713:
606:, both in synthetic and natural diamonds. Three major structures can be distinguished: substitutional Ni, nickel-vacancy and nickel-vacancy complex decorated by one or more substitutional nitrogen atoms. The "nickel-vacancy" structure, also called "semi-divacancy" is specific for most large impurities in diamond and silicon (e.g., tin in silicon). Its production mechanism is generally accepted as follows: large nickel atom incorporates substitutionally, then expels a nearby carbon (creating a neighboring vacancy), and shifts in-between the two sites.
536:(CVD) techniques in an atmosphere rich in hydrogen (typical hydrogen/carbon ratio >100), under strong bombardment of growing diamond by the plasma ions. As a result, CVD diamond is always rich in hydrogen and lattice vacancies. In polycrystalline films, much of the hydrogen may be located at the boundaries between diamond 'grains', or in non-diamond carbon inclusions. Within the diamond lattice itself, hydrogen-vacancy and hydrogen-nitrogen-vacancy complexes have been identified in negative charge states by
812:. Similar to single interstitials, divacancies do not produce photoluminescence. Divacancies, in turn, anneal out at ~900 °C creating multivacancy chains detected by EPR and presumably hexavacancy rings. The latter should be invisible to most spectroscopies, and indeed, they have not been detected thus far. Annealing of vacancies changes diamond color from green to yellow-brown. Similar mechanism (vacancy aggregation) is also believed to cause brown color of plastically deformed natural diamonds.
30:
19:
463:, so its structure is well justified from the analysis of the EPR spectrum P2. This defect produces a characteristic absorption and luminescence line at 415 nm and thus does not induce color on its own. However, the N3 center is always accompanied by the N2 center, having an absorption line at 478 nm (and no luminescence). As a result, diamonds rich in N3/N2 centers are yellow in color.
776:
910:
882:
447:
926:. A and B centers upon trapping a vacancy create corresponding 2N-V (H3 and H2 centers, where H2 is simply a negatively charged H3 center) and the neutral 4N-2V (H4 center). The H2, H3 and H4 centers are important because they are present in many natural diamonds and their optical absorption can be strong enough to alter the diamond color (H3 or H4 – yellow, H2 – green).
576:
565:
418:
382:
313:
630:
852:
897:. Laboratory experiments demonstrated that annealing of type-IaB diamond at high temperatures and pressures (>2600 °C) results in break-up of the platelets and formation of dislocation loops and voidites, i.e. that voidites are a result of thermal degradation of platelets. Contrary to platelets, voidites do contain much nitrogen, in the molecular form.
742:
937:
A similar mechanism is expected for nickel, for which both substitutional and semi-divacancy configurations are reliably identified (see subsection "nickel and cobalt" above). In an unpublished study, diamonds rich in substitutional nickel were electron irradiated and annealed, with following careful
724:
has never been observed in diamond and is considered unstable. Its interaction with a regular carbon lattice atom produces a "split-interstitial", a defect where two carbon atoms share a lattice site and are covalently bonded with the carbon neighbors. This defect has been thoroughly characterized by
872:
Platelets produce sharp absorption peaks at 1359–1375 and 330 cm in IR absorption spectra; remarkably, the position of the first peak depends on the platelet size. As with dislocations, a broad photoluminescence centered at ~1000 nm was associated with platelets by direct observation in an
514:
Phosphorus could be intentionally introduced into diamond grown by chemical vapor deposition (CVD) at concentrations up to ~0.01%. Phosphorus substitutes carbon in the diamond lattice. Similar to nitrogen, phosphorus has one more electron than carbon and thus acts as a donor; however, the ionization
373:
The C center produces a characteristic infrared absorption spectrum with a sharp peak at 1344 cm and a broader feature at 1130 cm. Absorption at those peaks is routinely used to measure the concentration of single nitrogen. Another proposed way, using the UV absorption at ~260 nm, has
917:
Most important is the interaction of vacancies and interstitials with nitrogen. Carbon interstitials react with substitutional nitrogen producing a bond-centered nitrogen interstitial showing strong IR absorption at 1450 cm. Vacancies are efficiently trapped by the A, B and C nitrogen centers.
394:
The A center is probably the most common defect in natural diamonds. It consists of a neutral nearest-neighbor pair of nitrogen atoms substituting for the carbon atoms. The A center produces UV absorption threshold at ~4 eV (310 nm, i.e. invisible to eye) and thus causes no coloration. Diamond
3527:
Trusheim, Matthew E.; Pingault, Benjamin; Wan, Noel H.; Gündoğan, Mustafa; De Santis, Lorenzo; Debroux, Romain; Gangloff, Dorian; Purser, Carola; Chen, Kevin C.; Walsh, Michael; Rose, Joshua J.; Becker, Jonas N.; Lienhard, Benjamin; Bersin, Eric; Paradeisanos, Ioannis; Wang, Gang; Lyzwa, Dominika;
590:
When diamonds are grown by the high-pressure high-temperature technique, nickel, cobalt, chromium or some other metals are usually added into the growth medium to facilitate catalytically the conversion of graphite into diamond. As a result, metallic inclusions are formed. Besides, isolated nickel
438:
Similar to the A centers, B centers do not induce color, and no UV or visible absorption can be attributed to the B centers. Early assignment of the N9 absorption system to the B center have been disproven later. The B center has a characteristic IR absorption spectrum (see the infrared absorption
301:
The most common impurity in diamond is nitrogen, which can comprise up to 1% of a diamond by mass. Previously, all lattice defects in diamond were thought to be the result of structural anomalies; later research revealed nitrogen to be present in most diamonds and in many different configurations.
341:
form. Most synthetic diamonds produced by high-pressure high-temperature (HPHT) technique contain a high level of nitrogen in the C form; nitrogen impurity originates from the atmosphere or from the graphite source. One nitrogen atom per 100,000 carbon atoms will produce yellow color. Because the
546:
In natural diamonds, several hydrogen-related IR absorption peaks are commonly observed; the strongest ones are located at 1405, 3107 and 3237 cm (see IR absorption figure above). The microscopic structure of the corresponding defects is yet unknown and it is not even certain whether or not
430:
There is a general consensus that B center (sometimes called B1) consists of a carbon vacancy surrounded by four nitrogen atoms substituting for carbon atoms. This model is consistent with other experimental results, but there is no direct spectroscopic data corroborating it. Diamonds where most
123:
Whereas some acronyms are logical, such as N3 (N for natural, i.e. observed in natural diamond) or H3 (H for heated, i.e. observed after irradiation and heating), many are not. In particular, there is no clear distinction between the meaning of labels GR (general radiation), R (radiation) and TR
762:
Most high-energy particles, beside displacing carbon atom from the lattice site, also pass it enough surplus energy for a rapid migration through the lattice. However, when relatively gentle gamma irradiation is used, this extra energy is minimal. Thus the interstitials remain near the original
672:
Around the year 2000, there was a wave of attempts to dope synthetic CVD diamond films by sulfur aiming at n-type conductivity with low activation energy. Successful reports have been published, but then dismissed as the conductivity was rendered p-type instead of n-type and associated not with
638:
Silicon is a common impurity in diamond films grown by chemical vapor deposition and it originates either from silicon substrate or from silica windows or walls of the CVD reactor. It was also observed in natural diamonds in dispersed form. Isolated silicon defects have been detected in diamond
864:
via their luminescence. For a long time, platelets were tentatively associated with large nitrogen complexes — nitrogen sinks produced as a result of nitrogen aggregation at high temperatures of the diamond synthesis. However, the direct measurement of nitrogen in the platelets by
688:
The easiest way to produce intrinsic defects in diamond is by displacing carbon atoms through irradiation with high-energy particles, such as alpha (helium), beta (electrons) or gamma particles, protons, neutrons, ions, etc. The irradiation can occur in the laboratory or in nature (see
442:
Many optical peaks in diamond accidentally have similar spectral positions, which causes much confusion among gemologists. Spectroscopists use the whole spectrum rather than one peak for defect identification and consider the history of the growth and processing of individual diamond.
680:) for isolated sulfur defects in diamond. The corresponding center called W31 has been observed in natural type-Ib diamonds in small concentrations (parts per million). It was assigned to a sulfur-vacancy complex – again, as in case of nickel and silicon, a semi-divacancy site.
701:) and remaining lattice vacancies. An important difference between the vacancies and interstitials in diamond is that whereas interstitials are mobile during the irradiation, even at liquid nitrogen temperatures, however vacancies start migrating only at temperatures ~700 °C.
2805:
Nadolinny, V. A.; Yelisseyev, A. P.; Baker, J. M.; Newton, M. E.; Twitchen, D. J.; Lawson, S. C.; Yuryeva, O. P.; Feigelson, B. N. (1999). "A study of C hyperfine structure in the EPR of nickel-nitrogen-containing centres in diamond and correlation with their optical properties".
766:
Vacancy-di-interstitial pairs have been also produced, though by electron irradiation and through a different mechanism: Individual interstitials migrate during the irradiation and aggregate to form di-interstitials; this process occurs preferentially near the lattice vacancies.
595:, optical absorption and photoluminescence spectra, and the concentration of isolated nickel can reach 0.01%. This fact is by all means unusual considering the large difference in size between carbon and transition metal atoms and the superior rigidity of the diamond lattice.
649:
constitute minor fraction of total silicon. It is believed (though no proof exists) that much silicon substitutes for carbon thus becoming invisible to most spectroscopic techniques because silicon and carbon atoms have the same configuration of the outer electronic shells.
643:. Similar to other large impurities, the major form of silicon in diamond has been identified with a Si-vacancy complex (semi-divacancy site). This center is a deep donor having an ionization energy of 2 eV, and thus again is unsuitable for electronic applications.
609:
Although the physical and chemical properties of cobalt and nickel are rather similar, the concentrations of isolated cobalt in diamond are much smaller than those of nickel (parts per billion range). Several defects related to isolated cobalt have been detected by
377:
Acceptor defects in diamond ionize the fifth nitrogen electron in the C center converting it into C+ center. The latter has a characteristic IR absorption spectrum with a sharp peak at 1332 cm and broader and weaker peaks at 1115, 1046 and 950 cm.
2263:
Kociniewski, T.; Barjon, J.; Pinault, M. -A.; Jomard, F.; Lusson, A.; Ballutaud, D.; Gorochov, O.; Laroche, J. M.; Rzepka, E.; Chevallier, J.; Saguy, C. (2006). "N-type CVD diamond doped with phosphorus using the MOCVD technology for dopant incorporation".
475:. Only one percent of natural diamonds are of this type, and most are blue to grey. Boron is an acceptor in diamond: boron atoms have one less available electron than the carbon atoms; therefore, each boron atom substituting for a carbon atom creates an
112:
There is a tradition in diamond spectroscopy to label a defect-induced spectrum by a numbered acronym (e.g. GR1). This tradition has been followed in general with some notable deviations, such as A, B and C centers. Many acronyms are confusing though:
1304:
d’Haenens-Johansson, U.; Edmonds, A.; Newton, M.; Goss, J.; Briddon, P.; Baker, J.; Martineau, P.; Khan, R.; Twitchen, D.; Williams, S. D. (2010). "EPR of a defect in CVD diamond involving both silicon and hydrogen that shows preferential alignment".
212:. However, introducing any defect (even "very symmetrical", such as N-N substitutional pair) breaks the crystal symmetry resulting in defect-induced infrared absorption, which is the most common tool to measure the defect concentrations in diamond.
933:
In contrast, silicon does react with vacancies, creating the described above optical absorption at 738 nm. The assumed mechanism is trapping of migrating vacancy by substitutional silicon resulting in the Si-V (semi-divacancy) configuration.
413:
The A center shows an IR absorption spectrum with no sharp features, which is distinctly different from that of the C or B centers. Its strongest peak at 1282 cm is routinely used to estimate the nitrogen concentration in the A form.
792:, which gives diamond green, or sometimes even green–blue color (in pure diamond). The characteristic feature of this absorption is a series of sharp lines called GR1-8, where GR1 line at 741 nm is the most prominent and important.
292:
centers (OK1 and N3) have been initially assigned to nitrogen–oxygen complexes, and later to titanium-related complexes. However, the assignment is indirect and the corresponding concentrations are rather low (few parts per million).
531:
Hydrogen is one of the most technological important impurities in semiconductors, including diamond. Hydrogen-related defects are very different in natural diamond and in synthetic diamond films. Those films are produced by various
4410:
33:
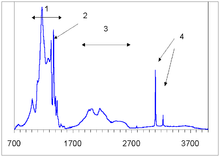
Infrared absorption spectrum of type IaB diamond. (1) region of nitrogen impurities absorption (here mostly due to the B-centers), (2) platelets peak, (3) self-absorption of diamond lattice, (4) hydrogen peaks at 3107 and 3237
905:
Extrinsic and intrinsic defects can interact producing new defect complexes. Such interaction usually occurs if a diamond containing extrinsic defects (impurities) is either plastically deformed or is irradiated and annealed.
