757:. Interestingly, this structure also shows how the DUB activity is coupled to the substrate recognition by the proteasomal AAA-ATPase. In contrast to Rpn11, USP14 and UCH37 are the DUBs that do not always associated with the proteasome. In cells, about 10-40% of the proteasomes were found to have USP14 associated. Both Ubp6/USP14 and UCH37 are largely activated by the proteasome and exhibit a very low DUB activity alone. Once activated, USP14 was found to suppress proteasome function by its DUB activity and by inducing parallel pathways of proteasome conformational transitions, one of which turned out to directly prohibit substrate insertion into the AAA-ATPase, as intuitively observed by time-resolved cryogenic electron microscopy. It appears that USP14 regulates proteasome function at multiple checkpoints by both catalytically competing with Rpn11 and allosterically reprogramming the AAA-ATPase states, which is rather unexpected for a DUB. These observations imply that the proteasome regulation may depend on its dynamic transitions of conformational states.
888:. Degradation occurs within the central chamber formed by the association of the two β rings and normally does not release partially degraded products, instead reducing the substrate to short polypeptides typically 7–9 residues long, though they can range from 4 to 25 residues, depending on the organism and substrate. The biochemical mechanism that determines product length is not fully characterized. Although the three catalytic β subunits have a common mechanism, they have slightly different substrate specificities, which are considered chymotrypsin-like, trypsin-like, and peptidyl-glutamyl peptide-hydrolyzing (PHGH)-like. These variations in specificity are the result of interatomic contacts with local residues near the active sites of each subunit. Each catalytic β subunit also possesses a conserved lysine residue required for proteolysis.
443:
homolog of these ATPases exists in archaea, called PAN (proteasome-activating nucleotidase). The association of the 19S and 20S particles requires the binding of ATP to the 19S ATPase subunits, and ATP hydrolysis is required for the assembled complex to degrade folded and ubiquitinated proteins. Note that only the step of substrate unfolding requires energy from ATP hydrolysis, while ATP-binding alone can support all the other steps required for protein degradation (e.g., complex assembly, gate opening, translocation, and proteolysis). In fact, ATP binding to the ATPases by itself supports the rapid degradation of unfolded proteins. However, while ATP hydrolysis is required for unfolding only, it is not yet clear whether this energy may be used in the coupling of some of these steps.
462:) that assemble to a heterohexameric ring of the order Rpt1/Rpt2/Rpt6/Rpt3/Rpt4/Rpt5. This ring is a trimer of dimers: Rpt1/Rpt2, Rpt6/Rpt3, and Rpt4/Rpt5 dimerize via their N-terminal coiled-coils. These coiled-coils protrude from the hexameric ring. The largest regulatory particle non-ATPases Rpn1 and Rpn2 bind to the tips of Rpt1/2 and Rpt6/3, respectively. The ubiquitin receptor Rpn13 binds to Rpn2 and completes the base sub-complex. The lid covers one half of the AAA-ATPase hexamer (Rpt6/Rpt3/Rpt4) and, unexpectedly, directly contacts the 20S via Rpn6 and to lesser extent Rpn5. The subunits Rpn9, Rpn5, Rpn6, Rpn7, Rpn3, and Rpn12, which are structurally related among themselves and to subunits of the
510:(i.e., HbYX motif). The ATPases C-termini bind into pockets in the top of the 20S, and tether the ATPase complex to the 20S proteolytic complex, thus joining the substrate unfolding equipment with the 20S degradation machinery. Binding of these C-termini into these 20S pockets by themselves stimulates opening of the gate in the 20S in much the same way that a "key-in-a-lock" opens a door. The precise mechanism by which this "key-in-a-lock" mechanism functions has been structurally elucidated in the context of human 26S proteasome at near-atomic resolution, suggesting that the insertion of five C-termini of ATPase subunits Rpt1/2/3/5/6 into the 20S surface pockets are required to fully open the 20S gate.
434:, whose substrate specificity is altered relative to the normal proteasome. Recently an alternative proteasome was identified in human cells that lack the α3 core subunit. These proteasomes (known as the α4-α4 proteasomes) instead form 20S core particles containing an additional α4 subunit in place of the missing α3 subunit. These alternative 'α4-α4' proteasomes have been known previously to exist in yeast. Although the precise function of these proteasome isoforms is still largely unknown, cells expressing these proteasomes show enhanced resistance to toxicity induced by metallic ions such as cadmium.
492:
488:
structural changes of the AAA-ATPase module. Some of the substrate-bound conformations bear high similarity to the substrate-free ones, but they are not entirely identical, particularly in the AAA-ATPase module. Prior to the 26S assembly, the 19S regulatory particle in a free form has also been observed in seven conformational states. Notably, all these conformers are somewhat different and present distinct features. Thus, the 19S regulatory particle can sample at least 20 conformational states under different physiological conditions.
458:. In 2016, three independent efforts have determined the first near-atomic resolution structure of the human 26S proteasome in the absence of substrates by cryo-EM. In 2018, a major effort has elucidated the detailed mechanisms of deubiquitylation, initiation of translocation and processive unfolding of substrates by determining seven atomic structures of substrate-engaged 26S proteasome simultaneously. In the heart of the 19S, directly adjacent to the 20S, are the AAA-ATPases (
447:
303:
22:
1482:
1297:. Oxidized proteins, which often form large amorphous aggregates in the cell, can be degraded directly by the 20S core particle without the 19S regulatory cap and do not require ATP hydrolysis or tagging with ubiquitin. However, high levels of oxidative damage increases the degree of cross-linking between protein fragments, rendering the aggregates resistant to proteolysis. Larger numbers and sizes of such highly oxidized aggregates are associated with
838:
31:
372:) form a gate that blocks unregulated access of substrates to the interior cavity. The inner two rings each consist of seven β subunits and in their N-termini contain the protease active sites that perform the proteolysis reactions. Three distinct catalytic activities were identified in the purified complex: chymotrypsin-like, trypsin-like and peptidylglutamyl-peptide hydrolyzing. The size of the proteasome is relatively conserved and is about 150
1888:
627:
1380:, are the primary producers of peptides which are optimal in size and composition for MHC binding. These proteins whose expression increases during the immune response include the 11S regulatory particle, whose main known biological role is regulating the production of MHC ligands, and specialized β subunits called β1i, β2i, and β5i with altered substrate specificity. The complex formed with the specialized β subunits is known as the
736:
244:, a protein that had no known function. It was then discovered that a previously identified protein associated with proteolytic degradation, known as ATP-dependent proteolysis factor 1 (APF-1), was the same protein as ubiquitin. The proteolytic activities of this system were isolated as a multi-protein complex originally called the multi-catalytic proteinase complex by Sherwin Wilk and Marion Orlowski. Later, the
1463:
950:
753:
in cells. Rpn11 is an intrinsic, stoichiometric subunit of the 19S regulatory particle and is essential for the function of 26S proteasome. The DUB activity of Rpn11 is enhanced in the proteasome as compared to its monomeric form. How Rpn11 removes a ubiquitin chain en bloc from a protein substrate was captured by an atomic structure of the substrate-engaged human proteasome in a conformation named E
1188:, but the proteasome also plays important and diverse roles in the apoptotic process. The involvement of the proteasome in this process is indicated by both the increase in protein ubiquitination, and of E1, E2, and E3 enzymes that is observed well in advance of apoptosis. During apoptosis, proteasomes localized to the nucleus have also been observed to translocate to outer membrane
1038:. Mitotic cyclins, which persist in the cell for only a few minutes, have one of the shortest life spans of all intracellular proteins. After a CDK-cyclin complex has performed its function, the associated cyclin is polyubiquitinated and destroyed by the proteasome, which provides directionality for the cell cycle. In particular, exit from
680:(E3) recognizes the specific protein to be ubiquitinated and catalyzes the transfer of ubiquitin from E2 to this target protein. A target protein must be labeled with at least four ubiquitin monomers (in the form of a polyubiquitin chain) before it is recognized by the proteasome lid. It is therefore the E3 that confers
1554:. Lactacystin covalently modifies the amino-terminal threonine of catalytic β subunits of the proteasome, particularly the β5 subunit responsible for the proteasome's chymotrypsin-like activity. This discovery helped to establish the proteasome as a mechanistically novel class of protease: an amino-terminal
1168:
expression. The cellular consequences of ARF activation depend on the plant type and developmental stage, but are involved in directing growth in roots and leaf veins. The specific response to ARF derepression is thought to be mediated by specificity in the pairing of individual ARF and Aux/IAA proteins.
899:, are synthesized as inactive precursors whose ubiquitination and subsequent proteasomal degradation converts them to an active form. Such activity requires the proteasome to cleave the substrate protein internally, rather than processively degrading it from one terminus. It has been suggested that long
774:
in the overall proteolysis reaction depends on the specific substrate; for some proteins, the unfolding process is rate-limiting, while deubiquitination is the slowest step for other proteins. The extent to which substrates must be unfolded before translocation is suggested to be around 20 amino acid
752:
Ubiquitin chains conjugated to a protein targeted for proteasomal degradation are normally removed by any one of the three proteasome-associated deubiquitylating enzymes (DUBs), which are Rpn11, Ubp6/USP14 and UCH37. This process recycles ubiquitin and is essential to maintain the ubiquitin reservoir
1195:
Proteasome inhibition has different effects on apoptosis induction in different cell types. In general, the proteasome is not required for apoptosis, although inhibiting it is pro-apoptotic in most cell types that have been studied. Apoptosis is mediated through disrupting the regulated degradation
731:
protein identified to date. Ubiquitin contains seven lysine residues to which another ubiquitin can be ligated, resulting in different types of polyubiquitin chains. Chains in which each additional ubiquitin is linked to lysine 48 of the previous ubiquitin have a role in proteasome targeting, while
335:
and ubiquitin binding sites; it is this structure that recognizes polyubiquitinated proteins and transfers them to the catalytic core. An alternative form of regulatory subunit called the 11S particle can associate with the core in essentially the same manner as the 19S particle; the 11S may play a
6886:
O'Connor OA, Wright J, Moskowitz C, Muzzy J, MacGregor-Cortelli B, Stubblefield M, Straus D, Portlock C, Hamlin P, Choi E, Dumetrescu O, Esseltine D, Trehu E, Adams J, Schenkein D, Zelenetz AD (February 2005). "Phase II clinical experience with the novel proteasome inhibitor bortezomib in patients
765:
After a protein has been ubiquitinated, it is recognized by the 19S regulatory particle in an ATP-dependent binding step. The substrate protein must then enter the interior of the 20S subunit to come in contact with the proteolytic active sites. Because the 20S particle's central channel is narrow
505:
The 19S regulatory particle is responsible for stimulating the 20S to degrade proteins. A primary function of the 19S regulatory ATPases is to open the gate in the 20S that blocks the entry of substrates into the degradation chamber. The mechanism by which the proteasomal ATPase open this gate has
482:
The 19S regulatory particle within the 26S proteasome holoenzyme has been observed in six strongly differing conformational states in the absence of substrates to date. A hallmark of the AAA-ATPase configuration in this predominant low-energy state is a staircase- or lockwasher-like arrangement of
355:
The number and diversity of subunits contained in the 20S core particle depends on the organism; the number of distinct and specialized subunits is larger in multicellular than unicellular organisms and larger in eukaryotes than in prokaryotes. All 20S particles consist of four stacked heptameric
327:
containing one 20S protein subunit and two 19S regulatory cap subunits. The core is hollow and provides an enclosed cavity in which proteins are degraded; openings at the two ends of the core allow the target protein to enter. Each end of the core particle associates with a 19S regulatory subunit
1167:
repressors known as Aux/IAA proteins for proteasomal degradation. These proteins are ubiquitinated by SCFTIR1, or SCF in complex with the auxin receptor TIR1. Degradation of Aux/IAA proteins derepresses transcription factors in the auxin-response factor (ARF) family and induces ARF-directed gene
818:
have been shown to inhibit substrate unfolding, decreasing the efficiency of proteasomal degradation; this results in the release of partially degraded byproducts, possibly due to the decoupling of the ATP hydrolysis and unfolding steps. Such glycine-alanine repeats are also found in nature, for
487:
but absence of substrate three alternative, less abundant conformations of the 19S are adopted primarily differing in the positioning of the lid with respect to the AAA-ATPase module. In the presence of ATP-γS or a substrate, considerably more conformations have been observed displaying dramatic
442:
The 19S particle in eukaryotes consists of 19 individual proteins and is divisible into two subassemblies, a 9-subunit base that binds directly to the α ring of the 20S core particle, and a 10-subunit lid. Six of the nine base proteins are ATPase subunits from the AAA Family, and an evolutionary
1281:
patches on the surface of misfolded proteins and recruits E3 ubiquitin ligases such as CHIP to tag the proteins for proteasomal degradation. The CHIP protein (carboxyl terminus of Hsp70-interacting protein) is itself regulated via inhibition of interactions between the E3 enzyme CHIP and its E2
688:
The mechanism by which a polyubiquitinated protein is targeted to the proteasome is not fully understood. A few high-resolution snapshots of the proteasome bound to a polyubiquitinated protein suggest that ubiquitin receptors might be coordinated with deubiquitinase Rpn11 for initial substrate
592:
damages the proteasome's ability to assemble. The assembly of the half-proteasomes, in turn, is initiated by the assembly of the α subunits into their heptameric ring, forming a template for the association of the corresponding pro-β ring. The assembly of α subunits has not been characterized.
567:
during the assembly of the 20S particle to expose the proteolytic active site. The 20S particle is assembled from two half-proteasomes, each of which consists of a seven-membered pro-β ring attached to a seven-membered α ring. The association of the β rings of the two half-proteasomes triggers
5707:
Tarrason Risa, Gabriel; Hurtig, Fredrik; Bray, Sian; Hafner, Anne E.; Harker-Kirschneck, Lena; Faull, Peter; Davis, Colin; Papatziamou, Dimitra; Mutavchiev, Delyan R.; Fan, Catherine; Meneguello, Leticia; Arashiro
Pulschen, Andre; Dey, Gautam; Culley, Siân; Kilkenny, Mairi; Souza, Diorge P.;
1744:. Additionally, evidence is accumulating that the UPS plays an essential role in malignant transformation. UPS proteolysis plays a major role in responses of cancer cells to stimulatory signals that are critical for the development of cancer. Accordingly, gene expression by degradation of
1669:
The proteasome and its subunits are of clinical significance for at least two reasons: (1) a compromised complex assembly or a dysfunctional proteasome can be associated with the underlying pathophysiology of specific diseases, and (2) they can be exploited as drug targets for therapeutic
474:, is placed at the mouth of the AAA-ATPase hexamer, ideally positioned to remove ubiquitin moieties immediately before translocation of substrates into the 20S. The second ubiquitin receptor identified to date, Rpn10, is positioned at the periphery of the lid, near subunits Rpn8 and Rpn9.
1693:. Proteasome defects lead to reduced proteolytic activity and the accumulation of damaged or misfolded proteins, which may contribute to neurodegenerative disease, cardiovascular diseases, inflammatory responses and autoimmune diseases, and systemic DNA damage responses leading to
1670:
interventions. More recently, more effort has been made to consider the proteasome for the development of novel diagnostic markers and strategies. An improved and comprehensive understanding of the pathophysiology of the proteasome should lead to clinical applications in the future.
995:, whose gene products are a multimeric protease arranged in a two-layered ring and an ATPase. The hslV protein has been hypothesized to resemble the likely ancestor of the 20S proteasome. In general, HslV is not essential in bacteria, and not all bacteria possess it, whereas some
534:
but not of complete proteins. It is presumed that this is because the complex cannot unfold larger substrates. This structure is also known as PA28, REG, or PA26. The mechanisms by which it binds to the core particle through the C-terminal tails of its subunits and induces α-ring
940:
have also been reported, although p53 is also subject to ubiquitin-dependent degradation. Finally, structurally abnormal, misfolded, or highly oxidized proteins are also subject to ubiquitin-independent and 19S-independent degradation under conditions of cellular stress.
7187:
Verdoes M, Florea BI, Menendez-Benito V, Maynard CJ, Witte MD, van der Linden WA, van den
Nieuwendijk AM, Hofmann T, Berkers CR, van Leeuwen FW, Groothuis TA, Leeuwenburgh MA, Ovaa H, Neefjes JJ, Filippov DV, van der Marel GA, Dantuma NP, Overkleeft HS (November 2006).
613:
ring. To date it is still under debate whether the base complex assembles separately, whether the assembly is templated by the 20S core particle, or whether alternative assembly pathways exist. In addition to the four assembly chaperones, the deubiquitinating enzyme
596:
Only recently, the assembly process of the 19S regulatory particle has been elucidated to considerable extent. The 19S regulatory particle assembles as two distinct subcomponents, the base and the lid. Assembly of the base complex is facilitated by four assembly
364:
homologous to β subunits. They are assembled with their N-termini adjacent to that of the β subunits. The outer two rings in the stack consist of seven α subunits each, which serve as docking domains for the regulatory particles and the alpha subunits N-termini
684:
specificity to this system. The number of E1, E2, and E3 proteins expressed depends on the organism and cell type, but there are many different E3 enzymes present in humans, indicating that there is a huge number of targets for the ubiquitin proteasome system.
1216: — are prevented from undergoing apoptosis on exposure to proteasome inhibitors. The mechanism for this effect is not clear, but is hypothesized to be specific to cells in quiescent states, or to result from the differential activity of the pro-apoptotic
495:
Three distinct conformational states of the 26S proteasome. The conformations are hypothesized to be responsible for recruitment of the substrate, its irreversible commitment, and finally processing and translocation into the core particle, where degradation
539:
to open the 20S gate suggest a similar mechanism for the 19S particle. The expression of the 11S particle is induced by interferon gamma and is responsible, in conjunction with the immunoproteasome β subunits, for the generation of peptides that bind to the
903:
on these proteins' surfaces serve as the proteasomal substrates and enter the central cavity, while the majority of the protein remains outside. Similar effects have been observed in yeast proteins; this mechanism of selective degradation is known as
1592:. Clinical results also seem to justify use of proteasome inhibitor combined with chemotherapy, for B-cell acute lymphoblastic leukemia Proteasome inhibitors can kill some types of cultured leukemia cells that are resistant to glucocorticoids.
221:, which lack lysosomes, suggested the presence of a second intracellular degradation mechanism. This was shown in 1978 to be composed of several distinct protein chains, a novelty among proteases at the time. Later work on modification of
25:
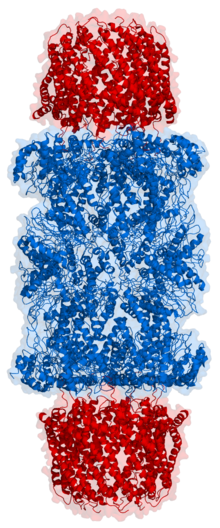
Cartoon representation of a proteasome. Its active sites are sheltered inside the tube (blue). The caps (red; in this case, 11S regulatory particles) on the ends regulate entry into the destruction chamber, where the protein is
1401:
and by close contacts with a region called the "B pocket" on the MHC surface. Many MHC class I alleles prefer hydrophobic C-terminal residues, and the immunoproteasome complex is more likely to generate hydrophobic C-termini.
6963:
Lambrou GI, Papadimitriou L, Chrousos GP, Vlahopoulos SA (April 2012). "Glucocorticoid and proteasome inhibitor impact on the leukemic lymphoblast: multiple, diverse signals converging on a few key downstream regulators".
1820:). The UPS is also involved in the regulation of inflammatory responses. This activity is usually attributed to the role of proteasomes in the activation of NF-κB which further regulates the expression of pro inflammatory
1097:) are the two key regulators of cyclin degradation and checkpoint control; the SCF itself is regulated by the APC via ubiquitination of the adaptor protein, Skp2, which prevents SCF activity before the G1-S transition.
8130:
Egerer K, Kuckelkorn U, Rudolph PE, Rückert JC, Dörner T, Burmester GR, Kloetzel PM, Feist E (October 2002). "Circulating proteasomes are markers of cell damage and immunologic activity in autoimmune diseases".
306:
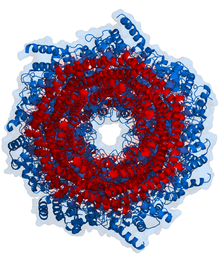
Schematic diagram of the proteasome 20S core particle viewed from one side. The α subunits that make up the outer two rings are shown in green, and the β subunits that make up the inner two rings are shown in
6030:
Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S, Elliott PJ (June 1999). "Proteasome inhibitors: a novel class of potent and effective antitumor agents".
794:
before translocation. While energy is needed for substrate unfolding, it is not required for translocation. The assembled 26S proteasome can degrade unfolded proteins in the presence of a non-hydrolyzable
529:
20S proteasomes can also associate with a second type of regulatory particle, the 11S regulatory particle, a heptameric structure that does not contain any ATPases and can promote the degradation of short
1546:, was the first non-peptidic proteasome inhibitor discovered and is widely used as a research tool in biochemistry and cell biology. Lactacystin was licensed to Myogenics/Proscript, which was acquired by
1006:
Sequence analysis suggests that the catalytic β subunits diverged earlier in evolution than the predominantly structural α subunits. In bacteria that express a 20S proteasome, the β subunits have high
766:
and gated by the N-terminal tails of the α ring subunits, the substrates must be at least partially unfolded before they enter the core. The passage of the unfolded substrate into the core is called
376:(Å) by 115 Å. The interior chamber is at most 53 Å wide, though the entrance can be as narrow as 13 Å, suggesting that substrate proteins must be at least partially unfolded to enter.
