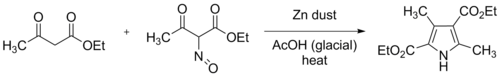111:
369:-Dialkyl 2-oximinoacetoacetamides also were found to give pyrroles when reacted under Knorr conditions with 3-substituted-2,4-pentanediones, in yields comparable to the corresponding esters (around 45%). However, when unsymmetrical diketones were used, it was found that the acetyl group from the acetoacetamide was retained in the product, and one of the acyl groups from the diketone had been lost. This same mechanism occurs to a minor extent in the acetoacetate ester systems, and had previously been detected radiochemically by Harbuck and
162:
305:-dialkyl acetoacetamides in the synthesis. Even thioesters have been successfully prepared, using the method. As for the nitrosation of β-ketoesters, despite the numerous literature specifications of tight temperature control on the nitrosation, the reaction behaves almost like a titration, and the mixture can be allowed to reach even 40 °C without significantly impacting the final yield.
316:
352:
most important in the repertory. Yields were significantly improved, by the use of preformed diethyl aminomalonate (prepared by the hydrogenolysis of diethyl oximinomalonate in ethanol, over Pd/C), and adding a mixture of diethyl aminomalonate and the β-diketone to actively boiling glacial acetic acid.
351:
George
Kleinspehn reported that the Fischer–Fink connectivity could be forced to occur exclusively, by the use of diethyl oximinomalonate in the synthesis, with 2,4-pentanedione, or its 3-alkyl substituted derivatives. Yields were high, around 60%, and this synthesis eventually came to be one of the
335:
and Emmy Fink found that
Zanetti's synthesis from 2,4-pentanedione and ethyl 2-oximinoacetoacetate gave ethyl 3,5-dimethylpyrrole-2-carboxylate as a trace byproduct. Similarly, 3-ketobutyraldehyde diethyl acetal led to the formation of ethyl 5-methylpyrrole-2-carboxylate. Both of these products
336:
resulted from the loss of the acetyl group from the inferred ethyl 2-aminoacetoacetate intermediate. An important product of the
Fischer-Fink synthesis was ethyl 4,5-dimethylpyrrole-2-carboxylate, made from ethyl 2-oximinoacetoacetate and 2-methyl-3-oxobutanal, in turn made by the
200:, and then pouring the resulting solution into water will hydrolyze the 4-ester group selectively. The 5-methyl group can be variously oxidized to chloromethyl, aldehyde, or carboxylic acid functionality by the use of stoichiometric
360:
by the use of unsymmetrical β-diketones (such as 3-alkyl substituted 2,4-hexanediones), which preferentially reacted initially at the less hindered acetyl group and afforded the corresponding 5-methylpyrrole-2-carboxylate esters.
355:
Meanwhile, Johnson had extended the
Fischer-Fink synthesis by reacting 2-oximinoacetoacetate esters (ethyl, benzyl, or tertiary-butyl), with 3-alkyl substituted 2,4-pentanediones. The Kleinspehn synthesis was extended under
328:
There are a number of important syntheses of pyrroles that are operated in the manner of the Knorr
Synthesis, despite having mechanisms of very different connectivity between the starting materials and the pyrrolic product.
169:
Modern practice is to add the oxime solution resulting from the nitrosation and the zinc dust gradually to a well-stirred solution of ethyl acetoacetate in glacial acetic acid. The reaction is
1932:
606:
Corwin, Alsoph H.; Bailey, William A.; Viohl, Paul (1942). "Structural
Investigations upon a Substituted Dipyrrylmethane. An Unusual Melting Point-Symmetry Relationship".
173:, and the mixture can reach the boiling point, if external cooling is not applied. The resulting product, diethyl 3,5-dimethylpyrrole-2,4-dicarboxylate, has been called
4147:
123:
The mechanism requires zinc and acetic acid as catalysts. It will proceed at room temperature. Because α-aminoketones self-condense very easily, they must be prepared
204:
in glacial acetic acid. Alternatively, the nitrogen atom can be alkylated. The two ester positions can be more smoothly differentiated by incorporating
635:
Oikawa, Yuji; Sugano, Kiyoshi; Yonemitsu, Osamu (1978). "Meldrum's acid in organic synthesis. 2. A general and versatile synthesis of β-keto esters".
4306:
3263:
3208:
247:, and the alcohol of one's choice. Ethyl and benzyl esters are easily made thereby, and the reaction is noteworthy in that even the highly hindered
3976:
4296:
3318:
261:(2,4-pentanedione) in reaction with ethyl 2-oximinoacetoacetate. The result was ethyl 4-acetyl-3,5-dimethylpyrrole-2-carboxylate, where "OEt" = R
3468:
2102:
470:
398:
4311:
4197:
61:
3971:
1797:
3073:
994:
3643:
2843:
1587:
3808:
3738:
3718:
3213:
2380:
1842:
856:
2261:
1817:
794:
608:
765:
1030:
3563:
4301:
4041:
3991:
2395:
308:
The mechanism of the Knorr pyrrole synthesis begins with condensation of the amine and ketone to give an imine. The imine then
3498:
4137:
3946:
3603:
3583:
3543:
2350:
1293:
4132:
4062:
3961:
3618:
3473:
3103:
2948:
2558:
2185:
1962:
4212:
3996:
3308:
2798:
2473:
3018:
4207:
3921:
3783:
3573:
3538:
373:. Most of the above-described syntheses have application in the synthesis of porphyrins, bile pigments, and dipyrrins.
315:
4097:
4036:
3568:
3483:
3453:
3433:
3298:
3293:
2668:
2593:
2236:
2190:
2057:
1318:
825:(1985). "Pyrrole chemistry. An improved synthesis of ethyl pyrrole-2-carboxylate esters from diethyl aminomalonate".
110:
4202:
4162:
4112:
3788:
3588:
3338:
3268:
1757:
1328:
933:
901:
827:
637:
3403:
2296:
2017:
239:
and "Et") can be varied by the application of appropriate β-ketoesters readily made by a synthesis emanating from
4291:
3956:
3798:
3668:
3663:
3478:
2953:
2863:
2453:
2385:
2276:
1852:
1607:
1532:
987:
3713:
4242:
4127:
4026:
3966:
3613:
3408:
3368:
3343:
3253:
2713:
1747:
1677:
1313:
1243:
2833:
429:
4232:
3818:
3708:
3328:
3053:
2838:
2783:
2628:
2588:
2420:
2175:
1892:
1742:
731:
4192:
3753:
3198:
4227:
4142:
4117:
4092:
4077:
4001:
3916:
3813:
3773:
3638:
3593:
3358:
2903:
2888:
2753:
2543:
2211:
1967:
1647:
1622:
1592:
1183:
670:
4177:
4122:
4067:
3778:
3698:
3598:
3313:
3278:
3123:
3013:
2728:
2723:
2548:
2508:
2405:
2216:
2180:
2032:
2022:
1877:
1737:
1597:
1547:
1542:
1517:
1477:
1423:
1188:
1178:
1153:
158:
group to the amine. This reduction consumes two equivalents of zinc and four equivalents of acetic acid.
854:
Bullock, E.; Johnson, A. W.; Markham, E.; Shaw, K. B. (1958). "287. A synthesis of coproporphyrin III".
161:
4152:
3853:
3658:
3093:
2978:
2658:
2633:
2573:
2430:
2165:
1872:
1652:
1617:
1522:
1213:
1148:
980:
962:
54:
3443:
1707:
4252:
4157:
4011:
3891:
3863:
3833:
3748:
3678:
3633:
3608:
3528:
3428:
3388:
3083:
2703:
2693:
2618:
2142:
2002:
1997:
1977:
1662:
1459:
1438:
1398:
1323:
665:
1143:
4217:
4107:
4087:
3951:
3793:
3703:
3673:
3653:
3548:
3503:
3333:
3243:
3173:
3058:
3048:
2878:
2435:
2375:
2340:
2147:
2127:
2087:
1862:
1732:
1697:
1657:
1408:
1248:
1238:
1168:
967:
525:"2,4-Dimethyl-3,5-dicarbethoxypyrrole (2,4-Pyrroledicarboxylic acid, 3,5-dimethyl-, diethyl ester)"
3683:
792:
Kleinspehn, George G. (1955). "A Novel Route to
Certain 2-Pyrrolecarboxylic Esters and Nitriles".
4316:
4046:
3896:
3838:
3763:
3463:
3413:
3273:
3238:
3178:
3108:
2663:
2410:
2122:
2042:
1937:
1897:
1802:
1687:
1672:
1582:
1572:
1233:
1158:
1113:
274:
1448:
312:
to an enamine, followed by cyclization, elimination of water, and isomerization to the pyrrole.
