804:
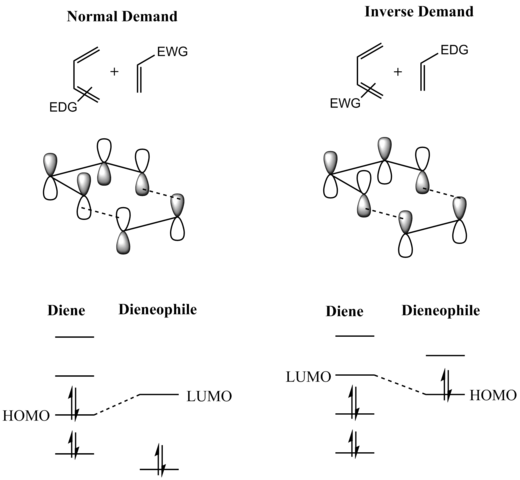
lowering the energy of the dienophile's LUMO and consequently, enhancing the normal electron demand orbital interaction. The Lewis acid binds via a donor-acceptor interaction to the dienophile and via that mechanism polarizes occupied orbital density away from the reactive C=C double bond of the dienophile towards the Lewis acid. This reduced occupied orbital density on C=C double bond of the dienophile will, in turn, engage in a less repulsive closed-shell-closed-shell orbital interaction with the incoming diene, reducing the destabilizing steric Pauli repulsion and hence lowers the Diels–Alder reaction barrier. In addition, the Lewis acid catalyst also increases the asynchronicity of the Diels–Alder reaction, making the occupied π-orbital located on the C=C double bond of the dienophile asymmetric. As a result, this enhanced asynchronicity leads to an extra reduction of the destabilizing steric Pauli repulsion as well as a diminishing pressure on the reactants to deform, in other words, it reduced the destabilizing activation strain (also known as distortion energy). This working catalytic mechanism is known as
77:
982:
549:
216:
1008:
279:
812:
indeed Lewis acid catalysts strengthen the normal electron demand orbital interaction by lowering the LUMO of the dienophile, but, they simultaneously weaken the inverse electron demand orbital interaction by also lowering the energy of the dienophile's HOMO. These two counteracting phenomena effectively cancel each other, resulting in nearly unchanged orbital interactions when compared to the corresponding uncatalyzed Diels–Alder reactions and making this not the active mechanism behind Lewis acid-catalyzed Diels–Alder reactions.
1113:
943:
884:
1073:
1047:
421:
918:
299:
coefficients gives the "ortho" product as seen in case 1 in the figure below. A diene substituted at C2 as in case 2 below has the largest HOMO coefficient at C1, giving rise to the "para" product. Similar analyses for the corresponding inverse-demand scenarios gives rise to the analogous products as seen in cases 3 and 4. Examining the canonical mesomeric forms above, it is easy to verify that these results are in accord with expectations based on consideration of electron density and polarization.
303:
516:
1103:
629:
428:
1183:
1165:
1146:
676:
undergoes Diels–Alder reaction with a diene introducing such a functionality onto the product molecule. A series of reactions then follow to transform the functionality into a desirable group. The end product cannot be made in a single DA step because equivalent dienophile is either unreactive or inaccessible. An example of such approach is the use of
800:, can catalyze Diels–Alder reactions by binding to the dienophile. Traditionally, the enhanced Diels-Alder reactivity is ascribed to the ability of the Lewis acid to lower the LUMO of the activated dienophile, which results in a smaller normal electron demand HOMO-LUMO orbital energy gap and hence more stabilizing orbital interactions.
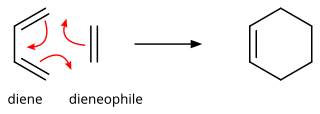
185:/suprafacial interaction of a 4π electron system (the diene structure) with a 2π electron system (the dienophile structure), an interaction that leads to a transition state without an additional orbital symmetry-imposed energetic barrier and allows the Diels–Alder reaction to take place with relative ease.
168:° for a typical Diels–Alder reaction, the microscopic reverse of a Diels–Alder reaction becomes favorable at high temperatures, although this is of synthetic importance for only a limited range of Diels–Alder adducts, generally with some special structural features; this reverse reaction is known as the
811:
The original rationale behind Lewis acid-catalyzed Diels–Alder reactions is incorrect, because besides lowering the energy of the dienophile's LUMO, the Lewis acid also lowers the energy of the HOMO of the dienophile and hence increases the inverse electron demand LUMO-HOMO orbital energy gap. Thus,
624:
systems by elimination of the 1-methoxy substituent after deprotection of the enol silyl ether. Other synthetically useful derivatives of
Danishefsky's diene include 1,3-alkoxy-1-trimethylsiloxy-1,3-butadienes (Brassard dienes) and 1-dialkylamino-3-trimethylsiloxy-1,3-butadienes (Rawal dienes). The
310:
In general, with respect to the energetically most well-matched HOMO-LUMO pair, maximizing the interaction energy by forming bonds between centers with the largest frontier orbital coefficients allows the prediction of the main regioisomer that will result from a given diene-dienophile combination.
223:
The "prevailing opinion" is that most Diels–Alder reactions proceed through a concerted mechanism; the issue, however, has been thoroughly contested. Despite the fact that the vast majority of Diels–Alder reactions exhibit stereospecific, syn addition of the two components, a diradical intermediate
211:
orbital and electron-donating substituents on the dienophile raise the energy of its filled π orbital sufficiently that the interaction between these two orbitals becomes the most energetically significant stabilizing orbital interaction. Regardless of which situation pertains, the HOMO and LUMO of
199:
as the highest occupied molecular orbital (HOMO) with the electron-deficient dienophile's π* as the lowest unoccupied molecular orbital (LUMO). However, the HOMO–LUMO energy gap is close enough that the roles can be reversed by switching electronic effects of the substituents on the two components.
298:
positions of disubstituted arenes. For example, in a normal-demand scenario, a diene bearing an electron-donating group (EDG) at C1 has its largest HOMO coefficient at C4, while the dienophile with an electron withdrawing group (EWG) at C1 has the largest LUMO coefficient at C2. Pairing these two
192:(FMO) makes plain why this is so. (The same conclusion can be drawn from an orbital correlation diagram or a Dewar-Zimmerman analysis.) For the more common "normal" electron demand Diels–Alder reaction, the more important of the two HOMO/LUMO interactions is that between the electron-rich diene's
180:
The reaction is an example of a concerted pericyclic reaction. It is believed to occur via a single, cyclic transition state, with no intermediates generated during the course of the reaction. As such, the Diels–Alder reaction is governed by orbital symmetry considerations: it is classified as a
458:
situation based on the relative orientation of the two separate components when they react with each other. In the context of the Diels–Alder reaction, the transition state in which the most significant substituent (an electron-withdrawing and/or conjugating group) on the dienophile is oriented
274:
Frontier molecular orbital theory has also been used to explain the regioselectivity patterns observed in Diels–Alder reactions of substituted systems. Calculation of the energy and orbital coefficients of the components' frontier orbitals provides a picture that is in good accord with the more
803:
Recent studies, however, have shown that this rationale behind Lewis acid-catalyzed Diels–Alder reactions is incorrect. It is found that Lewis acids accelerate the Diels–Alder reaction by reducing the destabilizing steric Pauli repulsion between the interacting diene and dienophile and not by
696:=C=O), which would produce same product in one DA step. The problem is that ketene itself cannot be used in Diels–Alder reactions because it reacts with dienes in unwanted manner (by cycloaddition), and therefore "masked functionality" approach has to be used. Other such functionalities are
675:
In a normal demand Diels–Alder reaction, the dienophile has an electron-withdrawing group in conjugation with the alkene; in an inverse-demand scenario, the dienophile is conjugated with an electron-donating group. Dienophiles can be chosen to contain a "masked functionality". The dienophile
139:
in 1950. Through the simultaneous construction of two new carbon–carbon bonds, the Diels–Alder reaction provides a reliable way to form six-membered rings with good control over the regio- and stereochemical outcomes. Consequently, it has served as a powerful and widely applied tool for the
539:
attractions may play a part as well, and solvent can sometimes make a substantial difference in selectivity. The secondary orbital overlap explanation was first proposed by
Woodward and Hoffmann. In this explanation, the orbitals associated with the group in conjugation with the dienophile
348:: HOMO raising and LUMO lowering (Ph, vinyl)) are considered, resulting in a total of 18 possible combinations. The maximization of orbital interaction correctly predicts the product in all cases for which experimental data is available. For instance, in uncommon combinations involving
1160:
of the thiophenyl group to give the sulfoxide as below proceeded enantiospecifically due to the predefined stereochemistry of the propargylic alcohol. In this way, the single allene isomer formed could direct the Diels–Alder reaction to occur on only one face of the generated 'diene'.
3385:
Diels, O.; Alder, K. (1929). "Synthesen in der hydroaromatischen Reihe, III. Mitteilung: Synthese von
Terpenen, Camphern, hydroaromatischen und heterocyclischen Systemen. Mitbearbeitet von den Herren Wolfgang Lübbert, Erich Naujoks, Franz Querberitz, Karl Röhl, Harro Segeberg".
224:
has been postulated (and supported with computational evidence) on the grounds that the observed stereospecificity does not rule out a two-step addition involving an intermediate that collapses to product faster than it can rotate to allow for inversion of stereochemistry.
352:
groups on both diene and dienophile, a 1,3-substitution pattern may be favored, an outcome not accounted for by a simplistic resonance structure argument. However, cases where the resonance argument and the matching of largest orbital coefficients disagree are rare.
4039:
Danishefsky, S.; Hirama, M.; Fritsch, N.; Clardy, J. (1979). "Synthesis of disodium prephenate and disodium epiprephenate. Stereochemistry of prephenic acid and an observation on the base-catalyzed rearrangement of prephenic acid to p-hydroxyphenyllactic acid".
3451:
Diels, O.; Alder, K. (1929). "Synthesen in der hydroaromatischen Reihe, VI. Mitteilung, Kurt Alder und
Gerhard Stein: Über partiell hydrierte Naphtho- und Anthrachinone mit Wasserstoff in γ- bzw. δ-Stellung. (Mitbearbeitet von Paul Pries und Hans Winckler)".
1179:-quinodimethane, which reacted intermolecularly to give the tetracycline skeleton. The dienophile's free hydroxyl group is integral to the success of the reaction, as hydroxyl-protected variants did not react under several different reaction conditions.
1494:
Gajewski, J. J.; Peterson, K. B.; Kagel, J. R. (1987). "Transition-state structure variation in the Diels–Alder reaction from secondary deuterium kinetic isotope effects: The reaction of a nearly symmetrical diene and dienophile is nearly synchronous".
544:
transition state. Although the original explanation only invoked the orbital on the atom α to the dienophile double bond, Salem and Houk have subsequently proposed that orbitals on the α and β carbons both participate when molecular geometry allows.
606:-butyl-buta-1,3-diene, for example, is 27 times more reactive than simple butadiene. Conversely, a diene having bulky substituents at both C2 and C3 is less reactive because the steric interactions between the substituents destabilize the s-
3901:
Diels, O.; Schrum, H. (1937). "Synthesen in der hydroaromatischen Reihe,XXVII. "Dien-Synthesen"︁ stickstoffhaltiger
Heteroringe. 12. Über den Abbau der "gelben Substanz"︁ zu einem Isomeren des Norlupinans (1-Methyl-octahydro-indolizin)".
3707:
Diels, O.; Alder, K. (1933). "Synthesen in der hydroaromatischen Reihe, XVIII "Dien-Synthesen"︁ stickstoffhaltiger
Heteroringe. 6. Dien-Synthesen des Pyridins. Zur Kenntnis des Chinolizins, Indolizins, Norlupinans und Pseudolupinins".
3429:
Diels, O.; Alder, K. (1929). "Synthesen in der hydroaromatischen Reihe, V. Über Δ4-Tetrahydro-o-phthalsäure (Stellungnahme zu der
Mitteilung von E. H. Farmer und F. L. Warren: Eigenschaften konjugierter Doppelbindungen (VII)".
3794:
Diels, O.; Friedrichsen, W. (1934). "Synthesen in der hydroaromatischen Reihe, XXII. Über die
Anthracen–C4O3-Addukte, ihre Eignung zu Dien-Synthesen und ein neues Prinzip zur Synthese von Phtalsäuren und Dihydro-phtalsäuren".
3729:
Diels, O.; Alder, K. (1934). "Synthesen in der hydroaromatischen Reihe, XIX. "Dien-Synthesen"︁ stickstoffhaltiger
Heteroringe. 7. Zur Kenntnis der Primärprodukte bei den Dien-Synthesen des Pyridins, Chinolins und Chinaldins".
1557:
Goldstein, E.; Beno, B.; Houk, K. N. (1996). "Density
Functional Theory Prediction of the Relative Energies and Isotope Effects for the Concerted and Stepwise Mechanisms of the Diels−Alder Reaction of Butadiene and Ethylene".
212:
the components are in phase and a bonding interaction results as can be seen in the diagram below. Since the reactants are in their ground state, the reaction is initiated thermally and does not require activation by light.
3621:
Diels, O.; Alder, K. (1931). "Synthesen in der hydroaromatischen Reihe, XIV. Mitteilung. ("Dien-Synthesen"︁ stickstoffhaltiger Heteroringe. 2. Dien-Synthesen der Pyrrole mit Acetylen-dicarbonsäure und mit ihren Estern.)".
160:. The reaction has also been generalized to other ring sizes, although none of these generalizations have matched the formation of six-membered rings in terms of scope or versatility. Because of the negative values of Δ
3685:
Diels, O.; Alder, K. (1932). "Synthesen in der hydroaromatischen Reihe, XVII. Mitteilung. ("Dien-Synthesen"︁ stickstoffhaltiger Heteroringe. 5. Dien-Synthesen des Pyridins, Chinolins, Chinaldins und Isochinolins.)".
3879:
Diels, O.; Harms, J. (1935). "Synthesen in der hydroaromatischen Reihe, XXVI. "Dien-Synthesen"︁ stickstoffhaltiger Heteroringe. 11. Über die aus Isochinolin und Acetylen-dicarbonsäureester entstehenden Addukte".
3772:
Diels, O.; Meyer, R. (1934). "Synthesen in der hydroaromatischen Reihe, XXI. "Dien-Synthesen"︁ stickstoffhaltiger Heteroringe. 8. Über den Verlauf der Dien-Synthese des Pyridins in methylalkoholischer Lösung".
4538:
English Translation of Diels and Alder's seminal 1928 German article that won them the Nobel prize. English title: 'Syntheses of the hydroaromatic series'; German title "Synthesen in der hydroaromatischen
3407:
Diels, O.; Alder, K. (1929). "Synthesen in der hydroaromatischen Reihe, IV. Mitteilung: Über die Anlagerung von Maleinsäure-anhydrid an arylierte Diene, Triene und Fulvene (Mitbearbeitet von Paul Pries)".
3557:
Diels, O.; Alder, K. (1931). "Synthesen in der hydroaromatischen Reihe, XI. Mitteilung. ("Dien-Synthesen"︁ des Cyclopentadiens, Cyclo-hexadiens und Butadiens mit Acetylen-dicarbonsäure und ihren Estern".
625:
increased reactivity of these and similar dienes is a result of synergistic contributions from donor groups at C1 and C3, raising the HOMO significantly above that of a comparable monosubstituted diene.
247:
as solvent. Several explanations for this effect have been proposed, such as an increase in effective concentration due to hydrophobic packing or hydrogen-bond stabilization of the transition state.
3664:
Diels, O.; Alder, K. (1932). "Synthesen in der hydroaromatischen Reihe, XVI. Mitteilung. ("Dien-Synthesen"︁ stickstoffhaltiger Heteroringe. 4. Dien-Synthesen der Pyrrole, Imidazole und Pyrazole.)".
4121:
Nicolaou, K. C.; Yang, Z.; Liu, J. J.; Ueno, H.; Nantermet, P. G.; Guy, R. K.; Claiborne, C. F.; Renaud, J.; Couladouros, E. A.; Paulvannan, K.; Sorensen, E. J. (1994). "Total synthesis of taxol".
684:=CClCN). When reacted with a diene, this dienophile will introduce α-chloronitrile functionality onto the product molecule. This is a "masked functionality" which can be then hydrolyzed to form a
4094:
Martin, S. F.; Rueger, H.; Williamson, S. A.; Grzejszczak, S. (1987). "General strategies for the synthesis of indole alkaloids. Total synthesis of (±)-reserpine and (±)-α-yohimbine".
5502:
3099:
Johnson, J. S.; Evans, D. A. (2000). "Chiral bis(oxazoline) copper(II) complexes: Versatile catalysts for enantioselective cycloaddition, Aldol, Michael, and carbonyl Ene reactions".
302:
454:
Diels–Alder reactions in which adjacent stereocenters are generated at the two ends of the newly formed single bonds imply two different possible stereochemical outcomes. This is a
3002:
Corey, E. J.; Loh, T. P. (1991). "First application of attractive intramolecular interactions to the design of chiral catalysts for highly enantioselective Diels–Alder reactions".
527:
The most widely accepted explanation for the origin of this effect is a favorable interaction between the π systems of the dienophile and the diene, an interaction described as a
3923:
Diels, O.; Pistor, H. (1937). "Synthesen in der hydroaromatischen Reihe, XXVIII. "Dien-Synthesen"︁ stickstoffhaltiger Heteroringe. 13. α-Picolin und Acetylen-dicarbonsäureeste".
3600:
Diels, O.; Alder, K. (1931). "Synthesen in der hydroaromatischen Reihe, XIII. Mitteilung. ("Dien-Synthesen"︁ sauerstoffhaltiger Heteroringe. 3. Dien-Synthesen der Cumaline.)".
4253:
Gibbs, R. A.; Okamura, W. H. (1988). "A short enantioselective synthesis of (+)-sterpurene: Complete intramolecular transfer of central to axial to central chiral elements".