138:, the proteasome is a cylindrical complex containing a "core" of four stacked rings forming a central pore. Each ring is composed of seven individual proteins. The inner two rings are made of seven
790:
The gate formed by the α subunits prevents peptides longer than about four residues from entering the interior of the 20S particle. The ATP molecules bound before the initial recognition step are
7790:
Manaka H, Kato T, Kurita K, Katagiri T, Shikama Y, Kujirai K, Kawanami T, Suzuki Y, Nihei K, Sasaki H (May 1992). "Marked increase in cerebrospinal fluid ubiquitin in
Creutzfeldt–Jakob disease".
5414:
Gille C, Goede A, Schlöetelburg C, Preissner R, Kloetzel PM, Göbel UB, Frömmel C (March 2003). "A comprehensive view on proteasomal sequences: implications for the evolution of the proteasome".
1531:
by disrupting the regulated degradation of pro-growth cell cycle proteins. This approach of selectively inducing apoptosis in tumor cells has proven effective in animal models and human trials.
916:
Although most proteasomal substrates must be ubiquitinated before being degraded, there are some exceptions to this general rule, especially when the proteasome plays a normal role in the post-
406:
6603:
Fenteany G, Standaert RF, Lane WS, Choi S, Corey EJ, Schreiber SL (May 1995). "Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin".
6401:
Sharma N, Brandis KA, Herrera SK, Johnson BE, Vaidya T, Shrestha R, Debburman SK (2006). "alpha-Synuclein budding yeast model: toxicity enhanced by impaired proteasome and oxidative stress".
7747:
Ikeda K, Akiyama H, Arai T, Ueno H, Tsuchiya K, Kosaka K (July 2002). "Morphometrical reappraisal of motor neuron system of Pick's disease and amyotrophic lateral sclerosis with dementia".
6759:
Shah SA, Potter MW, McDade TP, Ricciardi R, Perugini RA, Elliott PJ, Adams J, Callery MP (2001). "26S proteasome inhibition induces apoptosis and limits growth of human pancreatic cancer".
5984:
Pitzer F, Dantes A, Fuchs T, Baumeister W, Amsterdam A (September 1996). "Removal of proteasomes from the nucleus and their accumulation in apoptotic blebs during programmed cell death".
5937:"Expression of a 26S proteasome ATPase subunit, MS73, in muscles that undergo developmentally programmed cell death, and its control by ecdysteroid hormones in the insect Manduca sexta"
154:
that recognize polyubiquitin tags attached to protein substrates and initiate the degradation process. The overall system of ubiquitination and proteasomal degradation is known as the
6677:
Fisher RI, Bernstein SH, Kahl BS, Djulbegovic B, Robertson MJ, de Vos S, Epner E, Krishnan A, Leonard JP, Lonial S, Stadtmauer EA, O'Connor OA, Shi H, Boral AL, Goy A (October 2006).
799:, but cannot degrade folded proteins, indicating that energy from ATP hydrolysis is used for substrate unfolding. Passage of the unfolded substrate through the opened gate occurs via
146:. These sites are located on the interior surface of the rings, so that the target protein must enter the central pore before it is degraded. The outer two rings each contain seven
6718:
Jakob C, Egerer K, Liebisch P, Türkmen S, Zavrski I, Kuckelkorn U, Heider U, Kaiser M, Fleissner C, Sterz J, Kleeberg L, Feist E, Burmester GR, Kloetzel PM, Sezer O (March 2007).
1776:
are all controlled by the UPS and thus involved in the development of various malignancies. Moreover, the UPS regulates the degradation of tumor suppressor gene products such as
5708:
Pellegrini, Luca; de Bruin, Robertus A. M.; Henriques, Ricardo; Snijders, Ambrosius P.; Šarić, Anđela; Lindås, Ann-Christin; Robinson, Nicholas P.; Baum, Buzz (7 August 2020).
1856:(NO). Additionally, the UPS also plays a role in inflammatory responses as regulators of leukocyte proliferation, mainly through proteolysis of cyclines and the degradation of
6924:"Bortezomib with chemotherapy is highly active in advanced B-precursor acute lymphoblastic leukemia: Therapeutic Advances in Childhood Leukemia & Lymphoma (TACL) Study"
4647:
Zhu Q, Wani G, Wang QE, El-mahdy M, Snapka RM, Wani AA (July 2005). "Deubiquitination by proteasome is coordinated with substrate translocation for proteolysis in vivo".
891:
Although the proteasome normally produces very short peptide fragments, in some cases these products are themselves biologically active and functional molecules. Certain
1580:
that decrease to normal levels in response to successful chemotherapy. Studies in animals have indicated that bortezomib may also have clinically significant effects in
770:
and necessarily occurs after deubiquitination. However, the order in which substrates are deubiquitinated and unfolded is not yet clear. Which of these processes is the
6142:"The cytoplasmic Hsp70 chaperone machinery subjects misfolded and endoplasmic reticulum import-incompetent proteins to degradation via the ubiquitin-proteasome system"
2537:
Löwe J, Stock D, Jap B, Zwickl P, Baumeister W, Huber R (April 1995). "Crystal structure of the 20S proteasome from the archaeon T. acidophilum at 3.4 A resolution".
1336:, the major protein component of Lewy bodies, under conditions of low proteasome activity. Impaired proteasomal activity may underlie cognitive disorders such as the
559:
The assembly of the proteasome is a complex process due to the number of subunits that must associate to form an active complex. The β subunits are synthesized with
282:
data revealing the stacked-ring structure of the proteasome became available in the mid-1980s, the first structure of the proteasome core particle was not solved by
696:(UBL) domain and one or more ubiquitin-associated (UBA) domains. The UBL domains are recognized by the 19S proteasome caps and the UBA domains bind ubiquitin via
5892:
Schwartz LM, Myer A, Kosz L, Engelstein M, Maier C (October 1990). "Activation of polyubiquitin gene expression during developmentally programmed cell death".
5623:
Bashir T, Dorrello NV, Amador V, Guardavaccaro D, Pagano M (March 2004). "Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase".
7701:
Chung KK, Dawson VL, Dawson TM (November 2001). "The role of the ubiquitin-proteasomal pathway in
Parkinson's disease and other neurodegenerative disorders".
6444:
Murata S, Sasaki K, Kishimoto T, Niwa S, Hayashi H, Takahama Y, Tanaka K (June 2007). "Regulation of CD8+ T cell development by thymus-specific proteasomes".
3057:
Kusmierczyk AR, Kunjappu MJ, Funakoshi M, Hochstrasser M (March 2008). "A multimeric assembly factor controls the formation of alternative 20S proteasomes".
700:. These receptor proteins may escort polyubiquitinated proteins to the proteasome, though the specifics of this interaction and its regulation are unclear.
8318:
1916:
356:
ring structures that are themselves composed of two different types of subunits; α subunits are structural in nature, whereas β subunits are predominantly
2336:
Arrigo AP, Tanaka, K, Goldberg F, Welch WJ (1988). "Identity of 19S prosome particle with the large multifunctional protease complex of mammalian cells".
506:
been recently elucidated. 20S gate opening, and thus substrate degradation, requires the C-termini of the proteasomal ATPases, which contains a specific
92:. Once a protein is tagged with a single ubiquitin molecule, this is a signal to other ligases to attach additional ubiquitin molecules. The result is a
656:
Proteins are targeted for degradation by the proteasome with covalent modification of a lysine residue that requires the coordinated reactions of three
1732:. As part of the ubiquitin–proteasome system (UPS), the proteasome maintains cardiac protein homeostasis and thus plays a significant role in cardiac
8206:
3247:
Lam YA, Lawson TG, Velayutham M, Zweier JL, Pickart CM (April 2002). "A proteasomal ATPase subunit recognizes the polyubiquitin degradation signal".
1673:
The proteasomes form a pivotal component for the ubiquitin–proteasome system (UPS) and corresponding cellular
Protein Quality Control (PQC). Protein
1273:
proteins have been implicated in increasing the activity of the ubiquitin-proteasome system, though they are not direct participants in the process.
787:
segments of sufficient size, either at the protein terminus or internally, has also been proposed to facilitate efficient initiation of degradation.
173:. The importance of proteolytic degradation inside cells and the role of ubiquitin in proteolytic pathways was acknowledged in the award of the 2004
4164:
Fukunaga K, Kudo T, Toh-e A, Tanaka K, Saeki Y (June 2010). "Dissection of the assembly pathway of the proteasome lid in
Saccharomyces cerevisiae".
8622:
5077:
Dick TP, Nussbaum AK, Deeg M, Heinemeyer W, Groll M, Schirle M, Keilholz W, Stevanović S, Wolf DH, Huber R, Rammensee HG, Schild H (October 1998).
928:
regions, are degraded in a ubiquitin-independent manner. The most well-known example of a ubiquitin-independent proteasome substrate is the enzyme
672:
residue in concert with the adenylylation of a second ubiquitin. This adenylylated ubiquitin is then transferred to a cysteine of a second enzyme,
1010:
to archaeal and eukaryotic β subunits, whereas the α sequence identity is much lower. The presence of 20S proteasomes in bacteria may result from
248:-dependent proteolytic complex that was responsible for ubiquitin-dependent protein degradation was discovered and was called the 26S proteasome.
150:
whose function is to maintain a "gate" through which proteins enter the barrel. These α subunits are controlled by binding to "cap" structures or
5026:
Groll M, Ditzel L, Löwe J, Stock D, Bochtler M, Bartunik HD, Huber R (April 1997). "Structure of 20S proteasome from yeast at 2.4 A resolution".
618:
also promotes base assembly, but it is not essential. The lid assembles separately in a specific order and does not require assembly chaperones.
397:, the β1, β2, and β5 subunits are catalytic; although they share a common mechanism, they have three distinct substrate specificities considered
2502:
Kopp F, Steiner R, Dahlmann B, Kuehn L, Reinauer H (August 1986). "Size and shape of the multicatalytic proteinase from rat skeletal muscle".
2158:
Ciehanover A, Hod Y, Hershko A (April 1978). "A heat-stable polypeptide component of an ATP-dependent proteolytic system from reticulocytes".
999:
possess both the 20S and the hslV systems. Many bacteria also possess other homologs of the proteasome and an associated ATPase, most notably
8920:
8594:
4948:
Seemüller E, Lupas A, Stock D, Löwe J, Huber R, Baumeister W (April 1995). "Proteasome from
Thermoplasma acidophilum: a threonine protease".
3301:
Beck F, Unverdorben P, Bohn S, Schweitzer A, Pfeifer G, Sakata E, Nickell S, Plitzko JM, Villa E, Baumeister W, Förster F (September 2012).
213:-filled interiors that can degrade and then recycle exogenous proteins and aged or damaged organelles. However, work by Joseph Etlinger and
1622:
Proteasome inhibitors have also shown promise in treating autoimmune diseases in animal models. For example, studies in mice bearing human
1184:, or programmed cell death. The resulting deconstruction of cellular components is primarily carried out by specialized proteases known as
8361:
1437:
3767:"Structure of the 26S proteasome with ATP-γS bound provides insights into the mechanism of nucleotide-dependent substrate translocation"
3150:"ATP binding to PAN or the 26S ATPases causes association with the 20S proteasome, gate opening, and translocation of unfolded proteins"
2816:
Wilk S, Orlowski M (March 1983). "Evidence that pituitary cation-sensitive neutral endopeptidase is a multicatalytic protease complex".
2293:
Wilk S, Orlowski M (November 1980). "Cation-sensitive neutral endopeptidase: isolation and specificity of the bovine pituitary enzyme".
251:
Much of the early work leading up to the discovery of the ubiquitin proteasome system occurred in the late 1970s and early 1980s at the
5853:"Coordinated induction of the ubiquitin conjugation pathway accompanies the developmentally programmed death of insect skeletal muscle"
1324:, through mechanisms that are not yet well understood. Decreased proteasome activity has been suggested as a cause of aggregation and
1223:. The ability of proteasome inhibitors to induce apoptosis in rapidly dividing cells has been exploited in several recently developed
3924:"The axial channel of the proteasome core particle is gated by the Rpt2 ATPase and controls both substrate entry and product release"
3102:"An archaebacterial ATPase, homologous to ATPases in the eukaryotic 26 S proteasome, activates protein breakdown by 20 S proteasomes"
7329:
Sulistio YA, Heese K (January 2015). "The
Ubiquitin–Proteasome System and Molecular Chaperone Deregulation in Alzheimer's Disease".
6656:
1425:. Increased levels of proteasome activity correlate with disease activity and have been implicated in autoimmune diseases including
8407:
1372:. Although constitutively expressed proteasomes can participate in this process, a specialized complex composed of proteins, whose
775:
residues by the atomic structure of the substrate-engaged 26S proteasome in the deubiquitylation-compatible state, but substantial
577:
1615:-like activity is somewhat enhanced. Studies in animal models suggest that ritonavir may have inhibitory effects on the growth of
3578:
Schweitzer A, Aufderheide A, Rudack T, Beck F, Pfeifer G, Plitzko JM, Sakata E, Schulten K, Förster F, Baumeister W (July 2016).
921:
615:
294:, revealing mechanisms by which the substrate is recognized, deubiquitylated, unfolded and degraded by the human 26S proteasome.
1304:
Dysregulation of the ubiquitin proteasome system may contribute to several neural diseases. It may lead to brain tumors such as
4774:
van der Lee R, Lang B, Kruse K, Gsponer J, Sánchez de Groot N, Huynen MA, Matouschek A, Fuxreiter M, Babu MM (September 2014).
1785:
6189:
Dai Q, Qian SB, Li HH, McDonough H, Borchers C, Huang D, Takayama S, Younger JM, Ren HY, Cyr DM, Patterson C (November 2005).
5486:"Cyclin B dissociation from CDK1 precedes its degradation upon MPF inactivation in mitotic extracts of Xenopus laevis embryos"
8375:
7573:
Karin M, Delhase M (February 2000). "The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling".
3420:
Lasker K, Förster F, Bohn S, Walzthoeni T, Villa E, Unverdorben P, Beck F, Aebersold R, Sali A, Baumeister W (January 2012).
1968:
2747:"Docking of the proteasomal ATPases' carboxyl termini in the 20S proteasome's alpha ring opens the gate for substrate entry"
2659:
Wang J, Maldonado MA (August 2006). "The ubiquitin-proteasome system and its role in inflammatory and autoimmune diseases".
4823:
Smith DM, Benaroudj N, Goldberg A (October 2006). "Proteasomes and their associated ATPases: a destructive combination".
3646:"Structural mechanism for nucleotide-driven remodeling of the AAA-ATPase unfoldase in the activated human 26S proteasome"
925:
7099:"Proteasome inhibition reduces superantigen-mediated T cell activation and the severity of psoriasis in a SCID-hu model"
6804:"The proteasome inhibitor bortezomib enhances the activity of docetaxel in orthotopic human pancreatic tumor xenografts"
5118:
Voges D, Zwickl P, Baumeister W (1999). "The 26S proteasome: a molecular machine designed for controlled proteolysis".
2373:"ATP serves two distinct roles in protein degradation in reticulocytes, one requiring and one independent of ubiquitin"
8346:
7048:
Laurent N, de Boüard S, Guillamo JS, Christov C, Zini R, Jouault H, Andre P, Lotteau V, Peschanski M (February 2004).
1700:
Research has implicated UPS defects in the pathogenesis of neurodegenerative and myodegenerative disorders, including
8913:
8587:
1600:
784:
4909:"Repeat sequence of Epstein–Barr virus-encoded nuclear antigen 1 protein interrupts proteasome substrate processing"
4289:
Risseeuw EP, Daskalchuk TE, Banks TW, Liu E, Cotelesage J, Hellmann H, Estelle M, Somers DE, Crosby WL (June 2003).
1986:"Distinct 19 S and 20 S subcomplexes of the 26 S proteasome and their distribution in the nucleus and the cytoplasm"
727:
demands on these genes to produce enough ubiquitin for the cell. It has been proposed that ubiquitin is the slowest-
470:(hence called PCI subunits) assemble to a horseshoe-like structure enclosing the Rpn8/Rpn11 heterodimer. Rpn11, the
271:
provided key conceptual insights, though Rose later downplayed his role in the discovery. The three shared the 2004
197:
Before the discovery of the ubiquitin–proteasome system, protein degradation in cells was thought to rely mainly on
8603:
1584:. Preclinical and early clinical studies have been started to examine bortezomib's effectiveness in treating other
1361:
541:
3965:"The 1.9 A structure of a proteasome-11S activator complex and implications for proteasome-PAN/PA700 interactions"
2101:"A soluble ATP-dependent proteolytic system responsible for the degradation of abnormal proteins in reticulocytes"
924:
into p50 via internal proteolysis is one major example. Some proteins that are hypothesized to be unstable due to
551:(human). It opens only one α subunit in the 20S gate and itself folds into a dome with a very small pore over it.
8982:
8435:
564:
4682:
Wenzel T, Baumeister W (March 1995). "Conformational constraints in protein degradation by the 20S proteasome".
845:. The α subunits are represented as green spheres and the β subunits as protein backbones colored by individual
5527:"Mitotic checkpoint slippage in humans occurs via cyclin B destruction in the presence of an active checkpoint"
467:
4500:
Xu P, Duong DM, Seyfried NT, Cheng D, Xie Y, Robert J, Rush J, Hochstrasser M, Finley D, Peng J (April 2009).
4123:
Sakata E, Stengel F, Fukunaga K, Zhou M, Saeki Y, Förster F, Baumeister W, Tanaka K, Robinson CV (June 2011).
9084:
5079:"Contribution of proteasomal beta-subunits to the cleavage of peptide substrates analyzed with yeast mutants"
1721:
1713:
1261:
that identify misfolded or unfolded proteins and target them for proteasomal degradation are expressed. Both
796:
291:
5802:
Weijers D, Benkova E, Jäger KE, Schlereth A, Hamann T, Kientz M, Wilmoth JC, Reed JW, Jürgens G (May 2005).
4502:"Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation"
1607:
infection. However, it has been shown to inhibit proteasomes as well as free proteases; to be specific, the
8906:
8580:
8400:
7960:
Predmore JM, Wang P, Davis F, Bartolone S, Westfall MV, Dyke DB, Pagani F, Powell SR, Day SM (March 2010).
3708:"Deep classification of a large cryo-EM dataset defines the conformational landscape of the 26S proteasome"
3706:
Unverdorben P, Beck F, Śledź P, Schweitzer A, Pfeifer G, Plitzko JM, Baumeister W, Förster F (April 2014).
1865:
1426:
876:. The mechanism of proteolysis by the β subunits of the 20S core particle is through a threonine-dependent
673:
96:
that is bound by the proteasome, allowing it to degrade the tagged protein. The degradation process yields
8572:
7007:
Schmidtke G, Holzhütter HG, Bogyo M, Kairies N, Groll M, de Giuli R, Emch S, Groettrup M (December 1999).
4330:
Elsasser S, Finley D (August 2005). "Delivery of ubiquitinated substrates to protein-unfolding machines".
2917:"The active sites of the eukaryotic 20 S proteasome and their involvement in subunit precursor processing"
1576:. Notably, multiple myeloma has been observed to result in increased proteasome-derived peptide levels in
1289:
proteins via the proteasome system. In particular, proteasomes localized to the nucleus are regulated by
1135:
Like eukaryotes, some archaea also use the proteasome to control cell cycle, specifically by controlling
661:
2195:"Isopeptide linkage between nonhistone and histone 2A polypeptides of chromosomal conjugate-protein A24"
1661:
inhibitors have also been developed to specifically label the active sites of the assembled proteasome.
830:
gene products bearing this sequence can stall the proteasome, helping the virus propagate by preventing
319:). The proteasome most exclusively used in mammals is the cytosolic 26S proteasome, which is about 2000
9342:
6679:"Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma"
2863:
2603:
Dong Y, Zhang S, Wu Z, Li X, Wang WL, Zhu Y, Stoilova-McPhie S, Lu Y, Finley D, Mao Y (November 2018).
1777:
1565:
1547:
1290:
1086:
8214:"Early work on the ubiquitin proteasome system, an interview with Aaron Ciechanover. Interview by CDD"
7474:"Targeting the ubiquitin–proteasome system in heart disease: the basis for new therapeutic strategies"
5277:"Determinants of proteasome recognition of ornithine decarboxylase, a ubiquitin-independent substrate"
2254:"Early work on the ubiquitin proteasome system, an interview with Aaron Ciechanover. Interview by CDD"
6720:"Circulating proteasome levels are an independent prognostic factor for survival in multiple myeloma"
3198:
Liu CW, Li X, Thompson D, Wooding K, Chang TL, Tang Z, Yu H, Thomas PJ, DeMartino GN (October 2006).
1589:
454:
In 2012, two independent efforts have elucidated the molecular architecture of the 26S proteasome by
5804:"Developmental specificity of auxin response by pairs of ARF and Aux/IAA transcriptional regulators"
5668:"The oncoprotein gankyrin negatively regulates both p53 and RB by enhancing proteasomal degradation"
9079:
8607:
6922:
Messinger YH, Gaynon PS, Sposto R, van der
Giessen J, Eckroth E, Malvar J, Bostrom BC (July 2012).
3476:
Chen S, Wu J, Lu Y, Ma YB, Lee BH, Yu Z, Ouyang Q, Finley DJ, Kirschner MW, Mao Y (November 2016).
1906:
1337:
1129:
1047:
724:
681:
455:
272:
174:
8249:"Early work on the ubiquitin proteasome system, an interview with Avram Hershko. Interview by CDD"
6497:"26S proteasomes and immunoproteasomes produce mainly N-extended versions of an antigenic peptide"
5240:
Asher G, Reuven N, Shaul Y (August 2006). "20S proteasomes and protein degradation "by default"".
4414:
Sharp PM, Li WH (1987). "Ubiquitin genes as a paradigm of concerted evolution of tandem repeats".
4004:
Witt S, Kwon YD, Sharon M, Felderer K, Beuttler M, Robinson CV, Baumeister W, Jap BK (July 2006).
