213:
1138:
225:
218:
1129:
230:
762:
1065:
313:
936:
306:
1010:
399:
406:
1022:
448:
441:
283:
1015:
42:
276:
1027:
31:
571:
Polarity is key in determining relative boiling point as strong intermolecular forces raise the boiling point. In the same manner, symmetry is key in determining relative melting point as it allows for better packing in the solid state, even if it does not alter the polarity of the molecule. Another
859:
is determined by the CIP rules; higher atomic numbers are given higher priority. For each of the two atoms in the double bond, it is necessary to determine the priority of each substituent. If both the higher-priority substituents are on the same side, the arrangement is
927:
Wavy single bonds are the standard way to represent unknown or unspecified stereochemistry or a mixture of isomers (as with tetrahedral stereocenters). A crossed double-bond has been used sometimes; it is no longer considered an acceptable style for general use by
606:
In the case of geometric isomers that are a consequence of double bonds, and, in particular, when both substituents are the same, some general trends usually hold. These trends can be attributed to the fact that the dipoles of the substituents in a
1541:
Craig, N. C.; Chen, A.; Suh, K. H.; Klee, S.; Mellau, G. C.; Winnewisser, B. P.; Winnewisser, M. (1997). "Contribution to the Study of the Gauche Effect. The
Complete Structure of the
736:
805:
are unambiguous in all cases, and therefore are especially useful for tri- and tetrasubstituted alkenes to avoid any confusion about which groups are being identified as
387:
isomer on the other hand, this does not occur because the two C−Cl bond moments cancel and the molecule has a net zero dipole moment (it does however have a non-zero
1305:
98:, that is, pairs of molecules which have the same formula but whose functional groups are in different orientations in three-dimensional space.
1989:
1509:
Bingham, Richard C. (1976). "The stereochemical consequences of electron delocalization in extended π systems. An interpretation of the
1949:
1665:
794:
766:
2008:
1493:
1468:
1440:
124:
where the two geometric forms easily interconvert, such as most open-chain single-bonded structures; instead, the terms "
376:
711:
1179:
isomer, the two Y ligands are adjacent to each other at 90°, as is true for the two chlorine atoms shown in green in
1290:
603:
alkenes, which are generally more polar and less symmetrical, have higher boiling points and lower melting points.
1864:
2077:
1388:
2031:
1658:
1362:
599:
alkenes, which are less polar and more symmetrical, have lower boiling points and higher melting points, and
335:
These differences can be very small, as in the case of the boiling point of straight-chain alkenes, such as
1969:
615:
isomer, the dipoles of the substituents will cancel out due to being on opposite sides of the molecule.
189:, whereas when the substituents are oriented in opposing directions, the diastereomer is referred to as
1845:
1767:
1582:
553:
74:" are from Latin: "this side of" and "the other side of", respectively. In the context of chemistry,
2067:
2048:
1651:
1574:
1336:
380:
121:
1227:
1940:
1705:
1244:
1054:
584:
isomer, has a melting point of 13.4 °C, making it a liquid at room temperature, while the
117:
24:
718:
isomers suffer a 1.10 kcal/mol stability penalty. Exceptions to this rule exist, such as
1887:
1827:
1239:
1199:
785:
notation should not be used for alkenes with two or more different substituents. Instead the
588:
isomer, elaidic acid, has the much higher melting point of 43 °C, due to the straighter
177:
or ring structures. In both cases the rotation of bonds is restricted or prevented. When the
212:
1891:
1266:
719:
482:
The differing properties of the two isomers of butenedioic acid are often very different.
20:
1171:, two isomers also exist. (Here M is a metal atom, and X and Y are two different types of
726:(FN=NF), and several other halogen- and oxygen-substituted ethylenes. In these cases, the
224:
8:
723:
649:
217:
982:
isomer is generally the more reactive of the two, being the only isomer that can reduce
2072:
1802:
1599:
1575:"Graphical representation of stereochemical configuration (IUPAC Recommendations 2006)"
1432:
1160:
1137:
919:)-2-chlorobut-2-ene (the chlorine and C4 are together because C1 and C4 are opposite).
703:
372:
356:
265:
229:
1187:
isomer shown at right, the two Cl atoms are on opposite sides of the central Co atom.
