240:
165:
46:
37:
24:
358:
591:, should be just as effective as the latter against "diabetes, arthritis, poliomyelitis, and even cancer". Even though there is no research supporting this claim (and Koch's glyoxylide preparations were found to be just distilled water), triquinoyl is still listed as an ingredient of some
846:
1346:
1363:
371:
674:
Schröder, Detlef; Schwarz, Helmut; Dua, Suresh; Blanksby, Stephen J.; Bowie, John H. (1999). "Mass spectrometric studies of the oxocarbons C
289:
122:
1232:
939:
896:
1442:
1274:
1260:
879:
366:
1226:
609:
531:
derivative of the six ketone groups with an additional two molecules of water—a solid that decomposes at 95 °C.
254:
1402:
1246:
806:
Person, Willis B.; Williams, Dale G. (1957). "Infrared
Spectra and the Structure of Leuconic Acid and Triquinoyl".
378:
1316:
932:
844:, Worne, Howard E., "Polycarbonyls", issued 1996-01-04, assigned to Natick Chemical Industries
197:
160:
1457:
218:
1288:
841:
925:
235:
524:
1462:
1447:
584:
900:
626:
568:
448:
64:
861:
768:
1452:
1127:
780:
691:
592:
206:
142:
8:
1437:
1197:
1183:
1169:
1141:
604:
98:
88:
784:
695:
534:
In 1966, Howard E. Worne of Natick
Chemical Industries patented compounds with formulas
239:
164:
1302:
1155:
1113:
1088:
1082:
1078:
580:
703:
523:
traded under the name "cyclohexanehexone octahydrate" or equivalent names is actually
1416:
1028:
823:
750:
707:
516:
452:
1053:
1008:
998:
815:
788:
742:
699:
656:
402:
312:
186:
1099:
1018:
988:
962:
502:
464:
441:
45:
1382:
1341:
1039:
967:
771:; Polborn, Kurt; Weigand, Jan J. (2005). "Dodecahydroxycyclohexane dihydrate".
349:
792:
1431:
1387:
885:(Interview). Interviewed by Ronald T. Ottes and Fred L. Lofsvold. p. 31.
827:
711:
153:
36:
451:
analog, and as of 1999 had only been observed as an ionized fragment during
1410:
1358:
754:
528:
447:
The compound is expected to be highly unstable, even less stable than the
1406:
1392:
421:
819:
660:
647:
Gunther Seitz; Peter Imming (1992). "Oxocarbons and pseudooxocarbons".
588:
506:
334:
133:
23:
746:
579:
In the late 1940s, William J. Hale claimed that "triquinoyl", being a
1397:
948:
433:
725:
Wyrwas, Richard B.; Jarrold, Caroline Chick (2006). "Production of C
556:, which can be described as the fusion of two or three molecules of
348:
Except where otherwise noted, data are given for materials in their
509:
463:
Cyclohexanehexone can be viewed as the neutral counterpart of the
121:
520:
437:
173:
429:
417:
501:
has been detected in mass spectrometry experiments, formed by
917:
467:
425:
111:
223:
899:. U. S. Food and Drug Administration. 1989. Archived from
673:
646:
629:, with same structure but with sulfur instead of oxygen.
897:"Import Alert #66-46 – Unapproved Version of Rodaquin"
767:
733:from Oligomerization of CO on Molybdenum Anions".
862:"Farmer Victorious—Money, Mart, and Mother Earth"
1429:
571:on a hot water solution of the parent compound.
185:
97:
805:
933:
724:
878:Goodrich, William W. (October 15–16, 1986).
505:of carbon monoxide through the formation of
263:InChI=1S/C6O6/c7-1-2(8)4(10)6(12)5(11)3(1)9
940:
926:
684:International Journal of Mass Spectrometry
567:, claimed to be produced by the action of
273:InChI=1/C6O6/c7-1-2(8)4(10)6(12)5(11)3(1)9
238:
163:
141:
205:
877:
735:Journal of the American Chemical Society
234:
1430:
880:"FDA Oral History Interview, Goodrich"
154:
921:
840:
574:
266:Key: PKRGYJHUXHCUCN-UHFFFAOYSA-N
859:
458:
1227:Ethylenetetracarboxylic dianhydride
718:
610:Ethylenetetracarboxylic dianhydride
276:Key: PKRGYJHUXHCUCN-UHFFFAOYAM
176:
13:
44:
35:
14:
1474:
808:The Journal of Physical Chemistry
356:
22:
1443:Hypothetical chemical compounds
352:(at 25 °C , 100 kPa).
