1421:
31:
148:
74:. Typically, because of the difficulty to isolate atoms in order to measure their radii separately, atomic radius is measured in a chemically bonded state; however theoretical calculations are simpler when considering atoms in isolation. The dependencies on environment, probe, and state lead to a multiplicity of definitions.
218:: In the simplest definition, half the minimum distance between the nuclei of two atoms of the element that are not otherwise bound by covalent or metallic interactions. The Van der Waals radius may be defined even for elements (such as metals) in which Van der Waals forces are dominated by other interactions. Because
1463:
Essentially, the atomic radius decreases across the periods due to an increasing number of protons. Therefore, there is a greater attraction between the protons and electrons because opposite charges attract, and more protons create a stronger charge. The greater attraction draws the electrons closer
203:, it was suggested that all atoms of the same element have the same radii. However, in 1923, when more crystal data had become available, it was found that the approximation of an atom as a sphere does not necessarily hold when comparing the same atom in different crystal structures.
1677:
The d-block contraction is less pronounced than the lanthanide contraction but arises from a similar cause. In this case, it is the poor shielding capacity of the 3d-electrons which affects the atomic radii and chemistries of the elements immediately following the first row of the
1464:
to the protons, decreasing the size of the particle. Therefore, the atomic radius decreases. Down the groups, atomic radius increases. This is because there are more energy levels and therefore a greater distance between protons and electrons. In addition,
248:
to other atoms, as deduced from the separation between the atomic nuclei in molecules. In principle, the distance between two atoms that are bound to each other in a molecule (the length of that covalent bond) should equal the sum of their covalent
285:. Although the model itself is now obsolete, the Bohr radius for the hydrogen atom is still regarded as an important physical constant, because it is equivalent to the quantum-mechanical most probable distance of the electron from the nucleus.
230:: the nominal radius of the ions of an element in a specific ionization state, deduced from the spacing of atomic nuclei in crystalline salts that include that ion. In principle, the spacing between two adjacent oppositely charged ions (the
1440:
in the nucleus. As the atomic number increases along each row of the periodic table, the additional electrons go into the same outermost shell; whose radius gradually contracts, due to the increasing nuclear charge. In a
108:
or electron clouds. Moreover, in condensed matter and molecules, the electron clouds of the atoms usually overlap to some extent, and some of the electrons may roam over a large region encompassing two or more atoms.
2961:
171:
For many purposes, atoms can be modeled as spheres. This is only a crude approximation, but it can provide quantitative explanations and predictions for many phenomena, such as the
58:. Since the boundary is not a well-defined physical entity, there are various non-equivalent definitions of atomic radius. Four widely used definitions of atomic radius are:
97:; and its value may be obtained through experimental measurements, or computed from theoretical models. The value of the radius may depend on the atom's state and context.
1432:
can be explained by the arrangement of electrons in shells of fixed capacity. The shells are generally filled in order of increasing radius, since the negatively
1468:
causes attraction to decrease, so remaining electrons can go farther away from the positively charged nucleus. Therefore, the size, or atomic radius, increases.
1581: = 70), are not particularly effective at shielding the increasing nuclear charge from the sub-shells further out. The elements immediately following the
1585:
have atomic radii which are smaller than would be expected and which are almost identical to the atomic radii of the elements immediately above them. Hence
306:(pm or 1×10 m), with an accuracy of about 5 pm. The shade of the box ranges from red to yellow as the radius increases; gray indicates lack of data.
3309:
Clementi, E.; Raimond, D. L.; Reinhardt, W. P. (1967). "Atomic
Screening Constants from SCF Functions. II. Atoms with 37 to 86 Electrons".
222:, the polarisability (which can usually be measured or calculated more easily) may be used to define the Van der Waals radius indirectly.
3356:
2892:
2818:
3215:
1636:, decrease in size of Ln ions increases the covalent character and decreases the basic character between Ln and OH ions in Ln(OH)
3156:
1456:; which explains why the size of atoms usually increases down each column. However, there is one notable exception, known as the
3293:
2906:
2875:
1452:
The increasing nuclear charge is partly counterbalanced by the increasing number of electrons, a phenomenon that is known as
117:
1460:: the 5d block of elements are much smaller than one would expect, due to the weak shielding of the 4f electrons.
100:
Electrons do not have definite orbits nor sharply defined ranges. Rather, their positions must be described as
104:
that taper off gradually as one moves away from the nucleus, without a sharp cutoff; these are referred to as
1656:
There is a regular decrease in their tendency to act as a reducing agent, with an increase in atomic number.
3311:
3242:
17:
3049:
3026:
101:
1471:
The following table summarizes the main phenomena that influence the atomic radius of an element:
3183:"On the Constitution of Atoms and Molecules, Part II. – Systems containing only a Single Nucleus"
3054:
3351:
3190:
3131:
3124:"On the Constitution of Atoms and Molecules, Part I. – Binding of Electrons by Positive Nuclei"
3050:"Quantum-Mechanical Relation between Atomic Dipole Polarizability and the van der Waals Radius"
2928:
1648:
can dissolve with difficulty in hot concentrated NaOH. Hence the order of size of Ln is given:
1554:
1457:
1662:
Consequently, these elements occur together in natural minerals and are difficult to separate.
1858:
1424:
A graph comparing the atomic radius of elements with atomic numbers 1–100. Accuracy of ±5 pm.
457:
200:
1449:
will go into the next outer shell, accounting for the sudden increase in the atomic radius.
