422:
minimized orientation of the sp center, display one face of an olefin outwards from the ring. Addition of reagents from the outside the olefin face and the ring (peripheral attack) is thus favored, while attack from across the ring on the inward diastereoface is disfavored. Ground state conformations dictate the exposed face of the reactive site of the macrocycle, thus both local and distant stereocontrol elements must be considered. The peripheral attack model holds well for several classes of macrocycles, though relies on the assumption that ground state geometries remain unperturbed in the corresponding
411:
536:
463:
632:
154:
550:
431:
452:
348:
entire structure. For example, in methyl cyclodecane, the ring can be expected to adopt the minimized conformation of boat-chair-boat. The figure below shows the energetic penalty between placing the methyl group at certain sites within the boat-chair-boat structure. Unlike canonical small ring systems, the cyclodecane system with the methyl group placed at the "corners" of the structure exhibits no preference for axial vs. equatorial positioning due to the presence of an unavoidable
373:
308:
516:
cladiell-11-ene-3,6,7-triol makes use of macrocyclic stereocontrol in the dihydroxylation of a trisubstituted olefin. Below is shown the synthetic step controlled by the ground state conformation of the macrocycle, allowing stereoselective dihydroxylation without the usage of an asymmetric reagent. This example of substrate controlled addition is an example of the peripheral attack model in which two centers on the molecule are added two at once in a concerted fashion.
326:
144:
38:
496:
480:
repulsive steric interactions provides the observed product by having the lowest barrier to a transition state for the reaction. Though no external attack by a reagent occurs, this reaction can be thought of similarly to those modeled with peripheral attack; the lowest energy conformation is the most likely to react for a given reaction.
368:
preferences of a molecule. In conjunction with remote substituent effects, local acyclic interactions can also play an important role in determining the outcome of macrocyclic reactions. The conformational flexibility of larger rings potentially allows for a combination of acyclic and macrocyclic stereocontrol to direct reactions.
474:
532:
directed using only ground state conformational preferences and the peripheral attack model. Reacting from the most stable boat-chair-boat conformation, asymmetric epoxidation of the cis-internal olefin can be achieved without using a reagent-controlled epoxidation method or a directed epoxidation with an allylic alcohol.
442:
337:
257:
231:
reaction, providing stereocontrol such as in the synthesis of miyakolide. Computational modeling can predict conformations of medium rings with reasonable accuracy, as Still used molecular mechanics modeling computations to predict ring conformations to determine potential reactivity and stereochemical outcomes.
297:
490:
The lowest energy conformations of macrocycles also influence intramolecular reactions involving transannular bond formation. In the intramolecular
Michael addition sequence below, the ground state conformation minimizes transannular interactions by placing the sp centers at the appropriate vertices,
468:
Conjugate addition to the E-enone below also follows the expected peripheral attack model to yield predominantly trans product. High selectivity in this addition can be attributed to the placement of sp centers such that transannular nonbonded interactions are minimized, while also placing the methyl
457:
However, 10-membered cyclic lactones display significant diastereoselectivity. The proximity of the methyl group to the ester linkage was directly correlated with the diastereomeric ratio of the reaction products, with placement at the 9 position (below) yielding the highest selectivity. In contrast,
347:
These ground-state conformational preferences are useful analogies to more highly functionalized macrocyclic ring systems, where local effects can still be governed to first approximation by energy minimized conformations even though the larger ring size allows more conformational flexibility of the
383:
The stereochemical result of a given reaction on a macrocycle capable of adopting several conformations can be modeled by a Curtin-Hammett scenario. In the diagram below, the two ground state conformations exist in an equilibrium, with some difference in their ground state energies. Conformation B
247:
nonbonded interactions within the ring. Medium rings (8-11 atoms) are the most strained with between 9-13 (kcal/mol) strain energy; analysis of the factors important in considering larger macrocyclic conformations can thus be modeled by looking at medium ring conformations. Conformational analysis
287:
Substitution positional preferences in the ground state conformer of methyl cyclooctane can be approximated using parameters similar to those for smaller rings. In general, the substituents exhibit preferences for equatorial placement, except for the lowest energy structure (pseudo A-value of -0.3
302:
These energetic differences can help rationalize the lowest energy conformations of 8 atom ring structures containing an sp center. In these structures, the chair-boat is the ground state model, with substitution forcing the structure to adopt a conformation such that non-bonded interactions are
479:
Similar to intermolecular reactions, intramolecular reactions can show significant stereoselectivity from the ground state conformation of the molecule. In the intramolecular Diels-Alder reaction depicted below, the lowest energy conformation yields the observed product. The structure minimizing
421:
Macrocyclic rings containing sp centers display a conformational preference for the sp centers to avoid transannular nonbonded interactions by orienting perpendicular to the plan of the ring. Clark W. Still proposed that the ground state conformations of macrocyclic rings, containing the energy
320:
cyclooctanes provided proof of conformational preferences in these medium rings. Significantly, calculated models matched the obtained X-ray data, indicating that computational modeling of these systems could in some cases quite accurately predict conformations. The increased sp character of the
230:
The degree to which a macrocyclic ring is either rigid or floppy depends significantly on the substitution of the ring and the overall size. Significantly, even small conformational preferences, such as those envisioned in floppy macrocycles, can profoundly influence the ground state of a given
531:
The synthesis of (±)-periplanone B is a prominent example of macrocyclic stereocontrol. Periplanone B is a sex pheromone of the
American female cockroach, and has been the target of several synthetic attempts. Significantly, two reactions on the macrocyclic precursor to (±)-periplanone B were
506:
These principles have been applied in multiple natural product targets containing medium and large rings. The syntheses of cladiell-11-ene-3,6,7- triol, (±)-periplanone B, eucannabinolide, and neopeltolide are all significant in their usage of macrocyclic stereocontrol en route to obtaining the
485:
367:
Similar principles guide the lowest energy conformations of larger ring systems. Along with the acyclic stereocontrol principles outlined below, subtle interactions between remote substituents in large rings, analogous to those observed for 8-10 membered rings, can influence the conformational
515:
The cladiellin family of marine natural products possesses interesting molecular architecture, generally containing a 9-membered medium-sized ring. The synthesis of (−)-cladiella-6,11-dien-3-ol allowed access to a variety of other members of the cladiellin family. Notably, the conversion to
581:
Neopeltolide was originally isolated from sponges near the
Jamaican coast and exhibits nanomolar cytoxic activity against several lines of cancer cells. The synthesis of the neopeltolide macrocyclic core displays a hydrogenation controlled by the ground state conformation of the macrocycle.
571:
234:
Reaction classes used in synthesis of natural products under the macrocyclic stereocontrol model for obtaining a desired stereochemistry include: hydrogenations such as in neopeltolide and (±)-methynolide, epoxidations such as in (±)-periplanone B and lonomycin A, hydroborations such as in
587:
436:
Early investigations of macrocyclic stereocontrol studied the alkylation of 8-membered cyclic ketones with varying substitution. In the example below, alkylation of 2-methylcyclooctanone occurred to yield the predominantly trans product. Proceeding from the lowest energy conformation of
545:
of the ketone was achieved, and can be modeled by peripheral attack of the sulfur ylide on the carbonyl group in a
Johnson-Corey-Chaykovsky reaction to yield the protected form of (±)-periplanone B. Deprotection of the alcohol followed by oxidation yielded the desired natural product.
458:
when the methyl group was placed at the 7 position, a 1:1 mixture of diastereomers was obtained. Placement of the methyl group at the 9-position in the axial position yields the most stable ground state conformation of the 10-membered ring leading to high diastereoselectivity.
521:
288:
kcal/mol in figure below) in which axial substitution is favored. The "pseudo A-value" is best treated as the approximate energy difference between placing the methyl substituent in the equatorial or axial positions. The most energetically unfavorable interaction involves
388:
to its transition state in a hypothetical reaction, thus the product formed is predominantly product B (P B) arising from conformation B via transition state B (TS B). The inherent preference of a ring to exist in one conformation over another provides a tool for
343:
Similar to cyclooctane, a cyclodecane ring exhibits several conformations with two lower energy conformations. The boat-chair-boat conformation is energetically minimized, while the chair-chair-chair conformation has significant eclipsing interactions.
401:, the free energy difference, which can, at some level, be estimated from conformational analysis. The free energy difference between the two transition states of each conformation on its path to product formation is given by ΔΔG. The value of ΔG
437:
2-methylcycloctanone, peripheral attack is observed from either one of the low energy (energetic difference of 0.5 (kcal/mol)) enolate conformations, resulting in a trans product from either of the two depicted transition state conformations.
566:. Significantly, the synthesis of eucannabinolide relied on the usage of molecular mechanics (MM2) computational modeling to predict the lowest energy conformation of the macrocycle to design substrate-controlled stereochemical reactions.
405:
between not just one, but many accessible conformations is the underlying energetic impetus for reactions occurring from the most stable ground state conformation and is the crux of the peripheral attack model outlined below.
226:
in the late 1970s and 1980s challenged this assumption, while several others found crystallographic data and NMR data that suggested macrocyclic rings were not the floppy, conformationally ill-defined species many assumed.
110:
macrocycles. The central challenge to macrocyclization is that ring-closing reactions do not favor the formation of large rings. Instead, small rings or polymers tend to form. This kinetic problem can be addressed by using
357:
303:
minimized from the parent structure. From the cyclooctene figure below, it can be observed that one face is more exposed than the other, foreshadowing a discussion of privileged attack angles (see peripheral attack).