76:hexaketocyclohexane, triquinoyl
947:
889:
871:
853:
834:
799:
761:
667:
640:
324:
318:
68:cyclohexane-1,2,3,4,5,6-hexone
1:
1376:Compounds derived from oxides
704:10.1016/S1387-3806(98)14208-2
633:
297:O=C1C(=O)C(=O)C(=O)C(=O)C1=O
7:
598:
485:. The singly charged anion
31:
10:
1479:
1375:
1334:
981:
955:
860:Hale, William J. (1949).
793:10.1107/S1600536805010007
346:
305:
285:
250:
81:
73:
63:
58:
30:
21:
773:Acta Crystallographica E
525:dodecahydroxycyclohexane
585:William Frederick Koch
49:
40:
627:Cyclohexanehexathione
569:ultraviolet radiation
449:cyclohexanehexathione
48:
39:
593:alternative medicine
820:10.1021/j150553a047
785:2005AcCrE..61O1393K
769:Klapötke, Thoman M.
741:(42): 13688–13689.
696:1999IJMSp.188...17S
661:10.1021/cr00014a004
605:Cyclopentanepentone
395:hexaketocyclohexane
342: g·mol
18:
1458:Conjugated ketones
1417:Peroxydicarbonates
1083:1,3-Dioxetanedione
1079:1,2-Dioxetanedione
779:(5): o1393–o1395.
575:Triquinoyl therapy
379:Infobox references
50:
41:
17:Cyclohexanehexone
16:
1425:
1424:
1223:Cyclohexanehexone
747:10.1021/ja0643927
517:X-ray diffraction
459:Related compounds
453:mass spectrometry
391:Cyclohexanehexone
387:Chemical compound
385:
384:
219:CompTox Dashboard
123:Interactive image
54:
53:
1470:
1326:
1312:
1298:
1284:
1270:
1256:
1242:
1220:
1207:
1193:
1179:
1165:
1151:
1137:
1123:
1109:
1095:
1076:
1063:
1049:
1035:
1024:
1014:
1004:
994:
973:
942:
935:
928:
919:
918:
912:
911:
909:
908:
893:
887:
886:
884:
875:
869:
868:
866:
857:
851:
850:
849:
845:
838:
832:
831:
814:(7): 1017–1018.
803:
797:
796:
765:
759:
758:
722:
716:
715:
671:
665:
664:
655:(6): 1227–1260.
649:Chemical Reviews
644:
622:
566:
555:
544:
500:
499:
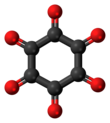
498:
495:
484:
483:
482:
479:
415:
403:organic compound
393:, also known as
369:
363:
360:
359:
341:
326:
320:
313:Chemical formula
243:
242:
227:
225:
209:
189:
178:
167:
156:
145:
125:
101:
32:
26:
19:
15:
1478:
1477:
1473:
1472:
1471:
1469:
1468:
1467:
1428:
1427:
1426:
1421:
1383:Metal carbonyls
1371:
1367:
1354:
1350:
1330:
1325:
1321:
1317:
1311:
1307:
1303:
1297:
1293:
1289:
1283:
1279:
1275:
1269:
1265:
1261:
1255:
1251:
1247:
1241:
1237:
1233:
1219:
1215:
1211:
1206:
1202:
1198:
1192:
1188:
1184:
1178:
1174:
1170:
1164:
1160:
1156:
1150:
1146:
1142:
1136:
1132:
1128:
1122:
1118:
1114:
1108:
1104:
1100:
1093:
1089:
1075:
1071:
1067:
1062:
1058:
1054:
1048:
1044:
1040:
1033:
1029:
1023:
1019:
1013:
1009:
1003:
999:
993:
989:
977:
972:
968:
951:
946:
916:
915:
906:
904:
895:
894:
890:
882:
876:
872:
864:
858:
854:
847:
839:
835:
804:
800:
766:
762:
732:
728:
723:
719:
681:
677:
672:
668:
645:
641:
636:
621:
617:
613:
612:, an isomer of
601:
577:
565:
561:
557:
554:
550:
546:
543:
539:
535:
503:oligomerization
496:
493:
492:
490:
486:
480:
477:
476:
474:
470:
461:
442:carbon monoxide
414:
410:
406:
388:
381:
376:
375:
374: ?)
