133:
81:
346:-coupling constant, the appearance of the NMR spectrum is unchanged if the sign of the coupling constant is reversed, although spectral lines at given positions may represent different transitions. The simple NMR spectrum therefore does not indicate the sign of the coupling constant, which there is no simple way of predicting.
175:
The multiplicity provides information on the number of centers coupled to the signal of interest, and their nuclear spin. For simple systems, as in H–H coupling in NMR spectroscopy, the multiplicity is one more than the number of adjacent protons which are magnetically nonequivalent to the protons of
341:
The value of each coupling constant also has a sign, and coupling constants of comparable magnitude often have opposite signs. If the coupling constant between two given spins is negative, the energy is lower when these two spins are parallel, and conversely if their coupling constant is positive.
251:
is related to the nuclear magnetic moments of the coupling partners. F, with a high nuclear magnetic moment, gives rise to large coupling to protons. Rh, with a very small nuclear magnetic moment, gives only small couplings to H. To correct for the effect of the nuclear magnetic moment (or
195:, which are called quadrupolar, can give rise to greater splitting, although in many cases coupling to quadrupolar nuclei is not observed. Many elements consist of nuclei with nuclear spin and without. In these cases, the observed spectrum is the sum of spectra for each
856:= 0.7 Hz). Such interaction came as a great surprise. The direct interaction between two magnetic dipoles depends on the relative position of two nuclei in such a way that when averaged over all possible orientations of the molecule it equals to zero.
215:
nuclei are either monoisotopic, e.g. P and F, or have very high natural abundance, e.g. H. An additional convenience is that C and O have no nuclear spin so these nuclei, which are common in organic molecules, do not cause splitting patterns in NMR.
176:
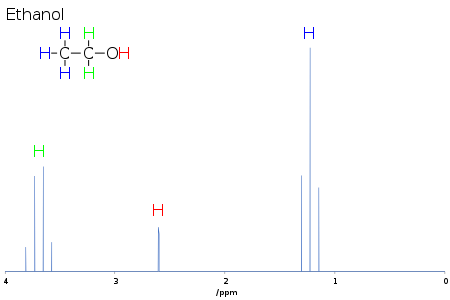
interest. For ethanol, each methyl proton is coupled to the two methylene protons, so the methyl signal is a triplet, while each methylene proton is coupled to the three methyl protons, so the methylene signal is a quartet.
786:
1611:
Blake, P. R.; Park, J.-B.; Adams, M. W. W.; Summers, M. F. (1992). "Novel observation of NH–S(Cys) hydrogen-bond-mediated scalar coupling in cadmium-113 substituted rubredoxin from
Pyrococcus furiosus".
62:-coupling provides information on the connectivity of chemical bonds. It is responsible for the often complex splitting of resonance lines in the NMR spectra of fairly simple molecules.
1160:
the sign of J may be either positive or negative. The spectrum has precisely the same appearance in either case, but lines at corresponding positions represent different transitions.
830:, a monotonic decay in the echo envelope is obtained. In the Hahn–Maxwell experiment, the decay was modulated by two frequencies: one frequency corresponded with the difference in
1642:
1285:
881:. The mechanism is the magnetic interaction between each nucleus and the electron spin of its own atom together with the exchange coupling of the electron spins with each other.
704:
1562:
Blake, P.; Lee, B.; Summers, M.; Adams, M.; Park, J.-B.; Zhou, Z.; Bax, A. (1992). "Quantitative measurement of small through-hydrogen-bond and 'through-space' H–Cd and H–Hg
739:
659:
353:-coupling constants, the relative signs of the two constants can be experimentally determined by a double resonance experiment. For example in the diethylthallium ion (C
1669:
Mallory, F. B.; et al. (2000). "Nuclear Spin−Spin
Coupling via Nonbonded Interactions. 8. 1 The Distance Dependence of Through-Space Fluorine−Fluorine Coupling".