561:
In the synthesis of the cytotoxic germacranolide sesquiterpene eucannabinolide, Still demonstrates the application of the peripheral attack model to the reduction of a ketone to set a new stereocenter using
283:
interactions (shown in blue), as well as torsional strain. The chair-chair conformation is the second most abundant conformation at room temperature, with a ratio of 96:4 chair-boat:chair-chair observed.
352:
interaction in both conformations. Significantly more intense interactions develop when the methyl group is placed in the axial position at other sites in the boat-chair-boat conformation.
469:
substitution in the more energetically favorable position for cyclodecane rings. This ground state conformation heavily biases conjugate addition to the less hindered diastereoface.
235:
9-dihydroerythronolide B, enolate alkylations such as in (±)-3-deoxyrosaranolide, dihydroxylations such as in cladiell-11-ene-3,6,7-triol, and reductions such as in eucannabinolide.
126:
are often generated in the presence of an alkali metal cation, which organizes the condensing components by complexation. An illustrative macrocyclization is the synthesis of (−)-
89:: Cyclic macromolecule or a macromolecular cyclic portion of a macromolecule. Note 1: A cyclic macromolecule has no end-groups but may nevertheless be regarded as a chain.
1640:
Marsault, Eric; Peterson, Mark L. (2011-04-14). "Macrocycles Are Great Cycles: Applications, Opportunities, and
Challenges of Synthetic Macrocycles in Drug Discovery".
983:
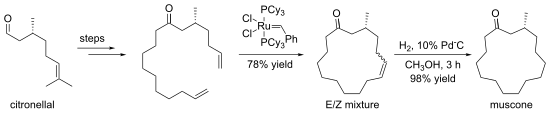
Kamat, V.P.; Hagiwara, H.; Katsumi, T.; Hoshi, T.; Suzuki, T.; Ando, M. (2000). "Ring
Closing Metathesis Directed Synthesis of (R)-(−)-Muscone from (+)-Citronellal".
289:
92:
Note 2: In the literature, the term macrocycle is sometimes used for molecules of low relative molecular mass that would not be considered macromolecules.
760:
Zhichang Liu; Siva
Krishna Mohan Nalluria; J. Fraser Stoddart (2017). "Surveying macrocyclic chemistry: from flexible crown ethers to rigid cyclophanes".
79:
317:
122:. Templates are ions, molecules, surfaces etc. that bind and pre-organize compounds, guiding them toward formation of a particular ring size. The
349:
199:
rings is well established in organic chemistry, in large part due to the axial/equatorial preferential positioning of substituents on the ring.
272:
268:
248:
of odd-membered rings suggests they tend to reside in less symmetrical forms with smaller energy differences between stable conformations.
276:
1079:
J. D. Dunitz. Perspectives in
Structural Chemistry (Edited by J. D. Dunitz and J. A. Ibers), Vol. 2, pp. l-70; Wiley, New York (1968)
922:
Gerbeleu, Nicolai V.; Arion, Vladimir B.; Burgess, John (2007). Nicolai V. Gerbeleu; Vladimir B. Arion; John
Burgess (eds.).
397:
are significant considerations in this scenario. The preference for one conformation over another can be characterized by ΔG
796:
393:
control of reactions by biasing the ring into a given configuration in the ground state. The energy differences, ΔΔG and ΔG
1572:
Choi, Kihang; Hamilton, Andrew D. (2003). "Macrocyclic anion receptors based on directed hydrogen bonding interactions".
624:
across hydrophobic membranes and solvents. The macrocycle envelops the ion with a hydrophobic sheath, which facilitates
243:
Macrocycles can access a number of stable conformations, with preferences to reside in those that minimize the number of
1029:
1724:
Iyoda, Masahiko; Yamakawa, Jun; Rahman, M. Jalilur (2011-11-04). "Conjugated
Macrocycles: Concepts and Applications".
447:
Unlike the cyclooctanone case, alkylation of 2-cyclodecanone rings does not display significant diastereoselectivity.
939:
873:"Macrocyclization Reactions: The Importance of Conformational, Configurational, and Template-Induced Preorganization"
832:
214:
Early assumptions towards macrocycles in synthetic chemistry considered them far too floppy to provide any degree of
1687:
Chambron, J-C.; Dietrich-Buchecker, C.; Hemmert, C.; Khemiss, A-K.; Mitchell, D.; Sauvage, J-P.; Weiss, J. (1990).
870:
356:
798:
IUPAC. Compendium of Polymer Terminology and Nomenclature, IUPAC Recommendations 2008 (the "Purple Book")
17:
679:
321:
cyclopropane rings favor them to be placed similarly such that they relieve non-bonded interactions.
