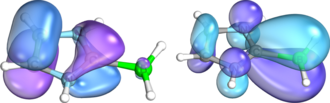502:
145:. In this class of molecules, the electron-deficient boron center has two valence electrons involved in sigma bonding with two ligands, while the third ligand is a two-electron donor such that the overall charge of the complex is +1. Depending on the nature of the ligands around the central boron, this positive charge can be localized on the boron center or delocalized across the entire molecule. Borenium ions can be made in a number of different ways and are of interest for applications in
232:
727:
635:
121:
170:
831:
481:, halides, and triflate are also possible. The synthetic viability of a borenium ion is often determined by its reactivity relative to its counterion. Halides are often unable to stabilize borenium ions, preferring instead to coordinate to the boron center to make a tetracoordinate species. A systematic evaluation of counterion effects on the synthetic viability of NHC-dicholoroborenium ions was conducted by Muthaiah and coworkers in 2013.
253:
216:
426:
28:
582:
It has been shown that the steric and electronic properties of the NHC ligand used in these borenium catalysts is of great importance to catalytic activity: NHCs that were too bulky prevented intermolecular hydride delivery and ligands that were highly electron donating weakened the borenium cation's
409:
Depending on the nature of the ligands around the central boron, this positive charge can be localized on the boron center or delocalized across the entire molecule. In some cases, pi-donating ligands arranged in the plane of the boron's empty p orbital can act to stabilize the electron deficiency of
260:
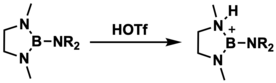
Aminoboranes can be protonated by various acids to make borenium ions. This synthetic method was developed in 1983 by Narula and Noth who used triflic acid to protonate 1,3-dimethyl-2-(dimethylamino)-1,3,2-diazaborolidine; however, they were unable to crystallize and structurally characterize this
239:
Displacement of a ligand from a neutral tricoordinate boron halide by a neutral donor such as pyridine results in the generation of a borenium cation. For this reaction to yield the desired borenium cation, the ligand must be a good leaving group and the neutral donor must have enough steric bulk
622:
by protonating a neutral oxazaborolidine with triflic acid. Corey and coworkers suggest that the stereoselectivity of this reaction is a result of aldehyde-catalyst association in the pre-transition state which governs stereoselectivity. The use of borenium ions as Diels–Alder catalysts has been
464:
Early crystal structures of borenium cations indicate that the corresponding anion is non-coordinating. Further studies have shown that the reactivity of borocations is highly tied to the identity of its counter ion. In catalytic applications, weakly coordinating anions have allowed for the most
304:. The electron-deficient nature of the boron center of many borenium ions has been confirmed by computational and experimental studies. A Natural Population Analysis treatment of many borenium ions show that the boron center does indeed carry a significant positive charge. For example, the BH
448:
method for energy decomposition analysis combined with the natural orbitals for chemical valence (NOCV) theory. This analysis showed a net pi-donating effect of the NHC ligand – in this case, the positive charge is delocalized over the entire pi system rather than localized on the boron.
1091:
Bentivegna, BriAnne; Mariani, Christine I.; Smith, Jason R.; Ma, Shuhua; Rheingold, Arnold L.; Brunker, Tim J. (2014-06-09). "Formation, Stability, and
Structures of Borenium and Boronium Cations Derived from Pentamethylazaferrocene–Boranes by Hydride or Chloride Abstraction Reactions".
456:
plane due to steric crowding. This nonplanar geometry leads to a reduction in pi-donation to the boron center, making it even more electron-deficient. It has been found that increased localization of charge on the boron increases the Lewis acidity of the borocation. The
528:
delivery during hydrogenation catalysis, borenium ions can be more potent catalysts than neutral boron species because they are effective hydride donors. Indeed, in 2012, Stephan and coworkers were able to develop a borenium-based FLP system capable of activating
1692:
Stahl, Timo; Müther, Kristine; Ohki, Yasuhiro; Tatsumi, Kazuyuki; Oestreich, Martin (2013-07-31). "Catalytic
Generation of Borenium Ions by Cooperative B–H Bond Activation: The Elusive Direct Electrophilic Borylation of Nitrogen Heterocycles with Pinacolborane".
1506:
Devillard, Marc; Brousses, Rémy; Miqueu, Karinne; Bouhadir, Ghenwa; Bourissou, Didier (2015-05-04). "A Stable but Highly
Reactive Phosphine‐Coordinated Borenium: Metal‐free Dihydrogen Activation and Alkyne 1,2‐Carboboration".
524:(FLP) chemistry of this type, borenium ions are inherently electrophilic and do not require electron-withdrawing ligands to perform these small-molecule activations. Because electron-withdrawing substituents can hamper
2130:
Arnold, Nicole; Braunschweig, Holger; Dewhurst, Rian D.; Hupp, Florian; Radacki, Krzysztof; Trumpp, Alexandra (2016-08-12). "Desymmetrizing
Electron-Deficient Diboranes(4): Diverse Products and Their Reactivity".
655:
reactions. In many examples of this reaction, a catalyst is used to activate a borane, producing a highly reactive borenium ion. The formation of this highly electrophilic species drives the formation of the
1615:
Corey, E. J.; Bakshi, Raman K.; Shibata, Saizo (1987-09-01). "Highly enantioselective borane reduction of ketones catalyzed by chiral oxazaborolidines. Mechanism and synthetic implications".