945:
940:
236:. Generally speaking two-bond coupling (i.e. H–C–H) is stronger than three-bond coupling (H–C–C–H). The magnitude of the coupling also provides information on the
124:-coupling provides three parameters: the multiplicity (the "number of lines"), the magnitude of the coupling (strong, medium, weak), and the sign of the coupling.
907:
The spin–spin coupling between nonbonded atoms in close proximity has sometimes been observed between fluorine, nitrogen, carbon, silicon and phosphorus atoms.
1733:
Zong, J.; Mague, J. T.; Welch, E. C.; Eckert, I. M.; Pascal Jr, R. A. (2013). "Sterically congested macrobicycles with heteroatomic bridgehead functionality".
950:
589:-coupling tensor, a real 3 × 3 matrix. It depends on molecular orientation, but in an isotropic liquid it reduces to a number, the so-called
1235:
412:) ring protons was shown to be positive because the splitting of the two peaks for each proton decreases with the applied electric field.
593:. In 1D NMR, the scalar coupling leads to oscillations in the free induction decay as well as splittings of lines in the spectrum.
120: = 1, which means that a given photon (in the radio frequency range) can affect ("flip") only one of the two nuclear spins.
96:-coupling can be visualized by a vector model for a simple molecule such as hydrogen fluoride (HF). In HF, the two nuclei have spin
744:
112:. Four states are possible, depending on the relative alignment of the H and F nuclear spins with the external magnetic field. The
1096:
Pregosin, P. S.; Rueegger, H. (2004). "Nuclear magnetic resonance spectroscopy". In McCleverty, Jon A.; Thomas J., Meyer (eds.).
51:
928:
606:
1655:
1490:
Hahn, E. L.; Maxwell, D. E. (1951). "Chemical Shift and Field
Independent Frequency Modulation of the Spin Echo Envelope".
1768:
233:
922:
1640:
Dingley, Andrew J.; Cordier, Florence; Grzesiek, Stephan (2001). "An introduction to hydrogen bond scalar couplings".
1297:
1245:
1212:
1181:
1138:
1113:
1080:
1055:
1030:
1219:
The double resonance technique has been successfully employed to determine the relative sign of coupling constants.
435:
199:. One of the great conveniences of NMR spectroscopy for organic molecules is that several important lighter spin
1454:
Gutowsky, H. S.; McCall, D. W.; Slichter, C. P. (1951). "Coupling among
Nuclear Magnetic Dipoles in Molecules".
810:
are applied to the spin ensemble at the nuclear resonance condition and are separated by a time interval of
1568:
934:
409:
664:
965:
860:
389:
307:
299:
For coupling of a C nucleus and a directly bonded proton, the dominant term in the coupling constant
985:
Hahn, E. L.; Maxwell, D. E. (1952). "Spin Echo
Measurements of Nuclear Spin Coupling in Molecules".
904:-couplings follow the same electron-mediated polarization mechanism as their covalent counterparts.
709:
1269:
Effects of a strong electric field on NMR spectra. The absolute sign of the spin coupling constant
632:
1697:
Zong, J.; Mague, J. T.; Kraml, C. M.; Pascal Jr, R. A. (2013). "A Congested in, in-Diphosphine".
477:= electron orbital–orbital, spin–orbital, spin–spin and electron-spin–external-field interactions
1359:
867:
proposed a mechanism that explained the observation and gave rise to an interaction of the form
741:, where they explained the presence of multiple resonance lines with an interaction of the form
626:
1237:
Optical, electric and magnetic properties of molecules. A review of the work of A.D.Buckingham
1735:
416:
311:
1536:
1500:
1464:
1421:
1371:
995:
916:
864:
384:
and
Lovering, who suggested the use of a strong electric field to align the molecules of a
318:
58:-coupling contains information about relative bond distances and angles. Most importantly,
1234:
Burnell, Elliott (1997). "12. Anisotropic NMR". In Clary, David C.; Orr, Brian J. (eds.).
8:
1526:
Ramsey, N. F.; Purcell, E. M. (1952). "Interactions between
Nuclear Spins in Molecules".