1547:
1515:
1489:
1464:
1436:
1128:
1101:
388:
79:
1603:
1315:
16:
Pairs of molecules with same chemical formula showing different spatial orientations
1591:
1555:
1523:
1428:
1319:
1310:
1104:
to have antitumor activity, and is now a chemotherapy drug known by the short name
756:
663:
1694:
1674:
761:
707:
264:
isomers have distinct physical properties. Their differing shapes influences the
1910:
691:
1116:) has no useful anticancer activity. Each isomer can be synthesized using the
375:
combine to give an overall molecular dipole, so that there are intermolecular
2061:
1314:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
1089:
1073:
887:
systems compare different groups on the alkene, it is not strictly true that
631:
95:
1595:
1323:
1291:
Charlton T. Lewis, Charles Short, A Latin
Dictionary (Clarendon Press, 1879)
1064:
1740:
1249:
1117:
998:
isomer cannot line its hydrogens up suitably to reduce the alkene, but the
577:
473:
431:
312:
182:
935:
592:
isomer being able to pack more tightly, and is solid at room temperature.
66:, describes certain arrangements of atoms within molecules. The prefixes "
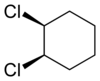
1113:
1009:
967:
419:
398:
305:
178:
174:
109:
occur both in organic molecules and in inorganic coordination complexes.
1630:
1625:
1620:
1527:
1513:
effect exhibited by 1,2-disubstituted ethylenes and related phenomena".
405:
1021:
652:
635:
573:
461:
1559:
447:
1689:
1271:
1105:
793:
notation is used based on the priority of the substituents using the
1423:
Ouellette, Robert J.; Rawn, J. David (2015). "Alkenes and
Alkynes".
440:
1922:
1014:
908:
363:
isomer in this case has a boiling point of 60.3 °C, while the
202:
1643:
282:
1026:
963:
336:
106:
41:
1218:
to each other. Metal carbonyl compounds can be characterized as
275:
1461:
Advanced
Organic Chemistry, Reactions, Mechanisms and structure
1261:
1172:
991:
987:
983:
851:
to each other. Whether a molecular configuration is designated
30:
1057:
with octahedral or square planar geometries can also exhibit
929:
907:-2-chlorobut-2-ene (the two methyl groups, C1 and C4, on the
611:
isomer will add up to give an overall molecular dipole. In a
136:
797:
for absolute configuration. The IUPAC standard designations
355:
isomers can be larger if polar bonds are present, as in the
690:
isomers. This difference is attributed to the unfavorable
82:(substituents) are on the same side of some plane, while
1484:
Williams, Dudley H.; Fleming, Ian (1989). "Table 3.27".
1210:) isomerism, in which different numbers of ligands are
835:) means "opposed" in the sense of "opposite". That is,
86:
conveys that they are on opposing (transverse) sides.
1068:
The two isomeric complexes, cisplatin and transplatin
670:(range: 12–18 Hz; typical: 15 Hz) than for
619:
isomers also tend to have lower densities than their
1100:-diamminedichloroplatinum(II), was shown in 1969 by
252:
153:
or geometric isomerism is classified as one type of
139:, "geometric isomerism" is an obsolete synonym of "
922:
674:(range: 0–12 Hz; typical: 8 Hz) isomers.
367:isomer has a boiling point of 47.5 °C. In the
955:isomerism can also occur in inorganic compounds.
2059:
1540:
932:but may still be required by computer software.
19:"Cis-trans" redirects here. For other uses, see
1483:
642:alkenes, in general, are more symmetrical than
193:. An example of a small hydrocarbon displaying
205:. 1,2-Dichlorocyclohexane is another example.
1659:
1422:
1190:A related type of isomerism in octahedral MX
830:
820:
572:example of this is the relationship between
978:isomerism. As with organic compounds, the
864:; if on opposite sides, the arrangement is
1666:
1652:
1566:
1486:Spectroscopic Methods in Organic Chemistry
1412:(60th ed.). 1979–1980. p. C-298.
268:, boiling, and especially melting points.
1626:IUPAC definition of "geometric isomerism"
1049:
1063:
934:
760:
181:are oriented in the same direction, the
40:
29:
1970:Pseudoasymmetric (pseudochiral) centers
1950:CIP (Cahn–Ingold–Prelog) priority rules
1572:
1508:
1454:
1452:
1354:
1002:isomer, being shaped differently, can.