3579:
Diels, O.; Alder, K. (1931). "Synthesen in der hydroaromatischen Reihe, XII. Mitteilung. ("Dien-Synthesen"︁ sauerstoffhaltiger Heteroringe. 2. Dien-Synthesen des Furans.)".
3816:
Diels, O.; Möller, F. (1935). "Synthesen in der hydroaromatischen Reihe, XXIII. "Dien-Synthesen"︁ stickstoffhaltiger Heteroringe. 9. Stilbazol und Acetylen-dicarbonester".
3643:
Diels, O.; Alder, K. (1931). "Synthesen in der hydroaromatischen Reihe, XV. Mitteilung. ("Dien-Synthesen"︁ stickstoffhaltiger Heteroringe. 3. Dien-Synthesen der Indole.)".
583:
component of the Diels–Alder reaction can be either open-chain or cyclic, and it can host many different types of substituents. It must, however, be able to exist in the s-
3134:
Ahrendt, K. A.; Borths, C. J.; MacMillan, D. W. C. (2000). "New Strategies for Organic Catalysis: The First Highly Enantioselective Organocatalytic Diels−Alder Reaction".
515:
3064:
Ryu, D. H.; Corey, E. J. (2003). "Triflimide activation of a chiral oxazaborolidine leads to a more general catalytic system for enantioselective Diels-Alder addition".
1922:
Wannere, Chaitanya S.; Paul, Ankan; Herges, Rainer; Houk, K. N.; Schaefer, Henry F.; Schleyer, Paul Von Ragué (2007). "The existence of secondary orbital interactions".
1000:. The Diels–Alder reaction establishes the relative stereochemistry of three contiguous stereocenters on the prostaglandin cyclopentane core. Activation by Lewis acidic
7717:
677:
1065:. Epoxidation from the less hindered α-face, followed by epoxide opening at the less hindered C18 afforded the desired stereochemistry at these positions, while the
1585:
Breslow, R.; Guo, T. (1988). "Diels-Alder reactions in nonaqueous polar solvents. Kinetic effects of chaotropic and antichaotropic agents and of β-cyclodextrin".
875:
intermediate which can then be trapped to form an aromatic product. This reaction allows the formation of heavily functionalized aromatic rings in a single step.
2698:
Hansen, Thomas; Vermeeren, Pascal; Yoshisada, Ryoji; Filippov, Dmitri V.; van der Marel, Gijsbert A.; Codée, Jeroen D. C.; Hamlin, Trevor A. (19 February 2021).
1142:
gave the second ring of the alkaloid core. The diene in this instance is notable as an example of a 1-amino-3-siloxybutadiene, otherwise known as a Rawal diene.
1099:. The stereospecificity of the Diels–Alder reaction in this instance allowed for the definition of four stereocenters that were carried on to the final product.
140:
introduction of chemical complexity in the synthesis of natural products and new materials. The underlying concept has also been applied to π-systems involving
3473:
Diels, O.; Alder, K. (1930). "Synthesen in der hydroaromatischen Reihe, VII. Mitteilung. (Mitbearbeitet von den Harren Ernst Petersen und Franz Querberitz.)".
1669:
Blokzijl, Wilfried; Engberts, Jan B. F. N. (1992). "Initial-State and Transition-State Effects on Diels–Alder Reactions in Water and Mixed Aqueous Solvents".
3858:
Diels, O.; Reese, J. (1935). "Synthesen in der hydroaromatischen Reihe, XXV Über die Addukte aus Acetylen-dicarbonsäureester und Hydrazo-Verbindungen (2)".
1696:
Ashby, E. C.; Chao, L.-C.; Neumann, H. M. (1973). "Organometallic reaction mechanisms. XII. Mechanism of methylmagnesium bromide addition to benzonitrile".
981:
649:
278:
962:
from 1928 to 1937. The first 19 articles were authored by Diels and Alder, while the later articles were authored by Diels and various other coauthors.
820:
Many methods have been developed for influencing the stereoselectivity of the Diels–Alder reaction, such as the use of chiral auxiliaries, catalysis by
2584:
1821:
Kobuke, Y.; Sugimoto, T.; Furukawa, J.; Fueno, T. (1972). "Role of attractive interactions in endo–exo stereoselectivities of Diels–Alder reactions".
6833:
6778:
1427:
Dewar, M. J.; Olivella, S.; Stewart, J. J. (1986). "Mechanism of the Diels-Alder reaction: Reactions of butadiene with ethylene and cyanoethylenes".
2975:
Evans, D. A.; Chapman, K. T.; Bisaha, J. (1988). "Asymmetric Diels–Alder cycloaddition reactions with chiral α,β-unsaturated N-acyloxazolidinones".
613:
Dienes with bulky terminal substituents (C1 and C4) decrease the rate of reaction, presumably by impeding the approach of the diene and dienophile.
7546:
4315:
Dauben, W. G.; Kessel, C. R.; Takemura, K. H. (1980). "Simple, efficient total synthesis of cantharidin via a high-pressure Diels–Alder reaction".
3751:
Diels, O.; Reese, J. (1934). "Synthesen in der hydroaromatischen Reihe, XX. Über die Anlagerung von Acetylen-dicarbonsäureester an Hydrazobenzol".
201:
480:
In cases where the dienophile has a single electron-withdrawing / conjugating substituent, or two electron-withdrawing / conjugating substituents
6888:
2747:
Tiekink, Eveline H.; Vermeeren, Pascal; Bickelhaupt, F. Matthias; Hamlin, Trevor A. (7 October 2021). "How Lewis Acids Catalyze Ene Reactions".
258:
and stereochemical relationship of substituents of the two components compared to each other are controlled by electronic effects. However, for
7038:
5672:
3029:
Corey, E. J.; Shibata, T.; Lee, T. W. (2002). "Asymmetric Diels-Alder reactions catalyzed by a triflic acid activated chiral oxazaborolidine".
1908:
958:
2015:
Craig, D.; Shipman, J. J.; Fowler, R. B. (1961). "The Rate of Reaction of Maleic Anhydride with 1,3-Dienes as Related to Diene Conformation".
7866:
7767:
1522:
Houk, K. N.; Lin, Y. T.; Brown, F. K. (1986). "Evidence for the concerted mechanism of the Diels–Alder reaction of butadiene with ethylene".
63:
7541:
5367:
3536:
Diels, O.; Alder, K. (1931). "Synthesen in der hydroaromatischen Reihe, X. Mitteilung: "Dien-Synthesen"︁ mit Pyrrol und seinen Homologen".
3494:
Diels, O.; Alder, K. (1931). "Synthesen in der hydroaromatischen Reihe, VIII. Mitteilung: Dien-Synthesen des Anthracens. Anthracen-Forme".
1775:
Bérubé, G.; DesLongchamps, P. (1987). "Stéréosélection acyclique-1,5: Synthèse de la chaîne latérale optiquement active de la vitamine E".
259:
4226:
Kozmin, S. A.; Rawal, V. H. (1998). "A General Strategy to Aspidosperma Alkaloids: Efficient, Stereocontrolled Synthesis of Tabersonine".
2939:
White, James D.; Shaw, Subrata (2011). "cis-2,5-Diaminobicyclooctane, a New Scaffold for Asymmetric Catalysis via Salen−Metal Complexes".
587:
conformation, since this is the only conformer that can participate in the reaction. Though butadienes are typically more stable in the s-
6643:
4564:
2890:
Vermeeren, Pascal; Tiezza, Marco Dalla; Dongen, Michelle; Fernández, Israel; Bickelhaupt, F. Matthias; Hamlin, Trevor A. (21 July 2021).
2477:
Vermeeren, Pascal; Tiezza, Marco Dalla; Dongen, Michelle; Fernández, Israel; Bickelhaupt, F. Matthias; Hamlin, Trevor A. (21 July 2021).
1848:
Williamson, K. L.; Hsu, Y.-F. L. (1970). "Stereochemistry of the Diels–Alder reaction. II. Lewis acid catalysis of syn-anti isomerism".
7213:
6413:
5157:
4067:
Wender, P. A.; Schaus, J. M.; White, A. W. (1980). "General methodology for cis-hydroisoquinoline synthesis: Synthesis of reserpine".
4004:
Corey, E. J.; Weinshenker, N. M.; Schaaf, T. K.; Huber, W. (1969). "Stereo-controlled synthesis of prostaglandins F-2a and E-2 (dl)".
3343:
Diels, O.; Alder, K. (1928). "Synthesen in der hydroaromatischen Reihe, I. Mitteilung: Anlagerungen von "Di-en"-kohlenwasserstoffen".
7378:
7308:
7288:
6783:
5950:
5412:
3515:
Diels, O.; Alder, K. (1931). "Synthesen in der hydroaromatischen Reihe, IX. Mitteilung: Synthese des Camphenilons und des Santens".
492:. As originally stated by Alder, the transition state that is preferred is the one with a "maximum accumulation of double bonds."
5831:
5387:
1322:
2069:
Savard, J.; Brassard, P. (1979). "Regiospecific syntheses of quinones using vinylketene acetals derived from unsaturated esters".
4600:
7133:
7611:
7561:
5965:
3837:
Diels, O.; Kech, H. (1935). "Synthesen in der hydroaromatischen Reihe, XXIV "Dien-Synthesen"︁ stickstoffhaltiger Heteroringe".
1152:(+)-Sterpurene can be prepared by asymmetric D-A reaction that featured a remarkable intramolecular Diels–Alder reaction of an
628:
215:
7068:
659:, being less aromatic (and therefore more reactive for Diels–Alder syntheses) in its central ring can form a 9,10 adduct with
7707:
7516:
7173:
7153:
7113:
5920:
4863:
2188:
1383:
1309:
Nicolaou, K. C.; Snyder, S. A.; Montagnon, T.; Vassilikogiannakis, G. (2002). "The Diels-Alder Reaction in Total Synthesis".
7702:
7632:
7531:
7188:
7043:
6673:
6518:
6128:
5755:
5532:
1344:
Atilla Tasdelen, Mehmet (2011). "Diels–Alder "click" reactions: recent applications in polymer and material science".
860:
4172:
Narasaka, K.; Shimada, S.; Osoda, K.; Iwasawa, N. (1991). "Phenylboronic Acid as a Template in the Diels-Alder Reaction".
7782:
7566:
6878:
6368:
6043:
1175:
was prepared with a Diels–Alder reaction. Thermally initiated, conrotatory opening of the benzocyclobutene generated the
227:
There is a notable rate enhancement when certain Diels–Alder reactions are carried out in polar organic solvents such as
6588:
488:
transition state is typically preferred, despite often being more sterically congested. This preference is known as the
7777:
7491:
7353:
7143:
7108:
4520:
4491:
4458:
4441:
Heintzelman, G. R.; Meigh, I. R.; Mahajan, Y. R.; Weinreb, S. W. (2005). "Diels-Alder Reactions of Imino Dienophiles".
4425:
4392:
4359:
3325:
1732:
1290:
1257:
7667:
7606:
7138:
7053:
7023:
7003:
6868:
6863:
6238:
6163:
5806:
5760:
5627:
4888:
2311:
2286:
1884:
952:
189:
2843:"Bifunctional Hydrogen Bond Donor-Catalyzed Diels–Alder Reactions: Origin of Stereoselectivity and Rate Enhancement"
2430:"Bifunctional Hydrogen Bond Donor-Catalyzed Diels–Alder Reactions: Origin of Stereoselectivity and Rate Enhancement"
286:
In general, the regioselectivity found for both normal and inverse electron-demand Diels–Alder reaction follows the
7772:
7732:
7682:
7358:
7158:
6908:
6838:
5327:
4898:
3977:
Woodward, R. B.; Sondheimer, F.; Taub, D.; Heusler, K.; McLamore, W. M. (1952). "The Total Synthesis of Steroids".
1642:
Breslow, R.; Rizzo, C. J. (1991). "Chaotropic salt effects in a hydrophobically accelerated Diels–Alder reaction".
851:
catalysis, and many other methodologies exist for effecting diastereo- and enantioselective Diels–Alder reactions.
833:
250:
The geometry of the diene and dienophile components each propagate into stereochemical details of the product. For
6973:
5866:
5587:
7871:
7526:
7368:
7238:
7233:
7048:
6523:
6433:
6023:
5955:
5846:
5422:
5177:
5102:
4557:
2096:
Kozmin, S. A.; Rawal, V. H. (1997). "Preparation and Diels−Alder Reactivity of 1-Amino-3-siloxy-1,3-butadienes".
7283:
7812:
7697:
7596:
7536:
7183:
6978:
6938:
6913:
6823:
6283:
5317:
5247:
4883:
4813:
1215:
1080:
169:
6403:
7802:
7388:
7278:
6898:
6623:
6408:
6353:
6198:
6158:
5990:
5745:
5462:
5312:
1157:
48:
7762:
7323:
6768:
7797:
7712:
7687:
7662:
7647:
7571:
7486:
7383:
7343:
7208:
7163:
6928:
6473:
6458:
6323:
6113:
5781:
5537:
5217:
5192:
5162:
4753:
3364:
Diels, O.; Alder, K. (1929). "Synthesen in der hydroaromatischen Reihe, II. Mitteilung: Über Cantharidin".
1220:
1001:
754:
742:
7747:
7692:
7637:
7348:
7268:
7168:
6883:
6848:
6693:
6583:
6298:
6293:
6118:
6078:
5975:
5786:
5750:
5602:
5592:
5447:
5307:
5167:
5117:
5112:
5087:
5047:
4993:
4758:
4748:
4723:
124:
7876:
7722:
7423:
7228:
6663:
6548:
6228:
6203:
6143:
6000:
5735:
5442:
5222:
5187:
5092:
4783:
4718:
4550:
2611:
540:
double-bond overlap with the interior orbitals of the diene, a situation that is possible only for the
484:
to each other, the outcome can often be predicted. In these "normal demand" Diels–Alder scenarios, the
56:
7013:
5277:
290:, so named, because the cyclohexene product bears substituents in positions that are analogous to the
7822:
7727:
7581:
7461:
7433:
7403:
7318:
7248:
7203:
7178:
7098:
6998:
6958:
6653:
6273:
6263:
6188:
5712:
5572:
5567:
5547:
5232:
5029:
5008:
4968:
4893:
1748:
Kirmse, W.; Mönch, D. (1991). "Umlagerungen von 1,4,4- und 2,2,5-Trimethylbicyclooct-6-yl-Kationen".
766:
4713:
3952:
7886:
7881:
7787:
7677:
7657:
7363:
7273:
7243:
7118:
7073:
6903:
6813:
6743:
6628:
6618:
6448:
6005:
5945:
5910:
5717:
5697:
5657:
5432:
5302:
5227:
4978:
4818:
4808:
4738:
2841:
Vermeeren, Pascal; Hamlin, Trevor A.; Bickelhaupt, F. Matthias; Fernández, Israel (17 March 2021).
2428:
Vermeeren, Pascal; Hamlin, Trevor A.; Bickelhaupt, F. Matthias; Fernández, Israel (17 March 2021).
2171:
Roush, W. R. (1991). "Intramolecular Diels–Alder Reactions". In Trost, B. M.; Flemming, I. (eds.).
136:
7253:
2649:
Vermeeren, Pascal; Brinkhuis, Francine; Hamlin, Trevor A.; Bickelhaupt, F. Matthias (April 2020).
2330:
Vermeeren, Pascal; Hamlin, Trevor A.; Fernández, Israel; Bickelhaupt, F. Matthias (6 April 2020).
7891:
7861:
7757:
7616:
7466:
7408:
7333:
7313:
7033:
6983:
6843:
6808:
6748:
6678:
6233:
5980:
5960:
5692:
5612:
5507:
5467:
5437:
5372:
5257:
5242:
5152:
5142:
4803:
4728:
4683:
3212:"Hexadehydro-Diels–Alder Reaction: Benzyne Generation via Cycloisomerization of Tethered Triynes"
1794:
Houk, K. N.; Luskus, L. J. (1971). "Influence of steric interactions on endo stereoselectivity".
1210:
1135:
1069:-fusion was achieved with hydrogenation, again proceeding primarily from the less hindered face.
5018:
306:
Regioselectivity in normal (1 and 2) and inverse (3 and 4) electron demand Diels-Alder reactions
7496:
7218:
6968:
6948:
6923:
6873:
6788:
6763:
6718:
6688:
6668:
6638:
6603:
6558:
6533:
6508:
6393:
6318:
6098:
5791:
5727:
5527:
5252:
5172:
4858:
4833:
4610:
4605:
1205:
617:
616:
An especially reactive diene is 1-methoxy-3-trimethylsiloxy-buta-1,3-diene, otherwise known as
594:
A bulky substituent at the C2 or C3 position can increase reaction rate by destabilizing the s-
365:. Stereochemical information of the diene and the dienophile are retained in the product, as a
244:
76:
523:
rule applies when there the electron-withdrawing groups on the dienophile are all on one side.
377:, resp.) relationship on the double bond of the dienophile give rise to substituents that are
7832:
7418:
7373:
7088:
7058:
7028:
6963:
6943:
6858:
6853:
6818:
6773:
6758:
6753:
6733:
6723:
6658:
6648:
6578:
6528:
6048:
5851:
5427:
5382:
5212:
5202:
4948:
4873:
4668:
4630:
4375:
Butz, L. W.; Rytina, A. W. (1949). "The Diels–Alder Reaction Quinones and Other Cyclenones".
4199:
Smith, A. B.; Sestelo, J. P.; Dormer, P. G. (1995). "Total Synthesis of (−)-Furaquinocin C".
2205:
1376:
1057:-fused D and E rings was formed by a Diels–Alder reaction. Intramolecular Diels–Alder of the
993:
922:
255:
153:
4878:
1007:
7601:
7551:
7501:
7481:
7471:
7328:
7303:
7018:
7008:
6893:
6708:
6703:
6633:
6418:
6218:
6178:
6108:
6073:
6028:
5995:
5861:
5836:
5816:
5637:
5597:
5557:
5522:
5452:
5207:
5077:
5052:
4590:
4130:
3956:
2539:
467:
transition state, it is oriented away from it. (There is a more general usage of the terms
2794:"Origin of rate enhancement and asynchronicity in iminium catalyzed Diels–Alder reactions"
2792:
Vermeeren, Pascal; Hamlin, Trevor A.; Fernández, Israel; Bickelhaupt, F. Matthias (2020).