1360:"N.M.R. studies of 3,3,3-trifluoropropyne dissolved in different nematic liquid crystals"
622:
381:
1540:
1504:
1468:
1425:
1375:
999:
171:
hydrogens are coupling with each other, resulting in a triplet and quartet respectively.
1593:
800:
72:
that is not affected by the strength of the magnetic field, so is always stated in Hz.
1715:
1671:
1614:
1585:
1293:
1241:
1208:
1177:
1134:
1109:
1105:
1076:
1051:
1026:
892:. Initially, it was surprising to observe such couplings across hydrogen bonds since
385:
232:
decreases rapidly with the number of bonds between the coupled nuclei, especially in
75:
20:
1597:
1744:
1707:
1679:
1651:
1622:
1577:
1544:
1508:
1472:
1429:
1406:"Anisotropies and Absolute Signs of the Indirect Spin–Spin Coupling Constants in CH
1379:
1333:
1321:
1101:
1003:
960:
241:
50:
that arises from hyperfine interactions between the nuclei and local electrons. In
1699:
1528:
1492:
1456:
987:
827:
602:
164:
113:
24:
822:, the maximum value of the echo signal is measured and plotted as a function of
132:
831:
807:
804:
401:
326:
237:
152:
144:
47:
1748:
1383:
1762:
1476:
897:
889:
610:
500:
415:
Another way to align molecules for NMR spectroscopy is to dissolve them in a
43:
1512:
1007:
1719:
1548:
1188:
there is no simple way of specifying whether J will be positive or negative
156:
1589:
419:
solvent. This method has also been used to determine the absolute sign of
155:
is not coupling with the other H atoms and appears as a singlet, but the
1626:
1358:
Buckingham, A. D.; Burnell, E. E.; de Lange, C. A.; Rest, A. J. (1968).
1337:
46:
connecting two spins. It is an indirect interaction between two nuclear
1581:
1272:
955:
799:
which indicates the existence of an interaction between two protons in
792:
400:-coupling if their signs are opposed. This method was first applied to
196:
1711:
1683:
1433:
396:-coupling if their signs are parallel and subtracts from the observed
329:
which are of the order of millihertz and are not normally resolvable.
1566:
couplings in metal-substituted rubredoxin from
Pyrococcus furiosus".
1405:
1656:
10.1002/1099-0534(2001)13:2<103::AID-CMR1001>3.0.CO;2-M
888:-couplings between magnetically active nuclei on both sides of the
148:
376:
The first experimental method to determine the absolute sign of a
76:
Vector model and manifestations for chemical structure assignments
1322:"Absolute Signs of Indirect Nuclear Spin-Spin Coupling Constants"
140:
838:, that was smaller and independent of magnetic field strength (
896:-couplings are usually associated with the presence of purely
486:= magnetic interactions between nuclear spin and electron spin
1357:
834:
between the two non-equivalent spins and a second frequency,
781:{\displaystyle A\mathbf {\mu } _{1}\cdot \mathbf {\mu } _{2}}
884:
In the 1990s, direct evidence was found for the presence of
609:, eliminating or selectively reducing the coupling effect.
80:
724:
692:
679:
647:
325:-coupling signals of the order of hertz usually dominate
151:
atoms in ethanol regarding NMR. The hydrogen (H) on the
946:
Nuclear magnetic resonance spectroscopy of nucleic acids
941:
Nuclear magnetic resonance spectroscopy of carbohydrates
1696:
803:. In the echo experiment, two short, intense pulses of
317:
Where the external magnetic field is very low, e.g. as
1639:
1453:
1286:"The Absolute Sign of the Spin-Spin Coupling Constant"
1271:, Transactions Faraday Society, 58, 2077-2081 (1962),
900:. However, it is now well established that the H-bond
1610:
747:
712:
667:
635:
1732:
1561:
1283:
240:
relating the coupling partners, as described by the
1153:
951:
Nuclear magnetic resonance spectroscopy of proteins
814:. The echo appears with a given amplitude at time 2
613:spectra are often recorded with proton decoupling.
