378:
sceptical of the original images. There were also immediate arguments as to the organisation of the filaments, whether the two sets (myosin and actin) of filaments were merely overlapping or continuous. It was only with the new electron microscope that Hugh Huxley confirmed the overlapping nature of the filaments in 1957. It was also from this publication that the existence of actin-myosin linkage (now called cross-bridge) was clearly shown. But he took another five years to provide evidence that the cross-bridge was a dynamic interaction between actin and myosin filaments. He obtained the actual molecular arrangement of the filaments using X-ray crystallography by teaming up with
127:
179:, he soon found that myosin B was associated with another protein, which they called actin, while myosin A was not. Straub purified actin in 1942, and Szent-Györgyi purified myosin A in 1943. It became apparent that myosin B was a combination of myosin A and actin, so that myosin A retained the original name, whereas they renamed myosin B as actomyosin. By the end of the 1940s Szent-Györgyi's team had postulated with evidence that contraction of actomyosin was equivalent to muscle contraction as a whole. But the notion was generally opposed, even from the likes of Nobel laureates such as
120:
390:, where the theory and its evidence were deliberated, that it became generally accepted. At the conference, as Koscak Maruyama later recalled, Hanson had to answer the criticisms by shouting, "I know I cannot explain the mechanism yet, but the sliding is a fact." The factual proofs came in the early 1980s when it could be demonstrated the actual sliding motion using novel sophisticated tools by different researchers.
300:
38:
405:
under the title "The
Mechanism of Muscular Contraction". According to his theory, filament sliding occurs by cyclic attachment and detachment of myosin on actin filaments. Contraction occurs when the myosin pulls the actin filament towards the centre of the A band, detaches from actin and creates a
398:
With substantial evidence, Hugh Huxley formally proposed the mechanism for sliding filament which is variously called swinging cross-bridge model, cross-bridge theory or cross-bridge model. (He himself preferred the name "swinging crossbridge model", because, as he recalled, "it was, after all, the
290:
at Woods Hole, Massachusetts, to use electron microscope there. There he met Hugh Huxley and Hanson with whom he shared data and information on their works. They parted with an agreement that they would keep in touch, and when their aim is achieved, they would publish together, if they ever "reached
235:
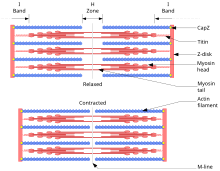
to study the details of those filaments as never done before. They soon discovered and confirmed the filament nature of muscle proteins. Myosin and actin form overlapping filaments, myosin filaments mainly constituting the A band (the dark region of a sarcomere), while actin filaments traverse both
338:
The second paper, by Hugh Huxley and Jean Hanson, is titled "Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation". It is more elaborate and was based on their study of rabbit muscle using phase contrast and electron microscopes. According to
172:. He demonstrated in 1942 that ATP was the source of energy for muscle contraction. He actually observed that muscle fibres containing myosin B shortened in the presence of ATP, but not with myosin A, the experience which he later described as "perhaps the most thrilling moment of my life." With
377:
In spite of strong evidence, the sliding filament theory did not gain any support for several years to come. Szent-Györgyi himself refused to believe that myosin filaments were confined to the thick filament (A band). F.O. Schmitt, whose electron microscope provided the best data, also remained
107:
Before the 1950s there were several competing theories on muscle contraction, including electrical attraction, protein folding, and protein modification. The novel theory directly introduced a new concept called cross-bridge theory (classically swinging cross-bridge, now mostly referred to as
196:
187:, who adhered to the prevailing dogma that myosin was a structural protein and not a functional enzyme. However, in one of his last contributions to muscle research, Szent-Györgyi demonstrated that actomyosin driven by ATP was the basic principle of muscle contraction.
321:
The first paper, written by Andrew Huxley and Rolf
Niedergerke, is titled "Interference microscopy of living muscle fibres". It was based on their study of frog muscle using interference microscope, which Andrew Huxley developed for the purpose. According to them:
116:) by attachment of myosin head on the actin filament, thereby forming a sort of cross-bridge between the two filaments. The sliding filament theory is a widely accepted explanation of the mechanism that underlies muscle contraction.
313:
under the common theme "Structural
Changes in Muscle During Contraction". Though their conclusions were fundamentally similar, their underlying experimental data and propositions were different.