3535:
Huang X, Luan B, Wu J, Shi Y (September 2016). "An atomic structure of the human 26S proteasome".
2463:"Early work on the ubiquitin proteasome system, an interview with Avram Hershko. Interview by CDD"
609:
subunits and their main function seems to be to ensure proper assembly of the heterohexameric AAA-
389:, all the α and all the β subunits are identical, whereas eukaryotic proteasomes such as those in
8393:
7190:"A fluorescent broad-spectrum proteasome inhibitor for labeling proteasomes in vitro and in vivo"
1857:
1737:
1717:
1365:
1341:
1254:
1109:
1027:
929:
867:
712:
471:
268:
4991:
Coux O, Tanaka K, Goldberg AL (1996). "Structure and functions of the 20S and 26S proteasomes".
4080:
Murata S, Yashiroda H, Tanaka K (February 2009). "Molecular mechanisms of proteasome assembly".
3422:"Molecular architecture of the 26S proteasome holocomplex determined by an integrative approach"
1876:(RA) predominantly exhibit circulating proteasomes which can be applied as clinical biomarkers.
1368:. These peptides are products of proteasomal degradation of proteins originated by the invading
9044:
9019:
8284:"Early work on the ubiquitin proteasome system, an interview with Irwin Rose. Interview by CDD"
5759:
Dharmasiri S, Estelle M (2002). "The role of regulated protein degradation in auxin response".
3873:
Lu Y, Wu J, Dong Y, Chen S, Sun S, Ma YB, Ouyang Q, Finley D, Kirschner MW, Mao Y (July 2017).
3765:Śledź P, Unverdorben P, Beck F, Pfeifer G, Schweitzer A, Förster F, Baumeister W (April 2013).
3010:"Plasticity in eucaryotic 20S proteasome ring assembly revealed by a subunit deletion in yeast"
2421:
2078:
1947:
Lodish H, Berk A, Matsudaira P, Kaiser CA, Krieger M, Scott MP, Zipursky SL, Darnell J (2004).
1705:
1701:
1551:
1317:
1313:
917:
827:
693:
484:
245:
8009:
Powell SR (July 2006). "The ubiquitin-proteasome system in cardiac physiology and pathology".
5370:"Ubiquitin conjugation is not required for the degradation of oxidized proteins by proteasome"
1641:
Labeling and inhibition of the proteasome is also of interest in laboratory settings for both
1386:. Another β5i variant subunit, β5t, is expressed in the thymus, leading to a thymus-specific "
895:
regulating the expression of specific genes, including one component of the mammalian complex
9283:
9069:
8962:
8539:
4776:"Intrinsically disordered segments affect protein half-life in the cell and during evolution"
1960:
1954:
1745:
1011:
800:
536:
283:
8319:"Targeting of nuclear factor-kappaB and proteasome by dithiocarbamate complexes with metals"
8087:
Ben-Neriah Y (January 2002). "Regulatory functions of ubiquitination in the immune system".
7876:
Mayer RJ (March 2003). "From neurodegeneration to neurohomeostasis: the role of ubiquitin".
491:
161:
The proteasomal degradation pathway is essential for many cellular processes, including the
9316:
8430:
6612:
6557:
6546:"Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21)"
6453:
5035:
4957:
4603:
4423:
4125:"The catalytic activity of Ubp6 enhances maturation of the proteasomal regulatory particle"
3778:
3719:
3657:
3591:
3489:
3376:
3314:
3256:
2546:
2206:
2112:
1873:
1725:
1516:
1457:
1430:
1205:
1164:
892:
831:
519:
105:
6851:
Schenkein D (June 2002). "Proteasome inhibitors in the treatment of B-cell malignancies".
4465:
Pickart CM, Fushman D (December 2004). "Polyubiquitin chains: polymeric protein signals".
576:
of the propeptides to expose the active site. These β interactions are mediated mainly by
8:
9347:
8942:
7657:
Checler F, da Costa CA, Ancolio K, Chevallier N, Lopez-Perez E, Marambaud P (July 2000).
6653:
6140:
Park SH, Bolender N, Eisele F, Kostova Z, Takeuchi J, Coffino P, Wolf DH (January 2007).
2794:
1901:
1649:
study of proteasomal activity in cells. The most commonly used laboratory inhibitors are
1393:
The strength of MHC class I ligand binding is dependent on the composition of the ligand
1270:
900:
715:
sequence and is found in all known eukaryotic organisms. The genes encoding ubiquitin in
598:
573:
523:
279:
8052:
Adams J (April 2003). "Potential for proteasome inhibition in the treatment of cancer".
6616:
6561:
6457:
5039:
5004:
4961:
4607:
4427:
3782:
3723:
3661:
3595:
3493:
3380:
3318:
3260:
2550:
2210:
2116:
1869:
9352:
8112:
8034:
7986:
7961:
7937:
7912:
7858:
7815:
7772:
7726:
7634:
7609:
7547:
7522:
7498:
7473:
7449:
7424:
7400:
7373:
7354:
7265:
7240:
7079:
6989:
6833:
6784:
6636:
6580:
6545:
6477:
6426:
6378:
6361:
6337:
6312:
6255:
6166:
6141:
6009:
5966:
5917:
5828:
5803:
5784:
5736:
5709:
5648:
5600:
5575:
5551:
5526:
5181:
5059:
4884:
4859:
4800:
4775:
4751:
4726:
4707:
4624:
4591:
4526:
4501:
4447:
4355:
4266:
4241:
4105:
3899:
3874:
3850:
3825:
3801:
3766:
3742:
3707:
3678:
3645:
3614:
3579:
3560:
3512:
3477:
3448:
3421:
3397:
3364:
3337:
3302:
3280:
3224:
3199:
3082:
3034:
3009:
2985:
2961:"Assembly of an Evolutionarily Conserved Alternative Proteasome Isoform in Human Cells"
2960:
2894:
2841:
2829:
2771:
2746:
2717:
2692:
2629:
2604:
2397:
2372:
2361:
2318:
2306:
2056:
1861:
1833:
1773:
1555:
1422:
1258:
1059:
968:
776:
771:
214:
104:
long, which can then be further degraded into shorter amino acid sequences and used in
8898:
8190:
8163:
8065:
7977:
7714:
7675:
7658:
7164:
7147:
7123:
7098:
6521:
6496:
6286:
5953:
5936:
5427:
5301:
5276:
5131:
4565:
4217:
4200:
4058:
3940:
3923:
3200:"ATP binding and ATP hydrolysis play distinct roles in the function of 26S proteasome"
2438:
2229:
2194:
2135:
2100:
2002:
1985:
1630:
after treatment with a proteasome inhibitor. Inhibitors also show positive effects in
1568:
and marketed as
Velcade, is the first proteasome inhibitor to reach clinical use as a
9337:
9266:
9104:
8987:
8471:
8445:
8338:
8305:
8270:
8235:
8195:
8140:
8104:
8069:
8026:
7991:
7962:"Ubiquitin proteasome dysfunction in human hypertrophic and dilated cardiomyopathies"
7942:
7893:
7850:
7807:
7803:
7764:
7718:
7680:
7639:
7590:
7552:
7503:
7454:
7405:
7346:
7311:
7306:
7289:
7270:
7221:
7169:
7128:
7071:
7050:"Effects of the proteasome inhibitor ritonavir on glioma growth in vitro and in vivo"
7030:
6981:
6945:
6904:
6868:
6825:
6776:
6741:
6700:
6628:
6585:
6526:
6469:
6418:
6383:
6342:
6290:
6247:
6212:
6171:
6122:
6081:
6040:
6001:
5997:
5958:
5909:
5905:
5874:
5833:
5776:
5741:
5689:
5640:
5605:
5556:
5507:
5466:
5462:
5431:
5391:
5347:
5306:
5257:
5222:
5173:
5135:
5100:
5051:
5008:
4973:
4930:
4889:
4840:
4805:
4756:
4699:
4664:
4629:
4569:
4531:
4482:
4439:
4396:
4391:
4374:
4347:
4312:
4307:
4290:
4271:
4222:
4181:
4146:
4097:
4062:
4027:
3986:
3945:
3904:
3855:
3806:
3747:
3683:
3619:
3552:
3517:
3453:
3402:
3342:
3272:
3229:
3171:
3123:
3074:
3039:
2990:
2938:
2886:
2833:
2776:
2722:
2668:
2634:
2562:
2519:
2515:
2484:
2443:
2422:"Purification of two high molecular weight proteases from rabbit reticulocyte lysate"
2402:
2353:
2310:
2275:
2234:
2175:
2171:
2140:
2048:
2007:
1964:
1949:
1709:
1581:
1353:
1309:
1233:
1066:
1007:
1000:
846:
841:
A cutaway view of the proteasome 20S core particle illustrating the locations of the
811:
260:
178:
135:
54:
8116:
7819:
7776:
7358:
7083:
6993:
6837:
6788:
6640:
6544:
Mallery DL, McEwan WA, Bidgood SR, Towers GJ, Johnson CM, James LC (November 2010).
6481:
6430:
6259:
6101:"Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties"
6013:
5970:
5921:
4711:
4359:
4109:
3564:
3363:
Lander GC, Estrin E, Matyskiela ME, Bashore C, Nogales E, Martin A (February 2012).
3086:
2898:
2845:
2060:
1681:
and degradation by the proteasome are important mechanisms in the regulation of the
1508:= yellow), surrounded by the local protein surface. The blue patch is the catalytic
651:
369:
336:
role in degradation of foreign peptides such as those produced after infection by a
8549:
8507:
8502:
8497:
8330:
8295:
8260:
8225:
8185:
8175:
8096:
8061:
8038:
8018:
7981:
7973:
7932:
7924:
7885:
7862:
7842:
7799:
7756:
7730:
7710:
7670:
7629:
7621:
7582:
7542:
7534:
7493:
7485:
7444:
7436:
7395:
7385:
7338:
7301:
7260:
7252:
7211:
7206:
7201:
7189:
7159:
7118:
7110:
7061:
7020:
6973:
6935:
6896:
6860:
6815:
6768:
6731:
6690:
6620:
6575:
6565:
6516:
6508:
6461:
6410:
6373:
6332:
6324:
6282:
6239:
6202:
6191:"Regulation of the cytoplasmic quality control protein degradation pathway by BAG2"
6161:
6153:
6112:
6071:
5993:
5948:
5901:
5864:
5823:
5815:
5788:
5768:
5731:
5721:
5679:
5652:
5632:
5595:
5587:
5546:
5538:
5497:
5458:
5423:
5381:
5337:
5296:
5288:
5249:
5212:
5201:"Productive RUPture: activation of transcription factors by proteasomal processing"
5185:
5165:
5127:
5090:
5063:
5043:
5000:
4965:
4920:
4879:
4871:
4832:
4795:
4787:
4746:
4738:
4691:
4656:
4619:
4611:
4561:
4521:
4513:
4474:
4451:
4431:
4386:
4339:
4302:
4291:"Protein interaction analysis of SCF ubiquitin E3 ligase subunits from Arabidopsis"
4261:
4253:
4212:
4173:
4136:
4089:
4054:
4017:
3976:
3935:
3894:
3886:
3845:
3837:
3796:
3786:
3737:
3727:
3673:
3665:
3609:
3599:
3544:
3507:
3497:
3443:
3433:
3392:
3384:
3332:
3322:
3284:
3264:
3219:
3211:
3161:
3113:
3066:
3029:
3021:
2980:
2972:
2928:
2878:
2825:
2766:
2758:
2712:
2704:
2624:
2616:
2554:
2511:
2474:
2433:
2392:
2384:
2345:
2322:
2302:
2265:
2224:
2214:
2167:
2130:
2120:
2038:
1997:
1573:
1475:
1445:
1382:
1377:
1312:
diseases that share aggregation of misfolded proteins as a common feature, such as
1090:
1015:
975:
The 20S proteasome is both ubiquitous and essential in eukaryotes and archaea. The
963:
807:
677:
641:
that serves as a molecular tag targeting proteins for degradation by the proteasome
463:
430:
425:
320:
170:
89:
8372:
6664:
5576:"Regulation of late G1/S phase transition and APC Cdh1 by reactive oxygen species"
4201:"Ubiquitin-activating enzyme. Mechanism and role in protein-ubiquitin conjugation"
1196:
of pro-growth cell cycle proteins. However, some cell lines — in particular,
263:
worked as a graduate student. Hershko's year-long sabbatical in the laboratory of
8967:
8455:
8440:
8379:
8180:
7889:
6940:
6923:
6736:
6719:
6660:
6099:
Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G (November 2006).
5217:
5200:
4791:
4141:
4124:
3981:
3964:
3890:
3215:
3166:
3149:
2976:
2762:
2708:
2365:
1911:
1789:
1387:
1373:
1333:
1197:
1014:, while the diversification of subunits among eukaryotes is ascribed to multiple
980:
547:
Yet another type of non-ATPase regulatory particle is the Blm10 (yeast) or PA200/
166:
81:
42:
9126:
8022:
7928:
7440:
7290:"New insights into proteasome function: from archaebacteria to drug development"
4660:
3875:"Conformational Landscape of the p28-Bound Human Proteasome Regulatory Particle"
2504:
Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology
1352:
The proteasome plays a straightforward but critical role in the function of the
8930:
8476:
8334:
7538:
7097:
Zollner TM, Podda M, Pien C, Elliott PJ, Kaufmann R, Boehncke WH (March 2002).
6550:
Proceedings of the National Academy of Sciences of the United States of America
6512:
6414:
5935:
Löw P, Bussell K, Dawson SP, Billett MA, Mayer RJ, Reynolds SE (January 1997).
5156:
Rape M, Jentsch S (May 2002). "Taking a bite: proteasomal protein processing".
4615:
4517:
4478:
4177:
3771:
Proceedings of the National Academy of Sciences of the United States of America
3712:
Proceedings of the National Academy of Sciences of the United States of America
3669:
3584:
Proceedings of the National Academy of Sciences of the United States of America
3482:
Proceedings of the National Academy of Sciences of the United States of America
3426:
Proceedings of the National Academy of Sciences of the United States of America
3307:
Proceedings of the National Academy of Sciences of the United States of America
2199:
Proceedings of the National Academy of Sciences of the United States of America
2105:
Proceedings of the National Academy of Sciences of the United States of America
1893:
1849:
1781:
1674:
1328:
formation in Parkinson's. This hypothesis is supported by the observation that
1320:, large insoluble aggregates of misfolded proteins can form and then result in
1177:
780:
676:(E2). In the last step, a member of a highly diverse class of enzymes known as
630:
507:
417:
324:
73:
7846:
7760:
7625:
7342:
7256:
7066:
7049:
6977:
6328:
5772:
5542:
4836:
4022:
4005:
3826:"Conformational switching of the 26S proteasome enables substrate degradation"
2620:
9331:
9213:
9173:
9148:
9064:
8977:
8952:
8534:
8529:
7390:
7025:
7008:
6820:
6803:
6695:
6678:
5819:
5449:
Bochtler M, Ditzel L, Groll M, Hartmann C, Huber R (1999). "The proteasome".
5095:
5078:
4875:
4860:"Glycine-alanine repeats impair proper substrate unfolding by the proteasome"
3118:
3101:
3025:
3008:
Velichutina I, Connerly PL, Arendt CS, Li X, Hochstrasser M (February 2004).
2933:
2916:
2745:
Smith DM, Chang SC, Park S, Finley D, Cheng Y, Goldberg AL (September 2007).
1921:
1829:
1741:
1398:
1321:
920:
processing of the protein. The proteasomal activation of NF-κB by processing
720:
410:
286:
until 1994. In 2018, the first atomic structures of the human 26S proteasome
256:
182:
77:
7523:"Protein quality control and metabolism: bidirectional control in the heart"
6900:
6624:
6570:
6465:
6273:
Davies KJ (2003). "Degradation of oxidized proteins by the 20S proteasome".
6157:
5869:
5852:
5726:
4969:
4592:"USP14-regulated allostery of the human proteasome by time-resolved cryo-EM"
4257:
3791:
3732:
3604:
3502:
3438:
3327:
2558:
9193:
9165:
9143:
9136:
9116:
9056:
8972:
8957:
8602:
8366:
8342:
8309:
8300:
8283:
8274:
8265:
8248:
8239:
8230:
8213:
8199:
8144:
8108:
8073:
8030:
7995:
7946:
7897:
7854:
7768:
7722:
7684:
7643:
7594:
7586:
7556:
7507:
7458:
7409:
7350:
7274:
7225:
7173:
7132:
7075:
7034:
6985:
6949:
6908:
6872:
6864:
6829:
6780:
6745:
6704:
6589:
6530:
6473:
6422:
6387:
6346:
6294:
6251:
6216:
6207:
6190:
6175:
6126:
6085:
6076:
6059:
6044:
5837:
5780:
5745:
5693:
5644:
5609:
5560:
5511:
5470:
5435:
5395:
5386:
5369:
5351:
5310:
5292:
5261:
5226:
5177:
5139:
4934:
4925:
4908:
4893:
4844:
4809:
4760:
4668:
4633:
4573:
4535:
4486:
4400:
4351:
4316:
4275:
4185:
4150:
4101:
4066:
4031:
4006:"Proteasome assembly triggers a switch required for active-site maturation"
3990:
3949:
3922:
Köhler A, Cascio P, Leggett DS, Woo KM, Goldberg AL, Finley D (June 2001).
3908:
3859:
3810:
3751:
3687:
3623:
3556:
3521:
3457:
3406:
3346:
3276:
3233:
3175:
3127:
3078:
3043:
2994:
2890:
2780:
2726:
2672:
2638:
2605:"Cryo-EM structures and dynamics of substrate-engaged human 26S proteasome"
2488:
2479:
2462:
2279:
2270:
2253:
2052:
1853:
1694:
1608:
1569:
1539:
1524:
1492:
proteasome. The bortezomib molecule is in the center colored by atom type (
1471:
1414:
1305:
1224:
1156:
1100:
Individual components of the 19S particle have their own regulatory roles.
1042:
requires the proteasome-dependent dissociation of the regulatory component
983:, also share homologs of the 20S proteasome, whereas most bacteria possess
873:
697:
459:
446:
428:. The proteasome assembled with these alternative subunits is known as the
414:
398:
385:
361:
218:
124:
58:
7811:
7610:"Quality control mechanisms in cellular and systemic DNA damage responses"
7489:
7315:
6632:
6005:
5962:
5913:
5878:
5104:
5055:
5012:
4977:
4858:
Hoyt MA, Zich J, Takeuchi J, Zhang M, Govaerts C, Coffino P (April 2006).
4703:
4443:
4226:
2942:
2837:
2566:
2523:
2447:
2406:
2388:
2357:
2314:
2219:
2011:
853:
residue in each subunit. Light blue chemical structures are the inhibitor
409:(PHGH). Alternative β forms denoted β1i, β2i, and β5i can be expressed in
9295:
9261:
9188:
9183:
9074:
8554:
8544:
6360:
McNaught KS, Jackson T, JnoBaptiste R, Kapustin A, Olanow CW (May 2006).
6117:
6100:
5710:"The proteasome controls ESCRT-III–mediated cell division in an archaeon"
5684:
5667:
5591:
5502:
5485:
5326:"p53 proteasomal degradation: poly-ubiquitination is not the whole story"
5169:
4742:
2238:
2179:
2144:
2125:
1686:
1678:
1658:
1650:
1577:
1534:
1278:
1094:
877:
842:
585:
581:
332:
302:
287:
143:
50:
8164:"The proteasome and the delicate balance between destruction and rescue"
6243:
5636:
5342:
5325:
4695:
4343:
3388:
3056:
2025:
Nassif, Nicholas D.; Cambray, Samantha E.; Kraut, Daniel A. (May 2014).
21:
16:
Protein complexes which degrade ubiquitin-tagged proteins by proteolysis
9230:
9223:
9208:
9203:
9198:
9153:
9099:
9089:
9039:
9034:
9024:
7833:
Mathews KD, Moore SA (January 2003). "Limb-girdle muscular dystrophy".
6962:
6802:
Nawrocki ST, Sweeney-Gotsch B, Takamori R, McConkey DJ (January 2004).
5253:
4435:
4375:"The ubiquitin-proteasome system: central modifier of plant signalling"
3148:
Smith DM, Kafri G, Cheng Y, Ng D, Walz T, Goldberg AL (December 2005).
2915:
Heinemeyer W, Fischer M, Krimmer T, Stachon U, Wolf DH (October 1997).
2882:
2027:"Slipping up: Partial substrate degradation by ATP-dependent proteases"
1845:
1817:
1813:
1809:
1801:
1793:
1682:
1623:
1561:
1485:
1481:
1467:
1394:
1250:
1228:
1051:
1035:
984:
933:
854:
837:
791:
708:
690:
560:
264:
234:
186:
162:
101:
30:
8100:
7216:
7146:
Elliott PJ, Pien CS, McCormack TA, Chapman ID, Adams J (August 1999).
7009:"How an inhibitor of the HIV-I protease modulates proteasome activity"
6801:
4045:
Krüger E, Kloetzel PM, Enenkel C (2001). "20S proteasome biogenesis".
