37:
809:
substrates cause an opposite change in spectral properties, a "reverse type I" spectrum, by processes that are as yet unclear. Inhibitors and certain substrates that bind directly to the heme iron give rise to the type II difference spectrum, with a maximum at 430 nm and a minimum at 390 nm (see inset graph in figure). If no reducing equivalents are available, this complex may remain stable, allowing the degree of binding to be determined from absorbance measurements
826:
661:
780:
774:
Depending on the substrate and enzyme involved, P450 enzymes can catalyze any of a wide variety of reactions. A hypothetical hydroxylation is illustrated. After the hydroxylated product has been released from the active site, the enzyme returns to its original state, with a water molecule returning
813:
C: If carbon monoxide (CO) binds to reduced P450, the catalytic cycle is interrupted. This reaction yields the classic CO difference spectrum with a maximum at 450 nm. However, the interruptive and inhibitory effects of CO varies upon different CYPs such that the CYP3A family is relatively less
808:
Binding of substrate is reflected in the spectral properties of the enzyme, with an increase in absorbance at 390 nm and a decrease at 420 nm. This can be measured by difference spectroscopies and is referred to as the "type I" difference spectrum (see inset graph in figure). Some
708:, on the side opposite to the axial thiolate. Substrate binding induces a change in the conformation of the active site, often displacing a water molecule from the distal axial coordination position of the heme iron, and changing the state of the heme iron from low-spin to high-spin.
791:
An alternative route for mono-oxygenation is via the "peroxide shunt" (path "S" in figure). This pathway entails oxidation of the ferric-substrate complex with oxygen-atom donors such as peroxides and hypochlorites. A hypothetical peroxide "XOOH" is shown in the
387:
is used synonymously. These names should never be used as according to the nomenclature convention (as they denote a P450 in family number 450). However, some gene or enzyme names for P450s are also referred to by historical names (e.g.
616:
The most common reaction catalyzed by cytochromes P450 is a monooxygenase reaction, e.g., insertion of one atom of oxygen into the aliphatic position of an organic substrate (RH), while the other oxygen atom is
1470:
Smith AT, Pazicni S, Marvin KA, Stevens DJ, Paulsen KM, Burstyn JN (April 2015). "Functional divergence of heme-thiolate proteins: a classification based on spectroscopic attributes".
200:
609:
2874:
1164:
652:
also rely on an Fe=O intermediate but lack hemes. Methane monooxygenase, which converts methane to methanol, are non-heme iron-and iron-copper-based enzymes.
281:, and participate in many biosyntheses. By hydroxylation, CYP450 enzymes convert xenobiotics into hydrophilic derivatives, which are more readily excreted.
786:
utilized by cytochrome P450 for conversion of hydrocarbons to alcohols via the action of "compound I", an iron(IV) oxide bound to a heme radical cation.
2204:
751:
The peroxo group formed in step 4 is rapidly protonated twice, releasing one molecule of water and forming the highly reactive species referred to as
3339:
457:
identity, while members of subfamilies must share at least 55% amino-acid identity. Nomenclature committees assign and track both base gene names (
2783:
132:
120:
2923:
498:
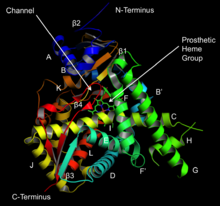
3095:
649:
3077:
3073:
2413:
2236:
839:
392:
for CYP102A1) or functional names, denoting the catalytic activity and the name of the compound used as substrate. Examples include
2762:
2282:
1265:
Sligar SG, Cinti DL, Gibson GG, Schenkman JB (October 1979). "Spin state control of the hepatic cytochrome P450 redox potential".
3346:
3055:
2579:
1523:
1303:
Rittle J, Green MT (November 2010). "Cytochrome P450 Compound I: Capture, Characterization, and C-H Bond
Activation Kinetics".
1366:
1098:
1071:
100:
1431:
Hopper CP, Zambrana PN, Goebel U, Wollborn J (June 2021). "A brief history of carbon monoxide and its therapeutic origins".
1187:
Meunier B, de Visser SP, Shaik S (September 2004). "Mechanism of oxidation reactions catalyzed by cytochrome p450 enzymes".
220:
3442:
2999:
2915:
461:
20:
722:
Molecular oxygen binds to the resulting ferrous heme center at the distal axial coordination position, initially giving a
3807:
3240:
2939:
929:
Danielson PB (December 2002). "The cytochrome P450 superfamily: biochemistry, evolution and drug metabolism in humans".
857:
Estabrook RW (December 2003). "A passion for P450s (Remembrances of the early history of research on cytochrome P450)".
755:(or just Compound I). This highly reactive intermediate was isolated in 2010, P450 Compound 1 is an iron(IV) oxo (or
3508:
2979:
723:
2214:
3318:
3116:
2957:
2836:
2535:
3302:
3109:
354:, a capital letter indicating the subfamily, and another numeral for the individual gene. The convention is to
208:
3664:
3013:
2983:
2943:
2931:
2229:
964:
3167:
3081:
3069:
677:
The active site of cytochrome P450 contains a heme-iron center. The iron is tethered to the protein via a
3219:
2919:
2587:
844:
1230:
Poulos TL, Finzel BC, Howard AJ (June 1987). "High-resolution crystal structure of cytochrome P450cam".
36:
3102:
3041:
3037:
3025:
3021:
3017:
1516:
687:. This cysteine and several flanking residues are highly conserved in known P450s, and have the formal
204:
3797:
3765:
3752:
3739:
3726:
3713:
3700:
3687:
3649:
3405:
3290:
3059:
730:
712:
502:
438:, lanosterol 14-α-demethylase, sometimes unofficially abbreviated to LDM according to its substrate (
340:
144:
113:
490:
Based on the nature of the electron transfer proteins, P450s can be classified into several groups:
149:
3787:
3659:
3613:
3556:
3474:
3212:
3159:
3154:
3009:
2860:
2257:
2222:
1591:
797:
783:
3561:
3261:
3134:
1669:
1613:
517:
485:
301:
289:
256:
1650:
453:
The current nomenclature guidelines suggest that members of new CYP families share at least 40%
3088:
3033:
3029:
2995:
2927:
2509:
405:
3582:
3501:
3325:
2831:
1509:
605:
587:
535:
1501:
3654:
3283:
3180:
3051:
2950:
2591:
1794:
1780:
1766:
1752:
1312:
1035:
Nelson DR (January 2011). "Progress in tracing the evolutionary paths of cytochrome P450".