3340:
Iwasaki, T.; Ishibashi, F.; Miyamoto, Y.; Doi, Y.; Kobayashi, S.; Miyazaki, T.; Tahara, K.; Jahnke, K. D.; Rogers, L. J.; Naydenov, B.; Jelezko, F.; Yamasaki, S.; Nagamachi, S.; Inubushi, T.; Mizuochi, N.; Hatano, M. (2015).
3039:
Aharonovich, Igor; Castelletto, Stefania; Johnson, Brett C.; McCallum, Jeffrey C.; Simpson, David A.; Greentree, Andrew D.; Prawer, Steven (2010). "Chromium single-photon emitters in diamond fabricated by ion implantation".
929:
Boron interacts with carbon interstitials forming a neutral boron–interstitial complex with a sharp optical absorption at 0.552 eV (2250 nm). No evidence is known so far (2009) for complexes of boron and vacancy.
1250:
Edmonds, A.; d’Haenens-Johansson, U.; Cruddace, R.; Newton, M.; Fu, K. -M.; Santori, C.; Beausoleil, R.; Twitchen, D.; Markham, M. (2012). "Production of oriented nitrogen-vacancy color centers in synthetic diamond".
779:
Pure diamonds, before and after irradiation and annealing. Clockwise from left bottom: 1) Initial (2×2 mm) 2–4) Irradiated by different doses of 2-MeV electrons 5–6) Irradiated by different doses and annealed at 800
859:
Most natural diamonds contain extended planar defects in the <100> lattice planes, which are called "platelets". Their size ranges from nanometers to many micrometers, and large ones are easily observed in an
633:
The semi-divacancy (impurity-vacancy) model for a large impurity in diamond (Ni, Co, Si, S, etc.), where a large pink impurity atom substitutes for two carbon atoms. Details on bonding with the diamond lattice are
3528:
Montblanch, Alejandro R-P.; Malladi, Girish; Bakhru, Hassaram; Ferrari, Andrea C.; Walmsley, Ian A.; Atatüre, Mete; Englund, Dirk (2020). "Transform-Limited
Photons from a Coherent Tin-Vacancy Spin in Diamond".
749:
The isolated split-interstitial moves through the diamond crystal during irradiation. When it meets other interstitials it aggregates into larger complexes of two and three split-interstitials, identified by
795:
The vacancy behaves as a deep electron donor/acceptor, whose electronic properties depend on the charge state. The energy level for the +/0 states is at 0.6 eV and for the 0/- states is at 2.5 eV above the
231:
Various elemental analyses of diamond reveal a wide range of impurities. They mostly originate, however, from inclusions of foreign materials in diamond, which could be nanometer-small and invisible in an
2520:
Fuchs, F.; Wild, C.; Schwarz, K.; MüLler-Sebert, W.; Koidl, P. (1995). "Hydrogen induced vibrational and electronic transitions in chemical vapor deposited diamond, identified by isotopic substitution".
4452:
3694:
Newton, M. E.; Campbell, B. A.; Twitchen, D. J.; Baker, J. M.; Anthony, T. R. (2002). "Recombination-enhanced diffusion of self-interstitial atoms and vacancy–interstitial recombination in diamond".
835:, in which the breaks occur between atoms of the same index. The dislocations produce dangling bonds which introduce energy levels into the band gap, enabling the absorption of light. Broadband blue
2626:
Teukam, Z. P.; Chevallier, J.; Saguy, C. C.; Kalish, R.; Ballutaud, D.; Barbé, M.; Jomard, F. O.; Tromson-Carli, A.; Cytermann, C.; Butler, J. E.; Bernard, M.; Baron, C. L.; Deneuville, A. (2003).
3305:
d'Haenens-Johansson, U.; Edmonds, A.; Green, B.; Newton, M.; Davies, G.; Martineau, P.; Khan, R.; Twitchen, D. (2011). "Optical properties of the neutral silicon split-vacancy center in diamond".
49:
are common. Such defects may be the result of lattice irregularities or extrinsic substitutional or interstitial impurities, introduced during or after the diamond growth. The defects affect the
2765:
Collins, A. T.; Kanda, H.; Isoya, J.; Ammerlaan, C. A. J.; Van Wyk, J. A. (1998). "Correlation between optical absorption and EPR in high-pressure diamond grown from a nickel solvent catalyst".
918:
The trapping rate is the highest for the C centers, 8 times lower for the A centers and 30 times lower for the B centers. The C center (single nitrogen) by trapping a vacancy forms the famous
543:
It is experimentally demonstrated that hydrogen passivates electrically active boron and phosphorus impurities. As a result of such passivation, shallow donor centers are presumably produced.
4818:
Lawson, S. C.; Davies, G.; Collins, A. T.; Mainwood, A. (1992). "The 'H2' optical transition in diamond: The effects of uniaxial stress perturbations, temperature and isotopic substitution".
302:
Most nitrogen enters the diamond lattice as a single atom (i.e. nitrogen-containing molecules dissociate before incorporation), however, molecular nitrogen incorporates into diamond as well.
3799:
Twitchen, D.; Newton, M.; Baker, J.; Tucker, O.; Anthony, T.; Banholzer, W. (1996). "Electron-paramagnetic-resonance measurements on the di-〈001〉-split interstitial center (R1) in diamond".
305:
Absorption of light and other material properties of diamond are highly dependent upon nitrogen content and aggregation state. Although all aggregate configurations cause absorption in the
653:
Germanium, tin and lead are normally absent in diamond, but they can be introduced during the growth or by subsequent ion implantation. Those impurities can be detected optically via the
483:. This allows red light absorption, and due to the small energy (0.37 eV) needed for the electron to leave the valence band, holes can be thermally released from the boron atoms to the
1414:
Lal, S.; Dallas, T.; Yi, S.; Gangopadhyay, S.; Holtz, M.; Anderson, F. (1996). "Defect photoluminescence in polycrystalline diamond films grown by arc-jet chemical-vapor deposition".
141:
describing the symmetry of crystals by absence of translations, and thus are much fewer in number. In diamond, only defects of the following symmetries have been observed thus far:
3466:
Iwasaki, Takayuki; Miyamoto, Yoshiyuki; Taniguchi, Takashi; Siyushev, Petr; Metsch, Mathias H.; Jelezko, Fedor; Hatano, Mutsuko (2017). "Tin-Vacancy
Quantum Emitters in Diamond".
240:. More essential are elements that can be introduced into the diamond lattice as isolated atoms (or small atomic clusters) during the diamond growth. By 2008, those elements are
2849:
Isoya, J.; Kanda, H.; Norris, J.; Tang, J.; Bowman, M. (1990). "Fourier-transform and continuous-wave EPR studies of nickel in synthetic diamond: Site and spin multiplicity".
584:
A micrograph (top) and UV-excited photoluminescence (bottom) from a synthetic diamond plate (width ~3 mm). Most of the yellow color and green emission originate from nickel.
208:
The defect symmetry allows predicting many optical properties. For example, one-phonon (infrared) absorption in pure diamond lattice is forbidden because the lattice has an
3729:
Hunt, D.; Twitchen, D.; Newton, M.; Baker, J.; Anthony, T.; Banholzer, W.; Vagarali, S. (2000). "Identification of the neutral carbon 〈100〉-split interstitial in diamond".
1340:
Hogg, R. A.; Takahei, K.; Taguchi, A.; Horikoshi, Y. (1996). "Preferential alignment of Er–2O centers in GaAs:Er,O revealed by anisotropic host-excited photoluminescence".
869:(an analytical technique of electron microscopy) revealed very little nitrogen. The currently accepted model of platelets is a large regular array of carbon interstitials.
439:
picture above) with a sharp peak at 1332 cm and a broader feature at 1280 cm. The latter is routinely used to estimate the nitrogen concentration in the B form.
4101:
Twitchen, D.; Newton, M.; Baker, J.; Anthony, T.; Banholzer, W. (1999). "Electron-paramagnetic-resonance measurements on the divacancy defect center R4/W6 in diamond".
3842:
Hunt, D.; Twitchen, D.; Newton, M.; Baker, J.; Kirui, J.; Van Wyk, J.; Anthony, T.; Banholzer, W. (2000). "EPR data on the self-interstitial complex O3 in diamond".
1915:
Tucker, O.; Newton, M.; Baker, J. (1994). "EPR and N14 electron-nuclear double-resonance measurements on the ionized nearest-neighbor dinitrogen center in diamond".
3004:
Lawson, S. C.; Kanda, H.; Watanabe, K.; Kiflawi, I.; Sato, Y.; Collins, A. T. (1996). "Spectroscopic study of cobalt-related optical centers in synthetic diamond".
309:, diamonds containing aggregated nitrogen are usually colorless, i.e. have little absorption in the visible spectrum. The four main nitrogen forms are as follows:
288:
produced during the diamond synthesis. Oxygen is believed to be a major impurity in diamond, but it has not been spectroscopically identified in diamond yet. Two
120:
Accidentally, the same labels were given to different centers detected by EPR and optical techniques (e.g., N3 EPR center and N3 optical center have no relation).
843:, however, it was noted that not all dislocations are luminescent, and there is no correlation between the dislocation type and the parameters of the emission.
4775:
Davies, G.; Nazare, M. H.; Hamer, M. F. (1976). "The H3 (2.463 eV) Vibronic Band in
Diamond: Uniaxial Stress Effects and the Breakdown of Mirror Symmetry".
3231:
Edmonds, A.; Newton, M.; Martineau, P.; Twitchen, D.; Williams, S. (2008). "Electron paramagnetic resonance studies of silicon-related defects in diamond".
4982:
Goss, J.; Jones, R.; Breuer, S.; Briddon, P.; Öberg, S. (1996). "The Twelve-Line 1.682 eV Luminescence Center in
Diamond and the Vacancy-Silicon Complex".
459:
The N3 center consists of three nitrogen atoms surrounding a vacancy. Its concentration is always just a fraction of the A and B centers. The N3 center is
3093:
Aharonovich, I.; Castelletto, S.; Simpson, D. A.; Greentree, A. D.; Prawer, S. (2010). "Photophysics of chromium-related diamond single-photon emitters".
1108:
506:
blue after exposure to shortwave ultraviolet light. Apart from optical absorption, boron acceptors have been detected by electron paramagnetic resonance.
3589:
Sakaguchi, I.; n.-Gamo, M.; Kikuchi, Y.; Yasu, E.; Haneda, H.; Suzuki, T.; Ando, T. (1999). "Sulfur: A donor dopant for n-type diamond semiconductors".
788:
is the most studied defect in diamond, both experimentally and theoretically. Its most important practical property is optical absorption, like in the
1610:
2477:
Glover, C.; Newton, M.; Martineau, P.; Twitchen, D.; Baker, J. (2003). "Hydrogen
Incorporation in Diamond: The Nitrogen-Vacancy-Hydrogen Complex".
2434:
Glover, C.; Newton, M. E.; Martineau, P. M.; Quinn, S.; Twitchen, D. J. (2004). "Hydrogen incorporation in diamond: The vacancy-hydrogen complex".
1837:
Lawson, S. C.; Fisher, D.; Hunt, D. C.; Newton, M. E. (1998). "On the existence of positively charged single-substitutional nitrogen in diamond".
4947:
Collins, A. T.; Allers, L.; Wort, C. J. H.; Scarsbrook, G. A. (1994). "The annealing of radiation damage in De Beers colourless CVD diamond".
2556:
Chevallier, J.; Theys, B.; Lusson, A.; Grattepain, C.; Deneuville, A.; Gheeraert, E. (1998). "Hydrogen-boron interactions in p-type diamond".
2591:
Chevallier, J.; Jomard, F.; Teukam, Z.; Koizumi, S.; Kanda, H.; Sato, Y.; Deneuville, A.; Bernard, M. (2002). "Hydrogen in n-type diamond".
2102:
Anderson, B.; Payne, J.; Mitchell, R.K. (ed.) (1998) "The spectroscope and gemology", p. 215, Robert Hale
Limited, Clerkwood House, London.
938:
optical measurements performed after each annealing step, but no evidence for creation or enhancement of Ni-vacancy centers was obtained.
1958:
Boyd, S. R.; Kiflawi, I.; Woods, G. S. (1994). "The relationship between infrared absorption and the a defect concentration in diamond".
