3172:
771:. These three types of inhibition result respectively from the inhibitor binding only to the enzyme E in the absence of substrate S, to the enzyme–substrate complex ES, or to both. The division of these classes arises from a problem in their derivation and results in the need to use two different binding constants for one binding event. It is further assumed that binding of the inhibitor to the enzyme results in 100% inhibition and fails to consider the possibility of partial inhibition. The common form of the inhibitory term also obscures the relationship between the inhibitor binding to the enzyme and its relationship to any other binding term be it the Michaelis–Menten equation or a dose response curve associated with ligand receptor binding. To demonstrate the relationship the following rearrangement can be made:
3255:
368:
1462:
33:
380:
2182:
2799:
3000:
777:
3720:
2981:
2785:
2817:
3548:
3292:
1457:{\displaystyle {\begin{aligned}{\cfrac {V_{\max }}{1+{\cfrac {\ce {}}{K_{i}}}}}&={V_{\max }}\left({\cfrac {K_{i}}{K_{i}+}}\right)&&{\text{multiply by }}{\cfrac {K_{i}}{K_{i}}}=1\\&={V_{\max }}\left({\cfrac {K_{i}+-}{K_{i}+}}\right)&&{\text{add }}-=0{\text{ to numerator}}\\&={V_{\max }}\left(1-{\cfrac {}{K_{i}+}}\right)&&{\text{simplify }}{\cfrac {K_{i}+}{K_{i}+}}=1\\&=V_{\max }-V_{\max }{\cfrac {\ce {}}{K_{i}+}}&&{\text{multiply out by }}V_{\max }\end{aligned}}}
3861:
3728:
4202:
274:, which are not essential to the organism that produces them, but provide the organism with an evolutionary advantage, in that they can be used to repel predators or competing organisms or immobilize prey. In addition, many drugs are small molecule enzyme inhibitors that target either disease-modifying enzymes in the patient or enzymes in pathogens which are required for the growth and reproduction of the pathogen.
2957:
where the kinases interact with their substrate proteins, and most proteins are present inside cells at concentrations much lower than the concentration of ATP. As a consequence, if two protein kinase inhibitors both bind in the active site with similar affinity, but only one has to compete with ATP, then the competitive inhibitor at the protein-binding site will inhibit the enzyme more effectively.
2618:, respectively. However, the modified Michaelis-Menten equation assumes that binding of the inhibitor to the enzyme has reached equilibrium, which may be a very slow process for inhibitors with sub-nanomolar dissociation constants. In these cases the inhibition becomes effectively irreversible, hence it is more practical to treat such tight-binding inhibitors as irreversible (see
4112:
337:("regular" orientation) inhibitors. The mechanism of orthosteric inhibition is simply to prevent substrate binding to the enzyme through direct competition which in turn prevents the enzyme from catalysing the conversion of substrates into products. Alternatively, the inhibitor can bind to a site remote from the enzyme active site. These are known as
3267:
of these inhibitors rapidly bind to the enzyme in a low-affinity EI complex and this then undergoes a slower rearrangement to a very tightly bound EI* complex (see the "irreversible inhibition mechanism" diagram). This kinetic behaviour is called slow-binding. This slow rearrangement after binding often involves a
7948:
Figure 1C: Clinical success of privileged protein family classes (% approved drugs targeting each target class): Reductase 7.62, Kinase 5.94, Protease 3.35, Hydrolase 2.76, NPTase 2.09, Transferase 1.92, Lyase 1.59, Isomerase 1.51, Phosphodiesterase 1.50, Cytochrome p450 0.84, Epigenetic eraser 0.33,
2713:
Substrate or product inhibition is where either an enzymes substrate or product also act as an inhibitor. This inhibition may follow the competitive, uncompetitive or mixed patterns. In substrate inhibition there is a progressive decrease in activity at high substrate concentrations, potentially from
3530:
protein in the pancreas. This inhibitor binds tightly to trypsin, preventing the trypsin activity that would otherwise be detrimental to the organ. Although the trypsin inhibitor is a protein, it avoids being hydrolysed as a substrate by the protease by excluding water from trypsin's active site and
3266:
Not all irreversible inhibitors form covalent adducts with their enzyme targets. Some reversible inhibitors bind so tightly to their target enzyme that they are essentially irreversible. These tight-binding inhibitors may show kinetics similar to covalent irreversible inhibitors. In these cases some
3184:
Irreversible inhibitors first form a reversible non-covalent complex with the enzyme (EI or ESI). Subsequently, a chemical reaction occurs between the enzyme and inhibitor to produce the covalently modified "dead-end complex" EI* (an irreversible covalent complex). The rate at which EI* is formed is
2690:
The mechanism of partially competitive inhibition is similar to that of non-competitive, except that the EIS complex has catalytic activity, which may be lower or even higher (partially competitive activation) than that of the enzyme–substrate (ES) complex. This inhibition typically displays a lower
599:
These four types of inhibition can also be distinguished by the effect of increasing the substrate concentration on the degree of inhibition caused by a given amount of inhibitor. For competitive inhibition the degree of inhibition is reduced by increasing , for noncompetitive inhibition the degree
580:
concentrations of substrate , this type of inhibition can be reduced (due to the competitive contribution), but not entirely overcome (due to the noncompetitive component). Although it is possible for mixed-type inhibitors to bind in the active site, this type of inhibition generally results from an
552:
but does not affect the binding of substrate. This type of inhibitor binds with equal affinity to the free enzyme as to the enzyme-substrate complex. It can be thought of as having the ability of competitive and uncompetitive inhibitors, but with no preference to either type. As a result, the extent
1630:
the effect of the inhibitor is a result of the percent of the enzyme population interacting with inhibitor. The only problem with this equation in its present form is that it assumes absolute inhibition of the enzyme with inhibitor binding, when in fact there can be a wide range of effects anywhere
753:
When an enzyme has multiple substrates, inhibitors can show different types of inhibition depending on which substrate is considered. This results from the active site containing two different binding sites within the active site, one for each substrate. For example, an inhibitor might compete with
3575:
and are so diverse that there are probably natural inhibitors for most metabolic processes. The metabolic processes targeted by natural poisons encompass more than enzymes in metabolic pathways and can also include the inhibition of receptor, channel and structural protein functions in a cell. For
2956:
inhibitors have chemical structures that are similar to ATP, one of the substrates of these enzymes. However, drugs that are simple competitive inhibitors will have to compete with the high concentrations of ATP in the cell. Protein kinases can also be inhibited by competition at the binding sites
2199:, the concentration at which the inhibitor half occupies the enzyme. In non-competitive inhibition the inhibitor can also bind to the enzyme-substrate complex, and the presence of bound substrate can change the affinity of the inhibitor for the enzyme, resulting in a second dissociation constant
3926:
is facilitated when an enzyme that is essential to the pathogen's survival is absent or very different in humans. Humans do not make peptidoglycan, therefore antibiotics that inhibit this process are selectively toxic to bacteria. Selective toxicity is also produced in antibiotics by exploiting
5139:
In some cases, the inhibitor may bind to a distinct site on the enzyme that is in allosteric communication with the substrate binding pocket. In many cases, allosteric, substrate competitive compounds result in conformational changes to the enzyme that change the ability of the enzyme to bind
579:
the inhibitor may bind to the enzyme whether or not the substrate has already bound. Hence mixed inhibition is a combination of competitive and noncompetitive inhibition. Furthermore, the affinity of the inhibitor for the free enzyme and the enzyme-substrate complex may differ. By increasing
3192:. Since formation of EI may compete with ES, binding of irreversible inhibitors can be prevented by competition either with substrate or with a second, reversible inhibitor. This protection effect is good evidence of a specific reaction of the irreversible inhibitor with the active site.
2455:
620:
scheme (shown in the "inhibition mechanism schematic" diagram), an enzyme (E) binds to its substrate (S) to form the enzyme–substrate complex ES. Upon catalysis, this complex breaks down to release product P and free enzyme. The inhibitor (I) can bind to either E or ES with the
3906:
inhibit the enzymes that produce and then cross-link the strands of this polymer together. This causes the cell wall to lose strength and the bacteria to burst. In the figure, a molecule of penicillin (shown in a ball-and-stick form) is shown bound to its target, the
391:
Competitive inhibitors usually bind to the active site. Non-competitive bind to a remote (allosteric) site. Uncompetitive inhibitors only bind once the substrate is bound, fully disrupting catalysis, and mixed inhibition is similar but with only partial disruption of
2739:(a change in shape) to a second more tightly held complex, EI*, but the overall inhibition process is reversible. This manifests itself as slowly increasing enzyme inhibition. Under these conditions, traditional Michaelis–Menten kinetics give a false value for
1875:
This term can then define the residual enzymatic activity present when the inhibitor is interacting with individual enzymes in the population. However the inclusion of this term has the added value of allowing for the possibility of activation if the secondary
2878:
As enzymes have evolved to bind their substrates tightly, and most reversible inhibitors bind in the active site of enzymes, it is unsurprising that some of these inhibitors are strikingly similar in structure to the substrates of their targets. Inhibitors of
3365:
can catalyse the decarboxylation of DFMO instead of ornithine (see the "DFMO inhibitor mechanism" diagram). However, this decarboxylation reaction is followed by the elimination of a fluorine atom, which converts this catalytic intermediate into a conjugated
2832:
Multi-substrate analogue inhibitors are high affinity selective inhibitors that can be prepared for enzymes that catalyse reactions with more than one substrate by capturing the binding energy of each of those substrate into one molecule. For example, in the
3408:
are often enzyme inhibitors that have evolved for use as toxic agents against predators, prey, and competing organisms. These natural toxins include some of the most poisonous substances known. Artificial inhibitors are often used as drugs, but can also be
6510:
Sibille E, Bana E, Chaouni W, Diederich M, Bagrel D, Chaimbault P (November 2012). "Development of a matrix-assisted laser desorption/ionization-mass spectrometry screening test to evidence reversible and irreversible inhibitors of CDC25 phosphatases".
2849:
cofactor together to produce thioglycinamide ribonucleotide dideazafolate (TGDDF), or enzymatically from the natural GAR substrate to yield GDDF. Here the subnanomolar dissociation constant (KD) of TGDDF was greater than predicted presumably due to
6298:
Enzyme inactivation is generally explained as a chemical process involving several phenomena like aggregation, dissociation into subunits, or denaturation (conformational changes), which occur simultaneously during the inactivation of a specific
3610:
leading to death and function for defence against predators or in hunting and capturing prey. Some of these natural inhibitors, despite their toxic attributes, are valuable for therapeutic uses at lower doses. An example of a neurotoxin are the
2718:
are followed. However, at higher concentrations, the second inhibitory site becomes occupied, inhibiting the enzyme. Product inhibition (either the enzyme's own product, or a product to an enzyme downstream in its metabolic pathway) is often a
3195:
The binding and inactivation steps of this reaction are investigated by incubating the enzyme with inhibitor and assaying the amount of activity remaining over time. The activity will be decreased in a time-dependent manner, usually following
3204:
gives the rate of inactivation at this concentration of inhibitor. This is done at several different concentrations of inhibitor. If a reversible EI complex is involved the inactivation rate will be saturable and fitting this curve will give
2030:
relating to the affinity of the enzyme for the substrate should in most cases relate to potential changes in the binding site of the enzyme which would directly result from enzyme inhibitor interactions. As such a term similar to the delta
3226:. Here, accurate measurement of the mass of the unmodified native enzyme and the inactivated enzyme gives the increase in mass caused by reaction with the inhibitor and shows the stoichiometry of the reaction. This is usually done using a
2015:
While this terminology results in a simplified way of dealing with kinetic effects relating to the maximum velocity of the
Michaelis–Menten equation, it highlights potential problems with the term used to describe effects relating to the
3373:
Since irreversible inhibition often involves the initial formation of a non-covalent enzyme inhibitor (EI) complex, it is sometimes possible for an inhibitor to bind to an enzyme in more than one way. For example, in the figure showing
1883:
term turns out to be higher than the initial term. To account for the possibly of activation as well the notation can then be rewritten replacing the inhibitor "I" with a modifier term (stimulator or inhibitor) denoted here as "X".
4154:
of databases of diverse molecules using computers, which are then followed by experimental confirmation of binding of the virtual screening hits. Complementary approaches that can provide new starting points for inhibitors include
120:
and may spontaneously leave the enzyme, allowing the enzyme to resume its function. Reversible inhibitors produce different types of inhibition depending on whether they bind to the enzyme, the enzyme-substrate complex, or both.
462:
the substrate and inhibitor cannot bind to the enzyme at the same time. This usually results from the inhibitor having an affinity for the active site of an enzyme where the substrate also binds; the substrate and inhibitor
4706:
3525:
protease, so it is important to inhibit the activity of trypsin in the pancreas to prevent the organ from digesting itself. One way in which the activity of trypsin is controlled is the production of a specific and potent
4134:
of the chemical reaction catalysed by the enzyme. The designed inhibitor often closely resembles the substrate, except that the portion of the substrate that undergoes chemical reaction is replaced by a chemically stable
2944:
is not based on a peptide and has no obvious structural similarity to a protein substrate. These non-peptide inhibitors can be more stable than inhibitors containing peptide bonds, because they will not be substrates for
3087:
Irreversible inhibition is different from irreversible enzyme inactivation. Irreversible inhibitors are generally specific for one class of enzyme and do not inactivate all proteins; they do not function by destroying
2010:
1870:
103:
An enzyme inhibitor stops ("inhibits") this process, either by binding to the enzyme's active site (thus preventing the substrate itself from binding) or by binding to another site on the enzyme such that the enzyme's
8173:
2263:
1739:
317:
blocks its activity as a protective mechanism against uncontrolled catalysis. The N‑terminal peptide is cleaved (split) from the zymogen enzyme precursor by another enzyme to release an active enzyme.
2585:
2171:
3501:
control helps maintain a steady concentration of ATP in the cell. However, metabolic pathways are not just regulated through inhibition since enzyme activation is equally important. With respect to PFK1,
8797:
349:
indistinguishable from competitive orthosteric inhibition) or alternatively stabilise binding of substrate to the enzyme but lock the enzyme in a conformation which is no longer catalytically active.
3111:
value. This is because the amount of active enzyme at a given concentration of irreversible inhibitor will be different depending on how long the inhibitor is pre-incubated with the enzyme. Instead,
782:
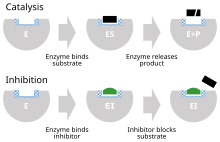
48:(P). Bottom: by binding to the enzyme, inhibitor (I) blocks binding of substrate. Binding site shown in blue checkerboard, substrate as black rectangle, and inhibitor as green rounded rectangle.
2918:
or intermediate of an enzyme-catalysed reaction. This ensures that the inhibitor exploits the transition state stabilising effect of the enzyme, resulting in a better binding affinity (lower
1467:
This rearrangement demonstrates that similar to the
Michaelis–Menten equation, the maximal rate of reaction depends on the proportion of the enzyme population interacting with its substrate.
4186:
of the enzyme in an inhibitor/enzyme complex to show how the molecule is binding to the active site, allowing changes to be made to the inhibitor to optimise binding in a process known as
2515:
6551:"Mechanism of the irreversible inactivation of mouse ornithine decarboxylase by alpha-difluoromethylornithine. Characterization of sequences at the inhibitor and coenzyme binding sites"
5306:
Walsh R, Martin E, Darvesh S (May 2007). "A versatile equation to describe reversible enzyme inhibition and activation kinetics: modeling beta-galactosidase and butyrylcholinesterase".
2854:
advantages gained and/or positive interactions acquired through the atoms linking the components. MAIs have also been observed to be produced in cells by reactions of pro-drugs such as
6488:
3699:. A less common class of toxins are toxic enzymes: these act as irreversible inhibitors of their target enzymes and work by chemically modifying their substrate enzymes. An example is
1631:
from 100% inhibition of substrate turn over to no inhibition. To account for this the equation can be easily modified to allow for different degrees of inhibition by including a delta
1625:
1545:
3005:
Irreversible inhibitors bind to the enzyme's binding site then undergo a chemical reaction to form a covalent enzyme-inhibitor complex (EI*). Binding site in blue, inhibitor in green.
8209:
3739:
The most common uses for enzyme inhibitors are as drugs to treat disease. Many of these inhibitors target a human enzyme and aim to correct a pathological condition. For instance,
4688:
124:
Enzyme inhibitors play an important role in all cells, since they are generally specific to one enzyme each and serve to control that enzyme's activity. For example, enzymes in a
6709:"Herpes simplex virus type 1 and human DNA polymerase interactions with 2'-deoxyguanosine 5'-triphosphate analogs. Kinetics of incorporation into DNA and induction of inhibition"
2625:
The effects of different types of reversible enzyme inhibitors on enzymatic activity can be visualised using graphical representations of the
Michaelis–Menten equation, such as
3096:
of all protein structure, but this is a non-specific effect. Similarly, some non-specific chemical treatments destroy protein structure: for example, heating in concentrated
266:
that inhibit upstream enzymes that produce those metabolites. This provides a negative feedback loop that prevents over production of metabolites and thus maintains cellular
4878:
3370:, a highly electrophilic species. This reactive form of DFMO then reacts with either a cysteine or lysine residue in the active site to irreversibly inactivate the enzyme.
421:
In contrast to irreversible inhibitors, reversible inhibitors generally do not undergo chemical reactions when bound to the enzyme and can be easily removed by dilution or
373:
Kinetic mechanisms for reversible inhibition. Substrate (S) binding to enzyme (E) in blue, catalysis releasing product (P) in red, inhibitor (I) binding to enzyme in green.
3246:
that can be analysed using a mass spectrometer. The peptide that changes in mass after reaction with the inhibitor will be the one that contains the site of modification.
293:
hibitors) which are produced by animals to protect against inappropriate enzyme activation and by plants to prevent predation. Another class of inhibitor proteins is the
8868:
Butterworth JF, IV, Mackey DC, Wasnick JD, eds. (2013). "Chapter 12. Cholinesterase
Inhibitors & Other Pharmacologic Antagonists to Neuromuscular Blocking Agents.".
8685:
Gentry BG, Bogner E, Drach JC (January 2019). "Targeting the terminase: An important step forward in the treatment and prophylaxis of human cytomegalovirus infections".
6631:
Stone SR, Morrison JF (February 1986). "Mechanism of inhibition of dihydrofolate reductases from bacterial and vertebrate sources by various classes of folate analogs".
5666:
Rozwarski DA, Grant GA, Barton DH, Jacobs WR, Sacchettini JC (January 1998). "Modification of the NADH of the isoniazid target (InhA) from
Mycobacterium tuberculosis".
8875:
3497:(PFK1). When ATP levels rise, ATP binds an allosteric site in PFK1 to decrease the rate of the enzyme reaction; glycolysis is inhibited and ATP production falls. This
8088:
Goldstein I, Burnett AL, Rosen RC, Park PW, Stecher VJ (January 2019). "The
Serendipitous Story of Sildenafil: An Unexpected Oral Therapy for Erectile Dysfunction".
6202:
Gehringer M, Laufer SA (June 2019). "Emerging and Re-Emerging
Warheads for Targeted Covalent Inhibitors: Applications in Medicinal Chemistry and Chemical Biology".
3388:
are bound in its active site. The top molecule is bound reversibly, but the lower one is bound covalently as it has reacted with an amino acid residue through its
7629:
Holmes CF, Maynes JT, Perreault KR, Dawson JF, James MN (November 2002). "Molecular enzymology underlying regulation of protein phosphatase-1 by natural toxins".
3125:
is the observed pseudo-first order rate of inactivation (obtained by plotting the log of % activity versus time) and is the concentration of inhibitor. The
3995:(AChE) is an enzyme found in animals, from insects to humans. It is essential to nerve cell function through its mechanism of breaking down the neurotransmitter
3258:
Chemical mechanism for irreversible inhibition of ornithine decarboxylase by DFMO. Pyridoxal 5'-phosphate (Py) and enzyme (E) are not shown. Adapted from Poulin
9208:
Gohlke H, Klebe G (August 2002). "Approaches to the description and prediction of the binding affinity of small-molecule ligands to macromolecular receptors".
3016:
bind to an enzyme, and this type of inhibition can therefore not be readily reversed. Irreversible inhibitors often contain reactive functional groups such as
3852:, which causes an erection. Since the drug decreases the activity of the enzyme that halts the signal, it makes this signal last for a longer period of time.
3345:
is an unusual type of irreversible inhibition where the enzyme converts the inhibitor into a reactive form in its active site. An example is the inhibitor of
8779:
7496:"A Systematic Review and Meta-Analysis of the Effectiveness of Acetylcholinesterase Inhibitors and Memantine in Treating the Cognitive Symptoms of Dementia"
5604:
Inglese J, Blatchly RA, Benkovic SJ (May 1989). "A multisubstrate adduct inhibitor of a purine biosynthetic enzyme with a picomolar dissociation constant".
3177:
Kinetic mechanism for irreversible inhibition. Substrate binding in blue, catalysis in red, inhibitor binding in green, inactivation reaction in dark green.
4826:
Cleland WW (February 1963). "The kinetics of enzyme-catalysed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory".
7543:
DeFrates LJ, Hoehns JD, Sakornbut EL, Glascock DG, Tew AR (January 2005). "Antimuscarinic intoxication resulting from the ingestion of moonflower seeds".
223:, meaning that only a minute amount of the inhibitor is required to inhibit the enzyme. A low concentration of the enzyme inhibitor reduces the risk for
6426:
Loo JA, DeJohn DE, Du P, Stevenson TI, Ogorzalek Loo RR (July 1999). "Application of mass spectrometry for target identification and characterization".
4370:
2911:
figure above. As this drug resembles the peptide that is the substrate of the HIV protease, it competes with the substrate in the enzyme's active site.
4123:
process, the first step of which is often the discovery of a new enzyme inhibitor. There are two principle approaches of discovering these inhibitors.
2231:, in which the inhibitor is titrated into a solution of enzyme and the heat released or absorbed is measured. However, the other dissociation constant
5569:
Schiffer CF, Burke JF, Besarab A, Lasker N, Simenhoff ML (January 1977). "Amylase/creatinine clearance fraction in patients on chronic hemodialysis".
