200:
739:
442:
437:
37:
551:
28:
560:
474:
806:– a genus of the large intestinal roundworm. In the early 1900s, it was a major remedy against intestinal parasites in humans, cats, dogs, goats, sheep, chickens, horses, and pigs, and it is still used in livestock, particularly in the Central American countries. The dosage was specified by the ascaridole content in the oil, which was traditionally determined with an
584:
oil and named by Hüthig in 1908. He found that when heated to between 130° and 150° C "there occurs, with sudden boiling in which the temperature momentarily rises to about 250°, a decomposition of an explosive character, occasionally accompanied by ignition. At the same time a very disagreeable
818:
methods. The worms and their larvae were killed by immersion in a solution of ascaridole in water (about 0.015 vol%) for 18 hours at 50 °F (10 °C) or 12 hours at 60 °F (16 °C) or 6 hours at 65 to 70 °F (18 to 21 °C). Meanwhile, such immersion did not damage the
1040:
Ascaridole is discussed in the section titled "Wormseed oil, American", pp. 109–119. Ascaridole is named on p. 111; its empirical formula is stated on p. 114; the "explosive character" of the decomposition on heating is mentioned on p. 115. Some of the text can be seen about nine minutes into
726:, it is unstable and prone to violent decomposition when heated to a temperature above 130 °C or treated with organic acids. When heated, it emits fumes which are poisonous and possibly carcinogenic. Ascaridole (organic peroxide) is forbidden to be shipped as listed in the
984:(yellowish pigmentation of the skin). Fatal doses of wormseed oil were reported as one teaspoon for a 14-month-old baby (at once) and daily administration of 1 mL over three weeks to a 2-year-old child. Ascaridole is also carcinogenic in rats.
685:
of 1.098 g/cm. Nelson also predicted the chemical structure of ascaridole which was almost correct, but had the peroxide bridge not along the molecular axis, but between the other, off-axis carbon atoms. This structure was corrected by
713:
with the diene system in the terpinene. Since 1945, this reaction has been adopted into the industry for large-scale production of ascaridole in
Germany. It was then used as an inexpensive drug against intestinal worms.
450:
417:
585:
skatol-like odour, difficult to define, is observed. In the course of the examination it was found that during the decomposition a gas is split off." He determined its chemical formula as C
722:
Ascaridole is a colorless liquid that is soluble in most organic solvents. It is toxic and has a pungent, unpleasant smell and taste. Like other pure, low molecular weight
629:. A detailed study was done by E. K. Nelson in 1911. He described the decomposition as apparently a molecular rearrangement, and found that it reacts with sulfuric,
529:(wormseed). It is a component of natural medicine, tonic drinks and food flavoring in Latin American cuisine. As part of the oil, ascaridole is used as an
521:, it is unstable and prone to rapid decomposition when heated or treated with organic acids. Ascaridole determines the specific flavor of the Chilean tree
487:
881:
896:
The usage of wormseed oil on humans is limited by the toxicity of ascaridole and has therefore been discouraged. In high doses, wormseed oil causes
517:
functional group. It is a colorless liquid with a pungent smell and taste that is soluble in most organic solvents. Like other low molecular weight
853:
2672:
318:
1586:
105:
1567:
1537:
1472:
1445:
1396:
1346:
1014:
2687:
1629:
2608:
482:
1153:
1031:
293:
2702:
494:
727:
2682:
2677:
2085:
784:
is about 1:4. It also changes through the year peaking around the time when the plant seeds become mature.
270:
195:
2635:
1607:
157:
2692:
2662:
1420:
441:
2629:
207:
1622:
1239:
710:
697:
and might be regarded as mimicking the natural production of ascaridole. The process starts from α-
436:
2657:
2040:
937:
800:(helminths) from the human body and plants. This property gave the name to the chemical, after
760:
1557:
1525:
1462:
1435:
1336:
1220:
1004:
2697:
2667:
1887:
1366:
429:
55:
1913:
1598:
1293:
1073:
279:
177:
8:
1615:
1284:(April–June 1944). "Die Synthese des Ascaridols" [The Synthesis of Ascaridoles].
857:
610:
81:
71:
1297:
1077:
1064:
Arbuzov, Yu. A. (1965). "The Diels–Alder
Reaction with Molecular Oxygen as Dienophile".
772:. The content of ascaridole in the plant depends on cultivation and is maximal when the
199:
1414:
1317:
1262:
1089:
811:
137:
117:
1563:
1533:
1468:
1441:
1402:
1392:
1342:
1309:
1190:
1117:
1093:
1010:
815:
630:
1266:
1085:
2227:
2003:
1943:
1501:
1321:
1301:
1254:
1198:
1162:
1125:
1081:
901:
723:
650:
575:
574:
Ascaridole was the first, and for a long time only, discovered naturally occurring
518:
514:
510:
341:
1391:. J.J. Keller & Associates. Neenah, Wis.: J.J. Keller & Associates. 2011.