333:
spectra (in which they are confusingly called P1 centers). C centers impart a deep yellow to brown color; these diamonds are classed as
4226:
Hanley, P. L.; Kiflawi, I.; Lang, A. R. (1977). "On
Topographically Identifiable Sources of Cathodoluminescence in Natural Diamonds".
4178:
Hounsome, L.; Jones, R.; Martineau, P.; Fisher, D.; Shaw, M.; Briddon, P.; Öberg, S. (2006). "Origin of brown coloration in diamond".
3659:
Baker, J.; Van Wyk, J.; Goss, J.; Briddon, P. (2008). "Electron paramagnetic resonance of sulfur at a split-vacancy site in diamond".
4374:
Goss, J.; Coomer, B.; Jones, R.; Fall, C.; Briddon, P.; Öberg, S. (2003). "Extended defects in diamond: The interstitial platelet".
4861:
Mita, Y.; Nisida, Y.; Suito, K.; Onodera, A.; Yazu, S. (1990). "Photochromism of H2 and H3 centres in synthetic type Ib diamonds".
4904:
Sa, E. S. D.; Davies, G. (1977). "Uniaxial Stress
Studies of the 2.498 eV (H4), 2.417 eV and 2.536 eV Vibronic Bands in Diamond".
3411:
Trusheim, Matthew E.; Wan, Noel H.; Chen, Kevin C.; et al. (February 21, 2019). "Lead-related quantum emitters in diamond".
502:
Boron-doped diamonds transmit light down to ~250 nm and absorb some red and infrared light (hence the blue color); they may
58:
1136:
Wyk, J. A. V. (1982). "Carbon-12 hyperfine interaction of the unique carbon of the P2 (ESR) or N3 (optical) centre in diamond".
808:
Upon annealing of pure diamond at ~700 °C, vacancies migrate and form divacancies, characterized by optical absorption and
1400:
219:, defects with symmetry lower than tetrahedral align to the direction of the growth. Such alignment has also been observed in
1180:
1827:
I. Kiflawi et al. "Infrared-absorption by the single nitrogen and a defect centers in diamond" Philos. Mag. B 69 (1994) 1141
4306:
948:
704:
Vacancies and interstitials can also be produced in diamond by plastic deformation, though in much smaller concentrations.
4213:
654:
96:
is used not only to identify the defects, but also to estimate their concentration; it can also distinguish natural from
2033:
Shiryaev, A. A.; Hutchison, M. T.; Dembo, K. A.; Dembo, A. T.; Iakoubovskii, K.; Klyuev, Y. A.; Naletov, A. M. (2001).
1377:"Characterization of Defects in as-Grown CVD Diamond Films and HPHT Diamond Powders by Electron Paramagnetic Resonance"
515:
energy of phosphorus (0.6 eV) is much smaller than that of nitrogen (1.7 eV) and is small enough for room-temperature
499:). Very few boron atoms are required for this to happen—a typical ratio is one boron atom per 1,000,000 carbon atoms.
5037:
4331:
Kiflawi, I.; Lang, A. R. (1977). "Polarised infrared cathodoluminescence from platelet defects in natural diamonds".
2166:
2161:
O'Donoghue, M. (2002) "Synthetic, imitation & treated gemstones", Elsevier
Butterworth-Heinemann, Great Britain.
2107:
1765:
658:
646:
519:. This important property of phosphorus in diamond favors electronic applications, such as UV light-emitting diodes (
280:
have been unambiguously detected in diamond, but they might originate from foreign inclusions. Detection of isolated
2969:
Twitchen, D.; Baker, J.; Newton, M.; Johnston, K. (2000). "Identification of cobalt on a lattice site in diamond".
1757:
4048:"Defects in electron irradiated boron-doped diamonds investigated by positron annihilation and optical absorption"
664:
Similar to N-V centers, Si-V, Ge-V, Sn-V and Pb-V complexes all have potential applications in quantum computing.
809:
751:
726:
690:
677:
640:
611:
599:
592:
591:
and cobalt atoms incorporate into diamond lattice, as demonstrated through characteristic hyperfine structure in
537:
407:
330:
289:
73:
2628:"Shallow donors with high n-type electrical conductivity in homoepitaxial deuterated boron-doped diamond layers"
1722:
Smith, W.; Sorokin, P.; Gelles, I.; Lasher, G. (1959). "Electron-Spin
Resonance of Nitrogen Donors in Diamond".
621:
A chromium-related optical center has been detected in diamond after ion implantation and subsequent annealing.
1059:
973:
873:
electron microscope. By studying this luminescence, it was deduced that platelets have a "bandgap" of ~1.7 eV.
50:
4560:
Kiflawi, I.; Bruley, J. (2000). "The nitrogen aggregation sequence and the formation of voidites in diamond".
2383:
Koizumi, S.; Watanabe, K.; Hasegawa, M.; Kanda, H. (2001). "Ultraviolet Emission from a Diamond pn Junction".
1008:
Collins, A. T. (2003). "The detection of colour-enhanced and synthetic gem diamonds by optical spectroscopy".
2068:
Boyd, S. R.; Kiflawi, I.; Woods, G. S. (1995). "Infrared absorption by the B nitrogen aggregate in diamond".
551:
in Australia is often associated with those hydrogen defects, but again, this assignment is yet unproven.
2309:"Lattice location of phosphorus in n-type homoepitaxial diamond films grown by chemical-vapor deposition"
922:, which can be neutral or negatively charged; the negatively charged state has potential applications in
831:
break between layers of atoms with different indices (those not lying directly above each other) and the
3410:
25:
of various colors grown by the high-pressure and high-temperature technique, the diamond size is ~2 mm.
3764:
Smith, H.; Davies, G.; Newton, M.; Kanda, H. (2004). "Structure of the self-interstitial in diamond".
2225:
Ammerlaan, C. A. J.; Kemp, R. V. (1985). "Magnetic resonance spectroscopy in semiconducting diamond".
823:
are the most common structural defect in natural diamond. The two major types of dislocations are the
4439:
1205:"Alignment of Ni- and Co-related centres during the growth of high-pressure–high-temperature diamond"
533:
216:
62:
712:
657:, tin-vacancy and lead-vacancy centers, respectively, which have similar properties to those of the
4732:
Mita, Y. (1996). "Change of absorption spectra in type-Ib diamond with heavy neutron irradiation".
1087:
4228:
Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences
2184:
Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences
729:(R2 center) and optical absorption, and unlike most other defects in diamond, it does not produce
547:
those defects originate in diamond or in foreign inclusions. Gray color in some diamonds from the
1590:
978:
953:
919:
492:
38:
4411:"The relationship between platelet size and the B′ infrared peak of natural diamonds revisited"
3624:
Kalish, R.; Reznik, A.; Uzan-Saguy, C.; Cytermann, C. (2000). "Is sulfur a donor in diamond?".
1082:
855:
Electron micrograph of platelets in diamond viewed normal to the cubic axis. Image width 1.5 μm
676:
So far (2009), there is only one reliable evidence (through hyperfine interaction structure in
4595:
Kiflawi, I.; Mainwood, A.; Kanda, H.; Fisher, D. (1996). "Nitrogen interstitials in diamond".
1880:
Davies, G. (1976). "The A nitrogen aggregate in diamond-its symmetry and possible structure".
1677:"Nitrogen incorporation in diamond films homoepitaxially grown by chemical vapour deposition"
1632:
Newton, M. E.; Baker, J. M. (1989). "N ENDOR of the OK1 centre in natural type Ib diamond".
4991:
4956:
4913:
4870:
4827:
4784:
4741:
4698:
4651:
4604:
4569:
4534:
4487:
4418:
4383:
4340:
4290:
4235:
4187:
4152:
4110:
4059:
4017:
3978:
3969:
Iakoubovskii, K.; Kiflawi, I.; Johnston, K.; Collins, A.; Davies, G.; Stesmans, A. (2003).
3935:
3893:
3851:
3808:
3773:
3738:
3703:
3668:
3633:
3598:
3547:
3485:
3364:
3314:
3279:
3240:
3194:
3159:
3112:
3059:
3013:
2978:
2943:
2908:
2858:
2815:
2774:
2736:
2697:
2639:
2600:
2565:
2530:
2486:
2443:
2392:
2357:
2320:
2273:
2234:
2191:
2128:
2077:
2042:
2007:
1967:
1924:
1889:
1846:
1794:
1731:
1688:
1641:
1563:
1517:
1470:
1423:
1388:
1349:
1314:
1270:
1216:
1145:
1074:
1017:
968:
496:
2182:
Collins, A. T. (1993). "The Optical and Electronic Properties of Semiconducting Diamond".
2119:
Thomaz, M. F.; Davies, G. (1978). "The Decay Time of N3 Luminescence in Natural Diamond".
8:
4071:
963:
894:
840:
721:
548:
354:"; that is, each substituting nitrogen has an extra electron to donate and forms a donor
101:
93:
85:
4995:
4960:
4917:
4874:
4831:
4788:
4745:
4702:
4655:
4608:
4573:
4538:
4491:
4422:
4387:
4344:
4294:
4239:
4191:
4156:
4114:
4063:
4021:
3982:
3947:
3939:
3897:
3855:
3812:
3777:
3742:
3707:
3672:
3637:
3602:
3551:
3489:
3368:
3318:
3283:
3244:
3198:
3163:
3116:
3063:
3017:
2982:
2947:
2912:
2862:
2819:
2778:
2740:
2701:
2643:
2604:
2569:
2534:
2490:
2447:
2396:
2361:
2324:
2277:
2238:
2195:
2132:
2081:
2046:
2011:
1971:
1928:
1893:
1850:
1798:
1735:
1692:
1645:
1567:
1521:
1474:
1427:
1392:
1353:
1318:
1274:
1220:
1149:
1078:
1021:
53:
and determine to which type a diamond is assigned; the most dramatic effects are on the
4929:
4886:
4843:
4800:
4714:
4667:
4503:
4444:
4356:
4251:
4083:
3951:
3571:
3537:
3509:
3475:
3422:
3385:
3354:
3342:
3128:
3102:
3075:
3049:
2831:
2663:
2416:
2289:
2207:
2144:
1862:
1810:
1704:
1657:
1533:
1486:
1286:
1260:
1232:
1100:
861:
673:
sulfur, but with residual boron, which is a highly efficient p-type dopant in diamond.
516:
233:
4663:
4581:
3715:
3171:
2827:
2786:
2709:
2612:
2246:
2054:
2019:
1998:
Collins, A. T. (1999). "Things we still don't know about optical centres in diamond".
1858:
1700:
1529:
1228:
1157:
1096:
1029:
763:
vacancies and form vacancy-interstitials pairs identified through optical absorption.
5007:
4968:
4890:
4882:
4847:
4839:
4757:
4718:
4710:
4686:
4671:
4639:
4620:
4522:
4507:
4475:
4448:
4255:
4140:
4075:
4005:
3970:
3923:
3881:
3824:
3563:
3513:
3501:
3440:
3413:
3390:
3267:
3210:
3147:
2896:
2874:
2835:
2724:
2685:
2655:
2502:
2459:
2408:
2369:
2211:
2162:
2103:
2034:
1940:
1901:
1866:
1814:
1806:
1782:
1761:
1708:
1676:
1661:
1653:
1537:
1505:
1490:
1482:
1458:
1439:
1376:
1290:
1236:
1204:
1176:
1104:
983:
923:
836:
730:
698:
615:
603:
97:
81:
22:
4933:
4804:
4047:
3955:
3575:
3185:
Clark, C.; Kanda, H.; Kiflawi, I.; Sittas, G. (1995). "Silicon defects in diamond".
3132:
3079:
2667:
2627:
2420:
2293:
2148:
1554:
Kaiser, W.; Bond, W. (1959). "Nitrogen, A Major Impurity in Common Type I Diamond".
435:; most gem diamonds contain a mixture of A and B centers, together with N3 centers.
5032:
4999:
4964:
4921:
4906:
Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences
4878:
4835:
4792:
4777:
Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences
4749:
4706:
4659:
4612:
4577:
4542:
4495:
4434:
4426:
4391:
4360:
4348:
4298:
4243:
4195:
4160:
4118:
4087:
4067:
4025:
3986:
3943:
3901:
3859:
3816:
3781:
3746:
3711:
3676:
3641:
3606:
3559:
3555:
3497:
3493:
3448:
3432:
3380:
3372:
3322:
3287:
3248:
3202:
3167:
3120:
3067:
3021:
2986:
2951:
2916:
2866:
2823:
2782:
2744:
2705:
2647:
2608:
2573:
2538:
2494:
2451:
2400:
2365:
2328:
2281:
2242:
2199:
2136:
2121:
Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences
2085:
2050:
2015:
1975:
1932:
1897:
1854:
1802:
1739:
1696:
1649:
1602:
1591:"Titanium-related color centers in diamond: a density functional theory prediction"
1571:
1525:
1478:
1431:
1396:
1357:
1322:
1278:
1224:
1153:
1092:
1025:
237:
220:
2934:
Watkins, G. (1975). "Defects in irradiated silicon: EPR of the tin-vacancy pair".