601:
549:
187:
800:, have been investigated with synthetic analogues, consisting of iron oxo heme complexes.
8:
3802:
3618:
3435:
3141:
2969:
2060:
2030:
1954:
1596:
347:
244:
156:
1316:
1140:
1115:
664:
The "Fe(V) intermediate" at the bottom left is a simplification: it is an Fe(IV) with a
3551:
2935:
2044:
1726:
1452:
1408:
1383:
1336:
1212:
1012:
985:
882:
665:
1384:"Oxygen Activation and Radical Transformations in Heme Proteins and Metalloporphyrins"
1090:
125:
2244:
2025:
1920:
1487:
1456:
1444:
1413:
1362:
1340:
1328:
1282:
1278:
1247:
1243:
1204:
1162:
1145:
1094:
1052:
1017:
946:
874:
293:
285:
195:
105:
1216:
886:
69:
3792:
3597:
3592:
3566:
3494:
1930:
1889:
1819:
1479:
1436:
1403:
1395:
1320:
1274:
1239:
1196:
1135:
1127:
1086:
1044:
1007:
997:
938:
866:
768:
265:
183:
161:
137:
3644:
3628:
3541:
3384:
2039:
1168:
1048:
465:
313:
81:
1399:
1131:
911:
458:
3682:
3623:
2692:
2086:
2081:
1440:
1116:"Direct Methane Oxidation by Copper- and Iron-Dependent Methane Monooxygenases"
831:
263:. However, they are not omnipresent; for example, they have not been found in
3781:
3587:
3546:
3467:
3371:
3332:
3254:
3247:
3233:
3193:
3174:
3148:
2191:
2150:
2065:
1872:
1654:
1637:
1576:
1002:
942:
738:
641:
506:
260:
1324:
3536:
2990:
2964:
2868:
2854:
2847:
2753:
2733:
2726:
2709:
2651:
2634:
2627:
2620:
2613:
2606:
2574:
2567:
2179:
2120:
1996:
1982:
1968:
1858:
1833:
1491:
1448:
1417:
1332:
1208:
1149:
1056:
1021:
950:
878:
870:
516:) can also contribute reducing power to this system after being reduced by
339:
encoding P450 enzymes, and the enzymes themselves, are designated with the
1251:
109:
3760:
3695:
3531:
2560:
2516:
2127:
1540:
1286:
760:
582:
539:
397:
369:
356:
351:
2245:
2174:
2132:
2035:
1558:
734:
705:
553:
454:
297:
278:
274:
93:
1483:
1200:
376:
nomenclature is the official naming convention, although occasionally
3734:
3708:
3460:
2661:
2249:
2115:
764:
716:
591:
305:
3417:
3377:
3364:
3309:
3276:
3226:
3204:
3199:
3185:
825:
681:
678:
645:
600:
which do not require external reducing power. Notable ones include
317:
76:
695:- - . In general, the P450 catalytic cycle proceeds as follows:
570:
in which both electrons required by the CYP come from cytochrome b
2886:
2813:
2803:
2793:
2776:
2766:
2758:
2746:
2742:
2738:
2719:
2671:
2644:
2599:
2595:
2583:
2474:
2469:
2464:
2439:
2434:
2418:
2338:
2333:
2313:
1532:
1171:
688:
435:
270:
88:
3747:
3517:
2553:
2549:
2539:
2525:
2521:
2489:
2484:
2479:
2459:
2454:
2449:
2444:
2408:
2403:
2398:
2378:
2373:
2368:
2363:
2358:
2353:
2348:
2343:
2328:
2323:
2318:
2308:
2303:
2287:
2277:
2272:
1549:
775:
to occupy the distal coordination position of the iron nucleus.
756:
684:
469:
393:
365:
325:
248:
215:
316:. Most P450s require a protein partner to deliver one or more
3721:
2840:
2702:
2389:
1601:
1531:
1361:(3rd ed.). New York: Kluwer Academic/Plenum Publishers.
1037:
Biochimica et
Biophysica Acta (BBA) - Proteins and Proteomics
767:
and thiolate ligands. Evidence for the alternative perferryl
711:
Substrate binding induces electron transfer from NAD(P)H via
648:
groups) use CYP enzymes, but many other hydroxylases exist.
618:
473:
309:
42:
1430:
660:
2894:
1710:
1698:
1693:
1625:
1536:
336:
321:
252:
177:
64:
3486:
1264:
779:
1940:
1681:
1469:
691:
signature consensus pattern - - x - - {F} - - {P} -
1359:
Cytochrome P450: structure, mechanism, and biochemistry
1072:"Electron transfer proteins of cytochrome P450 systems"
284:
P450s are, in general, the terminal oxidase enzymes in
1186:
821:
1356:
1267:
1229:
759:) species with an additional oxidizing equivalent
360:the name when referring to the gene. For example,
3779:
635:
1182:
1180:
1113:
729:A second electron is transferred, from either
259:that mostly, but not exclusively, function as
3502:
2230:
1517:
1063:
1352:
1350:
1177:
1381:
1302:
922:
3509:
3495:
2237:
2223:
1524:
1510:
1081:. Advances in Molecular and Cell Biology.
748:adduct to give a short-lived peroxo state.
650:Alpha-ketoglutarate-dependent hydroxylases
35:
2205:disorders of globin and globulin proteins
1407:
1347:
1298:
1296:
1139:
1114:Tucci FJ, Rosenzweig AC (February 2024).
1069:
1011:
1001:
928:
856:
840:Cytochrome P450 oxidoreductase deficiency
542:to transfer electrons from NADPH to P450.
41:Structure of lanosterol 14α-demethylase (
1223:
778:
659:
497:in which electrons are transferred from
1258:
3780:
1293:
1034:
983:
350:, followed by a number indicating the
292:. The term "P450" is derived from the
3490:
2218:
1505:
906:
904:
704:Substrate binds in proximity to the
364:is the gene that encodes the enzyme
269:. In mammals, these enzymes oxidize
21:Cytochrome P450 (individual enzymes)
796:Mechanistic details, including the
13:
850:
698:
14:
3819:
1382:Huang X, Groves JT (March 2018).
901:
479:
474:CYP Allele Nomenclature Committee
824:
505:(variously CPR, POR, or CYPOR).
372:(acetaminophen) metabolism. The
368:—one of the enzymes involved in
1463:
1424:
1375:
1357:Ortiz de Montellano PR (2005).