795:Cahn–Ingold–Prelog (CIP) priority rules
2060:
1360:
1072:For example, there are two isomers of
943:
734:isomer. This phenomenon is called the
1647:
1621:IUPAC definition of "stereoisomerism"
1458:
1410:CRC Handbook of Chemistry and Physics
1367:Virtual Textbook of Organic Chemistry
1120:to control which isomer is produced.
769:than chlorine, so this alkene is the
379:(or Keesom forces), which add to the
1449:
383:and raise the boiling point. In the
160:
1673:
13:
1488:(4th rev. ed.). McGraw-Hill.
1433:10.1016/B978-0-12-802444-7.00004-5
1311:Compendium of Chemical Terminology
994:, but for a different reason: the
712:heat of formation group additivity
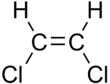
14:
2089:
1614:
253:Comparison of physical properties
1545:Rotamer of 1,2-Difluoroethane".
1334:
1136:
1127:
1025:
1020:
1013:
1008:
923:Undefined alkene stereochemistry
446:
439:
404:
397:
347:isomer. The differences between
311:
304:
281:
274:
228:
223:
216:
211:
1534:
1502:
1425:Principles of Organic Chemistry
847:has the higher-priority groups
839:has the higher-priority groups
743:
730:isomer is more stable than the
702:isomers have a less-exothermic
1477:
1416:
1402:
1381:
1328:
1299:
1284:
1:
1463:(3rd ed.). p. 111.
1277:
686:isomers are more stable than
343:isomer and 36 °C in the
339:, which is 37 °C in the
682:Usually for acyclic systems
677:
630:alkenes tend to have higher
7:
1369:. Michigan State University
1233:
1096:isomer, whose full name is
958:
694:of the substituents in the
10:
2094:
2032:Octahedral propeller twist
1768:Arene substitution pattern
1583:Pure and Applied Chemistry
1573:Brecher, Jonathan (2006).
754:
554:Acid dissociation constant
371:isomer the two polar C–Cl
120:are not used for cases of
18:
2046:
2030:
2007:
1988:
1968:
1948:
1939:
1921:
1909:
1886:
1863:
1844:
1826:
1801:
1766:
1739:
1713:
1706:Configuration descriptors
1704:
1681:
710:stability. In the Benson
486:Properties of isomers of
246:-1,2-dichlorocyclohexane
155:configurational isomerism
2049:Category:Stereochemistry
1361:Reusch, William (2010).
381:London dispersion forces
240:-1,2-dichlorocyclohexane
122:conformational isomerism
1941:Absolute configurations
1846:Three identical ligands
1596:10.1351/pac200678101897
1324:10.1351/goldbook.G02620
2078:Orientation (geometry)
2009:Relative configuration
1363:"Stereoisomers Part I"
1245:Descriptor (chemistry)
1069:
1055:Coordination complexes
1050:Coordination complexes
940:
939:Alkene stereochemistry
831:
821:
774:
638:in inert solvents, as
173:stereoisomers contain
49:
38:
25:trans (disambiguation)
1828:Syn and anti addition
1631:IUPAC definition of "
1459:March, Jerry (1985).
1389:"Chemicalland values"
1341:University of Calgary
1240:Chirality (chemistry)
1228:infrared spectroscopy
1067:
938:
765:Bromine has a higher
764:
542:water solubility, g/L
470:-9-octadecenoic acid
458:-9-octadecenoic acid
44:
33:
1858:(facies, meridonal)
1391:. Chemicalland21.com
1267:Structural isomerism
1161:octahedral complexes
825:) means "together".
720:1,2-difluoroethylene
706:, indicating higher
626:As a general trend,
377:dipole–dipole forces
329:-1,2-dichloroethene
21:cis (disambiguation)
1865:In carbon skeletons
1528:10.1021/ja00418a036
1427:. pp. 95–132.
1316:geometric isomerism
1183:-, at left. In the
1108:. In contrast, the
970:) can also exhibit
944:Inorganic chemistry
915:to each other) is (
724:1,2-difluorodiazene
698:isomer. Therefore,
506:
373:bond dipole moments
357:1,2-dichloroethenes
323:-1,2-dichloroethene
78:indicates that the
64:geometric isomerism
1088:, as explained by
1070:
941:
843:to each other and
775:
704:heat of combustion
692:steric interaction
653:coupling constants
580:; oleic acid, the
485:
428:-butenedioic acid
416:-butenedioic acid
185:is referred to as
179:substituent groups
50:
39:
2055:
2054:
2042:
2041:
1935:
1934:
1590:(10): 1897–1970.