2498:
2455:
4430:
3924:"Electron irradiation and the formation of vacancy–interstitial pairs in diamond"
503:
367:
89:
42:
5003:
4275:
3990:
3146:
Iakoubovskii, K.; Adriaenssens, G. J.; Dogadkin, N. N.; Shiryaev, A. A. (2001).
325:
The C center corresponds to electrically neutral single substitutional nitrogen
4395:
4199:
4164:
3905:
3785:
3680:
3436:
3326:
3252:
3124:
3071:
2920:
2748:
1589:
Czelej, K.; Ćwieka, K.; Śpiewadoi, Piotr; Kurzydłowskia, Krzysztof Jan (2018).
1326:
1282:
785:
694:
488:
351:
209:
4753:
4616:
4122:
3610:
3291:
3206:
2089:
1979:
1936:
1435:
215:
In synthetic diamond grown by the high-pressure high-temperature synthesis or
5026:
3863:
3820:
3750:
3444:
2955:
2870:
2577:
1743:
958:
828:
540:. In addition, numerous hydrogen-related IR absorption peaks are documented.
476:
460:
363:
54:
4546:
4499:
4302:
3452:
2404:
1575:
5011:
4925:
4796:
4521:
Chen, J. H.; Bernaerts, D.; Seo, J. W.; Van Tendeloo, G.; Kagi, H. (1998).
4247:
4079:
4029:
3567:
3505:
3394:
2659:
2506:
2463:
2412:
2285:
2203:
2140:
839:
has been reliably identified with dislocations by direct observation in an
797:
789:
484:
480:
355:
77:
69:
4761:
4624:
4214:
Investigation of band gap energy states at dislocations in natural diamond
3828:
3214:
2990:
2878:
1944:
1443:
893:
nanometer-sized clusters present in many natural diamonds, as revealed by
3304:
3148:"Optical characterization of some irradiation-induced centers in diamond"
2348:
Farrer, R. G. (1969). "On the substitutional nitrogen donor in diamond".
820:
403:
142:
138:
134:
4006:"Electron spin resonance study of perturbed di-interstitials in diamond"
3145:
1249:
284:
in diamond has later been re-interpreted in terms of micro-particles of
4476:"Characterization of platelet-related infrared luminescence in diamond"
4409:
Speich, L.; Kohn, S.C.; Wirth, R.; Bulanova, G.P.; Smith, C.B. (2017).
1606:
890:
257:
178:
150:
3376:
3092:
2333:
2308:
1783:"Optical transitions at the substitutional nitrogen centre in diamond"
1401:
10.1002/1521-396X(200108)186:2<199::AID-PSSA199>3.0.CO;2-R
900:
4352:
3645:
3038:
3025:
2542:
1361:
639:
lattice through the sharp optical absorption peak at 738 nm and
410:
spectrum W24, whose analysis unambiguously proves the N=N structure.
273:
194:
29:
4216:. Cavendish Laboratory, University of Cambridge; Cambridge, England.
2651:
1303:
3542:
3480:
3427:
3359:
745:
One of the configurations of the carbon di-interstitials in diamond
359:
343:
338:
306:
277:
249:
241:
158:
3968:
3922:
Kiflawi, I.; Collins, A. T.; Iakoubovskii, K.; Fisher, D. (2007).
3107:
3054:
2897:"Ni-vacancy defect in diamond detected by electron spin resonance"
1588:
1265:
471:
Diamonds containing boron as a substitutional impurity are termed
1506:"Comment on 'Evidence for a Fe-related defect centre in diamond'"
253:
170:
46:
4684:
3921:
3465:
18:
4273:
3623:
2555:
754:(R1 and O3 centers), optical absorption and photoluminescence.
406:, but if ionized by UV light or deep acceptors, it produces an
347:
337:
and are commonly known as "canary diamonds", which are rare in
269:
265:
261:
4274:
Kiflawi, I.; Bruley, J.; Luyten, W.; Van Tendeloo, G. (1998).
3230:
2804:
2262:
395:
containing nitrogen predominantly in the A form as classed as
236:. Also, virtually any element can be hammered into diamond by
3879:
2590:
2519:
2476:
1674:
775:
245:
4685:
Iakoubovskii, K.; Adriaenssens, G. J.; Nesladek, M. (2000).
4520:
4177:
3882:"Evidence for vacancy-interstitial pairs in Ib-type diamond"
2382:
1675:
Iakoubovskii, K.; Adriaenssens, G. J.; Vohra, Y. K. (2000).
851:
4946:
4817:
3693:
3588:
3526:
3339:
2968:
2625:
2032:
1339:
909:
881:
866:
446:
326:
285:
281:
4594:
4100:
3003:
2764:
2725:"Vibronic effects in the 1.4-eV optical center in diamond"
2433:
417:
381:
312:
3798:
575:
564:
520:
4637:
4473:
4276:"'Natural' and 'man-made' platelets in type-Ia diamonds"
3265:
2683:
1780:
1721:
1503:
1456:
697:(carbon atoms knocked off their normal lattice sites to
629:
133:
The symmetry of defects in crystals is described by the
4408:
4003:
3841:
3728:
3658:
3184:
1413:
885:
Electron micrograph showing several octahedral voidites
4981:
4860:
4638:
Iakoubovskii, Konstantin; Adriaenssens, Guy J (2001).
4141:"Dominant paramagnetic centers in O-implanted diamond"
4004:
Iakoubovskii, K.; Baker, J. M.; Newton, M. E. (2004).
3880:
Iakoubovskii, K.; Dannefaer, S.; Stesmans, A. (2005).
3763:
1836:
487:
even at room temperatures. These holes can move in an
4687:"Photochromism of vacancy-related centres in diamond"
4045:
3971:"Annealing of vacancies and interstitials in diamond"
2686:"Optical characterization of natural Argyle diamonds"
2306:
2035:"High-temperature high-pressure annealing of diamond"
741:
479:
in the band gap that can accept an electron from the
431:
nitrogen forms B centers are rare and are classed as
4138:
2848:
1459:"Evidence for a Fe-related defect centre in diamond"
1374:
1173:
Optical Properties of Diamond : A Data Handbook
4373:
3343:"Germanium-Vacancy Single Color Centers in Diamond"
1202:
901:
Interaction between intrinsic and extrinsic defects
624:
2722:
598:Numerous Ni-related defects have been detected by
68:The defects can be detected by different types of
4774:
4225:
1914:
757:
716:Model of the carbon split-interstitial in diamond
329:in the diamond lattice. These are easily seen in
5024:
2067:
1957:
1060:"Optical absorption and luminescence in diamond"
92:(IR), visible and UV parts of the spectrum. The
707:
117:Some symbols are too similar (e.g., 3H and H3).
4474:Iakoubovskii, K.; Adriaenssens, G. J. (2000).
2684:Iakoubovskii, K.; Adriaenssens, G. J. (2002).
2307:Hasegawa, M.; Teraji, T.; Koizumi, S. (2001).
2258:
2256:
1781:Iakoubovskii, K.; Adriaenssens, G. J. (2000).
1504:Iakoubovskii, K.; Adriaenssens, G. J. (2002).
1457:Iakoubovskii, K.; Adriaenssens, G. J. (2002).
1170:
554:
107:
4640:"Trapping of vacancies by defects in diamond"
4559:
4523:"Voidites in polycrystalline natural diamond"
2224:
3268:"Luminescence excitation spectra in diamond"
2894:
2118:
4330:
3333:
3266:Iakoubovskii, K.; Adriaenssens, G. (2000).
2253:
1993:
1991:
1989:
1631:
3226:
3224:
1553:
1003:
1001:
999:
4440:1983/34ba5767-e947-43d2-a4da-41dd88455f70
4438:
4134:
4132:
3541:
3479:
3426:
3384:
3358:
3106:
3053:
2679:
2677:
2332:
2227:Journal of Physics C: Solid State Physics
1882:Journal of Physics C: Solid State Physics
1549:
1547:
1264:
1203:Iakoubovskii, K.; Collins, A. T. (2004).
1138:Journal of Physics C: Solid State Physics
1086:
803:
736:
4903:
4046:Dannefaer, S.; Iakoubovskii, K. (2008).
2800:
2798:
2796:
2177:
2175:
1986:
1053:
1051:
1049:
1047:
1045:
1043:
1041:
1039:
908:
880:
850:
774:
740:
711:
628:
445:
416:
380:
366:can excite the donor electrons into the
311:
28:
17:
4402:
4269:
4267:
4265:
4139:Iakoubovskii, K.; Stesmans, A. (2002).
3221:
2933:
2376:
2181:
1997:
1756:Nassau, Kurt (1980) "Gems made by man"
1375:Iakoubovskii, K.; Stesmans, A. (2001).
1198:
1196:
1194:
1192:
1007:
996:
350:atoms they replace), they act as "deep
5025:
4312:from the original on February 12, 2019
4129:
3406:
3404:
2890:
2888:
2674:
2347:
1879:
1544:
1164:
1057:
691:Diamond enhancement – Irradiation
618:, but their structure is yet unknown.
88:, CL), and absorption of light in the
4041:
4039:
3917:
3915:
3875:
3873:
2793:
2760:
2758:
2723:Iakoubovskii, K.; Davies, G. (2004).
2172:
1776:
1774:
1036:
693:); it produces primary defects named
454:
4863:Journal of Physics: Condensed Matter
4820:Journal of Physics: Condensed Matter
4731:
4691:Journal of Physics: Condensed Matter
4644:Journal of Physics: Condensed Matter
4262:
4052:Journal of Physics: Condensed Matter
3928:Journal of Physics: Condensed Matter
2808:Journal of Physics: Condensed Matter
1839:Journal of Physics: Condensed Matter
1787:Journal of Physics: Condensed Matter
1681:Journal of Physics: Condensed Matter
1634:Journal of Physics: Condensed Matter
1510:Journal of Physics: Condensed Matter
1463:Journal of Physics: Condensed Matter
1209:Journal of Physics: Condensed Matter
1189:
1131:
1129:
949:Chemical vapor deposition of diamond
683:
425:
389:
374:later been discarded as unreliable.
320:
226:
3401:
2885:
1135:
770:
342:nitrogen atoms have five available
223:and thus is not unique to diamond.
13:
4455:from the original on June 30, 2023
4212:Kolodzie, A.T. and Bleloch, A.L.
4036:
3912:
3870:
2755:
1771:
1613:from the original on June 30, 2023
1114:from the original on July 23, 2018
913:Schematic of the H3 and H2 centers
128:
14:
5049:
1873:
1126:
370:, resulting in the yellow color.
