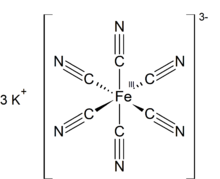874:
312:
191:
54:
555:
45:
706:
841:
711:
1048:
from color negatives and positives during processing, a process called bleaching. Because potassium ferricyanide bleaches are environmentally unfriendly, short-lived, and capable of releasing hydrogen cyanide gas if mixed with high concentrations and volumes of acid, bleaches using ferric
709:
1215:, potassium ferricyanide is used to detect ferrous iron in biological tissue. Potassium ferricyanide reacts with ferrous iron in acidic solution to produce the insoluble blue pigment, commonly referred to as Turnbull's blue or
873:
563:
535:
1572:
Nakajima, Y., Sato, Y., & Konishi, T. (2007). Antioxidant Small
Phenolic Ingredients in Inonotus obliquus (persoon) Pilat (Chaga). Chemical & Pharmaceutical Bulletin, 55(8), 1222–1276.
954:
Like other metal cyanides, solid potassium ferricyanide has a complicated polymeric structure. The polymer consists of octahedral centers crosslinked with K ions that are bound to the CN
1117:) in the presence of Fe ions, and which can therefore be used to detect metal oxidation that will lead to rust. It is possible to calculate the number of moles of Fe ions by using a
710:
128:
1189:
as a substitute) and water to formulate
Murakami's etchant. This etchant is used by metallographers to provide contrast between binder and carbide phases in cemented carbides.
1139:
Potassium ferricyanide is used to determine the ferric reducing power potential of a sample (extract, chemical compound, etc.). Such a measurement is used to determine of the
1235:
Potassium ferricyanide has low toxicity, its main hazard being that it is a mild irritant to the eyes and skin. However, under very strongly acidic conditions, highly toxic
2893:
649:
2909:
712:
2738:
2722:
2330:
2283:
2295:
2137:
2873:
2092:
1879:
1061:, potassium ferricyanide is used to reduce the size of color dots without reducing their number, as a kind of manual color correction called dot etching.
854:
1032:
processes involve the use of potassium ferricyanide.It is often used as a mild bleach in a concentration of 10g/L to reduce film or print density.
734:
1778:
1751:
361:
1227:
staining method. The material formed in the
Turnbull's blue reaction and the compound formed in the Prussian blue reaction are the same.
1642:"Comparative Studies on Prussian Blue or Diaquatetraamine-Cobalt(III) Promoted Hydrolysis of 4-Nitrophenylphosphate in Microemulsions"
645:
849:
1730:
1624:
1439:
1383:
Figgis, B. N.; Gerloch, M.; Mason, R. (1969). "The crystallography and paramagnetic anisotropy of potassium ferricyanide".
1132:(E°' ~ 436 mV at pH 7). As such, it can oxidize reduced cytochrome c (E°' ~ 247 mV at pH 7) in isolated mitochondria.
1771:
1599:
1346:
1314:
326:
1093:
Potassium ferricyanide is a used as an oxidant in organic chemistry. It is an oxidant for catalyst regeneration in
861:
2961:
2754:
720:
2437:
1546:
Oi, Ryu; Sharpless, K. Barry (1996). "3-[(1S)-1,2-Dihydroxyethyl]-1,5-Dihydro-3H-2,4-Benzodioxepine".
2571:
2475:
2267:
1582:
Dunbar, K. R.; Heintz, R. A. (1997). "Chemistry of
Transition Metal Cyanide Compounds: Modern Perspectives".
893:
633:
1515:
Gonzalez, Javier; Aurigemma, Christine; Truesdale, Larry (2002). "Synthesis of (+)-(1S,2R)- and (−)-(1R,2S)-
2946:
2941:
2523:
2461:
1764:
641:
607:
269:
186:
1677:
2499:
2487:
2011:
1106:
290:
629:
2801:
2533:
2406:
554:
2966:
2547:
1094:
1029:
198:
1349:[On a particular potassium iron cyanate, and on a new series of iron salts of cyanic acid].
2951:
2697:
2685:
2378:
677:
307:
2956:
2255:
2219:
2035:
1224:
1118:
484:
2661:
2653:
2609:
2585:
2307:
2231:
1966:
1824:
1263:
1082:
1074:
926:
808:
20:
1427:
2358:
2243:
2051:
2019:
1899:
1837:
896:
577:
547:
516:
66:
669:
2706:
2067:
1941:
1806:
1392:
1347:"Ueber ein besonderes Cyaneisenkalium, and über eine neue Reihe von blausauren Eisensalzen"
1078:
922:
665:
278:
168:
104:
1128:
In physiology experiments potassium ferricyanide provides a means increasing a solution's
653:
8:
2829:
2641:
2390:
2346:
2210:
2125:
2116:
2003:
1983:
1924:
1889:
1871:
1787:
1746:
1182:
94:
1396:
788:
311:
190:
148:
2857:
2673:
2449:
2366:
2275:
2149:
2104:
2083:
1929:
1829:
1819:
1801:
1734:
1408:
1070:
1619:(2nd ed.). Chicago: American Society of Clinical Pathologists. pp. 209–211.