605:irradiation, NMR spectra can be fully or partially
503:molecular state and frequent molecular collisions,
365:
Tl, this method showed that the methyl-thallium (CH
780:
733:
698:
653:
1284:Buckingham, A. D.; McLauchlan, K. A. (May 1963).
1760:
1095:
1154:Carrington, Alan; McLachlan, Andrew D. (1967).
1319:
1171:
1128:
495:= direct interaction of nuclei with each other
1525:
1403:
1320:Bernheim, R.A.; Lavery, B.J. (1 March 1967).
1050:(4th ed.). McGraw-Hill. pp. 223–4.
1046:Banwell, Colin N.; McCash, Elaine M. (1994).
1045:
392:of the two spins, which adds to the observed
373:-Tl) coupling constants have opposite signs.
349:However for some molecules with two distinct
247:For heteronuclear coupling, the magnitude of
88:-coupling for the molecule hydrogen fluoride.
408:-coupling constant between two adjacent (or
1489:
984:
380:-coupling constant was proposed in 1962 by
1229:
1227:
1198:
1196:
219:
426:
16:Type of coupling used in NMR spectroscopy
1447:
1326:Journal of the American Chemical Society
1176:(8th ed.). Macmillan. p. 530.
1133:(8th ed.). Macmillan. p. 528.
131:
79:
1668:
1233:
1224:
1193:
1172:Atkins, Peter; de Paula, Julio (2006).
1129:Atkins, Peter; de Paula, Julio (2006).
1098:Comprehensive Coordination Chemistry II
438:of a molecular system may be taken as:
1761:
1519:
1048:Fundamentals of Molecular Spectroscopy
517:are almost zero. The full form of the
147:. There are three different types of
84:Energy diagram showing the effects of
1207:. W.B.Saunders Company. p. 280.
1202:
1075:. W.B.Saunders Company. p. 218.
1070:
1020:
826:. If the spin ensemble consists of a
521:-coupling interaction between spins '
332:
1483:
1404:Krugh, T.R.; Bernheim, R.A. (1970).
1273:https://doi.org/10.1039/TF9625802077
1025:. W. B. Saunders. pp. 211–213.
252:equivalently the gyromagnetic ratio
1290:Proceedings of the Chemical Society
1267:Buckingham A.D. and Lovering E.G.,
256:), the "reduced coupling constant"
244:for three-bond coupling constants.
228:For H–H coupling, the magnitude of
13:
1156:Introduction to Magnetic Resonance
929:Magnetic dipole–dipole interaction
923:Exclusive correlation spectroscopy
116:of NMR spectroscopy dictate that Δ
14:
1780:
699:{\displaystyle {\ce {CH_3OPF_2}}}
1158:. Harper & Row. p. 47.
791:Independently, in October 1951,
143:plotted as signal intensity vs.
1726:
1690:
1662:
1633:
1604:
1555:
1397:
1351:
1313:
1277:
1261:
734:{\displaystyle {\ce {POCl_2F}}}
369:-Tl) and methylene-thallium (CH
314:of the bond at the two nuclei.
179:Nuclei with spins greater than
127:
40:indirect dipole–dipole coupling
1643:Concepts in Magnetic Resonance
1240:. Elsevier. pp. 327–334.
1165:
1147:
1122:
1106:10.1016/B0-08-043748-6/01061-6
1100:. Vol. 2. pp. 1–35.
1089:
1064:
1039:
1014:
978:
388:. The field produces a direct
1:
1205:Physical Methods in Chemistry
1073:Physical Methods in Chemistry
1023:Physical Methods in Chemistry
971:
795:and D. E. Maxwell reported a
654:{\displaystyle {\ce {HPF_6}}}
596:
342:For a molecule with a single
310:, which is a measure of the
7:
1414:Journal of Chemical Physics
910:
10:
1785:
1769:Nuclear magnetic resonance
1203:Drago, Russell S. (1977).