3926:
3648:
3398:
3378:
3353:
3303:
3218:
3193:
3148:
3118:
3098:
3068:
3033:
2988:
2963:
2938:
2823:
2748:
2528:
2221:
2157:
1957:
1682:
1602:
1288:
1263:
1040:
1035:
763:; Fink, Emmy (1948). "Über eine neue Pyrrolsynthese" [On a new synthesis of pyrroles].
4262:
3848:
3803:
3518:
3488:
3458:
3393:
3373:
3288:
3283:
3248:
3203:
3188:
3183:
3163:
3153:
3088:
3078:
3008:
2958:
2478:
2281:
1857:
1812:
1642:
1632:
1378:
1303:
1098:
1060:
42:
1308:
285:-butyl acetoacetates also work well in this system, and with close temperature control, the
215:
groups via the corresponding acetoacetate esters. Benzyl groups can be removed by catalytic
4031:
3981:
3931:
3911:
3901:
3758:
3733:
3448:
3438:
3323:
3138:
3133:
3063:
2848:
2648:
2608:
2538:
2503:
2458:
2425:
2291:
2266:
2246:
2067:
2027:
1987:
1952:
1882:
1637:
1507:
1482:
1020:
506:
337:
224:
8:
4237:
4222:
3868:
3843:
3828:
3823:
3553:
3508:
3493:
3383:
3363:
3258:
3143:
3128:
2973:
2918:
2908:
2873:
2638:
2513:
2488:
2400:
2256:
2241:
2226:
2047:
1992:
1762:
1612:
1557:
1428:
1343:
1203:
1128:
220:
143:
132:
4247:
2898:
2082:
1273:
100:
and a compound containing an electron-withdrawing group (e.g. an ester as shown) α to a
3986:
3936:
3906:
3768:
3558:
3348:
3233:
3168:
3158:
2923:
2853:
2818:
2813:
2793:
2788:
2733:
2643:
2493:
2355:
2345:
2251:
2037:
1982:
1912:
1832:
1727:
1627:
1562:
1487:
1333:
1198:
1133:
1118:
698:
434:
248:
139:
3723:
3043:
2928:
2893:
2858:
2803:
2758:
2673:
2653:
2603:
2598:
2568:
2553:
2463:
2370:
2306:
2271:
2097:
1972:
1847:
1772:
1752:
1667:
1502:
1497:
1443:
1353:
1258:
1218:
1173:
1055:
1050:
1015:
572:
529:
498:
244:
201:
79:
2718:
192:
Knorr's pyrrole can be derivatized in a number of useful manners. One equivalent of
4257:
4102:
4072:
4016:
3941:
3873:
3628:
3578:
3423:
3228:
3003:
2998:
2943:
2933:
2708:
2518:
2498:
2468:
2365:
2301:
2286:
2117:
2072:
2062:
2052:
1947:
1927:
1922:
1907:
1902:
1782:
1777:
1717:
1702:
1692:
1537:
1527:
1393:
1383:
1283:
1278:
1253:
1193:
1045:
1004:
942:
910:
861:
836:
803:
778:
774:
740:
707:
689:
646:
617:
581:
538:
479:
443:
407:
193:
1373:
196:
will saponify the 2-ester selectively. Dissolving Knorr's pyrrole in concentrated
4167:
3858:
3693:
3688:
2983:
2968:
2913:
2868:
2828:
2778:
2743:
2738:
2683:
2678:
2613:
2563:
2483:
2311:
2195:
2170:
2132:
2107:
2092:
2077:
2012:
1887:
1837:
1827:
1807:
1767:
1577:
1567:
1552:
1348:
1268:
1138:
1108:
1093:
1088:
931:; Harbuct, John W. (1971). "Mechanism of a modified Knorr pyrrole condensation".
309:
567:
524:
497:
Corwin, Alsoph Henry (1950). "The
Chemistry of Pyrrole and its Derivatives". In
142:, one of which was converted to ethyl 2-oximinoacetoacetate by dissolving it in
4172:
4082:
4021:
3113:
3023:
2993:
2768:
2623:
2360:
2137:
2007:
1822:
1792:
1492:
1388:
1163:
1025:
928:
370:
216:
147:
101:
972:
4285:
4182:
3883:
3728:
3623:
3418:
2808:
2773:
2763:
2698:
2688:
2578:
2415:
2231:
1942:
1917:
1787:
1433:
1418:
1403:
1298:
1228:
1208:
1123:
880:
822:
711:
585:
542:
483:
447:
411:
357:
345:
258:
240:
197:
273:= COOEt. The 4-acetyl group could easily be converted to a 4-ethyl group by
3223:
2583:
2335:
2112:
1712:
1512:
1363:
1358:
1223:
1078:
760:
563:
520:
461:
425:
389:
332:
32:
879:
Paine, John B.; Brough, Jonathan R.; Buller, Kathy K.; Erikson, Erika E.;
468:[On the formation of pyrrole derivatives from isonitrosoketones].
1722:
1368:
1338:
1103:
865:
946:
914:
840:
807:
650:
621:
297:-dialkyl pyrrole-2- and/or 4-carboxamides may be prepared by the use of
4006:
3533:
2883:
341:
209:
170:
745:
726:
465:
432:[Synthetic experiments with the ester of acetoacetic acid].
393:
257:
Levi and
Zanetti extended the Knorr synthesis in 1894 to the use of
1413:
1083:
278:
1073:
83:
205:
94:
668:[Synthesis of pyrrole compounds from nitrosoketones].
277:(hydrazine and alkali, heated); hydrogenolysis, or the use of
466:"Ueber die Bildung von Pyrrolderivaten aus Isonitrosoketonen"
223:, and tertiary-butyl groups can be removed by treatment with
155:
128:
90:
151:
853:
138:
The original Knorr synthesis employed two equivalents of
878:
146:, and slowly adding one equivalent of saturated aqueous
891:-dialkyl-2-pyrrolecarboxamides from 1,3-diketones and
727:"Preparation and reactions of some pyrrylthiol esters"
289:-butyl system gives a very high yield (close to 80%).
634:
4148:
Erlenmeyer–Plöchl azlactone and amino-acid synthesis
127:. The usual way of doing this is from the relevant
666:"Sintesi di composti pirrolici dai nitrosochetoni"
3209:Divinylcyclopropane-cycloheptadiene rearrangement
724:
688:
605:
4283:
725:Bullock, E.; Chen, T. S.; Loader, C. E. (1966).
1002:
927:
568:"Kryptopyrrole (Pyrrole, 2,4-dimethyl-3-ethyl)"
3469:Thermal rearrangement of aromatic hydrocarbons
2103:Thermal rearrangement of aromatic hydrocarbons
471:Berichte der deutschen chemischen Gesellschaft
430:"Synthetische Versuche mit dem Acetessigester"
399:Berichte der deutschen chemischen Gesellschaft
4198:Lectka enantioselective beta-lactam synthesis
1458:
988:
3977:Inverse electron-demand Diels–Alder reaction
1798:Heterogeneous metal catalyzed cross-coupling
820:
396:[Synthesis of pyrrole derivatives].
3319:Lobry de Bruyn–Van Ekenstein transformation
759:
663:
89:. The method involves the reaction of an α-
995:
981:
791:
460:
254:gives very high yields in this synthesis.
3809:Petrenko-Kritschenko piperidone synthesis
3264:Fritsch–Buttenberg–Wiechell rearrangement
857:Journal of the Chemical Society (Resumed)
744:
4307:Carbon-heteroatom bond forming reactions
3972:Intramolecular Diels–Alder cycloaddition
795:Journal of the American Chemical Society
609:Journal of the American Chemical Society
883:(1987). "Mechanism of the formation of
562:
519:
189:= Me represent this original reaction.
154:dust was then stirred in, reducing the
4297:Nitrogen heterocycle forming reactions
4284:
3992:Metal-centered cycloaddition reactions
3644:Debus–Radziszewski imidazole synthesis
1588:Bodroux–Chichibabin aldehyde synthesis
496:
4138:Diazoalkane 1,3-dipolar cycloaddition
4042:Vinylcyclopropane (5+2) cycloaddition
3947:Diazoalkane 1,3-dipolar cycloaddition
3719:Hurd–Mori 1,2,3-thiadiazole synthesis
3214:Dowd–Beckwith ring-expansion reaction
2381:Hurd–Mori 1,2,3-thiadiazole synthesis
1457:
1294:LFER solvent coefficients (data page)
976:
766:Zeitschrift für Physiologische Chemie
696:-Butylester von Pyrrolcarbonsäuren".