259:
2346:
2261:
2219:
1727:
973:
953:
841:
638:
754:) primarily originates from ascaridole. Ascaridole is also a major component of
738:
2612:
2566:
2479:
2474:
2464:
2441:
2403:
2393:
1974:
797:
706:
693:
The first laboratory synthesis was demonstrated in 1944 by Günther
Schenck and
534:
465:
1597:. Ithaca, NY: Cornell University, Department of Animal Science. Archived from
1042:
2651:
2557:
2523:
2518:
2469:
1699:
1406:
1313:
1166:
885:
769:
678:
674:
614:
402:
392:
188:
1258:
2571:
2489:
2408:
2398:
2341:
2324:
2309:
2278:
2238:
2208:
2134:
1813:
1793:
1281:
913:
873:
821:
793:
694:
687:
530:
1637:
2543:
2533:
2509:
2494:
2336:
2301:
2296:
2185:
2180:
2048:
2012:
1989:
1928:
1877:
1867:
1817:
1737:
1505:
969:
702:
634:
622:
580:
1202:
1129:
2528:
2446:
2372:
2331:
2319:
2314:
2200:
2190:
1979:
1932:
1305:
945:
897:
845:
777:
364:
302:
InChI=1S/C10H16O2/c1-8(2)10-6-4-9(3,5-7-10)11-12-10/h4,6,8H,5,7H2,1-3H3
208:
168:
36:
2155:
2064:
2017:
1948:
1788:
1643:
965:
933:
869:
865:
698:
1181:
1148:
1108:
513:
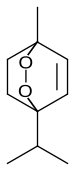
classified as a bicyclic monoterpenoid that has an unusual bridging
464:
Except where otherwise noted, data are given for materials in their
2499:
2484:
2456:
2385:
2351:
2150:
2022:
1923:
1897:
1882:
1833:
1823:
1783:
1708:
1647:
981:
941:
929:
925:
917:
909:
773:
768:) where it typically constitutes between 16 and 70% of the plant's
646:
642:
598:
234:
1335:
Brown, W. H.; Foote, C. S.; Iverson, B. L.; Anslyn, E. V. (2009).
810:
developed by Nelson in 1920. It was later substituted with modern
550:
104:
2377:
2367:
2288:
2109:
2104:
2032:
2027:
1969:
1964:
1918:
1892:
1828:
1767:
1762:
1688:
1679:
1639:
921:
877:
840:
The wormseed plant itself (Mexican tea) is traditionally used in
802:
755:
682:
526:
382:
246:
2538:
2080:
1938:
1872:
1838:
1809:
1722:
1032:"Commercial notes and scientific information on essential oils"
905:
861:
654:
626:
606:
602:
148:
1437:
Plantas
Competidoras: un componente más de los agroecosistemas
837:
and others at 70 °F (21 °C) for 15 hours or longer.
977:
957:
833:
827:
807:
747:
673:. The glycol is more stable than ascaridole and has a higher
522:
128:
94:
1364:
559:
27:
1530:
Food
Flavors: Formation, Analysis, and Packaging Influences
961:
949:
849:
781:
641:. Nelson showed that the new substance contained neither a
618:
225:
888:
practiced in North and South
America, China, and Turkey.
1334:
597:. Hüthig also noted the indifference of ascaridole to
2607:
Terpene synthase enzymes (many), having in common a
59:
1-Methyl-4-(1-methylethyl)-2,3-dioxabicyclooct-5-ene
1149:"Zur Kenntnis der Terpene und der Ätherischen Öle"
537:from plants, domestic animals and the human body.
1151:[Regarding Terpenes and Essential Oils].
407:40 °C (104 °F; 313 K) at 0.2 mmHg
2649:
701:which reacts with oxygen under the influence of
258:
80:
1685:Iridoid glycosides (iridoids bound to a sugar)
1528:. In Tucker, A. O.; Maciarella, M. J. (eds.).
1279:
1623:
1227:. Vol. 2. CUP Archive. pp. 446–452.
1218:
936:. Prolonged action induces depression of the
1526:"Some Toxic Culinary Herbs in North America"
1240:"Industrial Applications of Photochemistry"
1630:
1616:
1581:
1579:
1002:
730:Hazardous Materials Table 49 CFR 172.101.
198:
176:
1389:Hazardous materials compliance pocketbook
525:and is a major constituent of the oil of
397:3.3 °C (37.9 °F; 276.4 K)
278:
1562:. NRC Research Press. pp. 295–296.
1551:
1549:
1433:
1182:"A Chemical Investigation of the Oil of
1109:"A Chemical Investigation of the Oil of
1036:Semi-annual Report of Schimmel & Co.
746:The specific flavor of the Chilean tree
737:
1576:
1146:
1063:
2650:
1523:
1179:
1106:
1029:
1003:Lewis, R. J.; Lewis, R. J. Sr (2008).