859:Drug Metabolism and Disposition
803:
719:, converting Fe(III) to Fe(II).
331:
288:chains, broadly categorized as
1156:
1107:
1028:
986:"The cytochrome p450 homepage"
977:
957:
556:to transfer electrons to P450.
1:
1091:10.1016/S1569-2558(08)60339-2
894:
636:Related hydroxylation enzymes
172:Available protein structures:
1279:10.1016/0006-291X(79)91916-8
1244:10.1016/0022-2836(87)90190-2
1232:Journal of Molecular Biology
1049:10.1016/j.bbapap.2010.08.008
672:
655:
7:
3516:
1400:10.1021/acs.chemrev.7b00373
1132:10.1021/acs.chemrev.3c00727
845:Cytochrome P450 engineering
817:
10:
3824:
3808:Integral membrane proteins
1441:10.1016/j.niox.2021.04.001
1174:consensus pattern for P450
984:Nelson DR (October 2009).
531:Mitochondrial P450 systems
483:
324:(and eventually molecular
18:
3673:
3665:Michaelis–Menten kinetics
3637:
3606:
3575:
3524:
3452:
3427:
3356:
3126:
2904:
2823:
2681:
2498:
2427:
2387:
2296:
2265:
2200:
2167:
2141:
2106:
2099:
2074:
2053:
2018:
1911:
1851:
1812:
1745:
1734:
1725:
1648:
1573:
1566:
1557:
1548:
731:cytochrome P450 reductase
726:similar to oxy-myoglobin.
713:cytochrome P450 reductase
503:cytochrome P450 reductase
442:anosterol) and activity (
404:synthase, abbreviated to
312:state and complexed with
214:
194:
176:
171:
167:
155:
143:
131:
119:
99:
87:
75:
63:
55:
50:
34:
29:
3557:Diffusion-limited enzyme
1003:10.1186/1479-7364-4-1-59
943:10.2174/1389200023337054
798:oxygen rebound mechanism
784:Oxygen rebound mechanism
644:reactions (insertion of
459:Cytochrome P450 Homepage
304:of the enzyme (450
1325:10.1126/science.1193478
931:Current Drug Metabolism
494:Microsomal P450 systems
486:P450-containing systems
290:P450-containing systems
965:"NCBI sequence viewer"
871:10.1124/dmd.31.12.1461
787:
715:or another associated
669:
545:Bacterial P450 systems
3650:Eadie–Hofstee diagram
3583:Allosteric regulation
782:
663:
628:+ NADPH + H → ROH + H
610:allene oxide synthase
606:prostacyclin synthase
536:adrenodoxin reductase
19:Further information:
3660:Lineweaver–Burk plot
1070:Hanukoglu I (1996).
608:(CYP8), and CYP74A (
602:thromboxane synthase
594:is fused to the CYP.
586:species, in which a
580:originally found in
550:ferredoxin reductase
308:) when it is in the
2061:Glycated hemoglobin
2031:Carbaminohemoglobin
1317:2010Sci...330..933R
1079:Adv. Mol. Cell Biol
744:, reducing the Fe-O
666:radical heme ligand
590:-domain-containing
577:FMN/Fd/P450 systems
3619:Enzyme superfamily
3552:Enzyme promiscuity
1435:. 111–112: 45–63.
1167:2019-10-18 at the
788:
670:
464:2010-06-27 at the
302:absorption maximum
294:spectrophotometric
3775:
3774:
3484:
3483:
2212:
2211:
2163:
2162:
2159:
2158:
2095:
2094:
2026:Carboxyhemoglobin
2014:
2013:
1907:
1906:
1721:
1720:
1484:10.1021/cr500056m
1368:978-0-306-48324-0
1311:(6006): 933–937.
1201:10.1021/cr020443g
1100:978-0-7623-0113-3
912:"Cytochrome P450"
865:(12): 1461–1473.
597:P450 only systems
286:electron transfer
230:
229:
226:
225:
221:structure summary
3815:
3798:Pharmacokinetics
3655:Hanes–Woolf plot
3598:Enzyme activator
3593:Enzyme inhibitor
3567:Enzyme catalysis
3511:
3504:
3497:
3488:
3487:
2256:(Most belong to
2239:
2232:
2225:
2216:
2215:
2104:
2103:
1743:
1742:
1732:
1731:
1571:
1570:
1564:
1563:
1555:
1554:
1526:
1519:
1512:
1503:
1502:
1496:
1495:
1478:(7): 2532–2558.
1472:Chemical Reviews
1467:
1461:
1460:
1428:
1422:
1421:
1411:
1394:(5): 2491–2553.
1388:Chemical Reviews
1379:
1373:
1372:
1354:
1345:
1344:
1300:
1291:
1290:
1262:
1256:
1255:
1227:
1221:
1220:
1195:(9): 3947–3980.
1189:Chemical Reviews
1184:
1175:
1160:
1154:
1153:
1143:
1126:(3): 1288–1320.
