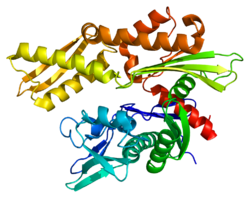
1623:. In order to properly fold non-native proteins, Hsp70 chaperones interact with the hydrophobic peptide segments of proteins in an ATP-controlled fashion. Though the exact mechanism still remains unclear, there are at least two alternative modes of action: kinetic partitioning and local unfolding. In kinetic partitioning, Hsp70s repetitively bind and release substrates in cycles that maintain low concentrations of free substrate. This effectively prevents aggregation while allowing free molecules to fold to the native state. In local unfolding, the binding and release cycles induce localized unfolding in the substrate, which helps to overcome kinetic barriers for folding to the native state. Ultimately, its role in protein folding contributes to its function in signal transduction, apoptosis, protein homeostasis, and cell growth and differentiation. Hsc70 is known to localize to the
4498:
4483:
4468:
4453:
4438:
4423:
4408:
4393:
4378:
4363:
368:
345:
1824:, and aging and cell senescence, as observed in centenarians subjected to heat shock challenge. In particular, Hsc70 plays a protective role in the aforementioned diseases, as well as in other neuropsychiatric disorders such as schizophrenia. Its protective role was further highlighted in a study that identified HSPA8 alongside other HSP70 proteins in a core sub-network of the wider chaperome interactome that functions as a proteostasis safeguard and that is repressed in aging brains and in the brains of Alzheimer's, Parkinson's and Huntington's disease patients.
4618:
4273:
4213:
4198:
4288:
267:
242:
1599:-binding domain. The substrate-binding domain consists of two subdomains, a two-layered β-sandwich subdomain (SBDβ) and an α-helical subdomain (SBDα), which are connected by the loop Lα,β. SBDβ contains the peptide binding pocket while SBDα serves as a lid to cover the substrate binding cleft. The ATP binding domain consists of four subdomains split into two lobes by a central ATP/ADP binding pocket. The two terminal domains are linked together by a conserved region referred to as loop LL,1, which is critical for
4243:
4603:
633:
626:
619:
4303:
4558:
4228:
4573:
4348:
4333:
4318:
4543:
4528:
4513:
4258:
374:
273:
4588:
31:
3274:
Stelzl U, Worm U, Lalowski M, Haenig C, Brembeck FH, Goehler H, Stroedicke M, Zenkner M, Schoenherr A, Koeppen S, Timm J, Mintzlaff S, Abraham C, Bock N, Kietzmann S, Goedde A, Toksöz E, Droege A, Krobitsch S, Korn B, Birchmeier W, Lehrach H, Wanker EE (Sep 2005). "A human protein-protein interaction
1799:
strategies for Hsp70s have been highly successful in animal models and progressed to clinical trials. One treatment, a Hsp72/AFP recombined vaccine, elicited robust protective immunity against AFP-expressing tumors in mice experiments. Therefore, the vaccine holds promise for treating hepatocellular
1682:
Human Hsc70 has 85% identity with human Hsp70 (SDSC workbench, blosom26 default analysis). The scientific community has long assumed that Hsp70 and Hsc70 have similar cellular roles, but this assumption proved incomplete. While Hsc70 also performed chaperone functions under normal conditions, unlike
3222:
Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, Berriz GF, Gibbons FD, Dreze M, Ayivi-Guedehoussou N, Klitgord N, Simon C, Boxem M, Milstein S, Rosenberg J, Goldberg DS, Zhang LV, Wong SL, Franklin G, Li S, Albala JS, Lim J, Fraughton C, Llamosas E, Cevik S, Bex C, Lamesch P,
1619:) family contains both heat-inducible and constitutively expressed members. The latter are called heat-shock cognate (Hsc) proteins. The heat shock 70 kDa protein 8 also known as Hsc70 belongs to the heat-shock cognate subgroup. This protein binds to nascent polypeptides to facilitate correct
57:
1547:
on chromosome 11. As a member of the heat shock protein 70 family and a chaperone protein, it facilitates the proper folding of newly translated and misfolded proteins, as well as stabilize or degrade mutant proteins. Its functions contribute to biological processes including
4497:
4482:
4467:
4452:
4437:
4422:
4407:
4392:
4377:
4362:
1727:
response. It is a mode of cell death defined by characteristic morphological, biochemical and molecular changes. It was first described as a "shrinkage necrosis", and then this term was replaced by apoptosis to emphasize its role opposite
1732:
in tissue kinetics. In later stages of apoptosis the entire cell becomes fragmented, forming a number of plasma membrane-bounded apoptotic bodies which contain nuclear and or cytoplasmic elements. The ultrastructural appearance of
1646:
Hsc70 additionally serves as a positive regulator of cell cycle transition and carcinogenesis. For example, Hsc70 regulates the nuclear accumulation of cyclin D1, which is a key player in G1 to S phase cell cycle transition.
1760:. This role leads to its involvement in many pathological processes, such as oncogenesis, neurodegeneration, and senescence. In particular, overexpression of HSP72 has been linked to the development some cancers, such as
3223:
Sikorski RS, Vandenhaute J, Zoghbi HY, Smolyar A, Bosak S, Sequerra R, Doucette-Stamm L, Cusick ME, Hill DE, Roth FP, Vidal M (Oct 2005). "Towards a proteome-scale map of the human protein-protein interaction network".
3877:
Abe T, Konishi T, Hirano T, Kasai H, Shimizu K, Kashimura M, Higashi K (Jan 1995). "Possible correlation between DNA damage induced by hydrogen peroxide and translocation of heat shock 70 protein into the nucleus".
4617:
4272:
3465:
Rasmussen HH, van Damme J, Puype M, Gesser B, Celis JE, Vandekerckhove J (Dec 1992). "Microsequences of 145 proteins recorded in the two-dimensional gel protein database of normal human epidermal keratinocytes".
4035:
Egerton M, Moritz RL, Druker B, Kelso A, Simpson RJ (Jul 1996). "Identification of the 70kD heat shock cognate protein (Hsc70) and alpha-actinin-1 as novel phosphotyrosine-containing proteins in T lymphocytes".
381:
280:
4212:
4197:
2810:
Bozidis P, Hyphantis T, Mantas C, Sotiropoulou M, Antypa N, Andreoulakis E, Serretti A, Mavreas V, Antoniou K (Apr 2014). "HSP70 polymorphisms in first psychotic episode drug-naïve schizophrenic patients".
4287:
2209:
Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, Zamzami N, Mak T, Jäättelä M, Penninger JM, Garrido C, Kroemer G (September 2001). "Heat-shock protein 70 antagonizes apoptosis-inducing factor".
3844:"70-kDa heat shock cognate protein interacts directly with the N-terminal region of the retinoblastoma gene product pRb. Identification of a novel region of pRb-mediating protein interaction"
3971:
Tavaria M, Gabriele T, Anderson RL, Mirault ME, Baker E, Sutherland G, Kola I (Sep 1995). "Localization of the gene encoding the human heat shock cognate protein, HSP73, to chromosome 11".
3620:"Characterization of the thermotolerant cell. II. Effects on the intracellular distribution of heat-shock protein 70, intermediate filaments, and small nuclear ribonucleoprotein complexes"
4506:: STRUCTURAL BASIS OF THE 70-KILODALTON HEAT SHOCK COGNATE PROTEIN ATP HYDROLYTIC ACTIVITY, II. STRUCTURE OF THE ACTIVE SITE WITH ADP OR ATP BOUND TO WILD TYPE AND MUTANT ATPASE FRAGMENT
4491:: STRUCTURAL BASIS OF THE 70-KILODALTON HEAT SHOCK COGNATE PROTEIN ATP HYDROLYTIC ACTIVITY, II. STRUCTURE OF THE ACTIVE SITE WITH ADP OR ATP BOUND TO WILD TYPE AND MUTANT ATPASE FRAGMENT
4476:: STRUCTURAL BASIS OF THE 70-KILODALTON HEAT SHOCK COGNATE PROTEIN ATP HYDROLYTIC ACTIVITY, II. STRUCTURE OF THE ACTIVE SITE WITH ADP OR ATP BOUND TO WILD TYPE AND MUTANT ATPASE FRAGMENT
4461:: STRUCTURAL BASIS OF THE 70-KILODALTON HEAT SHOCK COGNATE PROTEIN ATP HYDROLYTIC ACTIVITY, II. STRUCTURE OF THE ACTIVE SITE WITH ADP OR ATP BOUND TO WILD TYPE AND MUTANT ATPASE FRAGMENT
4446:: STRUCTURAL BASIS OF THE 70-KILODALTON HEAT SHOCK COGNATE PROTEIN ATP HYDROLYTIC ACTIVITY, II. STRUCTURE OF THE ACTIVE SITE WITH ADP OR ATP BOUND TO WILD TYPE AND MUTANT ATPASE FRAGMENT
4431:: STRUCTURAL BASIS OF THE 70-KILODALTON HEAT SHOCK COGNATE PROTEIN ATP HYDROLYTIC ACTIVITY, II. STRUCTURE OF THE ACTIVE SITE WITH ADP OR ATP BOUND TO WILD TYPE AND MUTANT ATPASE FRAGMENT
4416:: STRUCTURAL BASIS OF THE 70-KILODALTON HEAT SHOCK COGNATE PROTEIN ATP HYDROLYTIC ACTIVITY, II. STRUCTURE OF THE ACTIVE SITE WITH ADP OR ATP BOUND TO WILD TYPE AND MUTANT ATPASE FRAGMENT
4401:: STRUCTURAL BASIS OF THE 70-KILODALTON HEAT SHOCK COGNATE PROTEIN ATP HYDROLYTIC ACTIVITY, II. STRUCTURE OF THE ACTIVE SITE WITH ADP OR ATP BOUND TO WILD TYPE AND MUTANT ATPASE FRAGMENT
4386:: STRUCTURAL BASIS OF THE 70-KILODALTON HEAT SHOCK COGNATE PROTEIN ATP HYDROLYTIC ACTIVITY, II. STRUCTURE OF THE ACTIVE SITE WITH ADP OR ATP BOUND TO WILD TYPE AND MUTANT ATPASE FRAGMENT
4371:: STRUCTURAL BASIS OF THE 70-KILODALTON HEAT SHOCK COGNATE PROTEIN ATP HYDROLYTIC ACTIVITY, II. STRUCTURE OF THE ACTIVE SITE WITH ADP OR ATP BOUND TO WILD TYPE AND MUTANT ATPASE FRAGMENT
1795:
by complexing, and hence, stabilizing, oncofetal proteins and products and transporting them into intracellular sites, thereby promoting tumor cell proliferation. As a result, tumor
1752:
Hsp70 member proteins, including Hsp72, inhibit apoptosis by acting on the caspase-dependent pathway and against apoptosis-inducing agents such as tumor necrosis factor-α (TNFα),
3813:
Nunes SL, Calderwood SK (Aug 1995). "Heat shock factor-1 and the heat shock cognate 70 protein associate in high molecular weight complexes in the cytoplasm of NIH-3T3 cells".
