801:(MP) have been used to store data on tapes and magnetic strips but they have reached their limit for high capacity data storage. In order to increase their capacity by (25×) on data tape the MP had to increase the tape length by (45%) and track density by over (500%) which made it necessary to reduce the size of the individual particles. As the particles were reduced in size, the passivizing coating needed to prevent the oxidation and deterioration of the MP had to become thicker. This presented a problem for as the passivation coating got thicker it became harder to achieve an acceptable signal to noise ratio.
777:
characteristic of barium ferrite makes it a popular choice in motor and generator designs and also in loudspeaker applications. Ferrite magnets can be used in temperatures up to 300 °C, which makes it a perfect to be used in the applications mentioned above. Ferrite magnets are extremely good insulators and don't allow any electrical current to flow through them and they are brittle which shows their ceramic characteristics. Ferrite magnets also have good machining properties, which allows for the material to be cut in many shapes and sizes.
175:
100:
26:
814:
847:, whereby powdered barium ferrite is pressed into a mold, and then heated until it fuses together. The barium ferrite turns into a solid block while still retaining its magnetic properties. The magnets have an excellent resistance to demagnetization, allowing them to still be useful in speaker units over a long period of time.
862:
Developments in the field have also resulted in the reduction of the size of BaFe particles to about 20 nm. This contrasts with MP technology, which has problems shrinking the particles past 100 nm. Barium ferrite has better packing properties than most other metal particles because of the
826:
oxide materials to yield the coercivity values necessary to record. In recent decades, barium ferrite has replaced acicular oxides; without any dopants, the acicular oxides produce very low coercivity values, making the material very magnetically soft, while barium ferrite's higher coercivity levels
821:
Barium ferrite is used in applications such as recording media, permanent magnets, and magnetic stripe cards (credit cards, hotel keys, ID cards). Due to the stability of the material, it is able to be greatly reduced in size, making the packing density much greater. Earlier media devices utilized
804:
Barium ferrite completely out classes MP, mostly because Ba‑Fe is already in its oxidized state and so is not restricted in its size by a protective coating. Also due to its hexagonal pattern it is easier to organize compared to the unorganized rod like MP. Another factor is the difference in the
776:
When barium ferrite magnets increase in temperature, their high intrinsic coercivity improves, this is what makes it more resistant to thermal demagnetization. Ferrite magnets are the only type of magnets that become substantially more resistant to demagnetization as temperature increases. This
768:
Barium ferrite has been considered for long term data storage. The material has proven to be resistant to a number of different environmental stresses, including humidity and corrosion. Because ferrites are already oxidized it can not be oxidized any further. This is one reason ferrites are so
77:
1463:
789:
that are generally stable to moisture and corrosion-resistant. Ba‑Fe ferrite is an oxide, so it does not break down due to oxidation as much as a metal alloy might; giving Ba‑Fe a much greater life expectancy.
1044:
Watson, Mark L.; Beard, Robert A.; Kientz, Steven M.; Feebeck, Timothy W. (2008). "Investigation of
Thermal Demagnetization Effects in Data Recorded on Advanced Barium Ferrite Recording Media".
859:(LTO) tape storage media. Because of its high density, barium ferrite has led to data capacity improvements in both enterprise and LTO tapes over prior metal particle (MP) media technology.
1471:
805:
size of the particles, in MP the size ranges from 40 to 100 nm while the Ba‑Fe is only 20 nm. So the smallest MP particle is still double the size of the BaFe particles.
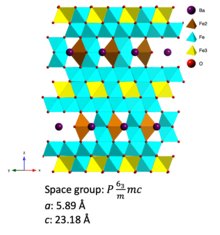
724:
1353:
489:. One area in particular it has found success in is long-term data storage; the material is magnetic, resistant to temperature change, corrosion and oxidization.
769:
resistant to corrosion. Barium ferrite also proved to be resistant to thermal demagnetization, another issue common with long-term storage. The
843:
Barium ferrite is a common material for speaker magnets. The materials can be formed into almost any shape and size using a process called
214:
1275:
Okazaki, Chisato; Mori, Saburo; Kanamaru, Fumikazu (1961). "Magnetic and crystallographical properties of hexagonal barium mono-ferrite,
863:
distinctive shape of the particles. This leads to better control over magnetic orientation and improved signal-to-noise characteristics.