1413:
Muthaiah, Senthilkumar; Do, Dinh Cao Huan; Ganguly, Rakesh; Vidović, Dragoslav (2013-11-25). "Counterion
Dependence on the Synthetic Viability of NHC-stabilized Dichloroborenium Cations".
280:
Borenium ions can also be made through other methods such as the addition of base to a dicoordinate borinium ion or by metathesis with salts with weakly coordinating anions such as Ag
316:
Natural Bond
Orbital analysis of a series of borenium ions calculated using the M06-2X level of theory and 6-311++G(d,p) basis set as described by Stojanovic and Stojanovic.
962:
Lee, Kyounghoon; Kirkvold, Clara; Vlaisavljevich, Bess; Daly, Scott R. (2018-11-05). "Ligand-Centered
Borenium Reactivity in Triaminoborane-Bridged Diphosphine Complexes".
1736:
Yin, Qin; Klare, Hendrik F. T.; Oestreich, Martin (2017). "Catalytic
Friedel–Crafts C−H Borylation of Electron-Rich Arenes: Dramatic Rate Acceleration by Added Alkenes".
440:(NHCs) can also be used to stabilize borenium ions through pi-conjugation, albeit acting as weaker pi-donors than neutral N-donors. The interaction energy between a BH
433:
fragment calculated as described by
Rezabal and Frison in 2015. The left structure shows loss of electron density; the right structure shows gain of electron density.
1891:
Shoji, Yoshiaki; Tanaka, Naoki; Mikami, Koichiro; Uchiyama, Masanobu; Fukushima, Takanori (2014-05-11). "A two-coordinate boron cation featuring C–B+–C bonding".
1164:
Corey, E. J.; Shibata, Takanori; Lee, Thomas W. (2002-04-01). "Asymmetric Diels−Alder
Reactions Catalyzed by a Triflic Acid Activated Chiral Oxazaborolidine".
1207:
Chen, Jiawei; Lalancette, Roger A.; Jäkle, Frieder (2013-05-02). "Synthesis and Lewis acid properties of a ferrocene-based planar-chiral borenium cation".
614:
and coworkers that N-protonation of non-Lewis acidic oxazaborolidines results in the generation of borenium ions which can catalyze the enantioselective
563:
Borenium ions have also been used catalytically for various hydrogenations. Stephan and coworkers were able to use a borenium ion catalyst to activate H
1463:
Farrell, Jeffrey M.; Hatnean, Jillian A.; Stephan, Douglas W. (2012-09-13). "Activation of Hydrogen and Hydrogenation Catalysis by a Borenium Cation".
610:
Further work on borenium ions generated from neutral oxazaborolidines has expanded the scope of their applications. In 2002, it was reported by
189:
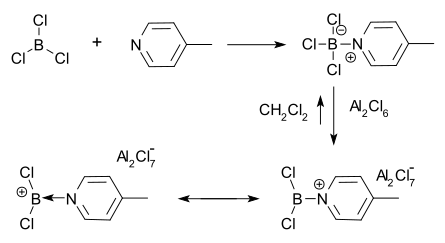
anion. The first borenium ion to be isolated and characterized was made by Ryschkewitsch and Wiggins in 1970 using this method. They found that
1999:
Piers, Warren E.; Bourke, Sara C.; Conroy, Korey D. (2005). "Borinium, Borenium, and Boronium Ions: Synthesis, Reactivity, and Applications".
664:
used a ruthenium(II) thiolate catalyst to generate borenium ions capable of effecting direct borylation of nitrogen-containing heterocycles.
223:
Similar to the halide abstraction method, borenium ions can be made through abstraction of a hydride from a tetracoordinate boron complex.
161:
Synthetic methods for preparing borenium ions include halide abstraction, nucleophilic dissociation, and protic addition to aminoboranes.
2034:
Vargas-Baca, Ignacio; Findlater, Michael; Powell, Adam; Vasudevan, Kalyan V.; Cowley, Alan H. (2008). "Boron di- and tri-cations".
747:
of polyalphaolefins (PAOs). While not yet widely adopted by industry, this technology could provide an alternative to the use of BF
1554:(2019-06-13). "Enantioselective reduction of N-alkyl ketimines with frustrated Lewis pair catalysis using chiral borenium ions".
264:
Protonation of non-Lewis acidic oxazaborolidines results in the generation of borenium ions that can be used as enantioselective
177:
Borenium ions can be made from tetracoordinate Lewis acid-base adducts of boron halides. In this method, halide abstraction by a
1053:
Clark, Ewan R.; Ingleson, Michael J. (2013-11-25). "+: A Borenium Cation That Is a Strong Boron- and Carbon-Based Lewis Acid".
465:
active borenium catalysts. A commonly used counter ion for borenium cations is tetrakis(pentafluorophenyl)borate, B(C
209:
1652:"Borenium ionic liquids as catalysts for Diels–Alder reaction: tuneable Lewis superacids for catalytic applications"
667:
In 2017, Oestreich and coworkers developed a metal-free method for effecting this transformation. In their work, B(C
300:. The structures of borenium ions generally have two short bonds and one longer bond which is characteristic of a
1132:
Narula, Chaitanya (1984). "Preparation and Characterization of Salts Containing Cations of Tricoordinate Boron".