53:
2841:
2813:
2789:
2781:
2629:
2202:
2194:
2169:
1991:
1971:
1957:
1949:
1894:
1884:
1850:
1845:
1814:
1710:
1620:
1595:
1435:
1310:
1151:
1133:
1058:
1000:
885:
782:
502:
1412:
1136:
is usually used as a reducing chemical in such experiments (E°' ~ −420 mV at pH 7).
1863:
1858:
1692:
1656:
1587:
1555:
1528:
1495:
1464:
1400:
1300:
1292:
1236:
1186:
1081:
where the mixture is known as Farmer's reducer; this can help offset problems from
448:
384:
242:
2186:
2178:
1915:
1385:
Proceedings of the Royal
Society of London. A. Mathematical and Physical Sciences
1162:
1129:
1110:
1041:
996:
621:
217:
1284:
1675:
1641:
978:
967:
906:
832:
613:
1591:
770:
2935:
1559:
1532:
1500:
1483:
1468:
1216:
1197:
1166:
1122:
1114:
823:
625:
437:
427:
179:
1053:
have been used in color processing since the 1972 introduction of the Kodak
1696:
1404:
1258:
1253:
1200:, the deep blue pigment in blue printing, is generated by the reaction of K
1054:
902:
899:
31:
27:
733:
44:
1140:
1021:
746:
657:
1305:
719:
637:
1296:
989:
726:
522:
508:
463:
399:
199:
159:
1756:
1519:-2-Phenylcyclohexanol via Sharpless Asymmetric Dihydroxylation (AD)".
412:
deep red crystals, sometimes small pellets, orange to dark red powder
1661:
1212:
1170:
1147:
1025:
1017:
958:. The K---NCFe linkages break when the solid is dissolved in water.
901:
ion. It is soluble in water and its solution shows some green-yellow
1154:
agent replacing an enzyme's natural electron transfer agent such as
831:
Except where otherwise noted, data are given for materials in their
1586:. Progress in Inorganic Chemistry. Vol. 45. pp. 283–391.
918:
692:
685:
127:
992:
727:
617:
469:
417:
229:
1159:
1155:
1105:
Potassium ferricyanide is also one of two compounds present in
1045:
955:
1676:
Verdaguer, M.; Galvez, N.; Garde, R.; Desplanches, C. (2002).
1069:
Ferricyanide is also used in black-and-white photography with
599:
1455:
Prill, E. A.; McElvain, S. M. (1935). "1-Methyl-2-Pyridone".
974:
673:
253:
139:
117:
295:
1752:
National
Pollutant Inventory – Cyanide compounds fact sheet
1050:
985:
970:
591:
474:
1731:
1581:
1514:
1372:(2nd ed.). New York: Dover Publications. p. 153.
1085:
of the negative, or brighten the highlights in the print.
1332:
The
Chemistry of Cyano Complexes of the Transition Metals
982:
817:
802:
1425:
1165:. It is an ingredient in commercially available blood
1146:
Potassium ferricyanide is a component of amperometric
929:. Potassium ferricyanide separates from the solution:
877:
Potassium ferricyanide when milled has lighter color
587:
26:"Prussian red" redirects here. For pigment based on
1382:
917:Potassium ferricyanide is manufactured by passing
583:
1484:"Syntheses of Substituted 2-Cyano-benzothiazoles"
2933:
595:
241:
1088:
708:
103:
1678:"Electrons at Work in Prussian Blue Analogues"
1289:Encyclopedia of Reagents for Organic Synthesis
1011:
681:
1772:
1614:
1454:
1329:
1481:
1282:
1649:International Journal of Molecular Sciences
1639:
1545:
1239:gas is evolved, according to the equation:
345:InChI=1S/6CN.Fe.3K/c6*1-2;;;;/q6*-1;+3;3*+1
335:InChI=1S/6CN.Fe.3K/c6*1-2;;;;/q6*-1;+3;3*+1
1779:
1765:
1617:Histotechnology: A Self-Instructional Text
661:
310:
189:
167:
1660:
1499:
1304:
1100:
277:
1482:Würfel, Hendryk; Jakobi, Dörthe (2018).
1219:. To detect ferric (Fe) iron, potassium
1181:Potassium ferricyanide is combined with
872:
1786:
1747:International Chemical Safety Card 1132
1121:, because of the very intense color of
306:
2934:
1344:
1040:Potassium ferricyanide was used as an
432:300 °C (572 °F; 573 K)
180:
1760:
1733:Effect of different parameters using
1432:The Focal Encyclopedia of Photography
338:Key: BYGOPQKDHGXNCD-UHFFFAOYSA-N
147:
1367:
892:. This bright red salt contains the
1426:Stroebel, L.; Zakia, R. D. (1993).