1546:
485:. Studies of this material date at least as far back as 1931, and it has found applications in magnetic card strips, speakers, and
996:
1416:
1361:
936:
Cao, H.B.; Zhao, Z.Y.; Lee, M.; Choi, E.S.; McGuire, M.A.; Sales, B.C.; Zhou, H.D.; Yan, J.-Q.; Mandrus, D.G. (1 June 2015).
835:
ID cards using barium ferrite are made with a magnetic fingerprint that identifies them, allowing readers to self-calibrate.
1445:
871:
The compound occurs in nature, although is exceedingly rare. It is called barioferrite and is related to pyrometamorphism.
1464:"FujiFilm barium-ferrite magnetic tape establishes world record in data density: 29.5 billion bits per square inch"
1400:
1256:
1223:
189:
327:
1539:
1088:
Rowley, S.E.; Chai, Yi-Sheng; Shen, Shi-Peng; Sun, Young; Jones, A.T.; Watts, B.E.; Scott, J.F. (17 May 2016).
938:"High pressure floating zone growth and structural properties of ferrimagnetic quantum paraelectric BaFe12O19"
898:
132:
95:
1987:
153:
2003:
1215:
893:
2039:
2013:
1532:
519:
coupled. This area of technology is usually considered to be an application of the related fields of
894:"Hexagonal ferrites: A review of the synthesis, properties and applications of hexaferrite ceramics"
827:
make the material magnetically hard and thus a superior choice for recording material applications.
170:
1967:
1690:
823:
696:
1090:"Uniaxial ferroelectric quantum criticality in multiferroic hexaferrites BaFe12O19 and SrFe12O19"
1622:
539:
1328:
1240:
1207:
466:. This area of technology is usually considered to be an application of the related fields of
1384:
524:
471:
1942:
1864:
1214:. Development and Prospective Applications of Nanoscience and Nanotechnology. Vol. 1.
1101:
1054:
1001:
959:
509:
405:
141:
53:
635:
with a hydroxide to chloride concentration ratio of 2:1. Nano-particles are prepared from
619:
A one-step hydrothermal process can be used to form crystals of barium ferrite, by mixing
174:
99:
8:
1795:
1642:
43:
1105:
1058:
963:
530:
A related family of industrially useful "hexagonal ferrites" are known, also containing
1894:
1695:
1302:
1192:
1180:
1130:
1089:
1070:
1017:
977:
949:
2044:
1566:
1420:
1396:
1252:
1219:
1135:
1117:
1046:
915:
770:
628:
520:
478:
467:
346:
121:
1074:
1021:
981:
1954:
1918:
1856:
1787:
1658:
1555:
1310:
1188:
1125:
1109:
1062:
1009:
967:
907:
856:
658:
647:
632:
624:
401:
350:
341:
308:
237:
25:
911:
1934:
1926:
1886:
1881:
1848:
1731:
1723:
1715:
1678:
672:
651:
620:
1910:
1902:
1876:
1831:
1819:
1759:
1739:
1707:
1666:
1650:
1606:
1594:
1586:
798:
747:
640:
636:
516:
456:
321:
1066:
2033:
1775:
1767:
1574:
1511:
1121:
919:
542:
framework of oxides. Furthermore, some of the oxygen centers are replaced by
486:
297:
88:
1392:
1843:
1807:
1248:
1139:
644:
409:
1493:
1314:
262:
1113:
1013:
972:
937:
1210:. In Khan, Sher Bahadar; Asiri, Abdullah M.; Akhtar, Kalsoom (eds.).
844:
1153:
Goto, Yasumasa; Takada, Toshio (1960). "Phase diagram of the system
813:
706:
320:
Except where otherwise noted, data are given for materials in their
1524:
1467:
1449:
1357:
954:
76:
1208:"Solvothermal / hydrothermal synthetic methods for nanomaterials"
786:
512:
459:
coupled, and one unit cell of BaM has a net magnetic moment of 40
287:
108:
643:, and sodium hydroxide. The typical preparation, however, is by
1962:
535:
531:
482:
66:
158:
1440:
1438:
994:
855:
Barium ferrite is used for enterprise level and commodity
1043:
995:
1435:
817:
Barium
Ferrite is used in tape drives and floppy disks.