826:
Other reported boron cations are dibora-dications (bis(borenium) dications), some examples are depicted below.
1842:
823:
Other non-classical boron cations are mononuclear boron di- and tri-cations with formula and , respectively.
493:. Their Lewis acidity is of the boron atom is determined by the electronic and steric effects of its ligands.
173:
Synthetic method involving halide abstraction used by Ryschkewitsch and Wiggins to synthesize a borenium ion.
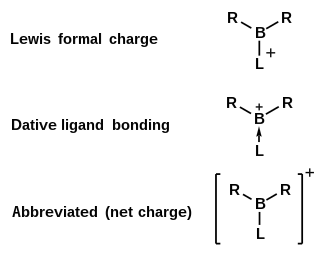
70:, where R represents a non-specific substituent. Being electron-deficient, boranylium ions form adducts with
679:
was used to activate catecholborane, generating a borenium ion capable of borylating various electron-rich
1651:
1550:
Mercea, Dan M.; Howlett, Michael G.; Piascik, Adam D.; Scott, Daniel J.; Steven, Alan; Ashley, Andrew E.;
2073:
Prokofjevs, Aleksandrs; Kampf, Jeff W.; Solovyev, Andrey; Curran, Dennis P.; Vedejs, Edwin (2013-10-10).
548:
with concomitant hydrogenolysis of a mesityl ligand. A second-order perturbation theory analysis of the
596:
592:
572:
458:
269:
219:
Mechanism of halide abstraction used by Ryschkewitsch and Wiggins to synthesize the first borenium ion.
1784:"Highly selective catalytic trans-hydroboration of alkynes mediated by borenium cations and B(C6F5)3"
411:
2180:
1306:
615:
461:
has been used by many researchers in this field to benchmark the Lewis acidities of these cations.
301:
265:
240:
that nucleophilic dissociation is favored over Lewis acid-base adduct formation with the neutral BR
17:
711:
hydroboration with a variety of arylacetylene substrates using a borenium ion electrophile and B(C
505:
Catalytic cycle for the hydrogenation of imines facilitated by a NHC-stabilized borenium catalyst.
1841:
Hogg, James M.; Ferrer-Ugalde, Albert; Coleman, Fergal; Swadźba-Kwaśny, Małgorzata (2019-08-08).
1255:"A theoretical study on borenium ion affinities toward ammonia, formaldehyde and chloride anions"
618:
of 1,3-dienes with 2-methacrolein or 2-bromoacrolein. This particular borenium ion could be made
509:
437:
575:
reduction of ketimines. In this example, enantioselectivity was afforded through the use of a
1944:"Cationic Tricoordinate Boron Intermediates: Borenium Chemistry from the Organic Perspective"
907:"Cationic Tricoordinate Boron Intermediates: Borenium Chemistry from the Organic Perspective"
680:
576:
544:
synthesized a naphthyl-bridged intramolecular borenium-containing FLP capable of activating H
521:
490:
415:
297:
178:
1900:
1266:
657:
549:
830:
74:. Boranylium ions have historical names that depend on the number of coordinated ligands:
8:
1904:
1270:
2107:
2074:
1976:
1943:
1873:
1818:
1783:
1597:
1345:
995:
939:
906:
190:
129:
52:
39:
molecule, an example of a borenium cation, a type of boranylium ion with three ligands.
1782:
McGough, John S.; Butler, Samuel M.; Cade, Ian A.; Ingleson, Michael J. (2016-04-26).
2156:
2148:
2112:
2094:
2055:
2047:
2016:
1981:
1963:
1924:
1916:
1877:
1865:
1823:
1805:
1761:
1753:
1718:
1710:
1674:
1632:
1601:
1589:
1581:
1532:
1524:
1488:
1480:
1440:
1395:
1387:
1337:
1329:
1284:
1232:
1224:
1189:
1181:
1109:
1070:
1035:
987:
979:
944:
926:
882:
611:
202:
146:
1349:
999:
2140:
2102:
2086:
2039:
2008:
1971:
1955:
1908:
1857:
1843:"Borenium Ionic Liquids as Alternative to BF3 in Polyalphaolefins (PAOs) Synthesis"
1813:
1795:
1745:
1702:
1666:
1624:
1571:
1563:
1516:
1472:
1430:
1422:
1379:
1321:
1274:
1216:
1173:
1141:
1101:
1062:
1027:
971:
934:
918:
874:
865:
Koelle, P.; Noeth, H. (1985-10-01). "The chemistry of borinium and borenium ions".
651:
Borenium ions have also been implicated as intermediates in electrophilic aromatic
595:
catalysts for a number of organic transformations. An early example of such is the
445:
198:
133:
56:
1861:
1307:"Estimating π binding energy of N-Heterocyclic carbenes: The role of polarization"
975:
429:
Contours of deformation density contributions from the pi orbitals of a NHC and BH
1551:
726:
634:
194:
1018:
Ryschkewitsch, George E.; Wiggins, J. W. (1970-03-01). "Trigonal boron cation".