1174:Atkins' Physical Chemistry
1131:Atkins' Physical Chemistry
1071:Drago, Russell S. (1977).
1021:Drago, Russell S. (1977).
935:Nuclear magnetic resonance
616:
260:is often discussed, where
1749:10.1016/j.tet.2013.10.018
1384:10.1080/00268976800100111
966:Residual dipolar coupling
539:on the same molecule is:
308:Fermi contact interaction
68:-coupling is a frequency
1477:10.1103/PhysRev.84.589.2
629:reported experiments on
1513:10.1103/PhysRev.84.1246
1008:10.1103/PhysRev.88.1070
42:) are mediated through
1549:10.1103/PhysRev.85.143
818:. For each setting of
782:
735:
700:
655:
417:nematic liquid crystal
172:
137:Example H NMR spectrum
89:
783:
736:
701:
656:
430:-coupling Hamiltonian
423:-coupling constants.
135:
83:
801:dichloroacetaldehyde
797:spin echo experiment
745:
710:
665:
633:
625:, D. W. McCall, and
1743:(48): 10316–10321.
1627:10.1021/ja00038a084
1541:1952PhRv...85..143R
1505:1951PhRv...84.1246H
1469:1951PhRv...84..589G
1426:1970JChPh..52.4942K
1376:1968MolPh..14..105B
1338:10.1021/ja00981a052
1000:1952PhRv...88.1070H
726:
694:
681:
649:
621:In September 1951,
234:saturated molecules
139:(1-dimensional) of
1582:10.1007/BF02192814
931:(dipolar coupling)
859:In November 1951,
778:
731:
714:
696:
682:
669:
651:
637:
173:
90:
36:spin-spin coupling
1712:10.1021/ol400728m
1684:10.1021/ja993032z
1678:(17): 4108–4116.
1672:J. Am. Chem. Soc.
1621:(12): 4931–4933.
1615:J. Am. Chem. Soc.
1434:10.1063/1.1672729
1364:Molecular Physics
917:Earth's field NMR
729:
717:
685:
672:
640:
319:Earth's field NMR
21:nuclear chemistry
1776:
1753:
1752:
1730:
1724:
1723:
1706:(9): 2179–2181.
1694:
1688:
1687:
1666:
1660:
1659:
1637:
1631:
1630:
1608:
1602:
1601:
1559:
1553:
1552:
1523:
1517:
1516:
1499:(6): 1246–1247.
1487:
1481:
1480:
1451:
1445:
1444:
1442:
1440:
1401:
1395:
1394:
1392:
1390:
1355:
1349:
1348:
1346:
1344:
1332:(5): 1279–1280.
1317:
1311:
1310:
1308:
1306:
1281:
1275:
1265:
1259:
1258:
1256:
1254:
1231:
1222:
1221:
1200:
1191:
1190:
1169:
1163:
1162:
1151:
1145:
1144:
1126:
1120:
1119:
1093:
1087:
1086:
1068:
1062:
1061:
1043:
1037:
1036:
1018:
1012:
1011:
982:
961:Relaxation (NMR)
855:
853:
852:
849:
846:
787:
785:
784:
779:
777:
776:
771:
762:
761:
756:
740:
738:
737:
732:
730:
727:
725:
722:
715:
705:
703:
702:
697:
695:
693:
690:
683:
680:
677:
670:
660:
658:
657:
652:
650:
648:
645:
638:
404:, for which the
390:dipolar coupling
294:
292:
291:
279:
276:
242:Karplus equation
214:
212:
211:
208:
205:
194:
192:
191:
188:
185:
111:
109:
108:
105:
102:
52:NMR spectroscopy
1784:
1783:
1779:
1778:
1777:
1775:
1774:
1773:
1759:
1758:
1757:
1756:
1731:
1727:
1695:
1691:
1667:
1663:
1638:
1634:
1609:
1605:
1560:
1556:
1524:
1520:
1488:
1484:
1452:
1448:
1438:
1436:
1409:
1402:
1398:
1388:
1386:
1356:
1352:
1342:
1340:
1318:
1314:
1304:
1302:
1300:
1282:
1278:
1266:
1262:
1252:
1250:
1248:
1232:
1225:
1215:
1201:
1194:
1184:
1170:
1166:
1152:
1148:
1141:
1127:
1123:
1116:
1094:
1090:
1083:
1069:
1065:
1058:
1044:
1040:
1033:
1019:
1015:
983:
979:
974:
913:
880:
873:
850:
847:
842:
841:
839:
828:magnetic moment
772:
767:
766:
757:
752:
751:
746:
743:
742:
723:
718:
713:
711:
708:
707:
691:
686:
678:
673:
668:
666:
663:
662:
646:
641:
636:
634:
631:
630:
619:
603:radio frequency
599:
591:scalar coupling
584:
572:
563:
554:
537:
528:
516:
509:
494:
485:
476:
465:
458:
451:
432:
372:
368:
364:
360:
356:
339:
327:chemical shifts
305:
289:
285:
280:
277:
271:
270:
268:
238:dihedral angles
226:
209:
206:
203:
202:
200:
189:
186:
183:
182:
180:
168:
160:
130:
114:selection rules
106:
103:
100:
99:
97:
78:
25:nuclear physics
17:
12:
11:
5:
1782:
1772:
1771:
1755:
1754:
1725:
1689:
1661:
1650:(2): 103–127.
1632:
1603:
1576:(5): 527–533.
1569:J. Biomol. NMR
1554:
1535:(1): 143–144.
1518:
1482:
1446:
1407:
1396:
1370:(2): 105–109.
1350:
1312:
1298:
1276:
1260:
1246:
1223:
1213:
1192:
1182:
1164:
1146:
1139:
1121:
1114:
1088:
1081:
1063:
1056:
1038:
1031:
1013:
994:(5): 1070–84.
976:
975:
973:
970:
969:
968:
963:
958:
953:
948:
943:
938:
932:
926:
920:
912:
909:
898:covalent bonds
878:
871:
832:chemical shift
808:magnetic field
805:radiofrequency
775:
770:
765:
760:
755:
750:
721:
689:
676:
644:
627:C. P. Slichter
623:H. S. Gutowsky
618:
615:
598:
595:
580:
574:
573:
568:
559:
550:
535:
526:
514:
507:
497:
496:
492:
487:
483:
478:
474:
468:
467:
463:
456:
449:
431:
425:
402:4-nitrotoluene
370:
366:
362:
358:
354:
338:
331:
303:
297:
296:
287:
283:
225:
218:
166:
158:
145:chemical shift
129:
126:
92:The origin of
77:
74:
44:chemical bonds
15:
9:
6:
4:
3:
2:
1781:
1770:
1767:
1766:
1764:
1750:
1746:
1742:
1738:
1737:
1729:
1721:
1717:
1713:
1709:
1705:
1702:
1701:
1693:
1685:
1681:
1677:
1674:
1673:
1665:
1657:
1653:
1649:
1645:
1644:
1636:
1628:
1624:
1620:
1617:
1616:
1607:
1599:
1595:
1591:
1587:
1583:
1579:
1575:
1571:
1570:
1565:
1558:
1550:
1546:
1542:
1538:
1534:
1531:
1530:
1522:
1514:
1510:
1506:
1502:
1498:
1495:
1494:
1486:
1478:
1474:
1470:
1466:
1463:(3): 589–90.