1448:. Data storage. Fujifilm Technologies. United States:
699:
931:
929:
1274:
1039:
1037:
1035:
1033:
1031:
718:
1087:
926:
2031:
120:
1028:
52:
1212:Nanomaterials and their Fascinating Attributes
935:
1540:
481:, has a high packing density, and is a metal
302:1,316 °C (2,401 °F; 1,589 K)
1547:
1533:
1152:
850:
173:
98:
1245:Magnetic Materials and Their Applications
1129:
971:
953:
553:ions. Formulas for these species include
140:
887:
885:
883:
812:
793:
773:is typically around 450 °C (723K).
1419:. Magnaworks Technology. Archived from
1270:
1268:
169:
2032:
891:
780:
222:O=O=O.O=O=O.O=O=O.O=O=O.O=O=O.O=O=O.O=
89:
1528:
1382:
1241:"Ceramic magnet materials (ferrites)"
1205:
997:"Studies on zinc and barium ferrites"
880:
866:
492:
201:Key: AJCDFVKYMIUXCR-UHFFFAOYSA-N
1554:
1329:"Characteristics of ferrite magnets"
1265:
1238:
1385:"Card-based identification systems"
830:
538:structure, these materials feature
111:
13:
1193:10.1111/j.1151-2916.1960.tb14330.x
838:
14:
2056:
1470:. 22 January 2010. Archived from
1417:"Hard ferrite (ceramic) magnets"
345:, abbreviated BaFe, BaM, is the
31:Barium ferrite crystal structure
24:
1504:
1486:
1456:
1409:
1376:
1346:
1321:
808:
324:(at 25 °C , 100 kPa).
1232:
1199:
1146:
1081:
988:
252:
243:
1:
912:10.1016/j.pmatsci.2012.04.001
899:Progress in Materials Science
874:
763:
719:{\displaystyle {\ce {->}}}
404:materials are components in
246:
7:
785:Barium ferrites are robust
534:. In contrast to the usual
477:Barium ferrite is a highly
10:
2061:
1446:"Barium ferrite: Overview"
1354:"Barium ferrite: Overview"
1216:Bentham Science Publishers
1206:Niazi, Shahida B. (2016).
892:Pullar, Robert C. (2012).
1562:
1389:Electronic Access Control
1067:10.1109/TMAG.2008.2001591
318:
230:
210:
185:
36:
23:
851:Tape Data Storage Media
1383:Honey, Gerard (2000).
818:
720:
540:hexagonal close-packed
816:
794:Mechanical properties
721:
525:solid state chemistry
472:solid state chemistry
415:BaFe is described as
406:magnetic stripe cards
1251:. pp. 291–294.
1218:. pp. 181–238.
1002:J. Electrochem. Soc.
697:
400:). This and related
198:InChI=1S/Ba.12Fe.19O
1315:10.1143/JPSJ.16.119
1239:Heck, Carl (1974).
1106:2016NatSR...625724R
1059:2008ITM....44.3568W
964:2015APLM....3f2512C
781:Chemical properties
712:
639:, barium chloride,
515:configuration, are
309:Solubility in water
274: g·mol
20:
1516:ima-mineralogy.org
1512:"List of Minerals"
1474:on 19 October 2020
1423:on 20 October 2018
1395:. pp. 47–55.
1303:J. Phys. Soc. Jpn.
1181:J. Am. Ceram. Soc.
1094:Scientific Reports
867:Natural occurrence
819:
716:
493:Chemical structure
328:Infobox references
18:
2027:
2026:
1466:(Press release).
1364:on 13 August 2017
1114:10.1038/srep25724
1053:(11): 3568–3571.
1047:IEEE Trans. Magn.
1014:10.1149/1.3497845
973:10.1063/1.4922934
771:Curie temperature
713:
710:
629:potassium nitrate
521:materials science
517:ferrimagnetically
479:magnetic material
468:materials science
457:ferrimagnetically
347:chemical compound
336:Chemical compound
334:
333:
154:CompTox Dashboard
78:Interactive image
2052:
2040:Barium compounds
2019:
2009:
1556:Barium compounds
1549:
1542:
1535:
1526:
1525:
1520:
1519:
1518:. 21 March 2011.
