2373:
2292:
2254:
2200:
2162:
2123:
2101:
2041:
2026:
2006:
1975:
1960:
1933:
1871:
1830:
1816:
1724:
1683:
1642:
1547:
1456:
1438:
1413:
1398:
1371:
1323:
1298:
1273:
1258:
1238:
1197:
1165:
1143:
1111:
1086:
1064:
1032:
992:
970:
938:
920:
878:
813:
798:
761:, apparent molar
671:
594:
537:
483:
433:
344:
312:is the number of
256:
169:
128:
114:
5831:
5824:Molar quantities
5698:Solubility chart
5525:Phase separation
5485:Aqueous solution
5457:
5450:
5443:
5434:
5433:
5394:
5391:
5385:
5379:
5373:
5370:
5364:
5351:
5345:
5342:
5336:
5322:
5316:
5315:
5295:
5289:
5282:
5276:
5273:
5257:
5253:
5202:Hydration energy
5197:Heat of dilution
5177:Regular solution
5152:
5150:
5149:
5144:
5142:
5140:
5139:
5130:
5129:
5128:
5112:
5107:
5106:
5101:
5100:
5092:
5088:
5087:
5082:
5036:
5034:
5033:
5028:
5026:
5025:
5017:
5016:
5008:
5004:
5003:
4998:
4988:
4986:
4985:
4980:
4978:
4977:
4972:
4971:
4963:
4959:
4958:
4953:
4937:
4935:
4934:
4929:
4927:
4925:
4924:
4915:
4906:
4879:
4847:
4842:
4841:
4836:
4835:
4827:
4823:
4822:
4817:
4801:
4799:
4798:
4793:
4791:
4789:
4788:
4779:
4770:
4714:
4709:
4708:
4703:
4702:
4694:
4690:
4689:
4684:
4674:
4672:
4671:
4666:
4664:
4662:
4661:
4652:
4643:
4587:
4582:
4581:
4576:
4575:
4567:
4563:
4562:
4557:
4535:
4533:
4532:
4527:
4513:
4512:
4507:
4506:
4498:
4494:
4493:
4488:
4485:
4484:
4472:
4471:
4466:
4465:
4457:
4453:
4452:
4447:
4444:
4443:
4431:
4430:
4425:
4424:
4416:
4412:
4411:
4406:
4403:
4402:
4375:
4374:
4362:
4361:
4349:
4348:
4336:
4335:
4330:
4329:
4321:
4317:
4316:
4311:
4293:
4291:
4290:
4285:
4283:
4281:
4268:
4267:
4255:
4254:
4242:
4241:
4231:
4230:
4229:
4213:
4208:
4207:
4202:
4201:
4193:
4189:
4188:
4183:
4165:
4164:
4152:
4151:
4139:
4138:
4126:
4125:
4117:
4114:
4113:
4108:
4095:
4093:
4092:
4087:
4085:
4084:
4068:
4066:
4065:
4060:
4044:
4042:
4041:
4036:
4034:
4032:
4031:
4030:
4018:
4017:
4007:
4006:
4005:
3989:
3984:
3983:
3978:
3977:
3969:
3965:
3964:
3959:
3950:
3949:
3937:
3936:
3924:
3923:
3915:
3912:
3911:
3906:
3890:
3888:
3887:
3882:
3880:
3879:
3878:
3877:
3845:
3844:
3832:
3815:
3814:
3809:
3808:
3799:
3777:
3775:
3774:
3769:
3764:
3763:
3754:
3753:
3748:
3747:
3739:
3735:
3734:
3729:
3723:
3722:
3713:
3712:
3707:
3706:
3698:
3694:
3693:
3688:
3682:
3681:
3672:
3671:
3666:
3665:
3657:
3512:
3510:
3509:
3504:
3502:
3500:
3496:
3491:
3490:
3489:
3476:
3461:
3445:
3444:
3432:
3431:
3418:
3410:
3405:
3404:
3403:
3390:
3373:
3365:
3357:
3352:
3351:
3350:
3337:
3320:
3315:
3304:
3299:
3298:
3265:
3263:
3262:
3257:
3255:
3254:
3220:
3218:
3217:
3212:
3207:
3205:
3197:
3196:
3195:
3190:
3189:
3181:
3177:
3176:
3171:
3164:
3156:
3155:
3150:
3149:
3141:
3137:
3136:
3131:
3125:
3124:
3119:
3118:
3109:
3093:
3091:
3090:
3085:
3083:
3082:
3077:
3076:
3068:
3061:
3060:
3055:
3054:
3046:
3042:
3041:
3036:
3026:
3024:
3023:
3018:
3016:
3015:
3010:
3009:
3000:
2994:
2993:
2988:
2987:
2978:
2968:
2966:
2965:
2960:
2952:
2951:
2939:
2938:
2922:
2920:
2919:
2914:
2912:
2911:
2902:
2901:
2896:
2886:
2884:
2883:
2878:
2876:
2875:
2855:
2853:
2852:
2847:
2844:
2843:
2834:
2833:
2828:
2827:
2819:
2815:
2814:
2809:
2803:
2802:
2793:
2792:
2787:
2786:
2778:
2769:
2768:
2767:
2762:
2756:
2755:
2750:
2744:
2743:
2718:
2716:
2715:
2710:
2708:
2707:
2702:
2701:
2693:
2689:
2688:
2683:
2668:
2666:
2665:
2660:
2658:
2657:
2650:
2649:
2627:
2626:
2619:
2617:
2616:
2615:
2602:
2594:
2592:
2591:
2582:
2581:
2576:
2575:
2566:
2553:
2551:
2550:
2545:
2543:
2542:
2537:
2536:
2528:
2517:
2515:
2514:
2509:
2507:
2506:
2497:
2496:
2491:
2490:
2481:
2475:
2474:
2465:
2464:
2459:
2458:
2449:
2433:
2431:
2430:
2425:
2423:
2422:
2410:
2409:
2404:
2403:
2394:
2388:
2387:
2375:
2374:
2369:
2368:
2359:
2336:
2334:
2333:
2328:
2326:
2325:
2324:
2323:
2301:
2300:
2293:
2291:
2290:
2289:
2276:
2268:
2266:
2265:
2256:
2255:
2250:
2249:
2240:
2234:
2233:
2232:
2231:
2209:
2208:
2201:
2199:
2198:
2197:
2184:
2176:
2174:
2173:
2164:
2163:
2158:
2157:
2148:
2135:
2133:
2132:
2127:
2125:
2124:
2119:
2118:
2109:
2103:
2102:
2097:
2096:
2087:
2069:
2067:
2066:
2061:
2059:
2058:
2049:
2048:
2043:
2042:
2034:
2027:
2025:
2024:
2012:
2007:
2005:
2004:
1995:
1994:
1985:
1983:
1982:
1977:
1976:
1968:
1961:
1959:
1958:
1946:
1941:
1940:
1935:
1934:
1926:
1922:
1921:
1916:
1900:
1898:
1897:
1892:
1889:
1888:
1879:
1878:
1873:
1872:
1864:
1860:
1859:
1854:
1848:
1847:
1838:
1837:
1832:
1831:
1823:
1814:
1813:
1812:
1807:
1801:
1800:
1795:
1789:
1788:
1754:
1752:
1751:
1746:
1732:
1731:
1726:
1725:
1717:
1713:
1712:
1707:
1704:
1703:
1691:
1690:
1685:
1684:
1676:
1672:
1671:
1666:
1663:
1662:
1650:
1649:
1644:
1643:
1635:
1631:
1630:
1625:
1622:
1621:
1594:
1593:
1581:
1580:
1568:
1567:
1555:
1554:
1549:
1548:
1540:
1536:
1535:
1530:
1513:
1511:
1510:
1505:
1503:
1502:
1493:
1492:
1467:
1465:
1464:
1459:
1457:
1449:
1444:
1440:
1439:
1436:
1431:
1419:
1414:
1406:
1399:
1397:
1396:
1384:
1379:
1378:
1373:
1372:
1364:
1360:
1359:
1354:
1334:
1332:
1331:
1326:
1324:
1319:
1318:
1309:
1304:
1300:
1299:
1296:
1291:
1279:
1274:
1266:
1259:
1251:
1246:
1245:
1240:
1239:
1231:
1227:
1226:
1221:
1208:
1206:
1205:
1200:
1198:
1196:
1195:
1194:
1181:
1180:
1171:
1166:
1164:
1163:
1151:
1149:
1145:
1144:
1141:
1136:
1127:
1126:
1117:
1112:
1107:
1106:
1097:
1087:
1085:
1084:
1072:
1070:
1066:
1065:
1062:
1057:
1048:
1047:
1038:
1033:
1028:
1027:
1026:
1014:
1013:
1003:
993:
991:
990:
978:
976:
972:
971:
968:
963:
954:
953:
944:
939:
931:
921:
919:
918:
909:
908:
907:
891:
886:
885:
880:
879:
871:
867:
866:
861:
845:
843:
842:
837:
832:
828:
827:
826:
814:
806:
799:
791:
739:
737:
736:
731:
728:
727:
718:
717:
712:
706:
705:
700:
694:
693:
684:
683:
669:
668:
667:
662:
656:
655:
650:
644:
643:
615:
613:
611:
610:
605:
602:
601:
596:
595:
587:
583:
582:
577:
558:
556:
554:
553:
548:
545:
544:
539:
538:
530:
526:
525:
520:
507:if it is assumed
504:
502:
500:
499:
494:
491:
490:
485:
484:
476:
472:
471:
466:
454:
452:
450:
449:
444:
441:
440:
435:
434:
426:
422:
421:
416:
400:
398:
396:
395:
390:
388:
387:
365:
363:
361:
360:
355:
352:
351:
346:
345:
337:
333:
332:
327:
311:
309:
307:
306:
301:
299:
298:
276:
274:
272:
271:
266:
264:
263:
258:
257:
249:
232:
230:
228:
227:
222:
220:
219:
198:
196:
195:
190:
187:
186:
177:
176:
171:
170:
162:
158:
157:
152:
146:
145:
136:
135:
130:
129:
121:
112:
111:
110:
105:
99:
98:
93:
87:
86:
5839:
5838:
5834:
5833:
5832:
5830:
5829:
5828:
5804:
5803:
5802:
5797:
5707:
5668:Solvation shell
5639:
5577:
5569:
5565:Miscibility gap
5550:Serial dilution
5545:Supersaturation
5495:Buffer solution
5466:
5461:
5418:Wayback Machine
5402:
5397:
5392:
5388:
5380:
5376:
5371:
5367:
5352:
5348:
5343:
5339:
5335:, 1950, p. 253.
5323:
5319:
5296:
5292:
5283:
5279:
5274:
5270:
5266:
5261:
5260:
5254:
5250:
5245:
5212:Solvation shell
5167:Volume fraction
5163:
5135:
5131:
5124:
5120:
5113:
5111:
5102:
5091:
5090:
5089:
5083:
5081:
5080:
5078:
5075:
5074:
5064:
5049:
5042:
5018:
5007:
5006:
5005:
4999:
4997:
4996:
4994:
4991:
4990:
4973:
4962:
4961:
4960:
4954:
4952:
4951:
4949:
4946:
4945:
4920:
4916:
4848:
4846:
4837:
4826:
4825:
4824:
4818:
4816:
4815:
4813:
4810:
4809:
4784:
4780:
4715:
4713:
4704:
4693:
4692:
4691:
4685:
4683:
4682:
4680:
4677:
4676:
4657:
4653:
4588:
4586:
4577:
4566:
4565:
4564:
4558:
4556:
4555:
4553:
4550:
4549:
4508:
4497:
4496:
4495:
4489:
4487:
4486:
4480:
4476:
4467:
4456:
4455:
4454:
4448:
4446:
4445:
4439:
4435:
4426:
4415:
4414:
4413:
4407:
4405:
4404:
4398:
4394:
4370:
4366:
4357:
4353:
4344:
4340:
4331:
4320:
4319:
4318:
4312:
4310:
4309:
4307:
4304:
4303:
4263:
4259:
4250:
4246:
4237:
4233:
4232:
4225:
4221:
4214:
4212:
4203:
4192:
4191:
4190:
4184:
4182:
4181:
4160:
4156:
4147:
4143:
4134:
4130:
4116:
4115:
4109:
4107:
4106:
4104:
4101:
4100:
4080:
4076:
4074:
4071:
4070:
4054:
4051:
4050:
4026:
4022:
4013:
4009:
4008:
4001:
3997:
3990:
3988:
3979:
3968:
3967:
3966:
3960:
3958:
3957:
3945:
3941:
3932:
3928:
3914:
3913:
3907:
3905:
3904:
3902:
3899:
3898:
3867:
3863:
3850:
3846:
3840:
3836:
3828:
3804:
3800:
3798:
3797:
3795:
3792:
3791:
3759:
3755:
3749:
3738:
3737:
3736:
3730:
3728:
3727:
3718:
3714:
3708:
3697:
3696:
3695:
3689:
3687:
3686:
3677:
3673:
3667:
3656:
3655:
3654:
3643:
3640:
3639:
3632:
3616:
3580:
3572:
3568:
3545:
3527:
3485:
3481:
3477:
3475:
3462:
3440:
3436:
3427:
3423:
3419:
3417:
3399:
3395:
3391:
3389:
3364:
3346:
3342:
3338:
3336:
3305:
3303:
3294:
3290:
3282:
3279:
3278:
3268:solvation shell
3250:
3246:
3244:
3241:
3240:
3235:
3227:
3198:
3191:
3180:
3179:
3178:
3172:
3170:
3169:
3165:
3163:
3151:
3140:
3139:
3138:
3132:
3130:
3129:
3114:
3110:
3108:
3107:
3105:
3102:
3101:
3078:
3067:
3066:
3065:
3056:
3045:
3044:
3043:
3037:
3035:
3034:
3032:
3029:
3028:
3005:
3001:
2999:
2998:
2983:
2979:
2977:
2976:
2974:
2971:
2970:
2947:
2943:
2934:
2930:
2928:
2925:
2924:
2907:
2903:
2897:
2895:
2894:
2892:
2889:
2888:
2871:
2867:
2865:
2862:
2861:
2839:
2835:
2829:
2818:
2817:
2816:
2810:
2808:
2807:
2798:
2794:
2788:
2777:
2776:
2775:
2763:
2758:
2757:
2751:
2749:
2748:
2739:
2735:
2727:
2724:
2723:
2703:
2692:
2691:
2690:
2684:
2682:
2681:
2679:
2676:
2675:
2645:
2641:
2628:
2622:
2621:
2620:
2611:
2607:
2603:
2595:
2593:
2587:
2586:
2571:
2567:
2565:
2564:
2562:
2559:
2558:
2538:
2527:
2526:
2525:
2523:
2520:
2519:
2502:
2498:
2486:
2482:
2480:
2479:
2470:
2466:
2454:
2450:
2448:
2447:
2439:
2436:
2435:
2418:
2414:
2399:
2395:
2393:
2392:
2383:
2379:
2364:
2360:
2358:
2357:
2346:
2343:
2342:
2319:
2315:
2302:
2296:
2295:
2294:
2285:
2281:
2277:
2269:
2267:
2261:
2260:
2245:
2241:
2239:
2238:
2227:
2223:
2210:
2204:
2203:
2202:
2193:
2189:
2185:
2177:
2175:
2169:
2168:
2153:
2149:
2147:
2146:
2144:
2141:
2140:
2114:
2110:
2108:
2107:
2092:
2088:
2086:
2085:
2083:
2080:
2079:
2076:
2054:
2050:
2044:
2033:
2032:
2031:
2020:
2016:
2011:
2000:
1996:
1990:
1986:
1984:
1978:
1967:
1966:
1965:
1954:
1950:
1945:
1936:
1925:
1924:
1923:
1917:
1915:
1914:
1912:
1909:
1908:
1884:
1880:
1874:
1863:
1862:
1861:
1855:
1853:
1852:
1843:
1839:
1833:
1822:
1821:
1820:
1808:
1803:
1802:
1796:
1794:
1793:
1784:
1780:
1772:
1769:
1768:
1762:
1727:
1716:
1715:
1714:
1708:
1706:
1705:
1699:
1695:
1686:
1675:
1674:
1673:
1667:
1665:
1664:
1658:
1654:
1645:
1634:
1633:
1632:
1626:
1624:
1623:
1617:
1613:
1589:
1585:
1576:
1572:
1563:
1559:
1550:
1539:
1538:
1537:
1531:
1529:
1528:
1526:
1523:
1522:
1498:
1494:
1488:
1484:
1473:
1470:
1469:
1448:
1432:
1427:
1418:
1405:
1404:
1400:
1392:
1388:
1383:
1374:
1363:
1362:
1361:
1355:
1353:
1352:
1350:
1347:
1346:
1341:
1314:
1310:
1308:
1292:
1287:
1278:
1265:
1264:
1260:
1250:
1241:
1230:
1229:
1228:
1222:
1220:
1219:
1217:
1214:
1213:
1190:
1186:
1182:
1176:
1172:
1170:
1159:
1155:
1150:
1137:
1132:
1122:
1118:
1116:
1102:
1098:
1096:
1095:
1091:
1080:
1076:
1071:
1058:
1053:
1043:
1039:
1037:
1022:
1018:
1009:
1005:
1004:
1002:
1001:
997:
986:
982:
977:
964:
959:
949:
945:
943:
930:
929:
925:
914:
910:
903:
899:
892:
890:
881:
870:
869:
868:
862:
860:
859:
857:
854:
853:
822:
818:
805:
804:
800:
790:
788:
785:
784:
771:
723:
719:
713:
708:
707:
701:
699:
698:
689:
685:
679:
675:
663:
658:
657:
651:
649:
648:
639:
635:
627:
624:
623:
597:
586:
585:
584:
578:
576:
575:
573:
570:
569:
567:
540:
529:
528:
527:
521:
519:
518:
516:
513:
512:
510:
486:
475:
474:
473:
467:
465:
464:
462:
459:
458:
456:
436:
425:
424:
423:
417:
415:
414:
412:
409:
408:
406:
383:
379:
377:
374:
373:
371:
347:
336:
335:
334:
328:
326:
325:
323:
320:
319:
317:
294:
290:
288:
285:
284:
282:
259:
248:
247:
246:
244:
241:
240:
238:
215:
211:
209:
206:
205:
203:
182:
178:
172:
161:
160:
159:
153:
151:
150:
141:
137:
131:
120:
119:
118:
106:
101:
100:
94:
92:
91:
82:
78:
70:
67:
66:
31:component in a
17:
12:
11:
5:
5837:
5827:
5826:
5821:
5816:
5799:
5798:
5796:
5795:
5790:
5785:
5780:
5775:
5770:
5765:
5760:
5755:
5750:
5745:
5740:
5735:
5733:Protic solvent
5730:
5725:
5717:
5715:
5709:
5708:
5706:
5705:
5700:
5695:
5690:
5685:
5680:
5678:Lattice energy
5675:
5670:
5665:
5660:
5655:
5649:
5647:
5641:
5640:
5638:
5637:
5632:
5627:
5622:
5617:
5612:
5607:
5602:
5597:
5592:
5587:
5581:
5579:
5571:
5570:
5568:
5567:
5562:
5557:
5552:
5547:
5542:
5537:
5532:
5530:Eutectic point
5527:
5522:
5517:
5512:
5507:
5502:
5497:
5492:
5490:Solid solution
5487:
5482:
5480:Ideal solution
5476:
5474:
5468:
5467:
5460:
5459:
5452:
5445:
5437:
5431:
5430:
5425:
5420:
5408:
5401:
5400:External links
5398:
5396:
5395:
5393:Apelblat p.320
5386:
5384:Apelblat p.320
5374:
5365:
5346:
5337:
5325:Herbert Harned
5317:
5290:
5277:
5267:
5265:
5262:
5259:
5258:
5247:
5246:
5244:
5241:
5240:
5239:
5234:
5229:
5224:
5219:
5214:
5209:
5204:
5199:
5194:
5189:
5184:
5179:
5174:
5172:Ideal solution
5169:
5162:
5159:
5154:
5153:
5138:
5134:
5127:
5123:
5119:
5116:
5110:
5105:
5098:
5095:
5086:
5062:
5047:
5040:
5024:
5021:
5014:
5011:
5002:
4976:
4969:
4966:
4957:
4939:
4938:
4923:
4919:
4914:
4911:
4905:
4902:
4899:
4896:
4893:
4890:
4887:
4884:
4878:
4875:
4872:
4869:
4866:
4863:
4860:
4857:
4854:
4851:
4845:
4840:
4833:
4830:
4821:
4803:
4802:
4787:
4783:
4778:
4775:
4769:
4766:
4763:
4760:
4757:
4754:
4751:
4748:
4745:
4742:
4739:
4736:
4733:
4730:
4727:
4724:
4721:
4718:
4712:
4707:
4700:
4697:
4688:
4660:
4656:
4651:
4648:
4642:
4639:
4636:
4633:
4630:
4627:
4624:
4621:
4618:
4615:
4612:
4609:
4606:
4603:
4600:
4597:
4594:
4591:
4585:
4580:
4573:
4570:
4561:
4538:
4537:
4525:
4522:
4519:
4516:
4511:
4504:
4501:
4492:
4483:
4479:
4475:
4470:
4463:
4460:
4451:
4442:
4438:
4434:
4429:
4422:
4419:
4410:
4401:
4397:
4393:
4390:
4387:
4384:
4381:
4378:
4373:
4369:
4365:
4360:
4356:
4352:
4347:
4343:
4339:
4334:
4327:
4324:
4315:
4296:
4295:
4280:
4277:
4274:
4271:
4266:
4262:
4258:
4253:
4249:
4245:
4240:
4236:
4228:
4224:
4220:
4217:
4211:
4206:
4199:
4196:
4187:
4180:
4177:
4174:
4171:
4168:
4163:
4159:
4155:
4150:
4146:
4142:
4137:
4133:
4129:
4123:
4120:
4112:
4083:
4079:
4058:
4047:
4046:
4029:
4025:
4021:
4016:
4012:
4004:
4000:
3996:
3993:
3987:
3982:
3975:
3972:
3963:
3956:
3953:
3948:
3944:
3940:
3935:
3931:
3927:
3921:
3918:
3910:
3876:
3873:
3870:
3866:
3862:
3859:
3856:
3853:
3849:
3843:
3839:
3835:
3831:
3827:
3824:
3821:
3818:
3812:
3807:
3803:
3767:
3762:
3758:
3752:
3745:
3742:
3733:
3726:
3721:
3717:
3711:
3704:
3701:
3692:
3685:
3680:
3676:
3670:
3663:
3660:
3653:
3650:
3647:
3631:
3628:
3615:
3612:
3579:
3576:
3570:
3566:
3544:
3541:
3526:
3523:
3515:
3514:
3499:
3494:
3488:
3484:
3480:
3474:
3471:
3468:
3465:
3460:
3457:
3454:
3451:
3448:
3443:
3439:
3435:
3430:
3426:
3422:
3416:
3413:
3408:
3402:
3398:
3394:
3388:
3385:
3382:
3379:
3376:
3371:
3368:
3363:
3360:
3355:
3349:
3345:
3341:
3335:
3332:
3329:
3326:
3323:
3318:
3314:
3311:
3308:
3302:
3297:
3293:
3289:
3286:
3253:
3249:
3233:
3226:
3223:
3222:
3221:
3210:
3204:
3201:
3194:
3187:
3184:
3175:
3168:
3162:
3159:
3154:
3147:
3144:
3135:
3128:
3122:
3117:
3113:
3081:
3074:
3071:
3064:
3059:
3052:
3049:
3040:
3013:
3008:
3004:
2997:
2991:
2986:
2982:
2958:
2955:
2950:
2946:
2942:
2937:
2933:
2910:
2906:
2900:
2874:
2870:
2858:
2857:
2842:
2838:
2832:
2825:
2822:
2813:
2806:
2801:
2797:
2791:
2784:
2781:
2774:
2766:
2761:
2754:
2747:
2742:
2738:
2734:
2731:
2706:
2699:
2696:
2687:
2671:
2670:
2656:
2653:
2648:
2644:
2640:
2637:
2634:
2631:
2625:
2614:
2610:
2606:
2601:
2598:
2590:
2585:
2579:
2574:
2570:
2541:
2534:
2531:
2505:
2501:
2494:
2489:
2485:
2478:
2473:
2469:
2462:
2457:
2453:
2446:
2443:
2421:
2417:
2413:
2407:
2402:
2398:
2391:
2386:
2382:
2378:
2372:
2367:
2363:
2356:
2353:
2350:
2341:one can write
2339:
2338:
2322:
2318:
2314:
2311:
2308:
2305:
2299:
2288:
2284:
2280:
2275:
2272:
2264:
2259:
2253:
2248:
2244:
2237:
2230:
2226:
2222:
2219:
2216:
2213:
2207:
2196:
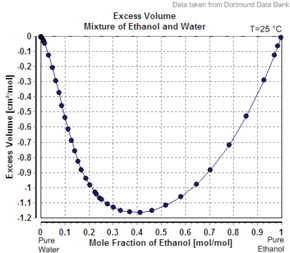
2192:
2188:
2183:
2180:
2172:
2167:
2161:
2156:
2152:
2122:
2117:
2113:
2106:
2100:
2095:
2091:
2075:
2072:
2071:
2070:
2057:
2053:
2047:
2040:
2037:
2030:
2023:
2019:
2015:
2010:
2003:
1999:
1993:
1989:
1981:
1974:
1971:
1964:
1957:
1953:
1949:
1944:
1939:
1932:
1929:
1920:
1902:
1901:
1887:
1883:
1877:
1870:
1867:
1858:
1851:
1846:
1842:
1836:
1829:
1826:
1819:
1811:
1806:
1799:
1792:
1787:
1783:
1779:
1776:
1761:
1758:
1757:
1756:
1744:
1741:
1738:
1735:
1730:
1723:
1720:
1711:
1702:
1698:
1694:
1689:
1682:
1679:
1670:
1661:
1657:
1653:
1648:
1641:
1638:
1629:
1620:
1616:
1612:
1609:
1606:
1603:
1600:
1597:
1592:
1588:
1584:
1579:
1575:
1571:
1566:
1562:
1558:
1553:
1546:
1543:
1534:
1515:
1514:
1501:
1497:
1491:
1487:
1483:
1480:
1477:
1455:
1452:
1447:
1443:
1435:
1430:
1426:
1422:
1417:
1412:
1409:
1403:
1395:
1391:
1387:
1382:
1377:
1370:
1367:
1358:
1339:
1336:
1335:
1322:
1317:
1313:
1307:
1303:
1295:
1290:
1286:
1282:
1277:
1272:
1269:
1263:
1257:
1254:
1249:
1244:
1237:
1234:
1225:
1210:
1209:
1193:
1189:
1185:
1179:
1175:
1169:
1162:
1158:
1154:
1148:
1140:
1135:
1131:
1125:
1121:
1115:
1110:
1105:
1101:
1094:
1090:
1083:
1079:
1075:
1069:
1061:
1056:
1052:
1046:
1042:
1036:
1031:
1025:
1021:
1017:
1012:
1008:
1000:
996:
989:
985:
981:
975:
967:
962:
958:
952:
948:
942:
937:
934:
928:
924:
917:
913:
906:
902:
898:
895:
889:
884:
877:
874:
865:
847:
846:
835:
831:
825:
821:
817:
812:
809:
803:
797:
794:
770:
767:
754:become equal.
741:
740:
726:
722:
716:
711:
704:
697:
692:
688:
682:
678:
674:
666:
661:
654:
647:
642:
638:
634:
631:
600:
593:
590:
581:
543:
536:
533:
524:
489:
482:
479:
470:
439:
432:
429:
420:
386:
382:
350:
343:
340:
331:
297:
293:
262:
255:
252:
218:
214:
200:
199:
185:
181:
175:
168:
165:
156:
149:
144:
140:
134:
127:
124:
117:
109:
104:
97:
90:
85:
81:
77:
74:
21:thermodynamics
15:
9:
6:
4:
3:
2:
5836:
5825:
5822:
5820:
5817:
5815:
5812:
5811:
5809:
5794:
5791:
5789:
5786:
5784:
5781:
5779:
5776:
5774:
5771:
5769:
5766:
5764:
5761:
5759:
5756:
5754:
5751:
5749:
5746:
5744:
5741:
5739:
5736:
5734:
5731:
5729:
5726:
5723:
5719:
5718:
5716:
5714:
5710:
5704:
5701:
5699:
5696:
5694:
5691:
5689:
5686:
5684:
5681:
5679:
5676:
5674:
5671:
5669:
5666:
5664:
5661:
5659:
5656:
5654:
5651:
5650:
5648:
5646:
5642:
5636:
5633:
5631:
5628:
5626:
5623:
5621:
5620:Mass fraction
5618:
5616:
5615:Mole fraction
5613:
5611:
5608:
5606:
5603:
5601:
5598:
5596:
5593:
5591:
5588:
5586:
5583:
5582:
5580:
5576:
5575:Concentration
5572:
5566:
5563:
5561:
5558:
5556:
5553:
5551:
5548:
5546:
5543:
5541:
5538:
5536:
5533:
5531:
5528:
5526:
5523:
5521:
5520:Phase diagram
5518:
5516:
5513:
5511:
5508:
5506:
5503:
5501:
5500:Flory–Huggins
5498:
5496:
5493:
5491:
5488:
5486:
5483:
5481:
5478:
5477:
5475:
5473:
5469:
5465:
5458:
5453:
5451:
5446:
5444:
5439:
5438:
5435:
5429:
5426:
5424:
5421:
5419:
5415:
5412:
5409:
5407:
5404:
5403:
5390:
5383:
5378:
5369:
5363:
5359:
5355:
5350:
5341:
5334:
5330:
5326:
5321:
5313:
5309:
5306:: 1235–1244.