1120:Chemical Reviews
1111:
1105:
1104:
1076:
1067:
1061:
1060:
1032:
1026:
1025:
1015:
1005:
981:
975:
974:
972:
971:
961:
955:
954:
926:
920:
919:
908:
890:
834:
829:
828:
266:Escherichia coli
233:Cytochromes P450
169:
168:
39:
27:
26:
16:Class of enzymes
3823:
3822:
3818:
3817:
3816:
3814:
3813:
3812:
3788:Cytochrome P450
3778:
3777:
3776:
3771:
3683:Oxidoreductases
3669:
3645:Enzyme kinetics
3633:
3629:List of enzymes
3602:
3571:
3542:Catalytic triad
3520:
3515:
3485:
3480:
3448:
3423:
3385:Halloween genes
3352:
3122:
2900:
2819:
2677:
2494:
2423:
2383:
2292:
2261:
2254:cytochrome P450
2243:
2213:
2208:
2196:
2185:Cytochrome P450
2155:
2137:
2091:
2070:
2049:
2040:Deoxyhemoglobin
2010:
2006:
2002:
1992:
1988:
1978:
1974:
1964:
1960:
1950:
1946:
1936:
1926:
1903:
1899:
1895:
1885:
1881:
1876:
1868:
1864:
1847:
1843:
1839:
1829:
1825:
1808:
1804:
1800:
1795:HbE Portland II
1790:
1786:
1776:
1772:
1762:
1758:
1737:
1717:
1644:
1575:Alpha locus on
1544:
1530:
1500:
1499:
1468:
1464:
1429:
1425:
1380:
1376:
1369:
1355:
1348:
1301:
1294:
1263:
1259:
1228:
1224:
1185:
1178:
1169:Wayback Machine
1161:
1157:
1112:
1108:
1101:
1074:
1068:
1064:
1033:
1029:
982:
978:
969:
967:
963:
962:
958:
927:
923:
910:
909:
902:
897:
853:
851:Further reading
830:
823:
820:
806:
753:P450 Compound 1
747:
742:
724:dioxygen adduct
701:
699:Catalytic cycle
675:
658:
638:
633:
631:
627:
573:
566:
562:
548:which employ a
527:
521:
515:
510:
488:
482:
466:Wayback Machine
426:
403:
391:
385:
334:
314:carbon monoxide
121:OPM superfamily
46:
30:Cytochrome P450
23:
17:
12:
11:
5:
3821:
3811:
3810:
3805:
3800:
3795:
3790:
3773:
3772:
3770:
3769:
3756:
3743:
3730:
3717:
3704:
3691:
3677:
3675:
3671:
3670:
3668:
3667:
3662:
3657:
3652:
3647:
3641:
3639:
3635:
3634:
3632:
3631:
3626:
3621:
3616:
3610:
3608:
3607:Classification
3604:
3603:
3601:
3600:
3595:
3590:
3585:
3579:
3577:
3573:
3572:
3570:
3569:
3564:
3559:
3554:
3549:
3544:
3539:
3534:
3528:
3526:
3522:
3521:
3514:
3513:
3506:
3499:
3491:
3482:
3481:
3479:
3478:
3471:
3464:
3456:
3454:
3450:
3449:
3447:
3446:
3439:
3431:
3429:
3425:
3424:
3422:
3421:
3414:
3382:
3381:
3380:
3368:
3360:
3358:
3354:
3353:
3351:
3350:
3343:
3336:
3329:
3322:
3315:
3314:
3313:
3306:
3294:
3287:
3280:
3273:
3272:
3271:
3268:
3258:
3251:
3244:
3237:
3230:
3223:
3216:
3209:
3208:
3207:
3202:
3190:
3189:
3188:
3183:
3171:
3164:
3163:
3162:
3157:
3145:
3138:
3130:
3128:
3124:
3123:
3121:
3120:
3113:
3106:
3099:
3092:
3085:
3063:
3045:
3003:
2987:
2973:
2961:
2954:
2947:
2908:
2906:
2902:
2901:
2899:
2898:
2891:
2890:
2889:
2879:
2878:
2877:
2865:
2864:
2863:
2851:
2844:
2827:
2825:
2821:
2820:
2818:
2817:
2807:
2797:
2787:
2780:
2770:
2750:
2730:
2723:
2713:
2706:
2696:
2685:
2683:
2679:
2678:
2676:
2675:
2665:
2655:
2648:
2638:
2631:
2624:
2617:
2610:
2603:
2571:
2564:
2557:
2543:
2529:
2513:
2502:
2500:
2496:
2495:
2493:
2492:
2487:
2482:
2477:
2472:
2467:
2462:
2457:
2452:
2447:
2442:
2437:
2431:
2429:
2425:
2424:
2422:
2421:
2416:
2411:
2406:
2401:
2395:
2393:
2385:
2384:
2382:
2381:
2376:
2371:
2366:
2361:
2356:
2351:
2346:
2341:
2336:
2331:
2326:
2321:
2316:
2311:
2306:
2300:
2298:
2294:
2293:
2291:
2290:
2285:
2280:
2275:
2269:
2267:
2263:
2262:
2242:
2241:
2234:
2227:
2219:
2210:
2209:
2201:
2198:
2197:
2195:
2194:
2189:
2188:
2187:
2182:
2171:
2169:
2165:
2164:
2161:
2160:
2157:
2156:
2154:
2153:
2147:
2145:
2139:
2138:
2136:
2135:
2130:
2125:
2124:
2123:
2112:
2110:
2101:
2097:
2096:
2093:
2092:
2090:
2089:
2087:Erythrocruorin
2084:
2078:
2076:
2072:
2071:
2069:
2068:
2063:
2057:
2055:
2051:
2050:
2048:
2047:
2045:Sulfhemoglobin
2042:
2033:
2028:
2022:
2020:
2016:
2015:
2012:
2011:
2009:
2008:
2004:
2000:
1994:
1990:
1986:
1980:
1976:
1972:
1966:
1962:
1958:
1952:
1948:
1944:
1938:
1934:
1928:
1924:
1917:
1915:
1909:
1908:
1905:
1904:
1902:
1901:
1897:
1893:
1887:
1883:
1879:
1874:
1870:
1866:
1862:
1855:
1853:
1849:
1848:
1846:
1845:
1841:
1837:
1831:
1827:
1823:
1816:
1814:
1810:
1809:
1807:
1806:
1802:
1798:
1792:
1788:
1784:
1781:HbE Portland I
1778:
1774:
1770:
1764:
1760:
1756:
1749:
1747:
1740:
1729:
1723:
1722:
1719:
1718:
1716:
1715:
1714:
1713:
1703:
1702:
1701:
1696:
1686:
1685:
1684:
1674:
1673:
1672:
1661:
1659:
1646:
1645:
1643:
1642:
1641:
1640:
1630:
1629:
1628:
1618:
1617:
1616:
1606:
1605:
1604:
1599:
1594:
1583:
1581:
1568:
1561:
1552:
1546:
1545:
1529:
1528:
1521:
1514:
1506:
1498:
1497:
1462:
1423:
1374:
1367:
1346:
1292:
1273:(3): 925–932.
1257:
1238:(3): 687–700.
1222:
1176:
1155:
1106:
1099:
1062:
1027:
990:Human Genomics
976:
956:
937:(6): 561–597.