1462:
1459:
1458:
1450:
1435:
1431:
1427:
1423:
1419:
1415:
1411:
1400:
1385:
1381:
1377:
1373:
1369:
1365:
1361:
1354:
1339:
1335:
1331:
1327:
1323:
1316:
1301:
1299:9780080538068
1295:
1291:
1287:
1280:
1274:
1270:
1264:
1249:
1247:0-444-82596-7
1243:
1239:
1238:
1230:
1228:
1220:
1216:
1214:0-7216-3184-3
1210:
1206:
1199:
1197:
1189:
1185:
1183:0-7167-8759-8
1179:
1175:
1168:
1161:
1157:
1150:
1142:
1140:0-7167-8759-8
1136:
1132:
1125:
1117:
1115:9780080437484
1111:
1107:
1103:
1099:
1092:
1084:
1082:0-7216-3184-3
1078:
1074:
1067:
1059:
1057:0-07-707976-0
1053:
1049:
1042:
1034:
1032:0-7216-3184-3
1028:
1024:
1017:
1009:
1005:
1001:
997:
993:
990:
989:
981:
977:
967:
964:
962:
959:
957:
954:
952:
949:
947:
944:
942:
939:
936:
933:
930:
927:
924:
921:
918:
915:
914:
908:
905:
903:
899:
895:
891:
890:hydrogen bond
887:
882:
877:
870:
866:
865:E. M. Purcell
862:
857:
845:
837:
833:
829:
825:
821:
817:
813:
809:
806:
802:
798:
794:
789:
773:
768:
763:
758:
753:
748:
719:
687:
674:
642:
628:
624:
614:
612:
611:Carbon-13 NMR
608:
604:
601:By selective
594:
592:
588:
583:
579:
571:
567:
562:
558:
553:
549:
545:
542:
541:
540:
538:
533:
529:
524:
520:
513:
506:
502:
491:
488:
482:
479:
473:
470:
469:
462:
455:
448:
444:
441:
440:
439:
437:
429:
424:
422:
418:
413:
411:
407:
403:
399:
395:
391:
387:
383:
379:
374:
352:
347:
345:
336:
330:
328:
324:
320:
315:
313:
309:
302:
290:
275:
266:
263:
262:
261:
259:
255:
250:
245:
243:
239:
235:
231:
223:
220:Magnitude of
217:
198:
177:
170:
162:
154:
150:
146:
142:
138:
134:
125:
123:
119:
115:
95:
87:
82:
73:
71:
67:
63:
61:
57:
53:
49:
45:
41:
37:
34:(also called
33:
31:
26:
22:
1740:
1734:
1728:
1703:
1698:
1692:
1675:
1670:
1664:
1647:
1641:
1635:
1618:
1613:
1606:
1573:
1567:
1563:
1557:
1532:
1527:
1521:
1496:
1491:
1485:
1460:
1455:
1449:
1437:. Retrieved
1420:(10): 4942.
1417:
1413:
1399:
1387:. Retrieved
1367:
1363:
1353:
1341:. Retrieved
1329:
1325:
1315:
1303:. Retrieved
1289:
1279:
1268:
1263:
1251:. Retrieved
1236:
1218:
1204:
1187:
1173:
1167:
1159:
1155:
1149:
1130:
1124:
1097:
1091:
1072:
1066:
1047:
1041:
1022:
1016:
991:
986:
980:
906:
901:
893:
885:
883:
875:
868:
861:N. F. Ramsey
858:
843:
835:
823:
819:
815:
811:
796:
790:
620:
600:
590:
586:
581:
577:
575:
569:
565:
560:
556:
551:
547:
543:
534:
531:
525:
522:
518:
511:
504:
498:
489:
480:
471:
460:
453:
446:
442:
433:
427:
420:
414:
405:
397:
393:
386:polar liquid
377:
375:
350:
348:
343:
340:
334:
322:
316:
300:
298:
281:
273:
264:
257:
253:
248:
246:
229:
227:
221:
178:
174:
136:
128:Multiplicity
121:
117:
93:
91:
85:
69:
65:
64:
59:
55:
39:
35:
29:
28:
18:
1736:Tetrahedron
436:Hamiltonian
312:s-character
1700:Org. Lett.
1529:Phys. Rev.
1493:Phys. Rev.
1457:Phys. Rev.