2381:"Origin of rate enhancement and asynchronicity in iminium catalyzed Diels–Alder reactions"
2379:
Vermeeren, Pascal; Hamlin, Trevor A.; Fernández, Israel; Bickelhaupt, F. Matthias (2020).
459:
towards the diene π system and slips under it as the reaction takes place is known as the
8:
7807:
7792:
7438:
7413:
7398:
7393:
7123:
7078:
7063:
6953:
6933:
6828:
6713:
6698:
6543:
6488:
6478:
6443:
6208:
6083:
6058:
5970:
5826:
5811:
5796:
5617:
5562:
5332:
5182:
5127:
4998:
4913:
4773:
4698:
2892:"Lewis Acid-Catalyzed Diels-Alder Reactions: Reactivity Trends across the Periodic Table"
2479:"Lewis Acid-Catalyzed Diels-Alder Reactions: Reactivity Trends across the Periodic Table"
1615:
Rideout, D. C.; Breslow, R. (1980). "Hydrophobic acceleration of Diels-Alder reactions".
1015:
837:
556:
536:
112:
7817:
6468:
5652:
4843:
4134:
2543:
749:
can be used, either as the dienophile or at various sites in the diene, to form various
7556:
7506:
7476:
7338:
7128:
6918:
6803:
6738:
6728:
6493:
6423:
6388:
6383:
6363:
6358:
6303:
6213:
6063:
5925:
5915:
5821:
5607:
5552:
5482:
5402:
5297:
5197:
5132:
5057:
4903:
4768:
4703:
4688:
4342:
Holmes, H. L. (1948). "The Diels–Alder Reaction Ethylenic and Acetylenic Dienophiles".
4154:
3290:
3236:
3211:
3187:
3162:
2916:
2891:
2867:
2842:
2818:
2793:
2774:
2724:
2699:
2675:
2650:
2631:
2560:
2527:
2503:
2478:
2454:
2429:
2405:
2380:
2356:
2331:
2180:
2153:
1955:
1902:
1273:
Holmes, H. L. (1948). "The Diels-Alder Reaction Ethylenic and Acetylenic Dienophiles".
1088:
797:
548:
468:
275:
straightforward analysis of the substituents' resonance effects, as illustrated below.
116:
2082:
7293:
6613:
6498:
6463:
6428:
6373:
6328:
6243:
6223:
6173:
6168:
6138:
6123:
6033:
5940:
5876:
5841:
5667:
5542:
5417:
5342:
5322:
5237:
5072:
5067:
5013:
4923:
4828:
4788:
4743:
4625:
4620:
4585:
4516:
4487:
4454:
4421:
4388:
4355:
4297:
4280:
Charest, M. G.; Siegel, D. R.; Myers, A. G. (2005). "Synthesis of (-)-tetracycline".
4146:
4021:
3321:
3282:
3241:
3192:
3116:
3081:
3046:
2957:
2921:
2872:
2823:
2778:
2729:
2680:
2635:
2623:
2615:
2565:
2508:
2459:
2410:
2361:
2307:
2282:
2259:
2184:
2157:
1947:
1939:
1890:
1880:
1728:
1539:
1444:
1379:
1326:
1286:
1253:
1087:
shown below suffered from poor yield and regioselectivity; however, when directed by
1061:
below with subsequent extrusion of carbon dioxide via a retro afforded the bicyclic
950:
The work by Diels and Alder is described in a series of 28 articles published in the
829:
821:
793:
789:
228:
93:
85:
6288:
3294:
1959:
7827:
7672:
7642:
7586:
7511:
7443:
7198:
7148:
6993:
6798:
6573:
6568:
6513:
6503:
6278:
6088:
6068:
6038:
5935:
5871:
5856:
5687:
5642:
5632:
5622:
5517:
5497:
5492:
5477:
5472:
5352:
5347:
5287:
5272:
5262:
5107:
5097:
4963:
4953:
4853:
4848:
4823:
4763:
4615:
4574:
4479:
4446:
4413:
4380:
4347:
4324:
4289:
4262:
4235:
4208:
4181:
4158:
4138:
4103:
4076:
4049:
4013:
3986:
3932:
3911:
3889:
3867:
3846:
3825:
3804:
3782:
3760:
3739:
3717:
3695:
3673:
3652:
3631:
3609:
3588:
3567:
3545:
3524:
3503:
3482:
3461:
3439:
3417:
3395:
3373:
3352:
3313:
3272:
3231:
3223:
3182:
3174:
3143:
3108:
3073:
3038:
3011:
2984:
2949:
2911:
2903:
2862:
2854:
2813:
2805:
2764:
2756:
2719:
2711:
2670:
2662:
2607:
2599:
2555:
2547:
2498:
2490:
2449:
2441:
2400:
2392:
2351:
2343:
2251:
2220:
2176:
2145:
2105:
2078:
2051:
2042:
Danishefsky, S.; Kitahara, T. (1974). "Useful diene for the Diels–Alder reaction".
2024:
1997:
1931:
1857:
1830:
1803:
1757:
1705:
1678:
1651:
1624:
1594:
1567:
1531:
1504:
1436:
1353:
1318:
1278:
1245:
1196:
Synthetic applications of the Diels–Alder reaction have been reviewed extensively.
1096:
997:
930:
660:
497:
263:
4943:
7737:
7428:
7263:
7258:
6553:
6538:
6483:
6438:
6398:
6348:
6313:
6308:
6253:
6248:
6183:
6133:
6053:
5881:
5765:
5740:
5702:
5677:
5662:
5647:
5582:
5457:
5407:
5397:
5377:
5337:
5147:
5137:
5122:
4918:
4838:
4708:
4678:
4663:
4658:
2941:
2603:
1139:
926:
899:
825:
701:
532:
455:
236:
232:
4483:
4450:
4417:
4384:
4351:
3227:
2583:
Hamlin, Trevor A.; Bickelhaupt, F. Matthias; Fernández, Israel (20 April 2021).
2136:
Ranganathan, S.; Ranganathan, D.; Mehrotra, A. K. (1977). "Ketene Equivalents".
1282:
1249:
942:
883:
420:
7742:
7652:
7591:
6683:
6593:
6563:
6338:
6193:
5930:
5707:
5577:
5392:
5362:
5062:
4958:
4733:
4595:
1112:
734:
641:
385:, resp.) on those same carbons with respect to the cyclohexene ring. Likewise,
362:
251:
204:, electron-withdrawing substituents on the diene lower the energy of its empty
145:
4542:
970:
The Diels–Alder reaction was one step in an early preparation of the steroids
917:
7855:
7752:
7453:
7298:
7193:
6988:
6378:
6343:
6333:
6268:
6258:
6148:
5985:
5801:
5512:
5487:
5357:
5003:
4988:
4973:
4868:
4798:
4778:
4693:
3936:
3915:
3893:
3871:
3850:
3829:
3808:
3786:
3764:
3743:
3721:
3699:
3677:
3656:
3635:
3613:
3592:
3571:
3549:
3528:
3507:
3486:
3465:
3443:
3421:
3399:
3377:
3356:
3317:
3261:"Recent advances of Diels–Alderases involved in natural product biosynthesis"
2619:
2263:
2224:
2001:
1943:
1894:
1761:
1109:
A Diels–Alder reaction is a key step in the synthesis of (-)-furaquinocin C.
1072:
1046:
1022:
990:
785:
636:
Unstable (and thus highly reactive) dienes can be synthetically useful, e.g.
621:
620:. It has particular synthetic utility as means of furnishing α,β–unsaturated
120:
32:
2715:
6793:
6153:
5905:
5682:
5282:
5082:
4933:
4928:
4793:
4648:
4301:
3286:
3245:
3196:
3120:
3085:
3050:
2961:
2925:
2907:
2876:
2858:
2827:
2760:
2733:
2684:
2666:
2627:
2569:
2512:
2494:
2463:
2445:
2414:
2365:
2347:
1951:
1543:
1448:
1330:
1172:
1102:
770:
738:
501:
4536:
4408:
Kloetzel, M. C. (1948). "The Diels–Alder Reaction with Maleic Anhydride".
4150:
4025:
1323:
10.1002/1521-3773(20020517)41:10<1668::AID-ANIE1668>3.0.CO;2-Z
1240:
Kloetzel, M. C. (1948). "The Diels–Alder Reaction with Maleic Anhydride".
5292:
4938:
4908:
4673:
4185:
3308:
Behr, Arno (2000). "Organometallic Compounds and Homogeneous Catalysis".
3161:
Hoye, T. R.; Baire, B.; Niu, D.; Willoughby, P. H.; Woods, B. P. (2012).
2149:
1308:
1190:
1119:
1037:
975:
848:
733:
are also known and are collectively called hetero-Diels–Alder reactions.
713:
697:
648:, require forcing conditions and/or highly reactive dienophiles, such as
645:
591:
conformation, for most cases energy difference is small (~2–5 kcal/mol).
182:
108:
4328:
4266:
4212:
4107:
4080:
4053:
4017:
3990:
3178:
3015:
2988:
2255:
2055:
2028:
1861:
1834:
1807:
1709:
1682:
1655:
1628:
1598:
1535:
1508:
1440:
1091:
the desired adduct could be obtained in 61% yield after cleavage of the
1083:. The intermolecular reaction of the hydroxy-pyrone and α,β–unsaturated
369:
addition with respect to each component. For example, substituents in a
7576:
7103:
6453:
3277:
3260:
2809:
2769:
2551:
2526:
Vermeeren, Pascal; Hamlin, Trevor A.; Bickelhaupt, F. Matthias (2021).
2396:
1357:
1019:
911:
903:
898:
The retro-Diels–Alder reaction is used in the industrial production of
891:
887:
Asymmetric Diels-Alder reaction is one step in the biosynthesis of the
781:
730:
656:
141:
132:
128:
4293:
4239:
3147:
3112:
3077:
3042:
2953:
2325:
2323:
2239:
2109:
1935:
1571:
4142:
1182:
1164:
1033:
971:
705:
664:
555:
Often, as with highly substituted dienes, very bulky dienophiles, or
509:
427:
1145:
4983:
4653:
2840:
2791:
2648:
2427:
2378:
2329:
2320:
1092:
1058:
1026:
762:
709:
505:
439:
240:
135:
in 1928. For the discovery of this reaction, they were awarded the
2206:"Iminium Ion-Based Diels–Alder Reactions: N-Benzyl-2-Azanorborene"
4643:
4093:
2746:
1126:
relative stereochemistry of the alkaloid core. Conversion of the
985:
Diels-Alder in the total synthesis of cortisone by R. B. Woodward
910:. The Diels–Alder reaction is also employed in the production of
907:
872:
844:
758:
719:
2697:
978:. The reaction involved the addition of butadiene to a quinone.
311:
In a more sophisticated treatment, three types of substituents (
262:
reactions, the conformational stability of the structure of the
4474:
Ciganek, E. (1984). "The Intramolecular Diels-Alder Reaction".
1153:
1131:
1062:
888:
864:
746:
689:
685:
644:
can be generated in situ. In contrast, stable dienes, such as
100:
4440:
4038:
2889:
2476:
2240:"Frontier molecular orbital theory of cycloaddition reactions"
2135:
496:
selectivity is typically higher for rigid dienophiles such as
119:. More specifically, it is classified as a thermally allowed
3976:
2700:"How Lewis Acids Catalyze Ring-Openings of Cyclohexene Oxide"
1084:
989:
Diels–Alder reactions were used in the original synthesis of
868:
580:
560:
149:
96:
2651:"How Alkali Cations Catalyze Aromatic Diels-Alder Reactions"
871:
are used instead of alkenes and dienes, forming an unstable
282:
Resonance structures of normal-demand dienes and dienophiles
4513:
Advanced Organic Chemistry: Part B: Reactions and Synthesis
4171:
4003:
2126:
p. 318-323. Editura Academiei Republicii Socialiste România
1820:
737:, for example, can successfully react with dienes to yield
2582:
2525:
2129:
1193:
in 1980 by Diels–Alder reaction, utilizing high pressure.
356:
1983:
1981:
808:, which is operative in a variety of organic reactions.
361:
Diels–Alder reactions, as concerted cycloadditions, are
1988:
Backer, H. J. (1939). "Le 2,3-Ditertiobutylbutadiène".
1079:
A pyranone was similarly used as the dienophile in the
688:. α-Chloroacrylonitrile dienophile is an equivalent of
632:
General form of Danishefsky, Brassard, and Rawal dienes
598:
conformation and forcing the diene into the reactive s-
181:
cycloaddition, indicating that it proceeds through the
3160:
3133:
1978:
878:
712:
functionalities (both are acetylene equivalents), and
202:
inverse (reverse) electron-demand Diels–Alder reaction
1921:
1493:
405:
substituents at these carbons of the product whereas
243:
for example is 700 times faster in water relative to
7718:
Erlenmeyer–Plöchl azlactone and amino-acid synthesis
4314:
1426:
1122:
was prepared by a Diels–Alder reaction to establish
1014:
A Diels–Alder reaction was used in the synthesis of
700:
substituents (yielding exocyclic double bonds after
450:
product ratio for this and various other dienophiles
4165:
4120:
2585:"The Pauli Repulsion-Lowering Concept in Catalysis"
2528:"Origin of asynchronicity in Diels–Alder reactions"
2041:
1774:
946:
The reaction discovered by Diels and Alder in 1928.
4279:
4198:
2974:
2014:
965:
563:as diene), steric effects can override the normal
6779:Divinylcyclopropane-cycloheptadiene rearrangement
4066:
3793:
2306:(2nd ed.). Oxford: Oxford University Press.
2279:Molecular orbitals and organic chemical reactions
1875:Woodward, R. B.; Hoffmann, R. (22 October 2013).
1556:
7853:
4511:Carey, Francis A.; Sundberg, Richard J. (2007).
2332:"How Lewis Acids Catalyze Diels–Alder Reactions"
1874:
1695:
1668:
761:compounds (R-N=O) can react with dienes to form
438:transition states for cyclopentadiene adding to
111:derivative. It is the prototypical example of a
4572:
3028:
1741:
1725:Frontier Orbital and Organic Chemical Reactions
1343:
7039:Thermal rearrangement of aromatic hydrocarbons
5673:Thermal rearrangement of aromatic hydrocarbons
3454:Berichte der Deutschen Chemischen Gesellschaft
3432:Berichte der Deutschen Chemischen Gesellschaft
3410:Berichte der Deutschen Chemischen Gesellschaft
3366:Berichte der Deutschen Chemischen Gesellschaft
3310:Ullmann's Encyclopedia of Industrial Chemistry
2068:
1614:
959:Berichte der deutschen chemischen Gesellschaft
720:Variants on the classical Diels–Alder reaction
7768:Lectka enantioselective beta-lactam synthesis
5028:
4558:
1847:
1689:
1610:
1608:
1304:
1302:
729:Diels–Alder reactions involving at least one
7547:Inverse electron-demand Diels–Alder reaction
5368:Heterogeneous metal catalyzed cross-coupling
4252:
3258:
3098:
2203:
1641:
1521:
1422:
1420:
1370:
902:. Cyclopentadiene is a precursor to various
547:
514:
419:
6889:Lobry de Bruyn–Van Ekenstein transformation
4225:
3922:
3900:
3815:
3210:Fluegel, Lucas L.; Hoye, Thomas R. (2021).
3209:
2612:1871.1/a0090b38-9ab8-4c32-9d9a-b3d5de4e5ed3
2095:
1747:
769:can be utilized as a dienophile to prepare
4565:
4551:
4374:
3878:
3857:
3771:
3750:
3728:
3706:
3684:
3663:
3642:
3620:
3599:
3578:
3556:
3535:
3514:
3493:
3472:
3450:
3428:
3406:
3384:
3363:
3342:
1990:Recueil des Travaux Chimiques des Pays-Bas
1907:: CS1 maint: location missing publisher (
1793:
1605:
1584:
1299:
854:
7379:Petrenko-Kritschenko piperidone synthesis
6834:Fritsch–Buttenberg–Wiechell rearrangement
3836:
3276:
3259:Minami, Atsushi; Oikawa, Hideaki (2016).
3235:
3186:
2938:
2915:
2866:
2817:
2768:
2723:
2674:
2559:
2502:
2453:
2404:
2355:
1777:Bulletin de la Société Chimique de France
1417:
1373:Modern Organic Synthesis: An Introduction
815:
7542:Intramolecular Diels–Alder cycloaddition
4407:
4317:Journal of the American Chemical Society
4282:Journal of the American Chemical Society
4255:Journal of the American Chemical Society
4228:Journal of the American Chemical Society
4201:Journal of the American Chemical Society
4096:Journal of the American Chemical Society
4069:Journal of the American Chemical Society
4042:Journal of the American Chemical Society
4006:Journal of the American Chemical Society
3979:Journal of the American Chemical Society
3154:
3136:Journal of the American Chemical Society
3066:Journal of the American Chemical Society
3063:
3031:Journal of the American Chemical Society
3004:Journal of the American Chemical Society
3001:
2977:Journal of the American Chemical Society
2281:. Chichester, West Sussex, U.K.: Wiley.