5305:
5301:
5294:
5287:
5281:
5272:
5268:
5252:
5248:
5238:
5235:
5233:
5230:
5228:
5225:
5223:
5220:
5218:
5215:
5213:
5210:
5208:
5205:
5203:
5200:
5198:
5195:
5193:
5190:
5188:
5185:
5183:
5180:
5178:
5175:
5173:
5170:
5168:
5165:
5164:
5158:
5136:
5132:
5125:
5121:
5117:
5114:
5108:
5103:
5093:
5084:
5073:
5072:
5071:
5067:
5065:
5058:
5054:
5050:
5043:
5022:
5019:
5009:
5000:
4974:
4964:
4955:
4942:
4921:
4917:
4909:
4903:
4900:
4897:
4894:
4891:
4888:
4885:
4882:
4876:
4873:
4870:
4867:
4864:
4861:
4855:
4852:
4849:
4843:
4838:
4828:
4819:
4808:
4807:
4806:
4785:
4781:
4773:
4767:
4764:
4761:
4758:
4755:
4752:
4749:
4746:
4743:
4740:
4737:
4734:
4731:
4728:
4722:
4719:
4716:
4710:
4705:
4695:
4686:
4658:
4654:
4646:
4640:
4637:
4634:
4631:
4628:
4625:
4622:
4619:
4616:
4613:
4610:
4607:
4604:
4601:
4595:
4592:
4589:
4583:
4578:
4568:
4559:
4548:
4547:
4546:
4543:
4523:
4520:
4517:
4514:
4509:
4499:
4490:
4481:
4477:
4473:
4468:
4458:
4449:
4440:
4436:
4432:
4427:
4417:
4408:
4399:
4395:
4391:
4385:
4382:
4379:
4376:
4371:
4367:
4363:
4358:
4354:
4350:
4345:
4341:
4332:
4322:
4313:
4302:
4301:
4300:
4278:
4275:
4272:
4269:
4264:
4260:
4256:
4251:
4247:
4243:
4238:
4234:
4226:
4222:
4218:
4215:
4209:
4204:
4194:
4185:
4178:
4172:
4169:
4166:
4161:
4157:
4153:
4148:
4144:
4140:
4135:
4131:
4118:
4110:
4099:
4098:
4097:
4081:
4077:
4056:
4027:
4023:
4019:
4014:
4010:
4002:
3998:
3994:
3991:
3985:
3980:
3970:
3961:
3954:
3946:
3942:
3938:
3933:
3929:
3916:
3908:
3897:
3896:
3895:
3892:
3874:
3871:
3868:
3864:
3860:
3857:
3854:
3851:
3841:
3837:
3829:
3825:
3816:
3805:
3801:
3789:
3785:
3781:
3760:
3756:
3750:
3740:
3731:
3724:
3719:
3715:
3709:
3699:
3690:
3683:
3678:
3674:
3668:
3658:
3651:
3648:
3637:
3627:
3625:
3621:
3611:
3609:
3604:
3601:
3597:
3596:mass percents
3593:
3584:
3575:
3573:
3562:
3558:
3554:
3550:
3536:
3531:
3522:
3520:
3492:
3486:
3482:
3478:
3472:
3469:
3463:
3455:
3452:
3449:
3446:
3441:
3437:
3428:
3424:
3420:
3414:
3406:
3400:
3396:
3392:
3386:
3383:
3377:
3374:
3369:
3366:
3361:
3353:
3347:
3343:
3339:
3333:
3330:
3324:
3321:
3316:
3312:
3309:
3306:
3300:
3295:
3291:
3287:
3284:
3277:
3276:
3275:
3273:
3269:
3251:
3247:
3239:
3232:
3208:
3202:
3192:
3182:
3173:
3160:
3157:
3152:
3142:
3133:
3126:
3115:
3111:
3100:
3099:
3098:
3095:
3079:
3069:
3062:
3057:
3047:
3038:
3006:
3002:
2995:
2984:
2980:
2956:
2953:
2948:
2944:
2940:
2935:
2931:
2908:
2904:
2898:
2872:
2868:
2840:
2836:
2830:
2820:
2811:
2804:
2799:
2795:
2789:
2779:
2772:
2764:
2759:
2752:
2745:
2740:
2736:
2732:
2729:
2722:
2721:
2720:
2704:
2694:
2685:
2654:
2651:
2646:
2642:
2638:
2635:
2632:
2629:
2612:
2608:
2599:
2583:
2572:
2568:
2557:
2556:
2555:
2539:
2529:
2503:
2499:
2487:
2483:
2476:
2471:
2467:
2455:
2451:
2444:
2441:
2419:
2415:
2411:
2400:
2396:
2389:
2384:
2380:
2376:
2365:
2361:
2354:
2351:
2348:
2320:
2316:
2312:
2309:
2306:
2303:
2286:
2282:
2273:
2257:
2246:
2242:
2235:
2228:
2224:
2220:
2217:
2214:
2211:
2194:
2190:
2181:
2165:
2154:
2150:
2139:
2138:
2137:
2115:
2111:
2104:
2093:
2089:
2055:
2051:
2045:
2035:
2028:
2021:
2017:
2013:
2008:
2001:
1997:
1991:
1987:
1979:
1969:
1962:
1955:
1951:
1947:
1942:
1937:
1927:
1918:
1907:
1906:
1905:
1885:
1881:
1875:
1865:
1856:
1849:
1844:
1840:
1834:
1824:
1817:
1809:
1804:
1797:
1790:
1785:
1781:
1777:
1774:
1767:
1766:
1765:
1742:
1739:
1736:
1733:
1728:
1718:
1709:
1700:
1696:
1692:
1687:
1677:
1668:
1659:
1655:
1651:
1646:
1636:
1627:
1618:
1614:
1610:
1604:
1601:
1598:
1595:
1590:
1586:
1582:
1577:
1573:
1569:
1564:
1560:
1551:
1541:
1532:
1521:
1520:
1519:
1499:
1495:
1489:
1485:
1481:
1478:
1475:
1453:
1450:
1445:
1441:
1433:
1428:
1424:
1420:
1415:
1410:
1407:
1401:
1393:
1389:
1385:
1380:
1375:
1365:
1356:
1345:
1344:
1343:
1320:
1315:
1311:
1305:
1301:
1293:
1288:
1284:
1280:
1275:
1270:
1267:
1261:
1255:
1252:
1247:
1242:
1232:
1223:
1212:
1211:
1191:
1187:
1183:
1177:
1173:
1167:
1160:
1156:
1152:
1146:
1138:
1133:
1129:
1123:
1119:
1113:
1108:
1103:
1099:
1092:
1088:
1081:
1077:
1073:
1067:
1059:
1054:
1050:
1044:
1040:
1034:
1029:
1023:
1019:
1015:
1010:
1006:
998:
994:
987:
983:
979:
973:
965:
960:
956:
950:
946:
940:
935:
932:
926:
922:
915:
911:
904:
900:
896:
893:
887:
882:
872:
863:
852:
851:
850:
833:
829:
823:
819:
815:
810:
807:
801:
795:
792:
783:
782:
781:
779:
776:
766:
764:
763:heat capacity
760:
755:
753:
749:
744:
724:
720:
714:
709:
702:
695:
690:
686:
680:
676:
672:
664:
659:
652:
645:
640:
636:
632:
629:
622:
621:
620:
617:
598:
588:
579:
564:
562:
541:
531:
522:
508:
487:
477:
468:
437:
427:
418:
403:
384:
380:
369:
348:
338:
329:
315:
295:
291:
280:
260:
250:
236:
216:
212:
183:
179:
173:
163:
154:
147:
142:
138:
132:
122:
115:
107:
102:
95:
88:
83:
79:
75:
72:
65:
64:
63:
61:
56:
54:
50:
46:
42:
38:
34:
30:
26:
22:
5683:Raoult's law
5635:Ternary plot
5630:Mixing ratio
5559:
5389:
5377:
5368:
5349:
5340:
5332:
5320:
5303:
5299:
5293:
5280:
5271:
5251:
5232:Ternary plot
5192:Block design
5155:
5068:
5060:
5056:
5053:mixing ratio
5045:
5038:
4943:
4940:
4804:
4542:pseudobinary
4541:
4539:
4297:
4048:
3893:
3787:
3635:
3633:
3617:
3605:
3589:
3553:electrolytes
3546:
3543:Electrolytes
3516:
3271:
3230:
3228:
3096:
2859:
2672:
2340:
2077:
1903:
1763:
1516:
1337:
848:
777:
772:
756:
745:
742:
618:
565:
560:
506:
404:
316:of solvent,
279:molar volume
201:
57:
52:
48:
24:
18:
5788:Lyonium ion
5703:Miscibility
5688:Henry's law
5382:Citric acid
5354:Citric acid
5329:Benton Owen
3624:salting out
3535:bulk volume
53:in solution
5808:Categories
5783:Amphiphile
5778:Lipophilic
5773:Hydrophile
5768:Hydrophobe
5645:Solubility
5540:Saturation
5510:Suspension
5264:References
5227:Salting in
3620:salting in
3563:−6.0, and
3521:as above.
