783:
790:
A liquid mixture containing two components is called a binary mixture. When a binary mixture is distilled, complete separation of the two components is rarely achieved. Typically, the overhead fraction from the distillation column consists predominantly of the more volatile component and some small
794:
A liquid mixture containing many components is called a multi-component mixture. When a multi-component mixture is distilled, the overhead fraction and the bottoms fraction typically contain much more than one or two components. For example, some intermediate products in an
1006:
227:
1034:
have been correlated empirically or theoretically in terms of temperature, pressure and phase compositions in the form of equations, tables or graph such as the well-known
791:
amount of the less volatile component and the bottoms fraction consists predominantly of the less volatile component and some small amount of the more volatile component.
777:
755:= 1 and separation of the two by distillation would be impossible under the given conditions because the compositions of the liquid and the vapor phase are the same (
753:
733:
671:
260:
49:
559:
499:
446:
389:
332:
631:
1058:
1032:
711:
691:
651:
603:
521:
468:
415:
358:
302:
282:
872:
1216:
111:
1571:
1290:
1567:
585:
When their liquid concentrations are equal, more volatile components have higher vapor pressures than less volatile components. Thus, a
1178:
1060:
values are widely used in the design of large-scale distillation columns for distilling multi-component mixtures in oil refineries,
1121:
863:(or a higher vapor pressure) and a heavier component means a component with a higher boiling point (or a lower vapor pressure).
1577:
1393:
833:
An overhead fraction containing predominantly the more volatile components ranging from methane (having one carbon atom) to
829:
having ten carbon atoms. For distilling such a mixture, the distillation column may be designed (for example) to produce:
1597:
1581:
1283:
1239:
1187:
1157:
31:
components from the less volatile components in a mixture. By convention, relative volatility is usually denoted as
1484:
851:
The designer would designate the key components governing the separation design to be propane as the so-called
23:
of the components in a liquid mixture of chemicals. This quantity is widely used in designing large industrial
1542:
1446:
1276:
337:
1355:
1462:
1011:
Large-scale industrial distillation is rarely undertaken if the relative volatility is less than 1.05.
866:
Thus, for the distillation of any multi-component mixture, the relative volatility is often defined as
1370:
1338:
54:
Relative volatilities are used in the design of all types of distillation processes as well as other
27:
processes. In effect, it indicates the ease or difficulty of using distillation to separate the more
1511:
1506:
1467:
1111:
1086:
1081:
73:
Relative volatilities are not used in separation or absorption processes that involve components
1530:
1420:
1126:
1069:
59:
1209:
1602:
1523:
1518:
1496:
1441:
1348:
1328:
762:
738:
718:
656:
245:
34:
782:
1405:
1388:
1299:
1096:
530:
477:
424:
367:
310:
28:
8:
1501:
1479:
1365:
1323:
1148:
1091:
608:
1489:
1380:
1313:
1197:
1043:
1017:
696:
676:
636:
588:
506:
453:
400:
343:
287:
267:
55:
840:
A bottoms fraction containing predominantly the less volatile components ranging from
1535:
1415:
1343:
1333:
1235:
1183:
1153:
1106:
74:
67:
1474:
1360:
1035:
82:
1436:
1116:
735:
is a unitless quantity. When the volatilities of both key components are equal,
1001:{\displaystyle \alpha ={\frac {(y_{LK}/x_{LK})}{(y_{HK}/x_{HK})}}=K_{LK}/K_{HK}}
1547:
1065:
564:
78:
20:
1591:
1400:
1101:
1061:
860:
394:
779:
increases above 1, separation by distillation becomes progressively easier.
796:
24:
222:{\displaystyle \alpha ={\frac {(y_{i}/x_{i})}{(y_{j}/x_{j})}}=K_{i}/K_{j}}
811:
786:
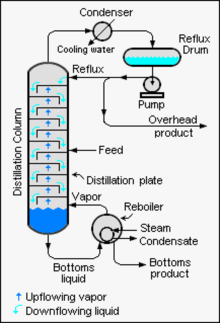
Schematic diagram of a typical large-scale industrial distillation column
693:
value of the more volatile component is in the numerator and the smaller
98:
1410:
1268:
859:. In that context, a lighter component means a component with a lower
1318:
1175:
841:
756:
102:
834:
815:
848:
Such a distillation column is typically called a depropanizer.