424:
388:
4312:Carbon-carbon bond forming reactions
2949:Sharpless asymmetric dihydroxylation
2186:Methoxymethylenetriphenylphosphorane
323:
319:Mechanism of Knorr pyrrole synthesis
3074:Allen–Millar–Trippett rearrangement
227:, or boiling aqueous acetic acid. R
13:
4213:Nitrone-olefin (3+2) cycloaddition
4208:Niementowski quinazoline synthesis
3997:Nitrone-olefin (3+2) cycloaddition
3922:Azide-alkyne Huisgen cycloaddition
3784:Niementowski quinazoline synthesis
3539:Azide-alkyne Huisgen cycloaddition
2844:Meerwein–Ponndorf–Verley reduction
2396:Leimgruber–Batcho indole synthesis
314:
177:ever since. In the Scheme above, R
160:
109:
14:
4328:
4037:Trimethylenemethane cycloaddition
3739:Johnson–Corey–Chaykovsky reaction
3604:Cadogan–Sundberg indole synthesis
3584:Bohlmann–Rahtz pyridine synthesis
3544:Baeyer–Emmerling indole synthesis
2351:Cadogan–Sundberg indole synthesis
1843:Johnson–Corey–Chaykovsky reaction
899:-dialkyloximinoacetoacetamides".
664:Zanetti, C. U.; Levi, E. (1894).
4133:Cook–Heilbron thiazole synthesis
3962:Hexadehydro Diels–Alder reaction
3789:Niementowski quinoline synthesis
3619:Cook–Heilbron thiazole synthesis
3564:Bischler–Möhlau indole synthesis
3474:Tiffeneau–Demjanov rearrangement
3104:Baker–Venkataraman rearrangement
2262:Horner–Wadsworth–Emmons reaction
1933:Mizoroki-Heck vs. Reductive Heck
1818:Horner–Wadsworth–Emmons reaction
1329:Neighbouring group participation
934:The Journal of Organic Chemistry
902:The Journal of Organic Chemistry
828:The Journal of Organic Chemistry
638:The Journal of Organic Chemistry
3669:Fiesselmann thiophene synthesis
3499:Westphalen–Lettré rearrangement
3479:Vinylcyclopropane rearrangement
3309:Kornblum–DeLaMare rearrangement
2954:Epoxidation of allylic alcohols
2864:Noyori asymmetric hydrogenation
2799:Kornblum–DeLaMare rearrangement
2474:Gallagher–Hollander degradation
921:
872:
847:
814:
785:
753:
718:
682:
4128:Chichibabin pyridine synthesis
3614:Chichibabin pyridine synthesis
3574:Blum–Ittah aziridine synthesis
3409:Ring expansion and contraction
1678:Cross dehydrogenative coupling
779:10.1515/bchm2.1948.283.3-4.152
657:
628:
599:
556:
513:
490:
454:
418:
394:"Synthese von Pyrrolderivaten"
382:
1:
4302:Heterocycle forming reactions
4098:Bischler–Napieralski reaction
4056:Heterocycle forming reactions
3709:Hemetsberger indole synthesis
3569:Bischler–Napieralski reaction
3484:Wagner–Meerwein rearrangement
3454:Sommelet–Hauser rearrangement
3434:Seyferth–Gilbert homologation
3299:Ireland–Claisen rearrangement
3294:Hofmann–Martius rearrangement
3054:2,3-sigmatropic rearrangement
2669:Corey–Winter olefin synthesis
2594:Barton–McCombie deoxygenation
2237:Corey–Winter olefin synthesis
2191:Seyferth–Gilbert homologation
2058:Seyferth–Gilbert homologation
732:Canadian Journal of Chemistry
692:; Hintermeier, Karl (1954). "
376:
82:that synthesizes substituted
4203:Lehmstedt–Tanasescu reaction
4163:Gabriel–Colman rearrangement
4118:Bucherer carbazole synthesis
4113:Borsche–Drechsel cyclization
4093:Bernthsen acridine synthesis
4078:Bamberger triazine synthesis
4063:Algar–Flynn–Oyamada reaction
3774:Nazarov cyclization reaction
3639:De Kimpe aziridine synthesis
3594:Bucherer carbazole synthesis
3589:Borsche–Drechsel cyclization
3359:Nazarov cyclization reaction
3339:Meyer–Schuster rearrangement
3269:Gabriel–Colman rearrangement
3019:Wolffenstein–Böters reaction
2904:Reduction of nitro compounds
2754:Grundmann aldehyde synthesis
2559:Algar–Flynn–Oyamada reaction
1968:Olefin conversion technology
1963:Nozaki–Hiyama–Kishi reaction
1758:Gabriel–Colman rearrangement
1648:Claisen-Schmidt condensation
1593:Bouveault aldehyde synthesis
671:La Gazzetta Chimica Italiana
7:
4178:Hantzsch pyridine synthesis
3957:Enone–alkene cycloadditions
3779:Nenitzescu indole synthesis
3699:Hantzsch pyridine synthesis
3664:Ferrario–Ackermann reaction
3314:Kowalski ester homologation
3279:Halogen dance rearrangement
3124:Benzilic acid rearrangement
2549:Akabori amino-acid reaction
2509:Von Braun amide degradation
2454:Barbier–Wieland degradation
2406:Nenitzescu indole synthesis
2386:Kharasch–Sosnovsky reaction
2277:Julia–Kocienski olefination
2181:Kowalski ester homologation
1878:Kowalski ester homologation
1853:Julia–Kocienski olefination
1608:Cadiot–Chodkiewicz coupling
1533:Aza-Baylis–Hillman reaction
1478:Acetoacetic ester synthesis
1189:Dynamic binding (chemistry)
1179:Conrotatory and disrotatory
1154:Charge remote fragmentation
956:
114:The Knorr pyrrole synthesis
10:
4333:
4243:Robinson–Gabriel synthesis
4193:Kröhnke pyridine synthesis
4027:Retro-Diels–Alder reaction
3967:Imine Diels–Alder reaction
3754:Kröhnke pyridine synthesis
3369:Newman–Kwart rearrangement
3344:Mislow–Evans rearrangement
3254:Fischer–Hepp rearrangement
3199:Di-π-methane rearrangement
2979:Stephen aldehyde synthesis
2714:Eschweiler–Clarke reaction
2431:Williamson ether synthesis
1748:Fujiwara–Moritani reaction
1653:Combes quinoline synthesis
1618:Carbonyl olefin metathesis
1319:More O'Ferrall–Jencks plot
1244:Grunwald–Winstein equation
1214:Electron-withdrawing group
1149:Catalytic resonance theory
963:Hantzsch pyrrole synthesis
594:, vol. 3, p. 513
551:, vol. 2, p. 202
150:, under external cooling.