7062:
2238:' is difficult to measure directly, since the enzyme-substrate complex is short-lived and undergoing a chemical reaction to form the product. Hence,
10115:
8336:
3323:(see the "DFP reaction" diagram). The enzyme hydrolyses the phosphorus–fluorine bond, but the phosphate residue remains bound to the serine in the
2220:' are the dissociation constants of the inhibitor for the enzyme and to the enzyme-substrate complex, respectively. The enzyme-inhibitor constant
2185:
Lineweaver–Burk diagrams of different types of reversible enzyme inhibitors. The arrow shows the effect of increasing concentrations of inhibitor.
4150:
of large libraries of structurally diverse compounds to identify hit molecules that bind to the enzyme. This method has been extended to include
3809:
An example of the structural similarity of some inhibitors to the substrates of the enzymes they target is seen in the figure comparing the drug
757:
Traditionally reversible enzyme inhibitors have been classified as competitive, uncompetitive, or non-competitive, according to their effects on
128:
may be inhibited by molecules produced later in the pathway, thus curtailing the production of molecules that are no longer needed. This type of
5639:
Inglese J, Benkovic SJ (1991). "Multisubstrate Adduct
Inhibitors of Glycinamide Ribonucleotide Transformylase: Synthetic and Enzyme Generated".
1890:
1750:
6983:
495:. Competitive inhibitors are often similar in structure to the real substrate (see for example the "methotrexate versus folate" figure in the
9994:
8358:
Zhang L, He J, Bai L, Ruan S, Yang T, Luo Y (July 2021). "Ribosome-targeting antibacterial agents: Advances, challenges, and opportunities".
6470:
6004:
Lew W, Chen X, Kim CU (June 2000). "Discovery and development of GS 4104 (oseltamivir): an orally active influenza neuraminidase inhibitor".
4023:
rather than cleaved. A large number of AChE inhibitors are used in both medicine and agriculture. Reversible competitive inhibitors, such as
2450:{\displaystyle V={\frac {V_{max}}{\alpha K_{m}+\alpha ^{\prime }}}={\frac {(1/\alpha ^{\prime })V_{max}}{(\alpha /\alpha ^{\prime })K_{m}+}}}
4541:
Boon L, Ugarte-Berzal E, Vandooren J, Opdenakker G (April 2020). "Protease propeptide structures, mechanisms of activation, and functions".
3400:
Enzyme inhibitors are found in nature and also produced artificially in the laboratory. Naturally occurring enzyme inhibitors regulate many
4072:
3711:
that inactivates ribosomes. Since ricin is a catalytic irreversible inhibitor, this allows just a single molecule of ricin to kill a cell.
3660:
Although many natural toxins are secondary metabolites, these poisons also include peptides and proteins. An example of a toxic peptide is
7432:
Abal M, Andreu JM, Barasoain I (June 2003). "Taxanes: microtubule and centrosome targets, and cell cycle dependent mechanisms of action".
9559:
9002:
7883:
6039:
Fischer PM (October 2003). "The design, synthesis and application of stereochemical and directional peptide isomers: a critical review".
4747:
4606:
2842:
7103:
6180:
1644:
4183:
2952:
In drug design it is important to consider the concentrations of substrates to which the target enzymes are exposed. For example, some
385:
Schematics for reversible inhibition. Binding site in blue, substrate in black, inhibitor in green, and allosteric site in light green.
4905:
3635:. Inhibition of this enzyme causes an uncontrolled increase in the acetylcholine neurotransmitter, muscular paralysis and then death.
2521:
9525:
6439:
600:
of inhibition is unchanged, and for uncompetitive (also called anticompetitive) inhibition the degree of inhibition increases with .
450:(the concentration of substrate resulting in half maximal enzyme activity) as the concentration of the enzyme's substrate is varied.
8231:
Buynak JD (September 2007). "Cutting and stitching: the cross-linking of peptidoglycan in the assembly of the bacterial cell wall".
7664:
Bischoff K (October 2001). "The toxicology of microcystin-LR: occurrence, toxicokinetics, toxicodynamics, diagnosis and treatment".
6912:"Two interacting binding sites for quinacrine derivatives in the active site of trypanothione reductase: a template for drug design"
6760:"Two interacting binding sites for quinacrine derivatives in the active site of trypanothione reductase: a template for drug design"
6123:
Bogoyevitch MA, Barr RK, Ketterman AJ (December 2005). "Peptide inhibitors of protein kinases-discovery, characterisation and use".
3821:, an enzyme that is potently inhibited by methotrexate. Methotrexate blocks the action of dihydrofolate reductase and thereby halts
2940:
However, not all inhibitors are based on the structures of substrates. For example, the structure of another HIV protease inhibitor
2051:
9221:
754:
substrate A for the first binding site, but be a non-competitive inhibitor with respect to substrate B in the second binding site.
9534:
Recommendations of the
Nomenclature Committee of the International Union of Biochemistry (NC-IUB) on enzyme inhibition terminology
7008:
Hiratake J (2005). "Enzyme inhibitors as chemical tools to study enzyme catalysis: rational design, synthesis, and applications".
4862:
3271:
as the enzyme "clamps down" around the inhibitor molecule. Examples of slow-binding inhibitors include some important drugs, such
467:
for access to the enzyme's active site. This type of inhibition can be overcome by sufficiently high concentrations of substrate (
6379:"Comparison of methods for analyzing kinetic data from mechanism-based enzyme inactivation: application to nitric oxide synthase"
2790:
TGDDF/GDDF multi-substrate adduct inhibitor. Substrate analogue in black, cofactor analogue in blue, non-cleavable linker in red.
3841:
6270:
Polakovič M, Vrabel P, Báleš V (January 1998). "Approaches for improved identification of mechanisms of enzyme inactivation".
334:
9385:
9093:
8996:
8791:
8762:
8737:
8469:
7877:
7708:
7381:
7356:
7245:
7177:
7097:
7056:
6977:
6859:
6830:
6607:
6482:
6320:
6291:
6254:
6174:
5823:
5512:
5247:
5222:
5197:
5161:
5083:
5016:
4983:
4955:
4872:
4741:
4700:
4600:
4439:
4364:
4276:
3886:
Drugs are also used to inhibit enzymes needed for the survival of pathogens. For example, bacteria are surrounded by a thick
3041:
116:
with the enzyme such that the enzyme is inhibited until the chemical bond is broken. By contrast, reversible inhibitors bind
8869:
2677:' accurately from such plots, it is advisable to estimate these constants using more reliable nonlinear regression methods.
7142:
5049:
4506:
Shapiro R, Vallee BL (February 1991). "Interaction of human placental ribonuclease with placental ribonuclease inhibitor".
5395:
Holdgate GA (July 2001). "Making cool drugs hot: isothermal titration calorimetry as a tool to study binding energetics".
5768:
Le Calvez PB, Scott CJ, Migaud ME (December 2009). "Multisubstrate adduct inhibitors: drug design and biological tools".
5373:
3829:
biosynthesis is selectively toxic to rapidly growing cells, therefore methotrexate is often used in cancer chemotherapy.
2714:
an enzyme having two competing substrate-binding sites. At low substrate, the high-affinity site is occupied and normal
9893:
9737:
9696:
8932:
Tan S, Evans R, Singh B (March 2006). "Herbicidal inhibitors of amino acid biosynthesis and herbicide-tolerant crops".
5874:
Agbowuro AA, Huston WM, Gamble AB, Tyndall JD (July 2018). "Proteases and protease inhibitors in infectious diseases".
3092:
but by specifically altering the active site of their target. For example, extremes of pH or temperature usually cause
5460:
Tseng SJ, Hsu JP (August 1990). "A comparison of the parameter estimating procedures for the
Michaelis-Menten model".
3107:
Irreversible inhibitors display time-dependent inhibition and their potency therefore cannot be characterised by an IC
10148:
9987:
9845:
9662:
5545:
3486:
3320:
2863:
17:
4143:
generally possess higher affinity for the enzyme compared to the substrate, and therefore are effective inhibitors.
341:("alternative" orientation) inhibitors. The mechanisms of allosteric inhibition are varied and include changing the
9854:
2460:
where the modifying factors α and α' are defined by the inhibitor concentration and its two dissociation constants
2228:
721:
Mixed-type inhibitors bind to both E and ES, but their affinities for these two forms of the enzyme are different (
3513:
Physiological enzyme inhibition can also be produced by specific protein inhibitors. This mechanism occurs in the
9768:
9552:
9158:
Lindquist RN (October 2013). "The design of enzyme inhibitors: Transition state analogues.". In Ariëns EJ (ed.).
7374:
Plant Protease Inhibitors: Significance in Nutrition, Plant Protection, Cancer Prevention and Genetic Engineering
3964:
2466:
302:
596:
or three-dimensional shape) of the enzyme so that the affinity of the substrate for the active site is reduced.
10427:
9814:
9721:
8493:
Voshavar C (2019). "Protease Inhibitors for the Treatment of HIV/AIDS: Recent Advances and Future Challenges".
8450:
Li G, Jing X, Pan, Zhang P, De Clercq E (2021). "Antiviral Classification". In Bamford D, Zuckerman MA (eds.).
4160:
3845:
3632:
7290:
4234:– a type of enzyme inhibitor that mimics the transition state of the chemical reaction catalysed by the enzyme
1556:
1476:
429:
that form a chemical bond with the enzyme, but the bond can be cleaved so the inhibition is fully reversible.
10304:
9908:
9742:
9503:
4352:
4156:
7193:
Okar DA, Lange AJ (1999). "Fructose-2,6-bisphosphate and control of carbohydrate metabolism in eukaryotes".
673:. The inhibitor affects substrate binding by increasing the enzyme's affinity for the substrate (decreasing
10432:
9980:
9941:
9933:
7467:
Hostettmann K, Borloz A, Urbain A, Marston A (2006). "Natural Product Inhibitors of Acetylcholinesterase".
3298:
with the lower molecule of an inhibitor bound irreversibly and the upper one reversibly. Created from Bond
8268:"Selective toxicity of antibacterial agents-still a valid concept or do we miss chances and ignore risks?"
8010:
McLornan DP, Pope JE, Gotlib J, Harrison CN (August 2021). "Current and future status of JAK inhibitors".
7044:
4115:
Robots are used for the high-throughput screening of chemical libraries to discover new enzyme inhibitors.
3758:
As of 2017, an estimated 29% of approved drugs are enzyme inhibitors of which approximately one-fifth are
3531:
destabilising the transition state. Other examples of physiological enzyme inhibitor proteins include the
567:
will remain the same as the actual binding of the substrate, by definition, will still function properly.
152:
produced by animals or plants are enzyme inhibitors that block the activity of crucial enzymes in prey or
9837:
9827:
9809:
9750:
9654:
9376:
Perez O, Pena J, Fernandez-Vega V, Scampavia L, Spicer T (2019). "Chapter 4: High Throughput Screening".
6810:
3231:
3093:
526:
9401:
Scarpino A, Ferenczy GG, Keserű GM (2020). "Covalent Docking in Drug Discovery: Scope and Limitations".
8316:
6076:"Small molecule substrate phosphorylation site inhibitors of protein kinases: approaches and challenges"
163:
are enzyme inhibitors that inhibit an aberrant human enzyme or an enzyme critical for the survival of a
10442:
10070:
9691:
9633:
9596:
9545:
5266:
Walsh R, Martin E, Darvesh S (December 2011). "Limitations of conventional inhibitor classifications".
3316:
3132:/ parameter is valid as long as the inhibitor does not saturate binding with the enzyme (in which case
3069:
2987:
545:
277:
In addition to small molecules, some proteins act as enzyme inhibitors. The most prominent example are
200:
8174:"Breaking down the cell wall: Strategies for antibiotic discovery targeting bacterial transpeptidases"
2925:) than substrate-based designs. An example of such a transition state inhibitor is the antiviral drug
10405:
10392:
10379:
10366:
10353:
10340:
10327:
10289:
9957:
9780:
4261:
Evaluation of Enzyme Inhibitors in Drug Discovery: A Guide for Medicinal Chemists and Pharmacologists
4231:
4215:
4147:
4140:
4139:
that resembles the transition state. Since the enzyme has evolved to stabilise the transition state,
4100:
3503:
2846:
617:
117:
9646:
7164:
Orencio-Trejo M, Utrilla J, Fernández-Sandoval MT, Huerta-Beristain G, Gosset G, Martinez A (2010).
5422:
Leatherbarrow RJ (December 1990). "Using linear and non-linear regression to fit biochemical data".
235:
in humans. Hence the discovery and refinement of enzyme inhibitors is an active area of research in
10299:
10253:
10196:
9913:
9775:
7775:
Sowa-Rogozińska N, Sominka H, Nowakowska-Gołacka J, Sandvig K, Słomińska-Wojewódzka M (June 2019).
6962:
5839:
Hsu JT, Wang HC, Chen GW, Shih SR (2006). "Antiviral drug discovery targeting to viral proteases".
4087:. Many other enzymes are inhibited by herbicides, including enzymes needed for the biosynthesis of
3887:
3763:
2736:
2626:
589:
508:
399:
342:
326:
81:
41:
7263:"Substrate variants versus transition state analogues as noncovalent reversible enzyme inhibitors"
10201:
9888:
9866:
9832:
9591:
9329:"Selections and screenings of DNA-encoded chemical libraries against enzyme and cellular targets"
8897:
Thapa S, Lv M, Xu H (2017). "Acetylcholinesterase: A Primary Target for Drugs and Insecticides".
8536:
de Leuw P, Stephan C (April 2018). "Protease inhibitor therapy for hepatitis C virus-infection".
6875:
Walsh CT (1984). "Suicide substrates, mechanism-based enzyme inactivators: recent developments".
5711:"Molecular basis for the high-affinity binding and stabilization of firefly luciferase by PTC124"
3968:
3849:
3818:
3684:
and is a known carcinogen that can also cause acute liver haemorrhage and death at higher doses.
3362:
3358:
2880:
2768:
407:
314:
7312:
Hartley RW (November 1989). "Barnase and barstar: two small proteins to fold and fit together".
6590:
Szedlacsek SE, Duggleby RG (1995). "[6] Kinetics of slow and tight-binding inhibitors".
10055:
9849:
9819:
9785:
9758:
9670:
9586:
7397:
Tan G, Gyllenhaal C, Soejarto DD (March 2006). "Biodiversity as a source of anticancer drugs".
3783:
3654:
3650:
3493:. A key step for the regulation of glycolysis is an early reaction in the pathway catalysed by
3482:
3328:
2867:
459:
294:
8976:
7865:
5101:"Enzyme-Inhibitor Interactions and a Simple, Rapid Method for Determining Inhibition Modality"
4729:
4588:
10222:
10141:
10075:
9898:
7826:
Hartley MR, Lord JM (September 2004). "Cytotoxic ribosome-inactivating lectins from plants".
7165:
7085:
6162:
4190:. This test and improve cycle is repeated until a sufficiently potent inhibitor is produced.
4080:
3976:
3787:
3507:
3268:
2630:
2190:
622:
609:
584:
effect where the inhibitor binds to a different site on an enzyme. Inhibitor binding to this
251:
Enzyme inhibitors are a chemically diverse set of substances that range in size from organic
232:
220:
97:
4909:
3848:. This signalling molecule triggers smooth muscle relaxation and allows blood flow into the
10294:
7595:
6338:"Profiling the specific reactivity of the proteome with non-directed activity-based probes"
5814:
Avendano C, Menendez JC (June 2015). "Chapter 2.5: Inhibitors of Dihydrofolate Reductase".
5722:
5675:
5469:
5356:
Strelow J, Dewe W, Iversen PW, Brooks PB, Radding JA, McGee J, et al. (October 2012).
5240:
Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems
4976:
Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems
4641:
4127:
3992:
3833:
3791:
3568:
3567:
Animals and plants have evolved to synthesise a vast array of poisonous products including
3434:
2250:
338:
271:
5074:
Voet D, Voet JG, Pratt CW (2016). "Chapter 12: Enzyme Kinetics, Inhibition, and Control".
4395:
Haefner B (June 2003). "Drugs from the deep: marine natural products as drug candidates".
4174:
for predicting the binding orientation and affinity of an inhibitor for an enzyme such as
8:
10437:
10258:
10090:
10080:
10004:
9763:
9678:
7908:
Santos R, Ursu O, Gaulton A, Bento AP, Donadi RS, Bologa CG, et al. (January 2017).
7872:(4th ed.). Philadelphia: Lippincott Williams & Wilkins. pp. 499–518 (502).
5917:
Qiu X, Liu ZP (2011). "Recent developments of peptidomimetic HIV-1 protease inhibitors".
5528:
Radzicka A, Wolfenden R (1995). "Transition state and multisubstrate analog inhibitors".
4187:
4179:
3952:
3494:
2888:
2884:
2838:
511:
the inhibitor binds only to the enzyme-substrate complex. This type of inhibition causes
199:. Since anti-pathogen inhibitors generally target only one enzyme, such drugs are highly
192:
188:
109:
85:
45:
7599:
6888:
5726:
5679:
5473:
5364:. Eli Lilly & Company and the National Center for Advancing Translational Sciences.
4645:
4630:"Analysis of equilibrium binding of an orthosteric tracer and two allosteric modulators"
4259:
Copeland RA (March 2013). "Why Enzymes as Drug Targets? Enzyme are Essential for Life".
3335:
of neurons, and consequently is a potent neurotoxin, with a lethal dose of less than 100
10191:
9601:
9472:
9445:
9426:
9358:
9304:
9279:
9140:
9061:
9048:
9028:
8957:
8845:
8820:
8710:
8667:
8654:
8629:
8605:
8580:
8561:
8518:
8460:
8427:
8402:
8383:
8292:
8267:
8201:
8113:
8035:
7987:
7962:
7934:
7909:
7803:
7776:
7752:
7725:
7568:
7525:
7218:
6938:
6911:
6910:
Saravanamuthu A, Vickers TJ, Bond CS, Peterson MR, Hunter WN, Fairlamb AH (July 2004).
6786:
6759:
6758:
Saravanamuthu A, Vickers TJ, Bond CS, Peterson MR, Hunter WN, Fairlamb AH (July 2004).
6684:
6667:
6475:
Enzyme Kinetics: Catalysis and Control: A Reference of Theory and Best-Practice Methods
6451:
6403:
6378:
6227:
6100:
6075:
5981:
5956:
5899:
5793:
5745:
5710:
5130:
4800:
4775:
4664:
4629:
4566:
4483:
4456:
4323:
4298:
3803:
3342:
593:
518:
to decrease (maximum velocity decreases as a result of removing activated complex) and
433:
263:
9414:
8977:"Major Synthetic Routes for Modern Herbicide Classes and Agrochemical Characteristics"
8910:
8506:
8023:
7607:
7238:
Fundamentals of enzymology : the cell and molecular biology of catalytic proteins
7163:
6822:
6725:
6708:
6567:
6550:
6354:
6337:
6283:
5652:
5481:
4408:
3171:
2999:
2841:, a potent Multi-substrate Adduct Inhibitor (MAI) to glycinamide ribonucleotide (GAR)
2798:
642:
Competitive inhibitors can bind to E, but not to ES. Competitive inhibition increases
10065:
9871:
9477:
9430:
9418:
9381:
9362:
9350:
9309:
9260:
9225:
9190:
9144:
9132:
9089:
9066:
8992:
8949:
8914:
8850:
8787:
8758:
8733:
8702:
8671:
8659:
8610:
8553:
8522:
8510:
8475:
8465:
8432:
8387:
8375:
8328:
8297:
8248:
8205:
8193:
8154:
8105:
8070:
8039:
8027:
7992:
7939:
7873:
7843:
7808:
7757:
7704:
7700:
7673:
7646:
7611:
7560:
7517:
7449:
7414:
7377:
7352:
7329:
7325:
7282:
7241:
7210:
7173:
7134:
7093:
7052:
7025:
6973:
6943:
6892:
6855:
6826:
6791:
6748:
6730:
6689:
6648:
6644:
6613:
6603:
6599:
6572:
6528:
6478:
6443:
6408:
6359:
6316:
6287:
6250:
6219:
6170:
6140:
6105:
6056:
6021:
5986:
5934:
5891:
5856:
5819:
5785:
5750:
5691:
5621:
5586:
5551:
5541:
5537:
5508:
5485:
5439:
5435:
5404:
5365:
5323:
5283:
5243:
5218:
5193:
5157:
5122:
5079:
5041:
5012:
4979:
4951:
4868:
4843:
4839:
4805:
4737:
4696:
4669:
4596:
4570:
4558:
4523:
4488:
4435:
4412:
4360:
4328:
4272:
4182:
can be used to assist in the optimisation process. New inhibitors are used to obtain
4175:
4151:
4036:
3944:
3908:
3874:
3744:
3692:
3669:
3645:
3560:
3527:
3458:
3380:
3304:
3223:
3197:
3097:
3089:
2724:
204:
129:
125:
73:
8714:
8698:
8133:"Erectile dysfunction: from biochemical pharmacology to advances in medical therapy"
8117:
7572:
7529:
7222:
6455:
6231:
5903:
5797:
5134:
3723:
The coenzyme folic acid (top) compared to the anti-cancer drug methotrexate (bottom)
2735:
Slow-tight inhibition occurs when the initial enzyme–inhibitor complex EI undergoes
379:
367:
10237:
10206:
10134:
10100:
10095:
10045:
9606:
9467:
9457:
9410:
9340:
9299:
9291:
9252:
9217:
9182:
9122:
9056:
9040:
8984:
8961:
8941:
8906:
8840:
8832:
8694:
8649:
8641:
8600:
8592:
8565:
8545:
8502:
8455:
8422:
8414:
8367:
8287:
8279:
8240:
8185:
8144:
8097:
8062:
8053:
McGuire JJ (2003). "Anticancer antifolates: current status and future directions".
8019:
7982:
7974:
7929:
7921:
7835:
7798:
7788:
7747:
7737:
7696:
7638:
7603:
7552:
7507:
7476:
7441:
7406:
7321:
7274:
7202:
7017:
6933:
6923:
6884:
6818:
6781:
6771:
6720:
6679:
6640:
6595:
6562:
6520:
6435:
6398:
6390:
6349:
6279:
6211:
6132:
6095:
6087:
6048:
6013:
5976:
5968:
5926:
5883:
5848:
5777:
5740:
5730:
5709:
Auld DS, Lovell S, Thorne N, Lea WA, Maloney DJ, Shen M, et al. (March 2010).