1758:Gemological Institute of America
625:Silicon, germanium, tin and lead
574:
563:
4975:
4940:
4897:
4854:
4811:
4768:
4725:
4678:
4631:
4588:
4553:
4514:
4467:
4367:
4324:
4219:
4206:
4171:
4094:
3997:
3962:
3835:
3792:
3757:
3722:
3687:
3652:
3617:
3582:
3520:
3459:
3298:
3259:
3178:
3139:
3086:
3032:
2997:
2962:
2927:
2842:
2716:
2619:
2584:
2549:
2513:
2470:
2427:
2341:
2300:
2218:
2155:
2112:
2096:
2061:
2026:
1951:
1908:
1830:
1821:
1750:
1715:
1668:
1625:
1582:
1497:
1450:
1407:
815:
810:electron paramagnetic resonance
752:electron paramagnetic resonance
727:electron paramagnetic resonance
678:electron paramagnetic resonance
641:electron paramagnetic resonance
612:electron paramagnetic resonance
600:electron paramagnetic resonance
593:electron paramagnetic resonance
538:electron paramagnetic resonance
408:electron paramagnetic resonance
362:. Light with energy above ~2.2
331:electron paramagnetic resonance
290:electron paramagnetic resonance
74:electron paramagnetic resonance
4527:Philosophical Magazine Letters
4480:Philosophical Magazine Letters
4072:10.1088/0953-8984/20/23/235225
3560:10.1103/PhysRevLett.124.023602
3498:10.1103/PhysRevLett.119.253601
1368:
1333:
1297:
1243:
1067:Reports on Progress in Physics
974:Material properties of diamond
758:Vacancy-interstitial complexes
51:material properties of diamond
1:
4949:Diamond and Related Materials
4582:10.1016/S0925-9635(99)00265-4
4562:Diamond and Related Materials
3948:10.1088/0953-8984/19/4/046216
3716:10.1016/S0925-9635(01)00623-9
3696:Diamond and Related Materials
3172:10.1016/S0925-9635(00)00361-7
3152:Diamond and Related Materials
2787:10.1016/S0925-9635(97)00270-7
2767:Diamond and Related Materials
2710:10.1016/S0925-9635(01)00533-7
2690:Diamond and Related Materials
2613:10.1016/S0925-9635(02)00063-8
2593:Diamond and Related Materials
2499:10.1103/PhysRevLett.90.185507
2456:10.1103/PhysRevLett.92.135502
2055:10.1016/S0921-4526(01)00750-5
2020:10.1016/S0925-9635(99)00013-8
2000:Diamond and Related Materials
1030:10.1016/S0925-9635(03)00262-0
1010:Diamond and Related Materials
990:
509:
4969:10.1016/0925-9635(94)90302-6
4431:10.1016/j.lithos.2017.02.010
2370:10.1016/0038-1098(69)90593-6
1760:, Santa Monica, California,
846:
708:Isolated carbon interstitial
7:
5004:10.1103/PhysRevLett.77.3041
4664:10.1088/0953-8984/13/26/316
3991:10.1016/j.physb.2003.09.005
3975:Physica B: Condensed Matter
2828:10.1088/0953-8984/11/38/314
2247:10.1088/0022-3719/18/13/009
2039:Physica B: Condensed Matter
1859:10.1088/0953-8984/10/27/016
1701:10.1088/0953-8984/12/30/106
1530:10.1088/0953-8984/14/21/401
1229:10.1088/0953-8984/16/39/022
1158:10.1088/0022-3719/15/27/007
1097:10.1088/0034-4885/42/10/001
941:
876:
555:Nickel, cobalt and chromium
526:
296:
108:Labeling of diamond centers
10:
5054:
4883:10.1088/0953-8984/2/43/002
4840:10.1088/0953-8984/4/13/008
4711:10.1088/0953-8984/12/2/308
4396:10.1103/PhysRevB.67.165208
4200:10.1103/PhysRevB.73.125203
4165:10.1103/PhysRevB.66.045406
3906:10.1103/PhysRevB.71.233201
3786:10.1103/PhysRevB.69.045203
3681:10.1103/PhysRevB.78.235203
3437:10.1103/PHYSREVB.99.075430
3327:10.1103/PhysRevB.84.245208
3253:10.1103/PhysRevB.77.245205
3125:10.1103/PhysRevA.81.043813
3072:10.1103/PhysRevB.81.121201
3006:Journal of Applied Physics
2921:10.1103/PhysRevB.70.205211
2749:10.1103/PhysRevB.70.245206
2350:Solid State Communications
1902:10.1088/0022-3719/9/19/005
1807:10.1088/0953-8984/12/6/102
1654:10.1088/0953-8984/1/51/024
1483:10.1088/0953-8984/14/4/104
1327:10.1103/PhysRevB.82.155205
1283:10.1103/PhysRevB.86.035201
450:Schematic of the N3 center
4754:10.1103/PhysRevB.53.11360
4617:10.1103/PhysRevB.54.16719
4123:10.1103/PhysRevB.59.12900
3611:10.1103/PhysRevB.60.R2139
3292:10.1103/PhysRevB.61.10174
3207:10.1103/PhysRevB.51.16681
2895:Iakoubovskii, K. (2004).
2090:10.1080/13642819508239089
1980:10.1080/01418639408240185
1937:10.1103/PhysRevB.50.15586
1436:10.1103/PhysRevB.54.13428
667:
602:, optical absorption and
534:chemical vapor deposition
421:Schematic of the B center
385:Schematic of the A center
316:Schematic of the C center
217:chemical vapor deposition
63:electronic band structure
5038:Crystallographic defects
4283:Philosophical Magazine B
3864:10.1103/PhysRevB.62.6587
3821:10.1103/PhysRevB.54.6988
3751:10.1103/PhysRevB.61.3863
2956:10.1103/PhysRevB.12.4383
2871:10.1103/PhysRevB.41.3905
2578:10.1103/PhysRevB.58.7966
2070:Philosophical Magazine B
1960:Philosophical Magazine B
1744:10.1103/PhysRev.115.1546
466:
84:, PL) or electron beam (
4984:Physical Review Letters
4547:10.1080/095008398178561
4500:10.1080/095008300403594
4303:10.1080/014186398258104
4010:Physica Status Solidi A
3626:Applied Physics Letters
3530:Physical Review Letters
3468:Physical Review Letters
2523:Applied Physics Letters
2479:Physical Review Letters
2436:Physical Review Letters
2405:10.1126/science.1060258
2313:Applied Physics Letters
2266:Physica Status Solidi A
1576:10.1103/PhysRev.115.857
1381:Physica Status Solidi A
1342:Applied Physics Letters
1171:Zaitsev, A. M. (2001).
979:Nitrogen-vacancy center
954:Crystallographic defect
920:nitrogen-vacancy center
493:electrically conductive
491:and render the diamond
137:. They differ from the
59:electrical conductivity
4926:10.1098/rspa.1977.0165
4797:10.1098/rspa.1976.0140
4248:10.1098/rsta.1977.0012
4030:10.1002/pssa.200405163
2286:10.1002/pssa.200671113
2204:10.1098/rsta.1993.0017
2141:10.1098/rspa.1978.0141
914:
886:
856:
804:Multivacancy complexes
781:
746:
737:Interstitial complexes
717:
635:
451:
422:
386:
317:
61:, as explained by the
35:
26:
2991:10.1103/PhysRevB.61.9
912:
884:
854:
778:
744:
715:
632:
449:
420:
384:
315:
32:
21:
4417:. 278–281: 419–426.
2041:. 308–310: 598–603.
1016:(10–11): 1976–1983.
969:Gemstone irradiation
497:p-type semiconductor
124:(type-II radiation).
4996:1996PhRvL..77.3041G
4961:1994DRM.....3..932C
4918:1977RSPSA.357..231S
4875:1990JPCM....2.8567M
4832:1992JPCM....4.3439L
4789:1976RSPSA.351..245D
4746:1996PhRvB..5311360M
4740:(17): 11360–11364.
4703:2000JPCM...12..189I
4656:2001JPCM...13.6015I
4609:1996PhRvB..5416719K
4603:(23): 16719–16726.
4574:2000DRM.....9...87K
4539:1998PMagL..77..135H
4492:2000PMagL..80..441A
4423:2017Litho.278..419S
4388:2003PhRvB..67p5208G
4345:1977Natur.267...36K
4295:1998PMagB..78..299K
4240:1977RSPTA.284..329H
4192:2006PhRvB..73l5203H
4157:2002PhRvB..66d5406I
4115:1999PhRvB..5912900T
4064:2008JPCM...20w5225D
4022:2004PSSAR.201.2516I
3983:2003PhyB..340...67I
3940:2007JPCM...19d6216K
3898:2005PhRvB..71w3201I
3856:2000PhRvB..62.6587H
3813:1996PhRvB..54.6988T
3778:2004PhRvB..69d5203S
3743:2000PhRvB..61.3863H
3708:2002DRM....11..618N
3673:2008PhRvB..78w5203B
3638:2000ApPhL..76..757K
3603:1999PhRvB..60.2139S
3552:2020PhRvL.124b3602T
3490:2017PhRvL.119y3601I
3369:2015NatSR...512882I
3319:2011PhRvB..84x5208D
3284:2000PhRvB..6110174I
3245:2008PhRvB..77x5205E
3199:1995PhRvB..5116681C
3193:(23): 16681–16688.
3164:2001DRM....10...18I
3117:2010PhRvA..81d3813A
3064:2010PhRvB..81l1201A
3018:1996JAP....79.4348L
2983:2000PhRvB..61....9T
2948:1975PhRvB..12.4383W
2913:2004PhRvB..70t5211I
2863:1990PhRvB..41.3905I
2820:1999JPCM...11.7357N
2779:1998DRM.....7..333C
2741:2004PhRvB..70x5206I
2702:2002DRM....11..125I
2644:2003NatMa...2..482T
2605:2002DRM....11.1566C
2570:1998PhRvB..58.7966C
2535:1995ApPhL..66..177F
2491:2003PhRvL..90r5507G
2448:2004PhRvL..92m5502G
2397:2001Sci...292.1899K
2391:(5523): 1899–1901.
2362:1969SSCom...7..685F
2325:2001ApPhL..79.3068H
2278:2006PSSAR.203.3136K
2239:1985JPhC...18.2623A
2196:1993RSPTA.342..233C
2133:1978RSPSA.362..405T
2082:1995PMagB..72..351B
2047:2001PhyB..308..598S
2012:1999DRM.....8.1455C
1972:1994PMagB..69.1149B
1929:1994PhRvB..5015586T
1923:(21): 15586–15596.
1894:1976JPhC....9L.537D
1851:1998JPCM...10.6171L
1799:2000JPCM...12L..77I
1736:1959PhRv..115.1546S
1693:2000JPCM...12L.519I
1646:1989JPCM....110549N
1568:1959PhRv..115..857K
1522:2002JPCM...14R.401I
1475:2002JPCM...14L..95I
1428:1996PhRvB..5413428L
1422:(19): 13428–13431.
1393:2001PSSAR.186..199I
1354:1996ApPhL..68.3317H
1319:2010PhRvB..82o5205D
1275:2012PhRvB..86c5201E
1221:2004JPCM...16.6897I
1150:1982JPhC...15L.981V
1079:1979RPPh...42.1605W
1058:Walker, J. (1979).
1022:2003DRM....12.1976C
964:Diamond enhancement
895:electron microscopy
841:electron microscope
523:, at 235 nm).
346:(one more than the
94:absorption spectrum
86:cathodoluminescence
3977:. 340–342: 67–75.
3347:Scientific Reports
2006:(8–9): 1455–1462.
1607:10.1039/C8TC00097B
915:
887:
862:optical microscope
857:
782:
747:
718:
699:interstitial sites
636:
517:thermal ionization
455:N3 nitrogen center
452:
423:
387:
318:
234:optical microscope
80:induced by light (
36:
27:
23:Synthetic diamonds
4990:(14): 3041–3044.
4734:Physical Review B
4597:Physical Review B
4376:Physical Review B
4180:Physical Review B
4145:Physical Review B
4103:Physical Review B
3886:Physical Review B
3844:Physical Review B
3807:(10): 6988–6998.
3801:Physical Review B
3766:Physical Review B
3731:Physical Review B
3661:Physical Review B
3591:Physical Review B
3414:Physical Review B
3377:10.1038/srep12882
3307:Physical Review B
3272:Physical Review B
3233:Physical Review B
3187:Physical Review B
3095:Physical Review A
3042:Physical Review B
2971:Physical Review B
2942:(10): 4383–4390.
2936:Physical Review B
2901:Physical Review B
2851:Physical Review B
2729:Physical Review B
2558:Physical Review B
2334:10.1063/1.1417514
2190:(1664): 233–244.
1917:Physical Review B
1888:(19): L537–L542.
1601:(19): 5261–5268.
1595:J. Mater. Chem. C
1416:Physical Review B
1307:Physical Review B
1253:Physical Review B
1182:978-3-540-66582-3
1144:(27): L981–L983.
1073:(10): 1605–1659.
984:Synthetic diamond
924:quantum computing
837:photoluminescence
731:photoluminescence
684:Intrinsic defects
659:Si-vacancy center
655:germanium-vacancy
616:photoluminescence
604:photoluminescence
426:B-nitrogen center
390:A-nitrogen center
321:C-nitrogen center
227:Extrinsic defects
102:enhanced diamonds
82:photoluminescence
5045:
5016:
5015:
4979:
4973:
4972:
4944:
4938:
4937:
4901:
4895:
4894:
4858:
4852:
4851:
4815:
4809:
4808:
4772:
4766:
4765:
4729:
4723:
4722:
4682:
4676:
4675:
4635:
4629:
4628:
4592:
4586:
4585:
4557:
4551:
4550:
4518:
4512:
4511:
4471:
4465:
4464:
4462:
4460:
4442:
4406:
4400:
4399:
4371:
4365:
4364:
4353:10.1038/267036a0
4328:
4322:
4321:
4319:
4317:
4311:
4280:
4271:
4260:
4259:
4223:
4217:
4210:
4204:
4203:
4175:
4169:
4168:
4136:
4127:
4126:
4098:
4092:
4091:
4043:
4034:
4033:
4001:
3995:
3994:
3966:
3960:
3959:
3919:
3910:
3909:
3877:
3868:
3867:
3839:
3833:
3832:
3796:
3790:
3789:
3761:
3755:
3754:
3726:
3720:
3719:
3691:
3685:
3684:
3656:
3650:
3649:
3646:10.1063/1.125885
3621:
3615:
3614:
3586:
3580:
3579:
3545:
3524:
3518:
3517:
3483:
3463:
3457:
3456:
3430:
3408:
3399:
3398:
3388:
3362:
3337:
3331:
3330:
3302:
3296:
3295:
3263:
3257:
3256:
3228:
3219:
3218:
3182:
3176:
3175:
3143:
3137:
3136:
3110:
3090:
3084:
3083:
3057:
3036:
3030:
3029:
3026:10.1063/1.361744
3001:
2995:
2994:
2966:
2960:
2959:
2931:
2925:
2924:
2892:
2883:
2882:
2857:(7): 3905–3913.