3589:
Lim MY, Davis N, Zhang JY, Bose HR (Mar 1990). "The v-rel oncogene product is complexed with cellular proteins including its proto-oncogene product and heat shock protein 70".
4242:
4602:
4658:
3371:"Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions"
5462:
1749:
development as programmed cell death and accompanies a variety of normal involutional processes in which it serves as a mechanism to remove "unwanted" cells.
4911:
3909:"Human immunodeficiency virus type 1 interaction with the membrane of CD4+ cells induces the synthesis and nuclear translocation of 70K heat shock protein"
2254:"Heat shock protein 72 suppresses apoptosis by increasing the stability of X-linked inhibitor of apoptosis protein in renal ischemia/reperfusion injury"
1246:
203:
4302:
1227:
1737:
is quite different, the main features being mitochondrial swelling, plasma membrane breakdown and cellular disintegration. Apoptosis occurs in many
1711:, an apoptotic cell undergoes structural changes including cell shrinkage, plasma membrane blebbing, nuclear condensation, and fragmentation of the
3090:"The MSG1 non-DNA-binding transactivator binds to the p300/CBP coactivators, enhancing their functional link to the Smad transcription factors"
3942:
Maruyama K, Sugano S (Jan 1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides".
1683:
canonical heat shock proteins, Hsc70 is constitutively expressed and performs functions related to normal cellular processes, such as protein
5321:
2017:
1999:
4651:
4557:
4227:
4572:
4626:: HIGH RESOLUTION SOLUTION STRUCTURE OF THE HEAT SHOCK COGNATE-70 KD SUBSTRATE BINDING DOMAIN OBTAINED BY MULTIDIMENSIONAL NMR TECHNIQUES
4281:: HIGH RESOLUTION SOLUTION STRUCTURE OF THE HEAT SHOCK COGNATE-70 KD SUBSTRATE BINDING DOMAIN OBTAINED BY MULTIDIMENSIONAL NMR TECHNIQUES
1588:
This gene encodes a 70kDa heat shock protein which is a member of the heat shock protein 70 (Hsp70) family. As a Hsp70 protein, it has a
54:
4347:
4332:
4317:
1462:
4542:
4527:
4512:
4257:
1469:
5210:
5205:
5180:
5170:
5165:
5155:
4221:: THREONINE 204 OF THE CHAPERONE PROTEIN HSC70 INFLUENCES THE STRUCTURE OF THE ACTIVE SITE BUT IS NOT ESSENTIAL FOR ATP HYDROLYSIS
4206:: THREONINE 204 OF THE CHAPERONE PROTEIN HSC70 INFLUENCES THE STRUCTURE OF THE ACTIVE SITE BUT IS NOT ESSENTIAL FOR ATP HYDROLYSIS
2560:"Differential effects of Hsc70 and Hsp70 on the intracellular trafficking and functional expression of epithelial sodium channels"
2306:"Crystal structure of the stress-inducible human heat shock protein 70 substrate-binding domain in complex with peptide substrate"
5226:
5160:
4644:
4296:: HOW POTASSIUM AFFECTS THE ACTIVITY OF THE MOLECULAR CHAPERONE HSC70. II. POTASSIUM BINDS SPECIFICALLY IN THE ATPASE ACTIVE SITE
3330:"CHIP is associated with Parkin, a gene responsible for familial Parkinson's disease, and enhances its ubiquitin ligase activity"
2048:
4587:
174:, HEL-33, HEL-S-72p, HSC54, HSC70, HSC71, HSP71, HSP73, HSPA10, LAP-1, LAP1, NIP71, heat shock protein family A (Hsp70) member 8
5457:
5256:
4065:
Lamian V, Small GM, Feldherr CM (Oct 1996). "Evidence for the existence of a novel mechanism for the nuclear import of Hsc70".
1864:
1640:
5499:
4177:
3842:
Inoue A, Torigoe T, Sogahata K, Kamiguchi K, Takahashi S, Sawada Y, Saijo M, Taya Y, Ishii S, Sato N, Kikuchi K (Sep 1995).
1982:
2846:
Brehme M, Voisine C, Rolland T, Wachi S, Soper JH, Zhu Y, Orton K, Villella A, Garza D, Vidal M, Ge H, Morimoto RI (2014).
3548:"Uncoating protein (hsc70) binds a conformationally labile domain of clathrin light chain LCa to stimulate ATP hydrolysis"
2763:"Chaperoning to the metabolic party: The emerging therapeutic role of heat-shock proteins in obesity and type 2 diabetes"
1961:
5474:
4149:
provides an overview of all the structure information available in the PDB for Human Heat shock cognate 71 kDa protein
367:
3784:
Benaroudj N, Batelier G, Triniolles F, Ladjimi MM (Nov 1995). "Self-association of the molecular chaperone HSC70".
3751:"Structural features of the precursor to mitochondrial aspartate aminotransferase responsible for binding to hsp70"
3424:"Inducible heat shock protein 70 kD and inducible nitric oxide synthase in hemorrhage/resuscitation-induced injury"
1565:
4667:
1986:
1965:
344:
4867:
3505:"Intracellular localization and partial amino acid sequence of a stress-inducible 40-kDa protein in HeLa cells"
1291:
5509:
4096:"Modification of two distinct COOH-terminal domains is required for murine p53 activation by bacterial Hsp70"
266:
241:
1272:
5504:
5080:
4002:"Effect of constitutive 70-kDa heat shock protein polymerization on its interaction with protein substrate"
1671:
183:
3041:"Functional analysis of the human CDC5L complex and identification of its components by mass spectrometry"
5025:
2848:"A conserved chaperome sub-network safeguards protein homeostasis in aging and neurodegenerative disease"
1856:
3088:
Yahata T, de
Caestecker MP, Lechleider RJ, Andriole S, Roberts AB, Isselbacher KJ, Shioda T (Mar 2000).
2131:
Wang X, Wang Q, Lin H, Li S, Sun L, Yang Y (Feb 2013). "HSP72 and gp96 in gastroenterological cancers".
380:
279:
2941:
Takayama S, Bimston DN, Matsuzawa S, Freeman BC, Aime-Sempe C, Xie Z, Morimoto RI, Reed JC (Aug 1997).
1635:
by aiding the unfolding and translocation of substrate proteins across the membrane into the lysosomal
373:
272:
4170:
1573:
170:
4965:
4140:
1761:
1603:. The unstructured region at the very end of the C-terminal is believed to be the docking site for
1445:
1390:
191:
5290:
1821:
1817:
1441:
1420:
1416:
1386:
1365:
1361:
5331:
2611:"Biochemical and Proteomic Analysis of Ubiquitination of Hsc70 and Hsp70 by the E3 Ligase CHIP"
1813:
1809:
1596:
4712:
1600:
255:
3503:
Hattori H, Liu YC, Tohnai I, Ueda M, Kaneda T, Kobayashi T, Tanabe K, Ohtsuka K (Feb 1992).
2662:"Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics"
4251:: HEAT-SHOCK COGNATE 70KD PROTEIN 44KD ATPASE N-TERMINAL MUTANT WITH CYS 17 REPLACED BY LYS
4163:
3713:
3232:
2558:
Goldfarb SB, Kashlan OB, Watkins JN, Suaud L, Yan W, Kleyman TR, Rubenstein RC (Apr 2006).
2317:
1792:
211:
3704:
Rensing SA, Maier UG (Jul 1994). "Phylogenetic analysis of the stress-70 protein family".
3663:"Structure and expression of a human gene coding for a 71 kd heat shock 'cognate' protein"
3297:
3132:
Hatakeyama T, Dai P, Harada Y, Hino H, Tsukahara F, Maru Y, Otsuji E, Takamatsu T (2013).
2504:
Hatakeyama T, Dai P, Harada Y, Hino H, Tsukahara F, Maru Y, Otsuji E, Takamatsu T (2013).
8:
4675:
4136:
3328:
Imai Y, Soda M, Hatakeyama S, Akagi T, Hashikawa T, Nakayama KI, Takahashi R (Jul 2002).
1674:
wherein it imparts selectivity to the proteins being degraded by this lysosomal pathway.
1658:-coated vesicles during transport of membrane components through the cell. It works with
1549:
4611:: THREE-DIMENSIONAL STRUCTURE OF THE ATPASE FRAGMENT OF A 70K HEAT-SHOCK COGNATE PROTEIN
3907:
Furlini G, Vignoli M, Re MC, Gibellini D, Ramazzotti E, Zauli G, La Placa M (Jan 1994).
3717:
3236:
2321:
1670:
is also an important protein involved in vesicle uncoating. Hsc70 is a key component of
4689:
3737:
3644:
3619:
3577:
3534:
3491:
3453:
3310:
3256:
3158:
3133:
2967:
2942:
2872:
2847:
2787:
2762:
2738:
2713:
2686:
2661:
2637:
2610:
2586:
2559:
2530:
2505:
2432:
2407:
2340:
2305:
2278:
2253:
2234:
2191:
2095:
2070:
1805:
1537:
215:
3687:
3662:
3395:
3370:
3346:
3329:
3134:"Connexin43 functions as a novel interacting partner of heat shock cognate protein 70"
3065:
3040:
3016:
2991:
2506:"Connexin43 functions as a novel interacting partner of heat shock cognate protein 70"
2481:
2456:
1832:
Hsc70 forms a chaperone complex by interacting with the heat shock protein of 40 kDa (
1719:. This is followed by fragmentation into apoptotic bodies that are quickly removed by
1639:. Through this pathway, Hsc70 also contributes to the degradation of the proapoptotic
1161:
1156:
1151:
1146:
1141:
1136:
1131:
1126:
1121:
1116:
1111:
1106:
1101:
1096:
1091:
1086:
1081:
1076:
1071:
1066:
1061:
1056:
1051:
1046:
1041:
1036:
1031:
1026:
1021:
1016:
1011:
1006:
1001:
996:
991:
986:
981:
976:
971:
955:
950:
945:
940:
935:
930:
925:
920:
915:
910:
905:
900:
895:
890:
885:
880:
875:
870:
865:
860:
855:
850:
845:
840:
835:
830:
825:
820:
815:
810:
805:
800:
795:
790:
785:
780:
764:
759:
754:
749:
744:
739:
734:
729:
724:
719:
714:
709:
704:
699:
694:
689:
684:
679:
674:
4991:
4146:
4117:
4082:
4053:
4023:
3988:
3959:
3955:
3930:
3895:
3865:
3830:
3801:
3772:
3729:
3692:
3649:
3606:
3602:
3569:
3564:
3547:
3526:
3483:
3445:
3400:
3351:
3302:
3248:
3204:
3163:
3111:
3070:
3021:
2992:"Tumor necrosis factor receptor 1 is an ATPase regulated by silencer of death domain"
2972:
2923:
2877:
2828:
2792:
2743:
2691:
2642:
2591:
2535:
2486:
2437:
2383:
2345:
2283:
2226:
2183:
2166:
Xilouri M, Stefanis L (Dec 2016). "Chaperone
Mediated Autophagy: Starve to Prosper".