921:
899:
898:
896:
893:
892:
891:
852:
849:
848:
847:
842:
836:
835:
832:Biology portal
819:
816:
805:
802:
794:
793:
777:
776:
772:
749:
745:
740:
727:
720:
709:
700:
697:
674:
671:
657:
654:
637:
634:
629:
625:
623:
614:
613:
598:
595:
578:
575:
571:
568:
564:
560:
557:
546:
543:
532:
529:
525:
519:
513:
508:
495:
484:Main article:
481:
480:Classification
478:
424:
401:
389:
383:
333:
330:
320:to reduce the
261:monooxygenases
228:
227:
224:
223:
218:
212:
211:
198:
192:
191:
181:
174:
173:
165:
164:
159:
153:
152:
147:
141:
140:
135:
129:
128:
123:
117:
116:
103:
97:
96:
91:
85:
84:
79:
73:
72:
67:
61:
60:
57:
53:
52:
48:
47:
40:
32:
31:
15:
9:
6:
4:
3:
2:
3820:
3809:
3806:
3804:
3801:
3799:
3796:
3794:
3791:
3789:
3786:
3785:
3783:
3767:
3763:
3762:
3757:
3754:
3750:
3749:
3744:
3741:
3737:
3736:
3731:
3728:
3724:
3723:
3718:
3715:
3711:
3710:
3705:
3702:
3698:
3697:
3692:
3689:
3685:
3684:
3679:
3678:
3676:
3672:
3666:
3663:
3661:
3658:
3656:
3653:
3651:
3648:
3646:
3643:
3642:
3640:
3636:
3630:
3627:
3625:
3624:Enzyme family
3622:
3620:
3617:
3615:
3612:
3611:
3609:
3605:
3599:
3596:
3594:
3591:
3589:
3588:Cooperativity
3586:
3584:
3581:
3580:
3578:
3574:
3568:
3565:
3563:
3560:
3558:
3555:
3553:
3550:
3548:
3547:Oxyanion hole
3545:
3543:
3540:
3538:
3535:
3533:
3530:
3529:
3527:
3523:
3519:
3512:
3507:
3505:
3500:
3498:
3493:
3492:
3489:
3477:
3476:
3472:
3470:
3469:
3465:
3463:
3462:
3458:
3457:
3455:
3451:
3445:
3444:
3440:
3438:
3437:
3433:
3432:
3430:
3426:
3420:
3419:
3415:
3412:
3408:
3407:
3402:
3398:
3394:
3390:
3386:
3383:
3379:
3376:
3375:
3374:
3373:
3369:
3367:
3366:
3362:
3361:
3359:
3355:
3349:
3348:
3344:
3342:
3341:
3337:
3335:
3334:
3330:
3328:
3327:
3323:
3321:
3320:
3316:
3312:
3311:
3307:
3305:
3304:
3300:
3299:
3298:
3295:
3293:
3292:
3288:
3286:
3285:
3281:
3279:
3278:
3274:
3269:
3266:
3265:
3264:
3263:
3259:
3257:
3256:
3252:
3250:
3249:
3245:
3243:
3242:
3238:
3236:
3235:
3231:
3229:
3228:
3224:
3222:
3221:
3217:
3215:
3214:
3210:
3206:
3203:
3201:
3198:
3197:
3196:
3195:
3191:
3187:
3184:
3182:
3179:
3178:
3177:
3176:
3172:
3170:
3169:
3165:
3161:
3158:
3156:
3153:
3152:
3151:
3150:
3146:
3144:
3143:
3139:
3137:
3136:
3132:
3131:
3129:
3125:
3119:
3118:
3114:
3112:
3111:
3107:
3105:
3104:
3100:
3098:
3097:
3093:
3091:
3090:
3086:
3083:
3079:
3075:
3071:
3067:
3064:
3061:
3057:
3053:
3049:
3046:
3043:
3039:
3035:
3031:
3027:
3023:
3019:
3015:
3011:
3007:
3004:
3001:
2997:
2993:
2992:
2988:
2985:
2981:
2977:
2974:
2971:
2967:
2966:
2962:
2960:
2959:
2955:
2953:
2952:
2948:
2945:
2941:
2937:
2933:
2929:
2925:
2921:
2917:
2913:
2910:
2909:
2907:
2903:
2897:
2896:
2892:
2888:
2885:
2884:
2883:
2880:
2876:
2873:
2872:
2871:
2870:
2866:
2862:
2859:
2858:
2857:
2856:
2852:
2850:
2849:
2845:
2842:
2838:
2834:
2833:
2829:
2828:
2826:
2822:
2815:
2811:
2808:
2805:
2801:
2798:
2795:
2791:
2788:
2786:
2785:
2781:
2778:
2774:
2771:
2768:
2764:
2760:
2756:
2755:
2751:
2748:
2744:
2740:
2736:
2735:
2731:
2729:
2728:
2724:
2721:
2717:
2714:
2712:
2711:
2707:
2704:
2700:
2697:
2694:
2690:
2687:
2686:
2684:
2680:
2673:
2669:
2666:
2663:
2659:
2656:
2654:
2653:
2649:
2646:
2642:
2639:
2637:
2636:
2632:
2630:
2629:
2625:
2623:
2622:
2618:
2616:
2615:
2611:
2609:
2608:
2604:
2601:
2597:
2593:
2589:
2585:
2581:
2577:
2576:
2572:
2570:
2569:
2565:
2563:
2562:
2558:
2555:
2551:
2547:
2544:
2541:
2537:
2533:
2530:
2527:
2523:
2519:
2518:
2514:
2511:
2507:
2504:
2503:
2501:
2497:
2491:
2488:
2486:
2483:
2481:
2478:
2476:
2473:
2471:
2468:
2466:
2463:
2461:
2458:
2456:
2453:
2451:
2448:
2446:
2443:
2441:
2438:
2436:
2433:
2432:
2430:
2426:
2420:
2417:
2415:
2412:
2410:
2407:
2405:
2402:
2400:
2397:
2396:
2394:
2391:
2386:
2380:
2377:
2375:
2372:
2370:
2367:
2365:
2362:
2360:
2357:
2355:
2352:
2350:
2347:
2345:
2342:
2340:
2337:
2335:
2332:
2330:
2327:
2325:
2322:
2320:
2317:
2315:
2312:
2310:
2307:
2305:
2302:
2301:
2299:
2295:
2289:
2286:
2284:
2281:
2279:
2276:
2274:
2271:
2270:
2268:
2264:
2259:
2255:
2251:
2247:
2240:
2235:
2233:
2228:
2226:
2221:
2220:
2217:
2207:
2206:
2199:
2193:
2192:Methemalbumin
2190:
2186:
2183:
2181:
2178:
2177:
2176:
2173:
2172:
2170:
2166:
2152:
2151:Leghemoglobin
2149:
2148:
2146:
2144:
2140:
2134:
2131:
2129:
2126:
2122:
2119:
2118:
2117:
2114:
2113:
2111:
2109:
2105:
2102:
2098:
2088:
2085:
2083:
2082:Chlorocruorin
2080:
2079:
2077:
2073:
2067:
2066:Methemoglobin
2064:
2062:
2059:
2058:
2056:
2052:
2046:
2043:
2041:
2037:
2036:Oxyhemoglobin
2034:
2032:
2029:
2027:
2024:
2023:
2021:
2017:
1998:
1995:
1984:
1981:
1970:
1967:
1956:
1953:
1942:
1939:
1932:
1929:
1922:
1919:
1918:
1916:
1914:
1910:
1891:
1888:
1877:
1871:
1860:
1857:
1856:
1854:
1850:
1835:
1832:
1821:
1818:
1817:
1815:
1811:
1796:
1793:
1782:
1779:
1768:
1765:
1754:
1751:
1750:
1748:
1744:
1741:
1739:
1733:
1730:
1728:
1724:
1712:
1709:
1708:
1707:
1704:
1700:
1697:
1695:
1692:
1691:
1690:
1687:
1683:
1680:
1679:
1678:
1675:
1671:
1668:
1667:
1666:
1663:
1662:
1660:
1658:
1656:
1652:
1647:
1639:
1636:
1635:
1634:
1631:
1627:
1624:
1623:
1622:
1619:
1615:
1612:
1611:
1610:
1607:
1603:
1600:
1598:
1595:
1593:
1590:
1589:
1588:
1585:
1584:
1582:
1580:
1578:
1572:
1569:
1565:
1562:
1560:
1556:
1553:
1551:
1547:
1542:
1538:
1535:that contain
1534:
1527:
1522:
1520:
1515:
1513:
1508:
1507:
1504:
1493:
1489:
1485:
1481:
1477:
1473:
1466:
1458:
1454:
1450:
1446:
1442:
1438:
1434:
1427:
1419:
1415:
1410:
1405:
1401:
1397:
1393:
1389:
1385:
1378:
1370:
1364:
1360:
1353:
1351:
1342:
1338:
1334:
1330:
1326:
1322:
1318:
1314:
1310:
1306:
1299:
1297:
1288:
1284:
1280:
1276:
1272:
1268:
1261:
1253:
1249:
1245:
1241:
1237:
1233:
1226:
1218:
1214:
1210:
1206:
1202:
1198:
1194:
1190:
1183:
1181:
1173:
1170:
1166:
1163:
1159:
1151:
1147:
1142:
1137:
1133:
1129:
1125:
1121:
1117:
1110:
1102:
1096:
1092:
1088:
1084:
1080:
1073:
1066:
1058:
1054:
1050:
1046:
1042:
1038:
1031:
1023:
1019:
1014:
1009:
1004:
999:
995:
991:
987:
980:
966:
960:
952:
948:
944:
940:
936:
932:
925:
917:
913:
907:
905:
900:
888:
884:
880:
876:
872:
868:
864:
860:
855:
854:
846:
843:
841:
838:
837:
833:
827:
822:
815:
812:
801:
799:
790:
789:
785:
781:
773:
770:
766:
762:
758:
754:
750:
743:
736:
732:
728:
725:
721:
718:
714:
710:
707:
703:
702:
696:
694:
690:
686:
683:
680:
667:
662:
653:
651:
647:
643:
642:hydroxylation
622:
620:
611:
607:
603:
599:
596:
593:
589:
585:
584:
579:
576:
569:
567:/P450 systems
558:
555:
551:
547:
544:
541:
537:
534:which employ
533:
530:
523:
511:
504:
500:
496:
493:
492:
491:
487:
477:
475:
471:
467:
463:
460:
456:
451:
450:ethylation).
449:
445:
441:
437:
433:
429:
423:
419:
415:
411:
407:
399:
395:
386:
379:
375:
371:
367:
363:
359:
358:
353:
349:
345:
342:
338:
329:
327:
323:
319:
315:
311:
307:
303:
299:
295:
291:
287:
282:
280:
276:
272:
268:
267:
262:
258:
254:
250:
246:
242:
238:
234:
222:
219:
217:
213:
210:
206:
202:
199:
197:
193:
189:
185:
182:
179:
175:
170:
166:
163:
160:
158:
154:
151:
148:
146:
142:
139:
136:
134:
130:
127:
124:
122:
118:
115:
111:
107:
104:
102:
98:
95:
92:
90:
86:
83:
80:
78:
74:
71:
68:
66:
62:
58:
54:
49:
44:
38:
33:
28:
25:
22:
3761:Translocases
3758:
3745:
3732:
3719:
3706:
3696:Transferases
3693:
3680:
3537:Binding site
3473:
3466:
3459:
3441:
3434:
3416:
3410:
3404:
3400:
3396:
3392:
3388:
3370:
3363:
3345:
3338:
3331:
3324:
3317:
3308:
3301:
3296:
3289:
3282:
3275:
3260:
3253:
3246:
3239:
3232:
3225:
3218:
3211:
3192:
3173:
3166:
3147:
3140:
3133:
3115:
3108:
3101:
3094:
3087:
3065:
3047:
3005:
2989:
2975:
2963:
2956:
2949:
2911:
2893:
2881:
2867:
2853:
2846:
2830:
2809:
2799:
2789:
2782:
2772:
2752:
2732:
2725:
2715:
2708:
2698:
2688:
2667:
2657:
2650:
2640:
2633:
2626:
2619:
2612:
2605:
2573:
2566:
2559:
2545:
2531:
2515:
2505:
2253:
2202:
2184:
2180:Cytochrome b
2142:
2121:Metmyoglobin
2107:
1912:
1738:development:
1735:
1705:
1688:
1676:
1664:
1649:
1632:
1620:
1608:
1586:
1574:
1541:hemoproteins
1475:
1471:
1465:
1433:Nitric Oxide
1432:
1426:
1391:
1387:
1377:
1358:
1308:
1304:
1270:
1266:
1260:
1235:
1231:
1225:
1192:
1188:
1158:
1123:
1119:
1109:
1082:
1078:
1065:
1043:(1): 14–18.
1040:
1036:
1030:
996:(1): 59–65.
993:
989:
979:
968:. Retrieved
959:
934:
930:
924:
915:
862:
858:
810:
807:
804:Spectroscopy
795:
752:
739:cytochrome b
692:
676:
639:
615:
581:
518:cytochrome b
507:Cytochrome b
489:
452:
447:
443:
439:
431:
427:
421:
417:
413:
409:
381:
377:
373:
361:
355:
343:
335:
332:Nomenclature
296:peak at the
283:
264:
240:
236:
232:
231:
24:
3532:Active site
2246:Cytochromes
2128:Neuroglobin
2054:Other human
1767:HbE Gower 2
1753:HbE Gower 1
771:is lacking.