744:
660:, a key step in the electrophilic aromatic addition mechanism. wIn 2013, Stahl
512:(NHC)-stabilized borenium ions have been demonstrated to be potent metal-free H
571:
hydrogenation. A similar NHC-stabilized borenium ion was used to catalyze the
452:
In other cases the dative ligand has been observed to be twisted out of the BR
2174:
2152:
2098:
2051:
1967:
1920:
1869:
1809:
1757:
1714:
1678:
1636:
1585:
1528:
1484:
1444:
1391:
1333:
1288:
1228:
1185:
1113:
1074:
1039:
983:
930:
886:
696:
517:
501:
638:
Enantioselective Diels–Alder reaction catalyzed by a borenium ion generated
231:
2160:
2144:
2116:
2059:
2020:
2012:
1985:
1928:
1827:
1765:
1749:
1722:
1593:
1576:
1536:
1520:
1492:
1399:
1341:
1236:
1193:
991:
948:
766:
N). They have linear geometry at boron and are coordinatively unsaturated.
740:
624:
1942:
De Vries, Timothy S.; Prokofjevs, Aleksandrs; Vedejs, Edwin (2012-04-20).
905:
De Vries, Timothy S.; Prokofjevs, Aleksandrs; Vedejs, Edwin (2012-07-11).
599:. One proposed mechanism for this enantioselective reduction involves the
556:
activation showed a 281.8 kcal/mol interaction between the sigma bond of H
268:
catalysts. These N-protonated borenium species have been characterized by
807:
419:
2075:"Weakly Stabilized Primary Borenium Cations and Their Dicationic Dimers"
1650:
Matuszek, K.; Coffie, S.; Chrobok, A.; Swadźba-Kwaśny, M. (2017-03-06).
1628:
1145:
1031:
878:
1912:
1800:
1670:
1567:
1435:
1383:
1279:
1254:
1220:
778:
652:
296:
A number of borenium ions have been structurally characterized through
235:
Example of the use of nucleophilic displacement to make a borenium ion.
71:
2090:
1959:
1840:
1706:
1476:
1426:
1370:
Eisenberger, P.; Crudden, C. M. (2017-04-10). "Borocation catalysis".
1325:
1177:
1105:
1066:
922:
2043:
534:
252:
169:
150:
44:
414:(DFT) calculations of isolable borenium ions show that the strongly
120:
2033:
1649:
785:
751:, a toxic and corrosive gas, in the industrial synthesis of PAOs.
789:
525:
444:
fragment and various NHCs has been calculated using the extended
1505:
781:). Boronium ions are tetrahedral and coordinatively saturated.
700:
244:
starting material, as demonstrated by competition experiments.
215:
2129:
1253:
Stojanović, Milovan; Baranac-Stojanović, Marija (2015-09-08).
425:
2072:
961:
568:
1252:
730:
Mechanism of hydroboration with a borenium ion electrophile.
256:
Formation of a borenium ion by protonation of an aminoborane
552:(NBOs) of the intermediate in this reaction involved with H
1890:
1781:
1549:
762:
ions have the formula , where X is usual a bulky amide (R
27:
1941:
1412:
1090:
904:
520:
catalysts. Unlike the neutral boranes typically used in
496:
1691:
1086:
1084:
627:
as catalysts for the Diels–Alder reaction by Matuszek
1462:
1017:
691:
The electrophilicity of borenium ions can drive the
646:
208:. A positive charge on boron was then inferred from
124:
Various representations of bonding in borenium ions.
1206:
1081:
1614:
1369:
247:
1998:
1735:
2172:
707:were able to successfully accomplish metal-free
312:cation has a natural charge of +0.687 on boron.
164:
1163:
603:generation of a borenium-like species using BH
484:
1304:
1052:
815:(diammoniodihydroboronium tetrahydroborate).
586:
418:boron can be stabilized by pi-donation from
291:
226:
1850:ACS Sustainable Chemistry & Engineering
1305:Rezabal, Elixabete; Frison, Gilles (2015).
864:
734:
591:Borenium ions have been used as metal-free
560:and the 2p orbital of the cationic boron.
2106:
1975:
1817:
1799:
1575:
1434:
1278:
938:
477:; however, other counterions such as AlCl
2079:Journal of the American Chemical Society
1695:Journal of the American Chemical Society
1617:Journal of the American Chemical Society
1465:Journal of the American Chemical Society
1166:Journal of the American Chemical Society
1020:Journal of the American Chemical Society
818:
725:
633:
623:further extended to the use of borenium
500:
424:
251:
230:
214:
168:
119:
26:
2001:Angewandte Chemie International Edition
1738:Angewandte Chemie International Edition
1509:Angewandte Chemie International Edition
14:
2173:
1131:
784:A well-known example is . Reaction of
739:Borenium ions have been shown to form
533:stoichiometrically in the presence of
1777:
1775:
1458:
1456:
1454:
1365:
1363:
1361:
1359:
1300:
1298:
1248:
1246:
1013:
1011:
1009:
860:
858:
856:
854:
852:
850:
848:
846:
497:Hydrogen activation and FLP chemistry
185:results in a borenium cation and AlCl
1159:
1157:
1155:
1127:
1125:
1123:
900:
898:
896:
769:
754:
24:
1992:
1935:
1772:
1659:Catalysis Science & Technology
1451:
1356:
1314:Journal of Computational Chemistry
1295:
1243:
1006:
843:
25:
2192:
1152:
1120:
893:
647:Electrophilic aromatic borylation
642:by protonation with triflic acid.