5683:
5648:
5613:
5578:
5533:
5477:
5431:
5315:
5275:
5112:
5004:
4835:
4828:
Biochimica et Biophysica Acta (BBA) - Specialized Section on Enzymological Subjects
4795:
4787:
4659:
4649:
4593:
Real World Drug Discovery: A Chemist's Guide to Biotech and Pharmaceutical Research
4550:
4515:
4478:
4468:
4404:
4318:
4310:
4264:
4136:
4131:
4120:
3771:
3704:
3446:
3389:
3033:
3017:
2915:
2634:
576:
560:
will decrease due to the inability for the reaction to proceed as efficiently, but
216:
89:
9537:
9529:
8549:
7978:
4554:
608:
Reversible inhibition can be described quantitatively in terms of the inhibitor's
432:
Reversible inhibitors are generally categorized into four types, as introduced by
10284:
10268:
10181:
10020:
9876:
9683:
9295:
9256:
9127:
9110:
8988:
8418:
8189:
7839:
7051:(Third ed.). Philadelphia, Pa.: Elsevier Health Sciences. pp. 153–155.
6215:
6136:
5319:
4654:
4044:
3324:
2991:
2715:
2637:. An illustration is provided by the three Lineweaver–Burk plots depicted in the
2254:
2246:
613:
585:
549:
422:
346:
65:
9972:
8645:
7126:
6633:
Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology
5972:
5687:
5033:
5008:
4791:
4111:
2883:(DHFR) are prominent examples. Other examples of these substrate mimics are the
2808:
with substrate binding sites located in enzyme labelled as S2, S1, S1', and S2'.
10322:
10263:
10085:
10050:
9729:
9716:
9623:
9345:
9328:
9186:
8596:
8283:
8101:
7774:
7480:
7410:
6169:(Sixth ed.). Oxford, United Kingdom: Oxford University Press. p. 95.
5930:
5852:
5715:
Proceedings of the National Academy of Sciences of the United States of America
5357:
4225:
4207:
4096:
4020:
4016:
3948:
3916:
3748:
3696:
3695:(discussed in the "metabolic regulation" section above) that are found in some
3661:
3572:
3571:, peptides and proteins that can act as inhibitors. Natural toxins are usually
3552:
3462:
2953:
2900:
2249:
under various substrate and inhibitor concentrations, and fitting the data via
2227:
can be measured directly by various methods; one especially accurate method is
735:'). Thus, mixed-type inhibitors affect substrate binding (increase or decrease
481:
will increase as it takes a higher concentration of the substrate to reach the
474:
remains constant), i.e., by out-competing the inhibitor. However, the apparent
252:
228:
224:
137:
8945:
8836:
8479:
7278:
5781:
5582:
4268:
2652:. In the bottom diagram the non-competitive inhibition lines intersect on the
536:
which indicates a higher binding affinity). Uncompetitive inhibition is rare.
10421:
10227:
10186:
10110:
10035:
10025:
9859:
8066:
7642:
7445:
6524:
6052:
6017:
5117:
5100:
4228:– an enzyme inhibitor that is used to interfere with cell growth and division
4222:
that uses covalent enzyme inhibitors as reporters to monitor enzyme activity.
4028:
3996:
3891:
3752:
3751:
enzyme. This inhibition in turn suppresses the production of proinflammatory
3636:
3612:
3588:
3469:
acting as inhibitors and enhancers for the enzymes in that same pathway. The
3438:
3375:
3295:
3201:
3045:
2934:
2845:
was prepared synthetically by linking analogues of the GAR substrate and the
2641:
figure. In the top diagram the competitive inhibition lines intersect on the
659:
Uncompetitive inhibitors bind to ES. Uncompetitive inhibition decreases both
656:(the inhibitor does not hamper catalysis in ES because it cannot bind to ES).
649:(i.e., the inhibitor interferes with substrate binding), but does not affect
436:
in 1963. They are classified according to the effect of the inhibitor on the
403:
113:
7691:
Savage GP, Morrison SC (2003). "Trypsin inhibitors.". In Caballero B (ed.).
7206:
5735:
4473:
270:(steady internal conditions). Small molecule enzyme inhibitors also include
10176:
10105:
9568:
9481:
9422:
9354:
9313:
9264:
9229:
9194:
9136:
9084:
Ganguly AK, Alluri SS (12 September 2021). "Chapter 2: Enzyme Inhibitors".
8953:
8918:
8854:
8706:
8663:
8614:
8557:
8514:
8454:. Vol. 5 (4th ed.). Amsterdam: Academic Press. pp. 129–130.
8436:
8379:
8332:
8301:
8252:
8197:
8158:
8109:
8074:
8031:
7996:
7943:
7847:
7812:
7793:
7761:
7742:
7677:
7650:
7564:
7521:
7453:
7418:
7286:
7214:
7138:
7029:
6947:
6928:
6795:
6776:
6532:
6447:
6412:
6363:
6223:
6144:
6109:
6060:
6025:
5990:
5938:
5895:
5860:
5789:
5754:
5408:
5369:
5341:
Walsh R (May 2012). "Alternative perspectives of enzyme kinetic modeling".
5327:
5287:
5126:
5045:
4847:
4809:
4673:
4562:
4492:
4416:
4332:
4314:
4076:
4056:
3810:
3779:
3688:
3596:
3426:
3350:
3272:
3254:
3101:
2904:
2005:{\displaystyle V_{\max 1}-(V_{\max 1}-V_{\max 2}){\cfrac {\ce {}}{+K_{x}}}}
1865:{\displaystyle V_{\max 1}-(V_{\max 1}-V_{\max 2}){\cfrac {\ce {}}{+K_{i}}}}
322:
298:
240:
236:
184:
180:
9222:
10.1002/1521-3773(20020802)41:15<2644::AID-ANIE2644>3.0.CO;2-O
9070:
8407:
Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids
8149:
8132:
7724:
Polito L, Bortolotti M, Battelli MG, Calafato G, Bolognesi A (June 2019).
7615:
7333:
6896:
6734:
6693:
6652:
6617:
6576:
5695:
5625:
5555:
5489:
5443:
4527:
612:
to the enzyme and to the enzyme-substrate complex, and its effects on the
10400:
10335:
10171:
10040:
9706:
9462:
9446:"Mechanisms of Proteolytic Enzymes and Their Inhibition in QM/MM Studies"
7925:
5590:
4171:
4167:
4032:
4024:
3960:
3923:
3767:
3719:
3708:
3681:
3677:
3673:
3668:
mushroom. This is a potent enzyme inhibitor, in this case preventing the
3592:
3466:
3442:
3410:
3276:
3053:
2930:
2926:
2834:
2816:
2181:
415:
330:
267:
133:
93:
7963:"A comprehensive review of protein kinase inhibitors for cancer therapy"
6440:
10.1002/(SICI)1098-1128(199907)19:4<307::AID-MED4>3.0.CO;2-2
5617:
4519:
10030:
9052:
5279:
4219:
4092:
4084:
4068:
3932:
3903:
3899:
3895:
3837:
3826:
3814:
3732:
3616:
3603:
3577:
3474:
3470:
3422:
3061:
3025:
2941:
2823:
581:
411:
325:
of inhibitors on enzymes is most commonly the same site that binds the
310:
160:
8371:
8244:
7556:
7512:
7495:
7021:
6752:
6091:
5887:
4007:. This is somewhat unusual among neurotransmitters as most, including
3878:
3755:
and thus aspirin may be used to reduce pain, fever, and inflammation.
3404:
processes and are essential for life. In addition, naturally produced
3327:, deactivating it. Similarly, DFP also reacts with the active site of
3308:
32:
10374:
10348:
9799:
9508:, Database of enzymes giving lists of known inhibitors for each entry
7777:"Intracellular Transport and Cytotoxicity of the Protein Toxin Ricin"
7170:
Biosystems Engineering II: Linking Cellular Networks and Bioprocesses
6394:
5530:
Enzyme Kinetics and Mechanism Part D: Developments in Enzyme Dynamics
4736:(First ed.). Boca Raton, FL: Garland Science. pp. 531–580.
4052:
4048:
4040:
4008:
3988:
3972:
3822:
3795:
3665:
3607:
3430:
3418:
3414:
3401:
3354:
3346:
3280:
3227:
3077:
3037:
2946:
2896:
2855:
2805:
172:
105:
9512:
9044:
4540:
3547:
2767:) rate constants for inhibitor association with kinetics similar to
529:
and the effective elimination of the ES complex thus decreasing the
7262:
4695:(Fifth ed.). Oxford, UK: Oxford University Press. p. 90.
4201:
4012:
3956:
3928:
3775:
3640:
3628:
3514:
3498:
3490:
3332:
3235:
3081:
3073:
3057:
3021:
3013:
2892:
2851:
1734:{\displaystyle V_{\max }-\Delta V_{\max }{\cfrac {\ce {}}{+K_{i}}}}
690:
Non-competitive inhibitors have identical affinities for E and ES (
208:
203:
and generally produce few side effects in humans, provided that no
196:
176:
164:
153:
145:
141:
57:
8581:"Approved HIV reverse transcriptase inhibitors in the past decade"
4627:
553:
of inhibition depends only on the concentration of the inhibitor.
9499:
7172:. Berlin: Springer Science & Business Media. pp. 77–78.
5360:. In Markossian S, Grossman A, Brimacombe K, et al. (eds.).
5040:. Treasure Island (FL): StatPearls Publishing. p. 31424826.
4004:
4000:
3740:
3585:
3536:
3532:
3522:
3518:
3478:
3243:
3239:
345:(shape) of the enzyme such that it can no longer bind substrate (
306:
256:
69:
9375:
8818:
8403:"Bacterial fatty acid metabolism in modern antibiotic discovery"
7723:
6909:
6757:
6668:"Reaction of formaldehyde and of methanol with xanthine oxidase"
4043:
pesticides are also examples of reversible AChE inhibitors. The
2580:{\displaystyle \alpha ^{\prime }=1+{\frac {}{K_{i}^{\prime }}}.}
10387:
10157:
9638:
9243:
Koppitz M, Eis K (June 2006). "Automated medicinal chemistry".
8730:
Carbamate Insecticides: Chemistry, Biochemistry, and Toxicology
7542:
6836:
3799:
3759:
3639:
can also result from the inhibition of receptors; for example,
3624:
3620:
3556:
3405:
3065:
3049:
3029:
2980:
2859:
2590:
Thus, in the presence of the inhibitor, the enzyme's effective
2166:{\displaystyle K_{m1}-(K_{m1}-K_{m2}){\cfrac {\ce {}}{+K_{x}}}}
278:
207:
enzyme is found in humans. (This is often the case, since such
149:
61:
37:
8317:"How do antibiotics kill bacterial cells but not human cells?"
8131:
Maggi M, Filippi S, Ledda F, Magini A, Forti G (August 2000).
7466:
6961:
Pereira DM, Andrade C, Valentão P, Andrade PB (October 2017).
4263:(Second ed.). John Wiley & Sons, Inc. pp. 1–23.
4170:
to high affinity binders that efficiently inhibit the enzyme.
3517:, which synthesises many digestive precursor enzymes known as
3052:. The residues modified are those with side chains containing
10361:
9923:
9015:
Chapter 10.2.1: Sulfonylurea acetolactate synthase inhibitors
8819:
Farmakidis C, Pasnoor M, Dimachkie MM, Barohn RJ (May 2018).
7828:
Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics
7494:
Knight R, Khondoker M, Magill N, Stewart R, Landau S (2018).
6960:
6471:"Irreversible Enzyme Inhibition by Affinity Labelling Agents"
6125:
Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics
5192:(3rd ed.). Newark: John Wiley & Sons, Incorporated.
5003:(2nd ed.). Woodhead Publishing. pp. 126–152 (135).
4734:
The Molecules of Life : Physical and Chemical Principles
4088:
3727:
3700:
3581:
3367:
3234:
involves digestion of the native and modified protein with a
2784:
525:
to decrease (due to better binding efficiency as a result of
212:
168:
92:
a specific chemical reaction by binding the substrate to its
8780:"Classification and Uses of Organophosphates and Carbamates"
7493:
6549:
Poulin R, Lu L, Ackermann B, Bey P, Pegg AE (January 1992).
4359:. Hoboken, N.J.: John Wiley & Sons. pp. 1–24 (12).
4083:. Both enzymes are needed for plants to make branched-chain
3510:
are examples of metabolites that are allosteric activators.
10015:
8983:. Springer Science & Business Media. pp. 179–195.
7628:
6509:
5873:
5098:
4908:. NIH Center for Translational Therapeutics. Archived from
3860:
3291:
711:(i.e., it does not affect substrate binding) but decreases
414:. Multiple weak bonds between the inhibitor and the enzyme
140:. Enzyme inhibitors also control essential enzymes such as
77:
10126:
9173:
Scapin G (2006). "Structural biology and drug discovery".
8867:
8009:
7166:"Engineering the Escherichia coli Fermentative Metabolism"
5665:
5568:
5532:. Methods in Enzymology. Vol. 249. pp. 284–312.
4628:
Jakubík J, Randáková A, El-Fakahany EE, Doležal V (2019).
4429:
4299:"Control and regulation of pathways via negative feedback"
3790:
and hence these inhibitors are used to treat a variety of
3762:
inhibitors. A notable class of kinase drug targets is the
3048:
groups react with amino acid side chains to form covalent
8087:
7586:
Vetter J (January 1998). "Toxins of Amanita phalloides".
6817:. Methods in Enzymology. Vol. 11. pp. 686–702.
6594:. Methods in Enzymology. Vol. 249. pp. 144–80.
5076:
Fundamentals of Biochemistry: Life at the Molecular Level
2753:
can be obtained through more complex analysis of the on (
9160:
Drug Design: Medicinal Chemistry: A Series of Monographs
8755:
CNS Neurotransmitters and Neuromodulators: Acetylcholine
6972:(First ed.). Wiley-VCH Verlag GmbH & Co. KGaA.
6122:
5355:
5078:(Fifth ed.). Hoboken, NJ: Wiley. pp. 361–401.
5001:
Enzymes: Biochemistry, Biotechnology, Clinical Chemistry
3222:
Another method that is widely used in these analyses is
96:, a specialized area on the enzyme that accelerates the
9086:
Medicinal Chemistry: A Look at How Drugs Are Discovered
8130:
7695:(Second ed.). Academic Press. pp. 5878–5884.
5603:
4595:(1st ed.). Amsterdam: Elsevier. pp. 281–285.
4146:
The second way of discovering new enzyme inhibitors is
3104:
holding proteins together, releasing free amino acids.
2645:-axis, illustrating that such inhibitors do not affect
2129:
2109:
1968:
1948:
1828:
1808:
1697:
1677:
1583:
1563:
1503:
1483:
1398:
1378:
1302:
1269:
1219:
1199:
1081:
1034:
979:
960:
911:
892:
841:
821:
807:
788:
548:
the binding of the inhibitor to the enzyme reduces its
108:
of the reaction is blocked. Enzyme inhibitors may bind
9400:
7907:
6970:
Natural Products Targeting Clinically Relevant Enzymes
6808:
5308:
Biochimica et Biophysica Acta (BBA) - General Subjects
5099:
Buker SM, Boriack-Sjodin PA, Copeland RA (June 2019).
4941:
4939:
4937:
4935:
4933:
4931:
4929:
4927:
4730:"Molecular Recognition: The Thermodynamics of Binding"
4543:
Critical Reviews in Biochemistry and Molecular Biology
3817:. Folic acid is the oxidised form of the substrate of
2132:
2112:
1971:
1951:
1831:
1811:
1700:
1680:
1586:
1566:
1506:
1486:
1401:
1381:
1305:
1272:
1222:
1202:
1084:
1037:
982:
963:
914:
895:
844:
824:
810:
791:
8786:. Amsterdam: Elsevier Academic Press. pp. 5–24.
8784:
Toxicology of Organophosphate and Carbamate Compounds
7396:
6477:. San Diego, Calif.: Elsevier Academic. p. 542.
5502:
5217:. Hoboken, N.J.: Wiley-Interscience. pp. 61–69.
5213:
Marangoni AG (2003). "Reversible Enzyme Inhibition".
4727:
4454:
2524:
2469:
2266:
2054:
1893:
1753:
1647:
1559:
1550:
fraction of the enzyme population bound by inhibitor
1479:
1470:
fraction of the enzyme population bound by substrate
780:
313:
peptide that binds to the active site of enzyme that
7768:
7726:"Ricin: An Ancient Story for a Timeless Plant Toxin"
7124:
6548:
6425:
6269:
5770:
Journal of Enzyme Inhibition and Medicinal Chemistry
5767:
4197:
3782:
are another notable example of drug enzyme targets.
3770:; their over-activation may result in cancer. Hence
3465:
through a pathway is often regulated by a pathway's
2903:(peptide mimic) protease inhibitor containing three
443:(maximum reaction rate catalysed by the enzyme) and
9567:
7431:
6335:
5708:
4924:
4430:Gualerzi CO, Brandi L, Fabbretti A, Pon CL (2013).
4039:and in anaesthesia to reverse muscle blockade. The
3844:, the enzyme that degrades the signalling molecule
2245:' is usually measured indirectly, by observing the
742:) and hamper catalysis in the ES complex (decrease
9380:. Boca Raton, Florida: CRC Press. pp. 47–69.
9088:. Boca Raton, Florida: CRC Press. pp. 29–68.
7960:
7863:
7157:
7125:Greathouse B, Zahra F, Brady MF (September 2021).
6589:
6310:
5305:
5265:
5183:
5181:
5179:
5177:
5175:
5173:
4999:Palmer T, Bonner PL (2007). "Enzyme inhibition.".
4860:
3680:. This toxin can contaminate water supplies after
2914:Enzyme inhibitors are often designed to mimic the
2579:
2509:
2449:
2165:
2004:
1864:
1733:
1619:
1539:
1456:
305:. A special case of protein enzyme inhibitors are
10002:
8684:
7090:Chemical Pesticides Mode of Action and Toxicology
6854:(1st ed.). Philadelphia, PA: W.B. Saunders.
6665:
6336:Adam GC, Cravatt BF, Sorensen EJ (January 2001).
5527:
5105:SLAS Discovery: Advancing Life Sciences R & D
3840:(Viagra). This compound is a potent inhibitor of
3230:mass spectrometer. In a complementary technique,
2929:; this drug mimics the planar nature of the ring
2663:. However, since it can be difficult to estimate
148:that, if left unchecked, may damage a cell. Many
10419:
9280:"Fragment-based approaches to enzyme inhibition"
8812:
7460:
5813:
5809:
5807:
5031:
4582:
4580:
4357:Functional Metabolism: Regulation and Adaptation
1934:
1918:
1899:
1794:
1778:
1759:
1669:
1653:
1445:
1370:
1357:
1179:
1021:
879:
798:
418:combine to produce strong and specific binding.
112:or irreversibly. Irreversible inhibitors form a
9443:
8974:
8578:
7949:Total enzyme targets of approved drugs = 29.45%
7910:"A comprehensive map of molecular drug targets"
7133:. Treasure Island (FL): StatPearls Publishing.
6666:Pick FM, McGartoll MA, Bray RC (January 1971).
6201:
6156:
6154:
5950:
5948:
5838:
5638:
5503:Dixon M, Webb EC, Thorne CJ, Tipton KF (1979).
5170:
5154:Physical Chemistry with Biological Applications
4821:
4819:
4432:Antibiotics: Targets, Mechanisms and Resistance
4254:
4252:
4250:
4248:
3353:(DFMO), which is an analogue of the amino acid
704:'). Non-competitive inhibition does not change
9526:"Symbolism and Terminology in Enzyme Kinetics"
9394:
8871:Morgan & Mikhail's Clinical Anesthesiology
8621:
8535:
8486:
8449:
8357:
8171:
8165:
7954:
7903:
7901:
7859:
7857:
7690:
6195:
5761:
5507:(3rd ed.). London: Longman. p. 126.
5206:
4945:
4448:
4390:
4388:
4346:
4344:
4342:
3672:enzyme from transcribing DNA. The algal toxin
2656:-axis, showing these inhibitors do not affect
219:.) Medicinal enzyme inhibitors often have low
10142:
9988:
9553:
9517:National Center for Biotechnology Information
9083:
8979:. In Böger P, Wakabayashi K, Hirai K (eds.).
8931:
8925:
8627:
8529:
8259:
8003:
7042:
7001:
6630:
6592:Kinetics of slow and tight-binding inhibitors
6073:
5957:"Enzymatic Transition States and Drug Design"
5867:
5804:
5455:
5453:
5421:
5301:
5299:
5297:
5261:
5259:
5073:
4769:
4767:
4765:
4577:
4505:
4166:Hits from any of the above approaches can be
3864:The structure of a complex between penicillin
3703:, an extremely potent protein toxin found in
2746:, which is time–dependent. The true value of
398:Reversible inhibitors attach to enzymes with
10116:Quantitative structure–activity relationship
9333:Bioorganic & Medicinal Chemistry Letters
9326:
9271:
9029:"Overview of herbicide mechanisms of action"
8678:
8224:
8081:
7235:
6741:
6503:
6419:
6151:
5997:
5945:
5349:
5069:
5067:
5025:
4998:
4816:
4680:
4621:
4423:
4292:
4290:
4288:
4245:
4073:3-phosphoshikimate 1-carboxyvinyltransferase
3947:are effective in treating viral infections.
3072:, see the "DFP reaction" diagram), and also
2774:
2619:
2189:An enzyme inhibitor is characterised by its
718:(i.e., inhibitor binding hampers catalysis).
680:) as well as hampering catalysis (decreases
262:Small molecule inhibitors include essential
9450:International Journal of Molecular Sciences
9277:
9207:
9102:
8890:
8771:
8727:
8634:Cold Spring Harbor Perspectives in Medicine
8351:
7898:
7854:
7825:
7717:
7693:Encyclopedia of Food Sciences and Nutrition
7487:
7168:. In Cordes M, Wittmann C, Krull R (eds.).