1370:The Development of Modern Chemistry
1204:with ferrous (Fe) ions as well as K
1064:
1016:The compound has widespread use in
348:Key: BYGOPQKDHGXNCD-UHFFFAOYAG
232:
13:
1724:
1073:(hypo) to reduce the density of a
704:
14:
2978:
1740:
1711:"MSDS for potassium ferricyanide"
1685:Electrochemical Society Interface
1291:. New York: J. Wiley & Sons.
1192:
839:
553:
52:
43:
1703:
1669:
1633:
1608:
1584:Progress in Inorganic Chemistry
1575:
1566:
1539:
961:
905:. It was discovered in 1822 by
835:(at 25 °C , 100 kPa).
70:Potassium hexacyanoferrate(III)
1508:
1475:
1448:
1419:
1376:
1361:
1338:
1323:
1276:
1006:
912:
1:
1351:Journal für Chemie und Physik
1269:
966:The compound is also used to
369:..N#C(C#N)(C#N)(C#N)(C#N)C#N.
1434:. Focal Press. p. 297.
1089:Reagent in organic synthesis
1035:
949:
7:
1247:
1107:ferroxyl indicator solution
1012:Blueprint, cyanotype, toner
759:or concentration (LD, LC):
10:
2983:
1095:Sharpless dihydroxylations
404:329.24 g/mol
25:
18:
2774:
2430:
2323:
2162:
1908:
1794:
1592:10.1002/9780470166468.ch4
1334:. London: Academic Press.
1287:. In Paquette, L. (ed.).
1230:
1030:photographic print toning
829:
794:
777:2970 mg/kg (mouse, oral)
755:
534:
529:
495:
377:
357:
322:
87:
75:
65:
60:
51:
42:
1560:10.15227/orgsyn.073.0001
1533:10.15227/orgsyn.079.0093
1501:10.15227/orgsyn.095.0177
1469:10.15227/orgsyn.015.0041
1345:Gmelin, Leopold (1822).
1285:"Potassium Ferricyanide"
1176:
608:Precautionary statements
78:Red prussiate of Potash,
19:Not to be confused with
1223:is used instead in the
485:Magnetic susceptibility
38:Potassium ferricyanide
2962:Photographic chemicals
1697:10.1002/chin.200304218
1615:Carson, F. L. (1997).
1405:10.1098/rspa.1969.0031
1330:Sharpe, A. G. (1976).
1264:Potassium ferrocyanide
1143:property of a sample.
1101:Sensors and indicators
927:potassium ferrocyanide
882:Potassium ferricyanide
878:
809:Potassium ferrocyanide
715:
458:775 g/L ("hot water")
454:330 g/L ("cold water")
82:Potassium ferricyanide
21:potassium ferrocyanide
1283:Kwong, H.-L. (2004).
876:
714:
517:Coordination geometry
1640:Tafesse, F. (2003).
1225:Perls' Prussian blue
1079:gelatin silver print
697:(fire diamond)
468:slightly soluble in
2947:Iron(III) compounds
2942:Potassium compounds
1795:H, (pseudo)halogens
1788:Potassium compounds
1397:1969RSPSA.309...91F
1368:Ihde, A.J. (1984).
1243:6 H + → 6 HCN + Fe
1208:with ferric salts.
1183:potassium hydroxide
1113:) that turns blue (
449:Solubility in water
39:
1735:Cyclic Voltammetry
1428:"Farmer's Reducer"
1297:10.1002/047084289X
1071:sodium thiosulfate
1028:process). Several
888:with the formula K
879:
862:Infobox references
795:Related compounds
716:
491:+2290.0·10 cm/mol
37:
2929:
2928:
2431:transition metals
1626:978-0-89189-411-7
1548:Organic Syntheses
1521:Organic Syntheses
1488:Organic Syntheses
1457:Organic Syntheses
1441:978-0-240-51417-8
1152:electron transfer
1134:Sodium dithionite
1059:color lithography
1001:organic chemistry
886:chemical compound
870:Chemical compound
868:
867:
783:Safety data sheet
578:Hazard statements
503:Crystal structure
478:soluble in water
422:1.89 g/cm, solid
291:CompTox Dashboard
129:Interactive image
16:Chemical compound
2974:
2967:Oxidizing agents
2581:
2567:
2566:
2565:
2557:
2556:
2543:
2529:
2519:
2518:
2517:
2509:
2508:
2471:
1781:
1774:
1767:
1758:
1757:
1718:
1717:
1715:
1707:
1701:
1700:
1682:
1673:
1667:
1666:
1664:
1662:10.3390/i4060362
1646:
1637:
1631:
1630:
1612:
1606:
1605:
1579:
1573:
1570:
1564:
1563:
1543:
1537:
1536:
1512:
1506:
1505:
1503:
1479:
1473:
1472:
1452:
1446:
1445:
1423:
1417:
1416:
1391:(1496): 91–118.