2148:
2100:
163:
47:
3741:
3538:
3495:
3457:
3007:
2472:
2406:
Xie W, Zhang L, Jiao H, Guan L, Zha J, Li X, Wu M, Wang Z, Han J, You H (Jul 2015).
2238:
5251:
4955:
4107:
4074:
4045:
4013:
3980:
3951:
3920:
3887:
3855:
3822:
3793:
3762:
3721:
3682:
3674:
3639:
3631:
3598:
3581:
3559:
3516:
3475:
3435:
3390:
3382:
3341:
3314:
3292:
3284:
3260:
3240:
3194:
3153:
3145:
3101:
3060:
3052:
3011:
3003:
2962:
2954:
2913:
2867:
2859:
2820:
2782:
2774:
2733:
2725:
2681:
2673:
2632:
2622:
2581:
2571:
2525:
2517:
2476:
2468:
2427:
2419:
2375:
2335:
2325:
2273:
2265:
2218:
2175:
2140:
2090:
2082:
460:
391:
335:
290:
195:
3369:
Ballinger CA, Connell P, Wu Y, Hu Z, Thompson LJ, Yin LY, Patterson C (Jun 1999).
2902:"An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators"
2457:"Hsc70 regulates accumulation of cyclin D1 and cyclin D1-dependent protein kinase"
2423:
2195:
4680:
4636:
3181:
Sarkar S, Pollack BP, Lin KT, Kotenko SV, Cook JR, Lewis A, Pestka S (Dec 2001).
2863:
2778:
2627:
2379:
2330:
1930:
1636:
1620:
435:
219:
2022:
National Center for
Biotechnology Information, U.S. National Library of Medicine
2004:
National Center for
Biotechnology Information, U.S. National Library of Medicine
1695:
The Hsp70 member proteins are important apoptotic constituents. During a normal
5004:
4999:
3925:
3908:
3288:
3056:
2958:
2564:
Proceedings of the
National Academy of Sciences of the United States of America
1765:
1684:
536:
2824:
2729:
2179:
2144:
2086:
632:
625:
618:
5493:
4112:
4095:
4018:
4001:
3860:
3843:
3767:
3750:
3678:
3479:
1845:
1773:
1753:
1738:
1700:
1699:
processes, or during cell injury (such as ischemia-reperfusion injury during
661:
4094:
Hansen S, Midgley CA, Lane DP, Freeman BC, Morimoto RI, Hupp TR (Nov 1996).
3106:
3089:
2576:
596:
474:
5360:
5342:
4311:: CRYSTAL STRUCTURE OF A BAG DOMAIN IN COMPLEX WITH THE HSC70 ATPASE DOMAIN
4078:
4049:
3984:
3891:
3826:
3749:
Lain B, Iriarte A, Mattingly JR, Moreno JI, Martinez-Carrion M (Oct 1995).
3635:
3449:
3404:
3355:
3306:
3252:
3208:
3199:
3182:
3167:
3115:
3074:
3025:
2881:
2832:
2796:
2747:
2646:
2595:
2539:
2490:
2441:
2387:
2349:
2287:
2230:
2187:
2152:
2104:
1808:
in cardiac muscle, as well damage from neurodegenerative diseases, such as
1800:
carcinoma. Alternatively, overexpression of Hsp70 can mitigate damage from
1769:
1742:
1724:
1716:
1667:
1604:
453:
232:
4121:
4086:
4057:
4027:
3992:
3963:
3934:
3899:
3869:
3834:
3805:
3776:
3733:
3696:
3653:
3610:
3573:
3530:
3487:
3440:
3386:
3183:"hTid-1, a human DnaJ protein, modulates the interferon signaling pathway"
2976:
2927:
2918:
2901:
2695:
2366:
Majeski AE, Dice JF (2004). "Mechanisms of chaperone-mediated autophagy".
2269:
1509:
1504:
5436:
5304:
1777:
1757:
1696:
1561:
1336:
1317:
30:
3797:
3244:
2677:
2408:"Chaperone-mediated autophagy prevents apoptosis by degrading BBC3/PUMA"
2222:
5266:
4694:
3725:
3039:
Ajuh P, Kuster B, Panov K, Zomerdijk JC, Mann M, Lamond AI (Dec 2000).
1788:
1781:
1720:
1593:
1589:
1577:
352:
249:
199:
3521:
3504:
3149:
2521:
1132:
protein targeting to lysosome involved in chaperone-mediated autophagy
140:
136:
132:
128:
124:
120:
116:
112:
108:
104:
100:
96:
92:
88:
84:
80:
76:
5014:
4960:
3087:
1906:
1787:
for these cancers. Elevated Hsp70 levels in tumor cells may increase
1784:
1746:
1632:
1624:
1557:
1553:
1191:
419:
406:
318:
305:
207:
3423:
2252:
Zhang B, Rong R, Li H, Peng X, Xiong L, Wang Y, Yu X, Mao H (2015).
831:
clathrin-sculpted gamma-aminobutyric acid transport vesicle membrane
5294:
4927:
4919:
3221:
1801:
1734:
1663:
1655:
1628:
1493:
3783:
2809:
5045:
4932:
4892:
4823:
4818:
4813:
4808:
4803:
4758:
2133:
Clinica
Chimica Acta; International Journal of Clinical Chemistry
1796:
1729:
1704:
1303:
1258:
1176:
1172:
187:
5261:
5236:
5231:
5221:
5185:
5175:
5150:
5135:
5130:
5125:
5120:
5115:
5110:
5105:
5100:
5065:
5050:
4897:
4862:
4847:
4842:
4837:
4798:
4793:
4788:
4783:
4778:
4773:
4768:
4763:
4753:
4748:
4743:
4738:
4733:
4566:: CRYSTAL STRUCTURE OF THE C-TERMINAL 10-kDA SUBDOMAIN OF HSC70
4236:: HEAT-SHOCK COGNATE 70KD PROTEIN 44KD ATPASE N-TERMINAL 1NGE 3
2940:
1924:
1912:
1900:
1708:
1659:
1651:
1569:
1477:
1213:
641:
4581:: crystal structure of bovine hsc70(aa1-554)E213A/D214A mutant
4155:
3970:
3545:
2714:"Hsp70 chaperones: cellular functions and molecular mechanism"
2071:"Hsp70 chaperones: cellular functions and molecular mechanism"
1017:
chaperone-mediated autophagy translocation complex disassembly
5452:
5442:
5432:
5417:
5399:
5394:
5389:
5384:
5316:
5276:
5241:
5215:
5200:
5195:
5190:
5145:
5140:
5095:
5090:
5085:
5055:
5040:
4937:
4906:
4887:
4877:
4872:
4857:
4852:
4832:
4728:
4721:
4717:
4707:
4702:
3841:
3464:
2553:
2551:
2549:
2455:
Diehl JA, Yang W, Rimerman RA, Xiao H, Emili A (March 2003).
2304:
Zhang P, Leu JI, Murphy ME, George DL, Marmorstein R (2014).
1936:
1894:
1841:
1837:
1833:
1616:
4356:: 70KD HEAT SHOCK COGNATE PROTEIN ATPASE DOMAIN, K71E MUTANT
4341:: 70KD HEAT SHOCK COGNATE PROTEIN ATPASE DOMAIN, K71A MUTANT
4326:: 70KD HEAT SHOCK COGNATE PROTEIN ATPASE DOMAIN, K71M MUTANT
2208:
5467:
5447:
5427:
5422:
5372:
5355:
5311:
5299:
5281:
5271:
5070:
5060:
5035:
5030:
4975:
4970:
3546:
DeLuca-Flaherty C, McKay DB, Parham P, Hill BL (Sep 1990).
2557:
1918:
1888:
1882:
1876:
1870:
1849:
1544:
3748:
2546:
4551:: E175S MUTANT OF BOVINE 70 KILODALTON HEAT SHOCK PROTEIN
4536:: D206S MUTANT OF BOVINE 70 KILODALTON HEAT SHOCK PROTEIN
4521:: D199S MUTANT OF BOVINE 70 KILODALTON HEAT SHOCK PROTEIN
3906:
2845:
2760:
1712:
1643:
under normal conditions, thus conferring cytoprotection.
4266:: T13S MUTANT OF BOVINE 70 KILODALTON HEAT SHOCK PROTEIN
4093:
4034:
3273:
3038:
1092:
negative regulation of supramolecular fiber organization
443:
3327:
3180:
3131:
2943:"BAG-1 modulates the chaperone activity of Hsp70/Hsc70"
2503:
1122:
positive regulation by host of viral genome replication
3876:
2609:
Soss SE, Rose KL, Hill S, Jouan S, Chazin WJ (2015).
2454:
1568:. It has been associated with an extensive number of
1002:
positive regulation of mRNA splicing, via spliceosome
608:
4596:: T13G MUTANT OF THE ATPASE FRAGMENT OF BOVINE HSC70
3502:
3368:
2303:
4064:
4038:
3880:
3815:
1077:
negative regulation of transcription, DNA-templated
4666:
3999:
3275:network: a resource for annotating the proteome".
1978:
1976:
1974:
1957:
1955:
1953:
3660:
3588:
2608:
1848:), and the Bcl2-associated athanogene 1 protein (
1087:regulation of protein-containing complex assembly
390:
289:
5491:
2659:
2405:
3812:
3127:
3125:
2899:
2401:
2399:
2397:
2361:
2359:
2299:
2297:
2251:
2165:
2126:
2124:
2122:
2120:
2118:
2116:
2114:
2049:"Entrez Gene: HSPA8 heat shock 70kDa protein 8"
1971:
1950:
16:Protein-coding gene in the species Homo sapiens
3941:
2895:
2893:
2891:
2761:Henstridge DC, Whitham M, Febbraio MA (2014).
1983:GRCm38: Ensembl release 89: ENSMUSG00000015656
1631:, where it participates in chaperone-mediated
1027:chaperone cofactor-dependent protein refolding
4652:
4171:
2803:
2707:
2705:
2497:
2130:
1745:processes. It plays an important role during
3703:
3362:
3321:
3267:
3215:
3174:
3122:
3081:
3032:
2983:
2754:
2394:
2356:
2294:
2245:
2111:
2064:
2062:
2060:
2058:
1677:
3617:
2934:
2888:
2365:
2202:
1962:GRCh38: Ensembl release 89: ENSG00000109971
4659:
4645:
4178:
4164:
2711:
2702:
2660:Kerr JF, Wyllie AH, Currie AR (Aug 1972).
2068:
1707:) or during developments and processes in
987:regulation of transcription, DNA-templated
4139:at the U.S. National Library of Medicine
4111:
4017:
4000:Gao B, Eisenberg E, Greene L (Jul 1996).