143:
1518:"Classics in Stereoselective Synthesis". Carreira, Erick M.; Kvaerno, Lisbet. Weinheim: Wiley-VCH,
850:"Cyclic and Macrocyclic OrganicCompounds – a Personal Review in Honor of Professor Leopold Ružička"
186:
625:
279:. Cyclooctane prefers to reside in a chair-boat conformation, minimizing the number of eclipsing
182:
135:
795:
R. G. Jones; J. Kahovec; R. Stepto; E. S. Wilks; M. Hess; T. Kitayama; W. V. Metanomski (2008).
1688:
112:
985:
267:. Spectroscopic methods have determined that cyclooctane possesses three main conformations:
1686:
957:"Macrocyclic Polyethers: Dibenzo-18-Crown-6 Polyether and Dicyclohexyl-18-Crown-6 Polyether"
871:
Vicente Martí-Centelles; Mrituanjay D. Pandey; M. Isabel Burguete; Santiago V. Luis (2015).
1770:
157:
617:. These rings arise from multistep biosynthetic processes that also feature macrocycles.
410:
8:
759:
211:
elements providing enough conformational influence to direct the outcome of a reaction.
1712:
1154:
1129:
691:
674:
28:
1585:
1128:
Kamenik, Anna S.; Lessel, Uta; Fuchs, Julian E.; Fox, Thomas; Liedl, Klaus R. (2018).
998:
737:
712:
535:
1749:
1741:
1665:
1657:
1622:
1159:
1025:
961:
935:
904:
828:
777:
742:
385:
204:
192:
119:
1716:
1733:
1704:
1696:
1649:
1612:
1581:
1149:
1141:
1130:"Peptidic Macrocycles - Conformational Sampling and Thermodynamic Characterization"
1017:
994:
927:
894:
884:
820:
769:
732:
724:
423:
56:
27:
For the molecular effect giving increased stability to coordination complexes, see
1021:
631:
1601:"Design, Properties and Recent Application of Macrocycles in Medicinal Chemistry"
728:
549:
462:
390:
203:
stereocontrol models the substitution and reactions of medium and large rings in
170:
1392:
Anet, F.A.L.; St. Jacques, M.; Henrichs, P.M.; Cheng, A.K.; Krane, J.; Wong, L.
956:
889:
872:
814:
153:
667:
602:
473:
223:
219:
215:
178:
115:, whereby intramolecular processes are favored relative to polymerizations.
1764:
1745:
1661:
1145:
621:
441:
336:
1708:
1617:
1600:
430:
1753:
1737:
1669:
1626:
1163:
982:
908:
781:
451:
256:
244:
72:
41:
931:
824:
746:
325:
307:
794:
605:. Many metallocofactors are bound to macrocyclic ligands, which include
542:
495:
264:
208:
196:
131:
123:
60:
899:
372:
773:
643:
Macrocycles are often bioactive and could be useful for drug delivery.
636:
384:
is lower in energy than conformation A, and while possessing a similar
296:
64:
1653:
606:
598:
107:
68:
45:
817:
Modern Supramolecular Chemistry: Strategies for Macrocycle Synthesis
597:
One important application are the many macrocyclic antibiotics, the
815:
François Diederich; Peter J. Stang; Rik R. Tykwinski, eds. (2008).
662:
657:
652:
484:
263:
Conformational analysis of medium rings begins with examination of
1286:
Mulzer, J.; Kirstein, H.M.; Buschmann, J.; Lehmann, C.; Luger, P.
570:
614:
586:
127:
37:
610:
280:
849:
292:
at the vertex of the boat portion of the ring (6.1 kcal/mol).
520:
313:
713:"Chemistry and Biology of the Polyene Macrolide Antibiotics"
378:
1176:
Evans, D. A.; Ripin, D.H.B.; Halstead, D.P.; Campos, K. R.
1011:
48:
antibiotic, is one of many naturally occurring macrocycles.
75:. Macrocycles describe a large, mature area of chemistry.
1551:
Scheerer, J.R.; Lawrence, J.F.; Wang, G.C.; Evans, D.A.
1266:
Evans, D.A.; Ratz, A.M.; Huff, B.E.; and Sheppard, G.S.
1012:
Paul R. Ortiz de Montellano (2008). "Hemes in Biology".
59:
of twelve or more atoms. Classical examples include the
102:
The formation of macrocycles by ring-closure is called
55:
are often described as molecules and ions containing a
1127:
1689:"Interlacing molecular threads on transition metals"
921:
1723:
1355:Still, W.C.; Murata, S.; Revial, G.; Yoshihara, K.