6809:Cohen JA, Oosterbaan RA, Berends F (1967).
4992:
4385:
4339:
4059:irreversibly inhibit acetylcholinesterase.
3778:are frequently used to treat malignancies.
3433:. Other artificial enzyme inhibitors block
2510:{\displaystyle \alpha =1+{\frac {}{K_{i}}}}
603:
10149:
10135:
9995:
9981:
9560:
9546:
9320:
9242:
7961:Kannaiyan R, Mahadevan D (December 2018).
7500:Dementia and Geriatric Cognitive Disorders
7346:
7127:"Acetylcholinesterase Inhibitors Toxicity"
7092:. Boca Raton: CRC Press. pp. 73–114.
6868:
6376:
6247:Chemical reagents for protein modification
6067:
6003:
5450:
5334:
5294:
5256:
5187:
5092:
5032:Delaune KP, Alsayouri K (September 2021).
4900:
4898:
4896:
4762:
4589:"Chapter 7.2.1: Competition and Allostery"
3943:Drugs that inhibit enzymes needed for the
3766:which are essential enzymes that regulate
3580:(taxol), an organic molecule found in the
2960:
2858:or enzyme inhibitor ligands (for example,
2045:should be appropriate in most situations:
9471:
9461:
9344:
9303:
9157:
9126:
9060:
8896:
8844:
8653:
8604:
8459:
8426:
8394:
8291:
8148:
7986:
7933:
7802:
7792:
7751:
7741:
7511:
7240:(3rd ed.). Oxford University Press.
7192:
7083:
6937:
6927:
6811:"[81] Organophosphorus compounds"
6785:
6775:
6724:
6683:
6566:
6544:
6542:
6402:
6353:
6099:
5980:
5744:
5734:
5212:
5116:
5064:
4799:
4773:
4663:
4653:
4586:
4482:
4472:
4322:
4285:
3676:is also a peptide and is an inhibitor of
3457:Enzyme inhibition is a common feature of
2720:
2176:
1620:{\displaystyle {\cfrac {\ce {}}{+K_{i}}}}
1540:{\displaystyle {\cfrac {\ce {}}{+K_{m}}}}
9162:. Vol. 5. Elsevier. pp. 24–80.
8630:"Antivirals Targeting the Neuraminidase"
8579:Li G, Wang Y, De Clercq E (April 2022).
8572:
8492:
7663:
7007:
6963:"Natural products as enzyme inhibitors."
6244:
5910:
5459:
5394:
5358:"Mechanism of Action Assays for Enzymes"
4969:
4967:
4728:Kuriyan J, Konforti B, Wemmer D (2012).
4534:
4455:Sanrattana W, Maas C, de Maat S (2019).
4258:
4110:
3859:
3726:
3718:
3687:Proteins can also be natural poisons or
3546:
3535:inhibitor of the bacterial ribonuclease
3290:
3253:
2685:
2180:
352:
31:
9521:Database of drugs and enzyme inhibitors
8757:(1st ed.). Boca Raton: CRC Press.
8400:
8265:
8178:European Journal of Medicinal Chemistry
8052:
7864:Finkel R, Cubeddu LX, Clark MA (2009).
7311:
7086:"Chapter 5: Specific Enzyme Inhibitors"
6849:
6706:
6160:
6038:
5954:
5816:Medicinal Chemistry of Anticancer Drugs
5190:Enzyme Kinetics: Principles and Methods
5151:
4893:
4825:
4686:
4394:
4350:
4303:Journal of the Royal Society, Interface
4106:
3615:, from the plant species in the family
3452:
3384:, two molecules of an inhibitor called
3319:(DFP) is an example of an irreversible
2986:Reaction of the irreversible inhibitor
2887:, a therapeutically effective class of
2804:Peptide-based HIV-1 protease inhibitor
2708:
14:
10420:
9172:
8314:
8230:
7585:
6539:
6468:
6167:An Introduction to Medicinal Chemistry
6074:Breen ME, Soellner MB (January 2015).
5916:
5034:"Physiology: Noncompetitive Inhibitor"
4881:from the original on 26 September 2009
4693:An Introduction to Medicinal Chemistry
3842:cGMP specific phosphodiesterase type 5
3064:groups; these include the amino acids
10130:
9976:
9541:
9108:
8777:
8752:
8495:Current Topics in Medicinal Chemistry
7349:Natural Products as Enzyme Inhibitors
7260:
6874:
6311:Price N, Hames B, Rickwood D (1996).
6041:Current Protein & Peptide Science
5340:
5237:
5052:from the original on 28 November 2021
4973:
4964:
4950:(4th ed.). Weinheim: Wiley-VCH.
4861:Berg J, Tymoczko J, Stryer L (2002).
4776:"The Taxonomy of Covalent Inhibitors"
4296:
4119:New drugs are the products of a long
3975:, and terminase inhibitors targeting
3743:is a widely used drug that acts as a
3664:, which is found in relatives of the
3521:. Many of these are activated by the
2822:Nonpeptidic HIV-1 protease inhibitor
246:
9444:Elsässer B, Goettig P (March 2021).
9327:Satz AL, Kuai L, Peng X (May 2021).
9026:
8628:Gubareva L, Mohan T (January 2022).
7371:
7267:Bioorganic & Medicinal Chemistry
7145:from the original on 17 January 2023
7049:Dale's Pharmacology Condensed E-Book
6989:from the original on 5 December 2022
5343:Medicinal Chemistry and Drug Design.
5242:(New ed.). Wiley–Interscience.
4978:(New ed.). Wiley-Interscience.
4689:"Chapter 7: Enzymes as Drug Targets"
3927:differences in the structure of the
2949:and are less likely to be degraded.
2933:in the reaction of the viral enzyme
27:Molecule that blocks enzyme activity
9278:Ciulli A, Abell C (December 2007).
8899:Mini Reviews in Medicinal Chemistry
8172:Cochrane SA, Lohans CT (May 2020).
7967:Expert Review of Anticancer Therapy
7866:"Chapter 41: Antiinfammatory Drugs"
7045:"Venoms, toxins, poisons and herbs"
6916:The Journal of Biological Chemistry
6889:10.1146/annurev.bi.53.070184.002425
6764:The Journal of Biological Chemistry
6713:The Journal of Biological Chemistry
6555:The Journal of Biological Chemistry
98:most difficult step of the reaction
24:
9506:from the original on 1 April 2022.
8975:Hirai K, Uchida A, Ohno R (2012).
8461:10.1016/B978-0-12-814515-9.00126-0
8212:from the original on 28 March 2023
7886:from the original on 28 March 2023
7293:from the original on 28 March 2023
7106:from the original on 28 March 2023
7065:from the original on 25 April 2013
6685:10.1111/j.1432-1033.1971.tb01215.x
6183:from the original on 28 March 2023
5215:Enzyme Kinetics: A Modern Approach
5156:. Benjamin/Cummings. p. 437.
4609:from the original on 28 March 2023
4434:. Hoboken: John Wiley & Sons.
4373:from the original on 28 March 2023
3890:made of a net-like polymer called
3655:muscarinic acetylcholine receptors
3542:
3378:from the human protozoan parasite
2862:) with cellular cofactors such as
2567:
2530:
2414:
2363:
2324:
1661:
539:
25:
10454:
9492:
9415:10.2174/1381612824999201105164942
9033:Environmental Health Perspectives
9005:from the original on 27 June 2022
8911:10.2174/1389557517666170120153930
8878:from the original on 30 June 2022
8800:from the original on 21 July 2022
8538:Expert Opinion on Pharmacotherapy
8507:10.2174/1568026619666190619115243
8339:from the original on 9 April 2022
8137:European Journal of Endocrinology
6491:from the original on 20 July 2022
5376:from the original on 15 June 2022
4709:from the original on 20 July 2022
4353:"Principles of Metabolic Control"
3591:and inhibits their assembly into
3164:Irreversible inhibition mechanism
2864:nicotinamide adenine dinucleotide
9437:
9369:
9284:Current Opinion in Biotechnology
9236:
9201:
9166:
9151:
9077:
9020:
8981:Herbicide Classes in Development
8968:
8861:
8821:"Treatment of Myasthenia Gravis"
8746:
8721:
8443:
8401:Yao J, Rock CO (November 2017).
8308:
8124:
6672:European Journal of Biochemistry
4750:from the original on 7 June 2022
4457:"SERPINs-From Trap to Treatment"
4200:
4075:, other herbicides, such as the
3965:reverse-transcriptase inhibitors
3286:
3185:called the inactivation rate or
3170:
2998:
2979:
2908:
2815:
2797:
2783:
2721:regulatory feature in metabolism
2680:
2229:isothermal titration calorimetry
2038:term proposed above to modulate
502:
378:
366:
132:is an important way to maintain
9519:. National Library of Medicine.
8699:10.1016/j.antiviral.2018.11.005
8046:
7819:
7684:
7666:Veterinary and Human Toxicology
7657:
7622:
7579:
7536:
7425:
7390:
7365:
7340:
7305:
7254:
7229:
7186:
7118:
7077:
7036:
6954:
6903:
6843:
6802:
6700:
6659:
6624:
6583:
6462:
6370:
6329:
6304:
6263:
6238:
6116:
6032:
5832:
5702:
5659:
5632:
5597:
5562:
5521:
5496:
5415:
5388:
5231:
5145:
4948:Fundamentals of Enzyme Kinetics
4854:
4721:
4035:, are used in the treatment of
3915:R61 (the protein is shown as a
3633:acetylcholinesterase inhibitors
3395:
3249:
309:that contain an autoinhibitory
9378:Drug Discovery and Development
7914:Nature Reviews. Drug Discovery
7701:10.1016/B0-12-227055-X/00934-2
7314:Trends in Biochemical Sciences
6315:. BIOS Scientific Publishers.
6204:Journal of Medicinal Chemistry
5606:Journal of Medicinal Chemistry
5462:Journal of Theoretical Biology
5424:Trends in Biochemical Sciences
4499:
4161:DNA Encoded Chemical Libraries
3931:in bacteria, or how they make
3855:
3846:cyclic guanosine monophosphate
3563:that interfere with digestion.
3437:, an enzyme which breaks down
2553:
2547:
2491:
2485:
2441:
2435:
2419:
2398:
2393:
2387:
2368:
2347:
2335:
2329:
2298:
2292:
2142:
2134:
2121:
2115:
2103:
2071:
1981:
1973:
1960:
1954:
1942:
1910:
1841:
1833:
1820:
1814:
1802:
1770:
1710:
1702:
1689:
1683:
1596:
1588:
1575:
1569:
1516:
1508:
1495:
1489:
1424:
1416:
1390:
1384:
1328:
1320:
1295:
1287:
1245:
1237:
1212:
1204:
1149:
1141:
1135:
1127:
1107:
1099:
1074:
1066:
1060:
1052:
937:
929:
833:
827:
616:of the enzyme. In the classic
453:
427:covalent reversible inhibitors
360:Inhibition mechanism schematic
40:(E) accelerates conversion of
13:
1:
9403:Current Pharmaceutical Design
9175:Current Pharmaceutical Design
8874:(5th ed.). McGraw Hill.
8550:10.1080/14656566.2018.1454428
8055:Current Pharmaceutical Design
8024:10.1016/S0140-6736(21)00438-4
7979:10.1080/14737140.2018.1527688
7608:10.1016/S0041-0101(97)00074-3
7545:The Annals of Pharmacotherapy
7016:(4). New York, N.Y.: 209–28.
6877:Annual Review of Biochemistry
6823:10.1016/S0076-6879(67)11085-9
6726:10.1016/S0021-9258(19)47263-3
6568:10.1016/S0021-9258(18)48472-4
6355:10.1016/S1074-5521(00)90060-7
6284:10.1016/S0921-0423(98)80013-0
5841:Current Pharmaceutical Design
5653:10.1016/S0040-4020(01)81773-7
5482:10.1016/S0022-5193(05)80481-3
5403:(1): 164–6, 168, 170 passim.
4867:. W. H. Freeman and Company.
4774:Tuley A, Fast W (June 2018).
4555:10.1080/10409238.2020.1742090
4409:10.1016/s1359-6446(03)02713-2
4238:
4157:fragment-based lead discovery
4062:
3982:
3938:
3242:. This will produce a set of
3153:is the rate of inactivation.
2730:
301:in one of the tightest known
9296:10.1016/j.copbio.2007.09.003
9257:10.1016/j.drudis.2006.04.005
9128:10.1016/j.drudis.2021.01.006
9111:"Drug discovery for enzymes"
8989:10.1007/978-3-642-59416-8_10
8728:Kuhr RJ, Dorough HW (1976).
8419:10.1016/j.bbalip.2016.09.014
8190:10.1016/j.ejmech.2020.112262
7840:10.1016/j.bbapap.2004.06.004
7326:10.1016/0968-0004(89)90104-7
7236:Price NC, Stevens L (1999).
7043:Page C, Pitchford S (2021).
6707:Reardon JE (November 1989).
6645:10.1016/0167-4838(86)90067-1
6600:10.1016/0076-6879(95)49034-5
6216:10.1021/acs.jmedchem.8b01153
6137:10.1016/j.bbapap.2005.07.025
5955:Schramm VL (November 2018).
5818:. Elsevier. pp. 54–58.
5538:10.1016/0076-6879(95)49039-6
5436:10.1016/0968-0004(90)90295-M
5320:10.1016/j.bbagen.2007.01.001
4840:10.1016/0926-6569(63)90226-8
4655:10.1371/journal.pone.0214255
4126:The first general method is
3825:biosynthesis. This block of
3602:Many natural poisons act as
3495:phosphofructokinase‑1
3279:, and the activated form of
3156:
2847:N-10-formyl tetrahydrofolate
303:protein–protein interactions
7:
10156:
8646:10.1101/cshperspect.a038455
8585:Acta Pharmaceutica Sinica B
8266:Dalhoff A (February 2021).
7631:Current Medicinal Chemistry
7434:Current Cancer Drug Targets
6249:(3rd ed.). CRC Press.
6006:Current Medicinal Chemistry
5973:10.1021/acs.chemrev.8b00369
5919:Current Medicinal Chemistry
5688:10.1126/science.279.5347.98
5571:Annals of Internal Medicine
5009:10.1533/9780857099921.2.126
4792:10.1021/acs.biochem.8b00315
4193:
4188:structure-based drug design
4184:crystallographic structures
3784:Inhibitors of Janus kinases
3473:is a classic example. This
3232:peptide mass fingerprinting
2873:
10:
10459:
9738:Dihydropteroate synthetase
9597:Non-competitive inhibition
9346:10.1016/j.bmcl.2021.127851
9187:10.2174/138161206777585201
8597:10.1016/j.apsb.2021.11.009
8360:Medicinal Research Reviews
8315:Mobley H (13 March 2006).
8284:10.1007/s15010-020-01536-y
8102:10.1016/j.sxmr.2018.06.005
7481:10.2174/138527206776894410
7411:10.2174/138945006776054942
6428:Medicinal Research Reviews
6377:Maurer T, Fung HL (2000).
5931:10.2174/092986711797287566
5876:Medicinal Research Reviews
5853:10.2174/138161206776361110
4297:Sauro HM (February 2017).
4141:transition state analogues
3317:Diisopropylfluorophosphate
3200:. Fitting these data to a
2988:diisopropylfluorophosphate
546:non-competitive inhibition
496:
10313:
10305:Michaelis–Menten kinetics
10277:
10246:
10215:
10164:
10011:
9958:Steroidogenesis inhibitor
9950:
9922:
9798:
9705:
9622:
9615:
9579:
8946:10.1007/s00726-005-0254-1
8837:10.1016/j.ncl.2018.01.011
8753:Stone TW (October 2020).
7469:Current Organic Chemistry
7279:10.1016/j.bmc.2004.05.041
6272:Progress in Biotechnology
6163:"Enzymes as Drug Targets"
5782:10.3109/14756360902843809
5583:10.7326/0003-4819-86-1-65
4946:Cornish-Bowden A (2012).
4269:10.1002/9781118540398.ch1
4232:Transition state analogue
4216:Activity-based proteomics
4148:high-throughput screening
4101:oxidative phosphorylation
3764:receptor tyrosine kinases
3504:fructose 2,6-bisphosphate
3351:α-difluoromethylornithine
3118:/ values are used, where
2775:Multi-substrate analogues
2255:Michaelis–Menten equation
400:non-covalent interactions
10197:Diffusion-limited enzyme
9894:Matrix metalloproteinase
9697:Ribonucleotide reductase
9592:Uncompetitive inhibition
8732:. Cleveland: CRC Press.
8452:Encyclopedia of Virology
8067:10.2174/1381612033453712
7920:(1): 19–34 (Figure 1C).
7643:10.2174/0929867023368827
7446:10.2174/1568009033481967
7261:Smyth TP (August 2004).
6525:10.1016/j.ab.2012.08.006
6053:10.2174/1389203033487054
6018:10.2174/0929867003374886
5118:10.1177/2472555219829898
4019:, are absorbed from the
3969:neuraminidase inhibitors
3786:block the production of
3714:
3643:from deadly nightshade (
3012:Irreversible inhibitors
2965:
2909:"competitive inhibition"
2737:conformational isomerism
2639:Lineweaver–Burk diagrams
604:Quantitative description
570:
527:Le Chatelier's principle
509:uncompetitive inhibition
408:hydrophobic interactions
333:inhibitors are known as
10071:Lipinski's rule of five
9663:Dihydrofolate reductase
9109:Rufer AC (April 2021).
8090:Sexual Medicine Reviews
7351:. Singapore: Springer.
7207:10.1002/biof.5520100101
6513:Analytical Biochemistry
6342:Chemistry & Biology
5736:10.1073/pnas.0909141107
4474:10.3389/fmed.2019.00025
4130:based on mimicking the
3999:into its constituents,
3991:are enzyme inhibitors.
3832:A common treatment for
3819:dihydrofolate reductase
3573:small organic molecules
3376:trypanothione reductase
3363:Ornithine decarboxylase
3359:African trypanosomiasis
3357:, and is used to treat
3296:Trypanothione reductase
2961:Irreversible inhibitors
2881:dihydrofolate reductase
2769:irreversible inhibition
295:ribonuclease inhibitors
9759:Nucleotidyltransferase
9587:Competitive inhibition
7794:10.3390/toxins11060350
7743:10.3390/toxins11060324
7347:Maheshwari VL (2022).
6929:10.1074/jbc.M403187200
6777:10.1074/jbc.M403187200
5345:(16). InTech: 357–372.
4355:. In Storey KB (ed.).
4315:10.1098/rsif.2016.0848
4172:Computer-based methods
4116:
3945:replication of viruses
3883:
3788:inflammatory cytokines
3736:
3724:
3670:RNA polymerase II
3651:competitive antagonist
3649:) that functions as a
3564:
3329:acetylcholine esterase
3313:
3263:
2868:adenosine triphosphate
2581:
2511:
2451:
2186:
2177:Dissociation constants
2167:
2006:
1866:
1735:
1621:
1541:
1458:
623:dissociation constants
460:competitive inhibition
233:adverse drug reactions
221:dissociation constants
49:
10428:Biochemical reactions
10290:Eadie–Hofstee diagram
10223:Allosteric regulation
10076:Lipophilic efficiency
9769:Reverse transcriptase
9027:Duke SO (July 1990).
8782:. In Gupta RC (ed.).
8150:10.1530/eje.0.1430143
5362:Assay Guidance Manual
5188:Bisswanger H (2017).
4906:"Types of Inhibition"
4587:Rydzewski RM (2010).
4461:Frontiers in Medicine
4114:
4095:and the processes of
4081:acetolactate synthase
3977:human cytomegalovirus
3863:
3792:inflammatory diseases
3730:
3722:
3569:secondary metabolites
3550:
3361:(sleeping sickness).
3294:
3269:conformational change
3257:
2723:and can be a form of
2686:Partially competitive
2582:
2512:
2452:
2191:dissociation constant
2184:
2168:
2007:
1867:
1736:
1622:
1542:
1459:
1437:multiply out by
353:Reversible inhibitors
329:of the enzyme. These
272:secondary metabolites
35:
10300:Lineweaver–Burk plot
9815:Acetylcholinesterase
9722:Thymidylate synthase
9463:10.3390/ijms22063232
9245:Drug Discovery Today
9115:Drug Discovery Today
8233:ACS Chemical Biology
7926:10.1038/nrd.2016.230
7399:Current Drug Targets
7376:. Berlin: Springer.
7084:Stenersen J (2004).
6839:on 28 February 2018.
6245:Lundblad RL (2004).
6080:ACS Chemical Biology
5647:(14–15): 2351–2364.
5111:(5): 515–522 (516).
4397:Drug Discovery Today
4128:rational drug design
4107:Discovery and design
3993:Acetylcholinesterase
3967:targeting HIV/AIDS,
3834:erectile dysfunction
3678:protein phosphatases
3453:Metabolic regulation
3435:acetylcholinesterase
2889:antiretroviral drugs
2870:(ATP) respectively.
2709:Substrate or product
2698:, but an unaffected
2522:
2467:
2264:
2251:nonlinear regression
2052:
1891:
1751:
1645:
1557:
1477:
778:
425:. A special case is
10433:Medicinal chemistry
10091:New chemical entity
10081:Mechanism of action
10005:medicinal chemistry
9909:Histone deacetylase
9743:Farnesyltransferase
8321:Scientific American
7600:1998Txcn...36...13V
6922:(28): 29493–29500.
6850:Brenner GM (2000).
6719:(32): 19039–19044.
6278:. Elsevier: 77–82.
6161:Patrick GL (2017).
5967:(22): 11194–11258.
5727:2010PNAS..107.4878A
5680:1998Sci...279...98R
5618:10.1021/jm00125a002
5474:1990JThBi.145..457T
5268:Integrative Biology
5152:Laidler KJ (1978).
4912:on 8 September 2011
4687:Patrick GL (2013).
4646:2019PLoSO..1414255J
4520:10.1021/bi00222a030
4351:Plaxton WC (2004).