3924:
3859:
3766:
3686:
3643:
3563:
3520:
3439:
3394:
3345:
3296:
3198:
3157:
3105:
3064:
3015:
2966:
2917:
2871:
2786:
2737:
2685:
2636:
2626:
2585:
2575:
2529:
2480:
2431:
2339:
2329:
2277:
2094:
2055:
2989:
2043:
2041:
2039:
2037:
2035:
2033:
2031:
1690:
1592:protein substrate-binding domain and an
2900:Takayama S, Xie Z, Reed JC (Jan 1999).
1844:), the hsc70-hsp90 organizing protein (
1067:regulation of cellular response to heat
1037:regulation of protein complex stability
5492:
745:protein-macromolecule adaptor activity
4640:
4159:
3661:Dworniczak B, Mirault ME (Jul 1987).
3421:
2159:
2028:
1836:), the heat shock protein of 90 kDa (
695:C3HC4-type RING finger domain binding
395:
356:
351:
294:
253:
248:
2718:Cellular and Molecular Life Sciences
2075:Cellular and Molecular Life Sciences
705:MHC class II protein complex binding
4100:The Journal of Biological Chemistry
4006:The Journal of Biological Chemistry
3848:The Journal of Biological Chemistry
3755:The Journal of Biological Chemistry
3187:The Journal of Biological Chemistry
3094:The Journal of Biological Chemistry
2906:The Journal of Biological Chemistry
1650:Another function of Hsc70 is as an
1147:cytokine-mediated signaling pathway
1117:modulation by host of viral process
13:
5475:Prokaryotic ubiquitin-like protein
3414:
1840:), the hsc70-interacting protein (
1666:from coated vesicles. In neurons,
871:lumenal side of lysosomal membrane
836:intracellular anatomical structure
765:clathrin-uncoating ATPase activity
750:protein folding chaperone activity
715:G protein-coupled receptor binding
14:
5521:
4130:
1540:that in humans is encoded by the
1526:heat shock cognate 71 kDa protein
4616:
4601:
4586:
4571:
4556:
4541:
4526:
4511:
4496:
4481:
4466:
4451:
4436:
4421:
4406:
4391:
4376:
4361:
4346:
4331:
4316:
4301:
4286:
4271:
4256:
4241:
4226:
4211:
4196:
3618:Welch WJ, Mizzen LA (Apr 1988).
725:ubiquitin protein ligase binding
631:
624:
617:
379:
372:
366:
343:
278:
271:
265:
240:
29:
4185:
3913:The Journal of General Virology
3008:10.1128/MCB.22.8.2536-2543.2002
2839:
2653:
2602:
2473:10.1128/mcb.23.5.1764-1774.2003
2448:
1827:
1007:cellular response to starvation
992:regulation of protein stability
5005:Mitochondrial targeting signal
4668:Posttranslational modification
3706:Journal of Molecular Evolution
3375:Molecular and Cellular Biology
3298:11858/00-001M-0000-0010-8592-0
2996:Molecular and Cellular Biology
2712:Mayer MP, Bukau B (Mar 2005).
2461:Molecular and Cellular Biology
2069:Mayer MP, Bukau B (Mar 2005).
2010:
1992:
977:mRNA splicing, via spliceosome
642:More reference expression data
597:More reference expression data
493:right hemisphere of cerebellum
1:
3347:10.1016/S1097-2765(02)00583-X
2424:10.1080/15548627.2015.1075688
1944:
1855:HSPA8 has also been shown to
982:late endosomal microautophagy
364:
263:
5500:Genes on human chromosome 11
5081:Ubiquitin-conjugating enzyme
3956:10.1016/0378-1119(94)90802-8
3603:10.1016/0042-6822(90)90195-W
3565:10.1016/0092-8674(90)90263-E
2990:Miki K, Eddy EM (Apr 2002).
2864:10.1016/j.celrep.2014.09.042
2779:10.1016/j.molmet.2014.08.003
2628:10.1371/journal.pone.0128240
2380:10.1016/j.biocel.2004.02.013
2331:10.1371/journal.pone.0103518
1780:, which led to its use as a
1672:chaperone-mediated autophagy
1583:
1097:regulation of protein import
1047:response to unfolded protein
1022:transcription, DNA-templated
997:regulation of mRNA stability
972:chaperone-mediated autophagy
911:ficolin-1-rich granule lumen
7:
5369:E2 SUMO-conjugating enzyme
5026:Ubiquitin-activating enzyme
3624:The Journal of Cell Biology
3509:Cell Structure and Function
1615:The heat shock protein 70 (
1610:
1560:, protein homeostasis, and
1522:Heat shock 70 kDa protein 8
10:
5526:
5352:E1 SUMO-activating enzyme
4067:Experimental Cell Research
3926:10.1099/0022-1317-75-1-193
3289:10.1016/j.cell.2005.08.029
2368:Int. J. Biochem. Cell Biol
1574:neurodegenerative diseases
1463:Chr 11: 123.06 – 123.06 Mb
1142:vesicle-mediated transport
1082:neurotransmitter secretion
690:phosphatidylserine binding
680:heat shock protein binding
5410:
5341:
5330:
5013:
4990:
4948:
4688:
4674:
4191:
2825:10.1016/j.lfs.2014.02.006
2730:10.1007/s00018-004-4464-6
2666:British Journal of Cancer
2180:10.1016/j.arr.2016.07.001
2145:10.1016/j.cca.2012.12.017
2087:10.1007/s00018-004-4464-6
2018:"Mouse PubMed Reference:"
2000:"Human PubMed Reference:"
1678:Hsc70 vs Hsp70 comparison
1508:
1503:
1499:
1492:
1476:
1457:
1438:
1434:
1413:
1409:
1402:
1383:
1379:
1358:
1354:
1347:
1334:
1330:
1315:
1311:
1302:
1289:
1285:
1270:
1266:
1257:
1244:
1240:
1225:
1221:
1212:
1197:
1190:
1186:
1170:
1157:Unfolded Protein Response
1152:cellular response to heat
1052:clathrin coat disassembly
956:ribonucleoprotein complex
760:misfolded protein binding
660:
656:
639:
616:
607:
594:
543:
534:
481:
472:
442:
434:
430:
413:
400:
363:
342:
333:
329:
312:
299:
262:
239:
230:
226:
181:
178:
168:
161:
156:
73:
68:
51:
46:
41:
37:
28:
23:
4966:Survival of motor neuron
4141:Medical Subject Headings
4113:10.1074/jbc.271.48.30922
4019:10.1074/jbc.271.28.16792
3861:10.1074/jbc.270.38.22571
3768:10.1074/jbc.270.42.24732
3480:10.1002/elps.11501301199
3057:10.1093/emboj/19.23.6569
2959:10.1093/emboj/16.16.4887
1762:hepatocellular carcinoma
1723:, thereby preventing an
1112:neutrophil degranulation
1032:regulation of cell cycle
811:ubiquitin ligase complex
685:unfolded protein binding
5332:Ubiquitin-like proteins
5291:Deubiquitinating enzyme
3107:10.1074/jbc.275.12.8825
2577:10.1073/pnas.0507903103
2168:Ageing Research Reviews
1822:spinocerebellar ataxias
1470:Chr 9: 40.71 – 40.72 Mb
1137:axo-dendritic transport
906:secretory granule lumen
4079:10.1006/excr.1996.0302
4050:10.1006/bbrc.1996.1082
3985:10.1006/geno.1995.1242
3892:10.1006/bbrc.1995.1078
3827:10.1006/bbrc.1995.2090
3679:10.1093/nar/15.13.5181
3667:Nucleic Acids Research
3636:10.1083/jcb.106.4.1117
3200:10.1074/jbc.M103683200
1654:in the disassembly of
489:superior frontal gyrus
397:9 A5.1|9 21.55 cM
3441:10.1038/sj.cr.7290247
3422:Kiang JG (Dec 2004).
3387:10.1128/mcb.19.6.4535
2919:10.1074/jbc.274.2.781
2270:10.3892/mmr.2014.2939
1793:resistance to therapy
1691:Clinical significance
1601:allosteric regulation
1162:slow axonal transport
1127:membrane organization
1042:ATP metabolic process
876:extracellular exosome
521:primary visual cortex
256:Chromosome 11 (human)
5510:Molecular chaperones
1818:Huntington's disease
946:postsynaptic cytosol
896:extracellular region
891:extracellular matrix
866:spliceosomal complex
358:Chromosome 9 (mouse)
69:List of PDB id codes
42:Available structures
5505:Heat shock proteins
4690:Heat shock proteins
3798:10.1021/bi00046a037
3718:1994JMolE..39...80R
3245:10.1038/nature04209
3237:2005Natur.437.1173R
2678:10.1038/bjc.1972.33
2322:2014PLoSO...9j3518Z
2223:10.1038/ncb0901-839
1814:Parkinson's disease
1810:Alzheimer's disease
1550:signal transduction
1107:protein methylation
941:presynaptic cytosol
901:extracellular space
796:blood microparticle
559:ganglionic eminence
513:ganglionic eminence
3726:10.1007/BF00178252
3138:Scientific Reports
2510:Scientific Reports
1538:heat shock protein
1292:ENSMUSG00000015656
965:Biological process
851:lysosomal membrane
774:Cellular component
675:nucleotide binding
668:Molecular function
579:hippocampus proper
501:right frontal lobe
5487:
5486:
5483:
5482:
4992:Protein targeting
4986:
4985:
4634:
4633:
3522:10.1247/csf.17.77
3150:10.1038/srep02719
2522:10.1038/srep02719
1687:and degradation.
1519:
1518:
1515:
1514:
1488:
1487:
1453:
1452:
1428:
1427:
1398:
1397:
1373:
1372:
1343:
1342:
1324:
1323:
1298:
1297:
1279:
1278:
1253:
1252:
1234:
1233:
1182:
1181:
1062:protein refolding
951:chaperone complex
755:chaperone binding
652:
651:
648:
647:
603:
602:
590:
589:
528:
527:
497:prefrontal cortex
426:
425:
325:
324:
220:HSPA8 - orthologs
152:
151:
148:
147:
52:Ortholog search:
5517:
5339:
5338:
5252:Ubiquitin ligase
5018:(ubiquitylation)
4956:Alpha crystallin
4686:
4685:
4661:
4654:
4647:
4638:
4637:
4620:
4605:
4590:
4575:
4560:
4545:
4530:
4515:
4500:
4485:
4470:
4455:
4440:
4425:
4410:
4395:
4380:
4365:
4350:
4335:
4320:
4305:
4290:
4275:
4260:
4245:
4230:
4215:
4200:
4180:
4173:
4166:
4157:
4156:
4125:
4115:
4090:
4061:
4031:
4021:
3996:
3967:
3938:
3928:
3903:
3873:
3863:
3838:
3809:
3792:(46): 15282–90.
3780:
3770:
3745:
3700:
3690:
3657:
3647:
3614:
3585:
3567:
3542:
3524:
3499:
3461:
3443:
3409:
3408:
3398:
3366:
3360:
3359:
3349:
3325:
3319:
3318:
3300:
3271:
3265:
3264:
3231:(7062): 1173–8.