1123:
1121:
854:Cyclic and Macrocyclic Organic Compounds, Kem. Ind
501:
1762:
1639:
1471:
1469:
1118:
710:
592:
510:
416:
106:. Pioneering work was reported for studies on
1514:
1512:
1466:
238:
1571:
1452:Schreiber, S. L.; Smith, D. B.; Schulte, G.
1332:Kim, H.; Lee, H.; Kim, J.; Kim, S.; Kim, D.
1134:Journal of Chemical Information and Modeling
635:The potassium (K) complex of the macrocycle
548:
491:while also minimizing diaxial interactions.
1375:Eliel, E.L., Wilen, S.H. and Mander, L.S. (
924:Template Synthesis of Macrocyclic Compounds
620:Macrocycles often bind ions and facilitate
1605:CHIMIA International Journal for Chemistry
1509:
1351:
1349:
1305:
1303:
1242:
1240:
1238:
1236:
222:control in a reaction. The experiments of
185:chemical reaction that is governed by the
177:refers to the directed outcome of a given
1616:
1219:Vedejs, E.; Buchanan, R.A.; Watanabe, Y.
1153:
1088:Anet, F. A. L.; Degen, P. J.; Yavari. I.
898:
888:
847:
736:
379:Reactivity and conformational preferences
118:Some macrocyclizations are favored using
1108:Casarini, D.; Lunazzi, L.; Mazzanti, A.
1055:
1053:
1051:
1049:
1047:
1045:
1043:
1041:
1016:. John Wiley & Sons. pp. 1–10.
976:
954:
630:
152:
134:. The 15-membered ring is generated by
36:
1726:Angewandte Chemie International Edition
1346:
1300:
1233:
160:, biosynthetic precursor to porphyrins.
14:
1763:
1598:
1328:
1326:
1014:Wiley Encyclopedia of Chemical Biology
362:
1381:Stereochemistry of Organic Compounds,
1195:
1193:
1038:
1005:
1432:Pawar, D.M.; Moody, E.M.; Noe, E.A.
1383:John Wiley and Sons, Inc., New York.
526:
34:Molecule with a large ring structure
1323:
24:
1680:
1633:
1190:
556:
534:
335:
255:
142:
25:
1782:
804:. RSC Publishing, Cambridge, UK.
585:
569:
519:
494:
483:
472:
461:
450:
440:
429:
409:
371:
355:
324:
306:
295:
164:
1592:
1565:
1545:
1525:
1489:
1446:
1426:
1406:
1386:
1369:
1280:
1260:
1213:
1170:
1102:
1082:
1073:
646:
576:
502:Prominent examples in synthesis
1642:Journal of Medicinal Chemistry
1599:Ermert, Philipp (2017-10-25).
1574:Coordination Chemistry Reviews
948:
915:
864:
841:
808:
788:
753:
704:
331:
251:
13:
1:
1586:10.1016/s0010-8545(02)00305-3
1412:Petasis, N. A.; Patane, M.A.
1022:10.1002/9780470048672.wecb221
999:10.1016/S0040-4020(00)00333-1
955:Pedersen, Charles J. (1988).
697:
729:10.1128/br.37.2.166-196.1973
711:Hamilton-Miller, JM (1973).
507:desired structural targets.
189:preference of a macrocycle.
147:Synthesis of muscone via RCM
97:
7:
1059:Still, W. C.; Galynker, I.
890:10.1021/acs.chemrev.5b00056
685:
593:Occurrence and applications
511:Cladiell-11-ene-3,6,7-triol
417:The peripheral attack model
10:
1787:
1309:Still, W.C.; Novack, V.J.
1199:Tu, W.; Floreancig, P. E.
971:, vol. 6, p. 395
239:Conformational preferences
26:
680:Macrocyclic stereocontrol
175:macrocyclic stereocontrol
1146:10.1021/acs.jcim.8b00097
762:Chemical Society Reviews
1709:10.1351/pac199062061027
1618:10.2533/chimia.2017.678
717:Bacteriological Reviews
136:ring-closing metathesis
113:high-dilution reactions
1738:10.1002/anie.201006198
640:
553:
539:
340:
260:
161:
148:
94:
49:
1201:Angew. Chem. Int. Ed.
932:10.1002/9783527613809
825:10.1002/9783527621484
634:
552:
538:
339:
259:
156:
146:
84:
40:
158:Uroporphyrinogen III
1732:(45): 10522–10553.
363:Larger ring systems
1531:Deslongchamps, P.
1475:Deslongchamps, P.
1110:Eur. J. Org. Chem.
848:H. Höcker (2009).
774:10.1039/c7cs00185a
692:Macrocyclic ligand
675:Effective molarity
641:
554:
540:
341:
290:axial substitution
261:
162:
149:
120:template reactions
50:
29:Macrocyclic effect
1654:10.1021/jm1012374
1553:J. Am. Chem. Soc.