1508:
1502:
1501:
1490:
1484:
1483:
1481:
1479:
1460:
1454:
1453:
1442:
1433:
1432:
1430:
1428:
1413:
1407:
1406:
1380:
1374:
1373:
1371:
1369:
1360:. Archived from
1350:
1344:
1343:
1341:
1339:
1325:
1319:
1318:
1299:
1298:
1297:
1289:
1288:
1278:
1272:
1263:
1262:
1236:
1230:
1229:
1203:
1197:
1196:
1177:
1176:
1175:
1167:
1166:
1156:
1150:
1144:
1143:
1133:
1085:
1079:
1078:
1041:
1026:
1025:
992:
986:
985:
975:
957:
933:
924:
923:
906:(7): 1191–1334.
889:
857:linear tape-open
831:Magnetic Stripes
758:
757:
756:
745:
744:
743:
735:
734:
725:
723:
722:
717:
715:
714:
711:
708:
702:
692:
691:
690:
682:
681:
669:
668:
667:
648:barium carbonate
633:sodium hydroxide
625:ferrous chloride
615:
614:
613:
605:
604:
594:
593:
592:
584:
583:
573:
572:
571:
563:
562:
552:
551:
550:
508:centers, with a
507:
506:
505:
462:
454:
453:
452:
443:
442:
441:
433:
432:
424:
423:
399:
398:
397:
396:
388:
387:
377:
372:
371:
370:
362:
361:
273:
271:
254:
248:
245:
238:Chemical formula
178:
177:
162:
160:
144:
124:
113:
102:
91:
80:
56:
28:
21:
17:
2060:
2059:
2055:
2054:
2053:
2051:
2050:
2049:
2030:
2029:
2028:
2023:
2018:
2014:
2008:
2004:
1999:
1995:
1991:
1983:
1979:
1975:
1971:
1958:
1950:
1946:
1938:
1930:
1922:
1914:
1906:
1898:
1890:
1872:
1868:
1860:
1852:
1839:
1835:
1827:
1823:
1815:
1811:
1803:
1799:
1791:
1783:
1779:
1771:
1763:
1755:
1751:
1743:
1735:
1727:
1719:
1711:
1703:
1699:
1686:
1682:
1674:
1670:
1662:
1654:
1646:
1638:
1634:
1630:
1626:
1618:
1614:
1610:
1602:
1598:
1590:
1582:
1578:
1570:
1558:
1553:
1523:
1510:
1509:
1505:
1492:
1491:
1487:
1477:
1475:
1462:
1461:
1457:
1444:
1443:
1436:
1426:
1424:
1415:
1414:
1410:
1403:
1381:
1377:
1367:
1365:
1352:
1351:
1347:
1337:
1335:
1327:
1326:
1322:
1296:
1293:
1292:
1291:
1287:
1284:
1283:
1282:
1280:
1276:
1273:
1266:
1259:
1237:
1233:
1226:
1204:
1200:
1174:
1171:
1170:
1169:
1165:
1162:
1161:
1160:
1158:
1154:
1151:
1147:
1086:
1082:
1042:
1029:
993:
989:
934:
927:
890:
881:
877:
869:
853:
841:
839:Speaker magnets
833:
811:
799:Metal particles
796:
783:
766:
755:
752:
751:
750:
748:
742:
739:
738:
737:
733:
730:
729:
728:
726:
707:
701:
700:
698:
695:
694:
689:
686:
685:
684:
680:
677:
676:
675:
673:
666:
663:
662:
661:
659:
652:iron(III) oxide
621:barium chloride
612:
609:
608:
607:
603:
600:
599:
598:
596:
591:
588:
587:
586:
582:
579:
578:
577:
575:
570:
567:
566:
565:
561:
558:
557:
556:
554:
549:
547:
546:
545:
543:
504:
502:
501:
500:
498:
495:
465:
460:
451:
449:
448:
447:
445:
440:
437:
436:
435:
431:
428:
427:
426:
422:
420:
419:
418:
416:
395:
392:
391:
390:
386:
383:
382:
381:
379:
375:
374:
369:
366:
365:
364:
360:
357:
356:
355:
353:
337:
330:
325:
311:
269:
267:
257:
251:
240:
226:
223:
218:
217:
206:
203:
202:
199:
193:
192:
181:
163:
156:
147:
127:
114:
83:
70:
59:
46:
32:
29:
19:Barium ferrite
12:
11:
5:
2058:
2048:
2047:
2042:
2025:
2024:
2022:
2021:
2016:
2011:
2006:
2001:
1997:
1993:
1989:
1985:
1981:
1977:
1973:
1969:
1965:
1960:
1956:
1952:
1948:
1944:
1940:
1936:
1932:
1928:
1924:
1920:
1916:
1912:
1908:
1904:
1900:
1896:
1892:
1888:
1884:
1879:
1874:
1870:
1866:
1862:
1858:
1854:
1850:
1846:
1841:
1837:
1833:
1829:
1825:
1821:
1817:
1813:
1809:
1805:
1801:
1797:
1793:
1789:
1785:
1781:
1777:
1773:
1769:
1765:
1761:
1757:
1753:
1749:
1745:
1741:
1737:
1733:
1729:
1725:
1721:
1717:
1713:
1709:
1705:
1701:
1697:
1693:
1688:
1684:
1680:
1676:
1672:
1668:
1664:
1660:
1656:
1652:
1648:
1644:
1640:
1636:
1632:
1628:
1624:
1620:
1616:
1612:
1608:
1604:
1600:
1596:
1592:
1588:
1584:
1580:
1576:
1572:
1568:
1563:
1560:
1559:
1552:
1551:
1544:
1537:
1529:
1522:
1521:
1503:
1494:"Barioferrite"
1485:
1455:
1434:
1408:
1401:
1375:
1345:
1320:
1294:
1285:
1264:
1257:
1231:
1224:
1198:
1187:(3): 150–153.
1172:
1163:
1145:
1080:
1027:
987:
925:
878:
876:
873:
868:
865:
852:
849:
840:
837:
832:
829:
810:
807:
795:
792:
782:
779:
765:
762:
761:
760:
753:
740:
731:
705:
687:
678:
664:
641:sodium citrate
637:ferric nitrate
610:
601:
589:
580:
568:
559:
548:
503:
494:
491:
487:magnetic tapes
463:
450:
438:
429:
421:
393:
384:
367:
358:
335:
332:
331:
326:
322:standard state
319:
316:
315:
312:
307:
304:
303:
300:
294:
293:
290:
284:
283:
280:
276:
275:
265:
259:
258:
255:
249:
241:
236:
233:
232:
228:
227:
225:
224:
221:
213:
212:
211:
208:
207:
205:
204:
200:
197:
196:
188:
187:
186:
183:
182:
180:
179:
171:DTXSID20923429
166:
164:
152:
149:
148:
146:
145:
137:
135:
129:
128:
126:
125:
117:
115:
107:
104:
103:
93:
85:
84:
82:
81:
73:
71:
64:
61:
60:
58:
57:
49:
47:
42:
39:
38:
34:
33:
30:
9:
6:
4:
3:
2:
2057:
2046:
2043:
2041:
2038:
2037:
2035:
2020:
2012:
2010:
2002:
2000:
1986:
1984:
1966:
1964:
1961:
1959:
1953:
1951:
1941:
1939:
1933:
1931:
1925:
1923:
1917:
1915:
1909:
1907:
1901:
1899:
1893:
1891:
1885:
1883:
1880:
1878:
1875:
1873:
1863:
1861:
1855:
1853:
1847:
1845:
1842:
1840:
1830:
1828:
1818:
1816:
1806:
1804:
1794:
1792:
1786:
1784:
1774:
1772:
1766:
1764:
1758:
1756:
1746:
1744:
1738:
1736:
1730:
1728:
1722:
1720:
1714:
1712:
1706:
1704:
1694:
1692:
1689:
1687:
1677:
1675:
1665:
1663:
1657:
1655:
1649:
1647:
1641:
1639:
1621:
1619:
1605:
1603:
1593:
1591:
1585:
1583:
1573:
1571:
1565:
1564:
1561:
1557:
1550:
1545:
1543:
1538:
1536:
1531:
1530:
1527:
1517:
1513:
1507:
1499:
1495:
1489:
1473:
1469:
1465:
1459:
1451:
1447:
1441:
1439:
1422:
1418:
1412:
1404:
1402:9780750644730
1398:
1394:
1390:
1386:
1379:
1363:
1359:
1355:
1349:
1334:
1330:
1324:
1316:
1312:
1308:
1305:
1304:
1271:
1269:
1260:
1258:9781483103174
1254:
1250:
1246:
1242:
1235:
1227:
1225:9781681081779
1221:
1217:
1213:
1209:
1202:
1194:
1190:
1186:
1183:
1182:
1149:
1141:
1137:
1132:
1127:
1123:
1119:
1115:
1111:
1107:
1103:
1099:
1095:
1091:
1084:
1076:
1072:
1068:
1064:
1060:
1056:
1052:
1049:
1048:
1040:
1038:
1036:
1034:
1032:
1023:
1019:
1015:
1011:
1008:(1): 95–106.