4253:Urech hydantoin synthesis
4233:Pomeranz–Fritsch reaction
4158:Fischer oxazole synthesis
4055:
3892:1,3-Dipolar cycloaddition
3882:
3864:Urech hydantoin synthesis
3834:Reissert indole synthesis
3819:Pomeranz–Fritsch reaction
3749:Knorr quinoline synthesis
3679:Fischer oxazole synthesis
3609:Camps quinoline synthesis
3529:1,3-Dipolar cycloaddition
3517:
3429:Semipinacol rearrangement
3404:Ramberg–Bäcklund reaction
3389:Piancatelli rearrangement
3329:McFadyen–Stevens reaction
3084:Alpha-ketol rearrangement
3032:
2839:McFadyen–Stevens reaction
2784:Kiliani–Fischer synthesis
2704:Elbs persulfate oxidation
2629:Bouveault–Blanc reduction
2589:Baeyer–Villiger oxidation
2527:
2444:
2421:Schotten–Baumann reaction
2324:
2297:Ramberg–Bäcklund reaction
2204:
2176:Kiliani–Fischer synthesis
2156:
2018:Ramberg–Bäcklund reaction
2003:Pinacol coupling reaction
1998:Piancatelli rearrangement
1893:Liebeskind–Srogl coupling
1743:Fujimoto–Belleau reaction
1466:
1460:List of organic reactions
1324:Negative hyperconjugation
1069:
1011:
505:. Vol. 1. New York:
499:Elderfield, Robert Cooley
118:
68:
48:
23:
4228:Pictet–Spengler reaction
4143:Einhorn–Brunner reaction
4108:Boger pyridine synthesis
4002:Oxo-Diels–Alder reaction
3917:Aza-Diels–Alder reaction
3814:Pictet–Spengler reaction
3714:Hofmann–Löffler reaction
3704:Hegedus indole synthesis
3674:Fischer indole synthesis
3549:Bartoli indole synthesis
3504:Willgerodt rearrangement
3334:McLafferty rearrangement
3244:Ferrier carbocyclization
3059:2,3-Wittig rearrangement
3049:1,2-Wittig rearrangement
2889:Parikh–Doering oxidation
2879:Oxygen rebound mechanism
2544:Adkins–Peterson reaction
2436:Yamaguchi esterification
2376:Hegedus indole synthesis
2341:Bartoli indole synthesis
2212:Bamford–Stevens reaction
2128:Weinreb ketone synthesis
2088:Stork enamine alkylation
1863:Knoevenagel condensation
1733:Ferrier carbocyclization
1623:Castro–Stephens coupling
1249:Hammett acidity function
1239:Free-energy relationship
1184:Curtin–Hammett principle
1169:Conformational isomerism
712:10.1002/cber.19540870818
586:10.15227/orgsyn.021.0067
543:10.15227/orgsyn.015.0017
484:10.1002/cber.19020350392
448:10.1002/jlac.18862360303
412:10.1002/cber.18840170220
24:Knorr pyrrole synthesis
4188:Knorr pyrrole synthesis
4123:Bucherer–Bergs reaction
4068:Allan–Robinson reaction
4047:Wagner-Jauregg reaction
3839:Ring-closing metathesis
3764:Larock indole synthesis
3744:Knorr pyrrole synthesis
3599:Bucherer–Bergs reaction
3464:Stieglitz rearrangement
3444:Skattebøl rearrangement
3414:Ring-closing metathesis
3274:Group transfer reaction
3239:Favorskii rearrangement
3179:Cornforth rearrangement
3109:Bamberger rearrangement
3014:Wolff–Kishner reduction
2834:Markó–Lam deoxygenation
2729:Fleming–Tamao oxidation
2724:Fischer–Tropsch process
2411:Oxymercuration reaction
2391:Knorr pyrrole synthesis
2217:Barton–Kellogg reaction
2123:Wagner-Jauregg reaction
2043:Ring-closing metathesis
2033:Reimer–Tiemann reaction
2023:Rauhut–Currier reaction
1938:Nef isocyanide reaction
1898:Malonic ester synthesis
1868:Knorr pyrrole synthesis
1803:High dilution principle
1738:Friedel–Crafts reaction
1673:Cross-coupling reaction
1598:Bucherer–Bergs reaction
1583:Blanc chloromethylation
1573:Blaise ketone synthesis
1548:Baylis–Hillman reaction
1543:Barton–Kellogg reaction
1518:Allan–Robinson reaction
1424:Woodward–Hoffmann rules
1159:Charge-transfer complex
275:Wolff-Kishner reduction
76:Knorr pyrrole synthesis
4292:Ring forming reactions
4153:Feist–Benary synthesis
3927:Bradsher cycloaddition
3897:4+4 Photocycloaddition
3854:Simmons–Smith reaction
3799:Paternò–Büchi reaction
3659:Feist–Benary synthesis
3649:Dieckmann condensation
3399:Pummerer rearrangement
3379:Oxy-Cope rearrangement
3354:Myers allene synthesis
3304:Jacobsen rearrangement
3219:Electrocyclic reaction
3194:Demjanov rearrangement
3149:Buchner ring expansion
3119:Beckmann rearrangement
3099:Aza-Cope rearrangement
3094:Arndt–Eistert reaction
3069:Alkyne zipper reaction
2989:Transfer hydrogenation
2964:Sharpless oxyamination
2939:Selenoxide elimination
2824:Lombardo methylenation
2749:Griesbaum coozonolysis
2659:Corey–Itsuno reduction
2634:Boyland–Sims oxidation
2574:Angeli–Rimini reaction
2222:Boord olefin synthesis
2166:Arndt–Eistert reaction
2158:Homologation reactions
1958:Nitro-Mannich reaction
1873:Kolbe–Schmitt reaction
1683:Cross-coupling partner
1603:Buchner ring expansion
1523:Arndt–Eistert reaction
1289:Kinetic isotope effect
1036:Rearrangement reaction
503:Heterocyclic Compounds
320:
166:
115:
4012:Pauson–Khand reaction
3849:Sharpless epoxidation
3804:Pechmann condensation
3684:Friedländer synthesis
3634:Davis–Beirut reaction
3489:Wallach rearrangement
3459:Stevens rearrangement
3394:Pinacol rearrangement
3374:Overman rearrangement
3289:Hofmann rearrangement
3284:Hayashi rearrangement
3249:Ferrier rearrangement
3204:Dimroth rearrangement
3189:Curtius rearrangement
3184:Criegee rearrangement
3164:Claisen rearrangement
3154:Carroll rearrangement
3089:Amadori rearrangement
3079:Allylic rearrangement
2959:Sharpless epoxidation
2694:Dess–Martin oxidation
2619:Bohn–Schmidt reaction
2479:Hofmann rearrangement
2282:Kauffmann olefination
2205:Olefination reactions
2143:Wurtz–Fittig reaction
1978:Palladium–NHC complex
1858:Kauffmann olefination
1813:Homologation reaction
1663:Corey–House synthesis
1643:Claisen rearrangement
1439:Yukawa–Tsuno equation
1399:Swain–Lupton equation
1379:Spherical aromaticity
1314:Möbius–Hückel concept
1099:Aromatic ring current
1061:Substitution reaction
318:
164:
113:
43:Ring forming reaction
4218:Paal–Knorr synthesis
4088:Barton–Zard reaction
4032:Staudinger synthesis
3982:Ketene cycloaddition
3952:Diels–Alder reaction
3932:Cheletropic reaction
3912:Alkyne trimerisation
3794:Paal–Knorr synthesis
3759:Kulinkovich reaction
3734:Jacobsen epoxidation
3654:Diels–Alder reaction
3449:Smiles rearrangement
3439:Sigmatropic reaction
3324:Lossen rearrangement
3174:Corey–Fuchs reaction
3139:Boekelheide reaction
3134:Bergmann degradation
3064:Achmatowicz reaction
2849:Methionine sulfoxide
2649:Clemmensen reduction
2609:Bergmann degradation
2539:Acyloin condensation
2504:Strecker degradation
2459:Bergmann degradation
2426:Ullmann condensation
2292:Peterson olefination
2267:Hydrazone iodination
2247:Elimination reaction
2148:Zincke–Suhl reaction
2068:Sonogashira coupling
2028:Reformatsky reaction
1988:Peterson olefination
1953:Nierenstein reaction
1883:Kulinkovich reaction
1698:Diels–Alder reaction
1658:Corey–Fuchs reaction
1638:Claisen condensation
1508:Alkyne trimerisation
1483:Acyloin condensation
1449:Σ-bishomoaromaticity
1409:Thorpe–Ingold effect
1021:Elimination reaction
968:Paal–Knorr synthesis
866:10.1039/JR9580001430
464:; Lange, H. (1902).
338:Claisen condensation
225:trifluoroacetic acid
165:Knorr 1886 synthesis
4238:Prilezhaev reaction
4223:Pellizzari reaction
3902:(4+3) cycloaddition
3869:Van Leusen reaction
3844:Robinson annulation
3829:Pschorr cyclization
3824:Prilezhaev reaction
3554:Bergman cyclization
3509:Wolff rearrangement
3494:Weerman degradation
3384:Pericyclic reaction
3364:Neber rearrangement
3259:Fries rearrangement
3144:Brook rearrangement
3129:Bergman cyclization
2974:Staudinger reaction
2919:Rosenmund reduction
2909:Reductive amination
2874:Oppenauer oxidation
2664:Corey–Kim oxidation
2639:Cannizzaro reaction
2514:Weerman degradation
2489:Isosaccharinic acid
2401:Mukaiyama hydration
2257:Hofmann elimination
2242:Dehydrohalogenation
2227:Chugaev elimination
2048:Robinson annulation
1993:Pfitzinger reaction
1763:Gattermann reaction
1708:Wulff–Dötz reaction
1688:Dakin–West reaction
1613:Carbonyl allylation
1558:Bergman cyclization
1344:Kennedy J. P. Orton
1264:Hammond's postulate
1234:Flippin–Lodge angle
1204:Electromeric effect
1129:Beta-silicon effect
1114:Baker–Nathan effect
947:10.1021/jo00805a030
915:10.1021/jo00227a010
841:10.1021/jo00350a033
808:10.1021/ja01611a043
651:10.1021/jo00404a066
622:10.1021/ja01258a007
221:palladium on carbon
144:glacial acetic acid
133:Neber rearrangement
3987:McCormack reaction
3937:Conia-ene reaction
3769:Madelung synthesis
3559:Biginelli reaction
3349:Mumm rearrangement
3234:Favorskii reaction
3169:Cope rearrangement
3159:Chan rearrangement
2924:Rubottom oxidation
2854:Miyaura borylation
2819:Lipid peroxidation
2814:Lindgren oxidation
2794:Kornblum oxidation
2789:Kolbe electrolysis
2734:Fukuyama reduction
2644:Carbonyl reduction
2494:Marker degradation
2356:Diazonium compound
2346:Boudouard reaction
2325:Carbon-heteroatom
2252:Grieco elimination
2038:Rieche formylation
1983:Passerini reaction
1913:Meerwein arylation
1833:Hydroxymethylation
1728:Favorskii reaction
1628:Chan rearrangement
1563:Biginelli reaction
1488:Aldol condensation
1334:2-Norbornyl cation
1309:Möbius aromaticity
1304:Markovnikov's rule
1199:Effective molarity
1144:Bürgi–Dunitz angle
1134:Bicycloaromaticity
699:Chemische Berichte
509:. pp. 287 ff.