2846:
2840:
2839:
2802:
2791:
2790:
2762:
2753:
2752:
2720:
2714:
2713:
2681:
2672:
2671:
2632:Nature Materials
2623:
2617:
2616:
2588:
2582:
2581:
2553:
2547:
2546:
2543:10.1063/1.113126
2517:
2511:
2510:
2474:
2468:
2467:
2431:
2425:
2424:
2380:
2374:
2373:
2345:
2339:
2338:
2336:
2304:
2298:
2297:
2260:
2251:
2250:
2222:
2216:
2215:
2179:
2170:
2159:
2153:
2152:
2116:
2110:
2100:
2094:
2093:
2065:
2059:
2058:
2030:
2024:
2023:
1995:
1984:
1983:
1955:
1949:
1948:
1912:
1906:
1905:
1877:
1871:
1870:
1834:
1828:
1825:
1819:
1818:
1778:
1769:
1754:
1748:
1747:
1719:
1713:
1712:
1672:
1666:
1665:
1629:
1623:
1622:
1620:
1618:
1586:
1580:
1579:
1551:
1542:
1541:
1501:
1495:
1494:
1454:
1448:
1447:
1411:
1405:
1404:
1372:
1366:
1365:
1362:10.1063/1.116043
1337:
1331:
1330:
1301:
1295:
1294:
1268:
1247:
1241:
1240:
1200:
1187:
1186:
1168:
1162:
1161:
1133:
1124:
1123:
1121:
1119:
1113:
1090:
1064:
1055:
1034:
1033:
1005:
771:Isolated vacancy
578:
567:
402:The A center is
238:ion implantation
221:gallium arsenide
210:inversion center
5053:
5052:
5048:
5047:
5046:
5044:
5043:
5042:
5023:
5022:
5019:
4980:
4976:
4945:
4941:
4902:
4898:
4859:
4855:
4816:
4812:
4773:
4769:
4730:
4726:
4683:
4679:
4636:
4632:
4593:
4589:
4558:
4554:
4519:
4515:
4472:
4468:
4458:
4456:
4407:
4403:
4372:
4368:
4329:
4325:
4315:
4313:
4309:
4278:
4272:
4263:
4224:
4220:
4211:
4207:
4176:
4172:
4137:
4130:
4099:
4095:
4044:
4037:
4002:
3998:
3967:
3963:
3920:
3913:
3878:
3871:
3840:
3836:
3797:
3793:
3762:
3758:
3727:
3723:
3692:
3688:
3657:
3653:
3622:
3618:
3587:
3583:
3525:
3521:
3464:
3460:
3409:
3402:
3338:
3334:
3303:
3299:
3264:
3260:
3229:
3222:
3183:
3179:
3144:
3140:
3091:
3087:
3037:
3033:
3002:
2998:
2967:
2963:
2932:
2928:
2893:
2886:
2847:
2843:
2803:
2794:
2763:
2756:
2721:
2717:
2682:
2675:
2652:10.1038/nmat929
2624:
2620:
2589:
2585:
2554:
2550:
2518:
2514:
2475:
2471:
2432:
2428:
2381:
2377:
2346:
2342:
2305:
2301:
2261:
2254:
2223:
2219:
2180:
2173:
2160:
2156:
2117:
2113:
2101:
2097:
2066:
2062:
2031:
2027:
1996:
1987:
1956:
1952:
1913:
1909:
1878:
1874:
1835:
1831:
1826:
1822:
1779:
1772:
1755:
1751:
1724:Physical Review
1720:
1716:
1673:
1669:
1630:
1626:
1616:
1614:
1587:
1583:
1556:Physical Review
1552:
1545:
1502:
1498:
1455:
1451:
1412:
1408:
1373:
1369:
1338:
1334:
1302:
1298:
1248:
1244:
1201:
1190:
1183:
1169:
1165:
1134:
1127:
1117:
1115:
1111:
1062:
1056:
1037:
1006:
997:
993:
988:
944:
903:
879:
849:
818:
806:
773:
760:
739:
710:
695:Frenkel defects
686:
670:
627:
588:
587:
586:
585:
581:
580:
579:
570:
569:
568:
557:
529:
512:
469:
457:
428:
392:
368:conduction band
323:
299:
229:
204:
200:
192:
188:
184:
176:
168:
164:
156:
148:
131:
129:Defect symmetry
110:
43:crystal lattice
12:
11:
5:
5051:
5041:
5040:
5035:
5018:
5017:
4974:
4939:
4896:
4853:
4810:
4767:
4724:
4677:
4630:
4587:
4552:
4513:
4466:
4401:
4382:(16): 165208.
4366:
4323:
4261:
4218:
4205:
4186:(12): 125203.
4170:
4128:
4093:
4058:(23): 235225.
4035:
3996:
3961:
3911:
3892:(23): 233201.
3869:
3834:
3791:
3756:
3721:
3686:
3667:(23): 235203.
3651:
3616:
3581:
3519:
3474:(25): 253601.
3458:
3400:
3332:
3313:(24): 245208.
3297:
3258:
3239:(24): 245205.
3220:
3177:
3138:
3085:
3048:(12): 121201.
3031:
2996:
2961:
2926:
2907:(20): 205211.
2884:
2841:
2792:
2754:
2735:(24): 245206.
2715:
2673:
2638:(7): 482–486.
2618:
2583:
2548:
2512:
2485:(18): 185507.
2469:
2442:(13): 135502.
2426:
2375:
2356:(9): 685–688.
2340:
2299:
2252:
2217:
2171:
2154:
2111:
2095:
2060:
2025:
1985:
1950:
1907:
1872:
1829:
1820:
1770:
1749:
1714:
1667:
1624:
1581:
1543:
1496:
1449:
1406:
1367:
1332:
1313:(15): 155205.
1296:
1242:
1188:
1181:
1163:
1125:
1088:10.1.1.467.443
1035:
994:
992:
989:
987:
986:
981:
976:
971:
966:
961:
956:
951:
945:
943:
940:
902:
899:
878:
875:
848:
845:
817:
814:
805:
802:
772:
769:
759:
756:
738:
735:
709:
706:
685:
682:
669:
666:
626:
623:
583:
582:
573:
572:
571:
562:
561:
560:
559:
558:
556:
553:
528:
525:
511:
508:
489:electric field
468:
465:
456:
453:
427:
424:
391:
388:
322:
319:
298:
295:
228:
225:
202:
198:
190:
186:
182:
174:
166:
162:
154:
146:
130:
127:
126:
125:
121:
118:
109:
106:
9:
6:
4:
3:
2:
5050:
5039:
5036:
5034:
5031:
5030:
5028:
5021:
5013:
5009:
5005:
5001:
4997:
4993:
4989:
4985:
4978:
4970:
4966:
4962:
4958:
4954:
4950:
4943:
4935:
4931:
4927:
4923:
4919:
4915:
4912:(1689): 231.
4911:
4907:
4900:
4892:
4888:
4884:
4880:
4876:
4872:
4868:
4864:
4857:
4849:
4845:
4841:
4837:
4833:
4829:
4825:
4821:
4814:
4806:
4802:
4798:
4794:
4790:
4786:
4783:(1665): 245.
4782:
4778:
4771:
4763:
4759:
4755:
4751:
4747:
4743:
4739:
4735:
4728:
4720:
4716:
4712:
4708:
4704:
4700:
4696:
4692:
4688:
4681:
4673:
4669:
4665:
4661:
4657:
4653:
4649:
4645:
4641:
4634:
4626:
4622:
4618:
4614:
4610:
4606:
4602:
4598:
4591:
4583:
4579:
4575:
4571:
4567:
4563:
4556:
4548:
4544:
4540:
4536:
4532:
4528:
4524:
4517:
4509:
4505:
4501:
4497:
4493:
4489:
4485:
4481:
4477:
4470:
4454:
4450:
4446:
4441:
4436:
4432:
4428:
4424:
4420:
4416:
4412:
4405:
4397:
4393:
4389:
4385:
4381:
4377:
4370:
4362:
4358:
4354:
4350:
4346:
4342:
4338:
4334:
4327:
4308:
4304:
4300:
4296:
4292:
4288:
4284:
4277:
4270:
4268:
4266:
4257:
4253:
4249:
4245:
4241:
4237:
4234:(1324): 329.
4233:
4229:
4222:
4215:
4209:
4201:
4197:
4193:
4189:
4185:
4181:
4174:
4166:
4162:
4158:
4154:
4151:(4): 045406.
4150:
4146:
4142:
4135:
4133:
4124:
4120:
4116:
4112:
4109:(20): 12900.
4108:
4104:
4097:
4089:
4085:
4081:
4077:
4073:
4069:
4065:
4061:
4057:
4053:
4049:
4042:
4040:
4031:
4027:
4023:
4019:
4015:
4011:
4007:
4000:
3992:
3988:
3984:
3980:
3976:
3972:
3965:
3957:
3953:
3949:
3945:
3941:
3937:
3934:(4): 046216.
3933:
3929:
3925:
3918:
3916:
3907:
3903:
3899:
3895:
3891:
3887:
3883:
3876:
3874:
3865:
3861:
3857:
3853:
3849:
3845:
3838:
3830:
3826:
3822:
3818:
3814:
3810:
3806:
3802:
3795:
3787:
3783:
3779:
3775:
3772:(4): 045203.
3771:
3767:
3760:
3752:
3748:
3744:
3740:
3736:
3732:
3725:
3717:
3713:
3709:
3705:
3701:
3697:
3690:
3682:
3678:
3674:
3670:
3666:
3662:
3655:
3647:
3643:
3639:
3635:
3631:
3627:
3620:
3612:
3608:
3604:
3600:
3596:
3592:
3585:
3577:
3573:
3569:
3565:
3561:
3557:
3553:
3549:
3544:
3539:
3536:(2): 023602.
3535:
3531:
3523:
3515:
3511:
3507:
3503:
3499:
3495:
3491:
3487:
3482:
3477:
3473:
3469:
3462:
3454:
3450:
3446:
3442:
3438:
3434:
3429:
3424:
3420:
3416:
3415:
3407:
3405:
3396:
3392:
3387:
3382:
3378:
3374:
3370:
3366:
3361:
3356:
3352:
3348:
3344:
3336:
3328:
3324:
3320:
3316:
3312:
3308:
3301:
3293:
3289:
3285:
3281:
3278:(15): 10174.
3277:
3273:
3269:
3262:
3254:
3250:
3246:
3242:
3238:
3234:
3227:
3225:
3216:
3212:
3208:
3204:
3200:
3196:
3192:
3188:
3181:
3173:
3169:
3165:
3161:
3157:
3153:
3149:
3142:
3134:
3130:
3126:
3122:
3118:
3114:
3109:
3104:
3101:(4): 043813.