3320:
3251:
3199:
3140:
3073:
2970:
2898:
2867:
1717:
334:
316:
215:
164:
59:
8:
1706:
The following table shows atomic radii computed from theoretical models, as published by
1672:
1618:
219:
188:
3324:
3255:
3203:
3144:
3077:
2974:
220:
Van der Waals interactions arise through quantum fluctuations of the atomic polarisation
3182:
3097:
3063:
2993:
2956:
1659:
The second and third rows of d-block transition elements are quite close in properties.
1445:, the outermost shell is completely filled; therefore, the additional electron of next
270:
3123:
3289:
3089:
3030:
2998:
2902:
2871:
2394:
1622:
993:
34:
Diagram of a helium atom, showing the electron probability density as shades of gray.
3101:
3048:
Federov, Dmitry V.; Sadhukhan, Mainak; Stöhr, Martin; Tkatchenko, Alexandre (2018).
199:
In 1920, shortly after it had become possible to determine the sizes of atoms using
112:
Under most definitions the radii of isolated neutral atoms range between 30 and 300
3328:
3259:
3207:
3148:
3085:
3081:
2988:
2978:
2937:
2843:
2838:
2446:
2306:
2169:
2032:
1965:
1898:
1873:
1807:
1793:
1679:
1633:
1465:
1453:
1045:
905:
768:
631:
564:
497:
472:
406:
399:
392:
180:
82:
78:
43:
2828:
1786:
1779:
1772:
1765:
1758:
1751:
1744:
1707:
1433:
385:
378:
371:
364:
357:
350:
343:
252:
241:
71:
67:
1420:
2962:
Proceedings of the
National Academy of Sciences of the United States of America
1735:
1560:
125:
105:
51:
3211:
3152:
2941:
1609:, and so forth. The effect of the lanthanide contraction is noticeable up to
3345:
2823:
2478:
1800:
1569:
1429:
1077:
256:
245:
121:
94:
3093:
3002:
2725:
2614:
2520:
1821:
1728:
1446:
1324:
1213:
1119:
420:
327:
299:
227:
63:
1628:
Due to lanthanide contraction, the 5 following observations can be drawn:
255:: the nominal radius of atoms of an element when joined to other atoms by
3285:
2833:
2795:
2781:
2774:
2562:
2534:
2527:
1814:
1632:
The size of Ln ions regularly decreases with atomic number. According to
1394:
1380:
1373:
1161:
1133:
1126:
413:
282:
262:
231:
2983:
2663:
2649:
2628:
2569:
2513:
2492:
2471:
2219:
2212:
2001:
1582:
1262:
1248:
1227:
1168:
1112:
1091:
1070:
818:
811:
600:
303:
266:
235:
133:
129:
90:
3332:
3263:
1533:
repulsive force acting on outermost shell electrons by inner electrons
2767:
2753:
2746:
2739:
2691:
2621:
2600:
2576:
2555:
2548:
2282:
2240:
2226:
2198:
2182:
2131:
2082:
2038:
1987:
1978:
1911:
1849:
1835:
1828:
1598:
1574:
1564:
1442:
1366:
1352:
1345:
1338:
1290:
1220:
1199:
1175:
1154:
1147:
881:
839:
825:
797:
781:
730:
681:
637:
586:
577:
510:
448:
434:
427:
176:
141:
113:
2923:
3068:
2802:
2711:
2642:
2635:
2541:
2452:
2429:
2422:
2401:
2380:
2352:
2345:
2331:
2275:
2175:
2145:
2075:
2068:
2061:
2054:
2015:
1948:
1934:
1879:
1610:
1602:
1586:
1401:
1310:
1241:
1234:
1140:
1051:
1028:
1021:
1000:
979:
951:
944:
930:
874:
774:
744:
674:
667:
660:
653:
614:
547:
533:
478:
274:
86:
55:
2788:
2732:
2718:
2684:
2670:
2656:
2506:
2499:
2485:
2415:
2373:
2359:
2338:
2312:
2254:
2233:
2205:
2191:
2159:
2152:
2138:
2124:
2045:
1994:
1904:
1842:
1691:
1683:
1606:
1594:
1590:
1387:
1331:
1317:
1283:
1269:
1255:
1105:
1098:
1084:
1014:
972:
958:
937:
911:
853:
832:
804:
790:
758:
751:
737:
723:
644:
593:
503:
441:
184:
172:
152:
147:
124:. Therefore, the radius of an atom is more than 10,000 times the
30:
3034:
3047:
2760:
2677:
2607:
2459:
2366:
2319:
2289:
2261:
2247:
2110:
2103:
2096:
2008:
1971:
1941:
1927:
1888:
1437:
1359:
1276:
1206:
1058:
965:
918:
888:
860:
846:
709:
702:
695:
607:
570:
540:
526:
487:
278:
265:: the radius of the lowest-energy electron orbit predicted by
50:, usually the mean or typical distance from the center of the
2436:
2296:
2022:
1920:
1035:
895:
621:
519:
137:
77:
Depending on the definition, the term may apply to atoms in
2408:
2387:
2117:
2089:
1955:
1007:
986:
716:
688:
554:
47:
1516:
attractive force acting on electrons by protons in nucleus
2268:
867:
3308:
1597:
has virtually the same atomic radius (and chemistry) as
238:
between them) should equal the sum of their ionic radii.
163:
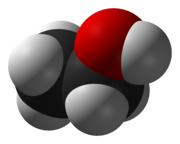
OH. Each atom is modeled by a sphere with the element's
1710:
and others in 1967. The values are in picometres (pm).
289:
244:: the nominal radius of the atoms of an element when
1415:
3240:Slater, J. C. (1964). "Atomic Radii in Crystals".