1533:J. Am. Chem. Soc.
1357:J. Am. Chem. Soc.
1334:J. Am. Chem. Soc.
1311:J. Am. Chem. Soc.
1288:J. Am. Chem. Soc.
1268:J. Am. Chem. Soc.
1248:J. Am. Chem. Soc.
1221:J. Am. Chem. Soc.
1178:J. Am. Chem. Soc.
993:(26): 4397–4403.
969:Collected Volumes
962:Organic Syntheses
883:(16): 8736–8834.
527:(±)-Periplanone B
426:of the reaction.
205:organic chemistry
16:(Redirected from
1778:
1757:
1720:
1697:Pure Appl. Chem.
1693:
1674:
1673:
1648:(7): 1961–2004.
1637:
1631:
1630:
1620:
1596:
1590:
1589:
1580:(1–2): 101–110.
1569:
1563:
1549:
1543:
1529:
1523:
1516:
1507:
1493:
1487:
1477:Pure Appl. Chem.
1473:
1464:
1450:
1444:
1430:
1424:
1410:
1404:
1390:
1384:
1373:
1367:
1353:
1344:
1330:
1321:
1307:
1298:
1284:
1278:
1264:
1258:
1244:
1231:
1217:
1211:
1197:
1188:
1174:
1168:
1167:
1157:
1125:
1116:
1106:
1100:
1086:
1080:
1077:
1071:
1057:
1036:
1035:
1009:
1003:
1002:
980:
974:
972:
965:
952:
946:
945:
919:
913:
912:
902:
892:
868:
862:
861:
845:
839:
838:
812:
806:
805:
803:
792:
786:
785:
768:(9): 2459–2478.
757:
751:
750:
740:
708:
589:
573:
523:
498:
487:
476:
465:
454:
444:
433:
424:transition state
413:
375:
359:
328:
310:
299:
21:
1786:
1785:
1781:
1780:
1779:
1777:
1776:
1775:
1761:
1760:
1691:
1683:
1681:Further reading
1678:
1677:
1638:
1634:
1611:(10): 678–702.
1597:
1593:
1570:
1566:
1550:
1546:
1530:
1526:
1517:
1510:
1494:
1490:
1474:
1467:
1451:
1447:
1431:
1427:
1411:
1407:
1391:
1387:
1374:
1370:
1354:
1347:
1331:
1324:
1308:
1301:
1285:
1281:
1265:
1261:
1245:
1234:
1218:
1214:
1198:
1191:
1175:
1171:
1126:
1119:
1107:
1103:
1087:
1083:
1078:
1074:
1058:
1039:
1032:
1010:
1006:
981:
977:
967:
953:
949:
942:
920:
916:
869:
865:
846:
842:
835:
813:
809:
801:
793:
789:
758:
754:
709:
705:
700:
688:
649:
595:
590:
579:
574:
565:
559:
557:Eucannabinolide
529:
524:
513:
504:
499:
488:
477:
466:
455:
445:
434:
419:
414:
404:
400:
396:
391:stereoselective
381:
376:
365:
360:
334:
329:
311:
300:
254:
241:
171:stereochemistry
167:
104:macrocylization
100:
95:
83:
35:
32:
23:
22:
15:
12:
11:
5:
1784:
1774:
1773:
1759:
1758:
1721:
1703:(6): 1027–34.
1682:
1679:
1676:
1675:
1632:
1591:
1564:
1544:
1542:, 13989-13995.
1524:
1508:
1495:Seeman, J. I.
1488:
1465:
1445:
1425:
1405:
1385:
1368:
1345:
1343:, 15851-15855.
1322:
1299:
1279:
1259:
1232:
1212:
1189:
1169:
1140:(5): 982–992.
1117:
1101:
1081:
1072:
1037:
1031:978-0470048672
1030:
1004:
975:
947:
940:
914:
863:
840:
833:
807:
787:
752:
723:(2): 166–196.