189:
1611:
1555:
1546:
1519:
1517:
1515:
1487:
1365:US Department of Agriculture (1972).
1360:
1358:
1142:
1140:
852:-containing food. It is also part of
305:Key: MGYMHQJELJYRQS-UHFFFAOYSA-N
156:
136:
1460:
1237:
1214:
1212:
998:
996:
844:for flavoring dishes and preventing
2673:Heterocyclic compounds with 2 rings
1481:
1009:. Wiley-Interscience. p. 114.
649:group and that upon reduction with
249:
233:
13:
2609:terpene synthase N terminal domain
1512:
1355:
1137:
1006:Hazardous Chemicals Desk Reference
819:roots and stems of plants such as
705:and light. Under these conditions
35:
26:
14:
2714:
1341:. Cengage Learning. p. 967.
1209:
993:
891:
792:Ascaridole is mainly used as an
558:
549:
472:
440:
435:
1467:. Plaza y Valdés. p. 323.
1454:
1427:
1381:
1328:
1273:
1221:"Chapter 5: Oxides: Ascaridole"
1086:10.1070/RC1965v034n08ABEH001512
787:
728:US Department of Transportation
709:is generated which reacts in a
468:(at 25 °C , 100 kPa).
1595:Medicinal Plants for Livestock
1532:. Elsevier. pp. 408–409.
1231:
1173:
1100:
1057:
1048:
1023:
1:
1054:(Hüthig, April 1908), p. 116.
987:
733:
717:
609:that characterized it as non-
2086:Geranylgeranyl pyrophosphate
1492:Oil. Part III. Ascaridole".
952:. Long-term effects include
7:
2688:Organic peroxide explosives
2636:Dimethylallyl pyrophosphate
22:
10:
2719:
568:α-Terpinene and ascaridole
540:
369:168.23 g/mol
2630:Isopentenyl pyrophosphate
2622:
2600:
2584:Norisoprenoids (modified)
2583:
2556:
2508:
2455:
2440:
2423:
2360:
2287:
2277:
2260:
2199:
2173:
2143:
2118:
2097:
2073:
2063:
2002:
1957:
1906:
1860:
1851:
1802:
1776:
1755:
1736:
1698:
1655:
462:
416:
411:
334:
326:O1OC2(\C=C/C1(C)CC2)C(C)C
314:
289:
64:
54:
49:
21:
2623:Activated isoprene forms
2424:Sesquarterpenes/oids (7)
1673:Monocyclic (single ring)
1589:Chenopodium ambrosioides
1167:10.1002/jlac.19123920104
766:Chenopodium ambrosioides
2703:Foul-smelling chemicals
2246:Secodehydroabietic acid
1461:Lang, A. L. A. (2003).
1259:10.1351/pac197541040535
856:and infusions to expel
578:. It was isolated from
2041:Farnesyl pyrophosphate
1524:Contis, E. T. (1998).
1440:. EUNED. p. 245.
1419:: CS1 maint: others (
1219:Nelson, E. K. (1947).
1180:Nelson, E. K. (1913).
1107:Nelson, E. K. (1911).
938:central nervous system
761:Dysphania ambrosioides
743:
40:
31:
2243:Sandaracopimaric acid
1888:Geranyl pyrophosphate
1030:Hüthig (April 1908).
741:
677:of about 64 °C,
39:
30:
2433:Tetraprenylcurcumene
1914:Grapefruit mercaptan
1506:10.1039/JR9380000829
1434:Garro Alfaro, J. E.
1367:"Technical Bulletin"
1147:Wallach, O. (1912).
980:, respectively) and
976:and proteins in the
944:which transits into
858:intestinal parasites
711:Diels–Alder reaction
681:of 272 °C, and
625:, ascaridole formed
613:. When reacted with
2683:Explosive chemicals
2678:Oxygen heterocycles
2216:Dehydroabietic acid
1682:(cyclopentane ring)
1488:Paget, H. (1938). "
1298:1944NW.....32..157G
1286:Naturwissenschaften
1203:10.1021/ja02190a009
1130:10.1021/ja02221a016
1078:1965RuCRv..34..558A
118:Beilstein Reference
18:
1676:Bicyclic (2 rings)
1556:Small, E. (2006).
1371:Technical Bulletin
1306:10.1007/BF01467891
1154:Liebigs Ann. Chem.
958:fluid accumulation
812:gas chromatography
744:
617:, or reduced with
495:Infobox references
41:
32:
16:
2693:Liquid explosives
2663:Organic peroxides
2645:
2644:
2593:7,8-dihydroionone
2552:
2551:
2419:
2418:
2256:
2255:
2224:Lambertianic acid
1998:
1997:
1847:
1846:
1662:Acyclic (linear,
1569:978-0-660-19073-0
1539:978-0-444-82590-2
1474:978-970-722-113-0
1447:978-9968-31-235-6
1398:978-1-60287-954-6
1348:978-0-495-38857-9
1338:Organic Chemistry
1238:Pape, M. (1975).