3229:The ratio
2969:, we have
5793:Lyate ion
5748:Solvation
5663:Solvation
5605:Normality
5256:reversed.
5157:mixture.
5118:−
5097:~
5013:~
5001:ϕ
4968:~
4956:ϕ
4853:−
4832:~
4820:ϕ
4720:−
4699:~
4687:ϕ
4593:−
4572:~
4560:ϕ
4503:~
4491:ϕ
4462:~
4450:ϕ
4421:~
4409:ϕ
4326:~
4314:ϕ
4219:−
4198:~
4186:ϕ
4122:~
4111:ϕ
3995:−
3974:~
3962:ϕ
3920:~
3909:ϕ
3872:≠
3834:∂
3823:∂
3811:¯
3744:~
3732:ϕ
3703:~
3691:ϕ
3662:~
3598:ethanol,
3456:ν
3453:−
3387:−
3378:
3370:ν
3362:−
3325:
3317:ν
3313:ν
3310:−
3292:γ
3288:
3248:γ
3200:∂
3186:~
3174:ϕ
3167:∂
3146:~
3134:ϕ
3121:¯
3073:¯
3063:≈
3051:~
3039:ϕ
3012:¯
2996:≈
2990:~
2954:≈
2941:≫
2899:ϕ
2860:the term
2824:~
2812:ϕ
2783:~
2753:ϕ
2698:~
2686:ϕ
2605:∂
2597:∂
2578:~
2533:~
2493:¯
2461:¯
2434:, and so
2406:¯
2371:¯
2279:∂
2271:∂
2252:¯
2187:∂
2179:∂
2160:¯
2121:¯
2099:¯
2039:~
2029:−
1973:~
1963:−
1931:~
1919:ϕ
1869:~
1857:ϕ
1828:~
1798:ϕ
1722:~
1710:ϕ
1681:~
1669:ϕ
1640:~
1628:ϕ
1545:~
1533:ϕ
1482:∑
1454:ρ
1425:ρ
1416:−
1411:ρ
1369:~
1357:ϕ
1321:ρ
1285:ρ
1276:−
1271:ρ
1236:~
1224:ϕ
1184:ρ
1130:ρ
1114:−
1109:ρ
1051:ρ
1035:−
1030:ρ
957:ρ
941:−
936:ρ
897:−
876:~
864:ϕ
796:ρ
703:ϕ
653:ϕ
592:~
580:ϕ
535:~
523:ϕ
481:~
469:ϕ
431:~
419:ϕ
342:~
330:ϕ
254:~
167:~
155:ϕ
126:~
96:ϕ
5763:Polarity
5722:Category
5610:Molality
5472:Solution
5414:Archived
5161:See also
3525:Examples
3519:molality
775:molality
759:enthalpy
748:dilution
561:apparent
49:apparent
29:solution
5713:Solvent
5515:Colloid
5505:Mixture
3636:ternary
3592:ethanol
3578:Alcohol
3270:number
614:
568:
563:value.
557:
511:
503:
457:
453:
407:
399:
372:
364:
318:
310:
283:
275:
239:
233:is the
231:
204:
60:solvent
33:mixture
5360:
4907:
4880:
4771:
4644:
4049:where
3559:−6.7,
3538:water.
2770:
1815:
670:
370:, and
368:solute
235:volume
202:where
113:
43:) per
41:volume
5535:Alloy
5243:Notes
4333:123..
4205:123..
3608:ideal
1552:123..
314:moles
27:of a
23:, an
5358:ISBN
5327:and
4675:and
3622:and
3561:LiOH
3557:NaOH
3549:NaCl
3493:55.5
3464:55.5
3407:55.5
3354:55.5
1376:12..
277:its
45:mole
5308:doi
5063:ijk
5059:or
4989:or
3891:.)
3790:as
19:In
5810::
5331:,
5304:51
5302:.
5126:01
5066:.
5048:jk
5044:,
5041:ij
4975:12
3981:12
3569:CO
3565:Na
3375:ln
3322:ln
3285:ln
3274::
3094:.
2719:,
2056:01
1468:,
1342::
5724:)
5720:(
5456:e
5449:t
5442:v
5314:.
5310::
5137:2
5133:n
5122:V
5115:V
5109:=
5104:2
5094:V
5085:c
5061:V
5057:V
5046:V
5039:V
5023:j
5020:i
5010:V
4965:V
4922:0
4918:n
4913:)
4910:2
4904:e
4901:t
4898:u
4895:l
4892:o
4889:s
4886:+
4883:1
4877:e
4874:t
4871:u
4868:l
4865:o
4862:s
4859:(
4856:V
4850:V
4844:=
4839:0
4829:V
4786:2
4782:n
4777:)
4774:1
4768:e
4765:t
4762:u
4759:l
4756:o
4753:s
4750:+
4747:t
4744:n
4741:e
4738:v
4735:l
4732:o
4729:s
4726:(
4723:V
4717:V
4711:=
4706:2
4696:V
4659:1
4655:n
4650:)
4647:2
4641:e
4638:t
4635:u
4632:l
4629:o
4626:s
4623:+
4620:t
4617:n
4614:e
4611:v
4608:l
4605:o
4602:s
4599:(
4596:V
4590:V
4584:=
4579:1
4569:V
4536:,
4524:.