2890:
1436:electrons are attracted by the positively charged
211:Widely used definitions of atomic radius include:
3275:
3273:
1653:There is a regular decrease in their ionic radii.
1428:The way the atomic radius varies with increasing
3343:
2861:
294:The following table shows empirically measured
3270:
2891:Basdevant, J.-L.; Rich, J.; Spiro, M. (2005).
269:of the atom (1913). It is only applicable to
3016:
3014:
3012:
2957:"On the Hypothesis of Constant Atomic Radii"
1701:
1617: = 78), after which it is masked by a
3041:
3009:
1522:increase along each period (left to right)
3067:
2992:
2982:
1548:
2884:
2819:Atomic radii of the elements (data page)
1419:
298:radii for the elements, as published by
146:
29:
3233:
3020:
2954:
1502:principal and azimuthal quantum numbers
183:, the arrangement of atoms and ions in
151:The approximate shape of a molecule of
14:
3344:
3302:
3239:
2924:"The arrangement of atoms in crystals"
1666:
3279:
3174:
2921:
2862:Cotton, F. A.; Wilkinson, G. (1988).
1563:, which is progressively filled from
271:atoms and ions with a single electron
3180:
3121:
3115:
1539:reduce the effect of nuclear charge
1536:number of electrons in inner shells
24:
310:inerd gas have no any capabalites
290:Empirically measured atomic radius
120:of a meter), or between 0.3 and 3
25:
3368:
1650:La > Ce > ..., ... > Lu.
1589:is in fact slightly smaller than
1416:Explanation of the general trends
1605:has an atomic radius similar to
46:is a measure of the size of its
3357:Properties of chemical elements
3221:from the original on 2008-12-09
3162:from the original on 2011-09-02
3023:The Nature of the Chemical Bond
2894:Fundamentals in Nuclear Physics
2801:
2794:
2787:
2780:
2773:
2766:
2759:
2752:
2745:
2738:
2731:
2724:
2717:
2710:
2690:
2683:
2676:
2669:
2662:
2655:
2648:
2641:
2634:
2627:
2620:
2613:
2606:
2599:
2575:
2568:
2561:
2554:
2547:
2540:
2533:
2526:
2519:
2512:
2505:
2498:
2491:
2484:
2477:
2470:
2458:
2451:
2435:
2421:
2414:
2407:
2400:
2393:
2386:
2379:
2372:
2365:
2358:
2351:
2344:
2337:
2330:
2318:
2311:
2295:
2288:
2281:
2274:
2267:
2260:
2253:
2246:
2239:
2232:
2225:
2218:
2211:
2204:
2197:
2190:
2181:
2174:
2158:
2151:
2144:
2137:
2130:
2123:
2116:
2109:
2102:
2095:
2088:
2081:
2074:
2067:
2060:
2053:
2044:
2037:
2021:
2014:
2007:
2000:
1993:
1986:
1977:
1970:
1954:
1947:
1940:
1933:
1926:
1919:
1910:
1903:
1887:
1878:
1400:
1393:
1386:
1379:
1372:
1365:
1358:
1351:
1344:
1337:
1330:
1323:
1316:
1309:
1289:
1282:
1275:
1268:
1261:
1254:
1247:
1240:
1233:
1226:
1219:
1212:
1205:
1198:
1174:
1167:
1160:
1153:
1146:
1139:
1132:
1125:
1118:
1111:
1104:
1097:
1090:
1083:
1076:
1069:
1057:
1050:
1034:
1027:
1020:
1013:
1006:
999:
992:
985:
978:
971:
964:
957:
950:
943:
936:
929:
917:
910:
894:
887:
880:
873:
866:
859:
852:
845:
838:
831:
824:
817:
810:
803:
796:
789:
780:
773:
757:
750:
743:
736:
729:
722:
715:
708:
701:
694:
687:
680:
673:
666:
659:
652:
643:
636:
620:
613:
606:
599:
592:
585:
576:
569:
553:
546:
539:
532:
525:
518:
509:
502:
486:
477:
132:), and less than 1/1000 of the
3086:10.1103/PhysRevLett.121.183401
2948:
2915:
2855:
2428:
206:
27:Measure of the size of an atom
13:
1:
2849:
2864:Advanced Inorganic Chemistry
2587:
2583:
2443:
1712:
1542:increases the atomic radius
1525:decreases the atomic radius
1508:increases the atomic radius
308:
7:
3312:Journal of Chemical Physics
3243:Journal of Chemical Physics
2812:
302:in 1964. The values are in
189:size and shape of molecules
175:of liquids and solids, the
10:
3373:
3282:Modern Inorganic Chemistry
2955:Wyckoff, R. W. G. (1923).
1984:
1917:
1885:
1867:
1670:
1640:, to the point that Yb(OH)
1552:
1409:
583:
516:
484:
466:
194:
54:to the outermost isolated
3212:10.1080/14786441308634993
3153:10.1080/14786441308634955
2942:10.1080/14786440808636111
1505:increase down each column
102:probability distributions
3027:Cornell University Press
1702:Calculated atomic radius
1559:The electrons in the 4f-
3055:Physical Review Letters
2901:. p. 13, fig 1.1.
3191:Philosophical Magazine
3132:Philosophical Magazine
2929:Philosophical Magazine
1555:Lanthanide contraction
1549:Lanthanide contraction
1458:lanthanide contraction
1425:
168:
35:
3280:Jolly, W. L. (1991).
2922:Bragg, W. L. (1920).