3841:
3548:
3070:
1611:-like activity of the proteasome is inhibited by ritonavir, while the
1054:
cells, "slippage" through the mitotic checkpoint leading to premature
9311:
9273:
9246:
9218:
9111:
9094:
9029:
9002:
8934:
8559:
7114:
6772:
5047:
3478:"Structural basis for dynamic regulation of the human 26S proteasome"
3365:"Complete subunit architecture of the proteasome regulatory particle"
3303:"Near-atomic resolution structural model of the yeast 26S proteasome"
2349:
2043:
2026:
1821:
1728:
and several rare forms of neurodegenerative diseases associated with
1690:
1627:
1596:
1528:
1509:
1421:
expression, proteasomal activity has been linked to inflammatory and
1410:
1325:
1246:
1209:
1181:
1121:
850:
740:
728:
716:
704:
665:
634:
605:(names for yeast/mammals). These assembly chaperones bind to the AAA-
569:
357:
290:
in complex with a polyubiquitylated protein substrate were solved by
241:
202:
128:
112:
85:
6921:
4093:
3268:
711:
long and was named due to its ubiquitous nature, as it has a highly
626:
9256:
9012:
8997:
8992:
8512:
8450:
7186:
4590:
Zhang S, Zou S, Yin D, Zhao L, Finley D, Wu Z, Mao Y (April 2022).
1887:
1733:
1729:
1543:
1497:
1418:
1369:
1201:
1189:
1105:
1101:
1082:
1070:
1043:
976:
885:
849:. The small pink spheres represent the location of the active-site
669:
602:
589:
421:
373:
312:
252:
226:
210:
198:
120:
66:
7425:"Proteotoxicity: an underappreciated pathology in cardiac disease"
6495:
Cascio P, Hilton C, Kisselev AF, Rock KL, Goldberg AL (May 2001).
6359:
3963:
Förster A, Masters EI, Whitby FG, Robinson H, Hill CP (May 2005).
1825:
1805:
1761:
1406:
1003:. This redundancy explains why the HslUV system is not essential.
896:
84:. Proteins are tagged for degradation with a small protein called
9278:
9178:
9131:
9007:
8522:
8517:
8416:
7374:"Ubiquitin-proteasome system involvement in Huntington's disease"
5622:
4240:
Thrower JS, Hoffman L, Rechsteiner M, Pickart CM (January 2000).
1689:
and differentiation, gene transcription, signal transduction and
1612:
1357:
1294:
1185:
1160:
1078:
1055:
1039:
996:
823:
815:
735:
638:
531:
402:
380:
237:
222:
116:
97:
46:
8011:
American Journal of Physiology. Heart and Circulatory Physiology
7917:
American Journal of Physiology. Heart and Circulatory Physiology
7663:
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease
7656:
7047:
6887:
with indolent non-Hodgkin's lymphoma and mantle cell lymphoma".
6885:
5413:
4239:
3580:"Structure of the human 26S proteasome at a resolution of 3.9 Å"
3100:
Zwickl P, Ng D, Woo KM, Klenk HP, Goldberg AL (September 1999).
1512:
residue whose activity is blocked by the presence of bortezomib.
9121:
8840:
8835:
8830:
8825:
8820:
8718:
7006:
6029:
5368:
Shringarpure R, Grune T, Mehlhase J, Davies KJ (January 2003).
5367:
4373:
Sadanandom A, Bailey M, Ewan R, Lee J, Nelis S (October 2012).
3577:
2914:
1841:
1837:
1635:
1631:
1616:
1585:
1501:
1493:
1441:
1217:
1213:
1031:
657:
610:
606:
394:
329:
230:
62:
8129:
6676:
6230:
Bader N, Grune T (2006). "Protein oxidation and proteolysis".
4727:"Defining the geometry of the two-component proteasome degron"
4724:
3705:
1108:, is one of the 19S subcomponents that also tightly binds the
949:
689:
targeting and engagement. Ubiquitin-receptor proteins have an
8887:
8871:
8866:
8861:
8856:
8815:
8810:
8805:
8800:
8795:
8790:
8785:
8780:
8775:
8759:
8754:
8749:
8744:
8739:
8734:
8713:
8708:
8703:
8698:
8693:
8688:
8683:
8678:
8673:
8657:
8652:
8647:
8642:
8637:
8632:
8627:
7241:"Perilous journey: a tour of the ubiquitin-proteasome system"
5850:
5706:
5205:
Biochimica et Biophysica Acta (BBA) - Molecular Cell Research
3362:
3300:
1769:
1765:
1757:
1753:
1724:, and motor neuron diseases, polyglutamine (PolyQ) diseases,
1654:
1520:
1505:
1489:
1462:
1448:
to direct the virion to the proteasome where it is degraded.
1329:
1298:
1286:
1274:
1266:
1262:
1152:
1148:
1136:
1125:
971:
is thought to resemble the ancestor of the modern proteasome.
881:
548:
390:
337:
7607:
5851:
Haas AL, Baboshina O, Williams B, Schwartz LM (April 1995).
5801:
3962:
2958:
1332:
models of Parkinson's are more susceptible to toxicity from
311:
The proteasome subcomponents are often referred to by their
8385:
8207:
The Yeast 26S Proteasome with list of subunits and pictures
7148:"Proteasome inhibition: A novel mechanism to combat asthma"
7145:
6400:
6098:
6060:"The role of the ubiquitin-proteasome pathway in apoptosis"
5983:
5448:
4773:
4725:
Inobe T, Fishbain S, Prakash S, Matouschek A (March 2011).
3764:
3419:
3007:
1440:
of antibody-bound virions. In this neutralisation pathway,
1117:
992:
988:
958:
954:
820:
366:
206:
7959:
6717:
6543:
5891:
5666:
Higashitsuji H, Liu Y, Mayer RJ, Fujita J (October 2005).
5665:
5076:
4288:
3644:
Zhu Y, Wang WL, Yu D, Ouyang Q, Lu Y, Mao Y (April 2018).
1946:
1470:(Boronated form of MG132), a proteasome inhibitor used in
1026:
Cell cycle progression is controlled by ordered action of
732:
other types of chains may be involved in other processes.
9251:
7913:"The ubiquitin proteasome system and myocardial ischemia"
6758:
6602:
6443:
6362:"Proteasomal dysfunction in sporadic Parkinson's disease"
6139:
5483:
4122:
3246:
2861:
1797:
1749:
1604:
1220:
1113:
937:
783:, are sufficient to inhibit degradation. The presence of
349:
7789:
7608:
Ermolaeva MA, Dakhovnik A, Schumacher B (January 2015).
6654:
United States Food and Drug Administration press release
6494:
5484:
Chesnel F, Bazile F, Pascal A, Kubiak JZ (August 2006).
2501:
2098:
1983:
1959:(5th ed.). New York: W.H. Freeman and CO. pp.
229:
modification of the histone protein by a bond between a
8928:
7096:
5934:
4947:
4372:
4163:
1285:
Similar mechanisms exist to promote the degradation of
1112:
CDK4 and plays a key role in recognizing ubiquitinated
668:
molecule. This is then transferred to E1's active-site
5451:
Annual Review of Biophysics and Biomolecular Structure
4822:
4003:
3921:
3823:
2959:
Padmanabhan A, Vuong SA, Hochstrasser M (March 2016).
2862:
Nandi D, Tahiliani P, Kumar A, Chandu D (March 2006).
1444:(a protein of the tripartite motif family) binds with
810:
is necessarily general, but somewhat dependent on the
88:. The tagging reaction is catalyzed by enzymes called
5573:
5117:
4857:
4242:"Recognition of the polyubiquitin proteolytic signal"
4079:
4044:
2954:
2952:
2419:
2335:
2076:
1163:
of plant growth, induces the targeting of a class of
1058:
exit can occur despite the delay of this exit by the
884:
molecule for deprotonation of the reactive threonine
123:. In eukaryotes, proteasomes are located both in the
7746:
6663:
13 May 2003. Access date 29 December 2006. See also
5025:
4499:
4199:
Haas AL, Warms JV, Hershko A, Rose IA (March 1982).
4198:
3197:
2744:
2370:
2157:
1984:
Peters JM, Franke WW, Kleinschmidt JA (March 1994).
1917:
Endoplasmic-reticulum-associated protein degradation
1883:
1405:
Due to its role in generating the activated form of
911:
6313:"The ubiquitin proteasome system in neuropathology"
5574:Havens CG, Ho A, Yoshioka N, Dowdy SF (June 2006).
5274:
4646:
4552:Pickart CM (November 2000). "Ubiquitin in chains".
4166:
Biochemical and Biophysical Research Communications
2160:
Biochemical and Biophysical Research Communications
1093:. The APC and the Skp1/Cul1/F-box protein complex (
906:
regulated ubiquitin/proteasome dependent processing
500:
72:Proteasomes are part of a major mechanism by which
8367:3D proteasome structures in the EM Data Bank(EMDB)
7287:
6188:
4990:
2949:
2536:
2024:
1948:
779:, and in particular nonlocal interactions such as
7700:
5758:
3147:
932:. Ubiquitin-independent mechanisms targeting key
393:contain seven distinct types of each subunit. In
9329:
7568:
7566:
5239:
4681:
3824:Matyskiela ME, Lander GC, Martin A (July 2013).
3099:
2690:
1772:, sterol-regulated element-binding proteins and
1245:In response to cellular stresses – such as
664:(known as E1) hydrolyzes ATP and adenylylates a
477:
217:in 1977 on ATP-dependent protein degradation in
7659:"Role of the proteasome in Alzheimer's disease"
7471:
6537:
5363:
5361:
4589:
3643:
2686:
2684:
2682:
1124:and has been shown to be overexpressed in some
645:
7742:
7740:
7696:
7694:
7152:The Journal of Allergy and Clinical Immunology
5844:
5268:
4464:
4329:
3701:
3699:
3697:
3534:
2192:
1626:found a reduction in the size of lesions from
1572:agent. Bortezomib is used in the treatment of
1293:and actively degrade inappropriately oxidized
1240:
760:
621:
518:"11S" redirects here. Not to be confused with
8914:
8588:
8401:
7563:
7422:
7328:
7288:Goldberg AL, Stein R, Adams J (August 1995).
6879:
6647:
6437:
5275:Zhang M, Pickart CM, Coffino P (April 2003).
2910:
2908:
2691:Stadtmueller, BM; Hill, CP (7 January 2011).
2658:
2602:
2420:Hough R, Pratt G, Rechsteiner M (June 1987).
2371:Tanaka K, Waxman L, Goldberg AL (June 1983).
1588:-related cancers, particularly some types of
1142:
1081:similarly involve proteasomal degradation of
880:. This mechanism may depend on an associated
513:
450:Cartoon representation of the 26S proteasome.
297:
8161:
7910:
7832:
7572:
7429:Journal of Molecular and Cellular Cardiology
7238:
5659:
5358:
4906:
3872:
2815:
2679:
2292:
1347:
1116:, via its affinity for the ubiquitin ligase
1065:Earlier cell cycle checkpoints such as post-
814:. Long sequences of alternating glycine and
601:, Hsm3/S5b, Nas2/p27, Rpn14/PAAF1, and Nas6/
8211:
7737:
7691:
7371:
6488:
5524:
5198:
5155:
3694:
2251:
1564:(Boronated MG132), a molecule developed by
1438:Intracellular antibody-mediated proteolysis
275:for their work in discovering this system.
225:led to the identification of an unexpected
8921:
8907:
8595:
8581:
8408:
8394:
8316:
8086:
7835:Current Neurology and Neuroscience Reports
7000:
6844:
6229:
5752:
5323:
4547:
4545:
3817:
3571:
3475:
2905:
2740:
2738:
2736:
2245:
1085:, whose ubiquitination is promoted by the
803:if the 19S cap is in the ATP-bound state.
437:
348:"20S" redirects here. For the decade, see
8299:
8264:
8229:
8189:
8179:
7985:
7936:
7674:
7633:
7546:
7497:
7448:
7399:
7389:
7305:
7264:
7215:
7205:
7163:
7122:
7065:
7024:
6939:
6850:
6819:
6795:
6735:
6694:
6596:
6579:
6569:
6520:
6377:
6336:
6306:
6304:
6206:
6165:
6116:
6075:
5952:
5868:
5827:
5735:
5725:
5683:
5599:
5550:
5501:
5385:
5341:
5300:
5216:
5094:
4924:
4883:
4799:
4750:
4623:
4585:
4583:
4525:
4390:
4306:
4265:
4216:
4140:
4021:
3980:
3939:
3898:
3849:
3830:Nature Structural & Molecular Biology
3800:
3790:
3741:
3731:
3677:
3613:
3603:
3537:Nature Structural & Molecular Biology
3511:
3501:
3447:
3437:
3396:
3358:
3356:
3336:
3326:
3223:
3165:
3117:
3059:Nature Structural & Molecular Biology
3033:
2984:
2932:
2787:
2770:
2716:
2628:
2478:
2437:
2396:
2269:
2228:
2218:
2151:
2134:
2124:
2099:Etlinger JD, Goldberg AL (January 1977).
2079:"Nobel Prize Awardees in Chemistry, 2004"
2042:
2001:
1599:, marketed as Norvir, was developed as a
1364:class I (MHC) proteins on the surface of
834:on the major histocompatibility complex.
7520:
7180:
7139:
7090:
7041:
6353:
6057:
6051:
6025:
6023:
5885:
5442:
5409:
5407:
5405:
4282:
4157:
3143:
3141:
3139:
3137:
2857:
2855:
2598:
2596:
2186:
1942:
1940:
1938:
1936:
1664:
1480:
1461:
1451:
1340:, and muscle and nerve diseases such as
948:
836:
734:
625:
490:
445:
301:
29:
20:
8246:
7472:Drews O, Taegtmeyer H (December 2014).
6670:
6092:
5795:
5477:
5151:
5149:
4551:
4542:
4413:
4323:
4233:
3956:
3639:
3637:
3635:
3633:
3471:
3469:
3467:
3296:
3294:
2733:
2654:
2652:
2650:
2648:
2594:
2592:
2590:
2588:
2586:
2584:
2582:
2580:
2578:
2576:
2460:
2454:
2072:
2070:
1474:that is particularly effective against
9330:
8008:
6711:
6394:
6310:
6301:
6272:
6266:
5977:
5616:
5567:
5317:
4816:
4675:
4580:
4116:
4073:
4038:
3413:
3353:
3193:
3191:
3189:
3187:
3185:
8902:
8576:
8389:
8362:Proteasome subunit nomenclature guide
8051:
7911:Calise J, Powell SR (February 2013).
7875:
7103:The Journal of Clinical Investigation
6752:
6223:
6020:
5518:
5402:
5233:
5192:
5111:
4900:
4407:
4082:Nature Reviews Molecular Cell Biology
3758:
3528:
3134:
2852:
2809:
1977:
1933:
1657:initially developed by Goldberg lab.
1021:
413:cells in response to exposure to pro-
407:peptidyl-glutamyl peptide-hydrolyzing
142:that contain three to seven protease
8281:
8162:Glickman MH, Adir N (January 2004).
6966:Molecular and Cellular Endocrinology
6133:
5146:
4851:
4192:
3997:
3630:
3464:
3291:
2645:
2573:
2530:
2495:
2193:Goldknopf IL, Busch H (March 1977).
2067:
1390:" whose function is as yet unclear.
483:the AAA-domains. In the presence of
343:
65:that help such reactions are called
7378:Frontiers in Molecular Neuroscience
7013:The Journal of Biological Chemistry
6195:The Journal of Biological Chemistry
6182:
5928:
5857:The Journal of Biological Chemistry
5374:The Journal of Biological Chemistry
5199:Rape M, Jentsch S (November 2004).
5083:The Journal of Biological Chemistry
5005:10.1146/annurev.bi.65.070196.004101
4913:The Journal of Biological Chemistry
4640:
4467:Current Opinion in Chemical Biology
4366:
4205:The Journal of Biological Chemistry
3182:
3106:The Journal of Biological Chemistry
2921:The Journal of Biological Chemistry
2661:Cellular & Molecular Immunology
2426:The Journal of Biological Chemistry
2286:
1990:The Journal of Biological Chemistry
1537:, a natural product synthesized by
1436:The proteasome is also involved in
1277:, on the other hand, binds exposed
868:threonine protease § mechanism
747:
456:single particle electron microscopy
315:sedimentation coefficient (denoted
80:of particular proteins and degrade
13:
8154:
7521:Wang ZV, Hill JA (February 2015).
7478:Antioxidants & Redox Signaling
6379:10.1212/01.wnl.0000221745.58886.2e
3866:
2830:10.1111/j.1471-4159.1983.tb08056.x
2307:10.1111/j.1471-4159.1980.tb07873.x
1786:von Hippel–Lindau tumor suppressor
233:side chain of the histone and the
14:
9364:
8355:
7978:10.1161/CIRCULATIONAHA.109.904557
7423:Sandri M, Robbins J (June 2014).
6403:Journal of Molecular Neuroscience
5525:Brito DA, Rieder CL (June 2006).
5132:10.1146/annurev.biochem.68.1.1015
4907:Zhang M, Coffino P (March 2004).
2864:"The ubiquitin-proteasome system"
912:Ubiquitin-independent degradation
111:Proteasomes are found inside all
34:Top view of the proteasome above.
8604:Proteasome endopeptidase complex
8212:Ciechanover A (September 2005).
8123:
8080:
8045:
8002:
7953:
7904:
7869:
7826:
7783:
7650:
7601:
7514:
7465:
7416:
7365:
7322:
7281:
7239:Kleiger G, Mayor T (June 2014).
7232:
6956:
6915:
6761:Journal of Cellular Biochemistry
5463:10.1146/annurev.biophys.28.1.295
5324:Asher G, Shaul Y (August 2005).
4392:10.1111/j.1469-8137.2012.04266.x
4308:10.1046/j.1365-313X.2003.01768.x
2252:Ciechanover A (September 2005).
1886:
1488:bound to the core particle in a
1362:major histocompatibility complex
785:intrinsically disordered protein
542:major histocompatibility complex
501:Regulation of the 20S by the 19S
8436:Post-translational modification
5700:
5070:
5019:
4984:
4941:
4767:
4718:
4493:
4458:
3915:
3240:
3093:
3050:
3001:
2413:
2329:
872:The proteasome functions as an
806:The mechanism for unfolding of
652:Ubiquitin § Ubiquitination
584:interactions between conserved
45:which degrade ubiquitin-tagged
8288:Cell Death and Differentiation
8253:Cell Death and Differentiation
8218:Cell Death and Differentiation
7207:10.1016/j.chembiol.2006.09.013
6064:Cell Death and Differentiation
5580:Molecular and Cellular Biology
4554:Trends in Biochemical Sciences
4416:Journal of Molecular Evolution
2467:Cell Death and Differentiation
2258:Cell Death and Differentiation
2092:
2077:Nobel Prize Committee (2004).
2018:
1788:(VHL), as well as a number of
1030:(CDKs), activated by specific
861:
1:
9085:Microtubule organizing center
8323:Current Pharmaceutical Design
8066:10.1016/s1359-6446(03)02647-3
7715:10.1016/s0166-2236(00)01998-6
7676:10.1016/s0925-4439(00)00039-9
7165:10.1016/S0091-6749(99)70369-6
7054:Molecular Cancer Therapeutics
6808:Molecular Cancer Therapeutics
6287:10.1016/S0300-9084(01)01250-0
6146:Molecular Biology of the Cell
5954:10.1016/S0014-5793(96)01413-5
5428:10.1016/S0022-2836(02)01470-5
5120:Annual Review of Biochemistry
4993:Annual Review of Biochemistry
4825:Journal of Structural Biology
4566:10.1016/S0968-0004(00)01681-9
4218:10.1016/S0021-9258(18)34958-5
4059:10.1016/S0300-9084(01)01241-X
3941:10.1016/S1097-2765(01)00274-X
2439:10.1016/S0021-9258(18)47564-3
2003:10.1016/S0021-9258(17)37345-3
1927:
1714:amyotrophic lateral sclerosis
1192:characteristic of apoptosis.
1180:can lead to the induction of
1159:that order the direction and
1139:-III-mediated cell division.
1034:that demarcate phases of the
565:post-translationally modified
478:Conformational changes of 19S
292:cryogenic electron microscopy
8415:
8247:Hershko A (September 2005).
8181:10.1371/journal.pbio.0020013
7890:10.1358/dnp.2003.16.2.829327
7878:Drug News & Perspectives
7804:10.1016/0304-3940(92)90854-z
7307:10.1016/1074-5521(95)90182-5
6941:10.1182/blood-2012-04-418640
6889:Journal of Clinical Oncology
6737:10.1182/blood-2006-04-016360
6683:Journal of Clinical Oncology
6665:FDA Velcade information page
6311:Lehman NL (September 2009).
5998:10.1016/0014-5793(96)00920-9
5906:10.1016/0896-6273(90)90080-Y
5416:Journal of Molecular Biology
5218:10.1016/j.bbamcr.2004.09.022
4792:10.1016/j.celrep.2014.07.055
4142:10.1016/j.molcel.2011.04.021
3982:10.1016/j.molcel.2005.04.016
3891:10.1016/j.molcel.2017.06.007
3216:10.1016/j.molcel.2006.08.025
3167:10.1016/j.molcel.2005.10.019
2977:10.1016/j.celrep.2016.02.068
2763:10.1016/j.molcel.2007.06.033
2709:10.1016/j.molcel.2010.12.020
2516:10.1016/0167-4838(86)90278-5
2461:Hershko A (September 2005).
2172:10.1016/0006-291X(78)91249-4
1780:(APC) in colorectal cancer,
1427:systemic lupus erythematosus
1308:. In some of the late-onset
1171:
944:
723:, possibly due to the heavy
674:ubiquitin-conjugating enzyme
646:Ubiquitination and targeting
192:
7:
8133:The Journal of Rheumatology
8023:10.1152/ajpheart.00062.2006
7929:10.1152/ajpheart.00604.2012
7441:10.1016/j.yjmcc.2013.12.015
7372:Ortega Z, Lucas JJ (2014).
4661:10.1016/j.yexcr.2005.03.031
2377:The Journal of Cell Biology
1879:
1241:Response to cellular stress
1176:Both internal and external
761:Unfolding and translocation
662:ubiquitin-activating enzyme
622:Protein degradation process
554:
156:ubiquitin–proteasome system
10:
9369:
8477:Protein structural domains
8335:10.2174/138161207782110390
7539:10.1016/j.cmet.2015.01.016
6058:Orlowski RZ (April 1999).