1191:J. Am. Chem. Soc.
1118:J. Am. Chem. Soc.
1016:978-0-470-18024-2
816:mass spectrometry
796:drug that expels
758:(or Mexican tea,
742:Mexican Tea plant
724:organic peroxides
659:ascaridole glycol
533:drug that expels
519:organic peroxides
503:Chemical compound
501:
500:
377:Colorless liquid
106:Interactive image
45:
44:
2710:
2453:
2452:
2285:
2284:
2262:Sesterterpenoids
2228:Levopimaric acid
2090:Geranyl-linalool
2071:
2070:
2004:Sesquiterpenoids
1944:Perillyl alcohol
1858:
1857:
1753:
1752:
1632:
1625:
1618:
1609:
1608:
1603:
1602:
1583:
1574:
1573:
1553:
1544:
1543:
1521:
1510:
1509:
1485:
1479:
1478:
1464:Ecología Química
1458:
1452:
1451:
1431:
1425:
1424:
1418:
1410:
1385:
1379:
1378:
1362:
1353:
1352:
1332:
1326:
1325:
1280:Schenck, G. O.;
1277:
1271:
1270:
1244:
1235:
1229:
1228:
1216:
1207:
1206:
1177:
1171:
1170:
1144:
1135:
1133:
1124:(8): 1404–1412.
1104:
1098:
1097:
1061:
1055:
1052:
1046:
1039:
1027:
1021:
1020:
1000:
902:mucous membranes
882:nervous diseases
651:iron(II) sulfate
639:phosphoric acids
576:organic peroxide
562:
553:
511:organic compound
485:
479:
476:
475:
444:
439:
342:Chemical formula
282:
262:
251:
237:
210:
202:
191:
180:
160:
140:
108:
84:
23:
19:
15:
2718:
2717:
2713:
2712:
2711:
2709:
2708:
2707:
2648:
2647:
2646:
2641:
2618:
2596:
2579:
2548:
2504:
2444:
2442:Tetraterpenoids
2436:
2415:
2356:
2347:Cholecalciferol
2273:
2270:Geranylfarnesol
2252:
2235:Neoabietic acid
2220:Isopimaric acid
2195:
2169:
2139:
2114:
2093:
2059:
1994:
1953:
1902:
1853:
1852:Monoterpenoids
1843:
1798:
1772:
1748:
1744:
1740:
1732:
1728:Isovaleric acid
1718:
1714:
1694:
1651:
1636:
1606:
1585:
1584:
1577:
1570:
1554:
1547:
1540:
1522:
1513:
1486:
1482:
1475:
1459:
1455:
1448:
1432:
1428:
1412:
1411:
1399:
1387:
1386:
1382:
1363:
1356:
1349:
1333:
1329:
1278:
1274:
1247:Pure Appl. Chem
1242:
1236:
1232:
1217:
1210:
1178:
1174:
1145:
1138:
1105:
1101:
1066:Russ. Chem. Rev
1062:
1058:
1053:
1049:
1028:
1024:
1017:
1001:
994:
990:
974:red blood cells
954:pulmonary edema
894:
842:Mexican cuisine
798:parasitic worms
790:
736:
720:
672:
668:
664:
657:, now known as
596:
592:
588:
572:
571:
570:
569:
565:
564:
563:
555:
554:
543:
535:parasitic worms
504:
497:
492:
491:
490: ?)