769:iron(V)-oxo
761:delocalized
735:ferredoxins
583:Rhodococcus
540:adrenodoxin
398:thromboxane
370:paracetamol
352:gene family
348:superfamily
341:root symbol
279:xenobiotics
275:fatty acids
251:containing
245:superfamily
133:OPM protein
51:Identifiers
3803:Metabolism
3782:Categories
3735:Isomerases
3709:Hydrolases
3576:Regulation
3453:CYP701-999
3428:CYP501-699
3357:CYP301-499
3127:CYP101-281
2250:oxygenases
2175:Cytochrome
2133:Cytoglobin
1913:pathology:
1736:stages of
1651:Beta locus
1559:Hemoglobin
970:2007-11-19
895:References
814:affected.
706:heme group
621:to water:
554:ferredoxin
455:amino-acid
298:wavelength
184:structures
157:Membranome
3614:EC number
3461:CYP704B22
2203:see also
2116:Myoglobin
2019:Compounds
1890:HbF/Fetal
1820:HbF/Fetal
1746:Embryonic
1727:Tetramers
1457:233205099
1341:206528205
1085:: 29–55.
765:porphyrin
763:over the
717:reductase
673:Structure
656:Mechanism
592:reductase
522:reductase
357:italicize
318:electrons
94:PDOC00081
82:IPR001128
3638:Kinetics
3562:Cofactor
3525:Activity
3475:CYP720A1
3443:CYP504B1
3418:CYP318A1
3411:CYP315A1
3406:CYP314A1
3401:CYP307A2
3397:CYP307A1
3393:CYP306A1
3389:CYP302A1
3365:CYP303A1
3340:CYP199A2
3319:CYP176A1
3277:CYP154C3
3241:CYP125A1
3227:CYP119A1
3220:CYP113A1
3168:CYP106A2
3142:CYP102A1
3135:CYP101A1
2905:CYP71-99
2824:CYP51-69
2682:CYP21-49
2075:Nonhuman
1567:Subunits
1533:Proteins
1492:25763468
1449:33838343
1418:29286645
1333:21071661
1217:33927145
1209:15352783
1165:Archived
1150:38305159
1141:10923174
1057:20736090
1022:19951895
951:12369887
916:InterPro
887:43655270
879:14625342
818:See also
811:in vitro
792:diagram.
682:thiolate
679:cysteine
646:hydroxyl
632:O + NADP
604:(CYP5),
462:Archived
430:ynthase
346:for the
271:steroids
257:cofactor
243:) are a
201:RCSB PDB
77:InterPro
3793:EC 1.14
3748:Ligases
3518:Enzymes
3347:CYP255A
3326:CYP183A
3291:CYP161C
3284:CYP158A
3117:CYP99A3
3110:CYP97C1
3103:CYP93E1
3096:CYP90C1
3089:CYP88D6
2958:CYP73A1
2951:CYP72A1
2499:CYP5-20
1550:Globins
1409:5855008
1313:Bibcode
1305:Science
1252:3656428
1172:PROSITE
1013:3500189
689:PROSITE
619:reduced
472:names (
436:CYP51A1
434:), and
310:reduced
300:of the
249:enzymes
150:cd00302
89:PROSITE
70:PF00067
3722:Lyases
3468:CYP710
3436:CYP503
3372:CYP305
3333:CYP197
3297:CYP170
3262:CYP152
3255:CYP147
3248:CYP139
3234:CYP123
3213:CYP111
3194:CYP109
3175:CYP107
3149:CYP105
2388:CYP3 (
2143:plant:
2108:human:
1602:pseudo
1490:
1455:
1447:
1416:
1406:
1365:
1339:
1331:
1287:228675
1285:
1250:
1215:
1207:
1148:
1138:
1097:
1055:
1020:
1010:
949:
885:
877:
757:ferryl
685:ligand
624:RH + O
552:and a
470:allele
468:) and
406:TBXAS1
394:CYP5A1
378:CYP450
366:CYP2E1
362:CYP2E1
326:oxygen
216:PDBsum
190:
180:
114:SUPFAM
56:Symbol
3674:Types
3066:CYP81
3048:CYP80
3006:CYP79
2991:CYP76
2976:CYP75
2965:CYP74
2912:CYP71
2895:CYP61
2882:CYP56
2869:CYP55
2855:CYP53
2848:CYP52
2832:CYP51
2810:CYP46
2800:CYP39
2790:CYP35
2784:CYP29
2773:CYP28
2754:CYP27
2734:CYP26
2727:CYP25
2716:CYP24
2710:CYP23
2699:CYP22
2689:CYP21
2668:CYP20
2658:CYP19
2652:CYP18
2641:CYP17
2635:CYP16
2628:CYP15
2621:CYP14
2614:CYP13
2607:CYP12
2575:CYP11
2568:CYP10
2390:CYP3A
2260:1.14)
2168:Other
2100:Other
1931:Barts
1852:Adult
1813:Fetal
1453:S2CID
1337:S2CID
1213:S2CID
1075:(PDF)
883:S2CID
737:, or
640:Many
563:R/cyb
499:NADPH
337:Genes
255:as a
237:P450s
110:SCOPe
101:SCOP2
43:CYP51
3766:list
3759:EC7
3753:list
3746:EC6
3740:list
3733:EC5
3727:list
3720:EC4
3714:list
3707:EC3
3701:list
3694:EC2
3688:list
3681:EC1
2561:CYP9
2546:CYP8
2532:CYP7
2517:CYP6
2506:CYP5
2428:CYP4
2297:CYP2
2266:CYP1
1711:HBE1
1699:HBG2
1694:HBG1
1626:HBQ1
1597:HBA2
1592:HBA1
1537:heme
1488:PMID
1445:PMID
1414:PMID
1363:ISBN
1329:PMID
1283:PMID
1248:PMID
1205:PMID
1146:PMID
1095:ISBN
1053:PMID
1041:1814
1018:PMID
947:PMID
875:PMID
538:and
524:(CYB
512:(cyb
501:via
420:ane
412:hrom
388:P450
322:iron
253:heme
241:CYPs
209:PDBj
205:PDBe
188:ECOD
178:Pfam
138:2bdm
106:2cpp
65:Pfam
59:p450
2944:BA1
2940:AV1
2936:AJ4
2475:F22
2470:F12
2465:F11
2440:A22
2435:A11
2419:A43
2414:A37
2339:C19
2334:C18
2314:A13
1997:HbO
1983:HbE
1969:HbC
1955:HbS
1941:HbD
1921:HbH
1873:HbA
1859:HbA
1834:HbA
1682:HBD
1670:HBB
1653:on
1638:HBM
1614:HBZ
1480:doi
1476:115
1437:doi
1404:PMC
1396:doi
1392:118
1321:doi
1309:330
1275:doi
1240:doi
1236:195
1197:doi
1193:104
1136:PMC
1128:doi
1124:124
1087:doi
1045:doi
1008:PMC
998:doi
939:doi
867:doi
588:FMN
559:CYB
528:R).