1380:
1374:
1373:
1365:
1359:
1358:
1342:
1336:
1335:
1327:
1321:
1320:
1308:
1280:
1237:hydrogen cyanide
1187:sodium hydroxide
1065:Farmer's reducer
995:, and as a mild
852:
846:
843:
842:
736:
729:
722:
707:
687:
683:
679:
675:
671:
667:
663:
659:
655:
651:
647:
643:
639:
635:
631:
627:
623:
619:
615:
601:
597:
593:
589:
585:
557:
385:Chemical formula
315:
314:
299:
297:
281:
245:
234:
218:Gmelin Reference
201:
193:
182:
171:
151:
131:
107:
56:
47:
40:
36:
2982:
2981:
2977:
2976:
2975:
2973:
2972:
2971:
2952:Cyano complexes
2932:
2931:
2930:
2925:
2921:
2917:
2913:
2905:
2901:
2897:
2889:
2885:
2881:
2877:
2869:
2865:
2861:
2853:
2849:
2845:
2837:
2833:
2825:
2821:
2817:
2809:
2805:
2797:
2793:
2785:
2770:
2766:
2762:
2758:
2750:
2746:
2742:
2734:
2730:
2726:
2718:
2714:
2710:
2701:
2693:
2689:
2681:
2677:
2669:
2665:
2657:
2649:
2645:
2637:
2633:
2625:
2621:
2617:
2613:
2605:
2601:
2593:
2589:
2580:
2576:
2572:
2564:
2561:
2560:
2559:
2555:
2552:
2551:
2550:
2548:
2542:
2538:
2534:
2528:
2524:
2516:
2513:
2512:
2511:
2507:
2504:
2503:
2502:
2500:
2495:
2491:
2483:
2479:
2470:
2466:
2462:
2457:
2453:
2445:
2441:
2426:
2422:
2418:
2414:
2410:
2402:
2398:
2394:
2386:
2382:
2374:
2370:
2362:
2354:
2350:
2342:
2338:
2334:
2319:
2315:
2311:
2303:
2299:
2291:
2287:
2279:
2271:
2263:
2259:
2251:
2247:
2239:
2235:
2227:
2223:
2214:
2206:
2198:
2190:
2182:
2173:
2158:
2153:
2145:
2141:
2133:
2129:
2120:
2112:
2108:
2100:
2096:
2087:
2079:
2075:
2071:
2063:
2059:
2055:
2047:
2043:
2039:
2031:
2027:
2023:
2015:
2007:
1999:
1995:
1987:
1979:
1975:
1961:
1953:
1945:
1937:
1933:
1919:
1904:
1875:
1867:
1854:
1841:
1833:
1810:
1790:
1785:
1743:
1727:
1725:Further reading
1722:
1721:
1713:
1709:
1708:
1704:
1680:
1674:
1670:
1644:
1638:
1634:
1627:
1613:
1609:
1602:
1580:
1576:
1571:
1567:
1544:
1540:
1513:
1509:
1480:
1476:
1453:
1449:
1442:
1424:
1420:
1381:
1377:
1366:
1362:
1343:
1339:
1328:
1324:
1317:
1281:
1277:
1272:
1250:
1233:
1207:
1203:
1195:
1179:
1163:glucose oxidase
1130:redox potential
1111:phenolphthalein
1103:
1091:
1067:
1042:oxidizing agent
1038:
1020:drawing and in
1014:
1009:
997:oxidizing agent
964:
952:
944:
940:
936:
915:
891:
871:
864:
859:
858:
857: ?)
848:
844:
840:
836:
820:
805:
774:
768:
741:
740:
739:
738:
731:
724:
717:
713:
705:
610:
580:
566:
550:
519:
505:
488:
477:
472:
457:
456:464 g/L (20 °C)
455:
451:
393:
387:
373:
370:
365:
364:
353:
350:
349:
346:
340:
339:
336:
330:
329:
318:
300:
293:
284:
264:
248:
235:
220:
211:
174:
154:
134:
121:
110:
97:
83:
81:
79:
71:
35:
24:
17:
12:
11:
5:
2980:
2970:
2969:
2964:
2959:
2957:Iron complexes
2954:
2949:
2944:
2927:
2926:
2924:
2923:
2919:
2915:
2911:
2907:
2903:
2899:
2895:
2891:
2887:
2883:
2879:
2875:
2871:
2867:
2863:
2859:
2855:
2851:
2847:
2843:
2839:
2835:
2831:
2827:
2823:
2819:
2815:
2811:
2807:
2803:
2799:
2795:
2791:
2787:
2783:
2778:
2776:
2772:
2771:
2769:
2768:
2764:
2760:
2756:
2752:
2748:
2744:
2740:
2736:
2732:
2728:
2724:
2720:
2716:
2712:
2708:
2704:
2699:
2695:
2691:
2687:
2683:
2679:
2675:
2671:
2667:
2663:
2659:
2655:
2651:
2647:
2643:
2639:
2635:
2631:
2627:
2623:
2619:
2615:
2611:
2607:
2603:
2599:
2595:
2591:
2587:
2583:
2578:
2574:
2569:
2562:
2553:
2545:
2540:
2536:
2531:
2526:
2521:
2514:
2505:
2497:
2493:
2489:
2485:
2481:
2477:
2473:
2468:
2464:
2459:
2455:
2451:
2447:
2443:
2439:
2434:
2432:
2428:
2427:
2425:
2424:
2420:
2416:
2412:
2408:
2404:
2400:
2396:
2392:
2388:
2384:
2380:
2376:
2372:
2368:
2364:
2360:
2356:
2352:
2348:
2344:
2340:
2336:
2332:
2327:
2325:
2321:
2320:
2318:
2317:
2313:
2309:
2305:
2301:
2297:
2293:
2289:
2285:
2281:
2277:
2273:
2269:
2265:
2261:
2257:
2253:
2249:
2245:
2241:
2237:
2233:
2229:
2225:
2221:
2217:
2212:
2208:
2204:
2200:
2196:
2192:
2188:
2184:
2180:
2176:
2171:
2166:
2164:
2160:
2159:
2157:
2156:
2151:
2147:
2143:
2139:
2135:
2131:
2127:
2123:
2118:
2114:
2110:
2106:
2102:
2098:
2094:
2090:
2085:
2081:
2077:
2073:
2069:
2065:
2061:
2057:
2053:
2049:
2045:
2041:
2037:
2033:
2029:
2025:
2021:
2017:
2013:
2009:
2005:
2001:
1997:
1993:
1989:
1985:
1981:
1977:
1973:
1969:
1964:
1959:
1955:
1951:
1947:
1943:
1939:
1935:
1931:
1927:
1922:
1917:
1912:
1910:
1906:
1905:
1903:
1902:
1897:
1892:
1887:
1882:
1877:
1873:
1869:
1865:
1861:
1856:
1852:
1848:
1843:
1839:
1835:
1831:
1827:
1822:
1817:
1812:
1808:
1804:
1798:
1796:
1792:
1791:
1784:
1783:
1776:
1769:
1761:
1755:
1754:
1749:
1742:
1741:External links
1739:
1738:
1737:
1726:
1723:
1720:
1719:
1702:
1668:
1655:(6): 362–370.
1632:
1625:
1607:
1600:
1574:
1565:
1538:
1507:
1474:
1447:
1440:
1418:
1375:
1360:
1337:
1322:
1315:
1274:
1273:
1271:
1268:
1267:
1266:
1261:
1256:
1249:
1246:
1245:
1244:
1232:
1229:
1205:
1201:
1194:
1191:
1178:
1175:
1167:glucose meters
1102:
1099:
1090:
1087:
1066:
1063:
1037:
1034:
1013:
1010:
1008:
1005:
979:electroplating
963:
960:
951:
948:
947:
946:
942:
938:
934:
914:
911:
907:Leopold Gmelin
889:
869:
866:
865:
860:
838:
837:
833:standard state
830:
827:
826:
821:
815:
812:
811:
806:
800:
797:
796:
792:
791:
786:
779:
778:
775:
766:
764:
761:
760:
753:
752:
751:Non-flammable
749:
743:
742:
732:
725:
718:
703:
702:
701:
700:
698:
689:
688:
650:P305+P351+P338
611:
606:
603:
602:
581:
576:
573:
572:
567:
562:
559:
558:
551:
546:
543:
542:
532:
531:
527:
526:
520:
515:
512:
511:
506:
501:
498:
497:
493:
492:
489:
483:
480:
479:
466:
460:
459:
452:
447:
444:
443:
440:
434:
433:
430:
424:
423:
420:
414:
413:
410:
406:
405:
402:
396:
395:
391:
388:
383:
380:
379:
375:
374:
372:
371:
368:
360:
359:
358:
355:
354:
352:
351:
347:
344:
343:
341:
337:
334:
333:
325:
324:
323:
320:
319:
317:
316:
303:
301:
289:
286:
285:
283:
282:
274:
272:
266:
265:
263:
262:
258:
256:
250:
249:
247:
246:
238:
236:
228:
225:
224:
221:
216:
213:
212:
210:
209:
205:
203:
195:
194:
184:
176:
175:
173:
172:
164:
162:
156:
155:
153:
152:
144:
142:
136:
135:
133:
132:
124:
122:
115:
112:
111:
109:
108:
100:
98:
93:
90:
89:
85:
84:
77:
73:
72:
69:
63:
62:
58:
57:
49:
48:
15:
9:
6:
4:
3:
2:
2979:
2968:
2965:
2963:
2960:
2958:
2955:
2953:
2950:
2948:
2945:
2943:
2940:
2939:
2937:
2922:
2908:
2906:
2892:
2890:
2872:
2870:
2856:
2854:
2840:
2838:
2828:
2826:
2812:
2810:
2800:
2798:
2788:
2786:
2780:
2779:
2777:
2773:
2767:
2753:
2751:
2737:
2735:
2721:
2719:
2705:
2703:
2696:
2694:
2684:
2682:
2672:
2670:
2660:
2658:
2652:
2650:
2640:
2638:
2628:
2626:
2608:
2606:
2596:
2594:
2584:
2582:
2570:
2568:
2546:
2544:
2532:
2530:
2522:
2520:
2498:
2496:
2486:
2484:
2474:
2472:
2460:
2458:
2448:
2446:
2436:
2435:
2433:
2429:
2423:
2405:
2403:
2389:
2387:
2377:
2375:
2365:
2363:
2357:
2355:
2345:
2343:
2329:
2328:
2326:
2322:
2316:
2306:
2304:
2294:
2292:
2282:
2280:
2274:
2272:
2266:
2264:
2254:
2252:
2242:
2240:
2230:
2228:
2218:
2216:
2209:
2207:
2201:
2199:
2193:
2191:
2185:
2183:
2177:
2175:
2168:
2167:
2165:
2161:
2155:
2148:
2146:
2136:
2134:
2124:
2122:
2115:
2113:
2103:
2101:
2091:
2089:
2082:
2080:
2066:
2064:
2050:
2048:
2034:
2032:
2018:
2016:
2010:
2008:
2002:
2000:
1990:
1988:
1982:
1980:
1970:
1968:
1965:
1963:
1956:
1954:
1948:
1946:
1940:
1938:
1928:
1926:
1923:
1921:
1914:
1913:
1911:
1907:
1901:
1898:
1896:
1893:
1891:
1888:
1886:
1883:
1881:
1878:
1876:
1870:
1868:
1862:
1860:
1857:
1855:
1849:
1847:
1844:
1842:
1836:
1834:
1828:
1826:
1823:
1821:
1818:
1816:
1813:
1811:
1805:
1803:
1800:
1799:
1797:
1793:
1789:
1782:
1777:
1775:
1770:
1768:
1763:
1762:
1759:
1753:
1750:
1748:
1745:
1744:
1736:
1732:
1729:
1728:
1712:
1706:
1698:
1694:
1690:
1686:
1679:
1672:
1663:
1658:
1654:
1650:
1643:
1636:
1628:
1622:
1618:
1611:
1603:
1601:9780470166468
1597:
1593:
1589:
1585:
1578:
1569:
1561:
1557:
1553:
1549:
1542:
1534:
1530:
1526:
1522:
1518:
1511:
1502:
1497:
1493:
1489:
1485:
1478:
1470:
1466:
1462:
1458:
1451:
1443:
1437:
1433:
1429:
1422:
1414:
1410:
1406:
1402:
1398:
1394:
1390:
1386:
1379:
1371:
1364:
1356:
1353:(in German).
1352:
1348:
1341:
1333:
1326:
1318:
1316:9780471936237
1312:
1307:
1302:
1298:
1294:
1290:
1286:
1279:
1275:
1265:
1262:
1260:
1257:
1255:
1252:
1251:
1242:
1241:
1240:
1238:
1228:
1226:
1222:
1218:
1217:Prussian blue
1214:
1209:
1199:
1198:Prussian blue
1193:Prussian blue
1190:
1188:
1184:
1174:
1172:
1168:
1164:
1161:
1157:
1153:
1149:
1144:
1142:
1137:
1135:
1131:
1126:
1124:
1123:Prussian blue
1120:
1116:
1115:Prussian blue
1112:
1108:
1098:
1096:
1086:
1084:
1080:
1076:
1072:
1062:
1060:
1056:
1052:
1047:
1043:
1033:
1031:
1027:
1023:
1019:
1004:
1002:
998:
994:
991:
987:
984:
980:
976:
972:
969:
959:
957:
932:
931:
930:
928:
924:
920:
910:
908:
904:
900:
898:
895:
887:
883:
875:
863:
856:
851:
834:
828:
825:
824:Prussian blue
822:
819:
814:
813:
810:
807:
804:
799:
798:
793:
790:
787:
784:
781:
780:
776:
772:
763:
762:
758:
754:
750:
748:
745:
744:
737:
730:
723:
699:
696:
695:
691:
690:
612:
609:
605:
604:
582:
579:
575:
574:
571:
568:
565:
561:
560:
556:
552:
549:
545:
544:
540:
538:
533:
528:
524:
521:
518:
514:
513:
510:
507:
504:
500:
499:
494:
490:
486:
482:
481:
476:
471:
467:
465:
462:
461:
453:
450:
446:
445:
441:
439:
438:Boiling point
436:
435:
431:
429:
428:Melting point
426:
425:
421:
419:
416:
415:
411:
408:
407:
403:
401:
398:
397:
389:
386:
382:
381:
376:
367:
366:
363:
356:
342:
332:
331:
328:
321:
313:
309:
308:DTXSID9031939
305:
304:
302:
292:
288:
287:
280:
276:
275:
273:
271:
268:
267:
260:
259:
257:
255:
252:
251:
244:
240:
239:
237:
231:
227:
226:
222:
219:
215:
214:
207:
206:
204:
202:
197:
196:
192:
188:
185:
183:
181:ECHA InfoCard
178:
177:
170:
166:
165:
163:
161:
158:
157:
150:
146:
145:
143:
141:
138:
137:
130:
126:
125:
123:
119:
114:
113:
106:
102:
101:
99:
96:
92:
91:
86:
80:Prussian red,
74:
68:
64:
59:
55:
50:
46:
41:
33:
29:
22:
2597:
1705:
1691:(3): 28–32.