481:
477:
473:
469:
453:
432:
358:
354:
350:
344:
330:
327:
322:
321:
310:
307:
306:
303:
297:
296:
285:
265:
252:
240:
220:
183:
163:
143:
120:
111:
98:
87:
74:
60:
12:
11:
5:
2716:
2706:
2705:
2700:
2695:
2690:
2685:
2680:
2675:
2670:
2665:
2660:
2643:
2642:
2640:
2639:
2633:
2626:
2624:
2620:
2619:
2617:
2616:
2613:protein domain
2604:
2602:
2598:
2597:
2595:
2594:
2591:
2587:
2585:
2581:
2580:
2578:
2577:
2574:
2569:
2567:Natural rubber
2563:
2561:
2558:Polyterpenoids
2554:
2553:
2550:
2549:
2547:
2546:
2541:
2536:
2531:
2526:
2521:
2515:
2513:
2506:
2505:
2503:
2502:
2497:
2492:
2487:
2482:
2480:Delta-Carotene
2477:
2475:Gamma-Carotene
2472:
2467:
2465:Alpha-Carotene
2461:
2459:
2450:
2438:
2437:
2435:
2434:
2431:
2427:
2425:
2421:
2420:
2417:
2416:
2414:
2413:
2412:
2411:
2406:
2404:Betulinic acid
2401:
2396:
2394:Oleanolic acid
2388:
2383:
2380:
2375:
2370:
2364:
2362:
2358:
2357:
2355:
2354:
2349:
2344:
2339:
2334:
2329:
2328:
2327:
2322:
2317:
2312:
2307:
2306:Citrostadienol
2304:
2293:
2291:
2282:
2275:
2274:
2272:
2271:
2267:
2265:
2258:
2257:
2254:
2253:
2251:
2250:
2249:Palustric acid
2247:
2244:
2241:
2236:
2233:
2230:
2225:
2222:
2217:
2214:
2211:
2205:
2203:
2197:
2196:
2194:
2193:
2188:
2183:
2177:
2175:
2171:
2170:
2168:
2167:
2164:
2161:
2158:
2153:
2147:
2145:
2141:
2140:
2138:
2137:
2132:
2129:
2122:
2120:
2116:
2115:
2113:
2112:
2107:
2101:
2099:
2095:
2094:
2092:
2091:
2088:
2083:
2077:
2075:
2068:
2061:
2060:
2058:
2057:
2054:
2051:
2046:
2043:
2038:
2035:
2030:
2025:
2020:
2015:
2009:
2007:
2000:
1999:
1996:
1995:
1993:
1992:
1987:
1982:
1977:
1975:Bornyl acetate
1972:
1967:
1961:
1959:
1955:
1954:
1952:
1951:
1946:
1941:
1936:
1926:
1921:
1916:
1910:
1908:
1904:
1903:
1901:
1900:
1895:
1890:
1885:
1880:
1875:
1870:
1864:
1862:
1855:
1849:
1848:
1845:
1844:
1842:
1841:
1836:
1831:
1826:
1821:
1806:
1804:
1800:
1799:
1797:
1796:
1791:
1786:
1780:
1778:
1774:
1773:
1771:
1770:
1765:
1759:
1757:
1750:
1746:
1742:
1734:
1733:
1731:
1730:
1725:
1720:
1716:
1712:
1705:
1703:
1700:Hemiterpenoids
1696:
1695:
1693:
1692:
1686:
1683:
1677:
1674:
1671:
1659:
1657:
1653:
1652:
1635:
1634:
1627:
1620:
1612:
1605:
1604:
1601:on 2006-02-21.
1575:
1568:
1559:Culinary Herbs
1545:
1538:
1511:
1500:(1): 829–833.
1480:
1473:
1453:
1446:
1426:
1397:
1380:
1354:
1347:
1327:
1292:(14–26): 157.
1272:
1253:(4): 535–558.
1230:
1208:
1172:
1136:
1099:
1056:
1047:
1022:
1015:
991:
989:
986:
893:
890:
789:
786:
735:
732:
719:
716:
707:singlet oxygen
670:
666:
662:
594:
590:
586:
567:
566:
557:
556:
548:
547:
546:
545:
544:
542:
539:
502:
499:
498:
493:
471:
470:
466:standard state
463:
460:
459:
454:
449:
446:
445:
433:
428:
425:
424:
414:
413:
409:
408:
405:
399:
398:
395:
389:
388:
385:
379:
378:
375:
371:
370:
367:
361:
360:
356:
352:
348:
345:
340:
337:
336:
332:
331:
329:
328:
325:
317:
316:
315:
312:
311:
309:
308:
304:
301:
300:
292:
291:
290:
287:
286:
284:
283:
275:
273:
267:
266:
264:
263:
255:
253:
245:
242:
241:
239:
238:
230:
228:
222:
221:
219:
218:
214:
212:
204:
203:
193:
185:
184:
182:
181:
173:
171:
165:
164:
162:
161:
153:
151:
145:
144:
142:
141:
133:
131:
125:
124:
121:
116:
113:
112:
110:
109:
101:
99:
92:
89:
88:
86:
85:
77:
75:
70:
67:
66:
62:
61:
58:
52:
51:
47:
46:
43:
42:
33:
9:
6:
4:
3:
2:
2715:
2704:
2701:
2699:
2696:
2694:
2691:
2689:
2686:
2684:
2681:
2679:
2676:
2674:
2671:
2669:
2666:
2664:
2661:
2659:
2658:Anthelmintics
2656:
2655:
2653:
2637:
2634:
2631:
2628:
2627:
2625:
2621:
2614:
2610:
2606:
2605:
2603:
2599:
2592:
2590:3-oxo-α-ionol
2589:
2588:
2586:
2582:
2575:
2573:
2570:
2568:
2565:
2564:
2562:
2559:
2555:
2545:
2542:
2540:
2537:
2535:
2532:
2530:
2527:
2525:
2524:Cryptoxanthin
2522:
2520:
2519:Canthaxanthin
2517:
2516:
2514:
2511:
2507:
2501:
2498:
2496:
2493:
2491:
2488:
2486:
2483:
2481:
2478:
2476:
2473:
2471:
2470:Beta-Carotene
2468:
2466:
2463:
2462:
2460:
2458:
2454:
2451:
2448:
2443:
2439:
2432:
2430:Ferrugicadiol
2429:
2428:
2426:
2422:
2410:
2407:
2405:
2402:
2400:
2397:
2395:
2392:
2391:
2389:
2387:
2384:
2382:Serratenediol
2381:
2379:
2376:
2374:
2371:
2369:
2366:
2365:
2363:
2359:
2353:
2350:
2348:
2345:
2343:
2340:
2338:
2335:
2333:
2330:
2326:
2323:
2321:
2318:
2316:
2313:
2311:
2308:
2305:
2303:
2300:
2299:
2298:
2295:
2294:
2292:
2290:
2286:
2283:
2280:
2279:Triterpenoids
2276:
2269:
2268:
2266:
2263:
2259:
2248:
2245:
2242:
2240:
2237:
2234:
2232:Mercusic acid
2231:
2229:
2226:
2223:
2221:
2218:
2215:
2213:Communic acid
2212:
2210:
2207:
2206:
2204:
2202:
2198:
2192:
2189:
2187:
2184:
2182:
2179:
2178:
2176:
2172:
2165:
2162:
2159:
2157:
2154:
2152:
2149:
2148:
2146:
2142:
2136:
2133:
2130:
2127:
2124:
2123:
2121:
2117:
2111:
2108:
2106:
2103:
2102:
2100:
2096:
2089:
2087:
2084:
2082:
2079:
2078:
2076:
2072:
2069:
2066:
2062:
2055:
2052:
2050:
2047:
2044:
2042:
2039:
2036:
2034:
2031:
2029:
2026:
2024:
2021:
2019:
2016:
2014:
2011:
2010:
2008:
2005:
2001:
1991:
1988:
1986:
1983:
1981:
1978:
1976:
1973:
1971:
1968:
1966:
1963:
1962:
1960:
1956:
1950:
1947:
1945:
1942:
1940:
1937:
1934:
1930:
1927:
1925:
1922:
1920:
1917:
1915:
1912:
1911:
1909:
1905:
1899:
1896:
1894:
1891:
1889:
1886:
1884:
1881:
1879:
1876:
1874:
1871:
1869:
1866:
1865:
1863:
1859:
1856:
1850:
1840:
1837:
1835:
1832:
1830:
1827:
1825:
1822:
1819:
1815:
1811:
1808:
1807:
1805:
1801:
1795:
1792:
1790:
1787:
1785:
1782:
1781:
1779:
1775:
1769:
1766:
1764:
1761:
1760:
1758:
1754:
1751:
1739:
1735:
1729:
1726:
1724:
1721:
1710:
1707:
1706:
1704:
1701:
1697:
1690:
1687:
1684:
1681:
1678:
1675:
1672:
1669:
1665:
1661:
1660:
1658:
1654:
1649:
1645:
1641:
1633:
1628:
1626:
1621:
1619:
1614:
1613:
1610:
1600:
1596:
1592:
1590:
1582:
1580:
1571:
1565:
1561:
1560:
1552:
1550:
1541:
1535:
1531:
1527:
1520:
1518:
1516:
1507:
1503:
1499:
1495:
1491:
1484:
1476:
1470:
1466:
1465:
1457:
1449:
1443:
1439:
1438:
1430:
1422:
1416:
1408:
1404:
1400:
1394:
1390:
1384:
1376:
1372:
1368:
1361:
1359:
1350:
1344:
1340:
1339:
1331:
1323:
1319:
1315:
1311:
1307:
1303:
1299:
1295:
1291:
1288:(in German).
1287:
1283:
1276:
1268:
1264:
1260:
1256:
1252:
1248:
1241:
1234:
1226:
1222:
1215:
1213:
1204:
1200:
1196:
1193:
1192:
1187:
1185:
1176:
1168:
1164:
1160:
1157:(in German).