826:
819:
808:
804:
800:
1229:
63:
822:
503:= the vapor–liquid equilibrium concentration of component
450:= the vapor–liquid equilibrium concentration of component
844:(having four carbon atoms) to decanes (ten carbon atoms).
799:
are multi-component liquid mixtures that may contain the
264:= the relative volatility of the more volatile component
1574:(scroll down to: 2.2.3 K-values and Relative Volatility)
77:
with each other (for example, the absorption of gaseous
713:
of the less volatile component is in the denominator.
653:
value for a less volatile component. That means that
1046:
1020:
875:
765:
741:
721:
699:
679:
659:
639:
611:
591:
533:
509:
480:
456:
427:
403:
370:
346:
313:
290:
270:
248:
114:
37:
1052:
1026:
1000:
771:
747:
727:
705:
685:
665:
645:
625:
597:
553:
515:
493:
462:
440:
409:
383:
352:
326:
296:
276:
254:
221:
43:
633:) for a more volatile component is larger than a
93:For a liquid mixture of two components (called a
1589:
1145:
1572:Norwegian University of Science and Technology
1230:Seader, J. D. & Henley, Ernest J. (1998).
1176:Perry, R.H. and Green, D.W. (Editors) (1997).
1284:
1122:Equilibrium flash of a multi-component liquid
1570:by Ivar J. Halvorsen and Sigurd Skogestad,
1223:
231:
1291:
1277:
1215:CS1 maint: multiple names: authors list (
62:processes that involve the contacting of
781:
105:, the relative volatility is defined as
1298:
1171:
1169:
1590:
1272:
1139:
1584:(scroll down to Relative Volatility)
1179:Perry's Chemical Engineers' Handbook
1166:
13:
14:
1614:
1582:University of Newcastle upon Tyne
1561:
1256:Chem. Eng. Prog. Symposium Series
66:and liquid phases in a series of
576:vapor-liquid distribution ratio
393:= the vapor–liquid equilibrium
284:to the less volatile component
1248:
958:
924:
919:
885:
855:and isobutane as the so-called
548:
534:
185:
157:
152:
124:
1:
1232:Separation Process Principles
1182:(7th ed.). McGraw-Hill.
1152:(1st ed.). McGraw-Hill.
1132:
1072:plants and other industries.
88:
7:
1075:
837:(having three carbon atoms)
673:≥ 1 since the larger
527:
474:
421:
364:
340:mole fraction of component
307:
242:
234:
19:is a measure comparing the
10:
1619:
1598:Engineering thermodynamics
1254:DePriester, C. L. (1953),
567:constant (also called the
562:
502:
449:
392:
335:
263:
1455:
1429:
1379:
1371:Thermodynamic equilibrium
1306:
1146:Kister, Henry Z. (1992).
1524:Distribution coefficient
1468:Hammett acidity function
1447:Liquid–liquid extraction
1356:Le Chatelier's principle
338:vapor–liquid equilibrium
81:in aqueous solutions of
1578:Distillation Principals
1087:Fractional distillation
1082:Continuous distillation
772:{\displaystyle \alpha }
748:{\displaystyle \alpha }
728:{\displaystyle \alpha }
666:{\displaystyle \alpha }
255:{\displaystyle \alpha }
44:{\displaystyle \alpha }
1485:Coordination complexes
1421:Thermodynamic activity
1127:Volatility (chemistry)
1070:natural gas processing
1054:
1028:
1002:
787:
773:
749:
729:
707:
687:
667:
647:
627:
599:
555:
517:
495:
464:
442:
411:
385:
354:
328:
298:
278:
256:
223:
45:
1497:Dissociation constant
1442:Equilibrium unfolding
1329:Equilibrium chemistry
1055:
1029:
1003:
785:
774:
750:
730:
708:
688:
668:
648:
628:
600:
556:
554:{\displaystyle (y/x)}
518:
496:
494:{\displaystyle x_{j}}
465:
443:
441:{\displaystyle y_{j}}
412:
386:
384:{\displaystyle x_{i}}
355:
329:
327:{\displaystyle y_{i}}
299:
279:
257:
224:
46:
1406:Predominance diagram
1389:Equilibrium constant
1112:McCabe–Thiele method
1097:Fractionation column
1044:
1018:
873:
763:
759:). As the value of
739:
719:
697:
677:
657:
637:
609:
589:
531:
523:in the liquid phase
507:
478:
454:
425:
417:in the liquid phase
401:
368:
344:
311:
288:
268:
246:
112:
35:
1568:Distillation Theory
1480:Binding selectivity
1456:Specific equilibria
1366:Reversible reaction
1324:Dynamic equilibrium
1300:Chemical equilibria
1234:. New York: Wiley.