365:
361:
357:
353:
339:
329:
323:
315:
301:
298:
293:
292:
281:
278:
277:
274:
268:
267:
264:
258:
257:
246:
228:
221:
212:
192:
179:
148:
128:
115:
104:
91:
77:
69:
12:
11:
5:
1476:
1466:
1465:
1463:Cycloalkanones
1460:
1455:
1450:
1448:Cyclic ketones
1445:
1440:
1423:
1422:
1420:
1419:
1414:
1403:Polycarbonates
1400:
1395:
1390:
1385:
1379:
1377:
1373:
1372:
1370:
1369:
1365:
1361:
1356:
1352:
1348:
1344:
1342:Graphite oxide
1338:
1336:
1332:
1331:
1329:
1328:
1323:
1319:
1314:
1309:
1305:
1300:
1295:
1291:
1286:
1281:
1277:
1272:
1267:
1263:
1258:
1253:
1249:
1244:
1239:
1235:
1230:
1217:
1213:
1209:
1204:
1200:
1195:
1190:
1186:
1181:
1176:
1172:
1167:
1162:
1158:
1153:
1148:
1144:
1139:
1134:
1130:
1125:
1120:
1116:
1111:
1106:
1102:
1097:
1091:
1086:
1073:
1069:
1065:
1060:
1056:
1051:
1046:
1042:
1037:
1031:
1026:
1021:
1016:
1011:
1006:
1001:
996:
991:
985:
983:
979:
978:
976:
975:
970:
965:
959:
957:
953:
952:
945:
944:
937:
930:
922:
914:
913:
888:
870:
852:
833:
798:
760:
730:
726:
717:
690:(1–2): 17–25.
679:
675:
666:
638:
637:
635:
632:
631:
630:
624:
619:
615:
607:
600:
597:
576:
573:
563:
559:
552:
548:
541:
537:
527:dihydrate—the
519:analysis, the
488:
472:
460:
457:
416:, the sixfold
412:
408:
386:
383:
382:
377:
355:
354:
350:standard state
347:
344:
343:
337:
331:
330:
327:
321:
316:
311:
308:
307:
303:
302:
300:
299:
296:
288:
287:
286:
283:
282:
280:
279:
275:
272:
271:
269:
265:
262:
261:
253:
252:
251:
248:
247:
245:
244:
236:DTXSID80200662
231:
229:
217:
214:
213:
211:
210:
202:
200:
194:
193:
191:
190:
182:
180:
172:
169:
168:
158:
150:
149:
147:
146:
138:
136:
130:
129:
127:
126:
118:
116:
109:
106:
105:
103:
102:
94:
92:
87:
84:
83:
79:
78:
75:
71:
70:
67:
61:
60:
56:
55:
52:
51:
42:
28:
27:
9:
6:
4:
3:
2:
1475:
1464:
1461:
1459:
1456:
1454:
1451:
1449:
1446:
1444:
1441:
1439:
1436:
1435:
1433:
1418:
1415:
1412:
1411:Tricarbonates
1408:
1404:
1401:
1399:
1396:
1394:
1391:
1389:
1388:Carbonic acid
1386:
1384:
1381:
1380:
1378:
1374:
1368:
1362:
1360:
1357:
1355:
1345:
1343:
1340:
1339:
1337:
1333:
1327:
1315:
1313:
1301:
1299:
1287:
1285:
1273:
1271:
1259:
1257:
1245:
1243:
1231:
1228:
1224:
1210:
1208:
1196:
1194:
1182:
1180:
1168:
1166:
1154:
1152:
1140:
1138:
1126:
1124:
1112:
1110:
1098:
1096:
1087:
1084:
1080:
1066:
1064:
1052:
1050:
1038:
1036:
1027:
1025:
1017:
1015:
1007:
1005:
997:
995:
987:
986:
984:
982:Exotic oxides
980:
974:
966:
964:
961:
960:
958:
956:Common oxides
954:
950:
943:
938:
936:
931:
929:
924:
923:
920:
903:on 2016-11-16
902:
898:
892:
881:
874:
863:
856:
843:
837:
829:
825:
821:
817:
813:
809:
802:
794:
790:
786:
782:
778:
774:
770:
764:
756:
752:
748:
744:
740:
736:
721:
713:
709:
705:
701:
697:
693:
689:
685:
670:
662:
658:
654:
650:
643:
639:
628:
625:
611:
608:
606:
603:
602:
596:
594:
590:
586:
582:
572:
570:
532:
530:
526:
522:
518:
515:According to
513:
511:
508:
504:
469:
466:
456:
454:
450:
445:
443:
439:
435:
431:
427:
423:
419:
405:with formula
404:
400:
396:
392:
380:
373:
368:
351:
345:
338:
336:
333:
332:
317:
314:
310:
309:
304:
295:
294:
291:
284:
270:
260:
259:
256:
249:
241:
237:
233:
232:
230:
220:
216:
215:
208:
204:
203:
201:
199:
196:
195:
188:
184:
183:
181:
175:
171:
170:
166:
162:
159:
157:
155:ECHA InfoCard
152:
151:
144:
140:
139:
137:
135:
132:
131:
124:
120:
119:
117:
113:
108:
107:
100:
96:
95:
93:
90:
86:
85:
80:
72:
66:
62:
57:
47:
43:
38:
34:
33:
29:
25:
20:
1407:Dicarbonates
1393:Bicarbonates
1222:
905:. Retrieved
901:the original
891:
873:
855:
836:
811:
807:
801:
776:
772:
763:
738:
734:
720:
687:
683:
682:(n = 3–6)".