1156:
1155:
1150:
1143:
1141:
1131:
1127:
1123:
1120:
1119:
1114:
1112:
1103:
1095:
1091:
1087:
1083:
1079:
1075:
1071:
1067:
1060:
1051:
1044:
1037:
1033:
1026:
1018:
1012:
1008:
1007:
999:
997:
992:
985:
983:
979:
975:
972:(presence of
971:
967:
963:
959:
955:
951:
947:
943:
939:
935:
931:
927:
923:
919:
915:
911:
907:
903:
899:
892:Health issues
889:
887:
886:folk medicine
883:
879:
875:
871:
867:
863:
859:
855:
851:
847:
843:
838:
836:
835:
830:
829:
824:
823:
817:
813:
809:
805:
804:
799:
795:
785:
783:
780:ratio in the
779:
775:
771:
770:essential oil
767:
763:
762:
757:
753:
752:Peumus boldus
749:
740:
731:
729:
725:
715:
712:
708:
704:
700:
696:
691:
689:
684:
680:
679:boiling point
676:
675:melting point
660:
656:
652:
648:
644:
640:
636:
632:
628:
624:
620:
616:
615:sulfuric acid
612:
608:
604:
600:
583:
582:
577:
561:
552:
538:
536:
532:
528:
524:
520:
516:
512:
509:is a natural
508:
496:
489:
484:
467:
461:
458:
455:
452:
448:
447:
443:
438:
434:
431:
427:
426:
422:
420:
415:
410:
406:
404:
403:Boiling point
401:
400:
396:
394:
393:Melting point
391:
390:
386:
384:
381:
380:
376:
373:
372:
368:
366:
363:
362:
346:
343:
339:
338:
333:
324:
323:
320:
313:
299:
298:
295:
288:
281:
277:
276:
274:
272:
269:
268:
261:
257:
256:
254:
248:
244:
243:
236:
232:
231:
229:
227:
224:
223:
216:
215:
213:
211:
206:
205:
201:
197:
194:
192:
190:ECHA InfoCard
187:
186:
179:
175:
174:
172:
170:
167:
166:
159:
155:
154:
152:
150:
147:
146:
139:
135:
134:
132:
130:
127:
126:
122:
119:
115:
114:
107:
103:
102:
100:
96:
91:
90:
83:
79:
78:
76:
73:
69:
68:
63:
57:
53:
48:
38:
34:
29:
25:
24:
20:
2698:Plant toxins
2668:Monoterpenes
2576:Gutta-balatá
2572:Gutta percha
2510:Xanthophylls
2490:Neurosporene
2409:Moronic acid
2399:Ursolic acid
2342:Testosterone
2325:Stigmasterol
2310:Cycloartenol
2297:Phytosterols
2239:Pimaric acid
2209:Abietic acid
2160:Manoyl oxide
2135:Salvinorin A
2125:
2065:Diterpenoids
1984:
1929:Thujaplicins
1854:(2,modified)
1794:Phellandrene
1738:Monoterpenes
1667:
1663:
1656:Basic forms:
1599:the original
1594:
1588:
1558:
1529:
1497:
1494:J. Chem. Soc
1493:
1489:
1483:
1463:
1456:
1436:
1429:
1388:
1383:
1374:
1370:
1337:
1330:
1289:
1285:
1275:
1250:
1246:
1233:
1225:The Terpenes
1224:
1194:
1189:
1183:
1175:
1161:(1): 49–75.
1158:
1152:
1134:See p. 1412.
1121:
1116:
1110:
1102:
1069:
1065:
1059:
1050:
1035:
1025:
1005:
928:, temporary
914:constipation
900:of skin and
895:
874:stomach ache
854:tonic drinks
839:
832:
826:
820:
801:
794:anthelmintic
791:
788:Applications
765:
759:
751:
745:
721:
695:Karl Ziegler
692:
688:Otto Wallach
658:
653:it formed a
631:hydrochloric
579:
573:
531:anthelmintic
506:
505:
456:
418:
158:ChEMBL467614
65:Identifiers
2544:Rubixanthin
2534:Astaxanthin
2495:Phytofluene
2447:Carotenoids
2337:Cholesterol
2332:Tocopherols
2302:Campesterol
2201:Resin acids
2186:Gibberellin
2181:Aphidicolin
2174:Tetracyclic
2049:Longifolene
2013:Artemisinin
1990:Umbellulone
1878:Citronellol
1868:Citronellal
1490:Chenopodium
1282:Ziegler, K.
1184:Chenopodium
1111:Chenopodium
970:albuminuria
946:convulsions
764:, formerly
703:chlorophyll
623:acetic acid
621:powder and
581:Chenopodium
527:Mexican tea
451:Signal word
387:1.010 g/cm
374:Appearance
335:Properties
196:100.007.408
17:Ascaridole
2652:Categories
2529:Zeaxanthin
2373:Lanosterol
2320:Sitosterol
2315:Sitostanol
2191:Paclitaxel
2098:Monocyclic
1985:Ascaridole
1980:Eucalyptol
1933:Hinokitiol
1907:Monocyclic
1777:Monocyclic
1644:terpenoids
1072:(8): 558.
1043:this video
988:References
898:irritation
860:and treat
846:flatulence
778:phosphorus
734:Occurrence
718:Properties
507:Ascaridole
430:Pictograms
365:Molar mass
280:1718D0GEVJ
169:ChemSpider
138:CHEBI:2866
93:3D model (
72:CAS Number
56:IUPAC name
2601:Synthesis
2457:Carotenes
2352:Ecdysones
2156:Forskolin
2144:Tricyclic
2131:Epimanool
2056:Nootkatin
2053:Muurolene
2045:Juniperol
2037:Chanootin
2018:Bisabolol
1949:Carvacrol
1789:Terpinene
1691:(4 rings)
1638:Types of
1415:cite book
1407:844215395
1314:0028-1042
1197:: 84–90.