4649:Experimental Cell Research
4616:10.1038/s41586-022-04671-8
4518:10.1016/j.cell.2009.01.041
4479:10.1016/j.cbpa.2004.09.009
4178:10.1016/j.bbrc.2010.05.061
3670:10.1038/s41467-018-03785-w
1778:adenomatous polyposis coli
1566:Millennium Pharmaceuticals
1548:Millennium Pharmaceuticals
1455:
1143:Regulation of plant growth
1087:anaphase promoting complex
926:intrinsically unstructured
865:
857:bound to the active sites.
649:
517:
514:Other regulatory particles
347:
298:Structure and organization
9304:
9239:
9164:
9055:
8941:
8880:
8849:
8768:
8727:
8666:
8615:
8490:
8464:
8423:
8317:Cvek B, Dvorak Z (2007).
8282:Rose I (September 2005).
7847:10.1007/s11910-003-0042-9
7761:10.1007/s00401-001-0513-5
7626:10.1016/j.arr.2014.12.009
7343:10.1007/s12035-014-9063-4
7257:10.1016/j.tcb.2013.12.003
7067:10.1158/1535-7163.129.3.2
6978:10.1016/j.mce.2012.01.003
6329:10.1007/s00401-009-0560-x
5543:10.1016/j.cub.2006.04.043
4837:10.1016/j.jsb.2006.04.012
4684:Nature Structural Biology
4023:10.1016/j.str.2006.05.019
2818:Journal of Neurochemistry
2621:10.1038/s41586-018-0736-4
2295:Journal of Neurochemistry
1722:Creutzfeldt–Jakob disease
1653:and the peptide aldehyde
1348:Role in the immune system
1338:autism spectrum disorders
953:The assembled complex of
826:; in particular, certain
9080:Prokaryotic cytoskeleton
8378:17 November 2020 at the
7391:10.3389/fnmol.2014.00077
7026:10.1074/jbc.274.50.35734
6821:10.1158/1535-7163.59.3.1
6696:10.1200/JCO.2006.07.9665
6659:19 February 2007 at the
6513:10.1093/emboj/20.10.2357
5820:10.1038/sj.emboj.7600659
5096:10.1074/jbc.273.40.25637
4876:10.1038/sj.emboj.7601058
3119:10.1074/jbc.274.37.26008
3026:10.1038/sj.emboj.7600059
2934:10.1074/jbc.272.40.25200
1907:DSS1/SEM1 protein family
1366:antigen-presenting cells
1130:hepatocellular carcinoma
1104:, a recently identified
1048:mitosis promoting factor
1028:cyclin-dependent kinases
386:Thermoplasma acidophilum
273:Nobel Prize in Chemistry
175:Nobel Prize in Chemistry
100:of about seven to eight
7703:Trends in Neurosciences
7614:Ageing Research Reviews
7294:Chemistry & Biology
7194:Chemistry & Biology
6901:10.1200/JCO.2005.02.050
6625:10.1126/science.7732382
6571:10.1073/pnas.1014074107
6466:10.1126/science.1141915
6158:10.1091/mbc.E06-04-0338
5870:10.1074/jbc.270.16.9407
5773:10.1023/A:1015203013208
5761:Plant Molecular Biology
5727:10.1126/science.aaz2532
4970:10.1126/science.7725107
4731:Nature Chemical Biology
3792:10.1073/pnas.1305782110
3733:10.1073/pnas.1403409111
3605:10.1073/pnas.1614614113
3503:10.1073/pnas.1614614113
3439:10.1073/pnas.1120559109
3328:10.1073/pnas.1213333109
2693:"Proteasome activators"
2559:10.1126/science.7725097
1738:ventricular hypertrophy
1342:inclusion body myopathy
1110:cyclin-dependent kinase
930:ornithine decarboxylase
660:. In the first step, a
637:, the highly conserved
563:"propeptides" that are
472:deubiquitinating enzyme
438:19S regulatory particle
328:that contains multiple
269:Fox Chase Cancer Center
8850:E (activator subunits)
8301:10.1038/sj.cdd.4401700
8266:10.1038/sj.cdd.4401709
8231:10.1038/sj.cdd.4401691
7587:10.1006/smim.2000.0210
7575:Seminars in Immunology
7331:Molecular Neurobiology
7245:Trends in Cell Biology
6865:10.3816/CLM.2002.n.011
6372:(10 Suppl 4): S37–49.
6208:10.1074/jbc.M507986200
6077:10.1038/sj.cdd.4400505
5387:10.1074/jbc.M206279200
4926:10.1074/jbc.M310449200
2871:Journal of Biosciences
2480:10.1038/sj.cdd.4401709
2271:10.1038/sj.cdd.4401691
1956:Molecular cell biology
1590:non-Hodgkin's lymphoma
1552:Takeda Pharmaceuticals
1513:
1478:
1466:Chemical structure of
1397:, as peptides bind by
1354:adaptive immune system
972:
858:
744:
642:
537:conformational changes
497:
451:
308:
35:
27:
9070:Intermediate filament
8963:Endoplasmic reticulum
8881:F (inhibitor subunit)
8540:Photoreceptor protein
7749:Acta Neuropathologica
7490:10.1089/ars.2013.5823
6317:Acta Neuropathologica
4258:10.1093/emboj/19.1.94
3650:Nature Communications
2389:10.1083/jcb.96.6.1580
2220:10.1073/pnas.74.3.864
1746:transcription factors
1665:Clinical significance
1517:Proteasome inhibitors
1484:
1465:
1452:Proteasome inhibitors
1360:are displayed by the
1012:lateral gene transfer
952:
893:transcription factors
840:
801:facilitated diffusion
738:
707:protein itself is 76
650:Further information:
629:
494:
449:
360:. The α subunits are
305:
284:X-ray crystallography
255:in the laboratory of
33:
24:
9317:Extracellular matrix
8431:Protein biosynthesis
8054:Drug Discovery Today
7792:Neuroscience Letters
6415:10.1385/JMN:28:2:161
6232:Biological Chemistry
6118:10.4161/cc.5.22.3448
5685:10.4161/cc.4.10.2107
5592:10.1128/MCB.00303-06
5503:10.4161/cc.5.15.3123
5293:10.1093/emboj/cdg158
5170:10.1038/ncb0502-e113
4743:10.1038/nchembio.521
2126:10.1073/pnas.74.1.54
1874:rheumatoid arthritis
1860:inhibitors. Lastly,
1726:muscular dystrophies
1718:Huntington's disease
1519:have effective anti-
1458:Proteasome inhibitor
1431:rheumatoid arthritis
1165:transcription factor
832:antigen presentation
588:whose disruption by
165:, the regulation of
152:regulatory particles
9020:Cytoplasmic granule
7709:(11 Suppl): S7–14.
7158:(2 Pt 1): 294–300.
6617:1995Sci...268..726F
6562:2010PNAS..10719985M
6556:(46): 19985–19990.
6458:2007Sci...316.1349M
6244:10.1515/BC.2006.169
5637:10.1038/nature02330
5343:10.4161/cc.4.8.1900
5158:Nature Cell Biology
5040:1997Natur.386..463G
4962:1995Sci...268..579S
4696:10.1038/nsb0395-199
4608:2022Natur.605..567Z
4428:1987JMolE..25...58S
4379:The New Phytologist
4344:10.1038/ncb0805-742
4332:Nature Cell Biology
3783:2013PNAS..110.7264S
3724:2014PNAS..111.5544U
3662:2018NatCo...9.1360Z
3596:2016PNAS..11312991C
3494:2016PNAS..11312991C
3488:(46): 12991–12996.
3389:10.1038/nature10774
3381:2012Natur.482..186L
3319:2012PNAS..10914870B
3261:2002Natur.416..763L
2551:1995Sci...268..533L
2211:1977PNAS...74..864G
2117:1977PNAS...74...54E
1902:The Proteolysis Map
1706:Parkinson's disease
1702:Alzheimer's disease
1603:and used to target
1423:autoimmune diseases
1318:Alzheimer's disease
1314:Parkinson's disease
1287:oxidatively damaged
1259:heat shock proteins
1128:cell types such as
1120:. Gankyrin is anti-
969:heat shock proteins
936:regulators such as
878:nucleophilic attack
812:amino acid sequence
698:three-helix bundles
280:electron microscopy
169:, and responses to
94:polyubiquitin chain
9045:Weibel–Palade body
8929:Structures of the
8616:A (alpha subunits)
5720:(6504): eaaz2532.
5254:10.1002/bies.20447
4436:10.1007/BF02100041
2883:10.1007/BF02705243
2795:"MEROPS Family T1"
1862:autoimmune disease
1834:adhesion molecules
1774:androgen receptors
1601:protease inhibitor
1556:threonine protease
1514:
1479:
1060:spindle checkpoint
1022:Cell cycle control
973:
967:. This complex of
859:
828:Epstein–Barr virus
777:tertiary structure
772:rate-limiting step
745:
643:
498:
452:
309:
215:Alfred L. Goldberg
82:misfolded proteins
36:
28:
9343:Protein complexes
9325:
9324:
9105:Spindle pole body
8896:
8895:
8667:B (beta subunits)
8570:
8569:
8472:Protein structure
8446:Protein targeting
8101:10.1038/ni0102-20
8089:Nature Immunology
6853:Clinical Lymphoma
6452:(5829): 1349–53.
6238:(10–11): 1351–5.
4602:(7910): 567–574.
4295:The Plant Journal
3885:(2): 322–333.e6.
3842:10.1038/nsmb.2616
3777:(18): 7264–7269.
3590:(28): 7816–7821.
3549:10.1038/nsmb.3273
3071:10.1038/nsmb.1389
1970:978-0-7167-4366-8
1768:, HIF-1α, MATα2,
1582:pancreatic cancer
1310:neurodegenerative
1282:binding partner.
1234:salinosporamide A
1067:restriction point
1008:sequence identity
847:polypeptide chain
808:globular proteins
678:ubiquitin ligases
424:, in particular,
344:20S core particle
261:Aaron Ciechanover
201:, membrane-bound
179:Aaron Ciechanover
90:ubiquitin ligases
55:chemical reaction
43:protein complexes
9360:
8923:
8916:
8909:
8900:
8899:
8597:
8590:
8583:
8574:
8573:
8550:Phycobiliprotein
8508:Globular protein
8503:Membrane protein
8498:List of proteins
8410:
8403:
8396:
8387:
8386:
8350:
8349:on 29 July 2012.
8345:. Archived from
8313:
8303:
8278:
8268:
8243:
8233:
8203:
8193:
8183:
8149:
8148:
8127:
8121:
8120:
8084:
8078:
8077:
8049:
8043:
8042:
8006:
8000:
7999:
7989:
7957:
7951:
7950:
7940:
7908:
7902:
7901:
7873:
7867:
7866:
7830:
7824:
7823:
7787:
7781:
7780:
7744:
7735:
7734:
7698:
7689:
7688:
7678:
7654:
7648:
7647:
7637:
7605:
7599:
7598:
7570:
7561:
7560:
7550:
7518:
7512:
7511:
7501:
7469:
7463:
7462:
7452:
7420:
7414:
7413:
7403:
7393:
7369:
7363:
7362:
7326:
7320:
7319:
7309:
7285:
7279:
7278:
7268:
7236:
7230:
7229:
7219:
7209:
7184:
7178:
7177:
7167:
7143:
7137:
7136:
7126:
7115:10.1172/JCI12736
7094:
7088:
7087:
7069:
7045:
7039:
7038:
7028:
7019:(50): 35734–40.
7004:
6998:
6997:
6960:
6954:
6953:
6943:
6919:
6913:
6912:
6883:
6877:
6876:
6848:
6842:
6841:
6823:
6799:
6793:
6792:
6773:10.1002/jcb.1150
6756:
6750:
6749:
6739:
6715:
6709:
6708:
6698:
6674:
6668:
6651:
6645:
6644:
6611:(5211): 726–31.
6600:
6594:
6593:
6583:
6573:
6541:
6535:
6534:
6524:
6501:The EMBO Journal
6492:
6486:
6485:
6441:
6435:
6434:
6398:
6392:
6391:
6381:
6357:
6351:
6350:
6340:
6308:
6299:
6298:
6270:
6264:
6263:
6227:
6221:
6220:
6210:
6201:(46): 38673–81.
6186:
6180:
6179:
6169:
6137:
6131:
6130:
6120:
6111:(22): 2592–601.
6096:
6090:
6089:
6079:
6055:
6049:
6048:
6027:
6018:
6017:
5981:
5975:
5974:
5956:
5932:
5926:
5925:
5889:
5883:
5882:
5872:
5848:
5842:
5841:
5831:
5808:The EMBO Journal
5799:
5793:
5792:
5756:
5750:
5749:
5739:
5729:
5704:
5698:
5697:
5687:
5663:
5657:
5656:
5620:
5614:
5613:
5603:
5571:
5565:
5564:
5554:
5537:(12): 1194–200.
5522:
5516:
5515:
5505:
5481:
5475:
5474:
5446:
5440:
5439:
5411:
5400:
5399:
5389:
5365:
5356:
5355:
5345:
5321:
5315:
5314:
5304:
5281:The EMBO Journal
5272:
5266:
5265:
5237:
5231:
5230:
5220:
5196:
5190:
5189:
5153:
5144:
5143:
5115:
5109:
5108:
5098:
5089:(40): 25637–46.
5074:
5068:
5067:
5048:10.1038/386463a0
5034:(6624): 463–71.
5023:
5017:
5016:
4988:
4982:
4981:
4956:(5210): 579–82.
4945:
4939:
4938:
4928:
4904:
4898:
4897:
4887:
4864:The EMBO Journal
4855:
4849:
4848:
4820:
4814:
4813:
4803:
4771:
4765:
4764:
4754:
4722:
4716:
4715:
4679:
4673:
4672:
4644:
4638:
4637:
4627:
4587:
4578:
4577:
4549:
4540:
4539:
4529:
4497:
4491:
4490:
4462:
4456:
4455:
4411:
4405:
4404:
4394:
4370:
4364:
4363:
4327:
4321:
4320:
4310:
4286:
4280:
4279:
4269:
4246:The EMBO Journal
4237:
4231:
4230:
4220:
4196:
4190:
4189:
4172:(4): 1048–1053.
4161:
4155:
4154:
4144:
4120:
4114:
4113:
4077:
4071:
4070:
4042:
4036:
4035:
4025:
4001:
3995:
3994:
3984:
3960:
3954:
3953:
3943:
3919:
3913:
3912:
3902:
3870:
3864:
3863:
3853:
3821:
3815:
3814:
3804:
3794:
3762:
3756:
3755:
3745:
3735:
3703:
3692:
3691:
3681:
3641:
3628:
3627:
3617:
3607:
3575:
3569:
3568:
3532:
3526:
3525:
3515:
3505:
3473:
3462:
3461:
3451:
3441:
3417:
3411:
3410:
3400:
3375:(7384): 186–91.
3360:
3351:
3350:
3340:
3330:
3298:
3289:
3288:
3244:
3238:
3237:
3227:
3195:
3180:
3179:
3169:
3145:
3132:
3131:
3121:
3112:(37): 26008–14.
3097:
3091:
3090:
3054:
3048:
3047:
3037:
3014:The EMBO Journal
3005:
2999:
2998:
2988:
2956:
2947:
2946:
2936:
2912:
2903:
2902:
2868:
2859:
2850:
2849:
2813:
2807:
2806:
2804:
2802:
2791:
2785:
2784:
2774:
2742:
2731:
2730:
2720:
2688:
2677:
2676:
2656:
2643:
2642:
2632:
2600:
2571:
2570:
2534:
2528:
2527:
2499:
2493:
2492:
2482:
2458:
2452:
2451:
2441:
2417:
2411:
2410:
2400:
2369:
2350:10.1038/331192a0
2344:(6152): 192–94.
2333:
2327:
2326:
2290:
2284:
2283:
2273:
2249:
2243:
2242:
2232:
2222:
2190:
2184:
2183:
2155:
2149:
2148:
2138:
2128:
2096:
2090:
2089:
2087:
2085:
2074:
2065:
2064:
2046:
2044:10.1002/iub.1271
2022:
2016:
2015:
2005:
1981:
1975:
1974:
1952:
1944:
1896:
1891:
1890:
1870:Sjögren syndrome
1574:multiple myeloma
1476:multiple myeloma
1446:immunoglobulin G
1399:hydrogen bonding
1383:immunoproteasome
1378:interferon gamma
1255:oxidative damage
1236:
1198:primary cultures
1091:ubiquitin ligase
1016:gene duplication
748:Deubiquitylation
719:are arranged in
431:immunoproteasome
426:interferon gamma
171:oxidative stress
9368:
9367:
9363:
9362:
9361:
9359:
9358:
9357:
9328:
9327:
9326:
9321:
9300:
9235:
9160:
9051:
8968:Golgi apparatus
8944:
8937:
8927:
8897:
8892:
8876:
8845:
8769:D (non-ATPases)
8764:
8723:
8662:
8611:
8601:
8571:
8566:
8530:Fibrous protein
8486:
8460:
8456:Protein methods
8441:Protein folding
8419:
8414:
8380:Wayback Machine
8358:
8353:
8329:(30): 3155–67.
8157:
8155:Further reading
8152:
8139:(10): 2045–52.
8128:
8124:
8085:
8081:
8050:
8046:
8007:
8003:
7972:(8): 997–1004.
7958:
7954:
7909:
7905:
7874:
7870:
7831:
7827:
7788:
7784:
7745:
7738:
7699:
7692:
7655:
7651:
7606:
7602:
7571:
7564:
7527:Cell Metabolism
7519:
7515:
7484:(17): 2322–43.
7470:
7466:
7421:
7417:
7370:
7366:
7327:
7323:
7286:
7282:
7237:
7233:
7200:(11): 1217–26.
7185:
7181:
7144:
7140:
7095:
7091:
7046:
7042:
7005:
7001:
6961:
6957:
6920:
6916:
6884:
6880:
6849:
6845:
6800:
6796:
6757:
6753:
6716:
6712:
6689:(30): 4867–74.
6675:
6671:
6661:Wayback Machine
6652:
6648:
6601:
6597:
6542:
6538:
6507:(10): 2357–66.
6493:
6489:
6442:
6438:
6399:
6395:
6358:
6354:
6309:
6302:
6281:(3–4): 301–10.
6271:
6267:
6228:
6224:
6187:
6183:
6138:
6134:
6097:
6093:
6056:
6052:
6039:(11): 2615–22.
6033:Cancer Research
6028:
6021:
5982:
5978:
5933:
5929:
5890:
5886:
5863:(16): 9407–12.
5849:
5845:
5814:(10): 1874–85.
5800:
5796:
5757:
5753:
5705:
5701:
5664:
5660:
5631:(6979): 190–3.
5621:
5617:
5586:(12): 4701–11.
5572:
5568:
5531:Current Biology
5523:
5519:
5496:(15): 1687–98.
5482:
5478:
5447:
5443:
5412:
5403:
5366:
5359:
5322:
5318:
5273:
5269:
5238:
5234:
5211:(1–3): 209–13.
5197:
5193:
5154:
5147:
5116:
5112:
5075:
5071:
5024:
5020:
4989:
4985:
4946:
4942:
4919:(10): 8635–41.
4905:
4901:
4856:
4852:
4821:
4817:
4772:
4768:
4723:
4719:
4680:
4676:
4645:
4641:
4588:
4581:
4550:
4543:
4498:
4494:
4463:
4459:
4412:
4408:
4371:
4367:
4328:
4324:
4287:
4283:
4238:
4234:
4197:
4193:
4162:
4158:
4121:
4117:
4094:10.1038/nrm2630
4078:
4074:
4053:(3–4): 289–93.
4043:
4039:
4002:
3998:
3961:
3957:
3920:
3916:
3871:
3867:
3822:
3818:
3763:
3759:
3704:
3695:
3642:
3631:
3576:
3572:
3533:
3529:
3474:
3465:
3418:
3414:
3361:
3354:
3313:(37): 14870–5.
3299:
3292:
3269:10.1038/416763a
3255:(6882): 763–7.
3245:
3241:
3196:
3183:
3146:
3135:
3098:
3094:
3055:
3051:
3006:
3002:
2971:(12): 2962–74.
2957:
2950:
2927:(40): 25200–9.
2913:
2906:
2866:
2860:
2853:
2814:
2810:
2800:
2798:
2793:
2792:
2788:
2743:
2734:
2689:
2680:
2657:
2646:
2615:(7737): 49–55.
2601:
2574:
2545:(5210): 533–9.
2535:
2531:
2500:
2496:
2459:
2455:
2432:(17): 8303–13.
2418:
2414:
2334:
2330:
2291:
2287:
2250:
2246:
2191:
2187:
2156:
2152:
2097:
2093:
2083:
2081:
2075:
2068:
2023:
2019:
1996:(10): 7709–18.
