79:
238:
and methyl iodide, which in turn affords, through carbonylation, acetyl iodide. Acetyl iodide reacts with acetate salts or acetic acid to give the anhydride. Rhodium iodides and lithium salts are employed as catalysts. Because acetic anhydride hydrolyzes, the conversion is conducted under anhydrous
634:
366:
Zoeller, J. R.; Agreda, V. H.; Cook, S. L.; Lafferty, N. L.; Polichnowski, S. W.; Pond, D. M. (1992). "Eastman
Chemical Company Acetic Anhydride Process".
276:
368:
202:
in a process that is similar to the
Monsanto acetic acid synthesis. Methyl acetate is used in place of methanol as a source of methyl iodide.
424:
467:
397:
482:
574:
78:
314:
Hartwig, J. F. Organotransition Metal
Chemistry, from Bonding to Catalysis; University Science Books: New York, 2010.
730:
62:
of 150–200 °C and gives a selectivity greater than 99%. It was developed in 1960 by the German chemical company
609:
604:
569:
619:
614:
599:
624:
502:
319:
417:
301:
114:
745:
675:
680:
492:
564:
528:
433:
410:
98:
332:
709:
594:
398:
https://web.archive.org/web/20050412040850/http://www.uyseg.org/catalysis/ethacid/ethacid2.htm
629:
180:
141:
584:
447:
340:
8:
704:
523:
497:
452:
118:
740:
518:
472:
176:
102:
750:
699:
670:
660:
589:
381:
365:
315:
295:
172:
55:
735:
559:
462:
457:
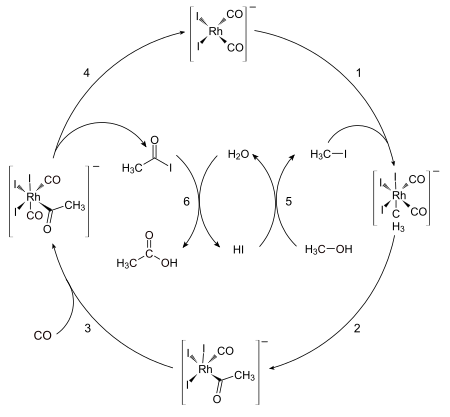
377:
348:
259:
191:
67:
538:
533:
235:
153:
137:
655:
554:
477:
352:
231:
199:
36:
724:
650:
263:
195:
161:
157:
145:
106:
28:
402:
579:
165:
59:
24:
691:
487:
87:
126:
51:
32:
156:
involves two non-organometallic steps: conversion of methanol to
40:
254:
Hosea Cheung, Robin S. Tanke, G. Paul
Torrence "Acetic Acid" in
140:
to form the six-coordinate dicarbonyl complex, which undergoes
133:
129:
122:
239:
conditions in contrast to the
Monsanto acetic acid synthesis.
186:
91:
63:
35:. The Monsanto process has largely been supplanted by the
635:
Arene complexes of univalent gallium, indium, and thallium
47:, which is more economical and environmentally friendly.
136:
complex . This five-coordinate complex then reacts with
333:"The Cativa Process for the Manufacture of Acetic Acid"
44:
117:species . This anion rapidly transforms, via the
70:in 1966, which introduced a new catalyst system.
722:
23:is an industrial method for the manufacture of
256:Ullmann's Encyclopedia of Industrial Chemistry
175:with respect to methyl iodide and . Hence the
418:
432:
330:
82:The catalytic cycle of the Monsanto process
519:Oxidative addition / reductive elimination
425:
411:
283:. Archived from the original on 2014-08-11
187:Tennessee Eastman acetic anhydride process
277:"Production method: The Monsanto process"
16:Method for the manufacture of acetic acid
468:Polyhedral skeletal electron pair theory
723:
406:
575:Transition metal fullerene complexes
179:of methyl iodide is proposed as the
13:
610:Transition metal carbyne complexes
605:Transition metal carbene complexes
570:Transition metal indenyl complexes
171:The reaction has been shown to be
77:
73:
14:
762:
620:Transition metal alkyne complexes
615:Transition metal alkene complexes
391:
625:Transition-metal allyl complexes
132:, affording the pentacoordinate
600:Transition metal acyl complexes
359:
324:
308:
269:
248:
1:
258:, 2002, Wiley-VCH, Weinheim.
242:
97:- (top of scheme). The first
382:10.1016/0920-5861(92)80188-S
43:-based process developed by
7:
676:Shell higher olefin process
483:Dewar–Chatt–Duncanson model
281:www.greener-industry.org.uk
234:converts methyl acetate to
50:This process operates at a
10:
767:
565:Cyclopentadienyl complexes
529:β-hydride elimination
503:Metal–ligand multiple bond
353:10.1595/003214000X44394105
160:and the hydrolysis of the
689:
643:
630:Transition metal carbides
547:
511:
440:
300:: CS1 maint: unfit URL (
731:Organometallic chemistry
434:Organometallic chemistry
264:10.1002/14356007.a01_045
595:Half sandwich compounds
710:Bioinorganic chemistry
90:active species is the
83:
681:Ziegler–Natta process
585:Metal tetranorbornyls
331:Jones, J. H. (2000).
181:rate-determining step
168:and hydrogen iodide.
142:reductive elimination
125:group to an adjacent
81:
690:Related branches of
448:Crystal field theory
341:Platinum Metals Rev.