3219:
3213:
3212:
3202:
3193:(52): 49034–42.
3178:
3172:
3171:
3161:
3129:
3120:
3119:
3109:
3085:
3079:
3078:
3068:
3045:The EMBO Journal
3036:
3030:
3029:
3019:
2987:
2981:
2980:
2970:
2947:The EMBO Journal
2938:
2932:
2931:
2921:
2897:
2886:
2885:
2875:
2858:(3): 1135–1150.
2843:
2837:
2836:
2807:
2801:
2800:
2790:
2758:
2752:
2751:
2741:
2709:
2700:
2699:
2689:
2657:
2651:
2650:
2640:
2630:
2606:
2600:
2599:
2589:
2579:
2555:
2544:
2543:
2533:
2501:
2495:
2494:
2484:
2452:
2446:
2445:
2435:
2418:(9): 1623–1635.
2403:
2392:
2391:
2363:
2354:
2353:
2343:
2333:
2301:
2292:
2291:
2281:
2249:
2243:
2242:
2206:
2200:
2199:
2163:
2157:
2156:
2128:
2109:
2108:
2098:
2066:
2053:
2052:
2045:
2026:
2025:
2014:
2008:
2007:
1996:
1990:
1980:
1969:
1959:
1501:
1500:
1472:
1465:
1448:
1432:
1431:
1423:
1407:
1406:
1403:RefSeq (protein)
1393:
1377:
1376:
1368:
1352:
1351:
1328:
1327:
1309:
1308:
1283:
1282:
1264:
1263:
1238:
1237:
1219:
1218:
1188:
1187:
740:cadherin binding
658:
657:
644:
635:
628:
621:
614:
613:
599:
555:ventricular zone
551:genital tubercle
539:
537:Top expressed in
532:
531:
505:ventricular zone
477:
475:Top expressed in
470:
469:
449:
448:
432:
431:
422:
409:
398:
383:
376:
370:
359:
347:
331:
330:
321:
308:
297:
282:
275:
269:
258:
244:
228:
227:
222:
173:
166:
143:
66:
65:
60:
39:
38:
33:
21:
20:
5525:
5524:
5520:
5519:
5518:
5516:
5515:
5514:
5490:
5489:
5488:
5479:
5406:
5381:E3 SUMO ligase
5345:
5334:
5326:
5017:
5009:
4982:
4944:
4923:
4915:
4693:
4681:protein folding
4679:
4670:
4665:
4635:
4630:
4627:
4621:
4612:
4606:
4597:
4591:
4582:
4576:
4567:
4561:
4552:
4546:
4537:
4531:
4522:
4516:
4507:
4501:
4492:
4486:
4477:
4471:
4462:
4456:
4447:
4441:
4432:
4426:
4417:
4411:
4402:
4396:
4387:
4381:
4372:
4366:
4357:
4351:
4342:
4336:
4327:
4321:
4312:
4306:
4297:
4291:
4282:
4276:
4267:
4261:
4252:
4246:
4237:
4231:
4222:
4216:
4207:
4201:
4187:
4184:
4153:
4133:
4128:
4106:(48): 30922–8.
4012:(28): 16792–7.
3854:(38): 22571–6.
3761:(42): 24732–9.
3673:(13): 5181–97.
3468:Electrophoresis
3417:
3415:Further reading
3412:
3367:
3363:
3326:
3322:
3272:
3268:
3220:
3216:
3179:
3175:
3130:
3123:
3100:(12): 8825–34.
3086:
3082:
3051:(23): 6569–81.
3037:
3033:
2988:
2984:
2953:(16): 4887–96.
2939:
2935:
2898:
2889:
2844:
2840:
2808:
2804:
2759:
2755:
2710:
2703:
2658:
2654:
2621:(5): e0128240.
2607:
2603:
2570:(15): 5817–22.
2556:
2547:
2502:
2498:
2453:
2449:
2404:
2395:
2374:(12): 2435–44.
2364:
2357:
2302:
2295:
2250:
2246:
2207:
2203:
2164:
2160:
2129:
2112:
2067:
2056:
2047:
2046:
2029:
2016:
2015:
2011:
1998:
1997:
1993:
1981:
1972:
1960:
1951:
1947:
1942:
1830:
1766:gastric cancers
1693:
1680:
1621:protein folding
1613:
1586:
1566:differentiation
1510:View/Edit Mouse
1505:View/Edit Human
1468:
1461:
1458:Location (UCSC)
1444:
1440:
1419:
1415:
1389:
1385:
1364:
1360:
1273:ENSG00000109971
1166:
1057:protein folding
1012:mRNA processing
960:
936:terminal bouton
856:lysosomal lumen
826:plasma membrane
769:
730:ATPase activity
700:protein binding
640:
630:
629:
623:
622:
595:
586:
583:proximal tubule
581:
577:
573:
569:
565:
561:
557:
553:
549:
535:
524:
519:
515:
511:
507:
503:
499:
495:
491:
487:
485:corpus callosum
473:
417:
404:
396:
386:
385:
384:
377:
357:
334:Gene location (
316:
303:
295:
285:
284:
283:
276:
254:
231:Gene location (
182:
169:
162:
75:
53:
17:
12:
11:
5:
5523:
5513:
5512:
5507:
5502:
5485:
5484:
5481:
5480:
5478:
5477:
5471:
5470:
5465:
5460:
5455:
5450:
5445:
5440:
5430:
5425:
5420:
5414:
5412:
5408:
5407:
5405:
5404:
5403:
5402:
5397:
5392:
5387:
5378:
5377:
5376:
5375:
5366:
5365:
5364:
5363:
5358:
5349:
5347:
5336:
5328:
5327:
5325:
5324:
5319:
5314:
5308:
5307:
5302:
5297:
5287:
5286:
5285:
5284:
5279:
5274:
5269:
5264:
5259:
5247:
5246:
5245:
5244:
5239:
5234:
5229:
5224:
5219:
5213:
5208:
5203:
5198:
5193:
5188:
5183:
5178:
5173:
5168:
5163:
5158:
5153:
5148:
5143:
5138:
5133:
5128:
5123:
5118:
5113:
5108:
5103:
5098:
5093:
5088:
5076:
5075:
5074:
5073:
5068:
5063:
5058:
5053:
5048:
5043:
5038:
5033:
5021:
5019:
5011:
5010:
5008:
5007:
5002:
5000:Signal peptide
4996:
4994:
4988:
4987:
4984:
4983:
4981:
4980:
4979:
4978:
4973:
4963:
4958:
4952:
4950:
4946:
4945:
4943:
4942:
4941:
4940:
4935:
4930:
4925:
4921:
4917:
4913:
4903:
4902:
4901:
4900:
4895:
4890:
4885:
4880:
4875:
4870:
4865:
4860:
4855:
4850:
4845:
4840:
4829:
4828:
4827:
4826:
4821:
4816:
4811:
4806:
4801:
4796:
4791:
4786:
4781:
4776:
4771:
4766:
4761:
4756:
4751:
4746:
4741:
4736:
4725:
4724:
4715:
4710:
4705:
4699:
4697:
4683:
4672:
4671:
4664:
4663:
4656:
4649:
4641:
4632:
4631:
4629:
4628:
4622:
4615:
4613:
4607:
4600:
4598:
4592:
4585:
4583:
4577:
4570:
4568:
4562:
4555:
4553:
4547:
4540:
4538:
4532:
4525:
4523:
4517:
4510:
4508:
4502:
4495:
4493:
4487:
4480:
4478:
4472:
4465:
4463:
4457:
4450:
4448:
4442:
4435:
4433:
4427:
4420:
4418:
4412:
4405:
4403:
4397:
4390:
4388:
4382:
4375:
4373:
4367:
4360:
4358:
4352:
4345:
4343:
4337:
4330:
4328:
4322:
4315:
4313:
4307:
4300:
4298:
4292:
4285:
4283:
4277:
4270:
4268:
4262:
4255:
4253:
4247:
4240:
4238:
4232:
4225:
4223:
4217:
4210:
4208:
4202:
4195:
4192:
4189:
4188:
4183:
4182:
4175:
4168:
4160:
4151:
4150:
4144:
4132:
4131:External links
4129:
4127:
4126:
4091:
4062:
4032:
3997:
3968:
3950:(1–2): 171–4.
3939:
3904:
3874:
3839:
3810:
3781:
3746:
3701:
3658:
3630:(4): 1117–30.
3615:
3586:
3543:
3500:
3462:
3418:
3416:
3413:
3411:
3410:
3381:(6): 4535–45.
3361:
3334:Molecular Cell
3320:
3266:
3214:
3173:
3121:
3080:
3031:
3002:(8): 2536–43.
2982:
2933:
2887:
2838:
2802:
2753:
2701:
2652:
2601:
2545:
2496:
2467:(5): 1764–74.
2447:
2393:
2355:
2316:(7): e103518.
2293:
2244:
2211:Nat. Cell Biol
2201:
2158:
2110:
2081:(6): 670–684.