2044:Journal of the American Chemical Society
2017:Journal of the American Chemical Society
1850:Journal of the American Chemical Society
1823:Journal of the American Chemical Society
1796:Journal of the American Chemical Society
1698:Journal of the American Chemical Society
1671:Journal of the American Chemical Society
1644:Journal of the American Chemical Society
1617:Journal of the American Chemical Society
1587:Journal of the American Chemical Society
1560:Journal of the American Chemical Society
1524:Journal of the American Chemical Society
1497:Journal of the American Chemical Society
1429:Journal of the American Chemical Society
1239:
941:
916:
882:
776:
426:
260:intramolecular Diels–Alder cycloaddition
219:FMO analysis of the Diels–Alder reaction
75:
4473:
2336:Angewandte Chemie International Edition
2301:
2276:
1722:
1477:
1475:
1462:
1460:
1458:
1407:
1405:
1403:
1401:
1399:
1397:
1395:
1311:Angewandte Chemie International Edition
1171:The tetracyclic core of the antibiotic
1053:In another synthesis of reserpine, the
1036:uses a Diels–Alder reaction to set the
357:Stereospecificity and stereoselectivity
7854:
7562:Metal-centered cycloaddition reactions
7214:Debus–Radziszewski imidazole synthesis
5158:Bodroux–Chichibabin aldehyde synthesis
4341:
3163:"The hexadehydro-Diels–Alder reaction"
2197:
1987:
1272:
512:, selectivity is not very pronounced.
463:transition state. In the alternative
80:Diels–Alder reaction, simplest example
7708:Diazoalkane 1,3-dipolar cycloaddition
7612:Vinylcyclopropane (5+2) cycloaddition
7517:Diazoalkane 1,3-dipolar cycloaddition
7289:Hurd–Mori 1,2,3-thiadiazole synthesis
6784:Dowd–Beckwith ring-expansion reaction
5951:Hurd–Mori 1,2,3-thiadiazole synthesis
5027:
4864:LFER solvent coefficients (data page)
4546:
3252:
2749:European Journal of Organic Chemistry
2204:Grieco, P. A.; Larsen, S. D. (1990).
2170:
1371:Zweifel, G. S.; Nantz, M. H. (2007).
1134:by Wittig olefination and subsequent
724:
667:, a weak dienophile, at 250 °C.
337:: HOMO and LUMO raising (Me, OMe, NMe
235:, and even in water. The reaction of
7867:Carbon-carbon bond forming reactions
6519:Sharpless asymmetric dihydroxylation
5756:Methoxymethylenetriphenylphosphorane
4515:(5th ed.). New York: Springer.
3307:
2932:
2238:Houk, Kendall N. (1 November 1975).
2237:
1877:The conservation of orbital symmetry
1472:
1455:
1392:
753:-heterocyclic compounds through the
254:reactions especially, the preferred
6644:Allen–Millar–Trippett rearrangement
3953:"The Nobel Prize in Chemistry 1950"
2532:Physical Chemistry Chemical Physics
879:Applications and natural occurrence
269:
104:
13:
7783:Nitrone-olefin (3+2) cycloaddition
7778:Niementowski quinazoline synthesis
7567:Nitrone-olefin (3+2) cycloaddition
7492:Azide-alkyne Huisgen cycloaddition
7354:Niementowski quinazoline synthesis
7109:Azide-alkyne Huisgen cycloaddition
6414:Meerwein–Ponndorf–Verley reduction
5966:Leimgruber–Batcho indole synthesis
4445:. Vol. 65. pp. 141–599.
2181:10.1016/B978-0-08-052349-1.00131-1
1924:Journal of Computational Chemistry
1181:
1163:
1144:
1111:
1101:
1071:
1045:
1018:, a biosynthetic precursor of the
1006:
980:
627:
301:
277:
266:can be an overwhelming influence.
214:
188:A consideration of the reactants'
152:, which furnish the corresponding
14:
7903:
7607:Trimethylenemethane cycloaddition
7309:Johnson–Corey–Chaykovsky reaction
7174:Cadogan–Sundberg indole synthesis
7154:Bohlmann–Rahtz pyridine synthesis
7114:Baeyer–Emmerling indole synthesis
5921:Cadogan–Sundberg indole synthesis
5413:Johnson–Corey–Chaykovsky reaction
4530:
4379:. Vol. 5. pp. 136–192.
3925:Justus Liebigs Annalen der Chemie
3904:Justus Liebigs Annalen der Chemie
3882:Justus Liebigs Annalen der Chemie
3860:Justus Liebigs Annalen der Chemie
3839:Justus Liebigs Annalen der Chemie
3818:Justus Liebigs Annalen der Chemie
3797:Justus Liebigs Annalen der Chemie
3775:Justus Liebigs Annalen der Chemie
3753:Justus Liebigs Annalen der Chemie
3732:Justus Liebigs Annalen der Chemie
3710:Justus Liebigs Annalen der Chemie
3688:Justus Liebigs Annalen der Chemie
3666:Justus Liebigs Annalen der Chemie
3645:Justus Liebigs Annalen der Chemie
3624:Justus Liebigs Annalen der Chemie
3602:Justus Liebigs Annalen der Chemie
3581:Justus Liebigs Annalen der Chemie
3560:Justus Liebigs Annalen der Chemie
3538:Justus Liebigs Annalen der Chemie
3517:Justus Liebigs Annalen der Chemie
3496:Justus Liebigs Annalen der Chemie
3475:Justus Liebigs Annalen der Chemie
3388:Justus Liebigs Annalen der Chemie
3345:Justus Liebigs Annalen der Chemie
2175:. Vol. 5. pp. 513–550.
953:Justus Liebigs Annalen der Chemie
670:
477:in stereochemical nomenclature.)
92:is a chemical reaction between a
7703:Cook–Heilbron thiazole synthesis
7532:Hexadehydro Diels–Alder reaction
7359:Niementowski quinoline synthesis
7189:Cook–Heilbron thiazole synthesis
7134:Bischler–Möhlau indole synthesis
7044:Tiffeneau–Demjanov rearrangement
6674:Baker–Venkataraman rearrangement
5832:Horner–Wadsworth–Emmons reaction
5503:Mizoroki-Heck vs. Reductive Heck
5388:Horner–Wadsworth–Emmons reaction
4899:Neighbouring group participation
4478:. Vol. 32. pp. 1–374.
4346:. Vol. 4. pp. 60–173.
2704:The Journal of Organic Chemistry
1277:. Vol. 4. pp. 60–173.
1043:framework of the D and E rings.
921:Typical route for production of
861:hexadehydro Diels–Alder reaction
826:small organic molecule catalysts
7239:Fiesselmann thiophene synthesis
7069:Westphalen–Lettré rearrangement
7049:Vinylcyclopropane rearrangement
6879:Kornblum–DeLaMare rearrangement
6524:Epoxidation of allylic alcohols
6434:Noyori asymmetric hydrogenation
6369:Kornblum–DeLaMare rearrangement
6044:Gallagher–Hollander degradation
4504:
4467:
4434:
4401:
4368:
4335:
4308:
4273:
4246:
4219:
4192:
4114:
4087:
4060:
4032:
3997:
3970:
3945:
3334:
3301:
3203:
3127:
3092:
3057:
3022:
2995:
2968:
2883:
2834:
2785:
2740:
2691:
2642:
2576:
2519:
2470:
2421:
2372:
2295:
2270:
2231:
2173:Comprehensive Organic Synthesis
2164:
2116:
2089:
2062:
2035:
2008:
1966:
1915:
1868:
1841:
1814:
1787:
1768:
1716:
1662:
1635:
1578:
1550:
1515:
1487:
1130:-aldehyde to its corresponding
966:Applications in total synthesis
741:rings, a reaction known as the
156:; this variant is known as the
7698:Chichibabin pyridine synthesis
7184:Chichibabin pyridine synthesis
7144:Blum–Ittah aziridine synthesis
6979:Ring expansion and contraction
5248:Cross dehydrogenative coupling
4412:. Vol. 4. pp. 1–59.
1364:
1337:
1266:
1244:. Vol. 4. pp. 1–59.
1233:
1:
7668:Bischler–Napieralski reaction
7626:Heterocycle forming reactions
7279:Hemetsberger indole synthesis
7139:Bischler–Napieralski reaction
7054:Wagner–Meerwein rearrangement
7024:Sommelet–Hauser rearrangement
7004:Seyferth–Gilbert homologation
6869:Ireland–Claisen rearrangement
6864:Hofmann–Martius rearrangement
6624:2,3-sigmatropic rearrangement
6239:Corey–Winter olefin synthesis
6164:Barton–McCombie deoxygenation
5807:Corey–Winter olefin synthesis
5761:Seyferth–Gilbert homologation
5628:Seyferth–Gilbert homologation
3101:Accounts of Chemical Research
2896:Chemistry: A European Journal
2847:Chemistry: A European Journal
2592:Accounts of Chemical Research
2483:Chemistry: A European Journal
2434:Chemistry: A European Journal
2244:Accounts of Chemical Research
2083:10.1016/S0040-4039(01)86747-2
1226:
7773:Lehmstedt–Tanasescu reaction
7733:Gabriel–Colman rearrangement
7688:Bucherer carbazole synthesis
7683:Borsche–Drechsel cyclization
7663:Bernthsen acridine synthesis
7648:Bamberger triazine synthesis
7633:Algar–Flynn–Oyamada reaction
7344:Nazarov cyclization reaction
7209:De Kimpe aziridine synthesis
7164:Bucherer carbazole synthesis
7159:Borsche–Drechsel cyclization
6929:Nazarov cyclization reaction
6909:Meyer–Schuster rearrangement
6839:Gabriel–Colman rearrangement
6589:Wolffenstein–Böters reaction
6474:Reduction of nitro compounds
6324:Grundmann aldehyde synthesis
6129:Algar–Flynn–Oyamada reaction
5538:Olefin conversion technology
5533:Nozaki–Hiyama–Kishi reaction
5328:Gabriel–Colman rearrangement
5218:Claisen-Schmidt condensation
5163:Bouveault aldehyde synthesis
2604:10.1021/acs.accounts.1c00016
2098:Journal of Organic Chemistry
1189:Takemura et al. synthesized
663:at 80 °C and even with
574:
567:selectivity in favor of the
318:: HOMO and LUMO lowering (CF
175:
127:. It was first described by
7:
7748:Hantzsch pyridine synthesis
7527:Enone–alkene cycloadditions
7349:Nenitzescu indole synthesis
7269:Hantzsch pyridine synthesis
7234:Ferrario–Ackermann reaction
6884:Kowalski ester homologation
6849:Halogen dance rearrangement
6694:Benzilic acid rearrangement
6119:Akabori amino-acid reaction
6079:Von Braun amide degradation
6024:Barbier–Wieland degradation
5976:Nenitzescu indole synthesis
5956:Kharasch–Sosnovsky reaction
5847:Julia–Kocienski olefination
5751:Kowalski ester homologation
5448:Kowalski ester homologation
5423:Julia–Kocienski olefination
5178:Cadiot–Chodkiewicz coupling
5103:Aza-Baylis–Hillman reaction
5048:Acetoacetic ester synthesis
4759:Dynamic binding (chemistry)
4749:Conrotatory and disrotatory
4724:Charge remote fragmentation
4484:10.1002/0471264180.or032.01
4451:10.1002/0471264180.or065.02
4418:10.1002/0471264180.or004.01
4385:10.1002/0471264180.or005.03
4352:10.1002/0471264180.or004.02
3228:10.1021/acs.chemrev.0c00825
2655:Chemistry: An Asian Journal
1283:10.1002/0471264180.or004.02
1250:10.1002/0471264180.or004.01
1199:
413:-disubstituted dienes give
401:-disubstituted dienes give
190:frontier molecular orbitals
158:hetero-Diels–Alder reaction
10:
7908:
7813:Robinson–Gabriel synthesis
7763:Kröhnke pyridine synthesis
7597:Retro-Diels–Alder reaction
7537:Imine Diels–Alder reaction
7324:Kröhnke pyridine synthesis
6939:Newman–Kwart rearrangement
6914:Mislow–Evans rearrangement
6824:Fischer–Hepp rearrangement
6769:Di-π-methane rearrangement
6549:Stephen aldehyde synthesis
6284:Eschweiler–Clarke reaction
6001:Williamson ether synthesis
5318:Fujiwara–Moritani reaction
5223:Combes quinoline synthesis
5188:Carbonyl olefin metathesis
4889:More O'Ferrall–Jencks plot
4814:Grunwald–Winstein equation
4784:Electron-withdrawing group
4719:Catalytic resonance theory
4510:
3265:The Journal of Antibiotics
2302:Clayden, Jonathan (2012).
1972:
1481:
1466:
1411:
1377:W. H. Freeman and Co.
1216:Imine Diels–Alder reaction
1158:-sigmatropic rearrangement
937:
170:retro-Diels–Alder reaction
7823:Urech hydantoin synthesis
7803:Pomeranz–Fritsch reaction
7728:Fischer oxazole synthesis
7625:
7462:1,3-Dipolar cycloaddition
7452:
7434:Urech hydantoin synthesis
7404:Reissert indole synthesis
7389:Pomeranz–Fritsch reaction
7319:Knorr quinoline synthesis
7249:Fischer oxazole synthesis
7179:Camps quinoline synthesis
7099:1,3-Dipolar cycloaddition
7087:
6999:Semipinacol rearrangement
6974:Ramberg–Bäcklund reaction
6959:Piancatelli rearrangement
6899:McFadyen–Stevens reaction
6654:Alpha-ketol rearrangement
6602:
6409:McFadyen–Stevens reaction
6354:Kiliani–Fischer synthesis
6274:Elbs persulfate oxidation
6199:Bouveault–Blanc reduction
6159:Baeyer–Villiger oxidation
6097:
6014:
5991:Schotten–Baumann reaction
5894:
5867:Ramberg–Bäcklund reaction
5774:
5746:Kiliani–Fischer synthesis
5726:
5588:Ramberg–Bäcklund reaction
5573:Pinacol coupling reaction
5568:Piancatelli rearrangement
5463:Liebeskind–Srogl coupling
5313:Fujimoto–Belleau reaction
5036:
5030:List of organic reactions
4894:Negative hyperconjugation
4639:
4581:
1727:. Chichester, UK: Wiley.
767:Chlorosulfonyl isocyanate
70:
44:Organic Chemistry Portal
38:
23:
7798:Pictet–Spengler reaction
7713:Einhorn–Brunner reaction
7678:Boger pyridine synthesis
7572:Oxo-Diels–Alder reaction
7487:Aza-Diels–Alder reaction
7384:Pictet–Spengler reaction
7284:Hofmann–Löffler reaction
7274:Hegedus indole synthesis
7244:Fischer indole synthesis
7119:Bartoli indole synthesis
7074:Willgerodt rearrangement
6904:McLafferty rearrangement
6814:Ferrier carbocyclization
6629:2,3-Wittig rearrangement
6619:1,2-Wittig rearrangement
6459:Parikh–Doering oxidation
6449:Oxygen rebound mechanism
6114:Adkins–Peterson reaction
6006:Yamaguchi esterification
5946:Hegedus indole synthesis
5911:Bartoli indole synthesis
5782:Bamford–Stevens reaction
5698:Weinreb ketone synthesis
5658:Stork enamine alkylation
5433:Knoevenagel condensation
5303:Ferrier carbocyclization
5193:Castro–Stephens coupling
4819:Hammett acidity function
4809:Free-energy relationship
4754:Curtin–Hammett principle
4739:Conformational isomerism
3937:10.1002/jlac.19375300107
3916:10.1002/jlac.19375300106
3894:10.1002/jlac.19365250107
3872:10.1002/jlac.19355190113
3851:10.1002/jlac.19355190112
3830:10.1002/jlac.19355160104
3809:10.1002/jlac.19345130109
3787:10.1002/jlac.19345130108
3765:10.1002/jlac.19345110114
3744:10.1002/jlac.19345100106
3722:10.1002/jlac.19335050109
3700:10.1002/jlac.19324980103
3678:10.1002/jlac.19324980102
3657:10.1002/jlac.19314900113
3636:10.1002/jlac.19314900112
3614:10.1002/jlac.19314900111
3593:10.1002/jlac.19314900110
3572:10.1002/jlac.19314900109
3550:10.1002/jlac.19314860112
3529:10.1002/jlac.19314860111
3508:10.1002/jlac.19314860110
3487:10.1002/jlac.19304780109
3466:10.1002/cber.19290620872
3444:10.1002/cber.19290620830
3422:10.1002/cber.19290620829
3400:10.1002/jlac.19294700106
3378:10.1002/cber.19290620318
3357:10.1002/jlac.19284600106
3318:10.1002/14356007.a18_215
2225:10.15227/orgsyn.068.0206
2122:Margareta Avram (1983).