197:in the presence of the adduct of
829:
686:
583:ability to act as a Lewis acid.
275:
115:
2123:
2066:
2027:
1884:
1834:
1729:
1685:
1643:
1608:
1543:
1499:
1406:
422:substituents such as pyridine.
248:Protic addition to aminoboranes
2133:Chemistry - A European Journal
1200:
1046:
955:
13:
1:
1862:10.1021/acssuschemeng.9b03621
976:10.1021/acs.inorgchem.8b01601
836:
567:catalytically to be used for
165:Halide or hydride abstraction
777:ions have the formula (L =
156:
7:
485:Reactivity and applications
10:
2197:
743:capable of catalyzing the
587:Enantioselective catalysis
327:Occupancy of B 2p Orbital
489:Borenium ions are highly
412:Density functional theory
292:Structure and electronics
227:Nucleophilic dissociation
735:Polymerization catalysis
1556:Chemical Communications
1209:Chemical Communications
438:N-heterocyclic carbenes
2145:10.1002/chem.201602805
2013:10.1002/anie.200500402
1750:10.1002/anie.201611536
1521:10.1002/anie.201500959
731:
643:
597:Corey–Itsuno reduction
510:N-heterocyclic carbene
506:
459:Gutmann–Beckett method
434:
257:
236:
220:
174:
125:
40:
819:Related boron cations
729:
637:
550:natural bond orbitals
522:frustrated Lewis pair
504:
428:
298:x-ray crystallography
255:
234:
218:
172:
128:A borenium ion is an
123:
30:
658:Wheland intermediate
616:Diels–Alder reaction
324:Natural Charge on B
2139:(39): 13927–13934.
2085:(42): 15686–15689.
2036:Dalton Transactions
1905:2014NatCh...6..498S
1856:(17): 15044–15052.
1701:(30): 10978–10981.
1629:10.1021/ja00252a056
1552:Fuchter, Matthew J.
1471:(38): 15728–15731.
1372:Dalton Transactions
1271:2015RSCAd...575895S
1265:(93): 75895–75910.
1146:10.1021/ic00193a009
1134:Inorganic Chemistry
1032:10.1021/ja00709a079
970:(21): 13188–13200.
964:Inorganic Chemistry
879:10.1021/cr00069a004
703:. In 2016, McGough
540:In 2015, Devillard
317:
261:particular cation.
1913:10.1038/nchem.1948
1801:10.1039/C5SC04798F
1671:10.1039/C7CY00106A
1568:10.1039/C9CC02900A
1384:10.1039/C6DT04232E
1280:10.1039/C5RA13825F
1221:10.1039/C3CC41556B
732:
644:
507:
435:
315:
258:
237:
221:
191:aluminium chloride
175:
126:
41:
2091:10.1021/ja407458k
2007:(32): 5016–5036.
1960:10.1021/cr200133c
1744:(13): 3712–3717.
1707:10.1021/ja405925w
1623:(18): 5551–5553.
1562:(49): 7077–7080.
1515:(19): 5722–5726.
1477:10.1021/ja307995f
1427:10.1021/om400541q
1421:(22): 6718–6724.
1378:(15): 4874–4887.
1326:10.1002/jcc.23852
1215:(43): 4893–4895.
1178:10.1021/ja025848x
1172:(15): 3808–3809.
1140:(25): 4147–4152.
1106:10.1021/om500348u
1100:(11): 2820–2830.
1067:10.1021/om400463r
1061:(22): 6712–6717.
923:10.1021/cr200133c
607:as a Lewis acid.
407:
406:
147:organic synthesis
16:(Redirected from
2188:
2165:
2164:
2127:
2121:
2120:
2110:
2070:
2064:
2063:
2044:10.1039/b810575h
2031:
2025:
2024:
1996:
1990:
1989:
1979:
1954:(7): 4246–4282.
1948:Chemical Reviews
1939:
1933:
1932:
1893:Nature Chemistry
1888:
1882:
1881:
1847:
1838:
1832:
1831:
1821:
1803:
1794:(5): 3384–3389.
1788:Chemical Science
1779:
1770:
1769:
1733:
1727:
1726:
1689:
1683:
1682:
1665:(5): 1045–1049.
1656:
1647:
1641:
1640:
1612:
1606:
1605:
1579:
1547:
1541:
1540:
1503:
1497:
1496:
1460:
1449:
1448:
1438:
1410:
1404:
1403:
1367:
1354:
1353:
1311:
1302:
1293:
1292:
1282:
1250:
1241:
1240:
1204:
1198:
1197:
1161:
1150:
1149:
1129:
1118:
1117:
1088:
1079:
1078:
1050:
1044:
1043:
1026:(6): 1790–1791.
1015:
1004:
1003:
959:
953:
952:
942:
917:(7): 4246–4282.