435:Annalen der Chemie
321:
167:
140:ethyl acetoacetate
116:
4279:
4278:
4275:
4274:
4271:
4270:
4263:Wohl–Aue reaction
3907:6+4 Cycloaddition
3724:Iodolactonization
3044:1,2-rearrangement
3009:Wohl–Aue reaction
2929:Sabatier reaction
2894:Pinnick oxidation
2859:Mozingo reduction
2804:Leuckart reaction
2759:Haloform reaction
2674:Criegee oxidation
2654:Collins oxidation
2604:Benkeser reaction
2599:Bechamp reduction
2569:Andrussow process
2554:Alcohol oxidation
2464:Edman degradation
2371:Haloform reaction
2320:
2319:
2307:Takai olefination
2272:Julia olefination
2098:Takai olefination
1973:Olefin metathesis
1848:Julia olefination
1773:Grignard reaction
1753:Fukuyama coupling
1668:Coupling reaction
1633:Chan–Lam coupling
1503:Alkyne metathesis
1498:Alkane metathesis
1354:Phosphaethynolate
1259:George S. Hammond
1219:Electronic effect
1174:Conjugated system
1056:Stereospecificity
1051:Stereoselectivity
1016:Addition reaction
1005:organic reactions
909:(18): 3993–3997.
835:(26): 5598–5604.
645:(10): 2087–2088.
592:Collected Volumes
573:Organic Syntheses
549:Collected Volumes
530:Organic Syntheses
324:Related synthesis
202:sulfuryl chloride
80:chemical reaction
78:is a widely used
72:
71:
16:Chemical reaction
4324:
4258:Wenker synthesis
4248:Stollé synthesis
4103:Bobbitt reaction
4073:Auwers synthesis
4017:Povarov reaction
3942:Cyclopropanation
3880:
3879:
3874:Wenker synthesis
3629:Darzens reaction
3579:Bobbitt reaction
3424:Schmidt reaction
3229:Enyne metathesis
3004:Whiting reaction
2999:Wharton reaction
2944:Shapiro reaction
2934:Sarett oxidation
2899:Prévost reaction
2709:Emde degradation
2519:Wohl degradation
2499:Ruff degradation
2469:Emde degradation
2366:Grignard reagent
2302:Shapiro reaction
2287:McMurry reaction
2154:
2153:
2118:Ullmann reaction
2083:Stollé synthesis
2073:Stetter reaction
2063:Shapiro reaction
2053:Sakurai reaction
1948:Negishi coupling
1928:Minisci reaction
1923:Michael reaction
1908:McMurry reaction
1903:Mannich reaction
1783:Hammick reaction
1778:Grignard reagent
1718:Enyne metathesis
1703:Doebner reaction
1693:Darzens reaction
1538:Barbier reaction
1528:Auwers synthesis
1455:
1454:
1429:Woodward's rules
1394:Superaromaticity
1384:Spiroaromaticity
1284:Inductive effect
1279:Hyperconjugation
1254:Hammett equation
1194:Edwards equation
1046:Regioselectivity
997:
990:
983:
974:
973:
951:
950:
925:
919:
918:
876:
870:
869:
851:
845:
844:
821:Paine, John B.;
818:
812:
811:
802:(6): 1546–1548.
789:
783:
782:
773:(3–4): 152–161.
757:
751:
750:
748:
739:(9): 1007–1111.
722:
716:
715:
706:(8): 1167–1174.
686:
680:
679:
661:
655:
654:
632:
626:
625:
616:(6): 1267–1273.
603:
597:
595:
588:
560:
554:
552:
545:
517:
511:
510:
494:
488:
487:
478:(3): 2998–3008.
458:
452:
451:
422:
416:
415:
406:(2): 1635–1642.
386:
194:sodium hydroxide
64:
21:
20:
4332:
4331:
4327:
4326:
4325:
4323:
4322:
4321:
4282:
4281:
4280:
4267:
4168:Gewald reaction
4051:
3878:
3859:Skraup reaction
3694:Graham reaction
3689:Gewald reaction
3520:
3513:
3035:
3028:
2984:Swern oxidation
2969:Stahl oxidation
2914:Riley oxidation
2869:Omega oxidation
2829:Luche reduction
2779:Jones oxidation
2744:Glycol cleavage
2739:Ganem oxidation
2684:Davis oxidation
2679:Dakin oxidation
2614:Birch reduction
2564:Amide reduction
2530:
2523:
2484:Hooker reaction
2446:
2440:
2328:
2326:
2316:
2312:Wittig reaction
2200:
2196:Wittig reaction
2171:Hooker reaction
2152:
2133:Wittig reaction
2108:Thorpe reaction
2093:Suzuki reaction
2078:Stille reaction
2013:Quelet reaction
1888:Kumada coupling
1838:Ivanov reaction
1828:Hydrovinylation
1808:Hiyama coupling
1768:Glaser coupling
1578:Blaise reaction
1568:Bingel reaction
1553:Benary reaction
1470:
1468:
1462:
1453:
1349:Passive binding
1269:Homoaromaticity
1119:Baldwin's rules
1094:Antiaromaticity
1089:Anomeric effect
1065:
1007:
1001:
959:
954:
929:Rapoport, Henry
926:
922:
877:
873:
852:
848:
819:
815:
790:
786:
758:
754:
746:10.1139/v66-149
723:
719:
687:
683:
662:
658:
633:
629:
604:
600:
590:
561:
557:
547:
518:
514:
495:
491:
459:
455:
423:
419:
387:
383:
379:
326:
272:
268:
264:
238:
234:
230:
188:
184:
180:
175:Knorr's Pyrrole
121:
60:
17:
12:
11:
5:
4330:
4320:
4319:
4317:Name reactions
4314:
4309:
4304:
4299:
4294:
4277:
4276:
4273:
4272:
4269:
4268:
4266:
4265:
4260:
4255:
4250:
4245:
4240:
4235:
4230:
4225:
4220:
4215:
4210:
4205:
4200:
4195:
4190:
4185:
4180:
4175:
4173:Hantzsch ester
4170:
4165:
4160:
4155:
4150:
4145:
4140:
4135:
4130:
4125:
4120:
4115:
4110:
4105:
4100:
4095:
4090:
4085:
4083:Banert cascade
4080:
4075:
4070:
4065:
4059:
4057:
4053:
4052:
4050:
4049:
4044:
4039:
4034:
4029:
4024:
4022:Prato reaction
4019:
4014:
4009:
4004:
3999:
3994:
3989:
3984:
3979:
3974:
3969:
3964:
3959:
3954:
3949:
3944:
3939:
3934:
3929:
3924:
3919:
3914:
3909:
3904:
3899:
3894:
3888:
3886:
3877:
3876:
3871:
3866:
3861:
3856:
3851:
3846:
3841:
3836:
3831:
3826:
3821:
3816:
3811:
3806:
3801:
3796:
3791:
3786:
3781:
3776:
3771:
3766:
3761:
3756:
3751:
3746:
3741:
3736:
3731:
3726:
3721:
3716:
3711:
3706:
3701:
3696:
3691:
3686:
3681:
3676:
3671:
3666:
3661:
3656:
3651:
3646:
3641:
3636:
3631:
3626:
3621:
3616:
3611:
3606:
3601:
3596:
3591:
3586:
3581:
3576:
3571:
3566:
3561:
3556:
3551:
3546:
3541:
3536:
3531:
3525:
3523:
3515:
3514:
3512:
3511:
3506:
3501:
3496:
3491:
3486:
3481:
3476:
3471:
3466:
3461:
3456:
3451:
3446:
3441:
3436:
3431:
3426:
3421:
3416:
3411:
3406:
3401:
3396:
3391:
3386:
3381:
3376:
3371:
3366:
3361:
3356:
3351:
3346:
3341:
3336:
3331:
3326:
3321:
3316:
3311:
3306:
3301:
3296:
3291:
3286:
3281:
3276:
3271:
3266:
3261:
3256:
3251:
3246:
3241:
3236:
3231:
3226:
3221:
3216:
3211:
3206:
3201:
3196:
3191:
3186:
3181:
3176:
3171:
3166:
3161:
3156:
3151:
3146:
3141:
3136:
3131:
3126:
3121:
3116:
3114:Banert cascade
3111:
3106:
3101:
3096:
3091:
3086:
3081:
3076:
3071:
3066:
3061:
3056:
3051:
3046:
3040:
3038:
3034:Rearrangement
3030:
3029:
3027:
3026:
3024:Zinin reaction
3021:
3016:
3011:
3006:
3001:
2996:
2994:Wacker process
2991:
2986:
2981:
2976:
2971:
2966:
2961:
2956:
2951:
2946:
2941:
2936:
2931:
2926:
2921:
2916:
2911:
2906:
2901:
2896:
2891:
2886:
2881:
2876:
2871:
2866:
2861:
2856:
2851:
2846:
2841:
2836:
2831:
2826:
2821:
2816:
2811:
2806:
2801:
2796:
2791:
2786:
2781:
2776:
2771:
2769:Hydrogenolysis
2766:
2761:
2756:
2751:
2746:
2741:
2736:
2731:
2726:
2721:
2719:Étard reaction
2716:
2711:
2706:
2701:
2696:
2691:
2686:
2681:
2676:
2671:
2666:
2661:
2656:
2651:
2646:
2641:
2636:
2631:
2626:
2624:Bosch reaction
2621:
2616:
2611:
2606:
2601:
2596:
2591:
2586:
2581:
2576:
2571:
2566:
2561:
2556:
2551:
2546:
2541:
2535:
2533:
2529:Organic redox
2525:
2524:
2522:
2521:
2516:
2511:
2506:
2501:
2496:
2491:
2486:
2481:
2476:
2471:
2466:
2461:
2456:
2450:
2448:
2442:
2441:
2439:
2438:
2433:
2428:
2423:
2418:
2413:
2408:
2403:
2398:
2393:
2388:
2383:
2378:
2373:
2368:
2363:
2361:Esterification
2358:
2353:
2348:
2343:
2338:
2332:
2330:
2322:
2321:
2318:
2317:
2315:
2314:
2309:
2304:
2299:
2294:
2289:
2284:
2279:
2274:
2269:
2264:
2259:
2254:
2249:
2244:
2239:
2234:
2229:
2224:
2219:
2214:
2208:
2206:
2202:
2201:
2199:
2198:
2193:
2188:
2183:
2178:
2173:
2168:
2162:
2160:
2151:
2150:
2145:
2140:
2138:Wurtz reaction
2135:
2130:
2125:
2120:
2115:
2110:
2105:
2100:
2095:
2090:
2085:
2080:
2075:
2070:
2065:
2060:
2055:
2050:
2045:
2040:
2035:
2030:
2025:
2020:
2015:
2010:
2008:Prins reaction
2005:
2000:
1995:
1990:
1985:
1980:
1975:
1970:
1965:
1960:
1955:
1950:
1945:
1940:
1935:
1930:
1925:
1920:
1915:
1910:
1905:
1900:
1895:
1890:
1885:
1880:
1875:
1870:
1865:
1860:
1855:
1850:
1845:
1840:
1835:
1830:
1825:
1823:Hydrocyanation
1820:
1815:
1810:
1805:
1800:
1795:
1793:Henry reaction
1790:
1785:
1780:
1775:
1770:
1765:
1760:
1755:
1750:
1745:
1740:
1735:
1730:
1725:
1720:
1715:
1710:
1705:
1700:
1695:
1690:
1685:
1680:
1675:
1670:
1665:
1660:
1655:
1650:
1645:
1640:
1635:
1630:
1625:
1620:
1615:
1610:
1605:
1600:
1595:
1590:
1585:
1580:
1575:
1570:
1565:
1560:
1555:
1550:
1545:
1540:
1535:
1530:
1525:
1520:
1515:
1510:
1505:
1500:
1495:
1493:Aldol reaction
1490:
1485:
1480:
1474:
1472:
1467:Carbon-carbon
1464:
1463:
1452:
1451:
1446:
1444:Zaitsev's rule
1441:
1436:
1431:
1426:
1421:
1416:
1411:
1406:
1401:
1396:
1391:
1389:Steric effects
1386:
1381:
1376:
1371:
1366:
1361:
1356:
1351:
1346:
1341:
1336:
1331:
1326:
1321:
1316:
1311:
1306:
1301:
1296:
1291:
1286:
1281:
1276:
1271:
1266:
1261:
1256:
1251:
1246:
1241:
1236:
1231:
1226:
1221:
1216:
1211:
1206:
1201:
1196:
1191:
1186:
1181:
1176:
1171:
1166:
1161:
1156:
1151:
1146:
1141:
1136:
1131:
1126:
1121:
1116:
1111:
1106:
1101:
1096:
1091:
1086:
1081:
1076:
1070:
1067:
1066:
1064:
1063:
1058:
1053:
1048:
1043:
1041:Redox reaction
1038:
1033:
1028:
1026:Polymerization
1023:
1018:
1012:
1009:
1008:
1000:
999:
992:
985:
977:
971:
970:
965:
958:
955:
953:
952:
941:(6): 853–855.
920:
871:
846:
823:Dolphin, David
813:
784:
752:
717:
690:Treibs, Alfred
681:
674:(in Italian).
656:
627:
598:
555:
512:
489:
453:
442:(3): 290–332.