1423:
201:X-ray crystallography
150:
126:radius of its nucleus
33:
3021:Pauling, L. (1945).
216:Van der Waals radius
165:Van der Waals radius
60:Van der Waals radius
3325:1967JChPh..47.1300C
3256:1964JChPh..41.3199S
3204:1913PMag...26..476B
3145:1913PMag...26....1B
3078:2018PhRvL.121r3401F
2984:10.1073/pnas.9.2.33
2975:1923PNAS....9...33W
1673:d-block contraction
1667:d-block contraction
1619:relativistic effect
311:
1466:electron shielding
1426:
309:
179:of fluids through
169:
83:covalently bonding
36:
3333:10.1063/1.1712084
3295:978-0-07-112651-9
3264:10.1063/1.1725697
3250:(10): 3199–3205.
3181:Bohr, N. (1913).
3122:Bohr, N. (1913).
2908:978-0-387-01672-6
2877:978-0-471-84997-1
2810:
2809:
1865:
1724:
1680:transition metals
1623:inert-pair effect
1546:
1545:
1499:quantum mechanics
1491:effect on radius
1413:
1412:
464:
323:
277:, singly ionized
16:(Redirected from
3364:
3337:
3336:
3319:(4): 1300–1307.
3306:
3300:
3299:
3284:(2nd ed.).
3277:
3268:
3267:
3237:
3231:
3230:
3228:
3226:
3220:
3198:(153): 476–502.
3187:
3178:
3172:
3171:
3169:
3167:
3161:
3128:
3119:
3113:
3112:
3110:
3108:
3071:
3045:
3039:
3038:
3025:(2nd ed.).
3018:
3007:
3006:
2996:
2986:
2952:
2946:
2945:
2936:(236): 169–189.
2919:
2913:
2912:
2888:
2882:
2881:
2870:. p. 1385.
2866:(5th ed.).
2859:
2844:Kinetic diameter
2839:Steric hindrance
1863:
1722:
1713:
1483:increase with...
1474:
1473:
462:
321:
312:
246:covalently bound
181:molecular sieves
79:condensed matter
44:chemical element
21:
3372:
3371:
3367:
3366:
3365:
3363:
3362:
3361:
3342:
3341:
3340:
3307:
3303:
3296:
3278:
3271:
3238:
3234:
3224:
3222:
3218:
3185:
3179:
3175:
3165:
3163:
3159:
3126:
3120:
3116:
3106:
3104:
3046:
3042:
3019:
3010:
2953:
2949:
2920:
2916:
2909:
2889:
2885:
2878:
2860:
2856:
2852:
2829:Covalent radius
2815:
2805:
2798:
2791:
2784:
2777:
2770:
2763:
2756:
2749:
2742:
2735:
2728:
2721:
2714:
2707:
2694:
2687:
2680:
2673:
2666:
2659:
2652:
2645:
2638:
2631:
2624:
2617:
2610:
2603:
2596:
2579:
2572:
2565:
2558:
2551:
2544:
2537:
2530:
2523:
2516:
2509:
2502:
2495:
2488:
2481:
2474:
2467:
2462:
2455:
2439:
2432:
2425:
2418:
2411:
2404:
2397:
2390:
2383:
2376:
2369:
2362:
2355:
2348:
2341:
2334:
2327:
2322:
2315:
2299:
2292:
2285:
2278:
2271:
2264:
2257:
2250:
2243:
2236:
2229:
2222:
2215:
2208:
2201:
2194:
2185:
2178:
2162:
2155:
2148:
2141:
2134:
2127:
2120:
2113:
2106:
2099:
2092:
2085:
2078:
2071:
2064:
2057:
2048:
2041:
2025:
2018:
2011:
2004:
1997:
1990:
1981:
1974:
1958:
1951:
1944:
1937:
1930:
1923:
1914:
1907:
1891:
1882:
1862:
1721:
1708:Enrico Clementi
1704:
1690: = 31) to
1675:
1669:
1649:
1647:
1643:
1639:
1573: = 57) to
1557:
1551:
1496:electron shells
1418:
1404:
1397:
1390:
1383:
1376:
1369:
1362:
1355:
1348:
1341:
1334:
1327:
1320:
1313:
1306:
1293:
1286:
1279:
1272:
1265:
1258:
1251:
1244:
1237:
1230:
1223:
1216:
1209:
1202:
1195:
1178:
1171:
1164:
1157:
1150:
1143:
1136:
1129:
1122:
1115:
1108:
1101:
1094:
1087:
1080:
1073:
1066:
1061:
1054:
1038:
1031:
1024:
1017:
1010:
1003:
996:
989:
982:
975:
968:
961:
954:
947:
940:
933:
926:
921:
914:
898:
891:
884:
877:
870:
863:
856:
849:
842:
835:
828:
821:
814:
807:
800:
793:
784:
777:
761:
754:
747:
740:
733:
726:
719:
712:
705:
698:
691:
684:
677:
670:
663:
656:
647:
640:
624:
617:
610:
603:
596:
589:
580:
573:
557:
550:
543:
536:
529:
522:
513:
506:
490:
481:
461:
320:
292:
253:Metallic radius
242:Covalent radius
209:
197:
162:
158:
106:atomic orbitals
72:covalent radius
68:metallic radius
28:
23:
22:
15:
12:
11:
5:
3370:
3360:
3359:
3354:
3339:
3338:
3301:
3294:
3288:. p. 22.
3269:
3232:
3173:
3114:
3062:(18): 183401.