4180:molecular mechanics
4079:inhibit the enzyme
4071:is an inhibitor of
4047:pesticides such as
3953:protease inhibitors
3707:. This enzyme is a
3584:, binds tightly to
3100:will hydrolyse the
2895:. The structure of
2885:protease inhibitors
2839:purine biosynthesis
2571:
2131:
2111:
1970:
1950:
1830:
1810:
1699:
1679:
1585:
1565:
1505:
1485:
1400:
1380:
1304:
1271:
1221:
1201:
1083:
1036:
981:
962:
913:
894:
843:
823:
809:
790:
488:point, or half the
264:primary metabolites
217:genetically distant
193:protease inhibitors
189:rheumatic arthritis
179:. Examples include
84:are converted into
82:substrate molecules
10259:Enzyme superfamily
10192:Enzyme promiscuity
9942:Carbonic anhydrase
9934:Dopa decarboxylase
9602:Suicide inhibition
9251:(11–12): 561–568.
8825:Neurologic Clinics
8687:Antiviral Research
8018:(10302): 803–816.
6469:Purich DL (2010).
5280:10.1039/c1ib00053e
4117:
3911:from the bacteria
3884:
3737:
3725:
3693:trypsin inhibitors
3565:
3561:trypsin inhibitors
3471:glycolytic pathway
3461:control in cells.
3441:, and are used as
3386:quinacrine mustard
3343:Suicide inhibition
3321:protease inhibitor
3314:
3264:
3068:(that reacts with
3042:fluorophosphonates
2907:, as shown in the
2577:
2557:
2507:
2447:
2187:
2163:
2159:
2126:
2002:
1998:
1965:
1862:
1858:
1825:
1731:
1727:
1694:
1617:
1613:
1580:
1537:
1533:
1500:
1454:
1452:
1428:
1395:
1332:
1299:
1249:
1216:
1160: to numerator
1111:
1078:
995:
976:
941:
908:
862:
857:
838:
804:
594:tertiary structure
255:to macromolecular
247:Structural classes
74:chemical reactions
50:
10443:Enzyme inhibitors
10415:
10414:
10124:
10123:
10066:Ligand efficiency
9970:
9969:
9966:
9965:
9838:Alpha-glucosidase
9828:Polygalacturonase
9810:Phosphodiesterase
9751:GABA transaminase
9655:Monoamine oxidase
9639:HMG-CoA reductase
9573:enzyme inhibition
9409:(44): 5684–5699.
9387:978-1-315-11347-0
9216:(15): 2644–2676.
9210:Angewandte Chemie
9181:(17): 2087–2097.
9095:978-1-00-043721-8
8998:978-3-642-59416-8
8905:(17): 1665–1676.
8793:978-0-08-054310-9
8778:Gupta RC (2006).
8764:978-1-00-009898-3
8739:978-0-87819-052-2
8501:(18): 1571–1598.
8471:978-0-12-814516-6
8413:(11): 1300–1309.
8372:10.1002/med.21780
8245:10.1021/cb700182u
8061:(31): 2593–2613.
7973:(12): 1249–1270.
7879:978-0-7817-7155-9
7710:978-0-12-227055-0
7637:(22): 1981–1989.
7557:10.1345/aph.1D536
7513:10.1159/000486546
7383:978-3-540-00118-8
7358:978-981-19-0932-0
7273:(15): 4081–4088.
7247:978-0-19-850229-6
7179:978-3-642-13865-2
7099:978-0-203-64683-0
7058:978-0-7020-7819-4
7022:10.1002/tcr.20045
6979:978-3-527-34205-1
6861:978-0-7216-7757-6
6832:978-0-12-181860-9
6770:(28): 29493–500.
6609:978-0-12-182150-0
6484:978-0-12-380925-4
6322:978-0-12-564710-6
6293:978-0-444-82970-2
6256:978-0-8493-1983-9
6210:(12): 5673–5724.
6176:978-0-19-874969-1
6092:10.1021/cb5008376
5888:10.1002/med.21475
5847:(11): 1301–1314.
5825:978-0-444-62667-7
5721:(11): 4878–4883.
5514:978-0-470-20745-1
5274:(12): 1197–1201.
5249:978-0-471-30309-1
5238:Segel IH (1993).
5224:978-0-471-15985-8
5199:978-3-527-80647-8
5163:978-0-8053-5680-9
5085:978-1-118-91840-1
5018:978-1-904275-27-5
4985:978-0-471-30309-1
4974:Segel IH (1993).
4957:978-3-527-66549-5
4874:978-0-7167-4955-4
4786:(24): 3326–3337.
4743:978-1-135-08892-7
4702:978-0-19-969739-7
4602:978-0-08-091488-6
4441:978-3-527-65970-8
4366:978-0-471-67557-0
4278:978-1-118-48813-3
4176:molecular docking
4152:virtual screening
4037:myasthenia gravis
3850:corpus cavernosum
3772:kinase inhibitors
3745:suicide inhibitor
3731:The structure of
3646:Atropa belladonna
3528:trypsin inhibitor
3499:negative feedback
3477:pathway consumes
3459:metabolic pathway
3381:Trypanosoma cruzi
3224:mass spectrometry
3198:exponential decay
3098:hydrochloric acid
3090:protein structure
3038:phenyl sulfonates
3034:Michael acceptors
3018:nitrogen mustards
2725:negative feedback
2635:Hanes-Woolf plots
2572:
2505:
2445:
2339:
2161:
2140:
2130:
2120:
2110:
2000:
1979:
1969:
1959:
1949:
1860:
1839:
1829:
1819:
1809:
1729:
1708:
1698:
1688:
1678:
1615:
1594:
1584:
1574:
1564:
1535:
1514:
1504:
1494:
1484:
1438:
1430:
1422:
1399:
1389:
1379:
1334:
1326:
1303:
1293:
1270:
1264:
1251:
1243:
1220:
1210:
1200:
1161:
1147:
1133:
1125:
1113:
1105:
1082:
1072:
1058:
1035:
997:
980:
961:
955:
954:multiply by
943:
935:
912:
893:
864:
859:
842:
832:
822:
808:
789:
638:', respectively.
614:kinetic constants
130:negative feedback
126:metabolic pathway
60:that binds to an
18:Enzyme inhibition
16:(Redirected from
10450:
10295:Hanes–Woolf plot
10238:Enzyme activator
10233:Enzyme inhibitor
10207:Enzyme catalysis
10151:
10144:
10137:
10128:
10127:
10101:Pharmacokinetics
10096:Pharmacodynamics
10061:Enzyme inhibitor
10046:Drug development
9997:
9990:
9983:
9974:
9973:
9692:Xanthine oxidase
9634:Aldose reductase
9620:
9619:
9607:Mixed inhibition
9562:
9555:
9548:
9539:
9538:
9533:
9532:on 20 June 2006.
9528:. Archived from
9520:
9507:
9486:
9485:
9475:
9465:
9441:
9435:
9434:
9398:
9392:
9391:
9373:
9367:
9366:
9348:
9324:
9318:
9317:
9307:
9275:
9269:
9268:
9240:
9234:
9233:
9205:
9199:
9198:
9170:
9164:
9163:
9155:
9149:
9148:
9130:
9106:
9100:
9099:
9081:
9075:
9074:
9064:
9024:
9018:
9017:
9012:
9010:
8972:
8966:
8965:
8929:
8923:
8922:
8894:
8888:
8887:
8885:
8883:
8865:
8859:
8858:
8848:
8816:
8810:
8809:
8807:
8805:
8775:
8769:
8768:
8750:
8744:
8743:
8725:
8719:
8718:
8682:
8676:
8675:
8657:
8625:
8619:
8618:
8608:
8591:(4): 1567–1590.
8576:
8570:
8569:
8533:
8527:
8526:
8490:
8484:
8483:
8463:
8447:
8441:
8440:
8430:
8398:
8392:
8391:
8366:(4): 1855–1889.
8355:
8349:
8348:
8346:
8344:
8312:
8306:
8305:
8295:
8263:
8257:
8256:
8228:
8222:
8221:
8219:
8217:
8169:
8163:
8162:
8152:
8128:
8122:
8121:
8085:
8079:
8078:
8050:
8044:
8043:
8007:
8001:
8000:
7990:
7958:
7952:
7951:
7937:
7905:
7896:
7895:
7893:
7891:
7861:
7852:
7851:
7823:
7817:
7816:
7806:
7796:
7772:
7766:
7765:
7755:
7745:
7721:
7715:
7714:
7688:
7682:
7681:
7661:
7655:
7654:
7626:
7620:
7619:
7583:
7577:
7576:
7540:
7534:
7533:
7515:
7506:(3–4): 131–151.
7491:
7485:
7484:
7464:
7458:
7457:
7429:
7423:
7422:
7394:
7388:
7387:
7369:
7363:
7362:
7344:
7338:
7337:
7309:
7303:
7302:
7300:
7298:
7258:
7252:
7251:
7233:
7227:
7226:
7190:
7184:
7183:
7161:
7155:
7154:
7152:
7150:
7122:
7116:
7115:
7113:
7111:
7081:
7075:
7074:
7072:
7070:
7040:
7034:
7033:
7005:
6999:
6998:
6996:
6994:
6988:
6967:
6958:
6952:
6951:
6941:
6931:
6907:
6901:
6900:
6872:
6866:
6865:
6847:
6841:
6840:
6835:. Archived from
6815:Enzyme Structure
6806:
6800:
6799:
6789:
6779:
6755:
6745:
6739:
6738:
6728:
6704:
6698:
6697:
6687:
6663:
6657:
6656:
6628:
6622:
6621:
6587:
6581:
6580:
6570:
6546:
6537:
6536:
6507:
6501:
6500:
6498:
6496:
6466:
6460:
6459:
6423:
6417:
6416:
6406:
6395:10.1208/ps020108
6374:
6368:
6367:
6357:
6333:
6327:
6326:
6308:
6302:
6301:
6267:
6261:
6260:
6242:
6236:
6235:
6199:
6193:
6192:
6190:
6188:
6158:
6149:
6148:
6120:
6114:
6113:
6103:
6071:
6065:
6064:
6036:
6030:
6029:
6001:
5995:
5994:
5984:
5961:Chemical Reviews
5952:
5943:
5942:
5914:
5908:
5907:
5882:(4): 1295–1331.
5871:
5865:
5864:
5836:
5830:
5829:
5811:
5802:
5801:
5765:
5759:
5758:
5748:
5738:
5706:
5700:
5699:
5674:(5347): 98–102.
5663:
5657:
5656:
5636:
5630:
5629:
5601:
5595:
5594:
5566:
5560:
5559:
5525:
5519:
5518:
5500:
5494:
5493:
5457:
5448:
5447:
5419:
5413:
5412:
5392:
5386:
5385:
5383:
5381:
5353:
5347:
5346:
5338:
5332:
5331:
5303:
5292:
5291:
5263:
5254:
5253:
5235:
5229:
5228:
5210:
5204:
5203:
5185:
5168:
5167:
5149:
5143:
5142:
5120:
5096:
5090:
5089:
5071:
5062:
5061:
5059:
5057:
5029:
5023:
5022:
4996:
4990:
4989:
4971:
4962:
4961:
4943:
4922:
4921:
4919:
4917:
4902:
4891:
4890:
4888:
4886:
4858:
4852:
4851:
4823:
4814:
4813:
4803:
4771:
4760:
4759:
4757:
4755:
4725:
4719:
4718:
4716:
4714:
4684:
4678:
4677:
4667:
4657:
4625:
4619:
4618:
4616:
4614:
4584:
4575:
4574:
4538:
4532:
4531:
4514:(8): 2246–2255.
4503:
4497:
4496:
4486:
4476:
4452:
4446:
4445:
4427:
4421:
4420:
4392:
4383:
4382:
4380:
4378:
4348:
4337:
4336:
4326:
4294:
4283:
4282:
4256:
4210:
4205:
4204:
4137:functional group
4132:transition state
4121:drug development
3961:Hepatitis C
3881:
3872:transpeptidase (
3867:
3705:castor oil beans
3582:Pacific yew tree
3447:chemical warfare
3390:nitrogen mustard
3338:
3311:
3174:
3002:
2983:
2916:transition state
2819:
2801:
2787:
2586:
2584:
2583:
2578:
2573:
2570:
2565:
2556:
2545:
2534:
2533:
2516:
2514:
2513:
2508:
2506:
2504:
2503:
2494:
2483:
2456:
2454:
2453:
2448:
2446:
2444:
2431:
2430:
2418:
2417:
2408:
2396:
2386:
2385:
2367:
2366:
2357:
2345:
2340:
2338:
2328:
2327:
2315:
2314:
2301:
2291:
2290:
2274:
2172:
2170:
2169:
2164:
2162:
2160:
2158:
2157:
2156:
2141:
2138:
2127:
2125:
2124:
2118:
2107:
2102:
2101:
2086:
2085:
2067:
2066:
2011:
2009:
2008:
2003:
2001:
1999:
1997:
1996:
1995:
1980:
1977:
1966:
1964:
1963:
1957:
1946:
1941:
1940:
1925:
1924:
1906:
1905:
1871:
1869:
1868:
1863:
1861:
1859:
1857:
1856:
1855:
1840:
1837:
1826:
1824:
1823:
1817:
1806:
1801:
1800:
1785:
1784:
1766:
1765:
1740:
1738:
1737:
1732:
1730:
1728:
1726:
1725:
1724:
1709:
1706:
1695:
1693:
1692:
1686:
1675:
1673:
1672:
1657:
1656:
1626:
1624:
1623:
1618:
1616:
1614:
1612:
1611:
1610:
1595:
1592:
1581:
1579:
1578:
1572:
1561:
1546:
1544:
1543:
1538:
1536:
1534:
1532:
1531:
1530:
1515:
1512:
1501:
1499:
1498:
1492:
1481:
1463:
1461:
1460:
1455:
1453:
1449:
1448:
1439:
1436:
1433:
1431:
1429:
1427:
1423:
1420:
1412:
1411:
1396:
1394:
1393:
1387:
1376:
1374:
1373:
1361:
1360:
1345:
1335:
1333:
1331:
1327:
1324:
1316:
1315:
1300:
1298:
1294:
1291:
1283:
1282:
1267:
1265:
1262:
1259:
1257:
1253:
1252:
1250:
1248:
1244:
1241:
1233:
1232:
1217:
1215:
1211:
1208:
1197:
1184:
1183:
1182:
1166:
1162:
1159:
1148:
1145:
1134:
1131:
1126:
1123:
1120:
1118:
1114:
1112:
1110:
1106:
1103:
1095:
1094:
1079:
1077:
1073:
1070:
1059:
1056:
1048:
1047:
1032:
1026:
1025:
1024:
1008:
998:
996:
994:
993:
992:
977:
975:
974:
973:
958:
956:
953:
950:
948:
944:
942:
940:
936:
933:
925:
924:
909:
907:
906:
905:
890:
884:
883:
882:
865:
863:
861:
860:
858:
856:
855:
854:
839:
837:
836:
830:
819:
805:
803:
802:
801:
786:
618:Michaelis-Menten
577:mixed inhibition
382:
370:
315:intramolecularly
297:, which bind to
187:and in treating
54:enzyme inhibitor
21:
10458:
10457:
10453:
10452:
10451:
10449:
10448:
10447:
10418:
10417:
10416:
10411:
10323:Oxidoreductases
10309:
10285:Enzyme kinetics
10273:
10269:List of enzymes
10242:
10211:
10182:Catalytic triad
10160:
10155:
10125:
10120:
10021:Bioavailability
10007:
10001:
9971:
9962:
9946:
9918:
9794:
9781:Tyrosine kinase
9701:
9611:
9575:
9566:
9524:
9511:
9498:
9495:
9490:
9489:
9442:
9438:
9399:
9395:
9388:
9374:
9370:
9325:
9321:
9276:
9272:
9241:
9237:
9206:
9202:
9171:
9167:
9156:
9152:
9107:
9103:
9096:
9082:
9078:
9045:10.2307/3431034
9025:
9021:
9008:
9006:
8999:
8973:
8969:
8930:
8926:
8895:
8891:
8881:
8879:
8866:
8862:
8817:
8813:
8803:
8801:
8794:
8776:
8772:
8765:
8751:
8747:
8740:
8726:
8722:
8683:
8679:
8626:
8622:
8577:
8573:
8534:
8530:
8491:
8487:
8472:
8448:
8444:
8399:
8395:
8356:
8352:
8342:
8340:
8313:
8309:
8264:
8260:
8229:
8225:
8215:
8213:
8170:
8166:
8129:
8125:
8086:
8082:
8051:
8047:
8008:
8004:
7959:
7955:
7906:
7899:
7889:
7887:
7880:
7862:
7855:
7824:
7820:
7773:
7769:
7722:
7718:
7711:
7689:
7685:
7662:
7658:
7627:
7623:
7584:
7580:
7541:
7537:
7492:
7488:
7465:
7461:
7430:
7426:
7395:
7391:
7384:
7372:Birk Y (2003).
7370:
7366:
7359:
7345:
7341:
7320:(11): 450–454.
7310:
7306:
7296:
7294:
7259:
7255:
7248:
7234:
7230:
7191:
7187:
7180:
7162:
7158:
7148:
7146:
7123:
7119:
7109:
7107:
7100:
7082:
7078:
7068:
7066:
7059:
7041:
7037:
7010:Chemical Record
7006:
7002:
6992:
6990:
6986:
6980:
6965:
6959:
6955:
6908:
6904:
6873:
6869:
6862:
6848:
6844:
6833:
6807:
6803:
6747:
6746:
6742:
6705:
6701:
6664:
6660:
6629:
6625:
6610:
6588:
6584:
6547:
6540:
6508:
6504:
6494:
6492:
6485:
6467:
6463:
6424:
6420:
6375:
6371:
6334:
6330:
6323:
6313:Proteins LabFax
6309:
6305:
6294:
6268:
6264:
6257:
6243:
6239:
6200:
6196:
6186:
6184:
6177:
6159:
6152:
6121:
6117:
6072:
6068:
6037:
6033:
6002:
5998:
5953:
5946:
5925:(29): 4513–37.
5915:
5911:
5872:
5868:
5837:
5833:
5826:
5812:
5805:
5776:(6): 1291–318.
5766:
5762:
5707:
5703:
5664:
5660:
5637:
5633:
5602:
5598:
5567:
5563:
5548:
5526:
5522:
5515:
5501:
5497:
5458:
5451:
5430:(12): 455–458.
5420:
5416:
5393:
5389:
5379:
5377:
5354:
5350:
5339:
5335:
5304:
5295:
5264:
5257:
5250:
5236:
5232:
5225:
5211:
5207:
5200:
5186:
5171:
5164:
5150:
5146:
5097:
5093:
5086:
5072:
5065:
5055:
5053:
5030:
5026:
5019:
4997:
4993:
4986:
4972:
4965:
4958:
4944:
4925:
4915:
4913:
4904:
4903:
4894:
4884:
4882:
4875:
4859:
4855:
4824:
4817:
4772:
4763:
4753:
4751:
4744:
4726:
4722:
4712:
4710:
4703:
4685:
4681:
4640:(3): e0214255.