1007:
1004:
1003:
998:
991:
983:
979:
974:
969:
965:
961:
956:
951:
948:(6): 062512.
947:
943:
942:APL Materials
939:
932:
930:
921:
917:
913:
909:
905:
901:
900:
895:
888:
886:
884:
879:
872:
864:
860:
858:
848:
846:
836:
828:
825:
815:
806:
802:
800:
791:
788:
778:
774:
772:
759:
703:
693:
670:
657:
656:
655:
653:
649:
646:
642:
638:
634:
630:
626:
622:
617:
541:
537:
533:
528:
526:
522:
518:
514:
511:
490:
488:
484:
480:
475:
473:
469:
458:
413:
411:
407:
403:
352:
348:
344:
343:
329:
323:
317:
313:
310:
306:
305:
301:
299:
298:Melting point
296:
295:
291:
289:
286:
285:
281:
278:
277:
266:
264:
261:
260:
242:
239:
235:
234:
229:
220:
219:
216:
209:
195:
194:
191:
184:
176:
172:
168:
167:
165:
155:
151:
150:
143:
139:
138:
136:
134:
131:
130:
123:
119:
118:
116:
110:
106:
105:
101:
97:
94:
92:
90:ECHA InfoCard
87:
86:
79:
75:
74:
72:
68:
63:
62:
55:
51:
50:
48:
45:
41:
40:
35:
27:
22:
16:
1747:
1515:
1506:
1497:
1488:
1476:. Retrieved
1472:the original
1458:
1425:. Retrieved
1421:the original
1411:
1388:
1378:
1366:. Retrieved
1362:the original
1348:
1336:. Retrieved
1333:e-Magnets UK
1332:
1323:
1306:
1301:
1249:Butterworths
1244:
1234:
1211:
1201:
1184:
1179:
1148:
1100:(1): 25724.
1097:
1093:
1083:
1050:
1045:
1005:
1000:
990:
945:
941:
903:
897:
870:
861:
854:
842:
834:
820:
809:Applications
803:
797:
784:
775:
767:
618:
529:
496:
476:
455:centers are
414:
339:
338:
282:black solid
37:Identifiers
15:
410:loudspeaker
279:Appearance
231:Properties
96:100.031.782
2034:Categories
1498:mindat.org
1478:12 October
1427:8 December
1338:8 December
1309:(3): 119.
955:1503.02568
875:References
764:Properties
671:+ 6
378: : 6
314:insoluble
292:5.28 g/cm
263:Molar mass
142:4HT629NL8B
65:3D model (
54:12047-11-9
44:CAS Number
1368:13 August
1122:2045-2322
920:0079-6425
845:sintering
645:calcining
510:high-spin
412:magnets.