2054:
2027:
2009:
1991:
1970:
1948:
1946:
1943:
1941:
1940:
1934:
1928:
1922:
1916:
1910:
1904:
1898:
1892:
1886:
1880:
1874:
1868:
1861:
1829:
1826:
1774:breast cancers
1692:
1689:
1685:ubiquitylation
1679:
1676:
1612:
1609:
1585:
1582:
1524:also known as
1517:
1516:
1513:
1512:
1507:
1497:
1496:
1490:
1489:
1486:
1485:
1483:
1481:
1474:
1473:
1466:
1459:
1455:
1454:
1451:
1450:
1436:
1435:
1429:
1426:
1425:
1411:
1410:
1404:
1400:
1399:
1396:
1395:
1381:
1380:
1374:
1371:
1370:
1356:
1355:
1349:
1345:
1344:
1341:
1340:
1332:
1331:
1325:
1322:
1321:
1313:
1312:
1306:
1300:
1299:
1296:
1295:
1287:
1286:
1280:
1277:
1276:
1268:
1267:
1261:
1255:
1254:
1251:
1250:
1242:
1241:
1235:
1232:
1231:
1223:
1222:
1216:
1210:
1209:
1204:
1199:
1195:
1194:
1184:
1183:
1180:
1179:
1168:
1167:
1165:
1164:
1159:
1154:
1149:
1144:
1139:
1134:
1129:
1124:
1119:
1114:
1109:
1104:
1099:
1094:
1089:
1084:
1079:
1074:
1069:
1064:
1059:
1054:
1049:
1044:
1039:
1034:
1029:
1024:
1019:
1014:
1009:
1004:
999:
994:
989:
984:
979:
974:
968:
966:
962:
961:
959:
958:
953:
948:
943:
938:
933:
928:
923:
918:
913:
908:
903:
898:
893:
888:
883:
878:
873:
868:
863:
858:
853:
848:
843:
838:
833:
828:
823:
818:
813:
808:
806:focal adhesion
803:
798:
793:
788:
783:
777:
775:
771:
770:
768:
767:
762:
757:
752:
747:
742:
737:
732:
727:
722:
717:
712:
710:enzyme binding
707:
702:
697:
692:
687:
682:
677:
671:
669:
665:
664:
654:
653:
650:
649:
646:
645:
637:
636:
611:
605:
604:
601:
600:
592:
591:
588:
587:
585:
584:
580:
576:
572:
568:
564:
560:
556:
552:
548:
547:tail of embryo
544:
541:
540:
529:
526:
525:
523:
522:
518:
514:
510:
506:
502:
498:
494:
490:
486:
482:
479:
478:
466:
465:
457:
446:
440:
439:
436:RNA expression
428:
427:
424:
423:
415:
411:
410:
402:
399:
394:
388:
387:
378:
371:
365:
361:
360:
355:
349:
348:
340:
339:
327:
326:
323:
322:
314:
310:
309:
301:
298:
293:
287:
286:
277:
270:
264:
260:
259:
252:
246:
245:
237:
236:
224:
223:
180:
176:
175:
167:
159:
158:
154:
153:
150:
149:
146:
145:
71:
70:
62:
61:
50:
44:
43:
35:
34:
26:
25:
15:
9:
6:
4:
3:
2:
5522:
5511:
5508:
5506:
5503:
5501:
5498:
5497:
5495:
5476:
5473:
5472:
5469:
5466:
5464:
5461:
5459:
5456:
5454:
5451:
5449:
5446:
5444:
5441:
5438:
5434:
5431:
5429:
5426:
5424:
5421:
5419:
5416:
5415:
5413:
5409:
5401:
5398:
5396:
5393:
5391:
5388:
5386:
5383:
5382:
5380:
5379:
5374:
5371:
5370:
5368:
5367:
5362:
5359:
5357:
5354:
5353:
5351:
5350:
5348:
5346:(SUMOylation)
5344:
5340:
5337:
5333:
5329:
5323:
5320:
5318:
5315:
5313:
5310:
5309:
5306:
5303:
5301:
5298:
5296:
5292:
5289:
5288:
5283:
5280:
5278:
5275:
5273:
5270:
5268:
5265:
5263:
5260:
5258:
5255:
5254:
5253:
5249:
5248:
5243:
5240:
5238:
5235:
5233:
5230:
5228:
5225:
5223:
5220:
5217:
5214:
5212:
5209:
5207:
5204:
5202:
5199:
5197:
5194:
5192:
5189:
5187:
5184:
5182:
5179:
5177:
5174:
5172:
5169:
5167:
5164:
5162:
5159:
5157:
5154:
5152:
5149:
5147:
5144:
5142:
5139:
5137:
5134:
5132:
5129:
5127:
5124:
5122:
5119:
5117:
5114:
5112:
5109:
5107:
5104:
5102:
5099:
5097:
5094:
5092:
5089:
5087:
5084:
5083:
5082:
5078:
5077:
5072:
5069:
5067:
5064:
5062:
5059:
5057:
5054:
5052:
5049:
5047:
5044:
5042:
5039:
5037:
5034:
5032:
5029:
5028:
5027:
5023:
5022:
5020:
5016:
5012:
5006:
5003:
5001:
4998:
4997:
4995:
4993:
4989:
4977:
4974:
4972:
4969:
4968:
4967:
4964:
4962:
4959:
4957:
4954:
4953:
4951:
4947:
4939:
4936:
4934:
4931:
4929:
4926:
4924:
4918:
4916:
4910:
4909:
4908:
4905:
4904:
4899:
4896:
4894:
4891:
4889:
4886:
4884:
4881:
4879:
4876:
4874:
4871:
4869:
4866:
4864:
4861:
4859:
4856:
4854:
4851:
4849:
4846:
4844:
4841:
4839:
4836:
4835:
4834:
4831:
4830:
4825:
4822:
4820:
4817:
4815:
4812:
4810:
4807:
4805:
4802:
4800:
4797:
4795:
4792:
4790:
4787:
4785:
4782:
4780:
4777:
4775:
4772:
4770:
4767:
4765:
4762:
4760:
4757:
4755:
4752:
4750:
4747:
4745:
4742:
4740:
4737:
4735:
4732:
4731:
4730:
4727:
4726:
4723:
4719:
4716:
4714:
4711:
4709:
4706:
4704:
4701:
4700:
4698:
4696:
4691:
4687:
4684:
4682:
4677:
4673:
4669:
4662:
4657:
4655:
4650:
4648:
4643:
4642:
4639:
4625:
4619:
4614:
4610:
4604:
4599:
4595:
4589:
4584:
4580:
4574:
4569:
4565:
4559:
4554:
4550:
4544:
4539:
4535:
4529:
4524:
4520:
4514:
4509:
4505:
4499:
4494:
4490:
4484:
4479:
4475:
4469:
4464:
4460:
4454:
4449:
4445:
4439:
4434:
4430:
4424:
4419:
4415:
4409:
4404:
4400:
4394:
4389:
4385:
4379:
4374:
4370:
4364:
4359:
4355:
4349:
4344:
4340:
4334:
4329:
4325:
4319:
4314:
4310:
4304:
4299:
4295:
4289:
4284:
4280:
4274:
4269:
4265:
4259:
4254:
4250:
4244:
4239:
4235:
4229:
4224:
4220:
4214:
4209:
4205:
4199:
4194:
4193:
4190:
4181:
4176:
4174:
4169:
4167:
4162:
4161:
4158:
4154:
4148:
4145:
4142:
4138:
4137:Hsc70+Protein
4135:
4134:
4123:
4119:
4114:
4109:
4105:
4101:
4097:
4092:
4088:
4084:
4080:
4076:
4072:
4068:
4063:
4059:
4055:
4051:
4047:
4044:(3): 666–74.
4043:
4039:
4033:
4029:
4025:
4020:
4015:
4011:
4007:
4003:
3998:
3994:
3990:
3986:
3982:
3978:
3974:
3969:
3965:
3961:
3957:
3953:
3949:
3945:
3940:
3936:
3932:
3927:
3922:
3918:
3914:
3910:
3905:
3901:
3897:
3893:
3889:
3886:(2): 548–55.
3885:
3881:
3875:
3871:
3867:
3862:
3857:
3853:
3849:
3845:
3840:
3836:
3832:
3828:
3824:
3820:
3816:
3811:
3807:
3803:
3799:
3795:
3791:
3787:
3782:
3778:
3774:
3769:
3764:
3760:
3756:
3752:
3747:
3743:
3739:
3735:
3731:
3727:
3723:
3719:
3715:
3711:
3707:
3702:
3698:
3694:
3689:
3684:
3680:
3676:
3672:
3668:
3664:
3659:
3655:
3651:
3646:
3641:
3637:
3633:
3629:
3625:
3621:
3616:
3612:
3608:
3604:
3600:
3597:(1): 149–60.
3596:
3592:
3587:
3583:
3579:
3575:
3571:
3566:
3561:
3558:(5): 875–87.
3557:
3553:
3549:
3544:
3540:
3536:
3532:
3528:
3523:
3518:
3514:
3510:
3506:
3501:
3497:
3493:
3489:
3485:
3481:
3477:
3474:(12): 960–9.
3473:
3469:
3463:
3459:
3455:
3451:
3447:
3442:
3437:
3433:
3429:
3428:Cell Research
3425:
3420:
3419:
3406:
3402:
3397:
3392:
3388:
3384:
3380:
3376:
3372:
3365:
3357:
3353:
3348:
3343:
3339:
3335:
3331:
3324:
3316:
3312:
3308:
3304:
3299:
3294:
3290:
3286:
3283:(6): 957–68.
3282:
3278:
3270:
3262:
3258:
3254:
3250:
3246:
3242:
3238:
3234:
3230:
3226:
3218:
3210:
3206:
3201:
3196:
3192:
3188:
3184:
3177:
3169:
3165:
3160:
3155:
3151:
3147:
3143:
3139:
3135:
3128:
3126:
3117:
3113:
3108:
3103:
3099:
3095:
3091:
3084:
3076:
3072:
3067:
3062:
3058:
3054:
3050:
3046:
3042:
3035:
3027:
3023:
3018:
3013:
3009:
3005:
3001:
2997:
2993:
2986:
2978:
2974:
2969:
2964:
2960:
2956:
2952:
2948:
2944:
2937:
2929:
2925:
2920:
2915:
2911:
2907:
2903:
2896:
2894:
2892:
2883:
2879:
2874:
2869:
2865:
2861:
2857:
2853:
2849:
2842:
2834:
2830:
2826:
2822:
2818:
2814:
2813:Life Sciences
2806:
2798:
2794:
2789:
2784:
2780:
2776:
2773:(8): 781–93.
2772:
2768:
2764:
2757:
2749:
2745:
2740:
2735:
2731:
2727:
2724:(6): 670–84.
2723:
2719:
2715:
2708:
2706:
2697:
2693:
2688:
2683:
2679:
2675:
2672:(4): 239–57.
2671:
2667:
2663:
2656:
2648:
2644:
2639:
2634:
2629:
2624:
2620:
2616:
2612:
2605:
2597:
2593:
2588:
2583:
2578:
2573:
2569:
2565:
2561:
2554:
2552:
2550:
2541:
2537:
2532:
2527:
2523:
2519:
2515:
2511:
2507:
2500:
2492:
2488:
2483:
2478:
2474:
2470:
2466:
2462:
2458:
2451:
2443:
2439:
2434:
2429:
2425:
2421:
2417:
2413:
2409:
2402:
2400:
2398:
2389:
2385:
2381:
2377:
2373:
2369:
2362:
2360:
2351:
2347:
2342:
2337:
2332:
2327:
2323:
2319:
2315:
2311:
2307:
2300:
2298:
2289:
2285:
2280:
2275:
2271:
2267:
2264:(3): 1793–9.
2263:
2259:
2255:
2248:
2240:
2236:
2232:
2228:
2224:
2220:
2217:(9): 839–43.
2216:
2212:
2205:
2197:
2193:
2189:
2185:
2181:
2177:
2173:
2169:
2162:
2154:
2150:
2146:
2142:
2138:
2134:
2127:
2125:
2123:
2121:
2119:
2117:
2115:
2106:
2102:
2097:
2092:
2088:
2084:
2080:
2076:
2072:
2065:
2063:
2061:
2059:
2050:
2044:
2042:
2040:
2038:
2036:
2034:
2032:
2023:
2019:
2013:
2005:
2001:
1995:
1988:
1984:
1979:
1977:
1975:
1967:
1963:
1958:
1956:
1954:
1949:
1938:
1935:
1932:
1929:
1926:
1923:
1920:
1917:
1914:
1911:
1908:
1905:
1902:
1899:
1896:
1893:
1890:
1887:
1884:
1881:
1878:
1875:
1872:
1869:
1866:
1863:
1862:
1860:
1858:
1853:
1851:
1847:
1843:
1839:
1835:
1825:
1823:
1819:
1815:
1811:
1807:
1803:
1798:
1794:
1790:
1786:
1783:
1779:
1775:
1771:
1770:colon cancers
1767:
1763:
1759:
1755:
1754:staurosporine
1750:
1748:
1744:
1740:
1739:physiological
1736:
1731:
1726:
1722:
1718:
1714:
1710:
1706:
1702:
1701:heart attacks
1698:
1688:
1686:
1675:
1673:
1669:
1665:
1661:
1657:
1653:
1648:
1644:
1642:
1638:
1634:
1630:
1626:
1622:
1618:
1608:
1606:
1605:co-chaperones
1602:
1598:
1595:
1591:
1581:
1580:, and aging.