66:and improved by the
705:Inorganic chemistry
524:Migratory insertion
498:Agostic interaction
453:Ligand field theory
746:Chemical processes
590:Sandwich compounds
548:Types of compounds
473:Isolobal principle
177:oxidative addition
103:oxidative addition
84:
718:
717:
700:Organic chemistry
671:Olefin metathesis
661:Grignard reaction
560:Grignard reagents
758:
666:Monsanto process
463:d electron count
458:18-electron rule
427:
420:
413:
404:
403:
386:
385:
363:
357:
356:
337:
328:
322:
312:
306:
305:
299:
291:
289:
288:
273:
267:
252:
230:In this process
192:Acetic anhydride
68:Monsanto Company
45:BP Chemicals Ltd
21:Monsanto process
766:
765:
761:
760:
759:
757:
756:
755:
721:
720:
719:
714:
685:
639:
555:Gilman reagents
543:
539:Carbometalation
534:Transmetalation
507:
436:
431:
394:
389:
369:Catalysis Today
364:
360:
335:
329:
325:
313:
309:
293:
292:
286:
284:
275:
274:
270:
253:
249:
245:
236:lithium acetate
225:
221:
217:
213:
209:
194:is produced by
189:
154:catalytic cycle
151:
138:carbon monoxide
76:
74:Catalytic cycle
17:
12:
11:
5:
764:
754:
753:
748:
743:
738:
733:
716:
715:
713:
712:
707:
702:
696:
694:
687:
686:
684:
683:
678:
673:
668:
663:
658:
656:Cativa process
653:
647:
645:
641:
640:
638:
637:
632:
627:
622:
617:
612:
607:
602:
597:
592:
587:
582:
577:
572:
567:
562:
557:
551:
549:
545:
544:
542:
541:
536:
531:
526:
521:
515:
513:
509:
508:
506:
505:
500:
495:
490:
485:
480:
475:
470:
465:
460:
455:
450:
444:
442:
438:
437:
430:
429:
422:
415:
407:
401:
400:
393:
392:External links
390:
388:
387:
358:
323:
307:
268:
246:
244:
241:
232:lithium iodide
228:
227:
223:
219:
215:
211:
207:
200:methyl acetate
188:
185:
149:
115:hexacoordinate
113:- to form the
99:organometallic
75:
72:
37:Cativa process
15:
9:
6:
4:
3:
2:
763:
752:
749:
747:
744:
742:
739:
737:
734:
732:
729:
728:
726:
711:
708:
706:
703:
701:
698:
697:
695:
693:
688:
682:
679:
677:
674:
672:
669:
667:
664:
662:
659:
657:
654:
652:
651:Carbonylation
649:
648:
646:
642:
636:
633:
631:
628:
626:
623:
621:
618:
616:
613:
611:
608:
606:
603:
601:
598:
596:
593:
591:
588:
586:
583:
581:
578:
576:
573:
571:
568:
566:
563:
561:
558:
556:
553:
552:
550:
546:
540:
537:
535:
532:
530:
527:
525:
522:
520:
517:
516:
514:
510:
504:
501:
499:
496:
494:
491:
489:
486:
484:
481:
479:
478:π backbonding
476:
474:
471:
469:
466:
464:
461:
459:
456:
454:
451:
449:
446:
445:
443:
439:
435:
428:
423:
421:
416:
414:
409:
408:
405:
399:
396:
395:
383:
379:
375:
371:
370:
362:
354:
350:
347:(3): 94–105.
346:
343:
342:
334:
327:
321:
317:
311:
303:
297:
282:
278:
272:
265:
261:
257:
251:
247:
240:
237:
233:
218:+ CO → (CH
205:
204:
203:
201:
197:
196:carbonylation
193:
184:
182:
178:
174:
169:
167:
163:
162:acetyl iodide
159:
158:methyl iodide
155:
147:
146:acetyl iodide
143:
139:
135:
131:
128:
124:
120:
116:
112:
108:
107:methyl iodide
104:
100:
96:
93:
89:
88:catalytically
80:
71:
69:
65:
61:
57:
53:
48:
46:
42:
38:
34:
30:
29:carbonylation
27:by catalytic
26:
22:
665:
644:Applications
580:Metallocenes
376:(1): 73–91.
373:
367:
361:
344:
339:
326:
310:
285:. Retrieved
280:
271:
255:
250:
229:
190:
170:
152:C(O)I). The
110:
101:step is the
94:
85:
49:
39:, a similar
20:
18:
493:spin states
173:first-order
166:acetic acid
144:to release
60:temperature
25:acetic acid
725:Categories
441:Principles
320:189138953X
287:2014-08-27
243:References
741:Catalysis
692:chemistry
512:Reactions
488:Hapticity
119:migration
54:of 30–60
751:Monsanto
296:cite web
127:carbonyl
52:pressure
33:methanol
736:Rhodium
41:iridium
318:
134:acetyl
130:ligand
123:methyl
58:and a
336:(PDF)
121:of a
92:anion
316:ISBN
302:link
86:The
64:BASF
19:The
378:doi
349:doi
260:doi
222:CO)
198:of
164:to
148:(CH
111:cis
109:to
105:of
95:cis
56:atm
31:of
727::
374:13
372:.
345:44
338:.
298:}}
294:{{
279:.
214:CH
210:CO
206:CH
183:.
426:e
419:t
412:v
384:.
380::
355:.
351::
304:)
290:.
266:.
262::
226:O
224:2
220:3
216:3
212:2
208:3
150:3
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.