476:).
390:BM3
384:450
382:CYP
380:or
374:CYP
344:CYP
328:).
277:,
247:of
239:or
196:PDB
162:265
145:CDD
3784::
3409:,
3403:,
3399:,
3395:,
3391:,
3387:(
3378:M2
3310:B1
3303:A1
3270:B1
3267:A1
3205:E1
3200:B1
3186:G1
3181:A1
3160:D7
3155:A1
3082:E9
3080:,
3078:E7
3076:,
3074:E3
3072:,
3070:E1
3060:G2
3058:,
3056:B1
3054:,
3052:A1
3042:D4
3040:,
3038:D3
3036:,
3034:D2
3032:,
3030:D1
3028:,
3026:B3
3024:,
3022:B2
3020:,
3018:B1
3016:,
3014:A2
3012:,
3010:A1
3000:M7
2998:,
2996:B6
2984:B1
2982:,
2980:A1
2970:D1
2942:,
2938:,
2934:,
2932:Z6
2930:,
2928:C4
2926:,
2924:C3
2922:,
2920:C2
2918:,
2916:C1
2887:A1
2875:A1
2861:A1
2841:F1
2839:,
2837:A1
2814:A1
2804:A1
2794:B1
2777:A1
2767:C1
2765:,
2763:B1
2761:,
2759:A1
2747:C1
2745:,
2743:B1
2741:,
2739:A1
2720:A1
2703:A1
2693:A2
2672:A1
2662:A1
2645:A1
2600:C1
2598:,
2596:B3
2594:,
2592:B2
2590:,
2588:B1
2586:,
2584:A2
2582:,
2580:A1
2554:B1
2552:,
2550:A1
2540:B1
2538:,
2536:A1
2526:M2
2524:,
2522:G1
2510:A1
2490:Z1
2485:X1
2480:V2
2460:F8
2455:F3
2450:F2
2445:B1
2409:A7
2404:A5
2399:A4
2379:W1
2374:U1
2369:S1
2364:R1
2359:J2
2354:F1
2349:E1
2344:D6
2329:C9
2324:C8
2319:B6
2309:A7
2304:A6
2288:B1
2283:A5
2278:A2
2273:A1
2258:EC
2252::
2248:,
1999:(α
1985:(α
1971:(α
1957:(α
1943:(α
1933:(γ
1923:(β
1892:(α
1878:(α
1861:(α
1836:(α
1822:(α
1797:(ζ
1783:(ζ
1769:(α
1755:(ζ
1655:11
1577:16
1486:.
1474:.
1451:.
1443:.
1412:.
1402:.
1390:.
1386:.
1349:^
1335:.
1327:.
1319:.
1307:.
1295:^
1281:.
1271:90
1269:.
1246:.
1234:.
1211:.
1203:.
1191:.
1179:^
1144:.
1134:.
1122:.
1118:.
1093:.
1083:14
1077:.
1051:.
1039:.
1016:.
1006:.
992:.
988:.
945:.
933:.
914:.
903:^
881:.
873:.
863:31
861:.
733:,
612:).
396:,
306:nm
273:,
207:;
203:;
186:/
126:39
112:/
108:/
3768:)
3764:(
3755:)
3751:(
3742:)
3738:(
3729:)
3725:(
3716:)
3712:(
3703:)
3699:(
3690:)
3686:(
3510:e
3503:t
3496:v
3413:)
3084:)
3068:(
3062:)
3050:(
3044:)
3008:(
3002:)
2994:(
2986:)
2978:(
2972:)
2968:(
2946:)
2914:(
2843:)
2835:(
2816:)
2812:(
2806:)
2802:(
2796:)
2792:(
2779:)
2775:(
2769:)
2757:(
2749:)
2737:(
2722:)
2718:(
2705:)
2701:(
2695:)
2691:(
2674:)
2670:(
2664:)
2660:(
2647:)
2643:(
2602:)
2578:(
2556:)
2548:(
2542:)
2534:(
2528:)
2520:(
2512:)
2508:(
2392:)
2238:e
2231:t
2224:v
2038:/
2007:)
2005:2
2003:β
2001:2
1993:)
1991:2
1989:β
1987:2
1979:)
1977:2
1975:β
1973:2
1965:)
1963:2
1961:β
1959:2
1951:)
1949:2
1947:β
1945:2
1937:)
1935:4
1927:)
1925:4
1900:)
1898:2
1896:γ
1894:2
1886:)
1884:2
1882:δ
1880:2
1875:2
1869:)
1867:2
1865:β
1863:2
1844:)
1842:2
1840:β
1838:2
1830:)
1828:2
1826:γ
1824:2
1805:)
1803:2
1801:β
1799:2
1791:)
1789:2
1787:γ
1785:2
1777:)
1775:2
1773:ε
1771:2
1763:)
1761:2
1759:ε
1757:2
1706:ε
1689:γ
1677:δ
1665:β
1657::
1633:μ
1621:θ
1609:ζ
1587:α
1579::
1543:)
1539:(
1525:e
1518:t
1511:v
1494:.
1482::
1459:.
1439::
1420:.
1398::
1371:.
1343:.
1323::
1315::
1289:.
1277::
1254:.
1242::
1219:.
1199::
1152:.
1130::
1103:.
1089::
1059:.
1047::
1024:.
1000::
994:4
973:.
953:.
941::
935:3
918:.
889:.
869::
746:2
741:5
693:C
668:.
630:2
626:2
574:.
572:5
565:5
561:5
526:5
520:5
514:5
509:5
448:M
446:e
444:D
440:L
432:1
428:S
425:2
422:A
418:X
416:o
414:B
410:T
408:(
402:2
400:A
235:(
45:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.