1579:
1575:
1571:
1567:
1563:
1559:
1555:
1551:
1546:
1543:
1539:
1535:
1531:
1527:
1523:
1511:
1506:
1502:
1498:
1495:
1491:
1484:
1482:
1479:
1475:
1471:
1467:
1464:
1460:
1456:
1449:
1447:
1443:
1437:
1433:
1430:
1424:
1422:
1418:
1412:
1408:
1405:
1401:
1394:
1392:
1388:
1382:
1378:
1375:
1369:
1367:
1363:
1357:
1353:
1350:
1348:RefSeq (mRNA)
1346:
1339:
1338:
1333:
1329:
1326:
1320:
1319:
1314:
1310:
1307:
1305:
1301:
1294:
1293:
1288:
1284:
1281:
1275:
1274:
1269:
1265:
1262:
1260:
1256:
1249:
1248:
1243:
1239:
1236:
1230:
1229:
1224:
1220:
1217:
1215:
1211:
1208:
1205:
1203:
1200:
1196:
1193:
1189:
1185:
1178:
1174:
1169:
1163:
1160:
1158:
1155:
1153:
1150:
1148:
1145:
1143:
1140:
1138:
1135:
1133:
1130:
1128:
1125:
1123:
1120:
1118:
1115:
1113:
1110:
1108:
1105:
1103:
1100:
1098:
1095:
1093:
1090:
1088:
1085:
1083:
1080:
1078:
1075:
1073:
1072:viral process
1070:
1068:
1065:
1063:
1060:
1058:
1055:
1053:
1050:
1048:
1045:
1043:
1040:
1038:
1035:
1033:
1030:
1028:
1025:
1023:
1020:
1018:
1015:
1013:
1010:
1008:
1005:
1003:
1000:
998:
995:
993:
990:
988:
985:
983:
980:
978:
975:
973:
970:
969:
967:
964:
963:
957:
954:
952:
949:
947:
944:
942:
939:
937:
934:
932:
929:
927:
924:
922:
921:autophagosome
919:
917:
914:
912:
909:
907:
904:
902:
899:
897:
894:
892:
889:
887:
884:
882:
879:
877:
874:
872:
869:
867:
864:
862:
859:
857:
854:
852:
849:
847:
844:
842:
841:Prp19 complex
839:
837:
834:
832:
829:
827:
824:
822:
821:myelin sheath
819:
817:
814:
812:
809:
807:
804:
802:
799:
797:
794:
792:
791:late endosome
789:
787:
784:
782:
779:
778:
776:
773:
772:
766:
763:
761:
758:
756:
753:
751:
748:
746:
743:
741:
738:
736:
733:
731:
728:
726:
723:
721:
718:
716:
713:
711:
708:
706:
703:
701:
698:
696:
693:
691:
688:
686:
683:
681:
678:
676:
673:
672:
670:
667:
666:
663:
662:Gene ontology
659:
655:
643:
638:
634:
627:
620:
615:
612:
610:
606:
598:
593:
582:
578:
574:
570:
567:mesencephalon
566:
562:
558:
554:
550:
546:
545:
542:
538:
533:
530:
520:
516:
512:
508:
504:
500:
496:
492:
488:
484:
483:
480:
476:
471:
468:
467:
464:
462:
458:
456:
455:
451:
450:
447:
445:
441:
437:
433:
429:
421:
416:
412:
408:
403:
393:
389:
382:
375:
369:
362:
354:
350:
346:
341:
337:
332:
328:
320:
315:
311:
307:
302:
292:
288:
281:
274:
268:
261:
257:
251:
247:
243:
238:
234:
229:
225:
221:
217:
213:
209:
205:
201:
197:
193:
189:
185:
177:
172:
165:
160:
155:
144:
142:
138:
134:
130:
126:
122:
118:
114:
110:
106:
102:
98:
94:
90:
86:
82:
78:
72:
67:
64:
63:
59:
56:
49:
45:
40:
36:
32:
27:
22:
19:
5343:SUMO protein
4882:
4623:
4608:
4593:
4578:
4563:
4548:
4533:
4518:
4503:
4488:
4473:
4458:
4443:
4428:
4413:
4398:
4383:
4368:
4353:
4338:
4323:
4308:
4293:
4278:
4263:
4248:
4233:
4218:
4203:
4152:
4103:
4099:
4073:(1): 84–91.
4070:
4066:
4041:
4037:
4009:
4005:
3979:(1): 266–8.
3976:
3972:
3947:
3943:
3919:(1): 193–9.
3916:
3912:
3883:
3879:
3851:
3847:
3818:
3814:
3789:
3786:Biochemistry
3785:
3758:
3754:
3709:
3705:
3670:
3666:
3627:
3623:
3594:
3590:
3555:
3551:
3515:(1): 77–86.
3512:
3508:
3471:
3467:
3434:(6): 450–9.
3431:
3427:
3378:
3374:
3364:
3340:(1): 55–67.
3337:
3333:
3323:
3280:
3276:
3269:
3228:
3224:
3217:
3190:
3186:
3176:
3141:
3137:
3097:
3093:
3083:
3048:
3044:
3034:
2999:
2995:
2985:
2950:
2946:
2936:
2912:(2): 781–6.
2909:
2905:
2855:
2851:
2841:
2819:(2): 133–7.
2816:
2812:
2805:
2770:
2766:
2756:
2721:
2717:
2669:
2665:
2655:
2618:
2614:
2604:
2567:
2563:
2513:
2509:
2499:
2464:
2460:
2450:
2415:
2411:
2371:
2367:
2313:
2309:
2261:
2257:
2247:
2214:
2210:
2204:
2171:
2167:
2161:
2136:
2132:
2078:
2074:
2021:
2012:
2003:
1994:
1854:
1831:
1828:Interactions
1778:lung cancers
1751:
1743:pathological
1725:inflammatory
1694:
1681:
1668:synaptojanin
1649:
1645:
1614:
1587:
1541:
1533:
1529:
1525:
1521:
1520:
1446:NP_001351409
1439:
1414:
1391:NM_001364480
1384:
1359:
1335:
1316:
1290:
1271:
1245:
1226:
1206:
1201:
1102:RNA splicing
575:right kidney
517:right testis
459:
452:
317:123,063,230
304:123,057,489
179:External IDs
74:
18:
5437:neddylation
4703:Hsp10/GroES
4695:Chaperonins
4186:PDB gallery
3712:(1): 80–6.
2258:Mol Med Rep
1806:reperfusion
1758:doxorubicin
1697:embryologic
1562:cell growth
846:nucleoplasm
735:RNA binding
720:ATP binding
571:neural tube
418:40,721,383
405:40,712,280
157:Identifiers
5494:Categories
4729:Hsp40/DnaJ
4676:Chaperones
3821:(1): 1–6.
1989:, May 2017
1968:, May 2017
1945:References
1789:malignancy
1782:prognostic
1721:phagocytes
1662:to remove
1594:N-terminal
1590:C-terminal
1578:senescence
886:presynapse
816:melanosome
463:(ortholog)
200:HomoloGene
5015:Ubiquitin
4961:Clusterin
2767:Mol Metab
2412:Autophagy
2174:: 13–21.
1865:BBC Three
1747:embryonal
1641:BBC3/PUMA
1633:autophagy
1625:cytoplasm
1584:Structure
1558:autophagy
1554:apoptosis
1442:NP_112442
1421:NP_694881
1417:NP_006588
1387:NM_031165
1366:NM_153201
1362:NM_006597
1192:Orthologs
861:nucleolus
781:cytoplasm
208:GeneCards
5295:Ataxin 3
3973:Genomics
3742:37505045
3591:Virology
3539:21909960
3496:41855774
3458:21654486
3450:15625011
3405:10330192
3356:12150907
3307:16169070
3253:16189514
3209:11679576
3168:24056538
3144:: 2719.
3116:10722728
3075:11101529
3026:11909948
2882:25437566
2852:Cell Rep
2833:24548631
2797:25379403
2748:15770419
2647:26010904
2615:PLOS ONE
2596:16585520
2540:24056538
2516:: 2719.
2491:12588994
2442:26212789
2388:15325583
2350:25058147
2310:PLOS ONE
2288:25394481
2239:21164493
2231:11533664
2188:27484893
2153:23266770
2139:: 73–9.