2002:10.1002/recl.19390580712
1762:10.1002/cber.19911240136
1221:Aza-Diels–Alder reaction
1081:total synthesis of taxol
1002:cupric tetrafluoroborate
806:Pauli-lowering catalysis
755:aza-Diels–Alder reaction
743:oxo-Diels–Alder reaction
529:secondary orbital effect
137:Nobel Prize in Chemistry
125:Woodward–Hoffmann symbol
107:, to form a substituted
7758:Knorr pyrrole synthesis
7693:Bucherer–Bergs reaction
7638:Allan–Robinson reaction
7617:Wagner-Jauregg reaction
7409:Ring-closing metathesis
7334:Larock indole synthesis
7314:Knorr pyrrole synthesis
7169:Bucherer–Bergs reaction
7034:Stieglitz rearrangement
7014:Skattebøl rearrangement
6984:Ring-closing metathesis
6844:Group transfer reaction
6809:Favorskii rearrangement
6749:Cornforth rearrangement
6679:Bamberger rearrangement
6584:Wolff–Kishner reduction
6404:Markó–Lam deoxygenation
6299:Fleming–Tamao oxidation
6294:Fischer–Tropsch process
5981:Oxymercuration reaction
5961:Knorr pyrrole synthesis
5787:Barton–Kellogg reaction
5693:Wagner-Jauregg reaction
5613:Ring-closing metathesis
5603:Reimer–Tiemann reaction
5593:Rauhut–Currier reaction
5508:Nef isocyanide reaction
5468:Malonic ester synthesis
5438:Knorr pyrrole synthesis
5373:High dilution principle
5308:Friedel–Crafts reaction
5243:Cross-coupling reaction
5168:Bucherer–Bergs reaction
5153:Blanc chloromethylation
5143:Blaise ketone synthesis
5118:Baylis–Hillman reaction
5113:Barton–Kellogg reaction
5088:Allan–Robinson reaction
4994:Woodward–Hoffmann rules
4729:Charge-transfer complex
2716:10.1021/acs.joc.0c02955
1211:Wagner-Jauregg reaction
1136:ring-closing metathesis
855:Hexadehydro Diels–Alder
7872:Ring forming reactions
7723:Feist–Benary synthesis
7497:Bradsher cycloaddition
7467:4+4 Photocycloaddition
7424:Simmons–Smith reaction
7369:Paternò–Büchi reaction
7229:Feist–Benary synthesis
7219:Dieckmann condensation
6969:Pummerer rearrangement
6949:Oxy-Cope rearrangement
6924:Myers allene synthesis
6874:Jacobsen rearrangement
6789:Electrocyclic reaction
6764:Demjanov rearrangement
6719:Buchner ring expansion
6689:Beckmann rearrangement
6669:Aza-Cope rearrangement
6664:Arndt–Eistert reaction
6639:Alkyne zipper reaction
6559:Transfer hydrogenation
6534:Sharpless oxyamination
6509:Selenoxide elimination
6394:Lombardo methylenation
6319:Griesbaum coozonolysis
6229:Corey–Itsuno reduction
6204:Boyland–Sims oxidation
6144:Angeli–Rimini reaction
5792:Boord olefin synthesis
5736:Arndt–Eistert reaction
5728:Homologation reactions
5528:Nitro-Mannich reaction
5443:Kolbe–Schmitt reaction
5253:Cross-coupling partner
5173:Buchner ring expansion
5093:Arndt–Eistert reaction
4859:Kinetic isotope effect
4606:Rearrangement reaction
2908:10.1002/chem.202100522
2859:10.1002/chem.202004496
2761:10.1002/ejoc.202101107
2667:10.1002/asia.202000009
2495:10.1002/chem.202100522
2446:10.1002/chem.202004496
2348:10.1002/anie.201914582
1414:, Part B., pp. 474–526
1206:Bradsher cycloaddition
1186:
1168:
1149:
1116:
1106:
1076:
1050:
1011:
986:
947:
934:
895:
816:Asymmetric Diels–Alder
716:(ketene equivalents).
633:
552:
524:
504:; for others, such as
451:
424:
307:
283:
245:2,2,4-trimethylpentane
220:
103:, commonly termed the
81:
7582:Pauson–Khand reaction
7419:Sharpless epoxidation
7374:Pechmann condensation
7254:Friedländer synthesis
7204:Davis–Beirut reaction
7059:Wallach rearrangement
7029:Stevens rearrangement
6964:Pinacol rearrangement
6944:Overman rearrangement
6859:Hofmann rearrangement
6854:Hayashi rearrangement
6819:Ferrier rearrangement
6774:Dimroth rearrangement
6759:Curtius rearrangement
6754:Criegee rearrangement
6734:Claisen rearrangement
6724:Carroll rearrangement
6659:Amadori rearrangement
6649:Allylic rearrangement
6529:Sharpless epoxidation
6264:Dess–Martin oxidation
6189:Bohn–Schmidt reaction
6049:Hofmann rearrangement
5852:Kauffmann olefination
5775:Olefination reactions
5713:Wurtz–Fittig reaction
5548:Palladium–NHC complex
5428:Kauffmann olefination
5383:Homologation reaction
5233:Corey–House synthesis
5213:Claisen rearrangement
5009:Yukawa–Tsuno equation
4969:Swain–Lupton equation
4949:Spherical aromaticity
4884:Möbius–Hückel concept
4669:Aromatic ring current
4631:Substitution reaction
2277:Fleming, Ian (2009).
1469:, Part A., pp. 836–50
1185:
1167:
1148:
1115:
1105:
1075:
1049:
1010:
984:
945:
923:ethylidene norbornene
920:
886:
830:Evans' oxazolidinones
777:Lewis acid activation
678:α-chloroacrylonitrile
631:
551:
518:
430:
423:
305:
281:
218:
79:
24:Diels–Alder reaction
7788:Paal–Knorr synthesis
7658:Barton–Zard reaction
7602:Staudinger synthesis
7552:Ketene cycloaddition
7522:Diels–Alder reaction
7502:Cheletropic reaction
7482:Alkyne trimerisation
7364:Paal–Knorr synthesis
7329:Kulinkovich reaction
7304:Jacobsen epoxidation
7224:Diels–Alder reaction
7019:Smiles rearrangement
7009:Sigmatropic reaction
6894:Lossen rearrangement
6744:Corey–Fuchs reaction
6709:Boekelheide reaction
6704:Bergmann degradation
6634:Achmatowicz reaction
6419:Methionine sulfoxide
6219:Clemmensen reduction
6179:Bergmann degradation
6109:Acyloin condensation
6074:Strecker degradation
6029:Bergmann degradation
5996:Ullmann condensation
5862:Peterson olefination
5837:Hydrazone iodination
5817:Elimination reaction
5718:Zincke–Suhl reaction
5638:Sonogashira coupling
5598:Reformatsky reaction
5558:Peterson olefination
5523:Nierenstein reaction
5453:Kulinkovich reaction
5268:Diels–Alder reaction
5228:Corey–Fuchs reaction
5208:Claisen condensation
5078:Alkyne trimerisation
5053:Acyloin condensation
5019:Σ-bishomoaromaticity
4979:Thorpe–Ingold effect
4591:Elimination reaction
4186:10.1055/s-1991-28413
3957:The Nobel Foundation
2150:10.1055/s-1977-24362
1723:Fleming, I. (1990).
557:reversible reactions
90:Diels–Alder reaction
49:diels-alder-reaction
7808:Prilezhaev reaction
7793:Pellizzari reaction
7472:(4+3) cycloaddition
7439:Van Leusen reaction
7414:Robinson annulation
7399:Pschorr cyclization
7394:Prilezhaev reaction
7124:Bergman cyclization
7079:Wolff rearrangement
7064:Weerman degradation
6954:Pericyclic reaction
6934:Neber rearrangement
6829:Fries rearrangement
6714:Brook rearrangement
6699:Bergman cyclization
6544:Staudinger reaction
6489:Rosenmund reduction
6479:Reductive amination
6444:Oppenauer oxidation
6234:Corey–Kim oxidation
6209:Cannizzaro reaction
6084:Weerman degradation
6059:Isosaccharinic acid
5971:Mukaiyama hydration
5827:Hofmann elimination
5812:Dehydrohalogenation
5797:Chugaev elimination
5618:Robinson annulation
5563:Pfitzinger reaction
5333:Gattermann reaction
5278:Wulff–Dötz reaction
5258:Dakin–West reaction
5183:Carbonyl allylation
5128:Bergman cyclization
4914:Kennedy J. P. Orton
4834:Hammond's postulate
4804:Flippin–Lodge angle
4774:Electromeric effect
4699:Beta-silicon effect
4684:Baker–Nathan effect
4329:10.1021/ja00542a060
4267:10.1021/ja00220a069
4234:(51): 13523–13524.
4213:10.1021/ja00148a023
4207:(43): 10755–10756.
4135:1994Natur.367..630N
4108:10.1021/ja00254a036
4081:10.1021/ja00539a038
4054:10.1021/ja00517a039
4018:10.1021/ja01048a062
3991:10.1021/ja01137a001
3179:10.1038/nature11518
3016:10.1021/ja00023a066
2989:10.1021/ja00212a037
2902:(41): 10610–10620.
2544:2021PCCP...2320095V
2538:(36): 20095–20106.
2489:(41): 10610–10620.
2256:10.1021/ar50095a001
2071:Tetrahedron Letters
2056:10.1021/ja00832a031
2029:10.1021/ja01474a023
1862:10.1021/ja00728a022
1835:10.1021/ja00765a066
1808:10.1021/ja00747a052
1710:10.1021/ja00796a022
1683:10.1021/ja00039a074
1656:10.1021/ja00011a052
1629:10.1021/ja00546a048
1599:10.1021/ja00225a003
1536:10.1021/ja00263a059
1509:10.1021/ja00252a052
1441:10.1021/ja00279a018
1016:disodium prephenate
906:, which are common
618:Danishefsky's diene
559:(as in the case of
117:concerted mechanism
113:pericyclic reaction
7557:McCormack reaction
7507:Conia-ene reaction
7339:Madelung synthesis
7129:Biginelli reaction
6919:Mumm rearrangement
6804:Favorskii reaction
6739:Cope rearrangement
6729:Chan rearrangement
6494:Rubottom oxidation
6424:Miyaura borylation
6389:Lipid peroxidation
6384:Lindgren oxidation
6364:Kornblum oxidation
6359:Kolbe electrolysis
6304:Fukuyama reduction
6214:Carbonyl reduction
6064:Marker degradation
5926:Diazonium compound
5916:Boudouard reaction
5895:Carbon-heteroatom
5822:Grieco elimination
5608:Rieche formylation
5553:Passerini reaction
5483:Meerwein arylation
5403:Hydroxymethylation
5298:Favorskii reaction
5198:Chan rearrangement
5133:Biginelli reaction
5058:Aldol condensation
4904:2-Norbornyl cation
4879:Möbius aromaticity
4874:Markovnikov's rule
4769:Effective molarity
4714:Bürgi–Dunitz angle
4704:Bicycloaromaticity
3278:10.1038/ja.2016.67
2810:10.1039/D0SC02901G
2552:10.1039/D1CP02456F
2397:10.1039/D0SC02901G
1750:Chemische Berichte
1358:10.1039/C1PY00041A
1187:
1169:
1150:
1117:
1107:
1089:phenylboronic acid
1077:
1051:
1012:
987:
948:
935:
896:
822:chiral Lewis acids
798:aluminium chloride
725:Hetero-Diels–Alder
634:
553:
525:
452:
425:
308:
284:
221:
99:and a substituted
82:
7877:German inventions
7849:
7848:
7845:
7844:
7841:
7840:
7833:Wohl–Aue reaction
7477:6+4 Cycloaddition
7294:Iodolactonization
6614:1,2-rearrangement
6579:Wohl–Aue reaction
6499:Sabatier reaction
6464:Pinnick oxidation
6429:Mozingo reduction
6374:Leuckart reaction
6329:Haloform reaction
6244:Criegee oxidation
6224:Collins oxidation
6174:Benkeser reaction
6169:Bechamp reduction
6139:Andrussow process
6124:Alcohol oxidation
6034:Edman degradation
5941:Haloform reaction
5890:
5889:
5877:Takai olefination
5842:Julia olefination
5668:Takai olefination
5543:Olefin metathesis
5418:Julia olefination
5343:Grignard reaction
5323:Fukuyama coupling
5238:Coupling reaction
5203:Chan–Lam coupling
5073:Alkyne metathesis
5068:Alkane metathesis
4924:Phosphaethynolate
4829:George S. Hammond
4789:Electronic effect
4744:Conjugated system
4626:Stereospecificity
4621:Stereoselectivity
4586:Addition reaction
4575:organic reactions
4476:Organic Reactions
4443:Organic Reactions
4410:Organic Reactions
4377:Organic Reactions
4344:Organic Reactions
4323:(22): 6893–6894.
4294:10.1021/ja052151d
4261:(12): 4062–4063.
4240:10.1021/ja983198k
4180:(12): 1171–1172.
4102:(20): 6124–6134.
4075:(19): 6157–6159.
4048:(23): 7013–7018.
3985:(17): 4223–4251.
3173:(7419): 208–212.
3148:10.1021/ja000092s
3142:(17): 4243–4244.
3113:10.1021/ar960062n
3078:10.1021/ja035393r
3072:(21): 6388–6390.
3043:10.1021/ja025848x
3037:(15): 3808–3809.
3010:(23): 8966–8967.
2954:10.1021/ol2007378
2853:(16): 5180–5190.
2804:(31): 8105–8112.
2755:(37): 5275–5283.
2440:(16): 5180–5190.
2391:(31): 8105–8112.
2342:(15): 6201–6206.
2304:Organic chemistry
2213:Organic Syntheses
2190:978-0-08-052349-1
2110:10.1021/jo970438q
2104:(16): 5252–5253.
2077:(51): 4911–4914.
2050:(25): 7807–7808.
2023:(13): 2885–2891.
1936:10.1002/jcc.20532
1856:(25): 7385–7389.
1829:(10): 3633–3635.
1802:(18): 4606–4607.
1704:(15): 4896–4904.
1677:(13): 5440–5442.
1650:(11): 4340–4341.
1623:(26): 7816–7817.
1593:(17): 5613–5617.
1572:10.1021/ja9601494
1566:(25): 6036–6043.
1503:(18): 5545–5546.
1484:, Part A., p. 839
1435:(19): 5771–5779.
1385:978-0-7167-7266-8
1352:(10): 2133–2145.
1346:Polymer Chemistry
1317:(10): 1668–1698.
1275:Organic Reactions
1242:Organic Reactions
794:tin tetrachloride
790:boron trifluoride
229:dimethylformamide
86:organic chemistry
74:
73:
16:Chemical reaction
7899:
7828:Wenker synthesis
7818:Stollé synthesis
7673:Bobbitt reaction
7643:Auwers synthesis
7587:Povarov reaction
7512:Cyclopropanation
7450:
7449:
7444:Wenker synthesis
7199:Darzens reaction
7149:Bobbitt reaction
6994:Schmidt reaction
6799:Enyne metathesis
6574:Whiting reaction
6569:Wharton reaction
6514:Shapiro reaction
6504:Sarett oxidation
6469:Prévost reaction
6279:Emde degradation
6089:Wohl degradation
6069:Ruff degradation
6039:Emde degradation
5936:Grignard reagent
5872:Shapiro reaction
5857:McMurry reaction
5724:
5723:
5688:Ullmann reaction
5653:Stollé synthesis
5643:Stetter reaction
5633:Shapiro reaction
5623:Sakurai reaction
5518:Negishi coupling
5498:Minisci reaction
5493:Michael reaction
5478:McMurry reaction
5473:Mannich reaction
5353:Hammick reaction
5348:Grignard reagent
5288:Enyne metathesis
5273:Doebner reaction
5263:Darzens reaction
5108:Barbier reaction
5098:Auwers synthesis
5025:
5024:
4999:Woodward's rules
4964:Superaromaticity
4954:Spiroaromaticity
4854:Inductive effect
4849:Hyperconjugation
4824:Hammett equation
4764:Edwards equation
4616:Regioselectivity
4567:
4560:
4553:
4544:
4543:
4526:
4498:
4497:
4471:
4465:
4464:
4438:
4432:
4431:
4405:
4399:
4398:
4372:
4366:
4365:
4339:
4333:
4332:
4312:
4306:
4305:
4277:
4271:
4270:
4250:
4244:
4243:
4223:
4217:
4216:
4196:
4190:
4189:
4169:
4163:
4162:
4143:10.1038/367630a0
4118:
4112:
4111:
4091:
4085:
4084:
4064:
4058:
4057:
4036:
4030:
4029:
4001:
3995:
3994:
3974:
3968:
3967:
3965:
3963:
3949:
3943:
3940:
3919:
3897:
3875:
3854:
3833:
3812:
3790:
3768:
3747:
3725:
3703:
3681:
3660:
3639:
3617:
3596:
3575:
3553:
3532:
3511:
3490:
3469:
3460:(8): 2337–2372.
3447:
3438:(8): 2087–2090.
3425:
3416:(8): 2081–2087.
3403:
3381:
3360:
3338:
3332:
3331:
3305:
3299:
3298:
3280:
3256:
3250:
3249:
3239:
3222:(4): 2413–2444.
3207:
3201:
3200:
3190:
3158:
3152:
3151:
3131:
3125:
3124:
3096:
3090:
3089:
3061:
3055:
3054:
3026:
3020:
3019:
2999:
2993:
2992:
2983:(4): 1238–1256.
2972:
2966:
2965:
2936:
2930:
2929:
2919:
2887:
2881:
2880:
2870:
2838:
2832:
2831:
2821:
2798:Chemical Science
2789:
2783:
2782:
2772:
2744:
2738:
2737:
2727:
2710:(4): 3565–3573.
2695:
2689:
2688:
2678:
2661:(7): 1167–1174.
2646:
2640:
2639:
2598:(8): 1972–1981.