1439:27 January
1389:27 January
1343:27 January
1305:23 January
1253:23 January
988:Phys. Rev.
972:References
956:Proton NMR
793:E. L. Hahn
597:Decoupling
382:Buckingham
197:isotopomer
70:difference
32:-couplings
769:μ
764:⋅
754:μ
607:decoupled
337:-coupling
224:-coupling
153:−OH group
1763:Category
1720:23611689
1598:19420482
911:See also
333:Sign of
163:and the
1590:1422158
1537:Bibcode
1501:Bibcode
1465:Bibcode
1422:Bibcode
1372:Bibcode
1292:: 144.
996:Bibcode
925:(ECOSY)
919:(EFNMR)
854:
840:
617:History
585:is the
501:singlet
306:is the
293:
269:
213:
201:
193:
181:
141:ethanol
110:
98:
1718:
1596:
1588:
1296:
1244:
1211:
1180:
1137:
1112:
1079:
1054:
1029:
706:, and
576:where
499:For a
1594:S2CID
937:(NMR)
546:= 2π
410:ortho
48:spins
1716:PMID
1586:PMID
1441:2021
1391:2021
1345:2021
1307:2021
1294:ISBN
1255:2021
1242:ISBN
1209:ISBN
1178:ISBN
1135:ISBN
1110:ISBN
1077:ISBN
1052:ISBN
1027:ISBN
863:and
716:POCl
530:and
510:and
434:The
23:and
1745:doi
1708:doi
1680:doi
1676:122
1652:doi
1623:doi
1619:114
1578:doi
1545:doi
1509:doi
1473:doi
1430:doi
1380:doi
1334:doi
1102:doi
1004:doi
684:OPF
639:HPF
304:C–H
165:−CH
38:or
19:In
1765::
1741:69
1739:.
1714:.
1704:15
1648:13
1646:.
1592:.
1584:.
1572:.
1543:.
1533:85
1507:.
1497:84
1471:.
1461:84
1428:.
1418:52
1416:.
1412:.
1410:F"
1378:.
1368:14
1366:.
1362:.
1330:89
1328:.
1324:.
1288:.
1226:^
1217:.
1195:^
1186:.
1108:.
1002:.
992:88
851:2π
788:.
671:CH
661:,
582:jk
564:·
561:jk
555:·
459:+
452:+
445:=
321:,
282:hγ
272:4π
267:=
157:CH
54:,
27:,
1751:.
1747::
1722:.
1710::
1686:.
1682::
1658:.
1654::
1629:.
1625::
1600:.
1580::
1574:2
1564:J
1551:.
1547::
1539::
1515:.
1511::
1503::
1479:.
1475::
1467::
1443:.
1432::
1424::
1408:3
1393:.
1382::
1374::
1347:.
1336::
1309:.
1257:.
1143:.
1118:.
1104::
1085:.
1060:.
1035:.
1010:.
1006::
998::
902:J
894:J
886:J
879:2
876:I
874:·
872:1
869:I
848:/
844:J
836:J
824:τ
820:τ
816:τ
812:τ
774:2
759:1
749:A
728:F
720:2
688:2
675:3
643:6
587:J
578:J
570:k
566:I
557:J
552:j
548:I
544:H
536:k
532:I
527:j
523:I
519:J
515:3
512:D
508:1
505:D
493:3
490:D
484:2
481:D
475:1
472:D
466:,
464:3
461:D
457:2
454:D
450:1
447:D
443:H
428:J
421:J
406:J
398:J
394:J
378:J
371:2
367:3
363:2
361:)
359:5
357:H
355:2
351:J
344:J
335:J
323:J
301:J
295:.
288:y
286:γ
284:x
278:/
274:J
265:K
258:K
254:γ
249:J
230:J
222:J
210:2
207:/
204:1
190:2
187:/
184:1
169:−
167:2
161:−
159:3
149:H
122:J
118:I
107:2
104:/
101:1
94:J
86:J
66:J
60:J
56:J
30:J
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.