1560:10.1021/ja963819e
1548:J. Am. Chem. Soc.
1516:J. Am. Chem. Soc.
1495:978-0-07-707212-4
1470:978-0-471-85472-2
1442:978-0-12-802444-7
1337:"Stereochemistry"
1200:facial–meridional
1102:Barnett Rosenberg
1047:
1046:
966:(and the related
829:(from the German
819:(from the German
666:, are larger for
569:
568:
531:melting point, °C
480:
479:
389:quadrupole moment
333:
332:
250:
249:
161:Organic chemistry
80:functional groups
2085:
2024:
2023:
2018:
2017:
1990:Optical rotation
1946:
1945:
1711:
1710:
1668:
1661:
1654:
1645:
1644:
1608:
1607:
1579:
1570:
1564:
1563:
1538:
1532:
1531:
1506:
1500:
1499:
1481:
1475:
1474:
1456:
1447:
1446:
1420:
1414:
1413:
1406:
1400:
1399:
1397:
1396:
1385:
1379:
1378:
1376:
1374:
1358:
1352:
1351:
1349:
1347:
1332:
1326:
1303:
1297:
1288:
1140:
1131:
1029:
1024:
1017:
1012:
1005:
1004:
834:
824:
664:NMR spectroscopy
507:
505:
484:
450:
443:
408:
401:
394:
393:
315:
308:
285:
278:
271:
270:
232:
227:
220:
215:
208:
207:
62:, also known as
2093:
2092:
2088:
2087:
2086:
2084:
2083:
2082:
2068:Stereochemistry
2058:
2057:
2056:
2051:
2038:
2026:
2021:
2020:
2015:
2014:
2003:
1984:
1964:
1931:
1917:
1905:
1882:
1859:
1840:
1822:
1797:
1762:
1735:
1700:
1699:
1695:Racemic mixture
1677:
1675:Stereochemistry
1672:
1617:
1612:
1611:
1577:
1571:
1567:
1539:
1535:
1507:
1503:
1496:
1482:
1478:
1471:
1457:
1450:
1443:
1421:
1417:
1408:
1407:
1403:
1394:
1392:
1387:
1386:
1382:
1372:
1370:
1359:
1355:
1345:
1343:
1333:
1329:
1304:
1300:
1289:
1285:
1280:
1236:
1197:
1193:
1170:
1166:
1157:
1156:
1155:
1154:
1143:
1142:
1141:
1133:
1132:
1087:
1083:
1079:
1052:
961:
946:
925:
903:. For example,
899:corresponds to
891:corresponds to
813:to each other.
759:
753:
680:
662:), measured by
661:
559:
503:
499:
495:
471:
459:
429:
417:
255:
163:
28:
17:
12:
11:
5:
2091:
2081:
2080:
2075:
2070:
2053:
2052:
2047:
2044:
2043:
2040:
2039:
2036:
2034:
2028:
2027:
2013:
2011:
2005:
2004:
1995:(+)-, (−)- or
1994:
1992:
1986:
1985:
1974:
1972:
1966:
1965:
1954:
1952:
1943:
1937:
1936:
1933:
1932:
1927:
1925:
1919:
1918:
1915:
1913:
1911:Spiro compound
1907:
1906:
1896:
1894:
1884:
1883:
1869:
1867:
1861:
1860:
1850:
1848:
1842:
1841:
1832:
1830:
1824:
1823:
1814:
1812:
1799:
1798:
1772:
1770:
1764:
1763:
1752:
1750:
1737:
1736:
1726:
1724:
1708:
1702:
1701:
1698:
1697:
1692:
1686:
1685:
1683:
1679:
1678:
1671:
1670:
1663:
1656:
1648:
1642:
1641:
1628:
1623:
1616:
1615:External links
1613:
1610:
1609:
1565:
1533:
1522:(2): 535–540.
1501:
1494:
1476:
1469:
1448:
1441:
1415:
1401:
1380:
1353:
1327:
1298:
1282:
1281:
1279:
1276:
1275:
1274:
1269:
1264:
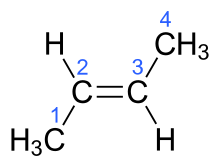
1259:
1247:
1242:
1235:
1232:
1195:
1191:
1168:
1164:
1145:
1144:
1135:
1134:
1126:
1125:
1124:
1123:
1122:
1085:
1081:
1077:
1051:
1048:
1045:
1044:
1038:
1031:
1030:
1018:
960:
957:
945:
942:
924:
921:
777:In principle,
755:Main article:
752:
742:
708:thermochemical
679:
676:
659:
632:melting points
623:counterparts.