2589:
2580:
2574:
2573:
2563:
2523:
2517:
2516:
2506:
2474:
2468:
2467:
2457:
2425:
2419:
2418:
2408:
2385:Chemical Science
2376:
2370:
2369:
2359:
2327:
2318:
2317:
2299:
2293:
2292:
2274:
2268:
2267:
2235:
2229:
2228:
2210:
2201:
2195:
2194:
2168:
2162:
2161:
2133:
2127:
2120:
2114:
2113:
2093:
2087:
2086:
2066:
2060:
2059:
2039:
2033:
2032:
2012:
2006:
2005:
1985:
1976:
1975:, Part A, p. 149
1970:
1964:
1963:
1919:
1913:
1912:
1906:
1898:
1872:
1866:
1865:
1845:
1839:
1838:
1818:
1812:
1811:
1791:
1785:
1784:
1772:
1766:
1765:
1745:
1739:
1738:
1720:
1714:
1713:
1693:
1687:
1686:
1666:
1660:
1659:
1639:
1633:
1632:
1612:
1603:
1602:
1582:
1576:
1575:
1554:
1548:
1547:
1519:
1513:
1512:
1491:
1485:
1479:
1470:
1464:
1453:
1452:
1424:
1415:
1409:
1390:
1389:
1368:
1362:
1361:
1341:
1335:
1334:
1306:
1297:
1296:
1270:
1264:
1263:
1237:
1173:(-)-tetracycline
1140:Schrock catalyst
1097:neopentyl glycol
931:vinyl norbornene
834:oxazaborolidines
661:maleic anhydride
653:-phenylmaleimide
602:conformation. 2-
498:maleic anhydride
270:Regioselectivity
264:transition state
66:
51:
21:
20:
7907:
7906:
7902:
7901:
7900:
7898:
7897:
7896:
7887:1928 in Germany
7882:1928 in science
7852:
7851:
7850:
7837:
7738:Gewald reaction
7621:
7448:
7429:Skraup reaction
7264:Graham reaction
7259:Gewald reaction
7090:
7083:
6605:
6598:
6554:Swern oxidation
6539:Stahl oxidation
6484:Riley oxidation
6439:Omega oxidation
6399:Luche reduction
6349:Jones oxidation
6314:Glycol cleavage
6309:Ganem oxidation
6254:Davis oxidation
6249:Dakin oxidation
6184:Birch reduction
6134:Amide reduction
6100:
6093:
6054:Hooker reaction
6016:
6010:
5898:
5896:
5886:
5882:Wittig reaction
5770:
5766:Wittig reaction
5741:Hooker reaction
5722:
5703:Wittig reaction
5678:Thorpe reaction
5663:Suzuki reaction
5648:Stille reaction
5583:Quelet reaction
5458:Kumada coupling
5408:Ivanov reaction
5398:Hydrovinylation
5378:Hiyama coupling
5338:Glaser coupling
5148:Blaise reaction
5138:Bingel reaction
5123:Benary reaction
5040:
5038:
5032:
5023:
4919:Passive binding
4839:Homoaromaticity
4689:Baldwin's rules
4664:Antiaromaticity
4659:Anomeric effect
4635:
4577:
4571:
4533:
4523:
4507:
4502:
4501:
4494:
4472:
4468:
4461:
4439:
4435:
4428:
4406:
4402:
4395:
4373:
4369:
4362:
4340:
4336:
4313:
4309:
4278:
4274:
4251:
4247:
4224:
4220:
4197:
4193:
4170:
4166:
4129:(6464): 630–4.
4119:
4115:
4092:
4088:
4065:
4061:
4037:
4033:
4002:
3998:
3975:
3971:
3961:
3959:
3951:
3950:
3946:
3339:
3335:
3328:
3306:
3302:
3257:
3253:
3208:
3204:
3159:
3155:
3132:
3128:
3097:
3093:
3062:
3058:
3027:
3023:
3000:
2996:
2973:
2969:
2937:
2933:
2888:
2884:
2839:
2835:
2790:
2786:
2745:
2741:
2696:
2692:
2647:
2643:
2587:
2581:
2577:
2524:
2520:
2475:
2471:
2426:
2422:
2377:
2373:
2328:
2321:
2314:
2300:
2296:
2289:
2275:
2271:
2250:(11): 361–369.
2236:
2232:
2208:
2202:
2198:
2191:
2169:
2165:
2134:
2130:
2124:Chimie organica
2121:
2117:
2094:
2090:
2067:
2063:
2040:
2036:
2013:
2009:
1986:
1979:
1971:
1967:
1920:
1916:
1900:
1899:
1887:
1873:
1869:
1846:
1842:
1819:
1815:
1792:
1788:
1773:
1769:
1746:
1742:
1735:
1721:
1717:
1694:
1690:
1667:
1663:
1640:
1636:
1613:
1606:
1583:
1579:
1555:
1551:
1520:
1516:
1492:
1488:
1480:
1473:
1465:
1456:
1425:
1418:
1410:
1393:
1386:
1369:
1365:
1342:
1338:
1307:
1300:
1293:
1271:
1267:
1260:
1238:
1234:
1229:
1202:
1032:A synthesis of
968:
940:
927:cyclopentadiene
900:cyclopentadiene
881:
857:
818:
779:
735:Carbonyl groups
727:
722:
702:Wittig reaction
695:
683:
673:
642:quinodimethanes
577:
490:Alder endo rule
456:stereoselective
359:
340:
329:
325:
321:
288:ortho-para rule
272:
237:cyclopentadiene
233:ethylene glycol
210:
198:
178:
62:
47:
17:
12:
11:
5:
7905:
7895:
7894:
7892:Name reactions
7889:
7884:
7879:
7874:
7869:
7864:
7862:Cycloadditions
7847:
7846:
7843:
7842:
7839:
7838:
7836:
7835:
7830:
7825:
7820:
7815:
7810:
7805:
7800:
7795:
7790:
7785:
7780:
7775:
7770:
7765:
7760:
7755:
7750:
7745:
7743:Hantzsch ester
7740:
7735:
7730:
7725:
7720:
7715:
7710:
7705:
7700:
7695:
7690:
7685:
7680:
7675:
7670:
7665:
7660:
7655:
7653:Banert cascade
7650:
7645:
7640:
7635:
7629:
7627:
7623:
7622:
7620:
7619:
7614:
7609:
7604:
7599:
7594:
7592:Prato reaction
7589:
7584:
7579:
7574:
7569:
7564:
7559:
7554:
7549:
7544:
7539:
7534:
7529:
7524:
7519:
7514:
7509:
7504:
7499:
7494:
7489:
7484:
7479:
7474:
7469:
7464:
7458:
7456:
7447:
7446:
7441:
7436:
7431:
7426:
7421:
7416:
7411:
7406:
7401:
7396:
7391:
7386:
7381:
7376:
7371:
7366:
7361:
7356:
7351:
7346:
7341:
7336:
7331:
7326:
7321:
7316:
7311:
7306:
7301:
7296:
7291:
7286:
7281:
7276:
7271:
7266:
7261:
7256:
7251:
7246:
7241:
7236:
7231:
7226:
7221:
7216:
7211:
7206:
7201:
7196:
7191:
7186:
7181:
7176:
7171:
7166:
7161:
7156:
7151:
7146:
7141:
7136:
7131:
7126:
7121:
7116:
7111:
7106:
7101:
7095:
7093:
7085:
7084:
7082:
7081:
7076:
7071:
7066:
7061:
7056:
7051:
7046:
7041:
7036:
7031:
7026:
7021:
7016:
7011:
7006:
7001:
6996:
6991:
6986:
6981:
6976:
6971:
6966:
6961:
6956:
6951:
6946:
6941:
6936:
6931:
6926:
6921:
6916:
6911:
6906:
6901:
6896:
6891:
6886:
6881:
6876:
6871:
6866:
6861:
6856:
6851:
6846:
6841:
6836:
6831:
6826:
6821:
6816:
6811:
6806:
6801:
6796:
6791:
6786:
6781:
6776:
6771:
6766:
6761:
6756:
6751:
6746:
6741:
6736:
6731:
6726:
6721:
6716:
6711:
6706:
6701:
6696:
6691:
6686:
6684:Banert cascade
6681:
6676:
6671:
6666:
6661:
6656:
6651:
6646:
6641:
6636:
6631:
6626:
6621:
6616:
6610:
6608:
6604:Rearrangement
6600:
6599:
6597:
6596:
6594:Zinin reaction
6591:
6586:
6581:
6576:
6571:
6566:
6564:Wacker process
6561:
6556:
6551:
6546:
6541:
6536:
6531:
6526:
6521:
6516:
6511:
6506:
6501:
6496:
6491:
6486:
6481:
6476:
6471:
6466:
6461:
6456:
6451:
6446:
6441:
6436:
6431:
6426:
6421:
6416:
6411:
6406:
6401:
6396:
6391:
6386:
6381:
6376:
6371:
6366:
6361:
6356:
6351:
6346:
6341:
6339:Hydrogenolysis
6336:
6331:
6326:
6321:
6316:
6311:
6306:
6301:
6296:
6291:
6289:Étard reaction
6286:
6281:
6276:
6271:
6266:
6261:
6256:
6251:
6246:
6241:
6236:
6231:
6226:
6221:
6216:
6211:
6206:
6201:
6196:
6194:Bosch reaction
6191:
6186:
6181:
6176:
6171:
6166:
6161:
6156:
6151:
6146:
6141:
6136:
6131:
6126:
6121:
6116:
6111:
6105:
6103:
6099:Organic redox
6095:
6094:
6092:
6091:
6086:
6081:
6076:
6071:
6066:
6061:
6056:
6051:
6046:
6041:
6036:
6031:
6026:
6020:
6018:
6012:
6011:
6009:
6008:
6003:
5998:
5993:
5988:
5983:
5978:
5973:
5968:
5963:
5958:
5953:
5948:
5943:
5938:
5933:
5931:Esterification
5928:
5923:
5918:
5913:
5908:
5902:
5900:
5892:
5891:
5888:
5887:
5885:
5884:
5879:
5874:
5869:
5864:
5859:
5854:
5849:
5844:
5839:
5834:
5829:
5824:
5819:
5814:
5809:
5804:
5799:
5794:
5789:
5784:
5778:
5776:
5772:
5771:
5769:
5768:
5763:
5758:
5753:
5748:
5743:
5738:
5732:
5730:
5721:
5720:
5715:
5710:
5708:Wurtz reaction
5705:
5700:
5695:
5690:
5685:
5680:
5675:
5670:
5665:
5660:
5655:
5650:
5645:
5640:
5635:
5630:
5625:
5620:
5615:
5610:
5605:
5600:
5595:
5590:
5585:
5580:
5578:Prins reaction
5575:
5570:
5565:
5560:
5555:
5550:
5545:
5540:
5535:
5530:
5525:
5520:
5515:
5510:
5505:
5500:
5495:
5490:
5485:
5480:
5475:
5470:
5465:
5460:
5455:
5450:
5445:
5440:
5435:
5430:
5425:
5420:
5415:
5410:
5405:
5400:
5395:
5393:Hydrocyanation
5390:
5385:
5380:
5375:
5370:
5365:
5363:Henry reaction
5360:
5355:
5350:
5345:
5340:
5335:
5330:
5325:
5320:
5315:
5310:
5305:
5300:
5295:
5290:
5285:
5280:
5275:
5270:
5265:
5260:
5255:
5250:
5245:
5240:
5235:
5230:
5225:
5220:
5215:
5210:
5205:
5200:
5195:
5190:
5185:
5180:
5175:
5170:
5165:
5160:
5155:
5150:
5145:
5140:
5135:
5130:
5125:
5120:
5115:
5110:
5105:
5100:
5095:
5090:
5085:
5080:
5075:
5070:
5065:
5063:Aldol reaction
5060:
5055:
5050:
5044:
5042:
5037:Carbon-carbon
5034:
5033:
5022:
5021:
5016:
5014:Zaitsev's rule
5011:
5006:
5001:
4996:
4991:
4986:
4981:
4976:
4971:
4966:
4961:
4959:Steric effects
4956:
4951:
4946:
4941:
4936:
4931:
4926:
4921:
4916:
4911:
4906:
4901:
4896:
4891:
4886:
4881:
4876:
4871:
4866:
4861:
4856:
4851:
4846:
4841:
4836:
4831:
4826:
4821:
4816:
4811:
4806:
4801:
4796:
4791:
4786:
4781:
4776:
4771:
4766:
4761:
4756:
4751:
4746:
4741:
4736:
4731:
4726:
4721:
4716:
4711:
4706:
4701:
4696:
4691:
4686:
4681:
4676:
4671:
4666:
4661:
4656:
4651:
4646:
4640:
4637:
4636:
4634:
4633:
4628:
4623:
4618:
4613:
4611:Redox reaction
4608:
4603:
4598:
4596:Polymerization
4593:
4588:
4582:
4579:
4578:
4570:
4569:
4562:
4555:
4547:
4541:
4540:
4532:
4531:External links
4529:
4528:
4527:
4522:978-0387683546
4521:
4506:
4503:
4500:
4499:
4493:978-0471264187
4492:
4466:
4460:978-0471264187
4459:
4433:
4427:978-0471264187
4426:
4400:
4394:978-0471264187
4393:
4367:
4361:978-0471264187
4360:
4334:
4307:
4288:(23): 8292–3.
4272:
4245:
4218:
4191:
4164:
4113:
4086:
4059:
4031:
4012:(20): 5675–7.
3996:
3969:
3944:
3942:
3941:
3920:
3898:
3876:
3855:
3834:
3813:
3791:
3769:
3748:
3726:
3704:
3682:
3661:
3640:
3618:
3597:
3576:
3554:
3533:
3512:
3491:
3470:
3448:
3426:
3404:
3382:
3372:(3): 554–562.
3361:
3333:
3327:978-3527306732
3326:
3300:
3271:(7): 500–506.
3251:
3202:
3153:
3126:
3107:(6): 325–335.
3091:
3056:
3021:
2994:
2967:
2948:(9): 2488–91.
2931:
2882:
2833:
2784:
2739:
2690:
2641:
2575:
2518:
2469:
2420:
2371:
2319:
2312:
2294:
2287:
2269:
2230:
2196:
2189:
2163:
2144:(5): 289–296.
2128:
2115:
2088:
2061:
2034:
2007:
1996:(7): 643–661.
1977:
1965:
1930:(1): 344–361.
1914:
1885:
1867:
1840:
1813:
1786:
1767:
1756:(1): 237–240.
1740:
1734:978-0471018193
1733:
1715:
1688:
1661:
1634:
1604:
1577:
1549:
1530:(3): 554–556.
1514:
1486:
1471:
1454:
1416:
1391:
1384:
1363:
1336:
1298:
1292:978-0471264187
1291:
1265:
1259:978-0471264187
1258:
1231:
1230:
1228:
1225:
1224:
1223:
1218:
1213:
1208:
1201:
1198:
1004:was required.
991:prostaglandins
967:
964:
939:
936:
880:
877:
856:
853:
817:
814:
778:
775:
726:
723:
721:
718:
693:
692:dienophile (CH
681:
672:
671:The dienophile
669:
610:conformation.