3040:
3008:
2947:
2914:
2907:
2883:
2876:
2853:
2851:
2848:
2847:
2846:
2841:
2836:
2831:
2826:
2821:
2814:
2811:
2808:
2807:
2800:
2793:
2786:
2779:
2772:
2765:
2758:
2751:
2744:
2737:
2730:
2723:
2716:
2709:
2704:
2702:
2700:
2697:
2696:
2689:
2682:
2675:
2668:
2661:
2654:
2647:
2640:
2633:
2626:
2619:
2612:
2605:
2598:
2593:
2591:
2589:
2586:
2585:
2582:
2581:
2574:
2567:
2560:
2553:
2546:
2539:
2532:
2525:
2518:
2511:
2504:
2497:
2490:
2483:
2476:
2469:
2464:
2457:
2450:
2442:
2441:
2434:
2427:
2420:
2413:
2406:
2399:
2392:
2385:
2378:
2371:
2364:
2357:
2350:
2343:
2336:
2329:
2324:
2317:
2310:
2302:
2301:
2294:
2287:
2280:
2273:
2266:
2259:
2252:
2245:
2238:
2231:
2224:
2217:
2210:
2203:
2196:
2189:
2187:
2180:
2173:
2165:
2164:
2157:
2150:
2143:
2136:
2129:
2122:
2115:
2108:
2101:
2094:
2087:
2080:
2073:
2066:
2059:
2052:
2050:
2043:
2036:
2028:
2027:
2020:
2013:
2006:
1999:
1992:
1985:
1983:
1976:
1969:
1961:
1960:
1953:
1946:
1939:
1932:
1925:
1918:
1916:
1909:
1902:
1894:
1893:
1886:
1884:
1877:
1869:
1868:
1866:
1854:
1853:
1846:
1839:
1832:
1825:
1818:
1811:
1804:
1797:
1790:
1783:
1776:
1769:
1762:
1755:
1748:
1741:
1739:
1732:
1725:
1703:
1700:
1671:Main article:
1668:
1665:
1664:
1663:
1660:
1657:
1654:
1651:
1645:
1641:
1637:
1553:Main article:
1550:
1547:
1544:
1543:
1540:
1537:
1534:
1531:
1527:
1526:
1523:
1520:
1517:
1514:
1513:nuclear charge
1510:
1509:
1506:
1503:
1500:
1497:
1493:
1492:
1489:
1484:
1481:
1478:
1417:
1414:
1411:
1410:
1407:
1406:
1399:
1392:
1385:
1378:
1371:
1364:
1357:
1350:
1343:
1336:
1329:
1322:
1315:
1308:
1303:
1301:
1299:
1296:
1295:
1288:
1281:
1274:
1267:
1260:
1253:
1246:
1239:
1232:
1225:
1218:
1211:
1204:
1197:
1192:
1190:
1188:
1185:
1184:
1181:
1180:
1173:
1166:
1159:
1152:
1145:
1138:
1131:
1124:
1117:
1110:
1103:
1096:
1089:
1082:
1075:
1068:
1063:
1056:
1049:
1041:
1040:
1033:
1026:
1019:
1012:
1005:
998:
991:
984:
977:
970:
963:
956:
949:
942:
935:
928:
923:
916:
909:
901:
900:
893:
886:
879:
872:
865:
858:
851:
844:
837:
830:
823:
816:
809:
802:
795:
788:
786:
779:
772:
764:
763:
756:
749:
742:
735:
728:
721:
714:
707:
700:
693:
686:
679:
672:
665:
658:
651:
649:
642:
635:
627:
626:
619:
612:
605:
598:
591:
584:
582:
575:
568:
560:
559:
552:
545:
538:
531:
524:
517:
515:
508:
501:
493:
492:
485:
483:
476:
468:
467:
465:
453:
452:
445:
438:
431:
424:
417:
410:
403:
396:
389:
382:
375:
368:
361:
354:
347:
340:
338:
331:
324:
291:
288:
287:
286:
260:
257:metallic bonds
250:
239:
224:
223:
208:
205:
196:
193:
160:
156:
95:excited states
26:
9:
6:
4:
3:
2:
3369:
3358:
3355:
3353:
3352:Atomic radius
3350:
3349:
3347:
3334:
3330:
3326:
3322:
3318:
3314:
3313:
3305:
3297:
3291:
3287:
3283:
3276:
3274:
3265:
3261:
3257:
3253:
3249:
3245:
3244:
3236:
3217:
3213:
3209:
3205:
3201:
3197:
3193:
3192:
3184:
3177:
3158:
3154:
3150:
3146:
3142:
3139:(151): 1–24.