1688:
1684:
1671:
1652:
1648:
1635:
1616:
1610:
1583:
1577:
1568:
1551:
1547:
1541:
1524:
1520:
1516:
1510:
1491:
1487:
1477:
1460:
1456:
1450:
1431:
1421:
1388:
1384:
1378:
1369:
1363:
1354:
1350:
1340:
1331:
1325:
1306:10261/236866
1288:
1278:
1259:Ferrocyanide
1254:Ferricyanide
1234:
1221:ferrocyanide
1220:
1210:
1196:
1180:
1158:as with the
1145:
1138:
1127:
1109:(along with
1104:
1092:
1083:overexposure
1068:
1055:C-41 process
1039:
1015:
965:
962:Applications
953:
916:
903:fluorescence
894:octahedrally
881:
880:
756:
693:
569:
536:
254:RTECS number
88:Identifiers
76:Other names
32:Venetian red
28:ferric oxide
1494:: 177–191.
1169:for use by
1141:antioxidant
1119:colorimeter
1022:photography
1007:Photography
913:Preparation
897:coordinated
771:median dose
757:Lethal dose
747:Flash point
564:Signal word
473:soluble in
442:decomposes
409:Appearance
378:Properties
187:100.033.916
149:CHEBI:30060
2936:Categories
2324:B, C group
2163:pnictogens
1909:chalcogens
1357:: 325–346.
1270:References
1148:biosensors
1044:to remove
990:laboratory
921:through a
548:Pictograms
523:octahedral
509:monoclinic
496:Structure
464:Solubility
400:Molar mass
279:U4MAF9C813
160:ChemSpider
116:3D model (
105:13746-66-2
95:CAS Number
67:IUPAC name
1213:histology
1171:diabetics
1036:Bleaching
1026:Cyanotype
1018:blueprint
950:Structure
678:P403+P233
670:P337+P313
666:P332+P313
646:P304+P340
642:P304+P312
638:P302+P352
634:P301+P312
539:labelling
261:LJ8225000
208:237-323-3
200:EC Number
1413:96689342
1248:See also
1075:negative
923:solution
919:chlorine
694:NFPA 704
530:Hazards
487:(χ)
2775:organic
1393:Bibcode
993:reagent
988:, as a
956:ligands
945:+ 2 KCl
884:is the
855:what is
853: (
818:cations
570:Warning
470:alcohol
418:Density
394:
230:PubChem
2602:Fe(CN)
2590:Fe(CN)
2454:Pt(CN)
1623:
1598:
1527:: 93.
1463:: 41.
1438:
1411:
1313:
1231:Safety
1160:enzyme
1156:oxygen
1150:as an
1046:silver
983:dyeing
968:harden
850:verify
847:
816:Other
803:anions
801:Other
785:(SDS)
525:at Fe
362:SMILES
223:21683
61:Names
30:, see
1714:(PDF)
1681:(PDF)
1645:(PDF)
1554:: 1.
1517:trans
1409:S2CID
1177:Other
1057:. In
977:, in
975:steel
941:→ 2 K
327:InChI
243:26250
169:24458
140:ChEBI
118:JSmol
2782:KHCO
2698:KCrO
2654:KMnO
2614:Fe(C
2539:ReBr
2525:KAsF
2492:ReCl
2480:PtCl
2442:PtCl
2359:KHCO
2300:HAsO
2276:KAsO
2012:KHSO
2004:KHSO
1984:KHSO
1900:KSCN
1895:KOCN
1890:KCNO
1851:KBrO
1838:KClO
1830:KClO
1825:KClO
1621:ISBN
1596:ISBN
1436:ISBN
1311:ISBN
1185:(or
1051:EDTA
986:wool
973:and
971:iron
937:+ Cl
789:MSDS
686:P501
682:P405
674:P362
662:P330
658:P321
654:P312
630:P280
626:P271
622:P270
618:P264
614:P261
600:H335
596:H332
592:H319
588:H315
584:H302
475:acid
270:UNII
2830:KHC
2802:KCF
2790:KCH
2690:CrO
2678:CrO
2666:CrO
2646:MnO
2634:FeO
2577:ZrF
2558:ReI
2510:ReF
2467:TiF
2383:SiF
2371:SiO
2312:AsO
2288:AsO
2268:KPF
2248:HPO
2203:KNO
2195:KNO
2179:KNH
2142:TeO
2130:TeO
2109:SeO
2097:SeO
1967:KHS
1925:KOH
1885:KCN
1880:KAt
1872:KIO
1864:KIO
1846:KBr
1820:KCl
1807:KHF
1693:doi
1657:doi
1588:doi
1556:doi
1529:doi
1496:doi
1465:doi
1401:doi
1389:309
1301:hdl
1293:doi
1211:In
1077:or
999:in
933:2 K
925:of
537:GHS
296:EPA
233:CID
2938::
2918:KO
2902:KO
2864:35
2860:18
2858:KC
2848:23
2844:12
2842:KC
2806:CO
2794:CO
2763:Cl
2759:Mo
2749:13
2743:Cr
2733:10
2727:Cr
2711:Cr
2702:Cl
2411:Al
2395:Al
2351:CO
2308:KH
2260:PO
2256:KH
2236:PO
2224:PO
2220:KH
2187:KN
2154:Po
2121:Te
2088:Se
1996:SO
1976:SO
1950:KO
1942:KO
1859:KI
1815:KH
1802:KF
1689:11
1687:.