576:
573:
417:substituents:
363:stereospecific
358:
355:
338:
327:
323:
319:
271:
268:
252:intermolecular
208:
196:
177:
174:
72:
71:
68:
67:
60:
53:
52:
45:
41:
40:
36:
35:
30:
29:Reaction type
26:
25:
15:
9:
6:
4:
3:
2:
7904:
7893:
7890:
7888:
7885:
7883:
7880:
7878:
7875:
7873:
7870:
7868:
7865:
7863:
7860:
7859:
7857:
7834:
7831:
7829:
7826:
7824:
7821:
7819:
7816:
7814:
7811:
7809:
7806:
7804:
7801:
7799:
7796:
7794:
7791:
7789:
7786:
7784:
7781:
7779:
7776:
7774:
7771:
7769:
7766:
7764:
7761:
7759:
7756:
7754:
7753:Herz reaction
7751:
7749:
7746:
7744:
7741:
7739:
7736:
7734:
7731:
7729:
7726:
7724:
7721:
7719:
7716:
7714:
7711:
7709:
7706:
7704:
7701:
7699:
7696:
7694:
7691:
7689:
7686:
7684:
7681:
7679:
7676:
7674:
7671:
7669:
7666:
7664:
7661:
7659:
7656:
7654:
7651:
7649:
7646:
7644:
7641:
7639:
7636:
7634:
7631:
7630:
7628:
7624:
7618:
7615:
7613:
7610:
7608:
7605:
7603:
7600:
7598:
7595:
7593:
7590:
7588:
7585:
7583:
7580:
7578:
7575:
7573:
7570:
7568:
7565:
7563:
7560:
7558:
7555:
7553:
7550:
7548:
7545:
7543:
7540:
7538:
7535:
7533:
7530:
7528:
7525:
7523:
7520:
7518:
7515:
7513:
7510:
7508:
7505:
7503:
7500:
7498:
7495:
7493:
7490:
7488:
7485:
7483:
7480:
7478:
7475:
7473:
7470:
7468:
7465:
7463:
7460:
7459:
7457:
7455:
7454:Cycloaddition
7451:
7445:
7442:
7440:
7437:
7435:
7432:
7430:
7427:
7425:
7422:
7420:
7417:
7415:
7412:
7410:
7407:
7405:
7402:
7400:
7397:
7395:
7392:
7390:
7387:
7385:
7382:
7380:
7377:
7375:
7372:
7370:
7367:
7365:
7362:
7360:
7357:
7355:
7352:
7350:
7347:
7345:
7342:
7340:
7337:
7335:
7332:
7330:
7327:
7325:
7322:
7320:
7317:
7315:
7312:
7310:
7307:
7305:
7302:
7300:
7299:Isay reaction
7297:
7295:
7292:
7290:
7287:
7285:
7282:
7280:
7277:
7275:
7272:
7270:
7267:
7265:
7262:
7260:
7257:
7255:
7252:
7250:
7247:
7245:
7242:
7240:
7237:
7235:
7232:
7230:
7227:
7225:
7222:
7220:
7217:
7215:
7212:
7210:
7207:
7205:
7202:
7200:
7197:
7195:
7194:Cycloaddition
7192:
7190:
7187:
7185:
7182:
7180:
7177:
7175:
7172:
7170:
7167:
7165:
7162:
7160:
7157:
7155:
7152:
7150:
7147:
7145:
7142:
7140:
7137:
7135:
7132:
7130:
7127:
7125:
7122:
7120:
7117:
7115:
7112:
7110:
7107:
7105:
7102:
7100:
7097:
7096:
7094:
7092:
7089:Ring forming
7086:
7080:
7077:
7075:
7072:
7070:
7067:
7065:
7062:
7060:
7057:
7055:
7052:
7050:
7047:
7045:
7042:
7040:
7037:
7035:
7032:
7030:
7027:
7025:
7022:
7020:
7017:
7015:
7012:
7010:
7007:
7005:
7002:
7000:
6997:
6995:
6992:
6990:
6989:Rupe reaction
6987:
6985:
6982:
6980:
6977:
6975:
6972:
6970:
6967:
6965:
6962:
6960:
6957:
6955:
6952:
6950:
6947:
6945:
6942:
6940:
6937:
6935:
6932:
6930:
6927:
6925:
6922:
6920:
6917:
6915:
6912:
6910:
6907:
6905:
6902:
6900:
6897:
6895:
6892:
6890:
6887:
6885:
6882:
6880:
6877:
6875:
6872:
6870:
6867:
6865:
6862:
6860:
6857:
6855:
6852:
6850:
6847:
6845:
6842:
6840:
6837:
6835:
6832:
6830:
6827:
6825:
6822:
6820:
6817:
6815:
6812:
6810:
6807:
6805:
6802:
6800:
6797:
6795:
6792:
6790:
6787:
6785:
6782:
6780:
6777:
6775:
6772:
6770:
6767:
6765:
6762:
6760:
6757:
6755:
6752:
6750:
6747:
6745:
6742:
6740:
6737:
6735:
6732:
6730:
6727:
6725:
6722:
6720:
6717:
6715:
6712:
6710:
6707:
6705:
6702:
6700:
6697:
6695:
6692:
6690:
6687:
6685:
6682:
6680:
6677:
6675:
6672:
6670:
6667:
6665:
6662:
6660:
6657:
6655:
6652:
6650:
6647:
6645:
6642:
6640:
6637:
6635:
6632:
6630:
6627:
6625:
6622:
6620:
6617:
6615:
6612:
6611:
6609:
6607:
6601:
6595:
6592:
6590:
6587:
6585:
6582:
6580:
6577:
6575:
6572:
6570:
6567:
6565:
6562:
6560:
6557:
6555:
6552:
6550:
6547:
6545:
6542:
6540:
6537:
6535:
6532:
6530:
6527:
6525:
6522:
6520:
6517:
6515:
6512:
6510:
6507:
6505:
6502:
6500:
6497:
6495:
6492:
6490:
6487:
6485:
6482:
6480:
6477:
6475:
6472:
6470:
6467:
6465:
6462:
6460:
6457:
6455:
6452:
6450:
6447:
6445:
6442:
6440:
6437:
6435:
6432:
6430:
6427:
6425:
6422:
6420:
6417:
6415:
6412:
6410:
6407:
6405:
6402:
6400:
6397:
6395:
6392:
6390:
6387:
6385:
6382:
6380:
6379:Ley oxidation
6377:
6375:
6372:
6370:
6367:
6365:
6362:
6360:
6357:
6355:
6352:
6350:
6347:
6345:
6344:Hydroxylation
6342:
6340:
6337:
6335:
6334:Hydrogenation
6332:
6330:
6327:
6325:
6322:
6320:
6317:
6315:
6312:
6310:
6307:
6305:
6302:
6300:
6297:
6295:
6292:
6290:
6287:
6285:
6282:
6280:
6277:
6275:
6272:
6270:
6269:DNA oxidation
6267:
6265:
6262:
6260:
6259:Deoxygenation
6257:
6255:
6252:
6250:
6247:
6245:
6242:
6240:
6237:
6235:
6232:
6230:
6227:
6225:
6222:
6220:
6217:
6215:
6212:
6210:
6207:
6205:
6202:
6200:
6197:
6195:
6192:
6190:
6187:
6185:
6182:
6180:
6177:
6175:
6172:
6170:
6167:
6165:
6162:
6160:
6157:
6155:
6152:
6150:
6149:Aromatization
6147:
6145:
6142:
6140:
6137:
6135:
6132:
6130:
6127:
6125:
6122:
6120:
6117:
6115:
6112:
6110:
6107:
6106:
6104:
6102:
6096:
6090:
6087:
6085:
6082:
6080:
6077:
6075:
6072:
6070:
6067:
6065:
6062:
6060:
6057:
6055:
6052:
6050:
6047:
6045:
6042:
6040:
6037:
6035:
6032:
6030:
6027:
6025:
6022:
6021:
6019:
6013:
6007:
6004:
6002:
5999:
5997:
5994:
5992:
5989:
5987:
5986:Reed reaction
5984:
5982:
5979:
5977:
5974:
5972:
5969:
5967:
5964:
5962:
5959:
5957:
5954:
5952:
5949:
5947:
5944:
5942:
5939:
5937:
5934:
5932:
5929:
5927:
5924:
5922:
5919:
5917:
5914:
5912:
5909:
5907:
5904:
5903:
5901:
5897:bond forming
5893:
5883:
5880:
5878:
5875:
5873:
5870:
5868:
5865:
5863:
5860:
5858:
5855:
5853:
5850:
5848:
5845:
5843:
5840:
5838:
5835:
5833:
5830:
5828:
5825:
5823:
5820:
5818:
5815:
5813:
5810:
5808:
5805:
5803:
5802:Cope reaction
5800:
5798:
5795:
5793:
5790:
5788:
5785:
5783:
5780:
5779:
5777:
5773:
5767:
5764:
5762:
5759:
5757:
5754:
5752:
5749:
5747:
5744:
5742:
5739:
5737:
5734:
5733:
5731:
5729:
5725:
5719:
5716:
5714:
5711:
5709:
5706:
5704:
5701:
5699:
5696:
5694:
5691:
5689:
5686:
5684:
5681:
5679:
5676:
5674:
5671:
5669:
5666:
5664:
5661:
5659:
5656:
5654:
5651:
5649:
5646:
5644:
5641:
5639:
5636:
5634:
5631:
5629:
5626:
5624:
5621:
5619:
5616:
5614:
5611:
5609:
5606:
5604:
5601:
5599:
5596:
5594:
5591:
5589:
5586:
5584:
5581:
5579:
5576:
5574:
5571:
5569:
5566:
5564:
5561:
5559:
5556:
5554:
5551:
5549:
5546:
5544:
5541:
5539:
5536:
5534:
5531:
5529:
5526:
5524:
5521:
5519:
5516:
5514:
5513:Nef synthesis
5511:
5509:
5506:
5504:
5501:
5499:
5496:
5494:
5491:
5489:
5488:Methylenation
5486:
5484:
5481:
5479:
5476:
5474:
5471:
5469:
5466:
5464:
5461:
5459:
5456:
5454:
5451:
5449:
5446:
5444:
5441:
5439:
5436:
5434:
5431:
5429:
5426:
5424:
5421:
5419:
5416:
5414:
5411:
5409:
5406:
5404:
5401:
5399:
5396:
5394:
5391:
5389:
5386:
5384:
5381:
5379:
5376:
5374:
5371:
5369:
5366:
5364:
5361:
5359:
5358:Heck reaction
5356:
5354:
5351:
5349:
5346:
5344:
5341:
5339:
5336:
5334:
5331:
5329:
5326:
5324:
5321:
5319:
5316:
5314:
5311:
5309:
5306:
5304:
5301:
5299:
5296:
5294:
5291:
5289:
5286:
5284:
5281:
5279:
5276:
5274:
5271:
5269:
5266:
5264:
5261:
5259:
5256:
5254:
5251:
5249:
5246:
5244:
5241:
5239:
5236:
5234:
5231:
5229:
5226:
5224:
5221:
5219:
5216:
5214:
5211:
5209:
5206:
5204:
5201:
5199:
5196:
5194:
5191:
5189:
5186:
5184:
5181:
5179:
5176:
5174:
5171:
5169:
5166:
5164:
5161:
5159:
5156:
5154:
5151:
5149:
5146:
5144:
5141:
5139:
5136:
5134:
5131:
5129:
5126:
5124:
5121:
5119:
5116:
5114:
5111:
5109:
5106:
5104:
5101:
5099:
5096:
5094:
5091:
5089:
5086:
5084:
5081:
5079:
5076:
5074:
5071:
5069:
5066:
5064:
5061:
5059:
5056:
5054:
5051:
5049:
5046:
5045:
5043:
5039:bond forming
5035:
5031:
5026:
5020:
5017:
5015:
5012:
5010:
5007:
5005:
5004:Y-aromaticity
5002:
5000:
4997:
4995:
4992:
4990:
4989:Walsh diagram
4987:
4985:
4982:
4980:
4977:
4975:
4974:Taft equation
4972:
4970:
4967:
4965:
4962:
4960:
4957:
4955:
4952:
4950:
4947:
4945:
4944:Σ-aromaticity
4942:
4940:
4937:
4935:
4932:
4930:
4927:
4925:
4922:
4920:
4917:
4915:
4912:
4910:
4907:
4905:
4902:
4900:
4897:
4895:
4892:
4890:
4887:
4885:
4882:
4880:
4877:
4875:
4872:
4870:
4869:Marcus theory
4867:
4865:
4862:
4860:
4857:
4855:
4852:
4850:
4847:
4845:
4844:Hückel's rule
4842:
4840:
4837:
4835:
4832:
4830:
4827:
4825:
4822:
4820:
4817:
4815:
4812:
4810:
4807:
4805:
4802:
4800:
4799:Evelyn effect
4797:
4795:
4792:
4790:
4787:
4785:
4782:
4780:
4779:Electron-rich
4777:
4775:
4772:
4770:
4767:
4765:
4762:
4760:
4757:
4755:
4752:
4750:
4747:
4745:
4742:
4740:
4737:
4735:
4732:
4730:
4727:
4725:
4722:
4720:
4717:
4715:
4712:
4710:
4707:
4705:
4702:
4700:
4697:
4695:
4694:Bema Hapothle
4692:
4690:
4687:
4685:
4682:
4680:
4677:
4675:
4672:
4670:
4667:
4665:
4662:
4660:
4657:
4655:
4652:
4650:
4647:
4645:
4642:
4641:
4638:
4632:
4629:
4627:
4624:
4622:
4619:
4617:
4614:
4612:
4609:
4607:
4604:
4602:
4599:
4597:
4594:
4592:
4589:
4587:
4584:
4583:
4580:
4576:
4568:
4563:
4561:
4556:
4554:
4549:
4548:
4545:
4537:
4535:
4534:
4524:
4518:
4514:
4509:
4508:
4495:
4489:
4485:
4481:
4477:
4470:
4462:
4456:
4452:
4448:
4444:
4437:
4429:
4423:
4419:
4415:
4411:
4404:
4396:
4390:
4386:
4382:
4378:
4371:
4363:
4357:
4353:
4349:
4345:
4338:
4330:
4326:
4322:
4318:
4311:
4303:
4299:
4295:
4291:
4287:
4283:
4276:
4268:
4264:
4260:
4256:
4249:
4241:
4237:
4233:
4229:
4222:
4214:
4210:
4206:
4202:
4195:
4187:
4183:
4179:
4175:
4168:
4160:
4156:
4152:
4148:
4144:
4140:
4136:
4132:
4128:
4124:
4117:
4109:
4105:
4101:
4097:
4090:
4082:
4078:
4074:
4070:
4063:
4055:
4051:
4047:
4043:
4035:
4027:
4023:
4019:
4015:
4011:
4007:
4000:
3992:
3988:
3984:
3980:
3973:
3958:
3954:
3948:
3938:
3934:
3930:
3926:
3921:
3917:
3913:
3909:
3905:
3899:
3895:
3891:
3887:
3883:
3877:
3873:
3869:
3865:
3861:
3856:
3852:
3848:
3844:
3840:
3835:
3831:
3827:
3823:
3819:
3814:
3810:
3806:
3802:
3798:
3792:
3788:
3784:
3780:
3776:
3770:
3766:
3762:
3758:
3754:
3749:
3745:
3741:
3737:
3733:
3727:
3723:
3719:
3715:
3711:
3705:
3701:
3697:
3693:
3689:
3683:
3679:
3675:
3671:
3667:
3662:
3658:
3654:
3650:
3646:
3641:
3637:
3633:
3629:
3625:
3619:
3615:
3611:
3607:
3603:
3598:
3594:
3590:
3586:
3582:
3577:
3573:
3569:
3565:
3561:
3555:
3551:
3547:
3543:
3539:
3534:
3530:
3526:
3522:
3518:
3513:
3509:
3505:
3501:
3497:
3492:
3488:
3484:
3480:
3476:
3471:
3467:
3463:
3459:
3455:
3449:
3445:
3441:
3437:
3433:
3427:
3423:
3419:
3415:
3411:
3405:
3401:
3397:
3393:
3389:
3383:
3379:
3375:
3371:
3367:
3362:
3358:
3354:
3350:
3346:
3341:
3340:
3337:
3329:
3323:
3319:
3315:
3311:
3304:
3296:
3292:
3288:
3284:
3279:
3274:
3270:
3266:
3262:
3255:
3247:
3243:
3238:
3233:
3229:
3225:
3221:
3217:
3213:
3206:
3198:
3194:
3189:
3184:
3180:
3176:
3172:
3168:
3164:
3157:
3149:
3145:
3141:
3137:
3130:
3122:
3118:
3114:
3110:
3106:
3102:
3095:
3087:
3083:
3079:
3075:
3071:
3067:
3060:
3052:
3048:
3044:
3040:
3036:
3032:
3025:
3017:
3013:
3009:
3005:
2998:
2990:
2986:
2982:
2978:
2971:
2963:
2959:
2955:
2951:
2947:
2944:
2943:
2935:
2927:
2923:
2918:
2913:
2909:
2905:
2901:
2897:
2893:
2886:
2878:
2874:
2869:
2864:
2860:
2856:
2852:
2848:
2844:
2837:
2829:
2825:
2820:
2815:
2811:
2807:
2803:
2799:
2795:
2788:
2780:
2776:
2771:
2766:
2762:
2758:
2754:
2750:
2743:
2735:
2731:
2726:
2721:
2717:
2713:
2709:
2705:
2701:
2694:
2686:
2682:
2677:
2672:
2668:
2664:
2660:
2656:
2652:
2645:
2637:
2633:
2629:
2625:
2621:
2617:
2613:
2609:
2605:
2601:
2597:
2593:
2586:
2579:
2571:
2567:
2562:
2557:
2553:
2549:
2545:
2541:
2537:
2533:
2529:
2522:
2514:
2510:
2505:
2500:
2496:
2492:
2488:
2484:
2480:
2473:
2465:
2461:
2456:
2451:
2447:
2443:
2439:
2435:
2431:
2424:
2416:
2412:
2407:
2402:
2398:
2394:
2390:
2386:
2382:
2375:
2367:
2363:
2358:
2353:
2349:
2345:
2341:
2337:
2333:
2326:
2324:
2315:
2313:9780199270293
2309:
2305:
2298:
2290:
2288:9780470746592
2284:
2280:
2273:
2265:
2261:
2257:
2253:
2249:
2245:
2241:
2234:
2226:
2222:
2218:
2214:
2207:
2200:
2192:
2186:
2182:
2178:
2174:
2167:
2159:
2155:
2151:
2147:
2143:
2139:
2132:
2125:
2119:
2111:
2107:
2103:
2099:
2092:
2084:
2080:
2076:
2072:
2065:
2057:
2053:
2049:
2045:
2038:
2030:
2026:
2022:
2018:
2011:
2003:
1999:
1995:
1991:
1984:
1982:
1974:
1969:
1961:
1957:
1953:
1949:
1945:
1941:
1937:
1933:
1929:
1925:
1918:
1910:
1904:
1896:
1892:
1888:
1886:9781483282046
1882:
1878:
1871:
1863:
1859:
1855:
1851:
1844:
1836:
1832:
1828:
1824:
1817:
1809:
1805:
1801:
1797:
1790:
1782:
1778:
1771:
1763:
1759:
1755:
1751:
1744:
1736:
1730:
1726:
1719:
1711:
1707:
1703:
1699:
1692:
1684:
1680:
1676:
1672:
1665:
1657:
1653:
1649:
1645:
1638:
1630:
1626:
1622:
1618:
1611:
1609:
1600:
1596:
1592:
1588:
1581:
1573:
1569:
1565:
1561:
1553:
1545:
1541:
1537:
1533:
1529:
1525:
1518:
1510:
1506:
1502:
1498:
1490:
1483:
1478:
1476:
1468:
1463:
1461:
1459:
1450:
1446:
1442:
1438:
1434:
1430:
1423:
1421:
1413:
1408:
1406:
1404:
1402:
1400:
1398:
1396:
1387:
1381:
1378:
1374:
1367:
1359:
1355:
1351:
1347:
1340:
1332:
1328:
1324:
1320:
1316:
1312:
1305:
1303:
1294:
1288:
1284:
1280:
1276:
1269:
1261:
1255:
1251:
1247:
1243:
1236:
1232:
1222:
1219:
1217:
1214:
1212:
1209:
1207:
1204:
1203:
1197:
1194:
1192:
1184:
1180:
1178:
1174:
1166:
1162:
1159:
1155:
1147:
1143:
1141:
1137:
1133:
1129:
1125:
1121:
1114:
1110:
1104:
1100:
1098:
1094:
1090:
1086:
1082:
1074:
1070:
1068:
1064:
1060:
1056:
1048:
1044:
1042:
1040:
1035:
1030:
1028:
1024:
1023:phenylalanine
1021:
1017:
1009:
1005:
1003:
999:
995:
992:
983:
979:
977:
973:
963:
961:
960:
955:
954:
944:
932:
928:
924:
919:
915:
913:
909:
905:
901:
893:
890:
885:
876:
874:
870:
866:
862:
852:
850:
846:
842:
840:
835:
831:
827:
823:
813:
809:
807:
801:
799:
795:
791:
787:
786:zinc chloride
783:
774:
772:
768:
764:
760:
756:
752:
748:
744:
740:
736:
732:
717:
715:
711:
707:
703:
699:
691:
687:
679:
668:
666:
662:
658:
654:
652:
647:
643:
639:
630:
626:
623:
622:cyclohexenone
619:
614:
611:
609:
605:
601:
597:
592:
590:
586:
582:
572:
570:
566:
562:
558:
550:
546:
543:
538:
537:van der Waals
534:
530:
522:
517:
513:
511:
507:
503:
499:
495:
491:
487:
483:
478:
476:
475:
471:
466:
462:
457:
449:
445:
441:
437:
433:
429:
422:
418:
416:
412:
408:
404:
400:
396:
392:
388:
384:
380:
376:
372:
368:
364:
354:
351:
347:
344:
336:
333:
317:
314:
304:
300:
297:
293:
289:
280:
276:
267:
265:
261:
257:
253:
248:
246:
242:
238:
234:
230:
225:
217:
213:
207:
203:
195:
191:
186:
184:
173:
171:
167:
163:
159:
155:
151:
147:
143:
138:
134:
130:
126:
122:
121:cycloaddition
118:
114:
110:
106:
102:
98:
95:
91:
87:
78:
69:
65:
61:
58:
55:
54:
50:
46:
43:
42:
37:
34:
33:Cycloaddition
31:
28:
27:
22:
19:
7521:
7223:
6794:Ene reaction
6154:Autoxidation
6015:Degradation
5906:Azo coupling
5683:Ugi reaction
5283:Ene reaction
5267:
5083:Alkynylation
4934:Polyfluorene
4929:Polar effect
4794:Electrophile
4709:Bredt's rule
4679:Baird's rule
4649:Alpha effect
4512:
4505:Bibliography
4475:
4469:
4442:
4436:
4409:
4403:
4376:
4370:
4343:
4337:
4320:
4316:
4310:
4285:
4281:
4275:
4258:
4254:
4248:
4231:
4227:
4221:
4204:
4200:
4194:
4177:
4173:
4167:
4126:
4122:
4116:
4099:
4095:
4089:
4072:
4068:
4062:
4045:
4041:
4034:
4009:
4005:
3999:
3982:
3978:
3972:
3960:. Retrieved
3947:
3928:
3924:
3907:
3903:
3885:
3881:
3863:
3859:
3842:
3838:
3821:
3817:
3800:
3796:
3778:
3774:
3756:
3752:
3735:
3731:
3713:
3709:
3691:
3687:
3669:
3665:
3648:
3644:
3627:
3623:
3605:
3601:
3584:
3580:
3563:
3559:
3541:
3537:
3520:
3516:
3499:
3495:
3478:
3474:
3457:
3453:
3435:
3431:
3413:
3409:
3391:
3387:
3369:
3365:
3348:
3344:
3336:
3309:
3303:
3268:
3264:
3254:
3219:
3215:
3205:
3170:
3166:
3156:
3139:
3135:
3129:
3104:
3100:
3094:
3069:
3065:
3059:
3034:
3030:
3024:
3007:
3003:
2997:
2980:
2976:
2970:
2945:
2940:
2934:
2899:
2895:
2885:
2850:
2846:
2836:
2801:
2797:
2787:
2752:
2748:
2742:
2707:
2703:
2693:
2658:
2654:
2644:
2595:
2591:
2578:
2535:
2531:
2521:
2486:
2482:
2472:
2437:
2433:
2423:
2388:
2384:
2374:
2339:
2335:
2303:
2297:
2278:
2272:
2247:
2243:
2233:
2216:
2212:
2199:
2172:
2166:
2141:
2137:
2131:
2123:
2118:
2101:
2097:
2091:
2074:
2070:
2064:
2047:
2043:
2037:
2020:
2016:
2010:
1993:
1989:
1968:
1927:
1923:
1917:
1879:. Weinheim.