3138:
3134:
3133:
3125:
3118:
3103:
3099:
3095:
3091:
3087:
3083:
3079:
3075:
3070:
3065:
3061:
3057:
3056:
3051:
3044:
3036:
3032:
3028:
3024:
3017:
3015:
3013:
3004:
3000:
2995:
2990:
2985:
2980:
2976:
2972:
2968:
2964:
2963:
2958:
2951:
2943:
2939:
2935:
2931:
2930:
2925:
2918:
2910:
2904:
2900:
2896:
2895:
2887:
2879:
2873:
2869:
2865:
2858:
2854:
2845:
2842:
2840:
2837:
2835:
2832:
2830:
2827:
2825:
2824:Chemical bond
2822:
2820:
2817:
2816:
2804:
2797:
2790:
2783:
2776:
2769:
2762:
2755:
2748:
2741:
2734:
2727:
2720:
2713:
2705:
2703:
2701:
2699:
2698:
2693:
2686:
2679:
2672:
2665:
2658:
2651:
2644:
2637:
2630:
2623:
2616:
2609:
2602:
2594:
2592:
2590:
2588:
2584:
2578:
2571:
2564:
2557:
2550:
2543:
2536:
2529:
2522:
2515:
2508:
2501:
2494:
2487:
2480:
2473:
2465:
2461:
2454:
2449:
2448:
2444:
2438:
2431:
2424:
2417:
2410:
2403:
2396:
2389:
2382:
2375:
2368:
2361:
2354:
2347:
2340:
2333:
2325:
2321:
2314:
2309:
2308:
2304:
2303:
2298:
2291:
2284:
2277:
2270:
2263:
2256:
2249:
2242:
2235:
2228:
2221:
2214:
2207:
2200:
2193:
2188:
2184:
2177:
2172:
2171:
2167:
2166:
2161:
2154:
2147:
2140:
2133:
2126:
2119:
2112:
2105:
2098:
2091:
2084:
2077:
2070:
2063:
2056:
2051:
2047:
2040:
2035:
2034:
2030:
2029:
2024:
2017:
2010:
2003:
1996:
1989:
1980:
1973:
1968:
1967:
1963:
1962:
1957:
1950:
1943:
1936:
1929:
1922:
1913:
1906:
1901:
1900:
1896:
1895:
1890:
1881:
1876:
1875:
1871:
1870:
1861:
1860:
1856:
1855:
1852:
1851:
1847:
1845:
1844:
1840:
1838:
1837:
1833:
1831:
1830:
1826:
1824:
1823:
1819:
1817:
1816:
1812:
1810:
1809:
1805:
1803:
1802:
1798:
1796:
1795:
1791:
1789:
1788:
1784:
1782:
1781:
1777:
1775:
1774:
1770:
1768:
1767:
1763:
1761:
1760:
1756:
1754:
1753:
1749:
1747:
1746:
1742:
1740:
1738:
1737:
1733:
1731:
1730:
1726:
1720:
1719:
1715:
1714:
1711:
1709:
1699:
1698: = 35).
1697:
1693:
1689:
1685:
1681:
1674:
1661:
1658:
1655:
1652:
1635:
1634:Fajans' rules
1631:
1630:
1629:
1626:
1624:
1621:known as the
1620:
1616:
1612:
1608:
1604:
1600:
1596:
1592:
1588:
1584:
1580:
1576:
1572:
1571:
1566:
1562:
1556:
1541:
1538:
1535:
1532:
1529:
1528:
1524:
1521:
1519:atomic number
1518:
1515:
1512:
1511:
1507:
1504:
1501:
1498:
1495:
1494:
1490:
1488:
1485:
1482:
1479:
1476:
1475:
1472:
1469:
1467:
1461:
1459:
1455:
1450:
1448:
1444:
1439:
1435:
1431:
1430:atomic number
1422:
1408:
1403:
1396:
1389:
1382:
1375:
1368:
1361:
1354:
1347:
1340:
1333:
1326:
1319:
1312:
1304:
1302:
1300:
1298:
1297:
1292:
1285:
1278:
1271:
1264:
1257:
1250:
1243:
1236:
1229:
1222:
1215:
1208:
1201:
1193:
1191:
1189:
1187:
1186:
1183:
1182:
1177:
1170:
1163:
1156:
1149:
1142:
1135:
1128:
1121:
1114:
1107:
1100:
1093:
1086:
1079:
1072:
1064:
1060:
1053:
1048:
1047:
1043:
1042:
1037:
1030:
1023:
1016:
1009:
1002:
995:
988:
981:
974:
967:
960:
953:
946:
939:
932:
924:
920:
913:
908:
907:
903:
902:
897:
890:
883:
876:
869:
862:
855:
848:
841:
834:
827:
820:
813:
806:
799:
792:
787:
783:
776:
771:
770:
766:
765:
760:
753:
746:
739:
732:
725:
718:
711:
704:
697:
690:
683:
676:
669:
662:
655:
650:
646:
639:
634:
633:
629:
628:
623:
616:
609:
602:
595:
588:
579:
572:
567:
566:
562:
561:
556:
549:
542:
535:
528:
521:
512:
505:
500:
499:
495:
494:
489:
480:
475:
474:
470:
469:
460:
459:
455:
454:
451:
450:
446:
444:
443:
439:
437:
436:
432:
430:
429:
425:
423:
422:
418:
416:
415:
411:
409:
408:
404:
402:
401:
397:
395:
394:
390:
388:
387:
383:
381:
380:
376:
374:
373:
369:
367:
366:
362:
360:
359:
355:
353:
352:
348:
346:
345:
341:
339:
337:
336:
332:
330:
329:
325:
319:
318:
314:
313:
307:
305:
301:
297:
284:
280:
276:
272:
268:
264:
261:
258:
254:
251:
247:
243:
240:
237:
233:
229:
226:
225:
221:
217:
214:
213:
212:
204:
202:
192:
190:
186:
182:
178:
174:
166:
154:
149:
145:
143:
139:
135:
131:
127:
123:
119:
115:
110:
107:
103:
98:
96:
92:
88:
84:
80:
75:
73:
69:
65:
61:
57:
53:
49:
45:
41:
40:atomic radius
32:
19:
3316:
3310:
3304:
3281:
3247:
3241:
3235:
3223:. Retrieved
3195:
3189:
3176:
3164:. Retrieved
3136:
3130:
3117:
3105:. Retrieved
3059:
3053:
3043:
3022:
2969:(2): 33–38.