3100:
3096:
3089:
3081:
3077:
3073:
3069:
3065:
3061:
3056:
3051:
3047:
3043:
3035:
3027:
3023:
3019:
3015:
3011:
3007:
3000:
2992:
2988:
2984:
2980:
2976:
2972:
2965:
2957:
2953:
2949:
2945:
2941:
2937:
2930:
2922:
2918:
2914:
2910:
2906:
2902:
2898:
2891:
2889:
2880:
2876:
2872:
2868:
2864:
2860:
2856:
2852:
2845:
2837:
2833:
2829:
2825:
2821:
2817:
2813:
2809:
2801:
2799:
2797:
2788:
2784:
2780:
2776:
2772:
2768:
2761:
2759:
2750:
2746:
2742:
2738:
2734:
2730:
2726:
2719:
2711:
2707:
2703:
2699:
2695:
2691:
2687:
2680:
2678:
2669:
2665:
2661:
2657:
2653:
2649:
2645:
2641:
2637:
2633:
2629:
2622:
2614:
2610:
2606:
2602:
2598:
2594:
2587:
2579:
2575:
2571:
2567:
2563:
2559:
2552:
2544:
2540:
2536:
2532:
2528:
2524:
2516:
2508:
2504:
2500:
2496:
2492:
2488:
2484:
2480:
2473:
2465:
2461:
2457:
2453:
2449:
2445:
2441:
2437:
2430:
2422:
2418:
2414:
2410:
2406:
2402:
2398:
2394:
2390:
2386:
2379:
2371:
2367:
2363:
2359:
2355:
2351:
2344:
2335:
2330:
2326:
2322:
2318:
2314:
2310:
2303:
2295:
2291:
2287:
2283:
2279:
2275:
2271:
2267:
2259:
2257:
2248:
2244:
2240:
2236:
2232:
2228:
2221:
2213:
2209:
2205:
2201:
2197:
2193:
2189:
2185:
2178:
2176:
2168:
2167:0-7506-3173-2
2164:
2158:
2150:
2146:
2142:
2138:
2134:
2130:
2127:(1710): 405.
2126:
2122:
2115:
2109:
2108:0-7198-0261-X
2105:
2099:
2091:
2087:
2083:
2079:
2075:
2071:
2064:
2056:
2052:
2048:
2044:
2040:
2036:
2029:
2021:
2017:
2013:
2009:
2005:
2001:
1994:
1992:
1990:
1981:
1977:
1973:
1969:
1965:
1961:
1954:
1946:
1942:
1938:
1934:
1930:
1926:
1922:
1918:
1911:
1903:
1899:
1895:
1891:
1887:
1883:
1876:
1868:
1864:
1860:
1856:
1852:
1848:
1844:
1840:
1833:
1824:
1816:
1812:
1808:
1804:
1800:
1796:
1792:
1788:
1784:
1777:
1775:
1767:
1766:0-87311-016-1
1763:
1759:
1753:
1745:
1741:
1737:
1733:
1729:
1725:
1718:
1710:
1706:
1702:
1698:
1694:
1690:
1686:
1682:
1678:
1671:
1663:
1659:
1655:
1651:
1647:
1643:
1640:(51): 10549.
1639:
1635:
1628:
1612:
1608:
1604:
1600:
1596:
1592:
1585:
1577:
1573:
1569:
1565:
1561:
1557:
1550:
1548:
1539:
1535:
1531:
1527:
1523:
1519:
1515:
1511:
1507:
1500:
1492:
1488:
1484:
1480:
1476:
1472:
1468:
1464:
1460:
1453:
1445:
1441:
1437:
1433:
1429:
1425:
1421:
1417:
1410:
1402:
1398:
1394:
1390:
1386:
1382:
1378:
1371:
1363:
1359:
1355:
1351:
1347:
1343:
1336:
1328:
1324:
1320:
1316:
1312:
1308:
1300:
1292:
1288:
1284:
1280:
1276:
1272:
1267:
1262:
1259:(3): 035201.
1258:
1254:
1246:
1238:
1234:
1230:
1226:
1222:
1218:
1214:
1210:
1206:
1199:
1197:
1195:
1193:
1184:
1178:
1174:
1167:
1159:
1155:
1151:
1147:
1143:
1139:
1132:
1130:
1110:
1106:
1102:
1098:
1094:
1089:
1084:
1080:
1076:
1072:
1068:
1061:
1054:
1052:
1050:
1048:
1046:
1044:
1042:
1040:
1031:
1027:
1023:
1019:
1015:
1011:
1004:
1002:
1000:
995:
985:
982:
980:
977:
975:
972:
970:
967:
965:
962:
960:
959:Diamond color
957:
955:
952:
950:
947:
946:
939:
935:
931:
927:
925:
921:
911:
907:
898:
896:
892:
889:Voidites are
883:
874:
870:
868:
863:
853:
844:
842:
838:
834:
830:
826:
822:
813:
811:
801:
799:
793:
791:
790:color centers
787:
777:
768:
764:
755:
753:
743:
734:
732:
728:
723:
714:
705:
702:
700:
696:
692:
681:
679:
674:
665:
662:
660:
656:
651:
648:
644:
642:
631:
622:
619:
617:
613:
607:
605:
601:
596:
594:
577:
566:
552:
550:
544:
541:
539:
535:
524:
522:
518:
507:
505:
500:
498:
494:
490:
486:
482:
478:
477:electron hole
474:
464:
462:
448:
444:
440:
436:
434:
419:
415:
411:
409:
405:
400:
398:
383:
379:
375:
371:
369:
365:
361:
357:
353:
349:
345:
340:
336:
332:
328:
314:
310:
308:
303:
294:
291:
287:
283:
279:
275:
271:
267:
263:
259:
255:
251:
247:
243:
239:
235:
224:
222:
218:
213:
211:
206:
196:
180:
172:
160:
152:
144:
140:
136:
122:
119:
116:
115:
114:
105:
103:
99:
95:
91:
87:
83:
79:
75:
71:
66:
64:
60:
56:
55:diamond color
52:
48:
44:
40:
39:Imperfections
31:
24:
20:
16:
5020:
4987:
4983:
4977:
4955:(4–6): 932.
4952:
4948:
4942:
4909:
4905:
4899:
4869:(43): 8567.
4866:
4862:
4856:
4826:(13): 3439.
4823:
4819:
4813:
4780:
4776:
4770:
4737:
4733:
4727:
4694:
4690:
4680:
4650:(26): 6015.
4647:
4643:
4633:
4600:
4596:
4590:
4565:
4561:
4555:
4530:
4526:
4516:
4483:
4479:
4469:
4457:. Retrieved
4414:
4404:
4379:
4375:
4369:
4339:(5606): 36.
4336:
4332:
4326:
4316:February 12,
4314:. Retrieved
4286:
4282:
4231:
4227:
4221:
4208:
4183:
4179:
4173:
4148:
4144:
4106:
4102:
4096:
4055:
4051:
4016:(11): 2516.
4013:
4009:
3999:
3974:
3964:
3931:
3927:
3889:
3885:
3850:(10): 6587.
3847:
3843:
3837:
3804:
3800:
3794:
3769:
3765:
3759:
3734:
3730:
3724:
3702:(3–6): 618.
3699:
3695:
3689:
3664:
3660:
3654:
3629:
3625:
3619:
3597:(4): R2139.
3594:
3590:
3584:
3533:
3529:
3522:
3471:
3467:
3461:
3418:
3412:
3350:
3346:
3335:
3310:
3306:
3300:
3275:
3271:
3261:
3236:
3232:
3190:
3186:
3180:
3155:
3151:
3141:
3098:
3094:
3088:
3045:
3041:
3034:
3009:
3005:
2999:
2974:
2970:
2964:
2939:
2935:
2929:
2904:
2900:
2854:
2850:
2844:
2814:(38): 7357.
2811:
2807:
2773:(2–5): 333.
2770:
2766:
2732:
2728:
2718:
2693:
2689:
2635:
2631:
2621:
2596:
2592:
2586:
2564:(12): 7966.
2561:
2557:
2551:
2526:
2522:
2515:
2482:
2478:
2472:
2439:
2435:
2429:
2388:
2384:
2378:
2353:
2349:
2343:
2319:(19): 3068.
2316:
2312:
2302:
2272:(12): 3136.
2269:
2265:
2233:(13): 2623.
2230:
2226:
2220:
2187:
2183:
2157:
2124:
2120:
2114:
2098:
2073:
2069:
2063:
2038:
2028:
2003:
1999:
1963:
1959:
1953:
1920:
1916:
1910:
1885:
1881:
1875:
1845:(27): 6171.
1842:
1838:
1832:
1823:
1790:
1786:
1752:
1727:
1723:
1717:
1687:(30): L519.
1684:
1680:
1670:
1637:
1633:
1627:
1617:November 24,
1615:. Retrieved
1598:
1594:
1584:
1559:
1555:
1516:(21): 5459.
1513:
1509:
1499:
1466:
1462:
1452:
1419:
1415:
1409:
1384:
1380:
1370:
1348:(23): 3317.
1345:
1341:
1335:
1310:
1306:
1299:
1256:
1252:
1245:
1215:(39): 6897.
1212:
1208:
1175:. Springer.
1172:
1166:
1141:
1137:
1118:February 12,
1116:. Retrieved
1070:
1066:
1013:
1009:
936:
932:
928:
916:
904:
888:
871:
858:
832:
824:
821:Dislocations
819:
816:Dislocations
807:
798:valence band
794:
783:
765:
761:
748:
722:interstitial
719:
703:
687:
675:
671:
663:
652:
647:Si-vacancies
645:
637:
620:
608:
597:
589:
545:
542:
530:
513:
504:phosphoresce
501:
485:valence band
481:valence band
472:
470:
461:paramagnetic
458:
441:
437:
432:
429:
412:
401:
396:
393:
376:
372:
356:energy level
334:
324:
304:
300:
268:and perhaps
230:
214:
207:
139:space groups
135:point groups
132:
111:
78:luminescence
72:, including
70:spectroscopy
67:
37:
15:
3737:(6): 3863.
3012:(8): 4348.
2599:(8): 1566.
1966:(6): 1149.
1730:(6): 1546.
833:shuffle set
827:, in which
549:Argyle mine
404:diamagnetic
358:within the
143:tetrahedral
5027:Categories
4697:(2): 189.
4533:(3): 135.
4486:(6): 441.
4459:August 11,
4289:(3): 299.
3632:(6): 757.
3543:1811.07777
3481:1708.03576
3453:Q105746117
3428:1805.12202
3360:1503.04938
2696:(1): 125.
2529:(2): 177.
2076:(3): 351.
1793:(6): L77.
1562:(4): 857.
1469:(4): L95.
1387:(2): 199.
991:References
891:octahedral
634:uncertain.
510:Phosphorus
258:phosphorus
179:monoclinic
151:tetragonal
4891:250878505
4848:250824280
4719:250820432
4672:250804678
4568:(1): 87.
4508:138243923
4449:131914804
4256:120959202
3514:206305034
3445:0163-1829
3353:: 12882.
3158:(1): 18.
3108:0909.1873
3055:1001.4373
2836:250759333
2212:202574625
1867:250841408
1815:250734213
1709:250888444
1662:250823139
1538:250752181
1491:250871851
1291:118609894
1266:1112.5757
1237:250836558
1105:250857323
1083:CiteSeerX
847:Platelets
825:glide set
784:Isolated
720:Isolated
495:(i.e., a
344:electrons
274:Manganese
195:triclinic
98:synthetic
5012:10062116
4934:98842822
4805:93034755
4453:Archived
4307:Archived
4080:21694316
3956:97742332
3576:53959613
3568:32004012
3506:29303349
3449:Wikidata
3395:26250337
3133:54887620
3080:56165312
2977:(1): 9.