4626:
4622:
4612:
4610:
4603:
4585:
4578:
4539:
4535:
4504:
4500:
4453:
4449:
4442:
4428:
4424:
4393:
4386:
4376:
4374:
4367:
4349:
4340:
4295:
4286:
4279:
4257:
4246:
4241:
4206:
4199:
4196:
4109:
4065:
4045:organophosphate
3985:
3949:Antiviral drugs
3941:
3873:
3865:
3858:
3804:Crohn's disease
3717:
3606:that can cause
3545:
3543:Natural poisons
3455:
3398:
3336:
3303:
3289:
3252:
3218:
3211:
3191:
3182:
3181:
3180:
3179:
3178:
3175:
3166:
3165:
3159:
3152:
3145:
3138:
3131:
3124:
3117:
3110:
3010:
3009:
3008:
3007:
3006:
3003:
2995:
2994:
2992:serine protease
2984:
2975:
2974:
2968:
2963:
2924:
2876:
2835:formyl transfer
2830:
2829:
2828:
2827:
2826:
2820:
2811:
2810:
2809:
2802:
2793:
2792:
2791:
2788:
2777:
2766:
2759:
2752:
2745:
2733:
2711:
2704:
2697:
2688:
2683:
2676:
2669:
2662:
2651:
2627:Lineweaver–Burk
2617:
2610:
2603:
2596:
2566:
2561:
2546:
2544:
2529:
2525:
2523:
2520:
2519:
2499:
2495:
2484:
2482:
2468:
2465:
2464:
2426:
2422:
2413:
2409:
2404:
2397:
2375:
2371:
2362:
2358:
2353:
2346:
2344:
2323:
2319:
2310:
2306:
2302:
2280:
2276:
2275:
2273:
2265:
2262:
2261:
2247:enzyme activity
2244:
2237:
2226:
2219:
2212:
2205:
2198:
2179:
2152:
2148:
2137:
2133:
2128:
2114:
2113:
2108:
2106:
2094:
2090:
2078:
2074:
2059:
2055:
2053:
2050:
2049:
2044:
2037:
2029:
2022:
1991:
1987:
1976:
1972:
1967:
1953:
1952:
1947:
1945:
1933:
1929:
1917:
1913:
1898:
1894:
1892:
1889:
1888:
1882:
1851:
1847:
1836:
1832:
1827:
1813:
1812:
1807:
1805:
1793:
1789:
1777:
1773:
1758:
1754:
1752:
1749:
1748:
1720:
1716:
1705:
1701:
1696:
1682:
1681:
1676:
1674:
1668:
1664:
1652:
1648:
1646:
1643:
1642:
1637:
1606:
1602:
1591:
1587:
1582:
1568:
1567:
1562:
1560:
1558:
1555:
1554:
1526:
1522:
1511:
1507:
1502:
1488:
1487:
1482:
1480:
1478:
1475:
1474:
1451:
1450:
1444:
1440:
1435:
1432:
1419:
1407:
1403:
1402:
1397:
1383:
1382:
1377:
1375:
1369:
1365:
1356:
1352:
1343:
1342:
1323:
1311:
1307:
1306:
1301:
1290:
1278:
1274:
1273:
1268:
1266:
1261:
1258:
1240:
1228:
1224:
1223:
1218:
1207:
1203:
1198:
1196:
1189:
1185:
1178:
1174:
1173:
1164:
1163:
1158:
1144:
1130:
1122:
1119:
1102:
1090:
1086:
1085:
1080:
1069:
1055:
1043:
1039:
1038:
1033:
1031:
1027:
1020:
1016:
1015:
1006:
1005:
988:
984:
983:
978:
969:
965:
964:
959:
957:
952:
949:
932:
920:
916:
915:
910:
901:
897:
896:
891:
889:
885:
878:
874:
873:
866:
850:
846:
845:
840:
826:
825:
820:
818:
811:
806:
797:
793:
792:
787:
785:
781:
779:
776:
775:
770:
763:
748:
741:
734:
727:
717:
710:
703:
696:
686:
679:
672:
665:
655:
648:
637:
630:
606:
586:allosteric site
573:
565:
558:
542:
540:Non-competitive
534:
523:
516:
505:
497:"Drugs" section
493:
486:
479:
472:
456:
448:
441:
396:
395:
394:
393:
388:
387:
386:
383:
375:
374:
371:
362:
361:
355:
253:small molecules
249:
64:and blocks its
28:
23:
22:
15:
12:
11:
5:
10456:
10446:
10445:
10440:
10435:
10430:
10413:
10412:
10410:
10409:
10396:
10383:
10370:
10357:
10344:
10331:
10317:
10315:
10311:
10310:
10308:
10307:
10302:
10297:
10292:
10287:
10281:
10279:
10275:
10274:
10272:
10271:
10266:
10261:
10256:
10250:
10248:
10247:Classification
10244:
10243:
10241:
10240:
10235:
10230:
10225:
10219:
10217:
10213:
10212:
10210:
10209:
10204:
10199:
10194:
10189:
10184:
10179:
10174:
10168:
10166:
10162:
10161:
10154:
10153:
10146:
10139:
10131:
10122:
10121:
10119:
10118:
10113:
10108:
10103:
10098:
10093:
10088:
10086:Mode of action
10083:
10078:
10073:
10068:
10063:
10058:
10056:Drug targeting
10053:
10051:Drug discovery
10048:
10043:
10038:
10033:
10028:
10023:
10018:
10012:
10009:
10008:
10000:
9999:
9992:
9985:
9977:
9968:
9967:
9964:
9963:
9961:
9960:
9954:
9952:
9948:
9947:
9945:
9944:
9937:
9936:
9929:
9927:
9920:
9919:
9917:
9916:
9914:Beta-lactamase
9911:
9904:
9903:
9902:
9901:
9896:
9891:
9881:
9880:
9879:
9874:
9864:
9863:
9862:
9857:
9841:
9840:
9835:
9830:
9823:
9822:
9817:
9812:
9805:
9803:
9796:
9795:
9793:
9792:
9791:
9790:
9789:
9788:
9776:Protein kinase
9773:
9772:
9771:
9766:
9754:
9753:
9746:
9745:
9740:
9733:
9732:
9725:
9724:
9719:
9712:
9710:
9703:
9702:
9700:
9699:
9694:
9687:
9686:
9681:
9674:
9673:
9666:
9665:
9658:
9657:
9650:
9649:
9642:
9641:
9636:
9629:
9627:
9624:Oxidoreductase
9617:
9613:
9612:
9610:
9609:
9604:
9599:
9594:
9589:
9583:
9581:
9577:
9576:
9565:
9564:
9557:
9550:
9542:
9536:
9535:
9522:
9509:
9494:
9493:External links
9491:
9488:
9487:
9436:
9393:
9386:
9368:
9319:
9270:
9235:
9200:
9165:
9150:
9121:(4): 875–886.
9101:
9094:
9076:
9019:
8997:
8967:
8940:(2): 195–204.
8924:
8889:
8860:
8831:(2): 311–337.
8811:
8792:
8770:
8763:
8745:
8738:
8720:
8677:
8640:(1): a038455.
8620:
8571:
8544:(6): 577–587.
8528:
8485:
8470:
8442:
8393:
8350:
8307:
8258:
8223:
8164:
8143:(2): 143–154.
8123:
8096:(1): 115–128.
8080:
8045:
8002:
7953:
7897:
7878:
7853:
7818:
7767:
7716:
7709:
7683:
7672:(5): 294–297.
7656:
7621:
7578:
7551:(1): 173–176.
7535:
7486:
7475:(8): 825–847.
7459:
7440:(3): 193–203.
7424:
7405:(3): 265–277.
7389:
7382:
7364:
7357:
7339:
7304:
7253:
7246:
7228:
7185:
7178:
7156:
7117:
7098:
7076:
7057:
7035:
7000:
6978:
6953:
6902:
6867:
6860:
6842:
6831:
6801:
6740:
6699:
6658:
6639:(3): 275–285.
6623:
6608:
6582:
6561:(1): 150–158.
6538:
6502:
6483:
6461:
6434:(4): 307–319.
6418:
6369:
6328:
6321:
6303:
6292:
6262:
6255:
6237:
6194:
6175:
6150:
6131:(1–2): 79–99.
6115:
6066:
6047:(5): 339–356.
6031:
6012:(6): 663–672.
5996:
5944:
5909:
5866:
5831:
5824:
5803:
5760:
5701:
5658:
5631:
5612:(5): 937–940.
5596:
5561:
5546:
5520:
5513:
5495:
5468:(4): 457–464.
5449:
5414:
5387:
5348:
5333:
5314:(5): 733–746.
5293:
5255:
5248:
5230:
5223:
5205:
5198:
5169:
5162:
5144:
5091:
5084:
5063:
5024:
5017:
4991:
4984:
4963:
4956:
4923:
4892:
4873:
4853:
4815:
4761:
4742:
4720:
4701:
4679:
4620:
4601:
4576:
4549:(2): 111–165.
4533:
4498:
4447:
4440:
4422:
4403:(12): 536–44.
4384:
4365:
4338:
4284:
4277:
4243:
4242:
4240:
4237:
4236:
4235:
4229:
4226:Antimetabolite
4223:
4218:– a branch of
4212:
4211:
4208:Biology portal
4195:
4192:
4108:
4105:
4097:photosynthesis
4067:The herbicide
4064:
4061:
4021:synaptic cleft
4017:norepinephrine
3984:
3981:
3955:used to treat
3940:
3937:
3917:ribbon diagram
3909:transpeptidase
3857:
3854:
3753:prostaglandins
3749:cyclooxygenase
3716:
3713:
3691:, such as the
3662:alpha-amanitin
3613:glycoalkaloids
3553:seed predation
3551:To discourage
3544:
3541:
3463:Metabolic flux
3454:
3451:
3397:
3394:
3349:biosynthesis,
3288:
3285:
3251:
3248:
3216:
3209:
3189:
3176:
3169:
3168:
3167:
3163:
3162:
3161:
3160:
3158:
3155:
3150:
3143:
3136:
3129:
3122:
3115:
3108:
3004:
2997:
2996:
2985:
2978:
2977:
2976:
2972:
2971:
2970:
2969:
2967:
2964:
2962:
2959:
2954:protein kinase
2922:
2901:peptidomimetic
2891:used to treat
2875:
2872:
2821:
2814:
2813:
2812:
2803:
2796:
2795:
2794:
2789:
2782:
2781:
2780:
2779:
2778:
2776:
2773:
2764:
2757:
2750:
2743:
2732:
2729:
2710:
2707:
2702:
2695:
2687:
2684:
2682:
2679:
2674:
2667:
2660:
2649:
2615:
2608:
2601:
2594:
2588:
2587:
2576:
2569:
2564:
2560:
2555:
2552:
2549:
2543:
2540:
2537:
2532:
2528:
2517:
2502:
2498:
2493:
2490:
2487:
2481:
2478:
2475:
2472:
2458:
2457:
2443:
2440:
2437:
2434:
2429:
2425:
2421:
2416:
2412:
2407:
2403:
2400:
2395:
2392:
2389:
2384:
2381:
2378:
2374:
2370:
2365:
2361:
2356:
2352:
2349:
2343:
2337:
2334:
2331:
2326:
2322:
2318:
2313:
2309:
2305:
2300:
2297:
2294:
2289:
2286:
2283:
2279:
2272:
2269:
2253:to a modified
2242:
2235:
2224:
2217:
2210:
2203:
2196:
2178:
2175:
2174:
2173:
2155:
2151:
2147:
2144:
2136:
2123:
2117:
2105:
2100:
2097:
2093:
2089:
2084:
2081:
2077:
2073:
2070:
2065:
2062:
2058:
2042:
2035:
2027:
2020:
2013:
2012:
1994:
1990:
1986:
1983:
1975:
1962:
1956:
1944:
1939:
1936:
1932:
1928:
1923:
1920:
1916:
1912:
1909:
1904:
1901:
1897:
1880:
1873:
1872:
1854:
1850:
1846:
1843:
1835:
1822:
1816:
1804:
1799:
1796:
1792:
1788:
1783:
1780:
1776:
1772:
1769:
1764:
1761:
1757:
1742:
1741:
1723:
1719:
1715:
1712:
1704:
1691:
1685:
1671:
1667:
1663:
1660:
1655:
1651:
1635:
1628:
1627:
1609:
1605:
1601:
1598:
1590:
1577:
1571:
1548:
1547:
1529:
1525:
1521:
1518:
1510:
1497:
1491:
1465:
1464:
1447:
1443:
1434:
1426:
1418:
1415:
1410:
1406:
1392:
1386:
1372:
1368:
1364:
1359:
1355:
1351:
1348:
1346:
1344:
1341:
1338:
1330:
1322:
1319:
1314:
1310:
1297:
1289:
1286:
1281:
1277:
1263:simplify
1260:
1256:
1247:
1239:
1236:
1231:
1227:
1214:
1206:
1195:
1192:
1188:
1181:
1177:
1172:
1169:
1167:
1165:
1157:
1154:
1151:
1143:
1140:
1137:
1129:
1121:
1117:
1109:
1101:
1098:
1093:
1089:
1076:
1068:
1065:
1062:
1054:
1051:
1046:
1042:
1030:
1023:
1019:
1014:
1011:
1009:
1007:
1004:
1001:
991:
987:
972:
968:
951:
947:
939:
931:
928:
923:
919:
904:
900:
888:
881:
877:
872:
869:
867:
853:
849:
835:
829:
817:
814:
800:
796:
784:
783:
768:
761:
751:
750:
746:
739:
732:
725:
719:
715:
708:
701:
694:
688:
684:
677:
670:
663:
657:
653:
646:
635:
628:
605:
602:
592:(that is, the
572:
569:
563:
556:
541:
538:
532:
521:
514:
504:
501:
491:
484:
477:
470:
455:
452:
446:
439:
404:hydrogen bonds
390:
389:
384:
377:
376:
372:
365:
364:
363:
359:
358:
357:
356:
354:
351:
248:
245:
195:used to treat
161:drug molecules
118:non-covalently
76:necessary for
72:that speed up
68:. Enzymes are
26:
9:
6:
4:
3:
2:
10455:
10444:
10441:
10439:
10436:
10434:
10431:
10429:
10426:
10425:
10423:
10407:
10403:
10402:
10397:
10394:
10390:
10389:
10384:
10381:
10377:
10376:
10371:
10368:
10364:
10363:
10358:
10355:
10351:
10350:
10345:
10342:
10338:
10337:
10332:
10329:
10325:
10324:
10319:
10318:
10316:
10312:
10306:
10303:
10301:
10298:
10296:
10293:
10291:
10288:
10286:
10283:
10282:
10280:
10276:
10270:
10267:
10265:
10264:Enzyme family
10262:
10260:
10257:
10255:
10252:
10251:
10249:
10245:
10239:
10236:
10234:
10231:
10229:
10228:Cooperativity
10226:
10224:
10221:
10220:
10218:
10214:
10208:
10205:
10203:
10200:
10198:
10195:
10193:
10190:
10188:
10187:Oxyanion hole
10185:
10183:
10180:
10178:
10175:
10173:
10170:
10169:
10167:
10163:
10159:
10152:
10147:
10145:
10140:
10138:
10133:
10132:
10129:
10117:
10114:
10112:
10111:Pharmacophore
10109:
10107:
10104:
10102:
10099:
10097:
10094:
10092:
10089:
10087:
10084:
10082:
10079:
10077:
10074:
10072:
10069:
10067:
10064:
10062:
10059:
10057:
10054:
10052:
10049:
10047:
10044:
10042:
10039:
10037:
10036:Drug delivery
10034:
10032:
10029:
10027:
10026:Chemogenomics
10024:
10022:
10019:
10017:
10014:
10013:
10010:
10006:
9998:
9993:
9991:
9986:
9984:
9979:
9978:
9975:
9959:
9956:
9955:
9953:
9951:Miscellaneous
9949:
9943:
9939:
9938:
9935:
9931:
9930:
9928:
9925:
9921:
9915:
9912:
9910:
9906:
9905:
9900:
9897:
9895:
9892:
9890:
9889:Enkephalinase
9887:
9886:
9885:
9882:
9878:
9875:
9873:
9870:
9869:
9868:
9867:Endopeptidase
9865:
9861:
9858:
9856:
9853:
9852:
9851:
9847:
9843:
9842:
9839:
9836:
9834:
9833:Neuraminidase
9831:
9829:
9825:
9824:
9821:
9818:
9816:
9813:
9811:
9807:
9806:
9804:
9801:
9797:
9787:
9784:
9783:
9782:
9779:
9778:
9777:
9774:
9770:
9767:
9765:
9762:
9761:
9760:
9756:
9755:
9752:
9748:
9747:
9744:
9741:
9739:
9735:
9734:
9731:
9727:
9726:
9723:
9720:
9718:
9714:
9713:
9711:
9708:
9704:
9698:
9695:
9693:
9689:
9688:
9685:
9682:
9680:
9676:
9675:
9672:
9668:
9667:
9664:
9660:
9659:
9656:
9652:
9651:
9648:
9644:
9643:
9640:
9637:
9635:
9631:
9630:
9628:
9625:
9621:
9618:
9614:
9608:
9605:
9603:
9600:
9598:
9595:
9593:
9590:
9588:
9585:
9584:
9582:
9578:
9574:
9570:
9563:
9558:
9556:
9551:
9549:
9544:
9543:
9540:
9531:
9527:
9523:
9518:
9514:
9510:
9505:
9501:
9497:
9496:
9483:
9479:
9474:
9469:
9464:
9459:
9455:
9451:
9447:
9440:
9432:
9428:
9424:
9420:
9416:
9412:
9408:
9404:
9397:
9389:
9383:
9379:
9372:
9364:
9360:
9356:
9352:
9347:
9342:
9338:
9334:
9330:
9323:
9315:
9311:
9306:
9301:
9297:
9293:
9290:(6): 489–96.
9289:
9285:
9281:
9274:
9266:
9262:
9258:
9254:
9250:
9246:
9239:
9231:
9227:
9223:
9219:
9215:
9211:
9204:
9196:
9192:
9188:
9184:
9180:
9176:
9169:
9161:
9154:
9146:
9142:
9138:
9134:
9129:
9124:
9120:
9116:
9112:
9105:
9097:
9091:
9087:
9080:
9072:
9068:
9063:
9058:
9054:
9050:
9046:
9042:
9038:
9034:
9030:
9023:
9016:
9004:
9000:
8994:
8990:
8986:
8982:
8978:
8971:
8963:
8959:
8955:
8951:
8947:
8943:
8939:
8935:
8928:
8920:
8916:
8912:
8908:
8904:
8900:
8893:
8877:
8873:
8872:
8864:
8856:
8852:
8847:
8842:
8838:
8834:
8830:
8826:
8822:
8815:
8799:
8795:
8789:
8785:
8781:
8774:
8766:
8760:
8756:
8749:
8741:
8735:
8731:
8724:
8716:
8712:
8708:
8704:
8700:
8696:
8692:
8688:
8681:
8673:
8669:
8665:
8661:
8656:
8651:
8647:
8643:
8639:
8635:
8631:
8624:
8616:
8612:
8607:
8602:
8598:
8594:
8590:
8586:
8582:
8575:
8567:
8563:
8559:
8555:
8551:
8547:
8543:
8539:
8532:
8524:
8520:
8516:
8512:
8508:
8504:
8500:
8496:
8489:
8481:
8477:
8473:
8467:
8462:
8457:
8453:
8446:
8438:
8434:
8429:
8424:
8420:
8416:
8412:
8408:
8404:
8397:
8389:
8385:
8381:
8377:
8373:
8369:
8365:
8361:
8354:
8338:
8334:
8330:
8326:
8322:
8318:
8311:
8303:
8299:
8294:
8289:
8285:
8281:
8277:
8273:
8269:
8262:
8254:
8250:
8246:
8242:
8238:
8234:
8227:
8211:
8207:
8203:
8199:
8195:
8191:
8187:
8183:
8179:
8175:
8168:
8160:
8156:
8151:
8146:
8142:
8138:
8134:
8127:
8119:
8115:
8111:
8107:
8103:
8099:
8095:
8091:
8084:
8076:
8072:
8068:
8064:
8060:
8056:
8049:
8041:
8037:
8033:
8029:
8025:
8021:
8017:
8013:
8006:
7998:
7994:
7989:
7984:
7980:
7976:
7972:
7968:
7964:
7957:
7950:
7945:
7941:
7936:
7931:
7927:
7923:
7919:
7915:
7911:
7904:
7902:
7885:
7881:
7875:
7871:
7867:
7860:
7858:
7849:
7845:
7841:
7837:
7834:(1–2): 1–14.
7833:
7829:
7822:
7814:
7810:
7805:
7800:
7795:
7790:
7786:
7782:
7778:
7771:
7763:
7759:
7754:
7749:
7744:
7739:
7735:
7731:
7727:
7720:
7712:
7706:
7702:
7698:
7694:
7687:
7679:
7675:
7671:
7667:
7660:
7652:
7648:
7644:
7640:
7636:
7632:
7625:
7617:
7613:
7609:
7605:
7601:
7597:
7593:
7589:
7582:
7574:
7570:
7566:
7562:
7558:
7554:
7550:
7546:
7539:
7531:
7527:
7523:
7519:
7514:
7509:
7505:
7501:
7497:
7490:
7482:
7478:
7474:
7470:
7463:
7455:
7451:
7447:
7443:
7439:
7435:
7428:
7420:
7416:
7412:
7408:
7404:
7400:
7393:
7385:
7379:
7375:
7368:
7360:
7354:
7350:
7343:
7335:
7331:
7327:
7323:
7319:
7315:
7308:
7292:
7288:
7284:
7280:
7276:
7272:
7268:
7264:
7257:
7249:
7243:
7239:
7232:
7224:
7220:
7216:
7212:
7208:
7204:
7200:
7196:
7189:
7181:
7175:
7171:
7167:
7160:
7144:
7140:
7136:
7132:
7128:
7121:
7105:
7101:
7095:
7091:
7087:
7080:
7064:
7060:
7054:
7050:
7046:
7039:
7031:
7027:
7023:
7019:
7015:
7011:
7004:
6985:
6981:
6975:
6971:
6964:
6957:
6949:
6945:
6940:
6935:
6930:
6925:
6921:
6917:
6913:
6906:
6898:
6894:
6890:
6886:
6882:
6878:
6871:
6863:
6857:
6853:
6846:
6838:
6834:
6828:
6824:
6820:
6816:
6812:
6805:
6797:
6793:
6788:
6783:
6778:
6773:
6769:
6765:
6761:
6754:
6750:
6744:
6736:
6732:
6727:
6722:
6718:
6714:
6710:
6703:
6695:
6691:
6686:
6681:
6677:
6673:
6669:
6662:
6654:
6650:
6646:
6642:
6638:
6634:
6627:
6619:
6615:
6611:
6605:
6601:
6597:
6593:
6586:
6578:
6574:
6569:
6564:
6560:
6556:
6552:
6545:
6543:
6534:
6530:
6526:
6522:
6518:
6514:
6506:
6490:
6486:
6480:
6476:
6472:
6465:
6457:
6453:
6449:
6445:
6441:
6437:
6433:
6429:
6422:
6414:
6410:
6405:
6400:
6396:
6392:
6388:
6384:
6383:AAPS PharmSci
6380:
6373:
6365:
6361:
6356:
6351:
6347:
6343:
6339:
6332:
6324:
6318:
6314:
6307:
6300:
6295:
6289:
6285:
6281:
6277:
6273:
6266:
6258:
6252:
6248:
6241:
6233:
6229:
6225:
6221:
6217:
6213:
6209:
6205:
6198:
6182:
6178:
6172:
6168:
6164:
6157:
6155:
6146:
6142:
6138:
6134:
6130:
6126:
6119:
6111:
6107:
6102:
6097:
6093:
6089:
6086:(1): 175–89.