1094:250895582
1038:: 12–120.
966:hematuria
934:blindness
870:dysentery
866:arthritis
699:terpinene
690:in 1912.
599:aldehydes
421:labelling
217:208-147-4
209:EC Number
2500:Phytoene
2485:Lycopene
2386:Squalane
2378:Saponins
2289:Steroids
2151:Cembrene
2128:-Abienol
2119:Bicyclic
2023:Cadinene
1958:Bicyclic
1924:p-Cymene
1898:Linalool
1883:Geraniol
1834:Sabinene
1824:Camphene
1803:Bicyclic
1784:Limonene
1768:Myrcenes
1709:Isoprene
1689:Steroids
1680:Iridoids
1648:isoprene
1640:terpenes
1267:24081588
982:jaundice
942:delirium
930:deafness
926:tinnitus
918:headache
910:vomiting
774:nitrogen
647:carbonyl
643:hydroxyl
515:peroxide
412:Hazards
82:512-85-6
2638:(DMAPP)
2368:Betulin
2166:Pimarol
2163:Pimaral
2110:Retinal
2105:Retinol
2074:Acyclic
2033:Cedrene
2028:Cadinol
1970:Borneol
1965:Camphor
1919:Menthol
1893:Halomon
1861:Acyclic
1829:Thujene
1763:Ocimene
1756:Acyclic
1322:8655604
1294:Bibcode
1074:Bibcode
960:in the
922:vertigo
878:malaria
803:Ascaris
756:epazote
683:density
611:alcohol
607:phenols
603:ketones
541:History
488:what is
486: (
383:Density
359:
247:PubChem
123:121382
2560:(many)
2539:Lutein
2390:Acids
2081:Phytol
1939:Thymol
1873:Citral
1839:Carene
1810:Pinene
1723:Prenol
1670:forms)
1650:units)
1646:(# of
1566:
1536:
1471:
1444:
1405:
1395:
1345:
1320:
1312:
1265:
1092:
1013:
968:, and
906:nausea
880:, and
862:asthma
655:glycol
645:nor a
635:nitric
627:cymene
483:verify
480:
457:Danger
319:SMILES
235:C09836
149:ChEMBL
50:Names
2632:(IPP)
2449:) (8)
2361:Other
1668:trans
1377:: 65.
1318:S2CID
1263:S2CID
1243:(PDF)
1186:. II"
1090:S2CID
978:urine
962:lungs
848:from
834:Sedum
828:Phlox
808:assay
748:boldo
637:, or
523:boldo
294:InChI
260:10545
178:10105
129:ChEBI
95:JSmol
1816:and
1749:)(2)
1666:and
1642:and
1564:ISBN
1534:ISBN
1469:ISBN
1442:ISBN
1421:link
1403:OCLC
1393:ISBN
1375:1441
1343:ISBN
1310:ISSN
1011:ISBN
950:coma
948:and
940:and
932:and
850:bean
822:Iris
814:and
782:soil
619:zinc
271:UNII
226:KEGG
2281:(6)
2264:(5)
2126:cis
2067:(4)
2006:(3)
1702:(1)
1664:cis
1502:doi
1498:392
1302:doi
1255:doi
1199:doi
1163:doi
1159:392
1126:doi
1082:doi
964:),
884:in
776:to
661:, C
605:or
419:GHS
250:CID
2654::
1747:16
1743:10
1741:(C
1711:(C
1593:.
1578:^
1548:^
1514:^
1496:.
1417:}}
1413:{{
1401:.
1373:.
1369:.
1357:^
1316:.
1308:.
1300:.
1290:32
1261:.
1251:41
1249:.
1245:.
1223:.
1211:^
1195:35
1188:.
1139:^
1122:33
1115:.
1088:.
1080:.
1070:34
1068:.
1034:.
995:^
924:,
920:,
916:,
912:,
908:,
904:,
876:,
872:,
868:,
864:,
831:,
825:,
667:18
663:10
633:,
601:,
591:16
587:10
423::
353:16
349:10
2615:)
2611:(
2512::
2445:(
1935:)
1931:(
1820:)
1818:β
1814:α
1812:(
1745:H
1719:)
1717:8
1715:H
1713:5
1631:e
1624:t
1617:v
1591:"
1587:"
1572:.
1542:.
1508:.
1504::
1477:.
1450:.
1423:)
1409:.
1351:.
1324:.
1304::
1296::
1269:.
1257::
1205:.
1201::
1169:.
1165::
1132:.
1128::
1113:"
1096:.
1084::
1076::
1045:.
1019:.
956:(
750:(
671:3
669:O
665:H
595:2
593:O
589:H
478:N
357:2
355:O
351:H
347:C
97:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.