2668:21797000
2660:12876564
2507:12786024
2464:15089622
2421:10675358
2413:11397942
2294:96415838
2149:98179513
1768:, p. 191
1611:Archived
1109:Archived
942:See also
877:Voidites
527:Hydrogen
473:type IIb
433:type IaB
397:type IaA
360:band gap
307:infrared
297:Nitrogen
278:tungsten
250:hydrogen
242:nitrogen
159:trigonal
90:infrared
5033:Diamond
4992:Bibcode
4957:Bibcode
4914:Bibcode
4871:Bibcode
4828:Bibcode
4785:Bibcode
4762:9982752
4742:Bibcode
4699:Bibcode
4652:Bibcode
4625:9985801
4605:Bibcode
4570:Bibcode
4535:Bibcode
4488:Bibcode
4419:Bibcode
4384:Bibcode
4361:4277090
4341:Bibcode
4291:Bibcode
4236:Bibcode
4188:Bibcode
4153:Bibcode
4111:Bibcode
4088:2988243
4060:Bibcode
4018:Bibcode
3979:Bibcode
3936:Bibcode
3894:Bibcode
3852:Bibcode
3829:9984317
3809:Bibcode
3774:Bibcode
3739:Bibcode
3704:Bibcode
3669:Bibcode
3634:Bibcode
3599:Bibcode
3548:Bibcode
3486:Bibcode
3386:4528202
3365:Bibcode
3315:Bibcode
3280:Bibcode
3241:Bibcode
3215:9978673
3195:Bibcode
3160:Bibcode
3113:Bibcode
3060:Bibcode
3014:Bibcode
2979:Bibcode
2944:Bibcode
2909:Bibcode
2879:9994206
2859:Bibcode
2816:Bibcode
2775:Bibcode
2737:Bibcode
2698:Bibcode
2640:Bibcode
2601:Bibcode
2566:Bibcode
2531:Bibcode
2487:Bibcode
2444:Bibcode
2393:Bibcode
2385:Science
2358:Bibcode
2321:Bibcode
2274:Bibcode
2235:Bibcode
2192:Bibcode
2169:, p. 52
2129:Bibcode
2078:Bibcode
2043:Bibcode
2008:Bibcode
1968:Bibcode
1945:9975922
1925:Bibcode
1890:Bibcode
1847:Bibcode
1795:Bibcode
1732:Bibcode
1689:Bibcode
1642:Bibcode
1564:Bibcode
1518:Bibcode
1471:Bibcode
1444:9985239
1424:Bibcode
1389:Bibcode
1350:Bibcode
1315:Bibcode
1271:Bibcode
1217:Bibcode
1146:Bibcode
1075:Bibcode
1018:Bibcode
786:vacancy
335:type Ib
254:silicon
171:rhombic
76:(EPR),
47:diamond
41:in the
5010:
4932:
4889:
4846:
4803:
4760:
4717:
4670:
4623:
4506:
4447:
4415:Lithos
4359:
4333:Nature
4254:
4086:
4078:
3954:
3827:
3574:
3566:
3512:
3504:
3451:
3443:
3393:
3383:
3213:
3131:
3078:
2877:
2834:
2666:
2658:
2505:
2462:
2419:
2411:
2292:
2210:
2165:
2147:
2106:
1943:
1865:
1813:
1764:
1707:
1660:
1536:
1489:
1442:
1289:
1235:
1179:
1103:
1085:
668:Sulfur
352:donors
348:carbon
270:sulfur
266:cobalt
262:nickel
193:) and
4930:S2CID
4887:S2CID
4844:S2CID
4801:S2CID
4715:S2CID
4668:S2CID
4504:S2CID
4445:S2CID
4357:S2CID
4310:(PDF)
4279:(PDF)
4252:S2CID
4084:S2CID
3952:S2CID
3572:S2CID
3538:arXiv
3510:S2CID
3476:arXiv
3423:arXiv
3421:(7).
3355:arXiv
3129:S2CID
3103:arXiv
3076:S2CID
3050:arXiv
2832:S2CID
2664:S2CID
2417:S2CID
2290:S2CID
2208:S2CID
2145:S2CID
1863:S2CID
1811:S2CID
1705:S2CID
1658:S2CID
1534:S2CID
1487:S2CID
1287:S2CID
1261:arXiv
1233:S2CID
1112:(PDF)
1101:S2CID
1063:(PDF)
829:bonds
467:Boron
327:atoms
246:boron
5008:PMID
4758:PMID
4621:PMID
4461:2019
4318:2019
4076:PMID
3825:PMID
3564:PMID
3502:PMID
3441:ISSN
3391:PMID
3211:PMID
2875:PMID
2656:PMID
2503:PMID
2460:PMID
2409:PMID
2163:ISBN
2104:ISBN
1941:PMID
1762:ISBN
1619:2020
1440:PMID
1177:ISBN
1120:2019
867:EELS
614:and
521:LEDs
286:ruby
282:iron
276:and
201:or C
57:and
5000:doi
4965:doi
4922:doi
4910:357
4879:doi
4836:doi
4793:doi
4781:351
4750:doi
4707:doi
4660:doi
4613:doi
4578:doi
4543:doi
4496:doi
4435:hdl
4427:doi
4392:doi
4349:doi
4337:267
4299:doi
4244:doi
4232:284
4196:doi
4161:doi
4119:doi
4068:doi
4026:doi
4014:201
3987:doi
3944:doi
3902:doi
3860:doi
3817:doi
3782:doi
3747:doi
3712:doi
3677:doi
3642:doi
3607:doi
3556:doi
3534:124
3494:doi
3472:119
3433:doi
3381:PMC
3373:doi
3323:doi
3288:doi
3249:doi
3203:doi
3168:doi
3121:doi
3068:doi
3022:doi
2987:doi
2952:doi
2917:doi
2867:doi
2824:doi
2783:doi
2745:doi
2706:doi
2648:doi
2609:doi
2574:doi
2539:doi
2495:doi
2452:doi
2401:doi
2389:292
2366:doi
2329:doi
2282:doi
2270:203
2243:doi
2200:doi
2188:342
2137:doi
2125:362
2086:doi
2051:doi
2016:doi
1976:doi
1933:doi
1898:doi
1855:doi
1803:doi
1740:doi
1728:115
1697:doi
1650:doi
1603:doi
1572:doi
1560:115
1526:doi
1479:doi
1432:doi
1397:doi
1385:186
1358:doi
1323:doi
1279:doi
1225:doi
1154:doi
1093:doi
1026:doi
780:°C.
339:gem
205:).
189:, C
185:, C
177:),
169:),
165:, C
157:),
149:),
100:or
45:of
5029::
5006:.
4998:.
4988:77
4986:.
4963:.
4951:.
4928:.
4920:.
4908:.
4885:.
4877:.
4865:.
4842:.
4834:.
4822:.
4799:.
4791:.
4779:.
4756:.
4748:.
4738:53
4736:.
4713:.
4705:.
4695:12
4693:.
4689:.
4666:.
4658:.
4648:13
4646:.
4642:.
4619:.
4611:.
4601:54
4599:.
4576:.
4564:.
4541:.
4531:77
4529:.
4525:.
4502:.
4494:.
4484:80
4482:.
4478:.
4451:.
4443:.
4433:.
4425:.
4413:.
4390:.
4380:67
4378:.
4355:.
4347:.
4335:.
4305:.
4297:.
4287:78
4285:.
4281:.
4264:^
4250:.
4242:.
4230:.
4194:.
4184:73
4182:.
4159:.
4149:66
4147:.
4143:.
4131:^
4117:.
4107:59
4105:.
4082:.
4074:.
4066:.
4056:20
4054:.
4050:.
4038:^
4024:.
4012:.
4008:.
3985:.
3973:.
3950:.
3942:.
3932:19
3930:.
3926:.
3914:^
3900:.
3890:71
3888:.
3884:.
3872:^
3858:.
3848:62
3846:.
3823:.
3815:.
3805:54
3803:.
3780:.
3770:69
3768:.
3745:.
3735:61
3733:.
3710:.
3700:11
3698:.
3675:.
3665:78
3663:.
3640:.
3630:76
3628:.
3605:.
3595:60
3593:.
3570:.
3562:.
3554:.
3546:.
3532:.
3508:.
3500:.
3492:.
3484:.
3470:.
3447:.
3439:.
3431:.
3419:99
3417:.
3403:^
3389:.
3379:.
3371:.
3363:.
3349:.
3345:.
3321:.
3311:84
3309:.
3286:.
3276:61
3274:.
3270:.
3247:.
3237:77
3235:.
3223:^
3209:.
3201:.
3191:51
3189:.
3166:.
3156:10
3154:.
3150:.
3127:.
3119:.
3111:.
3099:81
3097:.
3074:.
3066:.
3058:.
3046:81
3044:.
3020:.
3010:79
3008:.
2985:.
2975:61
2973:.
2950:.
2940:12
2938:.
2915:.
2905:70
2903:.
2899:.
2887:^
2873:.
2865:.
2855:41
2853:.
2830:.
2822:.
2812:11
2810:.
2795:^
2781:.
2769:.
2757:^
2743:.
2733:70
2731:.
2727:.
2704:.
2694:11
2692:.
2688:.
2676:^
2662:.
2654:.
2646:.
2634:.
2630:.
2607:.
2597:11
2595:.
2572:.
2562:58
2560:.
2537:.
2527:66
2525:.
2501:.
2493:.
2483:90
2481:.
2458:.
2450:.
2440:92
2438:.
2415:.
2407:.
2399:.
2387:.
2364:.
2352:.
2327:.
2317:79
2315:.
2311:.
2288:.
2280:.
2268:.
2255:^
2241:.
2231:18
2229:.
2206:.
2198:.
2186:.
2174:^
2143:.
2135:.
2123:.
2084:.
2074:72
2072:.
2049:.
2037:.
2014:.
2002:.
1988:^
1974:.
1964:69
1962:.
1939:.
1931:.
1921:50
1919:.
1896:.
1884:.
1861:.
1853:.
1843:10
1841:.
1809:.
1801:.
1791:12
1789:.
1785:.
1773:^
1738:.
1726:.
1703:.
1695:.
1685:12
1683:.
1679:.
1656:.
1648:.
1636:.
1609:.
1597:.
1593:.
1570:.
1558:.
1546:^
1532:.
1524:.
1514:14
1512:.
1508:.
1485:.
1477:.
1467:14
1465:.
1461:.
1438:.
1430:.
1420:54
1418:.
1395:.
1383:.
1379:.
1356:.
1346:68
1344:.
1321:.
1311:82
1309:.
1285:.
1277:.
1269:.
1257:86
1255:.
1231:.
1223:.
1213:16
1211:.
1207:.
1191:^
1152:.
1142:15
1140:.
1128:^
1107:.
1099:.
1091:.
1081:.
1071:42
1069:.
1065:.
1038:^
1024:.
1014:12
1012:.
998:^
800:.
733:.
661:.
399:.
364:eV
272:.
264:,
260:,
256:,
252:,
248:,
244:,
197:(C
187:1h
183:2h
181:(C
175:2v
173:(C
167:3v
163:3d
161:(D
155:2d
153:(D
145:(T
104:.
65:.
34:cm
5014:.
5002::
4994::
4971:.
4967::
4959::
4953:3
4936:.
4924::
4916::
4893:.
4881::
4873::
4867:2
4850:.
4838::
4830::
4824:4
4807:.
4795::
4787::
4764:.
4752::
4744::
4721:.
4709::
4701::
4674:.
4662::
4654::
4627:.
4615::
4607::
4584:.
4580::
4572::
4566:9
4549:.
4545::
4537::
4510:.
4498::
4490::
4463:.
4437::
4429::
4421::
4398:.
4394::
4386::
4363:.
4351::
4343::
4320:.
4301::
4293::
4258:.
4246::
4238::
4202:.
4198::
4190::
4167:.
4163::
4155::
4125:.
4121::
4113::
4090:.
4070::
4062::
4032:.
4028::
4020::
3993:.
3989::
3981::
3958:.
3946::
3938::
3908:.
3904::
3896::
3866:.
3862::
3854::
3831:.
3819::
3811::
3788:.
3784::
3776::
3753:.
3749::
3741::
3718:.
3714::
3706::
3683:.
3679::
3671::
3648:.
3644::
3636::
3613:.
3609::
3601::
3578:.
3558::
3550::
3540::
3516:.
3496::
3488::
3478::
3455:.
3435::
3425::
3397:.
3375::
3367::
3357::
3351:5
3329:.
3325::
3317::
3294:.
3290::
3282::
3255:.
3251::
3243::
3217:.
3205::
3197::
3174:.
3170::
3162::
3135:.
3123::
3115::
3105::
3082:.
3070::
3062::
3052::
3028:.
3024::
3016::
2993:.
2989::
2981::
2958:.
2954::
2946::
2923:.
2919::
2911::
2881:.
2869::
2861::
2838:.
2826::
2818::
2789:.
2785::
2777::
2771:7
2751:.
2747::
2739::
2712:.
2708::
2700::
2670:.
2650::
2642::
2636:2
2615:.
2611::
2603::
2580:.
2576::
2568::
2545:.
2541::
2533::
2509:.
2497::
2489::
2466:.
2454::
2446::
2423:.
2403::
2395::
2372:.
2368::
2360::
2354:7
2337:.
2331::
2323::
2296:.
2284::
2276::
2249:.
2245::
2237::
2214:.
2202::
2194::
2151:.
2139::
2131::
2092:.
2088::
2080::
2057:.
2053::
2045::
2022:.
2018::
2010::
2004:8
1982:.
1978::
1970::
1947:.
1935::
1927::
1904:.
1900::
1892::
1886:9
1869:.
1857::
1849::
1817:.
1805::
1797::
1746:.
1742::
1734::
1711:.
1699::
1691::
1664:.
1652::
1644::
1638:1
1621:.
1605::
1599:6
1578:.
1574::
1566::
1540:.
1528::
1520::
1493:.
1481::
1473::
1446:.
1434::
1426::
1403:.
1399::
1391::
1364:.
1360::
1352::
1329:.
1325::
1317::
1293:.
1281::
1273::
1263::
1239:.
1227::
1219::
1185:.
1160:.
1156::
1148::
1122:.
1095::
1077::
1032:.
1028::
1020::
203:S
199:1
191:2
147:d
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.