1982:
1978:
1971:
1945:
1934:
1930:
1912:Exosome complex
1892:
1885:
1882:
1790:proto-oncogenes
1677:and subsequent
1667:
1460:
1454:
1388:thymoproteasome
1350:
1243:
1232:
1227:agents such as
1174:
1151:, signaling by
1145:
1074:
1024:
981:Actinomycetales
947:
914:
870:
864:
781:disulfide bonds
763:
756:
750:
654:
648:
624:
557:
527:
516:
503:
480:
440:
353:
346:
300:
195:
167:gene expression
17:
12:
11:
5:
9366:
9356:
9355:
9350:
9345:
9340:
9323:
9322:
9320:
9319:
9314:
9308:
9306:
9302:
9301:
9299:
9298:
9293:
9288:
9287:
9286:
9281:
9271:
9270:
9269:
9264:
9259:
9249:
9243:
9241:
9240:Other internal
9237:
9236:
9234:
9233:
9228:
9227:
9226:
9221:
9216:
9211:
9206:
9201:
9196:
9191:
9186:
9176:
9170:
9168:
9162:
9161:
9159:
9158:
9157:
9156:
9151:
9141:
9140:
9139:
9134:
9129:
9124:
9114:
9109:
9108:
9107:
9102:
9097:
9092:
9082:
9077:
9072:
9067:
9061:
9059:
9053:
9052:
9050:
9049:
9048:
9047:
9042:
9037:
9032:
9027:
9017:
9016:
9015:
9010:
9005:
9000:
8995:
8990:
8980:
8975:
8970:
8965:
8960:
8955:
8949:
8947:
8939:
8938:
8926:
8925:
8918:
8911:
8903:
8894:
8893:
8891:
8890:
8884:
8882:
8878:
8877:
8875:
8874:
8869:
8864:
8859:
8853:
8851:
8847:
8846:
8844:
8843:
8838:
8833:
8828:
8823:
8818:
8813:
8808:
8803:
8798:
8793:
8788:
8783:
8778:
8772:
8770:
8766:
8765:
8763:
8762:
8757:
8752:
8747:
8742:
8737:
8731:
8729:
8725:
8724:
8722:
8721:
8716:
8711:
8706:
8701:
8696:
8691:
8686:
8681:
8676:
8670:
8668:
8664:
8663:
8661:
8660:
8655:
8650:
8645:
8640:
8635:
8630:
8625:
8619:
8617:
8613:
8612:
8600:
8599:
8592:
8585:
8577:
8568:
8567:
8565:
8564:
8563:
8562:
8557:
8552:
8542:
8537:
8532:
8527:
8526:
8525:
8520:
8515:
8505:
8500:
8494:
8492:
8488:
8487:
8485:
8484:
8479:
8474:
8468:
8466:
8462:
8461:
8459:
8458:
8453:
8448:
8443:
8438:
8433:
8427:
8425:
8421:
8420:
8413:
8412:
8405:
8398:
8390:
8384:
8383:
8371:Key points of
8369:
8364:
8357:
8356:External links
8354:
8352:
8351:
8314:
8279:
8259:(9): 1158–61.
8244:
8224:(9): 1167–77.
8209:
8204:
8158:
8156:
8153:
8151:
8150:
8122:
8079:
8044:
8001:
7952:
7923:(3): H337–49.
7903:
7868:
7825:
7782:
7736:
7690:
7649:
7620:(Pt A): 3–11.
7600:
7562:
7513:
7464:
7415:
7364:
7321:
7280:
7231:
7179:
7138:
7089:
7040:
6999:
6955:
6914:
6878:
6843:
6794:
6751:
6710:
6669:
6646:
6595:
6536:
6487:
6436:
6393:
6352:
6300:
6265:
6222:
6181:
6132:
6091:
6050:
6019:
5976:
5927:
5884:
5843:
5794:
5767:(3–4): 401–9.
5751:
5699:
5678:(10): 1335–7.
5658:
5615:
5566:
5517:
5476:
5457:(1): 295–317.
5441:
5422:(5): 1437–48.
5401:
5357:
5316:
5287:(7): 1488–96.
5267:
5232:
5191:
5145:
5126:(1): 1015–68.
5110:
5069:
5018:
4983:
4940:
4899:
4850:
4815:
4786:(6): 1832–44.
4766:
4717:
4690:(3): 199–204.
4674:
4639:
4579:
4541:
4492:
4457:
4406:
4365:
4322:
4281:
4232:
4191:
4156:
4135:(5): 637–649.
4129:Molecular Cell
4115:
4088:(2): 104–115.
4072:
4037:
4016:(7): 1179–88.
3996:
3969:Molecular Cell
3955:
3934:(6): 1143–52.
3928:Molecular Cell
3914:
3879:Molecular Cell
3865:
3836:(7): 781–788.
3816:
3757:
3718:(15): 5544–9.
3693:
3629:
3570:
3543:(9): 778–785.
3527:
3463:
3412:
3352:
3290:
3239:
3204:Molecular Cell
3181:
3154:Molecular Cell
3133:
3092:
3049:
3000:
2948:
2904:
2851:
2808:
2786:
2751:Molecular Cell
2732:
2697:Molecular Cell
2678:
2644:
2572:
2529:
2494:
2473:(9): 1158–61.
2453:
2412:
2328:
2301:(5): 1172–82.
2285:
2264:(9): 1167–77.
2244:
2185:
2150:
2091:
2066:
2037:(5): 309–317.
2017:
1976:
1969:
1931:
1929:
1926:
1925:
1924:
1919:
1914:
1909:
1904:
1898:
1897:
1894:Biology portal
1881:
1878:
1864:patients with
1850:prostaglandins
1782:retinoblastoma
1710:Pick's disease
1675:ubiquitination
1666:
1663:
1550:, now part of
1456:Main article:
1453:
1450:
1376:is induced by
1349:
1346:
1242:
1239:
1208:cells such as
1206:differentiated
1173:
1170:
1144:
1141:
1072:
1069:check between
1023:
1020:
946:
943:
913:
910:
863:
860:
762:
759:
754:
749:
746:
741:ubiquitination
721:tandem repeats
694:ubiquitin-like
647:
644:
631:Ribbon diagram
623:
620:
556:
553:
515:
512:
502:
499:
479:
476:
439:
436:
345:
342:
325:molecular mass
299:
296:
194:
191:
119:, and in some
108:new proteins.
15:
9:
6:
4:
3:
2:
9365:
9354:
9351:
9349:
9346:
9344:
9341:
9339:
9336:
9335:
9333:
9318:
9315:
9313:
9310:
9309:
9307:
9303:
9297:
9294:
9292:
9289:
9285:
9282:
9280:
9277:
9276:
9275:
9272:
9268:
9265:
9263:
9260:
9258:
9255:
9254:
9253:
9250:
9248:
9245:
9244:
9242:
9238:
9232:
9229:
9225:
9222:
9220:
9217:
9215:
9214:Proteinoplast
9212:
9210:
9207:
9205:
9202:
9200:
9197:
9195:
9192:
9190:
9187:
9185:
9182:
9181:
9180:
9177:
9175:
9174:Mitochondrion
9172:
9171:
9169:
9167:
9166:Endosymbionts
9163:
9155:
9152:
9150:
9149:Lamellipodium
9147:
9146:
9145:
9142:
9138:
9135:
9133:
9130:
9128:
9125:
9123:
9120:
9119:
9118:
9115:
9113:
9110:
9106:
9103:
9101:
9098:
9096:
9093:
9091:
9088:
9087:
9086:
9083:
9081:
9078:
9076:
9073:
9071:
9068:
9066:
9065:Microfilament
9063:
9062:
9060:
9058:
9054:
9046:
9043:
9041:
9038:
9036:
9033:
9031:
9028:
9026:
9023:
9022:
9021:
9018:
9014:
9011:
9009:
9006:
9004:
9001:
8999:
8996:
8994:
8991:
8989:
8986:
8985:
8984:
8981:
8979:
8978:Autophagosome
8976:
8974:
8971:
8969:
8966:
8964:
8961:
8959:
8956:
8954:
8953:Cell membrane
8951:
8950:
8948:
8946:
8943:Endomembrane
8940:
8936:
8932:
8924:
8919:
8917:
8912:
8910:
8905:
8904:
8901:
8889:
8886:
8885:
8883:
8879:
8873:
8870:
8868:
8865:
8863:
8860:
8858:
8855:
8854:
8852:
8848:
8842:
8839:
8837:
8834:
8832:
8829:
8827:
8824:
8822:
8819:
8817:
8814:
8812:
8809:
8807:
8804:
8802:
8799:
8797:
8794:
8792:
8789:
8787:
8784:
8782:
8779:
8777:
8774:
8773:
8771:
8767:
8761:
8758:
8756:
8753:
8751:
8748:
8746:
8743:
8741:
8738:
8736:
8733:
8732:
8730:
8726:
8720:
8717:
8715:
8712:
8710:
8707:
8705:
8702:
8700:
8697:
8695:
8692:
8690:
8687:
8685:
8682:
8680:
8677:
8675:
8672:
8671:
8669:
8665:
8659:
8656:
8654:
8651:
8649:
8646:
8644:
8641:
8639:
8636:
8634:
8631:
8629:
8626:
8624:
8621:
8620:
8618:
8614:
8609:
8605:
8598:
8593:
8591:
8586:
8584:
8579:
8578:
8575:
8561:
8558:
8556:
8553:
8551:
8548:
8547:
8546:
8543:
8541:
8538:
8536:
8535:Chromoprotein
8533:
8531:
8528:
8524:
8521:
8519:
8516:
8514:
8511:
8510:
8509:
8506:
8504:
8501:
8499:
8496:
8495:
8493:
8489:
8483:
8480:
8478:
8475:
8473:
8470:
8469:
8467:
8463:
8457:
8454:
8452:
8449:
8447:
8444:
8442:
8439:
8437:
8434:
8432:
8429:
8428:
8426:
8422:
8418:
8411:
8406:
8404:
8399:
8397:
8392:
8391:
8388:
8381:
8377:
8374:
8370:
8368:
8365:
8363:
8360:
8359:
8348:
8344:
8340:
8336:
8332:
8328:
8324:
8320:
8315:
8311:
8307:
8302:
8297:
8294:(9): 1162–6.
8293:
8289:
8285:
8280:
8276:
8272:
8267:
8262:
8258:
8254:
8250:
8245:
8241:
8237:
8232:
8227:
8223:
8219:
8215:
8210:
8208:
8205:
8201:
8197:
8192:
8187:
8182:
8177:
8173:
8169:
8165:
8160:
8159:
8146:
8142:
8138:
8134:
8126:
8118:
8114:
8110:
8106:
8102:
8098:
8094:
8090:
8083:
8075:
8071:
8067:
8063:
8060:(7): 307–15.
8059:
8055:
8048:
8040:
8036:
8032:
8028:
8024:
8020:
8017:(1): H1–H19.
8016:
8012:
8005:
7997:
7993:
7988:
7983:
7979:
7975:
7971:
7967:
7963:
7956:
7948:
7944:
7939:
7934:
7930:
7926:
7922:
7918:
7914:
7907:
7899:
7895:
7891:
7887:
7883:
7879:
7872:
7864:
7860:
7856:
7852:
7848:
7844:
7840:
7836:
7829:
7821:
7817:
7813:
7809:
7805:
7801:
7797:
7793:
7786:
7778:
7774:
7770:
7766:
7762:
7758:
7754:
7750:
7743:
7741:
7732:
7728:
7724:
7720:
7716:
7712:
7708:
7704:
7697:
7695:
7686:
7682:
7677:
7672:
7668:
7664:
7660:
7653:
7645:
7641:
7636:
7631:
7627:
7623:
7619:
7615:
7611:
7604:
7596:
7592:
7588:
7584:
7580:
7576:
7569:
7567:
7558:
7554:
7549:
7544:
7540:
7536:
7533:(2): 215–26.
7532:
7528:
7524:
7517:
7509:
7505:
7500:
7495:
7491:
7487:
7483:
7479:
7475:
7468:
7460:
7456:
7451:
7446:
7442:
7438:
7434:
7430:
7426:
7419:
7411:
7407:
7402:
7397:
7392:
7387:
7383:
7379:
7375:
7368:
7360:
7356:
7352:
7348:
7344:
7340:
7337:(2): 905–31.
7336:
7332:
7325:
7317:
7313:
7308:
7303:
7299:
7295:
7291:
7284:
7276:
7272:
7267:
7262:
7258:
7254:
7250:
7246:
7242:
7235:
7227:
7223:
7218:
7213:
7208:
7203:
7199:
7195:
7191:
7183:
7175:
7171:
7166:
7161:
7157:
7153:
7149:
7142:
7134:
7130:
7125:
7120:
7116:
7112:
7108:
7104:
7100:
7093:
7085:
7081:
7077:
7073:
7068:
7063:
7060:(2): 129–36.
7059:
7055:
7051:
7044:
7036:
7032:
7027:
7022:
7018:
7014:
7010:
7003:
6995:
6991:
6987:
6983:
6979:
6975:
6972:(2): 142–51.
6971:
6967:
6959:
6951:
6947:
6942:
6937:
6934:(2): 285–90.
6933:
6929:
6925:
6918:
6910:
6906:
6902:
6898:
6895:(4): 676–84.
6894:
6890:
6882:
6874:
6870:
6866:
6862:
6858:
6854:
6847:
6839:
6835:
6831:
6827:
6822:
6817:
6813:
6809:
6805:
6798:
6790:
6786:
6782:
6778:
6774:
6770:
6767:(1): 110–22.
6766:
6762:
6755:
6747:
6743:
6738:
6733:
6730:(5): 2100–5.
6729:
6725:
6721:
6714:
6706:
6702:
6697:
6692:
6688:
6684:
6680:
6673:
6666:
6662:
6658:
6655:
6650:
6642:
6638:
6634:
6630:
6626:
6622:
6618:
6614:
6610:
6606:
6599:
6591:
6587:
6582:
6577:
6572:
6567:
6563:
6559:
6555:
6551:
6547:
6540:
6532:
6528:
6523:
6518:
6514:
6510:
6506:
6502:
6498:
6491:
6483:
6479:
6475:
6471:
6467:
6463:
6459:
6455:
6451:
6447:
6440:
6432:
6428:
6424:
6420:
6416:
6412:
6409:(2): 161–78.
6408:
6404:
6397:
6389:
6385:
6380:
6375:
6371:
6367:
6363:
6356:
6348:
6344:
6339:
6334:
6330:
6326:
6323:(3): 329–47.
6322:
6318:
6314:
6307:
6305:
6296:
6292:
6288:
6284:
6280:
6276:
6269:
6261:
6257:
6253:
6249:
6245:
6241:
6237:
6233:
6226:
6218:
6214:
6209:
6204:
6200:
6196:
6192:
6185:
6177:
6173:
6168:
6163:
6159:
6155:
6152:(1): 153–65.
6151:
6147:
6143:
6136:
6128:
6124:
6119:
6114:
6110:
6106:
6102:
6095:
6087:
6083:
6078:
6073:
6070:(4): 303–13.
6069:
6065:
6061:
6054:
6046:
6042:
6038:
6034:
6026:
6024:
6015:
6011:
6007:
6003:
5999:
5995:
5991:
5987:
5980:
5972:
5968:
5964:
5960:
5955:
5950:
5946:
5942:
5938:
5931:
5923:
5919:
5915:
5911:
5907:
5903:
5899:
5895:
5888:
5880:
5876:
5871:
5866:
5862:
5858:
5854:
5847:
5839:
5835:
5830:
5825:
5821:
5817:
5813:
5809:
5805:
5798:
5790:
5786:
5782:
5778:
5774:
5770:
5766:
5762:
5755:
5747:
5743:
5738:
5733:
5728:
5723:
5719:
5715:
5711:
5703:
5695:
5691:
5686:
5681:
5677:
5673:
5669:
5662:
5654:
5650:
5646:
5642:
5638:
5634:
5630:
5626:
5619:
5611:
5607:
5602:
5597:
5593:
5589:
5585:
5581:
5577:
5570:
5562:
5558:
5553:
5548:
5544:
5540:
5536:
5532:
5528:
5521:
5513:
5509:
5504:
5499:
5495:
5491:
5487:
5480:
5472:
5468:
5464:
5460:
5456:
5452:
5445:
5437:
5433:
5429:
5425:
5421:
5417:
5410:
5408:
5406:
5397:
5393:
5388:
5383:
5379:
5375:
5371:
5364:
5362:
5353:
5349:
5344:
5339:
5336:(8): 1015–8.
5335:
5331:
5327:
5320:
5312:
5308:
5303:
5298:
5294:
5290:
5286:
5282:
5278:
5271:
5263:
5259:
5255:
5251:
5247:
5243:
5236:
5228:
5224:
5219:
5214:
5210:
5206:
5202:
5195:
5187:
5183:
5179:
5175:
5171:
5167:
5164:(5): E113–6.
5163:
5159:
5152:
5150:
5141:
5137:
5133:
5129:
5125:
5121:
5114:
5106:
5102:
5097:
5092:
5088:
5084:
5080:
5073:
5065:
5061:
5057:
5053:
5049:
5045:
5041:
5037:
5033:
5029:
5022:
5014:
5010:
5006:
5002:
4998:
4994:
4987:
4979:
4975:
4971:
4967:
4963:
4959:
4955:
4951:
4944:
4936:
4932:
4927:
4922:
4918:
4914:
4910:
4903:
4895:
4891:
4886:
4881:
4877:
4873:
4870:(8): 1720–9.
4869:
4865:
4861:
4854:
4846:
4842:
4838:
4834:
4830:
4826:
4819:
4811:
4807:
4802:
4797:
4793:
4789:
4785:
4781:
4777:
4770:
4762:
4758:
4753:
4748:
4744:
4740:
4736:
4732:
4728:
4721:
4713:
4709:
4705:
4701:
4697:
4693:
4689:
4685:
4678:
4670:
4666:
4662:
4658:
4655:(2): 436–51.
4654:
4650:
4643:
4635:
4631:
4626:
4621:
4617:
4613:
4609:
4605:
4601:
4597:
4593:
4586:
4584:
4575:
4571:
4567:
4563:
4560:(11): 544–8.
4559:
4555:
4548:
4546:
4537:
4533:
4528:
4523:
4519:
4515:
4512:(1): 133–45.
4511:
4507:
4503:
4496:
4488:
4484:
4480:
4476:
4473:(6): 610–16.
4472:
4468:
4461:
4453:
4449:
4445:
4441:
4437:
4433:
4429:
4425:
4421:
4417:
4410:
4402:
4398:
4393:
4388:
4384:
4380:
4376:
4369:
4361:
4357:
4353:
4349:
4345:
4341:
4337:
4333:
4326:
4318:
4314:
4309:
4304:
4301:(6): 753–67.
4300:
4296:
4292:
4285:
4277:
4273:
4268:
4263:
4259:
4255:
4252:(1): 94–102.
4251:
4247:
4243:
4236:
4228:
4224:
4219:
4214:
4211:(5): 2543–8.
4210:
4206:
4202:
4195:
4187:
4183:
4179:
4175:
4171:
4167:
4160:
4152:
4148:
4143:
4138:
4134:
4130:
4126:
4119:
4111:
4107:
4103:
4099:
4095:
4091:
4087:
4083:
4076:
4068:
4064:
4060:
4056:
4052:
4048:
4041:
4033:
4029:
4024:
4019:
4015:
4011:
4007:
4000:
3992:
3988:
3983:
3978:
3975:(5): 589–99.
3974:
3970:
3966:
3959:
3951:
3947:
3942:
3937:
3933:
3929:
3925:
3918:
3910:
3906:
3901:
3896:
3892:
3888:
3884:
3880:
3876:
3869:
3861:
3857:
3852:
3847:
3843:
3839:
3835:
3831:
3827:
3820:
3812:
3808:
3803:
3798:
3793:
3788:
3784:
3780:
3776:
3772:
3768:
3761:
3753:
3749:
3744:
3739:
3734:
3729:
3725:
3721:
3717:
3713:
3709:
3702:
3700:
3698:
3689:
3685:
3680:
3675:
3671:
3667:
3663:
3659:
3655:
3651:
3647:
3640:
3638:
3636:
3634:
3625:
3621:
3616:
3611:
3606:
3601:
3597:
3593:
3589:
3585:
3581:
3574:
3566:
3562:
3558:
3554:
3550:
3546:
3542:
3538:
3531:
3523:
3519:
3514:
3509:
3504:
3499:
3495:
3491:
3487:
3483:
3479:
3472:
3470:
3468:
3459:
3455:
3450:
3445:
3440:
3435:
3432:(5): 1380–7.
3431:
3427:
3423:
3416:
3408:
3404:
3399:
3394:
3390:
3386:
3382:
3378:
3374:
3370:
3366:
3359:
3357:
3348:
3344:
3339:
3334:
3329:
3324:
3320:
3316:
3312:
3308:
3304:
3297:
3295:
3286:
3282:
3278:
3274:
3270:
3266:
3262:
3258:
3254:
3250:
3243:
3235:
3231:
3226:
3221:
3217:
3213:
3209:
3205:
3201:
3194:
3192:
3190:
3188:
3186:
3177:
3173:
3168:
3163:
3160:(5): 687–98.
3159:
3155:
3151:
3144:
3142:
3140:
3138:
3129:
3125:
3120:
3115:
3111:
3107:
3103:
3096:
3088:
3084:
3080:
3076:
3072:
3068:
3065:(3): 237–44.
3064:
3060:
3053:
3045:
3041:
3036:
3031:
3027:
3023:
3020:(3): 500–10.
3019:
3015:
3011:
3004:
2996:
2992:
2987:
2982:
2978:
2974:
2970:
2966:
2962:
2955:
2953:
2944:
2940:
2935:
2930:
2926:
2922:
2918:
2911:
2909:
2900:
2896:
2892:
2888:
2884:
2880:
2877:(1): 137–55.
2876:
2872:
2865:
2858:
2856:
2847:
2843:
2839:
2835:
2831:
2827:
2823:
2819:
2812:
2796:
2790:
2782:
2778:
2773:
2768:
2764:
2760:
2757:(5): 731–44.
2756:
2752:
2748:
2741:
2739:
2737:
2728:
2724:
2719:
2714:
2710:
2706:
2702:
2698:
2694:
2687:
2685:
2683:
2674:
2670:
2667:(4): 255–61.