6085:
6081:
6077:
6070:
6062:
6058:
6054:
6050:
6046:
6042:
6035:
6027:
6023:
6019:
6015:
6011:
6007:
6000:
5992:
5988:
5983:
5978:
5974:
5970:
5966:
5962:
5958:
5951:
5949:
5940:
5936:
5932:
5928:
5924:
5920:
5913:
5905:
5901:
5897:
5893:
5889:
5885:
5881:
5877:
5870:
5862:
5858:
5854:
5850:
5846:
5842:
5835:
5827:
5821:
5817:
5810:
5808:
5799:
5795:
5791:
5787:
5783:
5779:
5775:
5771:
5764:
5756:
5752:
5747:
5742:
5737:
5732:
5728:
5724:
5720:
5716:
5712:
5705:
5697:
5693:
5689:
5685:
5681:
5677:
5673:
5669:
5662:
5654:
5650:
5646:
5642:
5635:
5627:
5623:
5619:
5615:
5611:
5607:
5600:
5592:
5588:
5584:
5580:
5576:
5572:
5565:
5557:
5553:
5549:
5547:9780121821500
5543:
5539:
5535:
5531:
5524:
5516:
5510:
5506:
5499:
5491:
5487:
5483:
5479:
5475:
5471:
5467:
5463:
5456:
5454:
5445:
5441:
5437:
5433:
5429:
5425:
5418:
5410:
5406:
5402:
5398:
5397:BioTechniques
5391:
5375:
5371:
5367:
5363:
5359:
5352:
5344:
5337:
5329:
5325:
5321:
5317:
5313:
5309:
5302:
5300:
5298:
5289:
5285:
5281:
5277:
5273:
5269:
5262:
5260:
5251:
5245:
5241:
5234:
5226:
5220:
5216:
5209:
5201:
5195:
5191:
5184:
5182:
5180:
5178:
5176:
5174:
5165:
5159:
5155:
5148:
5141:
5136:
5132:
5128:
5124:
5119:
5114:
5110:
5106:
5102:
5095:
5087:
5081:
5077:
5070:
5068:
5051:
5047:
5043:
5039:
5035:
5028:
5020:
5014:
5010:
5006:
5002:
4995:
4987:
4981:
4977:
4970:
4968:
4959:
4953:
4949:
4942:
4940:
4938:
4936:
4934:
4932:
4930:
4928:
4911:
4907:
4901:
4899:
4897:
4880:
4876:
4870:
4866:
4865:
4857:
4849:
4845:
4841:
4837:
4833:
4829:
4822:
4820:
4811:
4807:
4802:
4797:
4793:
4789:
4785:
4781:
4777:
4770:
4768:
4766:
4749:
4745:
4739:
4735:
4731:
4724:
4708:
4704:
4698:
4694:
4690:
4683:
4675:
4671:
4666:
4661:
4656:
4651:
4647:
4643:
4639:
4635:
4631:
4624:
4608:
4604:
4598:
4594:
4590:
4583:
4581:
4572:
4568:
4564:
4560:
4556:
4552:
4548:
4544:
4537:
4529:
4525:
4521:
4517:
4513:
4509:
4502:
4494:
4490:
4485:
4480:
4475:
4470:
4466:
4462:
4458:
4451:
4443:
4437:
4433:
4426:
4418:
4414:
4410:
4406:
4402:
4398:
4391:
4389:
4372:
4368:
4362:
4358:
4354:
4347:
4345:
4343:
4334:
4330:
4325:
4320:
4316:
4312:
4309:(127): 1–13.
4308:
4304:
4300:
4293:
4291:
4289:
4280:
4274:
4270:
4266:
4262:
4255:
4253:
4251:
4249:
4244:
4233:
4230:
4227:
4224:
4221:
4217:
4214:
4213:
4209:
4203:
4198:
4191:
4189:
4185:
4181:
4177:
4173:
4169:
4164:
4162:
4158:
4153:
4149:
4144:
4142:
4138:
4133:
4129:
4124:
4122:
4113:
4104:
4102:
4098:
4094:
4090:
4086:
4082:
4078:
4077:sulfonylureas
4074:
4070:
4060:
4058:
4054:
4050:
4046:
4042:
4038:
4034:
4030:
4029:physostigmine
4026:
4022:
4018:
4014:
4010:
4006:
4002:
3998:
3997:acetylcholine
3994:
3990:
3980:
3978:
3974:
3970:
3966:
3962:
3958:
3954:
3950:
3946:
3936:
3934:
3930:
3925:
3920:
3918:
3914:
3910:
3905:
3901:
3897:
3893:
3892:peptidoglycan
3889:
3880:
3876:
3871:
3862:
3853:
3851:
3847:
3843:
3839:
3835:
3830:
3828:
3824:
3820:
3816:
3812:
3807:
3805:
3801:
3797:
3794:in including
3793:
3789:
3785:
3781:
3780:Janus kinases
3777:
3773:
3769:
3765:
3761:
3756:
3754:
3750:
3746:
3742:
3734:
3729:
3721:
3712:
3710:
3706:
3702:
3698:
3694:
3690:
3689:antinutrients
3685:
3683:
3679:
3675:
3671:
3667:
3663:
3658:
3656:
3652:
3648:
3647:
3642:
3638:
3637:Neurotoxicity
3634:
3630:
3626:
3622:
3618:
3614:
3609:
3605:
3600:
3598:
3594:
3590:
3587:
3583:
3579:
3574:
3570:
3562:
3558:
3554:
3549:
3540:
3538:
3534:
3529:
3524:
3520:
3516:
3511:
3509:
3505:
3500:
3496:
3492:
3488:
3484:
3481:and produces
3480:
3476:
3472:
3468:
3464:
3460:
3450:
3448:
3444:
3440:
3439:acetylcholine
3436:
3432:
3428:
3427:disinfectants
3424:
3420:
3416:
3412:
3407:
3403:
3393:
3391:
3387:
3383:
3382:
3377:
3371:
3369:
3364:
3360:
3356:
3352:
3348:
3344:
3340:
3334:
3330:
3326:
3322:
3318:
3310:
3306:
3301:
3297:
3293:
3287:Some examples
3284:
3282:
3278:
3274:
3270:
3261:
3256:
3247:
3245:
3241:
3237:
3233:
3229:
3225:
3220:
3215:
3208:
3203:
3202:rate equation
3199:
3193:
3188:
3173:
3154:
3149:
3142:
3135:
3128:
3121:
3114:
3105:
3103:
3102:peptide bonds
3099:
3095:
3091:
3085:
3083:
3079:
3075:
3071:
3067:
3063:
3059:
3055:
3051:
3047:
3046:electrophilic
3043:
3039:
3035:
3031:
3027:
3023:
3019:
3015:
3001:
2993:
2990:(DFP) with a
2989:
2982:
2958:
2955:
2950:
2948:
2943:
2938:
2936:
2935:neuraminidase
2932:
2928:
2921:
2917:
2912:
2910:
2906:
2905:peptide bonds
2902:
2898:
2894:
2890:
2886:
2882:
2871:
2869:
2865:
2861:
2857:
2853:
2848:
2844:
2840:
2837:reactions of
2836:
2825:
2818:
2807:
2800:
2786:
2772:
2770:
2763:
2756:
2749:
2742:
2738:
2728:
2726:
2722:
2717:
2706:
2701:
2694:
2681:Special cases
2678:
2673:
2666:
2659:
2655:
2648:
2644:
2640:
2636:
2632:
2631:Eadie-Hofstee
2628:
2623:
2621:
2614:
2607:
2604:become (α/α')
2600:
2593:
2574:
2562:
2558:
2550:
2541:
2538:
2535:
2526:
2518:
2500:
2496:
2488:
2479:
2476:
2473:
2470:
2463:
2462:
2461:
2438:
2432:
2427:
2423:
2410:
2405:
2401:
2390:
2382:
2379:
2376:
2372:
2359:
2354:
2350:
2341:
2332:
2320:
2316:
2311:
2307:
2303:
2295:
2287:
2284:
2281:
2277:
2270:
2267:
2260:
2259:
2258:
2256:
2252:
2248:
2241:
2234:
2230:
2223:
2216:
2209:
2202:
2195:
2192:
2183:
2153:
2149:
2145:
2098:
2095:
2091:
2087:
2082:
2079:
2075:
2068:
2063:
2060:
2056:
2048:
2047:
2046:
2041:
2034:
2026:
2019:
1992:
1988:
1984:
1937:
1930:
1926:
1921:
1914:
1907:
1902:
1895:
1887:
1886:
1885:
1879:
1852:
1848:
1844:
1797:
1790:
1786:
1781:
1774:
1767:
1762:
1755:
1747:
1746:
1745:
1721:
1717:
1713:
1665:
1658:
1649:
1641:
1640:
1639:
1634:
1607:
1603:
1599:
1553:
1552:
1551:
1527:
1523:
1519:
1473:
1472:
1471:
1468:
1441:
1413:
1408:
1404:
1366:
1362:
1353:
1349:
1347:
1339:
1336:
1317:
1312:
1308:
1284:
1279:
1275:
1254:
1234:
1229:
1225:
1193:
1190:
1186:
1175:
1170:
1168:
1155:
1152:
1138:
1115:
1096:
1091:
1087:
1063:
1049:
1044:
1040:
1028:
1017:
1012:
1010:
1002:
999:
989:
985:
970:
966:
945:
926:
921:
917:
902:
898:
886:
875:
870:
868:
851:
847:
815:
812:
794:
774:
773:
772:
767:
760:
755:
745:
738:
731:
724:
720:
714:
707:
700:
693:
689:
683:
676:
669:
662:
658:
652:
645:
641:
640:
639:
634:
627:
624:
619:
615:
611:
601:
597:
595:
591:
587:
583:
578:
568:
566:
559:
551:
547:
537:
535:
528:
524:
517:
510:
503:Uncompetitive
500:
498:
494:
487:
480:
473:
466:
461:
451:
449:
442:
435:
430:
428:
424:
419:
417:
413:
409:
405:
401:
381:
369:
350:
348:
344:
340:
336:
332:
328:
324:
319:
316:
312:
308:
304:
300:
299:ribonucleases
296:
292:
288:
284:
280:
275:
273:
269:
265:
260:
258:
254:
244:
242:
238:
234:
230:
229:kidney damage
226:
222:
218:
214:
210:
206:
202:
198:
194:
190:
186:
182:
178:
174:
170:
166:
162:
157:
155:
151:
147:
143:
139:
135:
131:
127:
122:
119:
115:
114:chemical bond
111:
107:
101:
99:
95:
91:
87:
83:
79:
75:
71:
67:
63:
59:
55:
47:
43:
39:
34:
30:
19:
10401:Translocases
10398:
10385:
10372:
10359:
10346:
10336:Transferases
10333:
10320:
10232:
10177:Binding site
10106:Pharmacology
10060:
9883:
9850:Exopeptidase
9820:Ribonuclease
9786:Janus kinase
9671:Lipoxygenase
9647:5α-Reductase
9572:
9569:Pharmacology
9530:the original
9516:
9453:
9449:
9439:
9406:
9402:
9396:
9377:
9371:
9336:
9332:
9322:
9287:
9283:
9273:
9248:
9244:
9238:
9213:
9209:
9203:
9178:
9174:
9168:
9159:
9153:
9118:
9114:
9104:
9085:
9079:
9036:
9032:
9022:
9014:
9007:. Retrieved
8980:
8970:
8937:
8933:
8927:
8902:
8898:
8892:
8880:. Retrieved
8870:
8863:
8828:
8824:
8814:
8802:. Retrieved
8783:
8773:
8754:
8748:
8729:
8723:
8690:
8686:
8680:
8637:
8633:
8623:
8588:
8584:
8574:
8541:
8537:
8531:
8498:
8494:
8488:
8451:
8445:
8410:
8406:
8396:
8363:
8359:
8353:
8341:. Retrieved
8324:
8320:
8310:
8278:(1): 29–56.
8275:
8271:
8261:
8239:(9): 602–5.
8236:
8232:
8226:
8214:. Retrieved
8181:
8177:
8167:
8140:
8136:
8126:
8093:
8089:
8083:
8058:
8054:
8048:
8015:
8011:
8005:
7970:
7966:
7956:
7947:
7917:
7913:
7888:. Retrieved
7870:Pharmacology
7869:
7831:
7827:
7821:
7784:
7780:
7770:
7733:
7729:
7719:
7692:
7686:
7669:
7665:
7659:
7634:
7630:
7624:
7594:(1): 13–24.
7591:
7587:
7581:
7548:
7544:
7538:
7503:
7499:
7489:
7472:
7468:
7462:
7437:
7433:
7427:
7402:
7398:
7392:
7373:
7367:
7348:
7342:
7317:
7313:
7307:
7295:. Retrieved
7270:
7266:
7256:
7237:
7231:
7198:
7194:
7188:
7169:
7159:
7147:. Retrieved
7130:
7120:
7108:. Retrieved
7089:
7079:
7067:. Retrieved
7048:
7038:
7013:
7009:
7003:
6991:. Retrieved
6969:
6956:
6919:
6915:
6905:
6880:
6876:
6870:
6852:Pharmacology
6851:
6845:
6837:the original
6814:
6804:
6767:
6763:
6743:
6716:
6712:
6702:
6678:(1): 65–72.
6675:
6671:
6661:
6636:
6632:
6626:
6591:
6585:
6558:
6554:
6519:(1): 83–91.
6516:
6512:
6505:
6493:. Retrieved
6474:
6464:
6431:
6427:
6421:
6389:(1): 68–77.
6386:
6382:
6372:
6348:(1): 81–95.
6345:
6341:
6331:
6312:
6306:
6297:
6275:
6271:
6265:
6246:
6240:
6207:
6203:
6197:
6185:. Retrieved
6166:
6128:
6124:
6118:
6083:
6079:
6069:
6044:
6040:
6034:
6009:
6005:
5999:
5964:
5960:
5922:
5918:
5912:
5879:
5875:
5869:
5844:
5840:
5834:
5815:
5773:
5769:
5763:
5718:
5714:
5704:
5671:
5667:
5661:
5644:
5640:
5634:
5609:
5605:
5599:
5577:(1): 65–66.
5574:
5570:
5564:
5529:
5523:
5504:
5498:
5465:
5461:
5427:
5423:
5417:
5400:
5396:
5390:
5378:. Retrieved
5361:
5351:
5342:
5336:
5311:
5307:
5271:
5267:
5239:
5233:
5214:
5208:
5189:
5153:
5147:
5138:
5108:
5104:
5094:
5075:
5054:. Retrieved
5037:
5027:
5000:
4994:
4975:
4947:
4914:. Retrieved
4910:the original
4883:. Retrieved
4864:Biochemistry
4863:
4856:
4831:
4827:
4783:
4780:Biochemistry
4779:
4752:. Retrieved
4733:
4723:
4711:. Retrieved
4692:
4682:
4637:
4633:
4623:
4611:. Retrieved
4592:
4546:
4542:
4536:
4511:
4508:Biochemistry
4507:
4501:
4464:
4460:
4450:
4431:
4425:
4400:
4396:
4375:. Retrieved
4356:
4306:
4302:
4260:
4165:
4145:
4125:
4118:
4066:
4057:chlorpyrifos
3986:
3942:
3921:
3913:Streptomyces
3912:
3885:
3870:Streptomyces
3869:
3831:
3811:methotrexate
3808:
3757:
3738:
3686:
3682:algal blooms
3659:
3644:
3631:), that are
3601:
3597:cytoskeleton
3593:microtubules
3566:
3512:
3456:
3443:nerve agents
3411:insecticides
3399:
3396:Applications
3385:
3379:
3372:
3341:
3315:
3299:
3273:methotrexate
3265:
3259:
3250:Slow binding
3221:
3213:
3206:
3194:
3186:
3183:
3147:
3140:
3133:
3126:
3119:
3112:
3106:
3094:denaturation
3086:
3054:nucleophiles
3011:
2973:DFP reaction
2951:
2939:
2919:
2913:
2877:
2831:
2761:
2754:
2747:
2740:
2734:
2712:
2699:
2692:
2689:
2671:
2664:
2657:
2653:
2646:
2642:
2638:
2624:
2612:
2605:
2598:
2591:
2589:
2459:
2239:
2232:
2221:
2214:
2207:
2200:
2193:
2188:
2039:
2032:
2024:
2017:
2014:
1877:
1874:
1743:
1632:
1629:
1549:
1469:
1466:
765:
758:
756:
752:
743:
736:
729:
722:
712:
705:
698:
691:
681:
674:
667:
660:
650:
643:
632:
625:
607:
598:
590:conformation
588:changes the
574:
561:
554:
543:
530:
519:
512:
506:
489:
482:
475:
468:
464:
457:
444:
437:
431:
426:
420:
397:
343:conformation
323:binding site
320:
290:
286:
282:
276:
261:
250:
241:pharmacology
237:biochemistry
185:chemotherapy
181:methotrexate
158:
123:
102:
88:. An enzyme
53:
51:
29:
10172:Active site
10041:Drug design
9899:Oxytocinase
9707:Transferase
9456:(6): 3232.
9039:: 263–271.
8934:Amino Acids
8693:: 116–124.
7201:(1): 1–14.
7131:StatPearls
6883:: 493–535.
5641:Tetrahedron
4834:: 173–187.
4093:carotenoids
4085:amino acids
4033:neostigmine
4025:edrophonium
3933:fatty acids
3924:drug design
3922:Antibiotic
3896:antibiotics
3856:Antibiotics
3768:cell growth
3709:glycosidase
3674:microcystin
3604:neurotoxins
3467:metabolites
3325:active site
3277:allopurinol
3026:haloalkanes
2931:oxonium ion
2927:oseltamivir
2866:(NADH) and
2760:) and off (
454:Competitive
416:active site
412:ionic bonds
347:kinetically
335:orthosteric
331:active site
268:homeostasis
94:active site
90:facilitates
80:, in which
10438:Metabolism
10422:Categories
10375:Isomerases
10349:Hydrolases
10216:Regulation
10031:Drug class
10003:Topics in
9339:: 127851.
8480:1240584737
8184:: 112262.
7787:(6): 350.
7736:(6): 324.
7195:BioFactors
5140:substrate.
5038:StatPearls
4239:References
4220:proteomics
4069:glyphosate
4063:Herbicides
3989:pesticides
3983:Pesticides
3971:targeting
3939:Antivirals
3904:vancomycin
3900:penicillin
3868:G and the
3838:sildenafil
3827:nucleotide
3815:folic acid
3733:sildenafil
3619:(includes
3617:Solanaceae
3578:paclitaxel
3423:glyphosate
3419:herbicides
3062:sulfhydryl
3014:covalently
2947:peptidases
2942:tipranavir
2824:tipranavir
2731:Slow-tight
2611:and (1/α')
582:allosteric
392:catalysis.
339:allosteric
311:N-terminal
231:and other
191:) and the
167:such as a
110:reversibly
42:substrates
10254:EC number
9800:Hydrolase
9764:Integrase
9679:Aromatase
9616:Substrate
9513:"PubChem"
9431:226271153
9363:232059044
9145:231633284
8672:212651676
8523:195356119
8388:231761270
8327:(6): 98.
8272:Infection
8206:214809706
8040:237311419
6756:;
4885:31 August
4571:215772580
4168:optimised
4053:parathion
4049:malathion
4041:carbamate
4009:serotonin
3973:influenza
3929:ribosomes
3888:cell wall
3823:thymidine
3796:arthritis
3666:death cap
3608:paralysis
3576:example,
3475:catabolic
3431:triclosan
3415:malathion
3402:metabolic
3355:ornithine
3347:polyamine
3302:, 2004. (
3281:acyclovir
3228:MALDI-TOF
3157:Measuring
3078:threonine
3022:aldehydes
2897:ritonavir
2856:isoniazid
2806:ritonavir
2568:′
2531:′
2527:α
2471:α
2415:′
2411:α
2402:α
2364:′
2360:α
2325:′
2321:α
2304:α
2206:'. Hence
2088:−
2069:−
1927:−
1908:−
1787:−
1768:−
1662:Δ
1659:−
1363:−
1194:−
1139:−
1124:add
1064:−
327:substrate
209:pathogens
205:analogous
183:(used in
173:bacterium
154:predators
146:nucleases
142:proteases
106:catalysis
10278:Kinetics
10202:Cofactor
10165:Activity
9846:Protease
9504:Archived
9500:"BRENDA"
9482:33810118
9423:33155894
9355:33631371
9314:17959370
9265:16713909
9230:12203463
9195:16796557
9137:33454380
9003:Archived
8954:16547651
8919:28117022
8876:Archived
8855:29655452
8798:Archived
8715:53763831
8707:30472161
8664:32152244
8615:35847492
8558:29595065
8515:31237209
8437:27668701
8380:33501747
8337:Archived
8333:16711368
8302:33367978
8253:17894443
8210:Archived
8198:32248005
8159:10913932
8118:52945888
8110:30301707
8075:14529544
8032:34454676
7997:30259761
7944:27910877
7890:14 April
7884:Archived
7848:15450171
7813:31216687
7762:31174319
7678:11577938
7651:12369866
7573:36465515
7565:15572604
7530:13701908
7522:29734182
7454:12769688
7419:16515527
7297:14 April
7291:Archived
7287:15246086
7223:24586866
7215:10475585
7149:14 April
7143:Archived
7139:30571049
7110:14 April
7104:Archived
7063:Archived
7030:16041744
6984:Archived
6948:15102853
6796:15102853
6533:22902804
6489:Archived
6456:11766917
6448:10398927
6413:11741224
6364:11182321
6232:56480231
6224:30565923
6181:Archived
6145:16182621
6110:25494294
6061:14529528
6026:10702632
5991:30335982
5939:21864279
5904:25269012
5896:29149530
5861:16611117
5798:21808708
5790:19912064
5755:20194791
5409:11464510
5374:Archived
5370:22553872
5328:17307293
5288:22038120
5135:73480979
5127:30811960
5050:Archived
5046:31424826
4879:Archived
4848:14021668
4810:29689165
4748:Archived
4707:Archived
4674:30917186
4634:PLOS ONE
4607:Archived
4563:32290726
4493:30809526
4417:12821301
4377:14 April
4371:Archived
4333:28202588
4194:See also
4013:dopamine
3957:HIV/AIDS
3951:include
3898:such as
3882:)
3776:imatinib
3774:such as
3735:(Viagra)
3641:atropine
3629:eggplant
3559:contain
3519:zymogens
3515:pancreas
3491:pyruvate
3429:such as
3421:such as
3413:such as
3333:synapses
3312:)
3244:peptides
3238:such as
3236:protease
3146:) where
3082:tyrosine
3074:cysteine
3058:hydroxyl
3056:such as
3044:. These
2893:HIV/AIDS
2874:Examples
2852:entropic
2716:kinetics
550:activity
423:dialysis
402:such as
307:zymogens
289:rotease
257:proteins
201:specific
197:HIV/AIDS
177:parasite
165:pathogen
86:products
70:proteins
66:activity
58:molecule
46:products
10388:Ligases
10158:Enzymes
9872:Trypsin
9473:8004986
9305:4441723
9071:1980104
9062:1567841
9053:3431034
9009:27 June
8962:2358278
8882:9 April
8846:6690491
8804:21 July
8655:8725622
8606:9279714
8566:4489039
8428:5364071
8343:9 April
8293:7851017
8216:20 July
7988:6322661
7935:6314433
7804:6628406
7753:6628454
7616:9604278
7596:Bibcode
7588:Toxicon
7334:2696173
6993:3 April
6939:3491871
6897:6433782
6787:3491871
6735:2553730
6694:4322209
6653:3511964
6618:7791610
6577:1730582
6495:20 July
6404:2751003
6299:enzyme.