1683:.
1651:.
1647:.
1594:.
1552:73
1550:.
1525:79
1523:.
1492:95
1490:.
1486:.
1461:15
1459:.
1430:.
1407:.
1399:.
1387:.
1355:34
1309:.
1299:.
1173:.
1125:.
1097:.
1003:.
981:,
909:.
767:50
765:LD
684:,
680:,
676:,
672:,
668:,
664:,
660:,
656:,
652:,
648:,
644:,
640:,
636:,
632:,
628:,
624:,
620:,
616:,
598:,
594:,
590:,
586:,
541::
2920:4
2916:7
2914:H
2912:5
2910:C
2904:4
2900:6
2898:H
2896:4
2894:C
2888:4
2886:O
2884:2
2882:K
2880:2
2878:H
2876:3
2874:C
2868:2
2866:O
2862:H
2852:2
2850:O
2846:H
2836:4
2834:O
2832:2
2824:4
2822:O
2820:2
2818:C
2816:2
2814:K
2808:2
2804:3
2796:2
2792:3
2784:2
2765:8
2761:2
2757:4
2755:K
2747:O
2745:4
2741:2
2739:K
2731:O
2729:3
2725:2
2723:K
2717:7
2715:O
2713:2
2709:2
2707:K
2700:3
2692:8
2688:3
2686:K
2680:4
2676:2
2674:K
2668:4
2664:3
2662:K
2656:4
2648:4
2644:2
2642:K
2636:4
2632:2
2630:K
2624:3
2622:)
2620:4
2618:O
2616:2
2612:3
2610:K
2604:6
2600:3
2598:K
2592:6
2588:4
2586:K
2579:6
2575:2
2573:K
2563:6
2554:2
2549:K
2541:6
2537:2
2535:K
2527:6
2515:6
2506:2
2501:K
2494:6
2490:2
2488:K
2482:6
2478:2
2476:K
2469:6
2465:2
2463:K
2456:4
2452:2
2450:K
2444:4
2440:2
2438:K
2421:7
2419:O
2417:2
2415:B
2413:2
2409:2
2407:K
2401:4
2399:O
2397:2
2393:2
2391:K
2385:6
2381:2
2379:K
2373:3
2369:2
2367:K
2361:3
2353:3
2349:2
2347:K
2341:7
2339:O
2337:2
2335:K
2333:4
2331:B
2314:4
2310:2
2302:4
2298:2
2296:K
2290:4
2286:3
2284:K
2278:2
2270:6
2262:4
2258:2
2250:4
2246:2
2244:K
2238:4
2234:3
2232:K
2226:3
2222:2
2215:P
2213:3
2211:K
2205:3
2197:2
2189:3
2181:2
2174:N
2172:3
2170:K
2152:2
2150:K
2144:4
2140:2
2138:K
2132:3
2128:2
2126:K
2119:2
2117:K
2111:4
2107:2
2105:K
2099:3
2095:2
2093:K
2086:2
2084:K
2078:8
2076:O
2074:2
2072:S
2070:2
2068:K
2062:7
2060:O
2058:2
2056:S
2054:2
2052:K
2046:5
2044:O
2042:2
2040:S
2038:2
2036:K
2030:3
2028:O
2026:2
2024:S
2022:2
2020:K
2014:5
2006:4
1998:4
1994:2
1992:K
1986:3
1978:3
1974:2
1972:K
1962:S
1960:2
1958:K
1952:3
1944:2
1936:2
1934:O
1932:2
1930:K
1920:O
1918:2
1916:K
1874:4
1866:3
1853:3
1840:4
1832:3
1809:2
1780:e
1773:t
1766:v
1716:.
1699:.
1695::
1665:.
1659::
1653:4
1629:.
1604:.
1590::
1562:.
1558::
1535:.
1531::
1504:.
1498::
1471:.
1467::
1444:.
1415:.
1403::
1395::
1319:.
1303::
1295::
1206:4
1202:3
1024:(
943:3
939:2
935:4
890:3
845:N
773:)
769:(
735:0
728:0
721:1
392:3
390:K
298:)
294:(
120:)
34:.
23:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.