2966:
2960:
2950:
2933:
2927:
2917:
2893:
2886:
2863:
2857:
2445:
2305:
2168:
2031:
1964:
1897:
1872:
1857:
1848:
1841:
1834:
1827:
1820:
1813:
1806:
1799:
1792:
1785:
1778:
1771:
1764:
1757:
1750:
1743:
1734:
1727:
1716:
1705:
1695:
1687:
1676:
1627:
1614:
1578:
1568:
1558:
1486:
1470:
1462:
1451:
1447:alkali metal
1427:
1044:
904:
767:
630:
563:
496:
471:
456:
447:
440:
433:
426:
419:
412:
405:
398:
391:
384:
377:
370:
363:
356:
349:
342:
333:
326:
315:
300:J. C. Slater
295:
293:
228:Ionic radius
210:
198:
170:
111:
99:
76:
64:ionic radius
39:
37:
3286:McGraw-Hill
2834:Bond length
1583:lanthanides
283:positronium
263:Bohr radius
207:Definitions
136:of visible
118:trillionths
18:Atomic size
3346:Categories
3069:1803.11507
2850:References
1644:and Lu(OH)
304:picometers
273:, such as
267:Bohr model
236:ionic bond
187:, and the
134:wavelength
1599:zirconium
1575:ytterbium
1565:lanthanum
1530:shielding
1480:principle
1454:shielding
1443:noble gas
177:diffusion
140:(400–700
122:ångströms
87:molecules
3216:Archived
3157:Archived
3102:53564141
3094:30444421
3035:42034474
3003:16576657
2899:Springer
2813:See also
1723:(column)
1611:platinum
1603:tantalum
1587:lutetium
1561:subshell
322:(column)
296:covalent
275:hydrogen
185:crystals
89:, or in
56:electron
3321:Bibcode
3252:Bibcode
3200:Bibcode
3141:Bibcode
3074:Bibcode
2994:1085234
2971:Bibcode
2806:
2799:
2792:
2785:
2778:
2771:
2764:
2757:
2750:
2743:
2736:
2729:
2722:
2715:
2708:
2597:
2580:
2573:
2566:
2559:
2552:
2545:
2538:
2531:
2524:
2517:
2510:
2503:
2496:
2489:
2482:
2475:
2468:
2463:
2456:
2328:
1692:bromine
1684:gallium
1682:, from
1607:niobium
1595:hafnium
1591:yttrium
1487:tend to
1438:protons
1434:charged
1405:
1398:
1391:
1384:
1377:
1370:
1363:
1307:
1196:
1179:
1172:
1165:
1158:
1151:
1144:
1137:
1130:
1123:
1116:
1109:
1102:
1095:
1088:
1081:
1074:
1067:
1055:
1039:
1032:
927:
899:
762:
625:
558:
491:
234:of the
195:History
173:density
153:ethanol
91:ionized
52:nucleus
3292:
3225:8 June
3166:8 June
3100:
3092:
3033:
3001:
2991:
2905:
2874:
1859:Period
1601:, and
1477:factor
458:Period
281:, and
279:helium
249:radii.
232:length
128:(1–10
3219:(PDF)
3194:. 6.
3186:(PDF)
3160:(PDF)
3135:. 6.
3127:(PDF)
3107:9 May
3098:S2CID
3064:arXiv
2932:. 6.
2868:Wiley
1864:(row)
1718:Group
463:(row)
317:Group
138:light
42:of a
3290:ISBN
3227:2011
3168:2011
3109:2021
3090:PMID
3031:LCCN
2999:PMID
2903:ISBN
2872:ISBN
2695:222
2688:222
2681:226
2674:226
2667:228
2660:225
2653:233
2646:231
2639:238
2632:205
2625:206
2618:247
2611:210
2604:226
2440:120
2433:127
2426:135
2419:143
2412:154
2405:156
2398:171
2391:174
2384:177
2377:180
2370:185
2363:188
2356:193
2349:200
2342:208
2335:217
2323:253
2316:298
2300:108
2293:115
2286:123
2279:133
2272:145
2265:156
2258:161
2251:165
2244:169
2237:173
2230:178
2223:183
2216:190
2209:198
2202:206
2195:212
2186:219
2179:265
2149:103
2142:114
2135:125
2128:136
2121:142
2114:145
2107:149
2100:152
2093:156
2086:161
2079:166
2072:171
2065:176
2058:184
2049:194
2042:243
1998:111
1991:118
1982:145
1975:190
1915:112
1908:167
1356:175
1349:175
1342:175
1335:175
1328:180
1321:180
1314:195
1294:175
1287:175
1280:175
1273:175
1266:175
1259:175
1252:180
1245:185
1238:185
1231:185
1224:185
1217:185
1210:185
1203:195
1062:215
1025:190
1018:160
1011:180
1004:190
997:150
990:135
983:135
976:135
969:130
962:135
955:135
948:145
941:155
934:175
922:215
915:260
892:140
885:140
878:145
871:145
864:155
857:155
850:160
843:140
836:135
829:130
822:135
815:145
808:145
801:155
794:180
785:200
778:235
755:115
748:115
741:115
734:125
727:130
720:135
713:135
706:135
699:135
692:140
685:140
678:140
671:135
664:140
657:160
648:180
641:220
618:100
611:100
604:100
597:110
590:125
581:150
574:180
514:105
507:145
155:, CH
93:and
70:and
48:atom
38:The
3329:doi
3260:doi
3208:doi
3149:doi
3082:doi
3060:121
2989:PMC
2979:doi
2938:doi
2163:88
2156:94
2026:71
2019:79
2012:88
2005:98
1959:38
1952:42
1945:48
1938:56
1931:67
1924:87
1892:31
1883:53
551:50
544:60
537:65
530:70
523:85
482:25
144:).