2105:15770419
1985:–
1964:–
1857:interact
1802:ischemia
1735:necrosis
1664:clathrin
1656:clathrin
1629:lysosome
1611:Function
1494:Wikidata
1171:Sources:
931:dendrite
916:lysosome
801:membrane
5218:(CDC34)
4147:PDBe-KB
4122:8940078
4087:8892974
4058:8713105
4028:8663341
3993:8530083
3964:8125298
3935:7906708
3900:7826371
3870:7673249
3835:7639722
3806:7578144
3777:7559589
3734:7545947
3714:Bibcode
3697:3037489
3654:2966179
3645:2115010
3611:2155506
3582:9501568
3574:1975516
3531:1586970
3488:1286667
3315:8235923
3261:4427026
3233:Bibcode
3159:3779846
2977:9305631
2968:1170124
2928:9873016
2873:4255334
2788:4216407
2739:2773841
2696:4561027
2687:2008650
2638:4444009
2587:1458656
2531:3779846
2433:4590652
2341:4110032
2318:Bibcode
2279:4270332
2096:2773841
1987:Ensembl
1966:Ensembl
1797:vaccine
1730:mitosis
1717:nucleus
1705:strokes
1660:auxilin
1576:, cell
1570:cancers
1304:UniProt
1259:Ensembl
1198:Species
1177:QuickGO
881:nucleus
786:cytosol
438:pattern
296:11q24.1
164:Aliases
5262:Cullin
4143:(MeSH)
4120:
4085:
4056:
4026:
3991:
3962:
3933:
3898:
3868:
3833:
3804:
3775:
3740:
3732:
3695:
3688:305955
3685:
3652:
3642:
3609:
3580:
3572:
3537:
3529:
3494:
3486:
3456:
3448:
3403:
3396:104411
3393:
3354:
3313:
3305:
3259:
3251:
3225:Nature
3207:
3166:
3156:
3114:
3073:
3066:305846
3063:
3024:
3017:133739
3014:
2975:
2965:
2926:
2880:
2870:
2831:
2795:
2785:
2746:
2736:
2694:
2684:
2645:
2635:
2594:
2584:
2538:
2528:
2489:
2482:151693
2479:
2440:
2430:
2386:
2348:
2338:
2286:
2276:
2237:
2229:
2196:884595
2194:
2186:
2151:
2103:
2093:
1925:HSPBP1
1913:DNAJA3
1901:CITED1
1859:with:
1820:, and
1785:marker
1776:, and
1756:, and
1709:cancer
1652:ATPase
1480:search
1478:PubMed
1337:P63017
1318:P11142
1214:Entrez
609:BioGPS
196:105384
188:600816
5453:ATG12
5443:FAT10
5433:NEDD8
5418:ISG15
5411:Other
5400:PIAS4
5395:PIAS3
5390:PIAS2
5385:PIAS1
5335:(UBL)
5317:BIRC6
5277:FANCL
4949:Other
4938:TRAP1
4907:Hsp90
4833:Hsp70
4722:GroEL
4718:HSP60
4713:Hsp47
4708:Hsp27
3738:S2CID
3578:S2CID
3535:S2CID
3492:S2CID
3454:S2CID
3311:S2CID
3257:S2CID
2235:S2CID
2192:S2CID
1937:STUB1
1933:, and
1931:PARK2
1907:CCND1
1895:CDC5L
1838:Hsp90
1834:Hsp40
1637:lumen
1617:Hsp70
1542:HSPA8
1536:is a
1534:Hsp73
1530:Hsc70
1247:15481
1207:Mouse
1202:Human
1173:Amigo
563:ovary
509:gonad
461:Mouse
454:Human
401:Start
336:Mouse
300:Start
233:Human
212:HSPA8
204:68524
171:HSPA8
24:HSPA8
5468:UBL5
5458:FUB1
5448:ATG8
5428:UFM1
5423:URM1
5373:UBC9
5361:SAE2
5356:SAE1
5322:UFC1
5312:ATG3
5305:CYLD
5300:USP6
5282:UBR1
5272:MDM2
5071:SAE1
5066:NAE1
5061:ATG7
5056:UBA7
5051:UBA6
5046:UBA5
5041:UBA3
5036:UBA2
5031:UBA1
4976:SMN2
4971:SMN1
4624:7hsc
4609:3hsc
4594:2bup
4579:1yuw
4564:1ud0
4549:1qqo
4534:1qqn
4519:1qqm
4504:1ngj
4489:1ngi
4474:1ngh
4459:1ngg
4444:1ngf
4429:1nge
4414:1ngd
4399:1ngc
4384:1ngb
4369:1nga
4354:1kaz
4339:1kay
4324:1kax
4309:1hx1
4294:1hpm
4279:1ckr
4264:1bup
4249:1ba1
4234:1ba0
4219:1ats
4204:1atr
4118:PMID
4083:PMID
4054:PMID
4024:PMID
3989:PMID
3960:PMID
3944:Gene
3931:PMID
3896:PMID
3866:PMID
3831:PMID
3802:PMID
3773:PMID
3730:PMID
3693:PMID
3650:PMID
3607:PMID
3570:PMID
3552:Cell
3527:PMID
3484:PMID
3446:PMID
3401:PMID
3352:PMID
3303:PMID
3277:Cell
3249:PMID
3205:PMID
3164:PMID
3112:PMID
3071:PMID
3022:PMID
2973:PMID
2924:PMID
2878:PMID
2829:PMID
2793:PMID
2744:PMID
2692:PMID
2643:PMID
2592:PMID
2536:PMID
2487:PMID
2438:PMID
2384:PMID
2346:PMID
2284:PMID
2227:PMID
2184:PMID
2149:PMID
2101:PMID
1919:GJA1
1889:BAG4
1883:BAG3
1877:BAG2
1871:BAG1
1850:BAG1
1791:and
1741:and
1715:and
1703:and
1627:and
1564:and
1545:gene
1228:3312
926:axon
444:Bgee
392:Band
353:Chr.
291:Band
250:Chr.
184:OMIM
141:4KBQ
137:4H5W
133:4H5V
129:4H5T
125:4H5R
121:4H5N
117:3M3Z
113:3LDQ
109:4HWI
105:3FZM
101:3FZL
97:3FZK
93:3FZH
89:3FZF
85:3ESK
81:3AGZ
77:3AGY
58:RCSB
55:PDBe
5463:MUB
5267:CBL
5257:VHL
5250:E3
5079:E2
5024:E1
4893:12A
4824:C19
4819:C14
4814:C13
4809:C11
4804:C10
4759:B11
4108:doi
4104:271
4075:doi
4071:228
4046:doi
4042:224
4014:doi
4010:271
3981:doi
3952:doi
3948:138
3921:doi
3888:doi
3884:206
3856:doi
3852:270
3823:doi
3819:213
3794:doi
3763:doi
3759:270
3722:doi
3683:PMC
3675:doi
3640:PMC
3632:doi
3628:106
3599:doi
3595:175
3560:doi
3517:doi
3476:doi
3436:doi
3391:PMC
3383:doi
3342:doi
3293:hdl
3285:doi
3281:122
3241:doi
3229:437
3195:doi
3191:276
3154:PMC
3146:doi
3102:doi
3098:275
3061:PMC
3053:doi
3012:PMC
3004:doi
2963:PMC
2955:doi
2914:doi
2910:274
2868:PMC
2860:doi
2821:doi
2817:100
2783:PMC
2775:doi
2734:PMC
2726:doi
2682:PMC
2674:doi
2633:PMC
2623:doi
2582:PMC
2572:doi
2568:103
2526:PMC
2518:doi
2477:PMC
2469:doi
2428:PMC
2420:doi
2376:doi
2336:PMC
2326:doi
2274:PMC
2266:doi
2219:doi
2176:doi
2141:doi
2137:417
2091:PMC
2083:doi
1852:).
1846:HOP
1842:HIP
1713:DNA
1597:ATP
1532:or
1528:or
414:End
313:End
216:OMA
192:MGI
48:PDB
5496::
5293::
5237:V2
5232:V1
5222:R2
5216:R1
5211:Q2
5206:Q1
5186:L6
5181:L4
5176:L3
5171:L2
5166:L1
5156:J2
5151:J1
5136:G2
5131:G1
5126:E3
5121:E2
5116:E1
5111:D3
5106:D2
5101:D1
4933:ER
4898:14
4863:4L
4848:1L
4843:1B
4838:1A
4799:C7
4794:C6
4789:C5
4784:C3
4779:C1
4774:B9
4769:B6
4764:B4
4754:B2
4749:B1
4744:A3
4739:A2
4734:A1
4116:.
4102:.
4098:.
4081:.
4069:.
4052:.
4040:.
4022:.
4008:.
4004:.
3987:.
3977:29
3975:.
3958:.
3946:.
3929:.
3917:75
3915:.
3911:.
3894:.
3882:.
3864:.
3850:.
3846:.
3829:.
3817:.
3800:.
3790:34
3788:.
3771:.
3757:.
3753:.
3736:.
3728:.
3720:.
3710:39
3708:.
3691:.
3681:.
3671:15
3669:.
3665:.
3648:.
3638:.
3626:.
3622:.
3605:.
3593:.
3576:.
3568:.
3556:62
3554:.
3550:.
3533:.
3525:.
3513:17
3511:.
3507:.
3490:.
3482:.
3472:13
3470:.
3452:.
3444:.
3432:14
3430:.
3426:.
3399:.
3389:.
3379:19
3377:.
3373:.
3350:.
3338:10
3336:.
3332:.
3309:.
3301:.
3291:.
3279:.
3255:.
3247:.
3239:.
3227:.
3203:.
3189:.
3185:.
3162:.
3152:.
3140:.
3136:.
3124:^
3110:.
3096:.
3092:.
3069:.
3059:.
3049:19
3047:.
3043:.
3020:.
3010:.
3000:22
2998:.
2994:.
2971:.
2961:.
2951:16
2949:.
2945:.
2922:.
2908:.
2904:.
2890:^
2876:.
2866:.
2854:.
2850:.
2827:.
2815:.
2791:.
2781:.
2769:.
2765:.
2742:.
2732:.
2722:62
2720:.
2716:.
2704:^
2690:.
2680:.
2670:26
2668:.
2664:.
2641:.
2631:.
2619:10
2617:.
2613:.
2590:.
2580:.
2566:.
2562:.
2548:^
2534:.
2524:.
2512:.
2508:.
2485:.
2475:.
2465:23
2463:.
2459:.
2436:.
2426:.
2416:11
2414:.
2410:.
2396:^
2382:.
2372:36
2370:.
2358:^
2344:.
2334:.
2324:.
2312:.
2308:.
2296:^
2282:.
2272:.
2262:11
2260:.
2256:.
2233:.
2225:.
2213:.
2190:.
2182:.
2172:32
2170:.
2147:.
2135:.
2113:^
2099:.
2089:.
2079:62
2077:.
2073:.
2057:^
2030:^
2020:.
2002:.
1973:^
1952:^
1816:,
1812:,
1772:,
1768:,
1764:,
1607:.
1572:,
1556:,
1552:,
1175:/
420:bp
407:bp
319:bp
306:bp
214:;
210::
206:;
202::
198:;
194::
190:;
186::
139:,
135:,
131:,
127:,
123:,
119:,
115:,
111:,
107:,
103:,
99:,
95:,
91:,
87:,
83:,
79:,
5439:)
5435:(
5242:Z
5227:S
5201:O
5196:N
5191:M
5161:K
5146:I
5141:H
5096:C
5091:B
5086:A
4928:β
4922:2
4920:α
4914:1
4912:α
4888:9
4883:8
4878:7
4873:6
4868:5
4858:4
4853:2
4720:/
4692:/
4678:/
4660:e
4653:t
4646:v
4179:e
4172:t
4165:v
4124:.
4110::
4089:.
4077::
4060:.
4048::
4030:.
4016::
3995:.
3983::
3966:.
3954::
3937:.
3923::
3902:.
3890::
3872:.
3858::
3837:.
3825::
3808:.
3796::
3779:.
3765::
3744:.
3724::
3716::
3699:.
3677::
3656:.
3634::
3613:.
3601::
3584:.
3562::
3541:.
3519::
3498:.
3478::
3460:.
3438::
3407:.
3385::
3358:.
3344::
3317:.
3295::
3287::
3263:.
3243::
3235::
3211:.
3197::
3170:.
3148::
3142:3
3118:.
3104::
3077:.
3055::
3028:.
3006::
2979:.
2957::
2930:.
2916::
2884:.
2862::
2856:9
2835:.
2823::
2799:.
2777::
2771:3
2750:.
2728::
2698:.
2676::
2649:.
2625::
2598:.
2574::
2542:.
2520::
2514:3
2493:.
2471::
2444:.
2422::
2390:.
2378::
2352:.
2328::
2320::
2314:9
2290:.
2268::
2241:.
2221::
2215:3
2198:.
2178::
2155:.
2143::
2107:.
2085::
2051:.
2024:.
2006:.
1939:.
1927:,
1921:,
1915:,
1909:,
1903:,
1897:,
1891:,
1885:,
1879:,
1873:,
1867:,
1804:-
338:)
235:)
218::
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.