2666:
2662:
2655:
2653:
2651:
2649:
2640:
2636:
2631:
2626:
2622:
2618:
2614:
2610:
2606:
2599:
2597:
2595:
2593:
2591:
2589:
2587:
2585:
2583:
2581:
2579:
2577:
2568:
2564:
2560:
2556:
2552:
2548:
2544:
2540:
2533:
2525:
2521:
2517:
2513:
2510:(3): 253–60.
2509:
2505:
2498:
2490:
2486:
2481:
2476:
2472:
2468:
2464:
2457:
2449:
2445:
2440:
2435:
2431:
2427:
2423:
2416:
2408:
2404:
2399:
2394:
2390:
2386:
2383:(6): 1580–5.
2382:
2378:
2374:
2367:
2363:
2359:
2355:
2351:
2347:
2343:
2339:
2332:
2324:
2320:
2316:
2312:
2308:
2304:
2300:
2296:
2289:
2281:
2277:
2272:
2267:
2263:
2259:
2255:
2248:
2240:
2236:
2231:
2226:
2221:
2216:
2212:
2208:
2204:
2200:
2196:
2189:
2181:
2177:
2173:
2169:
2166:(4): 1100–5.
2165:
2161:
2154:
2146:
2142:
2137:
2132:
2127:
2122:
2118:
2114:
2110:
2106:
2102:
2095:
2080:
2073:
2071:
2062:
2058:
2054:
2050:
2045:
2040:
2036:
2032:
2028:
2021:
2013:
2009:
2004:
1999:
1995:
1991:
1987:
1980:
1972:
1966:
1962:
1958:
1957:
1951:
1943:
1941:
1939:
1937:
1932:
1923:
1922:JUNQ and IPOD
1920:
1918:
1915:
1913:
1910:
1908:
1905:
1903:
1900:
1899:
1895:
1889:
1884:
1877:
1875:
1871:
1867:
1863:
1859:
1855:
1851:
1847:
1843:
1839:
1835:
1831:
1827:
1823:
1819:
1815:
1811:
1807:
1803:
1799:
1795:
1791:
1787:
1783:
1779:
1775:
1771:
1767:
1763:
1759:
1755:
1751:
1747:
1743:
1742:heart failure
1739:
1735:
1731:
1727:
1723:
1719:
1715:
1711:
1707:
1703:
1698:
1696:
1692:
1688:
1684:
1680:
1676:
1671:
1662:
1660:
1656:
1652:
1648:
1644:
1639:
1637:
1633:
1629:
1625:
1620:
1618:
1614:
1610:
1606:
1602:
1598:
1595:The molecule
1593:
1591:
1587:
1583:
1579:
1575:
1571:
1567:
1563:
1559:
1557:
1553:
1549:
1545:
1542:
1541:
1536:
1532:
1530:
1526:
1522:
1518:
1511:
1507:
1503:
1499:
1495:
1491:
1487:
1483:
1477:
1473:
1469:
1464:
1459:
1449:
1447:
1443:
1439:
1434:
1432:
1428:
1424:
1420:
1417:regulator of
1416:
1412:
1408:
1403:
1400:
1396:
1391:
1389:
1385:
1384:
1379:
1375:
1371:
1367:
1363:
1359:
1355:
1345:
1343:
1339:
1335:
1331:
1327:
1323:
1322:neurotoxicity
1319:
1315:
1311:
1307:
1302:
1300:
1296:
1292:
1288:
1283:
1280:
1276:
1272:
1268:
1264:
1260:
1256:
1252:
1248:
1238:
1235:
1230:
1226:
1222:
1219:
1215:
1211:
1207:
1203:
1199:
1193:
1191:
1187:
1183:
1179:
1169:
1166:
1162:
1158:
1157:phytohormones
1154:
1150:
1140:
1138:
1133:
1131:
1127:
1123:
1119:
1115:
1111:
1107:
1103:
1098:
1096:
1092:
1089:(APC), an E3
1088:
1084:
1080:
1076:
1068:
1063:
1061:
1057:
1053:
1049:
1045:
1041:
1037:
1033:
1029:
1019:
1017:
1013:
1009:
1004:
1002:
1001:ClpP and ClpX
998:
994:
990:
986:
982:
978:
970:
966:
965:
960:
956:
951:
942:
939:
935:
931:
927:
923:
919:
918:translational
909:
907:
902:
898:
894:
889:
887:
883:
879:
875:
869:
856:
852:
848:
844:
839:
835:
833:
829:
825:
822:
817:
813:
809:
804:
802:
798:
793:
788:
786:
782:
778:
773:
769:
768:translocation
758:
742:
737:
733:
730:
726:
725:transcription
722:
718:
714:
710:
706:
701:
699:
695:
692:
686:
683:
679:
675:
671:
667:
663:
659:
653:
640:
636:
632:
628:
619:
617:
612:
608:
604:
600:
594:
591:
587:
586:alpha helices
583:
579:
575:
571:
566:
562:
552:
550:
545:
543:
538:
533:
525:
521:
511:
509:
493:
489:
486:
475:
473:
469:
465:
461:
457:
448:
444:
435:
433:
432:
427:
423:
419:
416:
412:
411:hematopoietic
408:
404:
400:
396:
392:
388:
387:
382:
377:
375:
371:
368:
363:
362:pseudoenzymes
359:
351:
341:
339:
334:
331:
326:
322:
318:
314:
304:
295:
293:
289:
285:
281:
276:
274:
270:
266:
262:
258:
257:Avram Hershko
254:
249:
247:
243:
239:
236:
232:
228:
224:
220:
219:reticulocytes
216:
212:
208:
204:
200:
190:
188:
184:
183:Avram Hershko
180:
176:
172:
168:
164:
159:
157:
153:
149:
145:
141:
137:
132:
130:
126:
122:
118:
114:
109:
107:
103:
99:
95:
91:
87:
83:
79:
78:concentration
76:regulate the
75:
70:
68:
64:
60:
59:peptide bonds
56:
52:
48:
44:
40:
32:
23:
19:
9290:
9194:Gerontoplast
9144:Pseudopodium
9137:Radial spoke
9117:Undulipodium
9057:Cytoskeleton
8973:Parenthesome
8481:
8347:the original
8326:
8322:
8291:
8287:
8256:
8252:
8221:
8217:
8171:
8168:PLOS Biology
8167:
8136:
8132:
8125:
8092:
8088:
8082:
8057:
8053:
8047:
8014:
8010:
8004:
7969:
7965:
7955:
7920:
7916:
7906:
7884:(2): 103–8.
7881:
7877:
7871:
7841:(1): 78–85.
7838:
7834:
7828:
7795:
7791:
7785:
7752:
7748:
7706:
7702:
7669:(1): 133–8.
7666:
7662:
7652:
7617:
7613:
7603:
7581:(1): 85–98.
7578:
7574:
7530:
7526:
7516:
7481:
7477:
7467:
7432:
7428:
7418:
7381:
7377:
7367:
7334:
7330:
7324:
7300:(8): 503–8.
7297:
7293:
7283:
7251:(6): 352–9.
7248:
7244:
7234:
7197:
7193:
7182:
7155:
7151:
7141:
7109:(5): 671–9.
7106:
7102:
7092:
7057:
7053:
7043:
7016:
7012:
7002:
6969:
6965:
6958:
6931:
6927:
6917:
6892:
6888:
6881:
6859:(1): 49–55.
6856:
6852:
6846:
6814:(1): 59–70.
6811:
6807:
6797:
6764:
6760:
6754:
6727:
6723:
6713:
6686:
6682:
6672:
6649:
6608:
6604:
6598:
6553:
6549:
6539:
6504:
6500:
6490:
6449:
6445:
6439:
6406:
6402:
6396:
6369:
6365:
6355:
6320:
6316:
6278:
6274:
6268:
6235:
6231:
6225:
6198:
6194:
6184:
6149:
6145:
6135:
6108:
6104:
6094:
6067:
6063:
6053:
6036:
6032:
5992:(1): 47–50.
5989:
5986:FEBS Letters
5985:
5979:
5947:(3): 345–9.
5944:
5941:FEBS Letters
5940:
5930:
5900:(4): 411–9.
5897:
5893:
5887:
5860:
5856:
5846:
5811:
5807:
5797:
5764:
5760:
5754:
5717:
5713:
5702:
5675:
5671:
5661:
5628:
5624:
5618:
5583:
5579:
5569:
5534:
5530:
5520:
5493:
5489:
5479:
5454:
5450:
5444:
5419:
5415:
5380:(1): 311–8.
5377:
5373:
5333:
5329:
5319:
5284:
5280:
5270:
5248:(8): 844–9.
5245:
5241:
5235:
5208:
5204:
5194:
5161:
5157:
5123:
5119:
5113:
5086:
5082:
5072:
5031:
5027:
5021:
4996:
4992:
4986:
4953:
4949:
4943:
4916:
4912:
4902:
4867:
4863:
4853:
4831:(1): 72–83.
4828:
4824:
4818:
4783:
4780:Cell Reports
4779:
4769:
4737:(3): 161–7.
4734:
4730:
4720:
4687:
4683:
4677:
4652:
4648:
4642:
4599:
4595:
4557:
4553:
4509:
4505:
4495:
4470:
4466:
4460:
4422:(1): 58–64.
4419:
4415:
4409:
4385:(1): 13–28.
4382:
4378:
4368:
4338:(8): 742–9.
4335:
4331:
4325:
4298:
4294:
4284:
4249:
4245:
4235:
4208:
4204:
4194:
4169:
4165:
4159:
4132:
4128:
4118:
4085:
4081:
4075:
4050:
4046:
4040:
4013:
4009:
3999:
3972:
3968:
3958:
3931:
3927:
3917:
3882:
3878:
3868:
3833:
3829:
3819:
3774:
3770:
3760:
3715:
3711:
3653:
3649:
3587:
3583:
3573:
3540:
3536:
3530:
3485:
3481:
3429:
3425:
3415:
3372:
3368:
3310:
3306:
3252:
3248:
3242:
3210:(1): 39–50.
3207:
3203:
3157:
3153:
3109:
3105:
3095:
3062:
3058:
3052:
3017:
3013:
3003:
2968:
2965:Cell Reports
2964:
2924:
2920:
2874:
2870:
2824:(3): 842–9.
2821:
2817:
2811:
2799:. Retrieved
2789:
2754:
2750:
2700:
2696:
2664:
2660:
2612:
2608:
2542:
2538:
2532:
2507:
2503:
2497:
2470:
2466:
2456:
2429:
2425:
2415:
2380:
2376:
2341:
2337:
2331:
2298:
2294:
2288:
2261:
2257:
2247:
2205:(3): 864–8.
2202:
2198:
2188:
2163:
2159:
2153:
2108:
2104:
2094:
2082:. Retrieved
2034:
2030:
2020:
1993:
1989:
1979:
1955:
1854:nitric oxide
1699:
1695:malignancies
1672:
1668:
1646:
1642:
1640:
1621:
1609:chymotrypsin
1594:
1570:chemotherapy
1560:
1540:Streptomyces
1538:
1533:
1525:cell culture
1523:activity in
1515:
1472:chemotherapy
1435:
1415:inflammatory
1404:
1392:
1381:
1351:
1306:astrocytomas
1303:
1284:
1244:
1225:chemotherapy
1194:
1175:
1146:
1134:
1099:
1064:
1050:complex. In
1025:
1005:
974:
962:
915:
905:
890:
874:endoprotease
871:
843:active sites
805:
789:
767:
764:
751:
702:
687:
655:
595:
578:salt bridges
558:
546:
528:
504:
481:
464:COP9 complex
460:AAA proteins
453:
441:
429:
415:inflammatory
399:chymotrypsin
384:
378:
354:
333:active sites
316:
310:
277:
250:
196:
160:
155:
151:
147:
144:active sites
139:
133:
110:
106:synthesizing
93:
71:
57:that breaks
38:
37:
18:
9296:Magnetosome
9262:Spliceosome
9189:Chromoplast
9184:Chloroplast
9075:Microtubule
8728:C (ATPases)
8555:Phytochrome
8545:Biliprotein
8095:(1): 20–6.
7966:Circulation
7798:(1): 47–9.
7755:(1): 21–8.
3656:(1): 1360.
2801:16 February
2703:(1): 8–19.
2111:(1): 54–8.
2084:11 December
1687:cell growth
1679:proteolysis
1659:Fluorescent
1651:lactacystin
1624:skin grafts
1578:blood serum
1535:Lactacystin
1527:, inducing
1334:α-synuclein
1279:hydrophobic
1106:oncoprotein
1095:SCF complex
961:(red) from
957:(blue) and
862:Proteolysis
819:example in
709:amino acids
582:hydrophobic
572:-dependent
524:11 (plural)
405:-like, and
321:kilodaltons
240:residue of
127:and in the
102:amino acids
51:proteolysis
39:Proteasomes
9348:Organelles
9332:Categories
9291:Proteasome
9284:Inclusions
9231:Nitroplast
9224:Apicoplast
9209:Elaioplast
9204:Amyloplast
9199:Leucoplast
9154:Filopodium
9100:Basal body
9090:Centrosome
9040:Peroxisome
9035:Glyoxysome
9025:Melanosome
8935:organelles
8606:subunits (
8482:Proteasome
8465:Structures
8373:proteasome
8174:(1): e13.
7217:1887/65477
6105:Cell Cycle
5672:Cell Cycle
5490:Cell Cycle
5330:Cell Cycle
4999:: 801–47.
2797:. EMBL-EBI
2031:IUBMB Life
1928:References
1846:P-selectin
1784:(Rb). and
1748:, such as
1683:cell cycle
1634:models of
1562:Bortezomib
1486:Bortezomib
1468:bortezomib
1409:, an anti-
1395:C-terminus
1374:expression
1356:. Peptide
1251:heat shock
1229:bortezomib
1210:thymocytes
1052:vertebrate
1036:cell cycle
985:heat shock
934:cell cycle
866:See also:
855:bortezomib
797:ATP analog
792:hydrolyzed
717:eukaryotes
691:N-terminal
599:chaperones
561:N-terminal
288:holoenzyme
265:Irwin Rose
235:C-terminal
203:organelles
187:Irwin Rose
163:cell cycle
148:α subunits
140:β subunits
113:eukaryotes
9353:Apoptosis
9312:Cell wall
9274:Cytoplasm
9247:Nucleolus
9219:Tannosome
9127:Flagellum
9112:Myofibril
9095:Centriole
9030:Microbody
9003:Phagosome
8610:3.4.25.1)
8560:Lipocalin
8424:Processes
6366:Neurology
6275:Biochimie
5242:BioEssays
4047:Biochimie
4010:Structure
1822:cytokines
1691:apoptosis
1628:psoriasis
1597:ritonavir
1529:apoptosis
1510:threonine
1411:apoptotic
1326:Lewy body
1271:chaperone
1247:infection
1202:quiescent
1182:apoptosis
1172:Apoptosis
1122:apoptotic
1046:from the
977:bacterial
945:Evolution
851:threonine
713:conserved
705:ubiquitin
682:substrate
666:ubiquitin
635:ubiquitin
574:autolysis
570:threonine
422:cytokines
374:angstroms
358:catalytic
323:(kDa) in
278:Although
242:ubiquitin
199:lysosomes
193:Discovery
136:structure
129:cytoplasm
86:ubiquitin
67:proteases
26:degraded.
9338:Proteins
9305:External
9257:Ribosome
9013:Acrosome
8998:Endosome
8993:Lysosome
8513:Globulin
8451:Proteome
8417:Proteins
8382:function
8376:Archived
8343:17979756
8310:16094392
8275:16094391
8240:16094393
8200:14737189
8145:12375310
8117:26973319
8109:11753406
8074:12654543
8031:16501026
7996:20159828
7947:23220331
7898:12792671
7855:12507416
7820:28190967
7777:22396490
7769:12070660
7723:11881748
7685:10899438
7644:25560147
7595:10723801
7557:25651176
7508:25133688
7459:24380730
7435:: 3–10.
7410:25324717
7359:14103185
7351:25561438
7275:24457024
7226:17114003
7174:10452747
7133:11877475
7084:24423806
7076:14985453
7035:10585454
6994:28749125
6986:22273806
6950:22653976
6909:15613699
6873:12141956
6838:38429730
6830:14749476
6789:21223980
6781:11400168
6746:17095627
6705:17001068
6657:Archived
6641:37779687
6590:21045130
6531:11350924
6482:37185716
6474:17540904
6431:27762513
6423:16679556
6388:16717251
6347:19597829
6295:11295490
6260:30385354
6252:17081106
6217:16169850
6176:17065559
6127:17106261
6086:10381632
6045:10363983
6014:29256092
5971:10873052
5922:33829749
5838:15889151
5781:12036263
5746:32764038
5694:16177571
5645:15014502
5610:16738333
5561:16782009
5512:16921258
5471:10410804
5436:12595256
5396:12401807
5352:16082197
5311:12660156
5262:16927316
5227:15571816
5178:11988749
5140:10872471
4935:14688254
4894:16601692
4845:16919475
4810:25220455
4761:21278740
4712:41599619
4669:15950624
4634:35477760
4574:11084366
4536:19345192
4487:15556404
4401:22897362
4360:21069699
4352:16056265
4317:12795696
4276:10619848
4186:20471955
4151:21658604
4110:21263837
4102:19165213
4067:11295488
4032:16843899
3991:15916965
3950:11430818
3909:28689658
3860:23770819
3811:23589842
3752:24706844
3688:29636472
3624:27791164
3565:21909333
3557:27428775
3522:27791164
3458:22307589
3407:22237024
3347:22927375
3277:11961560
3234:17018291
3176:16337593
3128:10473546
3087:21181637
3079:18278055
3044:14739934
2995:26997268
2899:21603835
2891:16595883
2846:23508675
2781:17803938
2727:21211719
2673:16978533
2639:30479383
2489:16094391
2280:16094393
2061:29860298
2053:24823973
1880:See also
1828:, IL-β,
1824:such as
1736:injury,
1734:ischemic
1730:dementia
1643:in vitro
1544:bacteria
1500:= blue,
1498:nitrogen
1496:= pink,
1419:cytokine
1413:and pro-
1370:pathogen
1358:antigens
1295:histones
1257: –
1186:caspases
1102:Gankyrin
1083:cyclin A
1044:cyclin B
1018:events.
997:protists
886:hydroxyl
729:evolving
670:cysteine
603:gankyrin
590:mutation
555:Assembly
532:peptides
420:such as
383:such as
313:Svedberg
259:, where
253:Technion
227:covalent
223:histones
211:protease
121:bacteria
98:peptides
47:proteins
9279:Cytosol
9179:Plastid
9132:Axoneme
9008:Vacuole
8988:Exosome
8983:Vesicle
8958:Nucleus
8523:Albumin
8518:Edestin
8039:7073263
7987:2857348
7938:3774499
7863:5780576
7812:1328965
7731:2211658
7635:4886828
7548:4317573
7499:4241867
7450:4011959
7401:4179678
7316:9383453
7266:4037451
6633:7732382
6613:Bibcode
6605:Science
6581:2993423
6558:Bibcode
6454:Bibcode
6446:Science
6338:2716447
6167:1751312
6006:8925925
5963:9009228
5914:2169771
5879:7721865
5829:1142592
5789:7669386
5737:7116001
5714:Science
5653:4401971
5601:1489138
5552:2749311
5186:7126477
5105:9748229
5064:4261663
5056:9087403
5036:Bibcode
5013:8811196
4978:7725107
4958:Bibcode
4950:Science
4885:1440830
4801:4358326
4752:3129032
4704:7773788
4625:9117149
4604:Bibcode
4527:2668214
4452:7929162
4444:3041010
4424:Bibcode
4267:1171781
4227:6277905
3900:5580496
3851:3712289
3802:3645540
3779:Bibcode
3743:3992697
3720:Bibcode
3679:5893597
3658:Bibcode
3615:5135334
3592:Bibcode
3513:5135334
3490:Bibcode
3449:3277140
3398:3285539
3377:Bibcode
3338:3443124
3315:Bibcode
3285:4421764
3257:Bibcode
3225:3951175
3035:1271798
2986:4828729
2943:9312134
2838:6338156
2772:2083707
2718:3040445
2630:6370054
2567:7725097
2547:Bibcode
2539:Science
2524:3524688
2448:3298229
2407:6304111
2398:2112434
2358:3277060
2323:9028201
2315:6778972
2207:Bibcode
2113:Bibcode
2012:8125997
1716:(ALS),
1647:in vivo
1619:cells.
1613:trypsin
1504:= red,
1214:neurons
1178:signals
1161:tropism
1079:S phase
1056:M phase
1040:mitosis
1032:cyclins
964:E. coli
908:(RUP).
824:fibroin
816:alanine
743:pathway
658:enzymes
639:protein
496:occurs.
418:signals
403:trypsin
401:-like,
395:mammals
381:archaea
370:PF10584
267:at the
238:glycine
125:nucleus
117:archaea
63:Enzymes
9122:Cilium
8945:system
8841:PSMD14
8836:PSMD13
8831:PSMD12
8826:PSMD11
8821:PSMD10
8719:PSMB10
8341:
8308:
8273:
8238:
8198:
8191:314468
8188:
8143:
8115:
8107:
8072:
8037:
8029:
7994:
7984:
7945:
7935:
7896:
7861:
7853:
7818:
7810:
7775:
7767:
7729:
7721:
7683:
7642:
7632:
7593:
7555:
7545:
7506:
7496:
7457:
7447:
7408:
7398:
7384:: 77.