669:
652:
648:
642:
578:
533:
529:geminal diol
514:
462:
446:
398:
394:
390:
389:
82:Identifiers
74:Other names
1453:Polyketones
465:rhodizonate
424:. It is an
422:cyclohexane
306:Properties
161:100.007.649
1438:Oxocarbons
1432:Categories
1398:Carbonates
949:Oxocarbons
907:2019-03-09
842:US 3227641
634:References
595:remedies.
589:glyoxylide
507:molybdenum
399:triquinoyl
335:Molar mass
207:7ZR8062LFD
134:ChemSpider
110:3D model (
89:CAS Number
65:IUPAC name
865:(reprint)
828:0022-3654
712:1387-3806
510:carbonyls
455:studies.
434:oxocarbon
1335:Polymers
755:17044687
599:See also
401:, is an
99:527-31-1
781:Bibcode
692:Bibcode
521:reagent
438:hexamer
372:what is
370: (
340:168.060
174:PubChem
848:
826:
753:
710:
581:trimer
430:carbon
418:ketone
367:verify
364:
290:SMILES
59:Names
883:(PDF)
468:anion
436:), a
426:oxide
255:InChI
187:68240
143:61541
112:JSmol
1409:and
1225:and
1081:and
824:ISSN
751:PMID
708:ISSN
545:and
432:(an
397:and
198:UNII
816:doi
789:doi
743:doi
739:128
700:doi
688:188
657:doi
587:'s
583:of
440:of
428:of
420:of
224:EPA
177:CID
1434::
1364:CO
1359:CO
1324:12
1320:12
1306:12
1292:12
1282:10
1278:10
1264:10
1020:CO
1010:CO
1000:CO
990:CO
969:CO
963:CO
822:.
812:61
810:.
787:.
777:61
775:.
749:.
737:.
706:.
698:.
686:.
653:92
651:.
553:10
549:14
538:10
512:.
478:2−
444:.
1413:)
1405:(
1366:2
1353:2
1351:O
1349:3
1347:C
1322:O
1318:C
1310:9
1308:O
1304:C
1296:6
1294:O
1290:C
1280:O
1276:C
1268:8
1266:O
1262:C
1254:9
1252:O
1250:9
1248:C
1240:8
1238:O
1236:8
1234:C
1229:)
1221:(
1218:6
1216:O
1214:6
1212:C
1205:5
1203:O
1201:5
1199:C
1191:2
1189:O
1187:5
1185:C
1177:6
1175:O
1173:4
1171:C
1163:4
1161:O
1159:4
1157:C
1149:2
1147:O
1145:4
1143:C
1135:6
1133:O
1131:3
1129:C
1121:3
1119:O
1117:3
1115:C
1107:2
1105:O
1103:3
1101:C
1094:O
1092:3
1090:C
1085:)
1077:(
1074:4
1072:O
1070:2
1068:C
1061:3
1059:O
1057:2
1055:C
1047:2
1045:O
1043:2
1041:C
1034:O
1032:2
1030:C
1022:6
1012:5
1002:4
992:3
971:2
941:e
934:t
927:v
910:.
867:.
830:.
818::
795:.
791::
783::
757:.
745::
731:6
729:O
727:6
714:.
702::
694::
680:n
678:O
676:n
663:.
659::
623:.
620:6
618:O
616:6
614:C
564:6
562:O
560:6
558:C
551:O
547:C
542:8
540:O
536:C
497:6
494:−
491:O
489:6
487:C
481:6
475:O
473:6
471:C
413:6
411:O
409:6
407:C
362:Y
328:6
325:O
322:6
319:C
226:)
222:(
114:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.