1149:Distillation Design
1092:Vacuum distillation
626:{\displaystyle y/x}
470:in the vapor phase
360:in the vapor phase
17:Relative volatility
1490:Macrocyclic effect
1314:Chemical stability
1208:has generic name (
1050:
1024:
998:
788:
769:
745:
725:
703:
683:
663:
643:
623:
595:
551:
513:
491:
460:
438:
407:
381:
350:
324:
294:
274:
252:
219:
68:equilibrium stages
41:
1580:by Ming T. Tham,
1558:
1557:
1536:Common-ion effect
1463:Acid dissociation
1416:Reaction quotient
1334:Equilibrium stage
1107:Theoretical plate
1053:{\displaystyle K}
1036:DePriester charts
1027:{\displaystyle K}
962:
706:{\displaystyle K}
686:{\displaystyle K}
646:{\displaystyle K}
598:{\displaystyle K}
583:
582:
579:) of a component
516:{\displaystyle j}
463:{\displaystyle j}
410:{\displaystyle i}
353:{\displaystyle i}
297:{\displaystyle j}
277:{\displaystyle i}
189:
1610:
1475:Binding constant
1361:Phase separation
1293:
1286:
1279:
1270:
1269:
1263:
1252:
1246:
1245:
1227:
1221:
1220:
1213:
1207:
1203:
1201:
1193:
1173:
1164:
1163:
1143:
1059:
1057:
1056:
1051:
1033:
1031:
1030:
1025:
1007:
1005:
1004:
999:
997:
996:
984:
979:
978:
963:
961:
957:
956:
944:
939:
938:
922:
918:
917:
905:
900:
899:
883:
778:
776:
775:
770:
754:
752:
751:
746:
734:
732:
731:
726:
712:
710:
709:
704:
692:
690:
689:
684:
672:
670:
669:
664:
652:
650:
649:
644:
632:
630:
629:
624:
619:
604:
602:
601:
596:
560:
558:
557:
552:
544:
522:
520:
519:
514:
500:
498:
497:
492:
490:
489:
469:
467:
466:
461:
447:
445:
444:
439:
437:
436:
416:
414:
413:
408:
390:
388:
387:
382:
380:
379:
359:
357:
356:
351:
333:
331:
330:
325:
323:
322:
303:
301:
300:
295:
283:
281:
280:
275:
261:
259:
258:
253:
232:
228:
226:
225:
220:
218:
217:
208:
203:
202:
190:
188:
184:
183:
174:
169:
168:
155:
151:
150:
141:
136:
135:
122:
83:sodium hydroxide
50:
48:
47:
42:
1618:
1617:
1613:
1612:
1611:
1609:
1608:
1607:
1588:
1587:
1564:
1559:
1554:
1507:Self-ionization
1451:
1437:Buffer solution
1425:
1375:
1302:
1297:
1267:
1266:
1253:
1249:
1242:
1228:
1224:
1214:
1205:
1204:
1195:
1194:
1190:
1174:
1167:
1160:
1144:
1140:
1135:
1117:Fenske equation
1078:
1066:chemical plants
1045:
1042:
1041:
1019:
1016:
1015:
989:
985:
980:
971:
967:
949:
945:
940:
931:
927:
923:
910:
906:
901:
892:
888:
884:
882:
874:
871:
870:
764:
761:
760:
740:
737:
736:
720:
717:
716:
698:
695:
694:
678:
675:
674:
658:
655:
654:
638:
635:
634:
615:
610:
607:
606:
590:
587:
586:
540:
532:
529:
528:
508:
505:
504:
485:
481:
479:
476:
475:
455:
452:
451:
432:
428:
426:
423:
422:
402:
399:
398:
375:
371:
369:
366:
365:
345:
342:
341:
318:
314:
312:
309:
308:
289:
286:
285:
269:
266:
265:
247:
244:
243:
213:
209:
204:
198:
194:
179:
175:
170:
164:
160:
156:
146:
142:
137:
131:
127:
123:
121:
113:
110:
109:
91:
36:
33:
32:
21:vapor pressures
12:
11:
5:
1616:
1606:
1605:
1600:
1586:
1585:
1575:
1563:
1562:External links
1560:
1556:
1555:
1553:
1552:
1551:
1550:
1540:
1539:
1538:
1528:
1527:
1526:
1516:
1515:
1514:
1504:
1499:
1494:
1493:
1492:
1482:
1477:
1472:
1471:
1470:
1459:
1457:
1453:
1452:
1450:
1449:
1444:
1439:
1433:
1431:
1427:
1426:
1424:
1423:
1418:
1413:
1408:
1403:
1398:
1397:
1396:
1385:
1383:
1377:
1376:
1374:
1373:
1368:
1363:
1358:
1353:
1352:
1351:
1346:
1336:
1331:
1326:
1321:
1316:
1310:
1308:
1304:
1303:
1296:
1295:
1288:
1281:
1273:
1265:
1264:
1247:
1240:
1222:
1188:
1165:
1158:
1137:
1136:
1134:
1131:
1130:
1129:
1124:
1119:
1114:
1109:
1104:
1099:
1094:
1089:
1084:
1077:
1074:
1049:
1023:
1014:The values of
1009:
1008:
995:
992:
988:
983:
977:
974:
970:
966:
960:
955:
952:
948:
943:
937:
934:
930:
926:
921:
916:
913:
909:
904:
898:
895:
891:
887:
881:
878:
857:heavy key (HK)
853:light key (LK)
846:
845:
838:
768:
744:
724:
702:
682:
662:
642:
622:
618:
614:
594:
581:
580:
561:
550:
547:
543:
539:
536:
525:
524:
512:
501:
488:
484:
472:
471:
459:
448:
435:
431:
419:
418:
406:
391:
378:
374:
362:
361:
349:
334:
321:
317:
305:
304:
293:
273:
262:
251:
240:
239:
236:
230:
229:
216:
212:
207:
201:
197:
193:
187:
182:
178:
173:
167:
163:
159:
154:
149:
145:
140:
134:
130:
126:
120:
117:
95:binary mixture
90:
87:
79:carbon dioxide
40:
9:
6:
4:
3:
2:
1615:
1604:
1601:
1599:
1596:
1595:
1593:
1583:
1579:
1576:
1573:
1569:
1566:
1565:
1549:
1546:
1545:
1544:
1541:
1537:
1534:
1533:
1532:
1529:
1525:
1522:
1521:
1520:
1517:
1513:
1510:
1509:
1508:
1505:
1503:
1500:
1498:
1495:
1491:
1488:
1487:
1486:
1483:
1481:
1478:
1476:
1473:
1469:
1466:
1465:
1464:
1461:
1460:
1458:
1454:
1448:
1445:
1443:
1440:
1438:
1435:
1434:
1432:
1428:
1422:
1419:
1417:
1414:
1412:
1409:
1407:
1404:
1402:
1401:Phase diagram
1399:
1395:
1394:determination
1392:
1391:
1390:
1387:
1386:
1384:
1382:
1378:
1372:
1369:
1367:
1364:
1362:
1359:
1357:
1354:
1350:
1347:
1345:
1342:
1341:
1340:
1337:
1335:
1332:
1330:
1327:
1325:
1322:
1320:
1317:
1315:
1312:
1311:
1309:
1305:
1301:
1294:
1289:
1287:
1282:
1280:
1275:
1274:
1271:
1261:
1257:
1251:
1243:
1241:0-471-58626-9
1237:
1233:
1226:
1218:
1211:
1206:|author=
1199:
1191:
1189:0-07-049841-5
1185:
1181:
1180:
1172:
1170:
1161:
1159:0-07-034909-6
1155:
1151:
1150:
1142:
1138:
1128:
1125:
1123:
1120:
1118:
1115:
1113:
1110:
1108:
1105:
1103:
1102:Phase diagram
1100:
1098:
1095:
1093:
1090:
1088:
1085:
1083:
1080:
1079:
1073:
1071:
1067:
1063:
1062:petrochemical
1047:
1039:
1037:
1021:
1012:
993:
990:
986:
981:
975:
972:
968:
964:
953:
950:
946:
941:
935:
932:
928:
914:
911:
907:
902:
896:
893:
889:
879:
876:
869:
868:
867:
864:
862:
861:boiling point
858:
854:
849:
843:
839:
836:
832:
831:
830:
828:
824:
821:
817:
814:ranging from
813:
810:
806:
802:
798:
792:
784:
780:
766:
758:
742:
722:
714:
700:
680:
660:
640:
620:
616:
612:
592:
578:
577:
572:
571:
566:
545:
541:
537:
526:
510:
486:
482:
473:
457:
433:
429:
420:
404:
397:of component
396:
395:mole fraction
376:
372:
363:
347:
339:
319:
315:
306:
291:
271:
249:
241:
237:
233:
214:
210:
205:
199:
195:
191:
180:
176:
171:
165:
161:
147:
143:
138:
132:
128:
118:
115:
108:
107:
106:
104:
100:
97:) at a given
96:
86:
84:
80:
76:
71:
69:
65:
61:
57:
52:
38:
30:
26:
22:
18:
1603:Distillation
1543:Vapor–liquid
1430:Applications
1262:, pages 1-43
1259:
1255:
1250:
1231:
1225:
1177:
1147:
1141:
1040:
1013:
1010:
865:
856:
852:
850:
847:
812:hydrocarbons
797:oil refinery
793:
789:
715:
584:
575:
574:
569:
568:
94:
92:
72:
53:
25:distillation
16:
15:
1548:Henry's law
1339:Free energy
818:having one
565:Henry's law
99:temperature
1592:Categories
1531:Solubility
1502:Hydrolysis
1411:Phase rule
1133:References
89:Definition
60:absorption
56:separation
1519:Partition
1349:Helmholtz
1319:Chelation
1198:cite book
877:α
842:isobutane
767:α
757:azeotrope
743:α
723:α
661:α
605:value (=
250:α
116:α
39:α
1512:of water
1307:Concepts
1076:See also
103:pressure
75:reacting
29:volatile
835:propane
827:decanes
816:methane
570:K value
238:
235:where:
1381:Models
1238:
1186:
1156:
820:carbon
809:alkyne
805:alkene
801:alkane
336:= the
1344:Gibbs
1258:, 7,
64:vapor
1236:ISBN
1217:link
1210:help
1184:ISBN
1154:ISBN
1064:and
823:atom
807:and
101:and
825:to
573:or
85:).
58:or
1594::
1260:49
1202::
1200:}}
1196:{{
1168:^
1068:,
1038:.
803:,
563:=
70:.
51:.
1292:e
1285:t
1278:v
1244:.
1219:)
1212:)
1192:.
1162:.
1048:K
1022:K
994:K
991:H
987:K
982:/
976:K
973:L
969:K
965:=
959:)
954:K
951:H
947:x
942:/
936:K
933:H
929:y
925:(
920:)
915:K
912:L
908:x
903:/
897:K
894:L
890:y
886:(
880:=
701:K
681:K
641:K
621:x
617:/
613:y
593:K
549:)
546:x
542:/
538:y
535:(
511:j
487:j
483:x
458:j
434:j
430:y
405:i
377:i
373:x
348:i
320:i
316:y
292:j
272:i
215:j
211:K
206:/
200:i
196:K
192:=
186:)
181:j
177:x
172:/
166:j
162:y
158:(
153:)
148:i
144:x
139:/
133:i
129:y
125:(
119:=
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.