702:
701:
699:
696:
695:
694:
687:
684:
683:
682:
677:
671:
670:
668:Molecular knot
665:
660:
655:
648:
645:
626:phase transfer
603:clarithromycin
594:
591:
584:
578:
575:
568:
563:
558:
555:
528:
525:
518:
512:
509:
503:
500:
493:
482:
471:
460:
449:
439:
428:
418:
415:
408:
402:
398:
394:
386:energy barrier
380:
377:
370:
364:
361:
354:
333:
330:
323:
318:functionalized
305:
294:
253:
250:
240:
237:
224:W. Clark Still
216:stereochemical
207:, with remote
187:conformational
183:intramolecular
179:intermolecular
166:
163:
151:
150:
99:
96:
78:
77:
33:
9:
6:
4:
3:
2:
1783:
1772:
1769:
1768:
1766:
1755:
1751:
1747:
1743:
1739:
1735:
1731:
1727:
1722:
1718:
1714:
1710:
1706:
1702:
1699:
1698:
1690:
1685:
1684:
1671:
1667:
1663:
1659:
1655:
1651:
1647:
1643:
1636:
1628:
1624:
1619:
1614:
1610:
1606:
1602:
1595:
1587:
1583:
1579:
1575:
1568:
1561:
1557:
1554:
1548:
1541:
1537:
1534:
1528:
1521:
1515:
1513:
1505:
1501:
1498:
1492:
1485:
1481:
1478:
1472:
1470:
1462:
1458:
1455:
1454:J. Org. Chem.
1449:
1442:
1438:
1435:
1434:J. Org. Chem.
1429:
1422:
1418:
1415:
1409:
1402:
1398:
1395:
1389:
1382:
1378:
1372:
1365:
1361:
1358:
1352:
1350:
1342:
1338:
1335:
1329:
1327:
1319:
1315:
1312:
1306:
1304:
1296:
1292:
1289:
1283:
1276:
1272:
1269:
1263:
1256:
1252:
1249:
1243:
1241:
1239:
1237:
1229:
1225:
1222:
1216:
1209:
1205:
1202:
1196:
1194:
1186:
1182:
1179:
1173:
1165:
1161:
1156:
1151:
1147:
1143:
1139:
1135:
1131:
1124:
1122:
1114:
1111:
1105:
1098:
1094:
1091:
1090:J. Org. Chem.
1085:
1076:
1069:
1065:
1062:
1056:
1054:
1052:
1050:
1048:
1046:
1044:
1042:
1033:
1027:
1023:
1019:
1015:
1008:
1000:
996:
992:
988:
987:
979:
970:
964:
963:
958:
951:
943:
941:9783527613809
937:
933:
929:
926:. Wiley-VCH.
925:
918:
910:
906:
901:
896:
891:
886:
882:
878:
874:
867:
859:
855:
851:
844:
836:
834:9783527621484
830:
826:
822:
819:. Wiley-VCH.
818:
811:
800:
799:
791:
783:
779:
775:
771:
767:
763:
756:
748:
744:
739:
734:
730:
726:
722:
718:
714:
707:
703:
693:
690:
689:
681:
678:
676:
673:
672:
669:
666:
664:
661:
659:
656:
654:
651:
650:
644:
638:
633:
629:
628:properties.
627:
623:
622:ion transport
618:
616:
612:
608:
604:
600:
588:
583:
572:
567:
551:
547:
544:
537:
533:
522:
517:
508:
497:
492:
486:
481:
475:
470:
464:
459:
453:
448:
443:
438:
432:
427:
425:
412:
407:
392:
387:
374:
369:
358:
353:
351:
350:gauche-butane
345:
338:
327:
322:
319:
315:
309:
304:
298:
293:
291:
285:
282:
278:
274:
270:
266:
258:
249:
246:
236:
232:
228:
225:
221:
220:regiochemical
217:
212:
210:
206:
202:
198:
194:
193:Stereocontrol
190:
188:
184:
180:
176:
172:
165:Stereocontrol
159:
155:
145:
141:
140:
139:
137:
133:
129:
125:
121:
116:
114:
109:
105:
93:
90:
88:
81:
76:
74:
73:cyclodextrins
70:
66:
62:
58:
54:
47:
43:
39:
30:
19:
1729:
1725:
1700:
1695:
1645:
1641:
1635:
1608:
1604:
1594:
1577:
1573:
1567:
1562:, 8968-8969.
1559:
1555:
1552:
1547:
1539:
1535:
1532:
1527:
1519:
1503:
1499:
1496:
1491:
1486:, 1831-1847.
1483:
1479:
1476:
1463:, 5994-5996.
1460:
1456:
1453:
1448:
1443:, 4586-4589.
1440:
1436:
1433:
1428:
1423:, 5757-5821.
1420:
1416:
1413:
1408:
1403:, 1629-1637.
1400:
1396:
1393:
1388:
1380:
1376:
1371:
1363:
1359:
1356:
1340:
1336:
1333:
1320:, 1148-1149.
1317:
1313:
1310:
1294:
1290:
1287:
1282:
1277:, 3448-3467.
1274:
1270:
1267:
1262:
1257:, 2493-2495.
1254:
1250:
1247:
1246:Still, W.C.
1230:, 8430-8438.
1227:
1223:
1220:
1215:
1210:, 4567-4571.
1207:
1203:
1200:
1187:, 6816-6826.
1184:
1180:
1177:
1172:
1137:
1133:
1115:, 2035-2056.