567:
566:
563:
560:
557:
550:
549:
546:
543:
539:
538:
535:
532:
528:
527:
524:
521:
517:
516:
513:
510:
501:
497:
478:
477:
465:
452:
451:
444:
436:
435:
423:
410:
409:
402:
331:
330:
324:
317:
316:
309:
301:
300:
294:
287:
286:
279:
266:dipole moments
254:
251:
248:
247:
241:
234:
233:
221:
162:
159:
135:According to
15:
9:
6:
4:
3:
2:
2090:
2079:
2076:
2074:
2071:
2069:
2066:
2065:
2063:
2050:
2045:
2035:
2033:
2029:
2012:
2010:
2006:
2002:
1998:
1993:
1991:
1987:
1982:
1978:
1973:
1971:
1967:
1962:
1958:
1953:
1951:
1947:
1944:
1942:
1938:
1930:
1926:
1924:
1920:
1914:
1912:
1908:
1903:
1899:
1895:
1893:
1889:
1885:
1880:
1876:
1872:
1868:
1866:
1862:
1857:
1853:
1849:
1847:
1843:
1839:
1835:
1831:
1829:
1825:
1821:
1817:
1813:
1811:
1809:
1805:
1800:
1795:
1791:
1787:
1783:
1779:
1775:
1771:
1769:
1765:
1760:
1756:
1751:
1749:
1747:
1743:
1738:
1733:
1729:
1725:
1723:
1721:
1717:
1712:
1709:
1707:
1703:
1696:
1693:
1691:
1688:
1687:
1684:
1680:
1676:
1669:
1664:
1662:
1657:
1655:
1650:
1649:
1646:
1640:
1638:
1634:
1629:
1627:
1624:
1622:
1619:
1618:
1605:
1601:
1597:
1593:
1589:
1585:
1584:
1576:
1569:
1561:
1557:
1553:
1550:
1549:
1544:
1537:
1529:
1525:
1521:
1518:
1517:
1512:
1505:
1497:
1491:
1487:
1480:
1472:
1466:
1462:
1455:
1453:
1444:
1438:
1434:
1430:
1426:
1419:
1411:
1405:
1390:
1384:
1368:
1364:
1357:
1342:
1338:
1331:
1325:
1321:
1317:
1313:
1312:
1307:
1302:
1296:
1292:
1287:
1283:
1273:
1270:
1268:
1265:
1263:
1260:
1258:
1256:
1252:
1248:
1246:
1243:
1241:
1238:
1237:
1231:
1229:
1225:
1221:
1217:
1213:
1209:
1205:
1201:
1198:complexes is
1188:
1186:
1182:
1178:
1174:
1163:of formula MX
1162:
1152:
1148:
1139:
1130:
1121:
1119:
1115:
1111:
1107:
1103:
1099:
1095:
1092:in 1893. The
1091:
1090:Alfred Werner
1075:
1074:square planar
1066:
1062:
1060:
1056:
1042:
1039:
1036:
1033:
1032:
1028:
1023:
1019:
1016:
1011:
1007:
1006:
1003:
1001:
997:
993:
989:
985:
981:
977:
973:
969:
965:
956:
954:
950:
937:
933:
931:
920:
918:
914:
911:backbone are
910:
906:
902:
898:
894:
890:
886:
882:
878:
874:
869:
867:
863:
858:
854:
850:
846:
842:
838:
833:
828:
823:
818:
814:
812:
808:
804:
800:
796:
792:
788:
784:
780:
772:
768:
763:
758:
750:
746:
741:
739:
738:
733:
729:
725:
721:
717:
713:
709:
705:
701:
697:
693:
689:
685:
675:
673:
669:
665:
658:
654:
651:
647:
645:
641:
637:
633:
629:
624:
622:
618:
614:
610:
604:
602:
598:
593:
591:
587:
583:
579:
575:
564:
561:
555:
552:
551:
547:
544:
541:
540:
536:
533:
530:
529:
525:
522:
519:
518:
515:fumaric acid
514:
511:
509:
508:
493:
489:
483:
475:
469:
466:
463:
457:
454:
453:
449:
445:
442:
438:
437:
433:
427:
424:
421:
415:
412:
411:
407:
403:
400:
396:
395:
392:
390:
386:
382:
378:
374:
370:
366:
362:
358:
354:
350:
346:
342:
338:
328:
325:
322:
319:
318:
314:
310:
307:
303:
302:
298:
295:
292:
289:
288:
284:
280:
277:
273:
272:
269:
267:
263:
259:
245:
242:
239:
236:
235:
231:
226:
222:
219:
214:
210:
209:
206:
204:
201:isomerism is
200:
196:
192:
188:
184:
180:
176:
172:
168:
158:
156:
152:
148:
146:
142:
138:
133:
131:
127:
123:
119:
116:
112:
108:
105:
101:
97:
96:stereoisomers
93:
89:
85:
81:
77:
73:
69:
65:
61:
59:
55:
47:
43:
36:
32:
26:
22:
2000:
1996:
1980:
1976:
1960:
1956:
1928:
1901:
1897:
1878:
1874:
1870:
1855:
1851:
1837:
1833:
1819:
1815:
1807:
1803:
1793:
1789:
1785:
1781:
1777:
1773:
1758:
1754:
1745:
1741:
1731:
1727:
1719:
1715:
1714:
1636:
1632:
1587:
1581:
1568:
1554:(20): 4789.
1551:
1546:
1542:
1536:
1519:
1514:
1510:
1504:
1485:
1479:
1460:
1424:
1418:
1409:
1404:
1393:. Retrieved
1383:
1371:. Retrieved
1366:
1356:
1344:. Retrieved
1340:
1330:
1309:
1301:
1294:
1286:
1254:
1250:
1223:
1219:
1215:
1211:
1207:
1203:
1189:
1184:
1180:
1176:
1158:
1150:
1146:
1118:trans effect
1109:
1097:
1093:
1071:
1058:
1053:
1040:
1034:
999:
995:
979:
975:
971:
968:diphosphenes
962:
952:
948:
947:
926:
916:
912:
904:
900:
896:
892:
888:
884:
880:
876:
872:
871:Because the
870:
865:
861:
856:
852:
848:
844:
840:
836:
826:
816:
815:
810:
806:
802:
798:
790:
786:
782:
778:
776:
770:
767:CIP priority
757:E–Z notation
748:
744:
735:
731:
727:
715:
699:
695:
687:
683:
681:
671:
667:
656:
648:
643:
639:
627:
625:
620:
616:
612:
608:
605:
600:
596:
594:
589:
585:
581:
578:elaidic acid
570:
491:
487:
481:
474:elaidic acid
467:
455:
432:fumaric acid
425:
413:
384:
368:
364:
360:
352:
348:
344:
340:
334:
326:
320:
296:
290:
261:
257:
256:
243:
237:
198:
194:
190:
186:
183:diastereomer
175:double bonds
170:
166:
165:Very often,
164:
154:
150:
149:
147:isomerism".
144:
140:
134:
132:" are used.
129:
125:
114:
110:
103:
99:
94:isomers are
91:
87:
83:
75:
71:
67:
63:
57:
53:
52:
51:
45:
34:
1335:Hunt, Ian.
1114:transplatin
1061:isomerism.
512:maleic acid
420:maleic acid
299:-2-pentene
118:descriptors
2062:Categories
1395:2010-06-22
1346:3 November
1293:Entry for
1278:References
1175:.) In the
737:cis effect
636:solubility
634:and lower
574:oleic acid
462:oleic acid
337:pent-2-ene
293:-2-pentene
48:-but-2-ene
37:-but-2-ene
2073:Isomerism
1888:Secondary
1810:isomerism
1722:isomerism
1690:Chirality
1272:Trans fat
1106:cisplatin
1059:cis-trans
1043:-diazene
909:but-2-ene
714:dataset,
678:Stability
646:alkenes.