7357:
7349:
7314:
7273:
7263:
7224:
7172:
7131:
7124:150886
7121:
7082:
7074:
7033:
6992:
6984:
6948:
6907:
6871:
6836:
6828:
6787:
6779:
6744:
6703:
6639:
6631:
6588:
6578:
6529:
6522:125470
6519:
6480:
6472:
6429:
6421:
6386:
6345:
6335:
6293:
6258:
6250:
6215:
6174:
6164:
6125:
6084:
6043:
6012:
6004:
5969:
5961:
5920:
5912:
5894:Neuron
5877:
5836:
5826:
5787:
5779:
5744:
5734:
5692:
5651:
5643:
5625:Nature
5608:
5598:
5559:
5549:
5510:
5469:
5434:
5394:
5350:
5309:
5302:152902
5299:
5260:
5225:
5184:
5176:
5138:
5103:
5062:
5054:
5028:Nature
5011:
4976:
4933:
4892:
4882:
4843:
4808:
4798:
4759:
4749:
4710:
4702:
4667:
4632:
4622:
4596:Nature
4572:
4534:
4524:
4485:
4450:
4442:
4399:
4358:
4350:
4315:
4274:
4264:
4225:
4184:
4149:
4108:
4100:
4065:
4030:
3989:
3948:
3907:
3897:
3858:
3848:
3809:
3799:
3750:
3740:
3686:
3676:
3622:
3612:
3563:
3555:
3520:
3510:
3456:
3446:
3405:
3395:
3369:Nature
3345:
3335:
3283:
3275:
3249:Nature
3232:
3222:
3174:
3126:
3085:
3077:
3042:
3032:
2993:
2983:
2941:
2897:
2889:
2844:
2836:
2779:
2769:
2725:
2715:
2671:
2637:
2627:
2609:Nature
2565:
2522:
2487:
2446:
2405:
2395:
2364:
2356:
2338:Nature
2321:
2313:
2278:
2239:265581
2237:
2230:430507
2227:
2180:666810
2178:
2145:264694
2143:
2136:393195
2133:
2059:
2051:
2010:
1967:
1848:) and
1842:VCAM-1
1838:ICAM-1
1636:asthma
1632:rodent
1617:glioma
1586:B-cell
1502:oxygen
1494:carbon
1442:TRIM21
1218:kinase
1153:auxins
1149:plants
987:genes
979:order
611:ATPase
607:ATPase
330:ATPase
231:lysine
207:acidic
9267:Vault
8888:PSMF1
8872:PSME4
8867:PSME3
8862:PSME2
8857:PSME1
8816:PSMD9
8811:PSMD8
8806:PSMD7
8801:PSMD6
8796:PSMD5
8791:PSMD4
8786:PSMD3
8781:PSMD2
8776:PSMD1
8760:PSMC6
8755:PSMC5
8750:PSMC4
8745:PSMC3
8740:PSMC2
8735:PSMC1
8714:PSMB9
8709:PSMB8
8704:PSMB7
8699:PSMB6
8694:PSMB5
8689:PSMB4
8684:PSMB3
8679:PSMB2
8674:PSMB1
8658:PSMA8
8653:PSMA7
8648:PSMA6
8643:PSMA5
8638:PSMA4
8633:PSMA3
8628:PSMA2
8623:PSMA1
8491:Types
8113:S2CID
8035:S2CID
7859:S2CID
7816:S2CID
7773:S2CID
7727:S2CID
7355:S2CID
7080:S2CID
6990:S2CID
6928:Blood
6834:S2CID
6785:S2CID
6724:Blood
6637:S2CID
6478:S2CID
6427:S2CID
6256:S2CID
6010:S2CID
5967:S2CID
5918:S2CID
5785:S2CID
5649:S2CID
5182:S2CID
5060:S2CID
4708:S2CID
4448:S2CID
4356:S2CID
4106:S2CID
3561:S2CID
3281:S2CID
3083:S2CID
2895:S2CID
2867:(PDF)
2842:S2CID
2366:97688
2362:S2CID
2319:S2CID
2057:S2CID
1961:66–72
1826:TNF-α
1770:STAT3
1766:c-Myc
1762:NF-κB
1758:c-Fos
1754:c-jun
1655:MG132
1521:tumor
1506:boron
1490:yeast
1407:NF-κB
1330:yeast
1299:aging
1275:Hsp70
1267:Hsp90
1263:Hsp27
1253:, or
1190:blebs
1155:, or
1137:ESCRT
1126:tumor
1075:phase
901:loops
897:NF-κB
882:water
616:Usp14
614:Ubp6/
549:PSME4
508:motif
391:yeast
338:virus
307:blue.
205:with
74:cells
8931:cell
8339:PMID
8306:PMID
8271:PMID
8236:PMID
8196:PMID
8141:PMID
8105:PMID
8070:PMID
8027:PMID
7992:PMID
7943:PMID
7894:PMID
7851:PMID
7808:PMID
7765:PMID
7719:PMID
7681:PMID
7667:1502
7640:PMID
7591:PMID
7553:PMID
7504:PMID
7455:PMID
7406:PMID
7347:PMID
7312:PMID
7271:PMID
7222:PMID
7170:PMID
7129:PMID
7072:PMID
7031:PMID
6982:PMID
6946:PMID
6905:PMID
6869:PMID
6826:PMID
6777:PMID
6742:PMID
6701:PMID
6629:PMID
6586:PMID
6527:PMID
6470:PMID
6419:PMID
6384:PMID
6343:PMID
6291:PMID
6248:PMID
6213:PMID
6172:PMID
6123:PMID
6082:PMID
6041:PMID
6002:PMID
5959:PMID
5910:PMID
5875:PMID
5834:PMID
5777:PMID
5742:PMID
5690:PMID
5641:PMID
5606:PMID
5557:PMID
5508:PMID
5467:PMID
5432:PMID
5392:PMID
5348:PMID
5307:PMID
5258:PMID
5223:PMID
5209:1695
5174:PMID
5136:PMID
5101:PMID
5052:PMID
5009:PMID
4974:PMID
4931:PMID
4890:PMID
4841:PMID
4806:PMID
4757:PMID
4700:PMID
4665:PMID
4630:PMID
4570:PMID
4532:PMID
4506:Cell
4483:PMID
4440:PMID
4397:PMID
4348:PMID
4313:PMID
4272:PMID
4223:PMID
4182:PMID
4147:PMID
4098:PMID
4063:PMID
4028:PMID
3987:PMID
3946:PMID
3905:PMID
3856:PMID
3807:PMID
3748:PMID
3684:PMID
3620:PMID
3553:PMID
3518:PMID
3454:PMID
3403:PMID
3343:PMID
3273:PMID
3230:PMID
3172:PMID
3124:PMID
3075:PMID
3040:PMID
2991:PMID
2939:PMID
2887:PMID
2834:PMID
2803:2019
2777:PMID
2723:PMID
2669:PMID
2635:PMID
2563:PMID
2520:PMID
2485:PMID
2444:PMID
2403:PMID
2354:PMID
2311:PMID
2276:PMID
2235:PMID
2176:PMID
2141:PMID
2086:2006
2049:PMID
2008:PMID
1965:ISBN
1872:and
1852:and
1830:IL-8
1740:and
1708:and
1645:and
1429:and
1316:and
1291:PARP
1265:and
1231:and
1212:and
1204:and
1118:MDM2
1077:and
993:hslU
991:and
989:hslV
959:hslU
955:hslV
922:p105
821:silk
739:The
703:The
580:and
468:eIF3
466:and
367:Pfam
209:and
185:and
115:and
53:, a
41:are
9252:RNA
8331:doi
8296:doi
8261:doi
8226:doi
8186:PMC
8176:doi
8097:doi
8062:doi
8019:doi
8015:291
7982:PMC
7974:doi
7970:121
7933:PMC
7925:doi
7921:304
7886:doi
7843:doi
7800:doi
7796:139
7757:doi
7753:104
7711:doi
7671:doi
7630:PMC
7622:doi
7583:doi
7543:PMC
7535:doi
7494:PMC
7486:doi
7445:PMC
7437:doi
7396:PMC
7386:doi
7339:doi
7302:doi
7261:PMC
7253:doi
7212:hdl
7202:doi
7160:doi
7156:104
7119:PMC
7111:doi
7107:109
7062:doi
7021:doi
7017:274
6974:doi
6970:351
6936:doi
6932:120
6897:doi
6861:doi
6816:doi
6769:doi
6732:doi
6728:109
6691:doi
6621:doi
6609:268
6576:PMC
6566:doi
6554:107
6517:PMC
6509:doi
6462:doi
6450:316
6411:doi
6374:doi
6333:PMC
6325:doi
6321:118
6283:doi
6240:doi
6236:387
6203:doi
6199:280
6162:PMC
6154:doi
6113:doi
6072:doi
5994:doi
5990:394
5949:doi
5945:400
5902:doi
5865:doi
5861:270
5824:PMC
5816:doi
5769:doi
5732:PMC
5722:doi
5718:369
5680:doi
5633:doi
5629:428
5596:PMC
5588:doi
5547:PMC
5539:doi
5498:doi
5459:doi
5424:doi
5420:326
5382:doi
5378:278
5338:doi
5297:PMC
5289:doi
5250:doi
5213:doi
5166:doi
5128:doi
5091:doi
5087:273
5044:doi
5032:386
5001:doi
4966:doi
4954:268
4921:doi
4917:279
4880:PMC
4872:doi
4833:doi
4829:156
4796:PMC
4788:doi
4747:PMC
4739:doi
4692:doi
4657:doi
4653:307
4620:PMC
4612:doi
4600:605
4562:doi
4522:PMC
4514:doi
4510:137
4475:doi
4432:doi
4387:doi
4383:196
4340:doi
4303:doi
4262:PMC
4254:doi
4213:doi
4209:257
4174:doi
4170:396
4137:doi
4090:doi
4055:doi
4018:doi
3977:doi
3936:doi
3895:PMC
3887:doi
3846:PMC
3838:doi
3797:PMC
3787:doi
3775:110
3738:PMC
3728:doi
3716:111
3674:PMC
3666:doi
3610:PMC
3600:doi
3588:113
3545:doi
3508:PMC
3498:doi
3486:113
3444:PMC
3434:doi
3430:109
3393:PMC
3385:doi
3373:482
3333:PMC
3323:doi
3311:109
3265:doi
3253:416
3220:PMC
3212:doi
3162:doi
3114:doi
3110:274
3067:doi
3030:PMC
3022:doi
2981:PMC
2973:doi
2929:doi
2925:272
2879:doi
2826:doi
2767:PMC
2759:doi
2713:PMC
2705:doi
2625:PMC
2617:doi
2613:565
2555:doi
2543:268
2512:doi
2508:872
2475:doi
2434:doi
2430:262
2393:PMC
2385:doi
2346:doi
2342:331
2303:doi
2266:doi
2225:PMC
2215:doi
2168:doi
2131:PMC
2121:doi
2039:doi
1998:doi
1994:269
1950:"3"
1866:SLE
1858:CDK
1818:ABL
1814:Mos
1810:Src
1806:Rel
1802:Myb
1798:Myc
1794:Raf
1750:p53
1605:HIV
1221:JNK
1200:of
1147:In
1114:p53
938:p53
633:of
522:or
520:S11
485:ATP
379:In
350:20s
246:ATP
177:to
134:In
49:by
9334::
8933:/
8608:EC
8337:.
8327:13
8325:.
8321:.
8304:.
8292:12
8290:.
8286:.
8269:.
8257:12
8255:.
8251:.
8234:.
8222:12
8220:.
8216:.
8194:.
8184:.
8170:.
8166:.
8137:29
8135:.
8111:.
8103:.
8091:.
8068:.
8056:.
8033:.
8025:.
8013:.
7990:.
7980:.
7968:.
7964:.
7941:.
7931:.
7919:.
7915:.
7892:.
7882:16
7880:.
7857:.
7849:.
7837:.
7814:.
7806:.
7794:.
7771:.
7763:.
7751:.
7739:^
7725:.
7717:.
7707:24
7705:.
7693:^
7679:.
7665:.
7661:.
7638:.
7628:.
7618:23
7616:.
7612:.
7589:.
7579:12
7577:.
7565:^
7551:.
7541:.
7531:21
7529:.
7525:.
7502:.
7492:.
7482:21
7480:.
7476:.
7453:.
7443:.
7433:71
7431:.
7427:.
7404:.
7394:.
7380:.
7376:.
7353:.
7345:.
7335:53
7333:.
7310:.
7296:.
7292:.
7269:.
7259:.
7249:24
7247:.
7243:.
7220:.
7210:.
7198:13
7196:.
7192:.
7168:.
7154:.
7150:.
7127:.
7117:.
7105:.
7101:.
7078:.
7070:.
7056:.
7052:.
7029:.
7015:.
7011:.
6988:.
6980:.
6968:.
6944:.
6930:.
6926:.
6903:.
6893:23
6891:.
6867:.
6855:.
6832:.
6824:.
6810:.
6806:.
6783:.
6775:.
6765:82
6763:.
6740:.
6726:.
6722:.
6699:.
6687:24
6685:.
6681:.
6635:.
6627:.
6619:.
6607:.
6584:.
6574:.
6564:.
6552:.
6548:.
6525:.
6515:.
6505:20
6503:.
6499:.
6476:.
6468:.
6460:.
6448:.
6425:.
6417:.
6407:28
6405:.
6382:.
6370:66
6368:.
6364:.
6341:.
6331:.
6319:.
6315:.
6303:^
6289:.
6279:83
6277:.
6254:.
6246:.
6234:.
6211:.
6197:.
6193:.
6170:.
6160:.
6150:18
6148:.
6144:.
6121:.
6107:.
6103:.
6080:.
6066:.
6062:.
6037:59
6035:.
6022:^
6008:.
6000:.
5988:.
5965:.
5957:.
5943:.
5939:.
5916:.
5908:.
5896:.
5873:.
5859:.
5855:.
5832:.
5822:.
5812:24
5810:.
5806:.
5783:.
5775:.
5765:49
5763:.
5740:.
5730:.
5716:.
5712:.
5688:.
5674:.
5670:.
5647:.
5639:.
5627:.
5604:.
5594:.
5584:26
5582:.
5578:.
5555:.
5545:.
5535:16
5533:.
5529:.
5506:.
5492:.
5488:.
5465:.
5455:28
5453:.
5430:.
5418:.
5404:^
5390:.
5376:.
5372:.
5360:^
5346:.
5332:.
5328:.
5305:.
5295:.
5285:22
5283:.
5279:.
5256:.
5246:28
5244:.
5221:.
5207:.
5203:.
5180:.
5172:.
5160:.
5148:^
5134:.
5124:68
5122:.
5099:.
5085:.
5081:.
5058:.
5050:.
5042:.
5030:.
5007:.
4997:65
4995:.
4972:.
4964:.
4952:.
4929:.
4915:.
4911:.
4888:.
4878:.
4868:25
4866:.
4862:.
4839:.
4827:.
4804:.
4794:.
4782:.
4778:.
4755:.
4745:.
4733:.
4729:.
4706:.
4698:.
4686:.
4663:.
4651:.
4628:.
4618:.
4610:.
4598:.
4594:.
4582:^
4568:.
4558:25
4556:.
4544:^
4530:.
4520:.
4508:.
4504:.
4481:.
4469:.
4446:.
4438:.
4430:.
4420:25
4418:.
4395:.
4381:.
4377:.
4354:.
4346:.
4334:.
4311:.
4299:34
4297:.
4293:.
4270:.
4260:.
4250:19
4248:.
4244:.
4221:.
4207:.
4203:.
4180:.
4168:.
4145:.
4133:42
4131:.
4127:.
4104:.
4096:.
4086:10
4084:.
4061:.
4051:83
4049:.
4026:.
4014:14
4012:.
4008:.
3985:.
3973:18
3971:.
3967:.
3944:.
3930:.
3926:.
3903:.
3893:.
3883:67
3881:.
3877:.
3854:.
3844:.
3834:20
3832:.
3828:.
3805:.
3795:.
3785:.
3773:.
3769:.
3746:.
3736:.
3726:.
3714:.
3710:.
3696:^
3682:.
3672:.
3664:.
3652:.
3648:.
3632:^
3618:.
3608:.
3598:.
3586:.
3582:.
3559:.
3551:.
3541:23
3539:.
3516:.
3506:.
3496:.
3484:.
3480:.
3466:^
3452:.
3442:.
3428:.
3424:.
3401:.
3391:.
3383:.
3371:.
3367:.
3355:^
3341:.
3331:.
3321:.
3309:.
3305:.
3293:^
3279:.
3271:.
3263:.
3251:.
3228:.
3218:.
3208:24
3206:.
3202:.
3184:^
3170:.
3158:20
3156:.
3152:.
3136:^
3122:.
3108:.
3104:.
3081:.
3073:.
3063:15
3061:.
3038:.
3028:.
3018:23
3016:.
3012:.
2989:.
2979:.
2969:14
2967:.
2963:.
2951:^
2937:.
2923:.
2919:.
2907:^
2893:.
2885:.
2875:31
2873:.
2869:.
2854:^
2840:.
2832:.
2822:40
2820:.
2775:.
2765:.
2755:27
2753:.
2749:.
2735:^
2721:.
2711:.
2701:41
2699:.
2695:.
2681:^
2663:.
2647:^
2633:.
2623:.
2611:.
2607:.
2575:^
2561:.
2553:.
2541:.
2518:.
2506:.
2483:.
2471:12
2469:.
2465:.
2442:.
2428:.
2424:.
2401:.
2391:.
2381:96
2379:.
2375:.
2360:.
2352:.
2340:.
2317:.
2309:.
2299:35
2297:.
2274:.
2262:12
2260:.
2256:.
2233:.
2223:.
2213:.
2203:74
2201:.
2197:.
2174:.
2164:81
2162:.
2139:.
2129:.
2119:.
2109:74
2107:.
2103:.
2069:^
2055:.
2047:.
2035:66
2033:.
2029:.
2006:.
1992:.
1988:.
1963:.
1953:.
1935:^
1868:,
1844:,
1840:,
1832:,
1816:,
1812:,
1808:,
1804:,
1800:,
1796:,
1764:,
1760:,
1756:,
1752:,
1720:,
1712:,
1704:,
1697:.
1685:,
1638:.
1558:.
1433:.
1344:.
1301:.
1249:,
1237:.
1132:.
1062:.
544:.
340:.
189:.
181:,
158:.
131:.
69:.
61:.
8922:e
8915:t
8908:v
8596:e
8589:t
8582:v
8409:e
8402:t
8395:v
8333::
8312:.
8298::
8277:.
8263::
8242:.
8228::
8202:.
8178::
8172:2
8147:.
8119:.
8099::
8093:3
8076:.
8064::
8058:8
8041:.
8021::
7998:.
7976::
7949:.
7927::
7900:.
7888::
7865:.
7845::
7839:3
7822:.
7802::
7779:.
7759::
7733:.
7713::
7687:.
7673::
7646:.
7624::
7597:.
7585::
7559:.
7537::
7510:.
7488::
7461:.
7439::
7412:.
7388::
7382:7
7361:.
7341::
7318:.
7304::
7298:2
7277:.
7255::
7228:.
7214::
7204::
7176:.
7162::
7135:.
7113::
7086:.
7064::
7058:3
7037:.
7023::
6996:.
6976::
6952:.
6938::
6911:.
6899::
6875:.
6863::
6857:3
6840:.
6818::
6812:3
6791:.
6771::
6748:.
6734::
6707:.
6693::
6667:.
6643:.
6623::
6615::
6592:.
6568::
6560::
6533:.
6511::
6484:.
6464::
6456::
6433:.
6413::
6390:.
6376::
6349:.
6327::
6297:.
6285::
6262:.
6242::
6219:.
6205::
6178:.
6156::
6129:.
6115::
6109:5
6088:.
6074::
6068:6
6047:.
6016:.
5996::
5973:.
5951::
5924:.
5904::
5898:5
5881:.
5867::
5840:.
5818::
5791:.
5771::
5748:.
5724::
5696:.
5682::
5676:4
5655:.
5635::
5612:.
5590::
5563:.
5541::
5514:.
5500::
5494:5
5473:.
5461::
5438:.
5426::
5398:.
5384::
5354:.
5340::
5334:4
5313:.
5291::
5264:.
5252::
5229:.
5215::
5188:.
5168::
5162:4
5142:.
5130::
5107:.
5093::
5066:.
5046::
5038::
5015:.
5003::
4980:.
4968::
4960::
4937:.
4923::
4896:.
4874::
4847:.
4835::
4812:.
4790::
4784:8
4763:.
4741::
4735:7
4714:.
4694::
4688:2
4671:.
4659::
4636:.
4614::
4606::
4576:.
4564::
4538:.
4516::
4489:.
4477::
4471:8
4454:.
4434::
4426::
4403:.
4389::
4362:.
4342::
4336:7
4319:.
4305::
4278:.
4256::
4229:.
4215::
4188:.
4176::
4153:.
4139::
4112:.
4092::
4069:.
4057::
4034:.
4020::
3993:.
3979::
3952:.
3938::
3932:7
3911:.
3889::
3862:.
3840::
3813:.
3789::
3781::
3754:.
3730::
3722::
3690:.
3668::
3660::
3654:9
3626:.
3602::
3594::
3567:.
3547::
3524:.
3500::
3492::
3460:.
3436::
3409:.
3387::
3379::
3349:.
3325::
3317::
3287:.
3267::
3259::
3236:.
3214::
3178:.
3164::
3130:.
3116::
3089:.
3069::
3046:.
3024::
2997:.
2975::
2945:.
2931::
2901:.
2881::
2848:.
2828::
2805:.
2783:.
2761::
2729:.
2707::
2675:.
2665:3
2641:.
2619::
2569:.
2557::
2549::
2526:.
2514::
2491:.
2477::
2450:.
2436::
2409:.
2387::
2368:.
2348::
2325:.
2305::
2282:.
2268::
2241:.
2217::
2209::
2182:.
2170::
2147:.
2123::
2115::
2088:.
2063:.
2041::
2014:.
2000::
1973:.
1836:(
1792:(
1269:—
1073:1
1071:G
755:B
526:.
365:(
352:.
317:S
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.