417:
380:
378:
375:
325:
322:
270:
266:
262:
252:-butyl alcohol
245:Meldrum's acid
241:acid chlorides
236:
232:
228:
217:hydrogenolysis
186:
182:
181:= COOEt, and R
178:
148:sodium nitrite
120:
117:
102:carbonyl group
70:
69:
66:
65:
58:
51:
50:
46:
45:
40:
39:Reaction type
36:
35:
30:
26:
25:
15:
9:
6:
4:
3:
2:
4329:
4318:
4315:
4313:
4310:
4308:
4305:
4303:
4300:
4298:
4295:
4293:
4290:
4289:
4287:
4264:
4261:
4259:
4256:
4254:
4251:
4249:
4246:
4244:
4241:
4239:
4236:
4234:
4231:
4229:
4226:
4224:
4221:
4219:
4216:
4214:
4211:
4209:
4206:
4204:
4201:
4199:
4196:
4194:
4191:
4189:
4186:
4184:
4183:Herz reaction
4181:
4179:
4176:
4174:
4171:
4169:
4166:
4164:
4161:
4159:
4156:
4154:
4151:
4149:
4146:
4144:
4141:
4139:
4136:
4134:
4131:
4129:
4126:
4124:
4121:
4119:
4116:
4114:
4111:
4109:
4106:
4104:
4101:
4099:
4096:
4094:
4091:
4089:
4086:
4084:
4081:
4079:
4076:
4074:
4071:
4069:
4066:
4064:
4061:
4060:
4058:
4054:
4048:
4045:
4043:
4040:
4038:
4035:
4033:
4030:
4028:
4025:
4023:
4020:
4018:
4015:
4013:
4010:
4008:
4005:
4003:
4000:
3998:
3995:
3993:
3990:
3988:
3985:
3983:
3980:
3978:
3975:
3973:
3970:
3968:
3965:
3963:
3960:
3958:
3955:
3953:
3950:
3948:
3945:
3943:
3940:
3938:
3935:
3933:
3930:
3928:
3925:
3923:
3920:
3918:
3915:
3913:
3910:
3908:
3905:
3903:
3900:
3898:
3895:
3893:
3890:
3889:
3887:
3885:
3884:Cycloaddition
3881:
3875:
3872:
3870:
3867:
3865:
3862:
3860:
3857:
3855:
3852:
3850:
3847:
3845:
3842:
3840:
3837:
3835:
3832:
3830:
3827:
3825:
3822:
3820:
3817:
3815:
3812:
3810:
3807:
3805:
3802:
3800:
3797:
3795:
3792:
3790:
3787:
3785:
3782:
3780:
3777:
3775:
3772:
3770:
3767:
3765:
3762:
3760:
3757:
3755:
3752:
3750:
3747:
3745:
3742:
3740:
3737:
3735:
3732:
3730:
3729:Isay reaction
3727:
3725:
3722:
3720:
3717:
3715:
3712:
3710:
3707:
3705:
3702:
3700:
3697:
3695:
3692:
3690:
3687:
3685:
3682:
3680:
3677:
3675:
3672:
3670:
3667:
3665:
3662:
3660:
3657:
3655:
3652:
3650:
3647:
3645:
3642:
3640:
3637:
3635:
3632:
3630:
3627:
3625:
3624:Cycloaddition
3622:
3620:
3617:
3615:
3612:
3610:
3607:
3605:
3602:
3600:
3597:
3595:
3592:
3590:
3587:
3585:
3582:
3580:
3577:
3575:
3572:
3570:
3567:
3565:
3562:
3560:
3557:
3555:
3552:
3550:
3547:
3545:
3542:
3540:
3537:
3535:
3532:
3530:
3527:
3526:
3524:
3522:
3519:Ring forming
3516:
3510:
3507:
3505:
3502:
3500:
3497:
3495:
3492:
3490:
3487:
3485:
3482:
3480:
3477:
3475:
3472:
3470:
3467:
3465:
3462:
3460:
3457:
3455:
3452:
3450:
3447:
3445:
3442:
3440:
3437:
3435:
3432:
3430:
3427:
3425:
3422:
3420:
3419:Rupe reaction
3417:
3415:
3412:
3410:
3407:
3405:
3402:
3400:
3397:
3395:
3392:
3390:
3387:
3385:
3382:
3380:
3377:
3375:
3372:
3370:
3367:
3365:
3362:
3360:
3357:
3355:
3352:
3350:
3347:
3345:
3342:
3340:
3337:
3335:
3332:
3330:
3327:
3325:
3322:
3320:
3317:
3315:
3312:
3310:
3307:
3305:
3302:
3300:
3297:
3295:
3292:
3290:
3287:
3285:
3282:
3280:
3277:
3275:
3272:
3270:
3267:
3265:
3262:
3260:
3257:
3255:
3252:
3250:
3247:
3245:
3242:
3240:
3237:
3235:
3232:
3230:
3227:
3225:
3222:
3220:
3217:
3215:
3212:
3210:
3207:
3205:
3202:
3200:
3197:
3195:
3192:
3190:
3187:
3185:
3182:
3180:
3177:
3175:
3172:
3170:
3167:
3165:
3162:
3160:
3157:
3155:
3152:
3150:
3147:
3145:
3142:
3140:
3137:
3135:
3132:
3130:
3127:
3125:
3122:
3120:
3117:
3115:
3112:
3110:
3107:
3105:
3102:
3100:
3097:
3095:
3092:
3090:
3087:
3085:
3082:
3080:
3077:
3075:
3072:
3070:
3067:
3065:
3062:
3060:
3057:
3055:
3052:
3050:
3047:
3045:
3042:
3041:
3039:
3037:
3031:
3025:
3022:
3020:
3017:
3015:
3012:
3010:
3007:
3005:
3002:
3000:
2997:
2995:
2992:
2990:
2987:
2985:
2982:
2980:
2977:
2975:
2972:
2970:
2967:
2965:
2962:
2960:
2957:
2955:
2952:
2950:
2947:
2945:
2942:
2940:
2937:
2935:
2932:
2930:
2927:
2925:
2922:
2920:
2917:
2915:
2912:
2910:
2907:
2905:
2902:
2900:
2897:
2895:
2892:
2890:
2887:
2885:
2882:
2880:
2877:
2875:
2872:
2870:
2867:
2865:
2862:
2860:
2857:
2855:
2852:
2850:
2847:
2845:
2842:
2840:
2837:
2835:
2832:
2830:
2827:
2825:
2822:
2820:
2817:
2815:
2812:
2810:
2809:Ley oxidation
2807:
2805:
2802:
2800:
2797:
2795:
2792:
2790:
2787:
2785:
2782:
2780:
2777:
2775:
2774:Hydroxylation
2772:
2770:
2767:
2765:
2764:Hydrogenation
2762:
2760:
2757:
2755:
2752:
2750:
2747:
2745:
2742:
2740:
2737:
2735:
2732:
2730:
2727:
2725:
2722:
2720:
2717:
2715:
2712:
2710:
2707:
2705:
2702:
2700:
2699:DNA oxidation
2697:
2695:
2692:
2690:
2689:Deoxygenation
2687:
2685:
2682:
2680:
2677:
2675:
2672:
2670:
2667:
2665:
2662:
2660:
2657:
2655:
2652:
2650:
2647:
2645:
2642:
2640:
2637:
2635:
2632:
2630:
2627:
2625:
2622:
2620:
2617:
2615:
2612:
2610:
2607:
2605:
2602:
2600:
2597:
2595:
2592:
2590:
2587:
2585:
2582:
2580:
2579:Aromatization
2577:
2575:
2572:
2570:
2567:
2565:
2562:
2560:
2557:
2555:
2552:
2550:
2547:
2545:
2542:
2540:
2537:
2536:
2534:
2532:
2526:
2520:
2517:
2515:
2512:
2510:
2507:
2505:
2502:
2500:
2497:
2495:
2492:
2490:
2487:
2485:
2482:
2480:
2477:
2475:
2472:
2470:
2467:
2465:
2462:
2460:
2457:
2455:
2452:
2451:
2449:
2443:
2437:
2434:
2432:
2429:
2427:
2424:
2422:
2419:
2417:
2416:Reed reaction
2414:
2412:
2409:
2407:
2404:
2402:
2399:
2397:
2394:
2392:
2389:
2387:
2384:
2382:
2379:
2377:
2374:
2372:
2369:
2367:
2364:
2362:
2359:
2357:
2354:
2352:
2349:
2347:
2344:
2342:
2339:
2337:
2334:
2333:
2331:
2327:bond forming
2323:
2313:
2310:
2308:
2305:
2303:
2300:
2298:
2295:
2293:
2290:
2288:
2285:
2283:
2280:
2278:
2275:
2273:
2270:
2268:
2265:
2263:
2260:
2258:
2255:
2253:
2250:
2248:
2245:
2243:
2240:
2238:
2235:
2233:
2232:Cope reaction
2230:
2228:
2225:
2223:
2220:
2218:
2215:
2213:
2210:
2209:
2207:
2203:
2197:
2194:
2192:
2189:
2187:
2184:
2182:
2179:
2177:
2174:
2172:
2169:
2167:
2164:
2163:
2161:
2159:
2155:
2149:
2146:
2144:
2141:
2139:
2136:
2134:
2131:
2129:
2126:
2124:
2121:
2119:
2116:
2114:
2111:
2109:
2106:
2104:
2101:
2099:
2096:
2094:
2091:
2089:
2086:
2084:
2081:
2079:
2076:
2074:
2071:
2069:
2066:
2064:
2061:
2059:
2056:
2054:
2051:
2049:
2046:
2044:
2041:
2039:
2036:
2034:
2031:
2029:
2026:
2024:
2021:
2019:
2016:
2014:
2011:
2009:
2006:
2004:
2001:
1999:
1996:
1994:
1991:
1989:
1986:
1984:
1981:
1979:
1976:
1974:
1971:
1969:
1966:
1964:
1961:
1959:
1956:
1954:
1951:
1949:
1946:
1944:
1943:Nef synthesis
1941:
1939:
1936:
1934:
1931:
1929:
1926:
1924:
1921:
1919:
1918:Methylenation
1916:
1914:
1911:
1909:
1906:
1904:
1901:
1899:
1896:
1894:
1891:
1889:
1886:
1884:
1881:
1879:
1876:
1874:
1871:
1869:
1866:
1864:
1861:
1859:
1856:
1854:
1851:
1849:
1846:
1844:
1841:
1839:
1836:
1834:
1831:
1829:
1826:
1824:
1821:
1819:
1816:
1814:
1811:
1809:
1806:
1804:
1801:
1799:
1796:
1794:
1791:
1789:
1788:Heck reaction
1786:
1784:
1781:
1779:
1776:
1774:
1771:
1769:
1766:
1764:
1761:
1759:
1756:
1754:
1751:
1749:
1746:
1744:
1741:
1739:
1736:
1734:
1731:
1729:
1726:
1724:
1721:
1719:
1716:
1714:
1711:
1709:
1706:
1704:
1701:
1699:
1696:
1694:
1691:
1689:
1686:
1684:
1681:
1679:
1676:
1674:
1671:
1669:
1666:
1664:
1661:
1659:
1656:
1654:
1651:
1649:
1646:
1644:
1641:
1639:
1636:
1634:
1631:
1629:
1626:
1624:
1621:
1619:
1616:
1614:
1611:
1609:
1606:
1604:
1601:
1599:
1596:
1594:
1591:
1589:
1586:
1584:
1581:
1579:
1576:
1574:
1571:
1569:
1566:
1564:
1561:
1559:
1556:
1554:
1551:
1549:
1546:
1544:
1541:
1539:
1536:
1534:
1531:
1529:
1526:
1524:
1521:
1519:
1516:
1514:
1511:
1509:
1506:
1504:
1501:
1499:
1496:
1494:
1491:
1489:
1486:
1484:
1481:
1479:
1476:
1475:
1473:
1469:bond forming
1465:
1461:
1456:
1450:
1447:
1445:
1442:
1440:
1437:
1435:
1434:Y-aromaticity
1432:
1430:
1427:
1425:
1422:
1420:
1419:Walsh diagram
1417:
1415:
1412:
1410:
1407:
1405:
1404:Taft equation
1402:
1400:
1397:
1395:
1392:
1390:
1387:
1385:
1382:
1380:
1377:
1375:
1374:Σ-aromaticity
1372:
1370:
1367:
1365:
1362:
1360:
1357:
1355:
1352:
1350:
1347:
1345:
1342:
1340:
1337:
1335:
1332:
1330:
1327:
1325:
1322:
1320:
1317:
1315:
1312:
1310:
1307:
1305:
1302:
1300:
1299:Marcus theory
1297:
1295:
1292:
1290:
1287:
1285:
1282:
1280:
1277:
1275:
1274:Hückel's rule
1272:
1270:
1267:
1265:
1262:
1260:
1257:
1255:
1252:
1250:
1247:
1245:
1242:
1240:
1237:
1235:
1232:
1230:
1229:Evelyn effect
1227:
1225:
1222:
1220:
1217:
1215:
1212:
1210:
1209:Electron-rich
1207:
1205:
1202:
1200:
1197:
1195:
1192:
1190:
1187:
1185:
1182:
1180:
1177:
1175:
1172:
1170:
1167:
1165:
1162:
1160:
1157:
1155:
1152:
1150:
1147:
1145:
1142:
1140:
1137:
1135:
1132:
1130:
1127:
1125:
1124:Bema Hapothle
1122:
1120:
1117:
1115:
1112:
1110:
1107:
1105:
1102:
1100:
1097:
1095:
1092:
1090:
1087:
1085:
1082:
1080:
1077:
1075:
1072:
1071:
1068:
1062:
1059:
1057:
1054:
1052:
1049:
1047:
1044:
1042:
1039:
1037:
1034:
1032:
1029:
1027:
1024:
1022:
1019:
1017:
1014:
1013:
1010:
1006:
998:
993:
991:
986:
984:
979:
978:
975:
969:
966:
964:
961:
960:
948:
944:
940:
936:
935:
930:
924:
916:
912:
908:
904:
903:
898:
894:
890:
886:
882:
875:
867:
863:
860:: 1430–1440.
859:
858:
850:
842:
838:
834:
830:
829:
824:
817:
809:
805:
801:
797:
796:
788:
780:
776:
772:
769:(in German).
768:
767:
762:
761:Fischer, Hans
756:
747:
742:
738:
734:
733:
728:
721:
713:
709:
705:
702:(in German).
701:
700:
695:
691:
685:
678:(1): 546–554.
677:
673:
672:
667:
660:
652:
648:
644:
640:
639:
631:
623:
619:
615:
611:
610:
602:
593:
587:
583:
579:
575:
574:
569:
565:
564:Fischer, Hans
559:
550:
544:
540:
536:
532:
531:
526:
522:
521:Fischer, Hans
516:
508:
504:
500:
493:
485:
481:
477:
474:(in German).
473:
472:
467:
463:
457:
449:
445:
441:
438:(in German).
437:
436:
431:
427:
426:Knorr, Ludwig
421:
413:
409:
405:
402:(in German).
401:
400:
395:
391:
390:Knorr, Ludwig
385:
381:
374:
372:
368:
364:
359:
358:David Dolphin
353:
349:
347:
346:ethyl formate
343:
339:
334:
330:
317:
313:
311:
306:
304:
300:
296:
292:
288:
284:
280:
276:
260:
259:acetylacetone
255:
253:
251:
246:
242:
235:(as well as R
226:
222:
218:
214:
212:
207:
203:
199:
198:sulfuric acid
195:
190:
176:
172:
163:
159:
157:
153:
149:
145:
141:
136:
134:
130:
126:
112:
108:
106:
103:
99:
96:
92:
88:
85:
81:
77:
67:
63:
59:
56:
53:
52:
47:
44:
41:
38:
37:
34:
31:
28:
27:
22:
19:
4187:
3743:
3224:Ene reaction
2584:Autoxidation
2445:Degradation
2390:
2336:Azo coupling
2113:Ugi reaction
1867:
1713:Ene reaction
1513:Alkynylation
1364:Polyfluorene
1359:Polar effect
1224:Electrophile
1139:Bredt's rule
1109:Baird's rule
1079:Alpha effect
938:
932:
923:
906:
900:
896:
892:
888:
884:
874:
855:
849:
832:
826:
816:
799:
793:
787:
770:
764:
755:
736:
730:
720:
703:
697:
693:
684:
675:
669:
659:
642:
636:
630:
613:
607:
601:
591:
577:
571:
558:
548:
534:
528:
515:
502:
492:
475:
469:
456:
439:
433:
420:
403:
397:
384:
366:
362:
354:
350:
333:Hans Fischer
331:
327:
310:tautomerizes
307:
302:
298:
294:
290:
286:
282:
281:. Benzyl or
256:
249:
210:
191:
174:
168:
137:
124:
122:
104:
97:
86:
75:
73:
62:RXNO:0000497
57:ontology ID
49:Identifiers
33:Ludwig Knorr
29:Named after
18:
1723:Ethenolysis
1369:Ring strain
1339:Nucleophile
1164:Clar's rule
1104:Aromaticity
881:Dolphin, D.
269:= Me, and R
4286:Categories
4007:Ozonolysis
3534:Annulation
2884:Ozonolysis
1003:Topics in
377:References
342:2-butanone
171:exothermic
131:, via the
3521:reactions
3036:reactions
2531:reactions
2447:reactions
2329:reactions
1471:reactions
462:Knorr, L.
1414:Vinylogy
1084:Annulene
1031:Reagents
957:See also
566:(1941).
523:(1935).
428:(1886).
392:(1884).
371:Rapoport
279:diborane
84:pyrroles
1074:A value
501:(ed.).
125:in situ
580:: 67.
537:: 17.
213:-butyl
206:benzyl
119:Method
95:ketone
507:Wiley
344:with
231:and R
219:over
156:oxime
129:oxime
91:amino
694:tert
287:tert
283:tert
250:tert
211:tert
152:Zinc
74:The
943:doi
911:doi
862:doi
837:doi
804:doi
775:doi
771:283
741:doi
708:doi
647:doi
618:doi
582:doi
539:doi
480:doi
444:doi
440:236
408:doi
340:of
265:= R
208:or
185:= R
105:(2)
98:(1)
87:(3)
55:RSC
4288::
939:36
937:.
907:52
905:.
833:50
831:.
800:77
798:.
737:44
735:.
729:.
704:87
676:24
643:43
641:.
614:64
612:.
589:;
578:21
576:.
570:.
546:;
535:15
533:.
527:.
476:35
404:17
348:.
243:,
135:.
107:.
996:e
989:t
982:v
949:.
945::
917:.
913::
897:N
895:,
893:N
889:N
887:,
885:N
868:.
864::
843:.
839::
810:.
806::
781:.
777::
749:.
743::
714:.
710::
653:.
649::
624:.
620::
596:.
584::
553:.
541::
486:.
482::
450:.
446::
414:.
410::
367:N
365:,
363:N
303:N
301:,
299:N
295:N
293:,
291:N
271:2
267:3
263:1
237:2
233:3
229:1
187:3
183:1
179:2
93:-
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.