349:with the
2045:Ferrites
1468:Fujifilm
1450:Fujifilm
1358:Fujifilm
1140:27185343
1075:22303270
1022:97566562
982:98312433
824:acicular
787:ceramics
704:→
122:16217742
1895:Ba(SCN)
1643:Ba(ClO)
1131:4869023
1102:Bibcode
1055:Bibcode
960:Bibcode
709:Δ
402:ferrite
351:formula
342:ferrite
340:Barium
288:Density
109:PubChem
1963:BaZnGa
1857:Ba(OH)
1796:Ba(MnO
1708:Ba(CN)
1696:Ba(ClO
1679:Ba(ClO
1595:Ba(BrO
1399:
1393:Newnes
1255:
1222:
1138:
1128:
1120:
1073:
1020:
980:
918:
822:doped
631:, and
595:, and
536:spinel
532:barium
444:. The
215:SMILES
2015:BaSiF
2005:BaGeF
1935:BaTiO
1927:BaSnO
1919:BaRuO
1887:BaSeO
1865:Ba(PO
1832:Ba(NO
1820:Ba(NO
1788:BaMnO
1776:Ba(IO
1740:BaFeO
1724:BaCrO
1691:BaClF
1607:Ba(CH
1575:Ba(BO
1071:S2CID
1018:S2CID
978:S2CID
950:arXiv
650:with
483:oxide
190:InChI
67:JSmol
1955:BaWO
1911:BaSO
1903:BaSO
1882:BaSe
1808:Ba(N
1748:BaFe
1716:BaCl
1659:BaCO
1623:Ba(C
1587:BaBr
1480:2020
1429:2013
1397:ISBN
1370:2017
1340:2013
1253:ISBN
1220:ISBN
1136:PMID
1118:ISSN
916:ISSN
727:BaFe
660:BaCO
597:BaFe
576:BaFe
555:BaFe
523:and
497:The
470:and
408:and
354:BaFe
272:.448
133:UNII
1998:7-x
1988:YBa
1974:1-x
1947:TiO
1877:BaS
1849:BaO
1844:BaO
1768:BaI
1760:BaH
1732:BaF
1667:BaC
1651:BaC
1567:BaB
1311:doi
1300:".
1277:BaO
1189:doi
1178:".
1155:BaO
1126:PMC
1110:doi
1063:doi
1010:doi
968:doi
908:doi
746:+
376:BaO
270:111
159:EPA
112:CID
2036::
1992:Cu
1976:Nb
1972:Ba
1968:Sr
1943:Ba
1611:CO
1514:.
1496:.
1437:^
1391:.
1387:.
1356:.
1331:.
1307:16
1281:Fe
1267:^
1247:.
1243:.
1185:43
1159:Fe
1134:.
1124:.
1116:.
1108:.
1096:.
1092:.
1069:.
1061:.
1051:44
1030:^
1016:.
1006:59
999:.
976:.
966:.
958:.
944:.
940:.
928:^
914:.
904:57
902:.
896:.
882:^
749:CO
741:19
732:12
674:Fe
654::
627:,
623:,
616:.
611:27
602:18
590:23
581:15
574:,
569:19
560:12
544:Ba
527:.
499:Fe
474:.
446:Fe
439:19
430:12
425:Fe
417:Ba
380:Fe
368:19
359:12
256:19
250:12
247:Fe
244:Ba
2017:6
2007:6
1996:O
1994:3
1990:2
1982:6
1980:O
1978:2
1970:2
1957:4
1949:4
1945:2
1937:3
1929:3
1921:3
1913:4
1905:3
1897:2
1889:4
1871:2
1869:)
1867:3
1859:2
1851:2
1838:2
1836:)
1834:3
1826:2
1824:)
1822:2
1814:2
1812:)
1810:3
1802:2
1800:)
1798:4
1790:4
1782:2
1780:)
1778:3
1770:2
1762:2
1754:4
1752:O
1750:2
1742:4
1734:2
1726:4
1718:2
1710:2
1702:2
1700:)
1698:4
1685:2
1683:)
1681:3
1673:4
1671:O
1669:2
1661:3
1653:2
1645:2
1637:2
1635:)
1633:2
1631:O
1629:7
1627:H
1625:5
1617:2
1615:)
1613:2
1609:3
1601:2
1599:)
1597:3
1589:2
1581:2
1579:)
1577:2
1569:6
1548:e
1541:t
1534:v
1500:.
1482:.
1452:.
1431:.
1405:.
1372:.
1342:.
1317:.
1313::
1295:3
1290:O
1286:2
1279:•
1261:.
1228:.
1195:.
1191::
1173:3
1168:O
1164:2
1157:-
1142:.
1112::
1104::
1098:6
1077:.
1065::
1057::
1024:.
1012::
984:.
970::
962::
952::
946:3
922:.
910::
754:2
736:O
688:3
683:O
679:2
665:3
606:O
585:O
564:O
513:d
464:B
461:μ
434:O
394:3
389:O
385:2
373:(
363:O
268:1
253:O
161:)
157:(
69:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.