6101:4301090
5982:6615489
5746:2841876
5723:Bibcode
5696:9417034
5676:Bibcode
5668:Science
5626:2709379
5556:7791615
5505:Enzymes
5490:2246896
5470:Bibcode
5444:2077683
5380:9 April
5056:3 April
4916:2 April
4801:6016374
4713:20 July
4665:6436737
4642:Bibcode
4613:20 July
4528:1998683
4484:6379291
4324:5332569
4163:(DEL).
4005:choline
4001:acetate
3894:. Many
3747:of the
3741:aspirin
3697:legumes
3653:of the
3595:in the
3586:tubulin
3557:legumes
3537:barnase
3533:barstar
3523:trypsin
3479:glucose
3406:poisons
3392:group.
3331:in the
3262:, 1992.
3240:trypsin
3050:adducts
3030:alkenes
2705:value.
610:binding
465:compete
434:Cleland
279:serpins
150:poisons
134:balance
44:(S) to
10362:Lyases
9926:(EC 4)
9802:(EC 3)
9709:(EC 2)
9669:1.13
9626:(EC 1)
9480:
9470:
9429:
9421:
9384:
9361:
9353:
9312:
9302:
9263:
9228:
9193:
9143:
9135:
9092:
9069:
9059:
9051:
8995:
8960:
8952:
8917:
8853:
8843:
8790:
8761:
8736:
8713:
8705:
8670:
8662:
8652:
8613:
8603:
8564:
8556:
8521:
8513:
8478:
8468:
8435:
8425:
8386:
8378:
8331:
8300:
8290:
8251:
8204:
8196:
8157:
8116:
8108:
8073:
8038:
8030:
8012:Lancet
7995:
7985:
7942:
7932:
7876:
7846:
7811:
7801:
7781:Toxins
7760:
7750:
7730:Toxins
7707:
7676:
7649:
7614:
7571:
7563:
7528:
7520:
7452:
7417:
7380:
7355:
7332:
7285:
7244:
7221:
7213:
7176:
7137:
7096:
7069:3 June
7055:
7028:
6976:
6946:
6936:
6895:
6858:
6829:
6794:
6784:
6733:
6692:
6651:
6616:
6606:
6575:
6531:
6481:
6454:
6446:
6411:
6401:
6362:
6319:
6290:
6253:
6230:
6222:
6187:3 June
6173:
6143:
6108:
6098:
6059:
6024:
5989:
5979:
5937:
5902:
5894:
5859:
5822:
5796:
5788:
5753:
5743:
5694:
5624:
5591:319722
5589:
5554:
5544:
5511:
5488:
5442:
5407:
5368:
5326:
5286:
5246:
5221:
5196:
5160:
5133:
5125:
5082:
5044:
5015:
4982:
4954:
4871:
4846:
4808:
4798:
4754:7 June
4740:
4699:
4672:
4662:
4599:
4569:
4561:
4526:
4491:
4481:
4467:: 25.
4438:
4415:
4363:
4331:
4321:
4275:
4089:lipids
4055:, and
4031:, and
4015:, and
3866:
3802:, and
3800:asthma
3760:kinase
3625:tomato
3621:potato
3589:dimers
3337:
3066:serine
2860:PTC124
2023:. The
1638:term.
213:humans
62:enzyme
38:enzyme
10314:Types
9924:Lyase
9884:Mixed
9877:Renin
9855:DPP-4
9690:1.17
9684:COX-2
9677:1.14
9580:Class
9427:S2CID
9359:S2CID
9141:S2CID
9049:JSTOR
8958:S2CID
8711:S2CID
8668:S2CID
8562:S2CID
8519:S2CID
8384:S2CID
8202:S2CID
8114:S2CID
8036:S2CID
7569:S2CID
7526:S2CID
7219:S2CID
6987:(PDF)
6966:(PDF)
6452:S2CID
6228:S2CID
5900:S2CID
5794:S2CID
5131:S2CID
4567:S2CID
3987:Many
3715:Drugs
3701:ricin
3425:, or
3368:imine
3300:et al
3260:et al
3210:inact
3190:inact
3151:inact
3144:inact
3080:, or
3040:, or
2966:Types
2843:TFase
2620:below
571:Mixed
225:liver
169:virus
159:Many
136:in a
56:is a
36:Top:
10406:list
10399:EC7
10393:list
10386:EC6
10380:list
10373:EC5
10367:list
10360:EC4
10354:list
10347:EC3
10341:list
10334:EC2
10328:list
10321:EC1
10016:ADME
9940:4.2
9932:4.1
9907:3.5
9844:3.4
9826:3.2
9808:3.1
9757:2.7
9749:2.6
9736:2.5
9730:PARP
9728:2.4
9717:COMT
9715:2.1
9661:1.5
9653:1.4
9645:1.3
9632:1.1
9478:PMID
9419:PMID
9382:ISBN
9351:PMID
9310:PMID
9261:PMID
9226:PMID
9191:PMID
9133:PMID
9090:ISBN
9067:PMID
9011:2022
8993:ISBN
8950:PMID
8915:PMID
8884:2022
8851:PMID
8806:2022
8788:ISBN
8759:ISBN
8734:ISBN
8703:PMID
8660:PMID
8611:PMID
8554:PMID
8511:PMID
8476:OCLC
8466:ISBN
8433:PMID
8411:1862
8376:PMID
8345:2022
8329:PMID
8298:PMID
8249:PMID
8218:2022
8194:PMID
8155:PMID
8106:PMID
8071:PMID
8028:PMID
7993:PMID
7940:PMID
7892:2022
7874:ISBN
7844:PMID
7832:1701
7809:PMID
7758:PMID
7705:ISBN
7674:PMID
7647:PMID
7612:PMID
7561:PMID
7518:PMID
7450:PMID
7415:PMID
7378:ISBN
7353:ISBN
7330:PMID
7299:2022
7283:PMID
7242:ISBN
7211:PMID
7174:ISBN
7151:2022
7135:PMID
7112:2022
7094:ISBN
7071:2022
7053:ISBN
7026:PMID
6995:2022
6974:ISBN
6944:PMID
6893:PMID
6856:ISBN
6827:ISBN
6792:PMID
6753:1GXF
6731:PMID
6690:PMID
6649:PMID
6614:PMID
6604:ISBN
6573:PMID
6529:PMID
6497:2022
6479:ISBN
6444:PMID
6409:PMID
6360:PMID
6317:ISBN
6288:ISBN
6251:ISBN
6220:PMID
6189:2022
6171:ISBN
6141:PMID
6129:1754
6106:PMID
6057:PMID
6022:PMID
5987:PMID
5935:PMID
5892:PMID
5857:PMID
5820:ISBN
5786:PMID
5751:PMID
5692:PMID
5622:PMID
5587:PMID
5552:PMID
5542:ISBN
5509:ISBN
5486:PMID
5440:PMID
5405:PMID
5382:2022
5366:PMID
5324:PMID
5312:1770
5284:PMID
5244:ISBN
5219:ISBN
5194:ISBN
5158:ISBN
5123:PMID
5080:ISBN
5058:2022
5042:PMID
5013:ISBN
4980:ISBN
4952:ISBN
4918:2012
4887:2017
4869:ISBN
4844:PMID
4806:PMID
4756:2022
4738:ISBN
4715:2022
4697:ISBN
4670:PMID
4615:2022
4597:ISBN
4559:PMID
4524:PMID
4489:PMID
4436:ISBN
4413:PMID
4379:2022
4361:ISBN
4329:PMID
4273:ISBN
4178:and
4159:and
4099:and
4091:and
4003:and
3959:and
3902:and
3879:1PWC
3627:and
3506:and
3489:and
3487:NADH
3339:mg.
3309:1GXF
3212:and
2899:, a
2670:and
2597:and
2213:and
764:and
666:and
410:and
321:The
285:ine
239:and
227:and
215:are
211:and
138:cell
78:life
9860:ACE
9468:PMC
9458:doi
9411:doi
9341:doi
9300:PMC
9292:doi
9253:doi
9218:doi
9183:doi
9123:doi
9057:PMC
9041:doi
8985:doi
8942:doi
8907:doi
8841:PMC
8833:doi
8695:doi
8691:161
8650:PMC
8642:doi
8601:PMC
8593:doi
8546:doi
8503:doi
8456:doi
8423:PMC
8415:doi
8368:doi
8325:294
8288:PMC
8280:doi
8241:doi
8186:doi
8182:194
8145:doi
8141:143
8098:doi
8063:doi
8020:doi
8016:398
7983:PMC
7975:doi
7930:PMC
7922:doi
7836:doi
7799:PMC
7789:doi
7748:PMC
7738:doi
7697:doi
7639:doi
7604:doi
7553:doi
7508:doi
7477:doi
7442:doi
7407:doi
7322:doi
7275:doi
7203:doi
7018:doi
6934:PMC
6924:doi
6920:279
6885:doi
6819:doi
6782:PMC
6772:doi
6768:279
6749:PDB
6721:doi
6717:264
6680:doi
6641:doi
6637:869
6596:doi
6563:doi
6559:267
6521:doi
6517:430
6436:doi
6399:PMC
6391:doi
6350:doi
6280:doi
6212:doi
6133:doi
6096:PMC
6088:doi
6049:doi
6014:doi
5977:PMC
5969:doi
5965:118
5927:doi
5884:doi
5849:doi
5778:doi
5741:PMC
5731:doi
5719:107
5684:doi
5672:279
5649:doi
5614:doi
5579:doi
5534:doi
5478:doi
5466:145
5432:doi
5316:doi
5276:doi
5113:doi
5005:doi
4836:doi
4796:PMC
4788:doi
4660:PMC
4650:doi
4551:doi
4516:doi
4479:PMC
4469:doi
4405:doi
4319:PMC
4311:doi
4265:doi
3919:).
3875:PDB
3836:is
3813:to
3508:ADP
3483:ATP
3445:in
3305:PDB
3137:obs
3130:obs
3123:obs
3116:obs
3070:DFP
3060:or
2765:off
2696:max
2650:max
2633:or
2622:).
2616:max
2602:max
2043:max
2036:max
1935:max
1919:max
1900:max
1881:max
1795:max
1779:max
1760:max
1744:or
1670:max
1654:max
1636:max
1446:max
1371:max
1358:max
1180:max
1022:max
880:max
799:max
769:max
747:max
716:max
685:max
671:max
654:max
631:or
575:In
557:max
544:In
515:max
507:In
499:).
492:max
471:max
458:In
440:max
283:ser
175:or
144:or
52:An
10424::
9848::
9571::
9515:.
9502:.
9476:.
9466:.
9454:22
9452:.
9448:.
9425:.
9417:.
9407:26
9405:.
9357:.
9349:.
9337:39
9335:.
9331:.
9308:.
9298:.
9288:18
9286:.
9282:.
9259:.
9249:11
9247:.
9224:.
9214:41
9212:.
9189:.
9179:12
9177:.
9139:.
9131:.
9119:26
9117:.
9113:.
9065:.
9055:.
9047:.
9037:87
9035:.
9031:.
9013:.
9001:.
8991:.
8956:.
8948:.
8938:30
8936:.
8913:.
8903:17
8901:.
8849:.
8839:.
8829:36
8827:.
8823:.
8796:.
8709:.
8701:.
8689:.
8666:.
8658:.
8648:.
8638:12
8636:.
8632:.
8609:.
8599:.
8589:12
8587:.
8583:.
8560:.
8552:.
8542:19
8540:.
8517:.
8509:.
8499:19
8497:.
8474:.
8464:.
8431:.
8421:.
8409:.
8405:.
8382:.
8374:.
8364:41
8362:.
8335:.
8323:.
8319:.
8296:.
8286:.
8276:49
8274:.
8270:.
8247:.
8235:.
8208:.
8200:.
8192:.
8180:.
8176:.
8153:.
8139:.
8135:.
8112:.
8104:.
8092:.
8069:.
8057:.
8034:.
8026:.
8014:.
7991:.
7981:.
7971:18
7969:.
7965:.
7946:.
7938:.
7928:.
7918:16
7916:.
7912:.
7900:^
7882:.
7868:.
7856:^
7842:.
7830:.
7807:.
7797:.
7785:11
7783:.
7779:.
7756:.
7746:.
7734:11
7732:.
7728:.
7703:.
7670:43
7668:.
7645:.
7633:.
7610:.
7602:.
7592:36
7590:.
7567:.
7559:.
7549:39
7547:.
7524:.
7516:.
7504:45
7502:.
7498:.
7473:10
7471:.
7448:.
7436:.
7413:.
7401:.
7328:.
7318:14
7316:.
7289:.
7281:.
7271:12
7269:.
7265:.
7217:.
7209:.
7199:10
7197:.
7141:.
7129:.
7102:.
7088:.
7061:.
7047:.
7024:.
7012:.
6982:.
6968:.
6942:.
6932:.
6918:.
6914:.
6891:.
6881:53
6879:.
6825:.
6813:.
6790:.
6780:.
6766:.
6762:.
6751::
6729:.
6715:.
6711:.
6688:.
6676:18
6674:.
6670:.
6647:.
6635:.
6612:.
6602:.
6571:.
6557:.
6553:.
6541:^
6527:.
6515:.
6487:.
6473:.
6450:.
6442:.
6432:19
6430:.
6407:.
6397:.
6385:.
6381:.
6358:.
6344:.
6340:.
6296:.
6286:.
6276:15
6274:.
6226:.
6218:.
6208:62
6206:.
6179:.
6165:.
6153:^
6139:.
6127:.
6104:.
6094:.
6084:10
6082:.
6078:.
6055:.
6043:.
6020:.
6008:.
5985:.
5975:.
5963:.
5959:.
5947:^
5933:.
5923:18
5921:.
5898:.
5890:.
5880:38
5878:.
5855:.
5845:12
5843:.
5806:^
5792:.
5784:.
5774:24
5772:.
5749:.
5739:.
5729:.
5717:.
5713:.
5690:.
5682:.
5670:.
5645:47
5643:.
5620:.
5610:32
5608:.
5585:.
5575:86
5573:.
5550:.
5540:.
5484:.
5476:.
5464:.
5452:^
5438:.
5428:15
5426:.
5401:31
5399:.
5372:.
5322:.
5310:.
5296:^
5282:.
5270:.
5258:^
5172:^
5137:.
5129:.
5121:.
5109:24
5107:.
5103:.
5066:^
5048:.
5036:.
5011:.
4966:^
4926:^
4895:^
4877:.
4842:.
4832:67
4830:.
4818:^
4804:.
4794:.
4784:57
4782:.
4778:.
4764:^
4746:.
4732:.
4705:.
4691:.
4668:.
4658:.
4648:.
4638:14
4636:.
4632:.
4605:.
4591:.
4579:^
4565:.
4557:.
4547:55
4545:.
4522:.
4512:30
4510:.
4487:.
4477:.
4463:.
4459:.
4411:.
4399:.
4387:^
4369:.
4341:^
4327:.
4317:.
4307:14
4305:.
4301:.
4287:^
4271:.
4247:^
4103:.
4051:,
4027:,
4011:,
3979:.
3963:,
3935:.
3877::
3806:.
3798:,
3657:.
3623:,
3599:.
3555:,
3539:.
3485:,
3449:.
3417:,
3307::
3283:.
3275:,
3219:.
3139:=
3109:50
3084:.
3076:,
3036:,
3032:,
3028:,
3024:,
3020:,
2937:.
2771:.
2758:on
2727:.
2629:,
2257:.
749:).
728:≠
697:=
687:).
406:,
291:in
259:.
243:.
171:,
156:.
100:.
10408:)
10404:(
10395:)
10391:(
10382:)
10378:(
10369:)
10365:(
10356:)
10352:(
10343:)
10339:(
10330:)
10326:(
10150:e
10143:t
10136:v
9996:e
9989:t
9982:v
9561:e
9554:t
9547:v
9484:.
9460::
9433:.
9413::
9390:.
9365:.
9343::
9316:.
9294::
9267:.
9255::
9232:.
9220::
9197:.
9185::
9147:.
9125::
9098:.
9073:.
9043::
8987::
8964:.
8944::
8921:.
8909::
8886:.
8857:.
8835::
8808:.
8767:.
8742:.
8717:.
8697::
8674:.
8644::
8617:.
8595::
8568:.
8548::
8525:.
8505::
8482:.
8458::
8439:.
8417::
8390:.
8370::
8347:.
8304:.
8282::
8255:.
8243::
8237:2
8220:.
8188::
8161:.
8147::
8120:.
8100::
8094:7
8077:.
8065::
8059:9
8042:.
8022::
7999:.
7977::
7924::
7894:.
7850:.
7838::
7815:.
7791::
7764:.
7740::
7713:.
7699::
7680:.
7653:.
7641::
7635:9
7618:.
7606::
7598::
7575:.
7555::
7532:.
7510::
7483:.
7479::
7456:.
7444::
7438:3
7421:.
7409::
7403:7
7386:.
7361:.
7336:.
7324::
7301:.
7277::
7250:.
7225:.
7205::
7182:.
7153:.
7114:.
7073:.
7032:.
7020::
7014:5
6997:.
6950:.
6926::
6899:.
6887::
6864:.
6821::
6798:.
6774::
6737:.
6723::
6696:.
6682::
6655:.
6643::
6620:.
6598::
6579:.
6565::
6535:.
6523::
6499:.
6458:.
6438::
6415:.
6393::
6387:2
6366:.
6352::
6346:8
6325:.
6282::
6259:.
6234:.
6214::
6191:.
6147:.
6135::
6112:.
6090::
6063:.
6051::
6045:4
6028:.
6016::
6010:7
5993:.
5971::
5941:.
5929::
5906:.
5886::
5863:.
5851::
5828:.
5800:.
5780::
5757:.
5733::
5725::
5698:.
5686::
5678::
5655:.
5651::
5628:.
5616::
5593:.
5581::
5558:.
5536::
5517:.
5492:.
5480::
5472::
5446:.
5434::
5411:.
5384:.
5330:.
5318::
5290:.
5278::
5272:3
5252:.
5227:.
5202:.
5166:.
5115::
5088:.
5060:.
5021:.
5007::
4988:.
4960:.
4920:.
4889:.
4850:.
4838::
4812:.
4790::
4758:.
4717:.
4676:.
4652::
4644::
4617:.
4573:.
4553::
4530:.
4518::
4495:.
4471::
4465:6
4444:.
4419:.
4407::
4401:8
4381:.
4335:.
4313::
4281:.
4267::
3217:i
3214:K
3207:k
3187:k
3148:k
3141:k
3134:k
3127:k
3120:k
3113:k
2923:i
2920:K
2762:k
2755:k
2751:i
2748:K
2744:i
2741:K
2703:m
2700:K
2693:V
2675:i
2672:K
2668:i
2665:K
2661:m
2658:K
2654:x
2647:V
2643:y
2613:V
2609:m
2606:K
2599:V
2595:m
2592:K
2575:.
2563:i
2559:K
2554:]
2551:I
2548:[
2542:+
2539:1
2536:=
2501:i
2497:K
2492:]
2489:I
2486:[
2480:+
2477:1
2474:=
2442:]
2439:S
2436:[
2433:+
2428:m
2424:K
2420:)
2406:/
2399:(
2394:]
2391:S
2388:[
2383:x
2380:a
2377:m
2373:V
2369:)
2355:/
2351:1
2348:(
2342:=
2336:]
2333:S
2330:[
2317:+
2312:m
2308:K
2299:]
2296:S
2293:[
2288:x
2285:a
2282:m
2278:V
2271:=
2268:V
2243:i
2240:K
2236:i
2233:K
2225:i
2222:K
2218:i
2215:K
2211:i
2208:K
2204:i
2201:K
2197:i
2194:K
2154:x
2150:K
2146:+
2143:]
2139:X
2135:[
2122:]
2119:X
2116:[
2104:)
2099:2
2096:m
2092:K
2083:1
2080:m
2076:K
2072:(
2064:1
2061:m
2057:K
2040:V
2033:V
2028:m
2025:K
2021:m
2018:K
1993:x
1989:K
1985:+
1982:]
1978:X
1974:[
1961:]
1958:X
1955:[
1943:)
1938:2
1931:V
1922:1
1915:V
1911:(
1903:1
1896:V
1878:V
1853:i
1849:K
1845:+
1842:]
1838:I
1834:[
1821:]
1818:I
1815:[
1803:)
1798:2
1791:V
1782:1
1775:V
1771:(
1763:1
1756:V
1722:i
1718:K
1714:+
1711:]
1707:I
1703:[
1690:]
1687:I
1684:[
1666:V
1650:V
1633:V
1608:i
1604:K
1600:+
1597:]
1593:I
1589:[
1576:]
1573:I
1570:[
1528:m
1524:K
1520:+
1517:]
1513:S
1509:[
1496:]
1493:S
1490:[
1442:V
1425:]
1421:I
1417:[
1414:+
1409:i
1405:K
1391:]
1388:I
1385:[
1367:V
1354:V
1350:=
1340:1
1337:=
1329:]
1325:I
1321:[
1318:+
1313:i
1309:K
1296:]
1292:I
1288:[
1285:+
1280:i
1276:K
1255:)
1246:]
1242:I
1238:[
1235:+
1230:i
1226:K
1213:]
1209:I
1205:[
1191:1
1187:(
1176:V
1171:=
1156:0
1153:=
1150:]
1146:I
1142:[
1136:]
1132:I
1128:[
1116:)
1108:]
1104:I
1100:[
1097:+
1092:i
1088:K
1075:]
1071:I
1067:[
1061:]
1057:I
1053:[
1050:+
1045:i
1041:K
1029:(
1018:V
1013:=
1003:1
1000:=
990:i
986:K
971:i
967:K
946:)
938:]
934:I
930:[
927:+
922:i
918:K
903:i
899:K
887:(
876:V
871:=
852:i
848:K
834:]
831:I
828:[
816:+
813:1
795:V
766:V
762:m
759:K
744:V
740:m
737:K
733:i
730:K
726:i
723:K
713:V
709:m
706:K
702:i
699:K
695:i
692:K
682:V
678:m
675:K
668:V
664:m
661:K
651:V
647:m
644:K
636:i
633:K
629:i
626:K
564:m
562:K
555:V
533:m
531:K
522:m
520:K
513:V
490:V
485:m
483:K
478:m
476:K
469:V
447:m
445:K
438:V
287:p
281:(
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.