911:Chemical Reviews
902:
891:
890:
867:Chemical Reviews
862:
833:
814:
770:Boronium cations
755:Borinium cations
593:enantioselective
573:enantioselective
446:transition state
318:
314:
199:4-methylpyridine
144:
143:
142:
134:chemical formula
132:cation with the
110:
109:
108:
98:
97:
96:
86:
85:
84:
69:
68:
67:
57:chemical formula
55:cation with the
21:
2196:
2195:
2191:
2190:
2189:
2187:
2186:
2185:
2181:Boron compounds
2171:
2170:
2169:
2168:
2128:
2124:
2071:
2067:
2032:
2028:
1997:
1993:
1940:
1936:
1889:
1885:
1845:
1839:
1835:
1780:
1773:
1734:
1730:
1690:
1686:
1654:
1648:
1644:
1613:
1609:
1548:
1544:
1504:
1500:
1461:
1452:
1415:Organometallics
1411:
1407:
1368:
1357:
1309:
1303:
1296:
1251:
1244:
1205:
1201:
1162:
1153:
1130:
1121:
1094:Organometallics
1089:
1082:
1055:Organometallics
1051:
1047:
1016:
1007:
960:
956:
903:
894:
863:
844:
839:
821:
811:
805:
801:
797:
793:
772:
765:
757:
750:
737:
723:as a catalyst.
722:
718:
714:
689:
678:
674:
670:
649:
606:
589:
566:
559:
555:
547:
532:
516:activation and
515:
499:
487:
480:
476:
472:
468:
455:
443:
432:
397:
393:
379:
375:
371:
357:
353:
339:
335:
311:
307:
294:
287:
283:
278:
250:
243:
229:
206:
195:dichloromethane
188:
184:
167:
159:
141:
139:
138:
137:
136:
118:
107:
105:
104:
103:
102:
95:
93:
92:
91:
90:
83:
81:
80:
79:
78:
66:
63:
62:
61:
59:
38:
34:
23:
22:
15:
12:
11:
5:
2194:
2184:
2183:
2167:
2166:
2122:
2065:
2038:(45): 6421–6.
2026:
1991:
1934:
1899:(6): 498–503.
1883:
1833:
1771:
1728:
1684:
1642:
1607:
1542:
1498:
1450:
1405:
1355:
1320:(8): 564–572.
1294:
1242:
1199:
1151:
1119:
1080:
1045:
1005:
954:
892:
873:(5): 399–418.
841:
840:
838:
835:
820:
817:
809:
803:
799:
795:
771:
768:
763:
756:
753:
748:
745:polymerization
736:
733:
720:
716:
712:
688:
685:
676:
672:
668:
648:
645:
604:
588:
585:
564:
557:
553:
545:
530:
513:
498:
495:
486:
483:
478:
474:
470:
466:
453:
441:
430:
405:
404:
401:
398:
395:
391:
387:
386:
383:
380:
377:
373:
369:
365:
364:
361:
358:
355:
351:
347:
346:
343:
340:
337:
333:
329:
328:
325:
322:
309:
305:
293:
290:
285:
281:
277:
274:
249:
246:
241:
228:
225:
212:spectroscopy.
204:
186:
182:
166:
163:
158:
155:
140:
117:
114:
113:
112:
106:
100:
94:
88:
82:
64:
36:
32:
9:
6:
4:
3:
2:
2193:
2182:
2179:
2178:
2176:
2162:
2158:
2154:
2150:
2146:
2142:
2138:
2134:
2126:
2118:
2114:
2109:
2104:
2100:
2096:
2092:
2088:
2084:
2080:
2076:
2069:
2061:
2057:
2053:
2049:
2045:
2041:
2037:
2030:
2022:
2018:
2014:
2010:
2006:
2002:
1995:
1987:
1983:
1978:
1973:
1969:
1965:
1961:
1957:
1953:
1949:
1945:
1938:
1930:
1926:
1922:
1918:
1914:
1910:
1906:
1902:
1898:
1894:
1887:
1879:
1875:
1871:
1867:
1863:
1859:
1855:
1851:
1844:
1837:
1829:
1825:
1820:
1815:
1811:
1807:
1802:
1797:
1793:
1789:
1785:
1778:
1776:
1767:
1763:
1759:
1755:
1751:
1747:
1743:
1739:
1732:
1724:
1720:
1716:
1712:
1708:
1704:
1700:
1696:
1688:
1680:
1676:
1672:
1668:
1664:
1660:
1653:
1646:
1638:
1634:
1630:
1626:
1622:
1618:
1611:
1603:
1599:
1595:
1591:
1587:
1583:
1578:
1577:10044/1/70396
1573:
1569:
1565:
1561:
1557:
1553:
1546:
1538:
1534:
1530:
1526:
1522:
1518:
1514:
1510:
1502:
1494:
1490:
1486:
1482:
1478:
1474:
1470:
1466:
1459:
1457:
1455:
1446:
1442:
1437:
1432:
1428:
1424:
1420:
1416:
1409:
1401:
1397:
1393:
1389:
1385:
1381:
1377:
1373:
1366:
1364:
1362:
1360:
1351:
1347:
1343:
1339:
1335:
1331:
1327:
1323:
1319:
1315:
1308:
1301:
1299:
1290:
1286:
1281:
1276:
1272:
1268:
1264:
1260:
1256:
1249:
1247:
1238:
1234:
1230:
1226:
1222:
1218:
1214:
1210:
1203:
1195:
1191:
1187:
1183:
1179:
1175:
1171:
1167:
1160:
1158:
1156:
1147:
1143:
1139:
1135:
1128:
1126:
1124:
1115:
1111:
1107:
1103:
1099:
1095:
1087:
1085:
1076:
1072:
1068:
1064:
1060:
1056:
1049:
1041:
1037:
1033:
1029:
1025:
1021:
1014:
1012:
1010:
1001:
997:
993:
989:
985:
981:
977:
973:
969:
965:
958:
950:
946:
941:
936:
932:
928:
924:
920:
916:
912:
908:
901:
899:
897:
888:
884:
880:
876:
872:
868:
861:
859:
857:
855:
853:
851:
849:
847:
842:
834:
832:
827:
824:
816:
813:
792:mainly gives
791:
787:
782:
780:
776:
767:
761:
752:
746:
742:
741:ionic liquids
728:
724:
710:
706:
702:
698:
697:hydroboration
694:
687:Hydroboration
684:
682:
665:
663:
659:
654:
641:
636:
632:
630:
626:
625:ionic liquids
621:
617:
613:
608:
602:
598:
594:
584:
580:
578:
574:
570:
561:
551:
543:
538:
536:
527:
523:
519:
518:hydrogenation
511:
503:
494:
492:
482:
462:
460:
450:
447:
439:
427:
423:
421:
417:
413:
402:
399:
389:
388:
384:
381:
367:
366:
362:
359:
349:
348:
344:
341:
331:
330:
326:
323:
321:Borenium Ion
320:
319:
313:
303:
299:
289:
276:Other methods
273:
271:
267:
262:
254:
245:
233:
224:
217:
213:
211:
207:
200:
196:
193:dissolved in
192:
180:
171:
162:
154:
152:
148:
135:
131:
122:
116:Borenium ions
101:
89:
77:
76:
75:
73:
58:
54:
50:
46:
29:
19:
2136:
2132:
2125:
2082:
2078:
2068:
2035:
2029:
2004:
2000:
1994:
1951:
1947:
1937:
1896:
1892:
1886:
1853:
1849:
1836:
1791:
1787:
1741:
1737:
1731:
1698:
1694:
1687:
1662:
1658:
1645:
1620:
1616:
1610:
1559:
1555:
1545:
1512:
1508:
1501:
1468:
1464:
1418:
1414:
1408:
1375:
1371:
1317:
1313:
1262:
1259:RSC Advances
1258:
1212:
1208:
1202:
1169:
1165:
1137:
1133:
1097:
1093:
1058:
1054:
1048:
1023:
1019:
967:
963:
957:
914:
910:
870:
866:
828:
825:
822:
783:
774:
773:
759:
758:
738:
708:
704:
692:
690:
681:heterocycles
666:
661:
650:
639:
628:
619:
609:
600:
590:
581:
579:NHC ligand.
562:
541:
539:
508:
491:Lewis acidic
488:
463:
451:
436:
416:Lewis acidic
408:
295:
279:
263:
259:
238:
222:
181:such as AlCl
176:
160:
127:
48:
42:
1436:10356/98250
612:E. J. Corey
410:the boron.
302:dative bond
266:Diels–Alder
72:Lewis bases
837:References
779:Lewis base
653:borylation
210:proton NMR
179:Lewis acid
111:: boronium
99:: borenium
87:: borinium
51:ion is an
49:boranylium
2153:0947-6539
2099:0002-7863
2052:1477-9226
1968:0009-2665
1921:1755-4330
1878:202069606
1870:2168-0485
1810:2041-6539
1758:1521-3773
1715:0002-7863
1679:2044-4761
1637:0002-7863
1602:206141629
1586:1364-548X
1529:1521-3773
1485:0002-7863
1445:0276-7333
1392:1477-9234
1334:1096-987X
1289:2046-2069
1229:1364-548X
1186:0002-7863
1114:0276-7333
1075:0276-7333
1040:0002-7863
984:0020-1669
931:0009-2665
887:0009-2665
631:in 2017.
535:phosphine
157:Synthesis
151:catalysis
130:inorganic
53:inorganic
45:chemistry
2175:Category
2161:27514500
2117:24087933
2060:19002329
2021:16086441
1986:22519545
1929:24848235
1828:29997833
1766:28244623
1723:23855894
1594:31149679
1537:25800957
1493:22931196
1400:28294211
1350:22398660
1342:25708019
1237:23571677
1194:11942799
1000:53036749
992:30351072
949:22519545
786:diborane
775:Boronium
760:Borinium
420:aromatic
18:Boronium
2108:3857331
1977:3394883
1901:Bibcode
1819:6006955
1267:Bibcode
940:3394883
808:[BH
790:ammonia
701:alkynes
640:in situ
620:in situ
601:in situ
526:hydride
400:+1.412
382:+1.087
360:+0.566
342:+0.687
284:] or Li
2159:
2151:
2115:
2105:
2097:
2058:
2050:
2019:
1984:
1974:
1966:
1927:
1919:
1876:
1868:
1826:
1816:
1808:
1764:
1756:
1721:
1713:
1677:
1635:
1600:
1592:
1584:
1535:
1527:
1491:
1483:
1443:
1398:
1390:
1348:
1340:
1332:
1287:
1235:
1227:
1192:
1184:
1112:
1073:
1038:
998:
990:
982:
947:
937:
929:
885:
794:[H
709:trans-
705:et al.