4521:.
4518:.
4515:+
4510:3
4500:V
4482:3
4478:b
4474:+
4469:2
4459:V
4441:2
4437:b
4433:+
4428:1
4418:V
4400:1
4396:b
4392:=
4389:)
4386:.
4383:.
4380:.
4377:+
4372:3
4368:b
4364:+
4359:2
4355:b
4351:+
4346:1
4342:b
4338:(
4323:V
4294:,
4279:.
4276:.
4273:.
4270:+
4265:3
4261:n
4257:+
4252:2
4248:n
4244:+
4239:1
4235:n
4227:0
4223:V
4216:V
4210:=
4195:V
4179:=
4176:)
4173:.
4170:.
4167:,
4162:3
4158:n
4154:,
4149:2
4145:n
4141:,
4136:1
4132:n
4128:(
4119:V
4082:0
4078:V
4057:V
4045:,
4028:2
4024:n
4020:+
4015:1
4011:n
4003:0
3999:V
3992:V
3986:=
3971:V
3955:=
3952:)
3947:2
3943:n
3939:,
3934:1
3930:n
3926:(
3917:V
3875:i
3869:j
3865:n
3861:,
3858:p
3855:,
3852:T
3848:)
3842:i
3838:n
3830:/
3826:V
3820:(
3817:=
3806:i
3802:V
3788:i
3766:)
3761:2
3757:n
3751:2
3741:V
3725:+
3720:1
3716:n
3710:1
3700:V
3684:+
3679:0
3675:n
3669:0
3659:V
3652:=
3649:V
3646:(
3571:3
3567:2
3513:,
3498:)
3487:a
3483:r
3479:b
3473:+
3470:1
3467:(
3459:)
3450:h
3447:+
3442:a
3438:r
3434:(
3429:a
3425:r
3421:b
3415:+
3412:)
3401:a
3397:r
3393:b
3384:1
3381:(
3367:h
3359:)
3348:a
3344:r
3340:b
3334:+
3331:1
3328:(
3307:h
3301:=
3296:s
3272:h
3252:s
3234:a
3231:r
3209:.
3203:b
3193:1
3183:V
3161:b
3158:+
3153:1
3143:V
3127:=
3116:1
3112:V
3080:1
3070:V
3058:1
3048:V
3007:0
3003:V
2985:0
2981:V
2957:0
2949:1
2945:n
2936:0
2932:n
2909:1
2905:V
2873:0
2869:V
2856:,
2841:1
2837:n
2831:1
2821:V
2805:+
2800:0
2796:n
2790:0
2780:V
2773:=
2765:1
2760:V
2746:+
2741:0
2737:V
2733:=
2730:V
2705:1
2695:V
2669:,
2655:0
2652:=
2647:1
2643:n
2639:,
2636:p
2633:,
2630:T
2624:)
2613:0
2609:n
2600:V
2589:(
2584:=
2573:0
2569:V
2540:0
2530:V
2504:1
2500:n
2488:1
2484:V
2477:+
2472:0
2468:n
2456:0
2452:V
2445:=
2442:V
2420:1
2416:n
2412:d
2401:1
2397:V
2390:+
2385:0
2381:n
2377:d
2366:0
2362:V
2355:=
2352:V
2349:d
2337:,
2321:0
2317:n
2313:,
2310:p
2307:,
2304:T
2298:)
2287:1
2283:n
2274:V
2263:(
2258:=
2247:1
2243:V
2236:,
2229:1
2225:n
2221:,
2218:p
2215:,
2212:T
2206:)
2195:0
2191:n
2182:V
2171:(
2166:=
2155:0
2151:V
2116:1
2112:V
2105:,
2094:0
2090:V
2052:r
2046:0
2036:V
2022:1
2018:n
2014:V
2009:=
2002:1
1998:n
1992:0
1988:n
1980:0
1970:V
1956:1
1952:n
1948:V
1943:=
1938:1
1928:V
1886:1
1882:n
1876:1
1866:V
1850:+
1845:0
1841:n
1835:0
1825:V
1818:=
1810:1
1805:V
1791:+
1786:0
1782:V
1778:=
1775:V
1755:,
1743:.
1740:.
1737:.
1734:+
1729:3
1719:V
1701:3
1697:b
1693:+
1688:2
1678:V
1660:2
1656:b
1652:+
1647:1
1637:V
1619:1
1615:b
1611:=
1608:)
1605:.
1602:.
1599:.
1596:+
1591:3
1587:b
1583:+
1578:2
1574:b
1570:+
1565:1
1561:b
1557:(
1542:V
1500:i
1496:M
1490:i
1486:y
1479:=
1476:M
1451:M
1446:+
1442:)
1434:0
1429:0
1421:1
1408:1
1402:(
1394:T
1390:b
1386:1
1381:=
1366:V
1340:T
1316:1
1312:M
1306:+
1302:)
1294:0
1289:0
1281:1
1268:1
1262:(
1256:b
1253:1
1248:=
1243:1
1233:V
1192:1
1188:n
1178:1
1174:m
1168:+
1161:1
1157:n
1153:1
1147:)
1139:0
1134:0
1124:0
1120:m
1104:0
1100:m
1093:(
1089:=
1082:1
1078:n
1074:1
1068:)
1060:0
1055:0
1045:0
1041:m
1024:0
1020:m
1016:+
1011:1
1007:m
999:(
995:=
988:1
984:n
980:1
974:)
966:0
961:0
951:0
947:m
933:m
927:(
923:=
916:1
912:n
905:0
901:V
894:V
888:=
883:1
873:V
834:.
830:)
824:1
820:M
816:+
811:b
808:1
802:(
793:1
778:b
725:1
721:m
715:1
710:v
696:+
691:0
687:m
681:0
677:v
673:=
665:1
660:V
646:+
641:0
637:V
633:=
630:V
599:0
589:V
542:1
532:V
488:1
478:V
438:1
428:V
385:1
381:n
349:1
339:V
296:0
292:n
261:0
251:V
217:0
213:V
184:1
180:n
174:1
164:V
148:+
143:0
139:n
133:0
123:V
116:=
108:1
103:V
89:+
84:0
80:V
76:=
73:V
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.