1876:
1870:
1853:
1849:
1843:
1826:
1822:
1816:
1799:
1795:
1789:
1780:
1776:
1770:
1753:
1749:
1743:
1724:
1718:
1701:
1697:
1691:
1674:
1670:
1664:
1647:
1643:
1637:
1620:
1616:
1590:
1586:
1580:
1563:
1559:
1552:
1527:
1523:
1517:
1500:
1496:
1489:
1432:
1428:
1372:
1366:
1349:
1345:
1339:
1314:
1310:
1274:
1268:
1241:
1235:
1195:
1188:
1176:
1170:
1151:
1127:
1123:
1118:
1108:
1078:
1066:
1054:
1052:
1038:
1031:
1013:
988:
969:
957:
951:
949:
897:
858:
838:
819:
810:
805:
802:
780:
771:Vince lactam
750:
739:dihydropyran
728:
714:nitro groups
674:
650:
637:
635:
615:
612:
607:
603:
599:
595:
593:
588:
584:
578:
568:
564:
554:
541:
528:
526:
520:
502:benzoquinone
493:
489:
485:
481:
479:
473:
469:
464:
460:
453:
447:
443:
435:
431:
414:
410:
406:
402:
398:
394:
390:
386:
382:
378:
374:
370:
366:
360:
349:
345:
342:
334:
331:
326:, CN, C(O)CH
315:
312:
309:
295:
291:
287:
285:
273:
249:
226:
222:
205:
193:
187:
179:
165:
161:
157:
154:heterocycles
89:
83:
64:RXNO:0000006
59:ontology ID
39:Identifiers
18:
5293:Ethenolysis
4939:Ring strain
4909:Nucleophile
4734:Clar's rule
4674:Aromaticity
3962:19 February
3866:: 147–157.
3845:: 140–146.
3803:: 145–155.
3781:: 129–145.
3759:: 168–182.
3716:: 103–150.
3651:: 277–294.
3630:: 267–276.
3608:: 257–266.
3587:: 243–257.
3566:: 236–242.
3544:: 211–225.
3523:: 202–210.
3502:: 191–202.
3481:: 137–154.
2770:2066/241097
1191:cantharidin
1120:Tabersonine
1020:amino acids
976:cholesterol
904:norbornenes
849:imidazoline
782:Lewis acids
704:), various
698:phosphonium
646:naphthalene
346:conjugating
316:withdrawing
183:suprafacial
142:heteroatoms
109:cyclohexene
7856:Categories
7577:Ozonolysis
7104:Annulation
6454:Ozonolysis
4573:Topics in
3738:: 87–128.
3394:: 62–103.
3351:: 98–122.
2942:Org. Lett.
1783:: 103–115.
1227:References
912:vitamin B6
892:lovastatin
841:-oxazoline
784:, such as
731:heteroatom
657:Anthracene
510:crotonates
256:positional
144:, such as
133:Kurt Alder
129:Otto Diels
105:dienophile
94:conjugated
7091:reactions
6606:reactions
6101:reactions
6017:reactions
5899:reactions
5041:reactions
4174:Synthesis
3931:: 87–98.
3910:: 68–86.
3888:: 73–94.
3824:: 45–61.
3694:: 16–49.
3216:Chem. Rev
2779:239089361
2636:232337915
2620:0001-4842
2264:0001-4842
2158:260335918
2138:Synthesis
1944:1096-987X
1903:cite book
1895:915343522
1034:reserpine
972:cortisone
706:sulfoxide
665:acetylene
575:The diene
531:, though
506:acrylates
176:Mechanism
146:carbonyls
4984:Vinylogy
4654:Annulene
4601:Reagents
4302:15941256
3672:: 1–15.
3295:30482282
3287:27301662
3246:33492939
3197:23060191
3121:10891050
3086:12785777
3051:11942799
2962:21462988
2926:33780068
2877:33169912
2828:34094173
2734:33538169
2685:32012430
2628:33759502
2570:34499069
2513:33780068
2464:33169912
2415:34094173
2366:31944503
1960:26096085
1952:17109435
1544:22175504
1449:22175326
1331:19750686
1200:See also
1093:boronate
1059:pyranone
1041:-decalin
1027:tyrosine
929:through
908:monomers
845:chelates
843:–copper
763:oxazines
710:sulfonyl
571:isomer.
440:acrolein
335:donating
241:butenone
4644:A value
4539:Reihe".
4159:4371975
4151:7906395
4131:Bibcode
4026:5808505
3237:8008985
3188:3538845
2917:8360170
2868:8049058
2819:8163289
2725:7901664
2676:7187256
2561:8457343
2540:Bibcode
2504:8360170
2455:8049058
2406:8163289
2357:7187354
2219:: 206.
1138:with a
938:History
873:benzyne
865:alkynes
859:In the
759:Nitroso
533:dipolar
164:° and Δ
115:with a
4519:
4490:
4457:
4424:
4391:
4358:
4300:
4157:
4149:
4123:Nature
4024:
3324:
3293:
3285:
3244:
3234:
3195:
3185:
3167:Nature
3119:
3084:
3049:
2960:
2924:
2914:
2875:
2865:
2826:
2816:
2777:
2732:
2722:
2683:
2673:
2634:
2626:
2618:
2568:
2558:
2511:
2501:
2462:
2452:
2413:
2403:
2364:
2354:
2310:
2285:
2262:
2187:
2156:
1958:
1950:
1942:
1893:
1883:
1731:
1542:
1447:
1382:
1329:
1289:
1256:
1156:. The
1154:allene
1132:alkene
1063:lactam
889:statin
869:diynes
824:, and
747:imines
745:, and
690:ketene
686:ketone
393:- and
200:In an
150:imines
101:alkene
88:, the
4155:S2CID
3291:S2CID
2775:S2CID
2632:S2CID
2588:(PDF)
2209:(PDF)
2154:S2CID
1973:Carey
1956:S2CID
1482:Carey
1467:Carey
1412:Carey
1095:with
1085:ester
925:from
796:, or
596:trans
589:trans
581:diene
561:furan
415:trans
411:trans
399:trans
395:trans
383:trans
375:trans
292:ortho
123:with
97:diene
4517:ISBN
4488:ISBN
4455:ISBN
4422:ISBN
4389:ISBN
4356:ISBN
4298:PMID
4178:1991
4147:PMID
4022:PMID
3964:2016
3322:ISBN
3283:PMID
3242:PMID
3193:PMID
3117:PMID
3082:PMID
3047:PMID
2958:PMID
2922:PMID
2873:PMID
2824:PMID
2753:2021
2730:PMID
2681:PMID
2624:PMID
2616:ISSN
2566:PMID
2509:PMID
2460:PMID
2411:PMID
2362:PMID
2308:ISBN
2283:ISBN
2260:ISSN
2185:ISBN
2142:1977
1948:PMID
1940:ISSN
1909:link
1891:OCLC
1881:ISBN
1729:ISBN
1540:PMID
1445:PMID
1380:ISBN
1327:PMID
1287:ISBN
1254:ISBN
1025:and
996:and
974:and
956:and
867:and
708:and
604:tert
579:The
565:endo
542:endo
535:and
521:endo
519:The
508:and
500:and
494:Endo
486:endo
472:and
470:endo
461:endo
444:endo
434:and
432:Endo
322:, NO
296:para
294:and
239:and
231:and
148:and
131:and
4480:doi
4447:doi
4414:doi
4381:doi
4348:doi
4325:doi
4321:102
4290:doi
4286:127
4263:doi
4259:110
4236:doi
4232:120
4209:doi
4205:117
4182:doi
4139:doi
4127:367
4104:doi
4100:109
4077:doi
4073:102
4050:doi
4046:101
4014:doi
3987:doi
3933:doi
3929:530
3912:doi
3908:530
3890:doi
3886:525
3868:doi
3864:519
3847:doi
3843:519
3826:doi
3822:516
3805:doi
3801:513
3783:doi
3779:513
3761:doi
3757:511
3740:doi
3736:510
3718:doi
3714:505
3696:doi
3692:498
3674:doi
3670:498
3653:doi
3649:490
3632:doi
3628:490
3610:doi
3606:490
3589:doi
3585:490
3568:doi
3564:490
3546:doi
3542:486
3525:doi
3521:486
3504:doi
3500:486
3483:doi
3479:478
3462:doi
3440:doi
3418:doi
3396:doi
3392:470
3374:doi
3353:doi
3349:460
3314:doi
3273:doi
3232:PMC
3224:doi
3220:121
3183:PMC
3175:doi
3171:490
3144:doi
3140:122
3109:doi
3074:doi
3070:125
3039:doi
3035:124
3012:doi
3008:113
2985:doi
2981:110
2950:doi
2912:PMC
2904:doi
2863:PMC
2855:doi
2814:PMC
2806:doi
2765:hdl
2757:doi
2720:PMC
2712:doi
2671:PMC
2663:doi
2608:hdl
2600:doi
2556:PMC
2548:doi
2499:PMC
2491:doi
2450:PMC
2442:doi
2401:PMC
2393:doi
2352:PMC
2344:doi
2252:doi
2221:doi
2177:doi
2146:doi
2106:doi
2079:doi
2052:doi
2025:doi
1998:doi
1932:doi
1858:doi
1831:doi
1804:doi
1758:doi
1754:124
1706:doi
1679:doi
1675:114
1652:doi
1648:113
1625:doi
1621:102
1595:doi
1591:110
1568:doi
1564:118
1532:doi
1528:108
1505:doi
1501:109
1437:doi
1433:108
1354:doi
1319:doi
1279:doi
1246:doi
1128:cis
1124:cis
1067:cis
1055:cis
1039:cis
994:F2α
839:bis
680:(CH
608:cis
600:cis
585:cis
569:exo
482:cis
474:exo
465:exo
448:exo
436:exo
407:cis
403:cis
391:cis
387:cis
379:cis
371:cis
367:syn
341:),
330:),
84:In
57:RSC
7858::
4486:.
4453:.
4420:.
4387:.
4354:.
4319:.
4296:.
4284:.
4257:.
4230:.
4203:.
4176:.
4153:.
4145:.
4137:.
4125:.
4098:.
4071:.
4044:.
4020:.
4010:91
4008:.
3983:74
3981:.
3955:.
3927:.
3906:.
3884:.
3862:.
3841:.
3820:.
3799:.
3777:.
3755:.
3734:.
3712:.
3690:.
3668:.
3647:.
3626:.
3604:.
3583:.
3562:.
3540:.
3519:.
3498:.
3477:.
3458:62
3456:.
3436:62
3434:.
3414:62
3412:.
3390:.
3370:62
3368:.
3347:.
3320:.
3312:.
3289:.
3281:.
3269:69
3267:.
3263:.
3240:.
3230:.
3218:.
3214:.
3191:.
3181:.
3169:.
3165:.
3138:.
3115:.
3105:33
3103:.
3080:.
3068:.
3045:.
3033:.
3006:.
2979:.
2956:.
2946:13
2920:.
2910:.
2900:27
2898:.
2894:.
2871:.
2861:.
2851:27
2849:.
2845:.
2822:.
2812:.
2802:11
2800:.
2796:.
2773:.
2763:.
2751:.
2728:.
2718:.
2708:86
2706:.
2702:.
2679:.
2669:.
2659:15
2657:.
2653:.
2630:.
2622:.
2614:.
2606:.
2596:54
2594:.
2590:.
2564:.
2554:.
2546:.
2536:23
2534:.
2530:.
2507:.
2497:.
2487:27
2485:.
2481:.
2458:.
2448:.
2438:27
2436:.
2432:.
2409:.
2399:.
2389:11
2387:.
2383:.
2360:.
2350:.
2340:59
2338:.
2334:.
2322:^
2258:.
2246:.
2242:.
2217:68
2215:.
2211:.
2183:.
2152:.
2140:.
2102:62
2100:.
2075:20
2073:.
2048:96
2046:.
2021:83
2019:.
1994:58
1992:.
1980:^
1954:.
1946:.
1938:.
1928:28
1926:.
1905:}}
1901:{{
1889:.
1854:92
1852:.
1827:94
1825:.
1800:93
1798:.
1779:.
1752:.
1702:95
1700:.
1673:.
1646:.
1619:.
1607:^
1589:.
1562:.
1538:.
1526:.
1499:.
1474:^
1457:^
1443:.
1431:.
1419:^
1394:^
1375:.
1348:.
1325:.
1315:41
1313:.
1301:^
1285:.
1252:.
1029:.
998:E2
914:.
863:,
847:,
836:,
832:,
828:.
792:,
788:,
773:.
765:.
757:.
655:.
442:;
172:.
4566:e
4559:t
4552:v
4525:.
4496:.
4482::
4463:.
4449::
4430:.
4416::
4397:.
4383::
4364:.
4350::
4331:.
4327::
4304:.
4292::
4269:.
4265::
4242:.
4238::
4215:.
4211::
4188:.
4184::
4161:.
4141::
4133::
4110:.
4106::
4083:.
4079::
4056:.
4052::
4028:.
4016::
3993:.
3989::
3966:.
3939:.
3935::
3918:.
3914::
3896:.
3892::
3874:.
3870::
3853:.
3849::
3832:.
3828::
3811:.
3807::
3789:.
3785::
3767:.
3763::
3746:.
3742::
3724:.
3720::
3702:.
3698::
3680:.
3676::
3659:.
3655::
3638:.
3634::
3616:.
3612::
3595:.
3591::
3574:.
3570::
3552:.
3548::
3531:.
3527::
3510:.
3506::
3489:.
3485::
3468:.
3464::
3446:.
3442::
3424:.
3420::
3402:.
3398::
3380:.
3376::
3359:.
3355::
3330:.
3316::
3297:.
3275::
3248:.
3226::
3199:.
3177::
3150:.
3146::
3123:.
3111::
3088:.
3076::
3053:.
3041::
3018:.
3014::
2991:.
2987::
2964:.
2952::
2928:.
2906::
2879:.
2857::
2830:.
2808::
2781:.
2767::
2759::
2736:.
2714::
2687:.
2665::
2638:.
2610::
2602::
2572:.
2550::
2542::
2515:.
2493::
2466:.
2444::
2417:.
2395::
2368:.
2346::
2316:.
2291:.
2266:.
2254::
2248:8
2227:.
2223::
2193:.
2179::
2160:.
2148::
2112:.
2108::
2085:.
2081::
2058:.
2054::
2031:.
2027::
2004:.
2000::
1962:.
1934::
1911:)
1897:.
1864:.
1860::
1837:.
1833::
1810:.
1806::
1781:1
1764:.
1760::
1737:.
1712:.
1708::
1685:.
1681::
1658:.
1654::
1631:.
1627::
1601:.
1597::
1574:.
1570::
1546:.
1534::
1511:.
1507::
1451:.
1439::
1388:.
1360:.
1356::
1350:2
1333:.
1321::
1295:.
1281::
1262:.
1248::
1177:o
933:.
894:.
751:N
694:2
682:2
651:N
640:-
638:o
446:/
409:,
397:,
389:,
381:(
373:(
350:X
343:C
339:2
332:X
328:3
324:2
320:3
313:Z
209:3
206:ψ
197:2
194:ψ
166:S
162:H
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.