1112:
1109:
1104:
1099:, 3021-3023.
1096:
1092:
1089:
1084:
1075:
1070:, 3981-3996.
1067:
1063:
1060:
1013:
1007:
990:
984:
978:
968:
960:
950:
923:
917:
900:10234/154905
880:
876:
866:
857:
853:
843:
816:
810:
797:
790:
765:
761:
755:
720:
716:
706:
647:Subdivisions
642:
619:
596:
580:
577:Neopeltolide
560:
541:
530:
514:
505:
489:
478:
467:
456:
446:
435:
420:
382:
366:
346:
342:
316:analysis of
312:
301:
286:
262:
245:transannular
242:
233:
229:
213:
200:
191:
174:
168:
124:crown ethers
117:
103:
101:
91:
86:
85:
61:crown ethers
52:
51:
42:Erythromycin
1771:Macrocycles
1414:Tetrahedron
1394:Tetrahedron
1061:Tetrahedron
986:Tetrahedron
543:Epoxidation
332:Cyclodecane
273:chair-chair
265:cyclooctane
252:Cyclooctane
209:stereogenic
201:Macrocyclic
197:cyclohexane
132:citronellal
65:calixarenes
53:Macrocycles
18:Macrocyclic
1522:. pp 1-16.
1497:Chem. Rev.
1366:, 625-627.
1297:, 910-923.
698:References
637:18-crown-6
607:porphyrins
599:macrolides
269:chair-boat
87:Macrocycle
82:definition
69:porphyrins
1746:1521-3773
1662:0022-2623
1506:, 83-134.
877:Chem. Rev
277:boat-boat
130:from (+)-
108:terpenoid
98:Synthesis
46:macrolide
1765:Category
1754:21960431
1717:21741762
1670:21381769
1627:29070413
1164:29652495
909:26248133
860:: 73–80.
782:28462968
686:See also
663:Catenane
658:Rotaxane
653:Cryptand
615:chlorins
1155:5974701
747:4578757
611:corrins
601:, e.g.
128:muscone
1752:
1744:
1715:
1668:
1660:
1625:
1162:
1152:
1028:
938:
907:
831:
780:
745:
738:413810
735:
613:, and
281:ethane
275:, and
71:, and
1713:S2CID
1692:(PDF)
802:(PDF)
314:X-ray
80:IUPAC
1750:PMID
1742:ISSN
1666:PMID
1658:ISSN
1623:PMID
1556:2007
1536:2008
1520:2009
1500:1983
1480:1992
1457:1989
1437:1999
1417:1992
1397:1974
1377:1994
1360:1983
1337:2006
1314:1984
1291:1991
1271:1995
1251:1979
1224:1989
1204:2009
1181:1999
1160:PMID
1113:2010
1093:1978
1064:1981
1026:ISBN
936:ISBN
905:PMID
829:ISBN
778:PMID
743:PMID
562:NaBH
195:for
57:ring
44:, a
1734:doi
1705:doi
1650:doi
1613:doi
1582:doi
1578:240
1560:129
1540:130
1364:105
1341:128
1318:106
1295:113
1275:117
1255:101
1228:111
1185:121
1150:PMC
1142:doi
1018:doi
995:doi
928:doi
895:hdl
885:doi
881:115
821:doi
770:doi
733:PMC
725:doi
218:or
181:or
169:In
1767::
1748:.
1740:.
1730:50
1728:.
1711:.
1701:62
1694:.
1664:.
1656:.
1646:54
1644:.
1621:.
1609:71
1607:.
1603:.
1576:.
1558:,
1511:^
1504:83
1502:,
1484:64
1482:,
1468:^
1461:54
1459:,
1441:64
1439:,
1421:48
1419:,
1401:30
1399:,
1379:)
1362:,
1348:^
1339:,
1325:^
1316:,
1302:^
1293:,
1273:,
1253:,
1235:^
1226:,
1208:48
1206:,
1192:^
1183:,
1158:.
1148:.
1138:58
1136:.
1132:.
1120:^
1097:43
1095:,
1068:37
1066:,
1040:^
1024:.
991:56
989:.
966:;
959:.
934:.
903:.
893:.
879:.
875:.
858:58
856:.
852:.
827:.
776:.
766:46
764:.
741:.
731:.
721:37
719:.
715:.
609:,
271:,
173:,
138:.
67:,
63:,
1756:.
1736::
1719:.
1707::
1672:.
1652::
1629:.
1615::
1588:.
1584::
1538:,
1166:.
1144::
1034:.
1020::
1001:.
997::
973:.
944:.
930::
911:.
897::
887::
837:.
823::
784:.
772::
749:.
727::
639:.
564:4
403:0
399:0
395:0
31:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.