203:but-2-ene
151:Cis–trans
60:isomerism
1923:Catenane
1892:tertiary
1748:notation
1639:isomers"
1604:97528124
1257:notation
1234:See also
1112:isomer (
1037:-diazene
964:Diazenes
959:Diazenes
832:entgegen
822:zusammen
751:notation
1881:, cyclo
1744:–
1373:7 April
1173:ligands
992:alkanes
988:alkynes
984:alkenes
650:Vicinal
500:CH=CHCO
128:" and "
107:isomers
70:" and "
2037:Λ-, Δ-
1929:catena
1682:Topics
1602:
1492:
1467:
1439:
1262:Isomer
1226:using
1149:- and
773:isomer
595:Thus,
526:white
490:- and
359:. The
1916:spiro
1786:ortho
1757:)-, (
1732:trans
1720:trans
1637:trans
1600:S2CID
1578:(PDF)
1306:IUPAC
1216:trans
1185:trans
1151:trans
1110:trans
1076:Pt(NH
1035:trans
996:trans
976:trans
953:trans
930:IUPAC
913:trans
905:trans
901:trans
877:trans
849:trans
811:trans
783:trans
732:trans
700:trans
684:trans
668:trans
640:trans
628:trans
617:Trans
613:trans
597:trans
590:trans
586:trans
565:3.03
523:white
520:color
492:trans
468:trans
426:trans
385:trans
365:trans
353:trans
345:trans
327:trans
297:trans
262:trans
238:trans
199:trans
191:trans
171:trans
145:trans
137:IUPAC
115:trans
104:trans
92:trans
84:trans
72:trans
58:trans
46:trans
1979:), (
1959:), (
1902:tert
1890:and
1838:anti
1816:endo
1804:Endo
1794:para
1790:meta
1543:Anti
1490:ISBN
1465:ISBN
1437:ISBN
1375:2015
1348:2023
1202:(or
1159:For
986:and
895:and
879:and
801:and
576:and
562:1.90
556:, pK
537:286
351:and
260:and
130:anti
113:and
102:and
23:and
2019:-,
1900:-,
1898:sec
1879:neo
1875:iso
1856:mer
1852:fac
1834:syn
1820:exo
1818:,
1808:exo
1784:- (
1780:-,
1776:-,
1730:-,
1728:cis
1716:cis
1633:cis
1592:doi
1556:doi
1552:119
1524:doi
1511:cis
1429:doi
1320:doi
1318:".
1295:cis
1224:mer
1222:or
1220:fac
1214:or
1212:cis
1208:mer
1204:fac
1181:cis
1177:cis
1147:cis
1098:cis
1094:cis
1041:cis
1000:cis
990:to
980:cis
972:cis
949:Cis
893:cis
873:cis
855:or
841:cis
809:or
807:cis
779:cis
728:cis
716:cis
696:cis
688:cis
672:cis
644:cis
621:cis
609:cis
601:cis
582:cis
545:788
534:130
488:cis
456:cis
414:cis
391:).
369:cis
361:cis
349:cis
341:cis
321:cis
291:cis
258:Cis
244:cis
195:cis
187:cis
167:cis
141:cis
126:syn
111:Cis
100:Cis
88:Cis
76:cis
68:cis
54:Cis
35:cis
2064::
2001:l-
1999:,
1997:d-
1877:,
1873:,
1854:,
1836:,
1792:,
1788:,
1761:)-
1598:.
1588:78
1586:.
1580:.
1520:98
1451:^
1435:.
1365:.
1339:.
1308:,
1230:.
1084:Cl
868:.
740:.
722:,
660:HH
558:a1
548:7
496:HO
476:)
434:)
157:.
2025:-
2022:L
2016:D
1983:)
1981:s
1977:r
1975:(
1963:)
1961:S
1957:R
1955:(
1904:-
1871:n
1806:-
1796:)
1782:p
1778:m
1774:o
1759:Z
1755:E
1753:(
1746:Z
1742:E
1734:-
1718:–
1667:e
1660:t
1653:v
1635:–
1606:.
1594::
1562:.
1558::
1530:.
1526::
1498:.
1473:.
1445:.
1431::
1398:.
1377:.
1350:.
1322::
1255:Z
1253:–
1251:E
1206:–
1196:3
1194:Y
1192:3
1169:2
1167:Y
1165:4
1153:-
1086:2
1082:2
1080:)
1078:3
974:–
951:–
917:Z
897:E
889:Z
885:Z
883:–
881:E
875:–
866:E
862:Z
857:Z
853:E
845:E
837:Z
827:E
817:Z
803:Z
799:E
791:Z
789:–
787:E
781:–
771:Z
749:Z
747:–
745:E
657:J
655:(
504:H
502:2
498:2
494:-
472:(
464:)
460:(
430:(
422:)
418:(
197:–
169:–
143:–
90:–
56:–
27:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.