662:et al.
629:et al.
577:chiral
542:et al.
403:0.289
385:0.167
363:0.460
345:0.023
1874:S2CID
1846:(PDF)
1655:(PDF)
1598:S2CID
1346:S2CID
1310:(PDF)
996:S2CID
788:with
693:trans
569:imine
2157:PMID
2149:ISSN
2113:PMID
2095:ISSN
2056:PMID
2048:ISSN
2017:PMID
1982:PMID
1964:ISSN
1925:PMID
1917:ISSN
1866:ISSN
1824:PMID
1806:ISSN
1762:PMID
1754:ISSN
1719:PMID
1711:ISSN
1675:ISSN
1633:ISSN
1590:PMID
1582:ISSN
1533:PMID
1525:ISSN
1489:PMID
1481:ISSN
1441:ISSN
1396:PMID
1388:ISSN
1338:PMID
1330:ISSN
1285:ISSN
1233:PMID
1225:ISSN
1190:PMID
1182:ISSN
1110:ISSN
1071:ISSN
1036:ISSN
988:PMID
980:ISSN
945:PMID
927:ISSN
883:ISSN
798:B(NH
368:B(CH
201:and
149:and
47:, a
2141:doi
2103:PMC
2087:doi
2083:135
2040:doi
2009:doi
1972:PMC
1956:doi
1952:112
1909:doi
1858:doi
1814:PMC
1796:doi
1746:doi
1703:doi
1699:135
1667:doi
1625:doi
1621:109
1572:hdl
1564:doi
1517:doi
1473:doi
1469:134
1431:hdl
1423:doi
1380:doi
1322:doi
1275:doi
1217:doi
1174:doi
1170:124
1142:doi
1102:doi
1063:doi
1028:doi
972:doi
935:PMC
919:doi
915:112
875:doi
699:of
350:BCl
288:].
270:NMR
203:BCl
43:In
2177::
2155:.
2147:.
2137:22
2135:.
2111:.
2101:.
2093:.
2081:.
2077:.
2054:.
2046:.
2015:.
2005:44
2003:.
1980:.
1970:.
1962:.
1950:.
1946:.
1923:.
1915:.
1907:.
1895:.
1872:.
1864:.
1852:.
1848:.
1822:.
1812:.
1804:.
1790:.
1786:.
1774:^
1760:.
1752:.
1742:56
1740:.
1717:.
1709:.
1697:.
1673:.
1661:.
1657:.
1631:.
1619:.
1596:.
1588:.
1580:.
1570:.
1560:55
1558:.
1531:.
1523:.
1513:54
1511:.
1487:.
1479:.
1467:.
1453:^
1439:.
1429:.
1419:32
1417:.
1394:.
1386:.
1376:46
1374:.
1358:^
1344:.
1336:.
1328:.
1318:36
1316:.
1312:.
1297:^
1283:.
1273:.
1261:.
1257:.
1245:^
1231:.
1223:.
1213:49
1211:.
1188:.
1180:.
1168:.
1154:^
1138:23
1136:.
1122:^
1108:.
1098:33
1096:.
1083:^
1069:.
1059:32
1057:.
1034:.
1024:92
1022:.
1008:^
994:.
986:.
978:.
968:57
966:.
943:.
933:.
925:.
913:.
909:.
895:^
881:.
871:85
869:.
845:^
683:.
537:.
394:NH
390:BF
376:NH
354:NH
336:NH
332:BH
308:NH
272:.
153:.
60:BR
35:NH
31:BH
2163:.
2143::
2119:.
2089::
2062:.
2042::
2023:.
2011::
1988:.
1958::
1931:.
1911::
1903::
1897:6
1880:.
1860::
1854:7
1830:.
1798::
1792:7
1768:.
1748::
1725:.
1705::
1681:.
1669::
1663:7
1639:.
1627::
1604:.
1574::
1566::
1539:.
1519::
1495:.
1475::
1447:.
1433::
1425::
1402:.
1382::
1352:.
1324::
1291:.
1277::
1269::
1263:5
1239:.
1219::
1196:.
1176::
1148:.
1144::
1116:.
1104::
1077:.
1065::
1042:.
1030::
1002:.
974::
951:.
921::
889:.
877::
812:]
810:4
806:]
804:2
802:)
800:3
796:2
764:2
749:3
721:3
719:)
717:5
715:F
713:6
695:-
677:3
675:)
673:5
671:H
669:6
605:3
565:2
558:2
554:2
546:2
531:2
529:H
514:2
479:4
475:4
473:)
471:5
469:F
467:6
454:3
442:2
431:2
396:3
392:2
378:3
374:2
372:)
370:3
356:3
352:2
338:3
334:2
310:3
306:2
286:4
282:4
242:3
205:3
187:4
183:3
65:2
37:3
33:2
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.