85:in
3348::
3327:.
3317:47
3315:.
3272:^
3258:.
3248:41
3246:.
3214:.
3206:.
3196:26
3188:.
3155:.
3147:.
3137:26
3129:.
3096:.
3088:.
3080:.
3072:.
3058:.
3052:.
3029:.
3011:^
2997:.
2987:.
2977:.
2965:.
2959:.
2934:40
2926:.
2897:.
2803:No
2796:Md
2789:Fm
2782:Es
2775:Cf
2768:Bk
2761:Cm
2754:Am
2747:Pu
2740:Np
2726:Pa
2719:Th
2712:Ac
2706:**
2692:Yb
2685:Tm
2678:Er
2671:Ho
2664:Dy
2657:Tb
2650:Gd
2643:Eu
2636:Sm
2629:Pm
2622:Nd
2615:Pr
2608:Ce
2601:La
2577:Og
2570:Ts
2563:Lv
2556:Mc
2549:Fl
2542:Nh
2535:Cn
2528:Rg
2521:Ds
2514:Mt
2507:Hs
2500:Bh
2493:Sg
2486:Db
2479:Rf
2472:Lr
2466:**
2460:Ra
2453:Fr
2437:Rn
2430:At
2423:Po
2416:Bi
2409:Pb
2402:Tl
2395:Hg
2388:Au
2381:Pt
2374:Ir
2367:Os
2360:Re
2346:Ta
2339:Hf
2332:Lu
2320:Ba
2313:Cs
2297:Xe
2283:Te
2276:Sb
2269:Sn
2262:In
2255:Cd
2248:Ag
2241:Pd
2234:Rh
2227:Ru
2220:Tc
2213:Mo
2206:Nb
2199:Zr
2183:Sr
2176:Rb
2160:Kr
2153:Br
2146:Se
2139:As
2132:Ge
2125:Ga
2118:Zn
2111:Cu
2104:Ni
2097:Co
2090:Fe
2083:Mn
2076:Cr
2062:Ti
2055:Sc
2046:Ca
2023:Ar
2016:Cl
1995:Si
1988:Al
1979:Mg
1972:Na
1956:Ne
1912:Be
1905:Li
1889:He
1850:18
1843:17
1836:16
1829:15
1822:14
1815:13
1808:12
1801:11
1794:10
1625:.
1593:,
1402:No
1395:Md
1388:Fm
1381:Es
1374:Cf
1367:Bk
1360:Cm
1353:Am
1346:Pu
1339:Np
1325:Pa
1318:Th
1311:Ac
1305:**
1291:Yb
1284:Tm
1277:Er
1270:Ho
1263:Dy
1256:Tb
1249:Gd
1242:Eu
1235:Sm
1228:Pm
1221:Nd
1214:Pr
1207:Ce
1200:La
1176:Og
1169:Ts
1162:Lv
1155:Mc
1148:Fl
1141:Nh
1134:Cn
1127:Rg
1120:Ds
1113:Mt
1106:Hs
1099:Bh
1092:Sg
1085:Db
1078:Rf
1071:Lr
1065:**
1059:Ra
1052:Fr
1036:Rn
1029:At
1022:Po
1015:Bi
1008:Pb
1001:Tl
994:Hg
987:Au
980:Pt
973:Ir
966:Os
959:Re
945:Ta
938:Hf
931:Lu
919:Ba
912:Cs
896:Xe
882:Te
875:Sb
868:Sn
861:In
854:Cd
847:Ag
840:Pd
833:Rh
826:Ru
819:Tc
812:Mo
805:Nb
798:Zr
782:Sr
775:Rb
759:Kr
752:Br
745:Se
738:As
731:Ge
724:Ga
717:Zn
710:Cu
703:Ni
696:Co
689:Fe
682:Mn
675:Cr
661:Ti
654:Sc
645:Ca
622:Ar
615:Cl
594:Si
587:Al
578:Mg
571:Na
555:Ne
511:Be
504:Li
488:He
449:18
442:17
435:16
428:15
421:14
414:13
407:12
400:11
393:10
191:.
159:CH
142:nm
130:fm
114:pm
81:,
66:,
62:,
3335:.
3331::
3323::
3298:.
3266:.
3262::
3254::
3229:.
3210::
3202::
3170:.
3151::
3143::
3111:.
3084::
3076::
3066::
3037:.
3005:.
2981::
2973::
2967:9
2944:.
2940::
2911:.
2880:.
2733:U
2595:*
2447:7
2353:W
2326:*
2307:6
2290:I
2192:Y
2170:5
2069:V
2039:K
2033:4
2009:S
2002:P
1966:3
1949:F
1942:O
1935:N
1928:C
1921:B
1899:2
1880:H
1874:1
1787:9
1780:8
1773:7
1766:6
1759:5
1752:4
1745:3
1736:2
1729:1
1696:Z
1694:(
1688:Z
1686:(
1646:3
1642:3
1638:3
1615:Z
1613:(
1579:Z
1577:(
1570:Z
1567:(
1332:U
1194:*
1046:7
952:W
925:*
906:6
889:I
791:Y
769:5
668:V
638:K
632:4
608:S
601:P
565:3
548:F
541:O
534:N
527:C
520:B
498:2
479:H
473:1
386:9
379:8
372:7
365:6
358:5
351:4
344:3
335:2
328:1
259:.
167:.
161:2
157:3
116:(
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.