148:, who extracted and named it in 1864. In 1939 a Russian husband and wife team Vladimir Alexandrovich Engelhardt and Militsa Nikolaevna Lyubimova discovered that myosin had an enzymatic (called
343:
the backbone of a muscle fibre is actin filaments which extend from the Z line up to one end of the H zone, where they are attached to an elastic component which they named "S filament";
361:
during contraction, actin filaments move into the A bands and the H zone is filled up reducing its stretch, the I bands shorten, the Z line comes in contact with the A bands; and
278:. Between March 1953 and January 1954 they executed their research. Huxley recollected that at the time the only person who ever thought of sliding filaments before 1953 was
207:
earned his PhD from the
University of Cambridge in 1952 on his research on the structure of muscle, Szent-Györgyi had turned his career into cancer research. Huxley went to
1614:
Yanagida, Toshio; Arata, Toshiaki; Oosawa, Fumio (1985). "Sliding distance of actin filament induced by a myosin crossbridge during one ATP hydrolysis cycle".
266:, and was looking for an associate who could properly dissect out muscle fibres. Upon recommendation of a close friend Robert Stämpfli, a German physician
112:) which explains the molecular mechanism of sliding filament. Cross-bridge theory states that actin and myosin form a protein complex (classically called
248:
Later, in 1996, Huxley regretted that he should have included Hanson in the formulation of his theory because it was based on their collaborative work.
240:"… f it is postulated that stretching of the muscle takes place, not by an extension of the filaments, but by a process in which the two sets of
1148:
Huxley, H.; Hanson, J. (1954). "Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation".
406:
force (stroke) to bind to the next actin molecule. This idea was subsequently proven in detail, and is more appropriately known as the
17:
262:) transmission (for which he and Hodgkin later won the Nobel Prize in Physiology or Medicine in 1963) in 1949 using his own design of
104:. It was originally conceived by Hugh Huxley in 1953. Andrew Huxley and Niedergerke introduced it as a "very attractive" hypothesis.
1332:
Huxley, HE (1963). "Electron microscope studies on the structure of natural and synthetic protein filaments from striated muscle".
651:
616:
435:
212:
161:
101:
57:
based on muscle proteins that slide past each other to generate movement. According to the sliding filament theory, the
130:
This model shows the four main and significant steps of the sliding filament theory as well as with a detailed visual.
355:
myosin and actin filaments lie side by side in the A band and in the absence of ATP they do not form cross-linkages;
236:
the A and I (light region) bands. Huxley was the first to suggest the sliding filament theory in 1953, stating:
215:
with a post-doctoral fellowship in
September 1952, where he was joined by another English post-doctoral fellow
1248:
Huxley, Hugh E. (2008). "Memories of early work on muscle contraction and regulation in the 1950s and 1960s".
387:
352:
if myosin filaments contract beyond the length of the A band, their ends fold up to form contraction bands;
287:
962:
Huxley, HE (1953). "Electron microscope studies of the organisation of the filaments in striated muscle".
258:
described as "wizard with scientific apparatus", had just discovered the mechanism of the nerve impulse (
346:
myosin filaments extend from one end of the A band through the H zone up to the other end of the A band;
307:
The sliding filament theory was born from two consecutive papers published on the 22 May 1954 issue of
271:
77:) during muscle contraction, while the two groups of filaments remain at relatively constant length.
1832:
270:
joined him at the
University of Cambridge in 1952. By then he realised that the conventionally used
1876:
283:
244:
past each other; extensibility will then be inhibited if the myosin and actin are linked together."
220:
358:
during stretching, only the I bands and H zone increase in length, while A bands remain the same;
326:
the I bands are composed of actin filaments, and the A bands principally of myosin filaments; and
275:
227:
to speculate that muscle proteins, particularly myosin, form structured filaments giving rise to
89:
157:
1886:
1827:
153:
173:
911:
Hanson, Jean; Huxley, Hugh E. (1953). "Structural basis of the cross-striations in muscle".
1733:
1678:
1623:
1523:
1380:
1157:
1048:
920:
725:
555:
349:
myosin filaments remain in relatively constant length during muscle stretch or contraction;
329:
during contraction, the actin filaments move into the A bands between the myosin filaments.
126:
1491:
1474:
199:
Structure of muscle fibre (sarcomere) under electron microscope with schematic explanation
8:
1881:
546:
Huxley, A.F.; Niedergerke, R. (1954). "Interference microscopy of living muscle fibres".
232:
180:
80:
The theory was independently introduced in 1954 by two research teams, one consisting of
1737:
1682:
1627:
1527:
1384:
1161:
1052:
1013:
924:
777:
760:
729:
559:
1853:
1800:
1757:
1647:
1547:
1450:
1425:
1406:
1309:
1284:
1225:
1200:
1181:
1125:
1100:
944:
870:
826:
801:
741:
693:
668:
622:
579:
528:
503:
407:
364:
the possible driving force of contraction is the actin-myosin linkages which depend on
208:
109:
54:
1591:
1566:
1345:
1845:
1792:
1749:
1706:
1701:
1666:
1639:
1596:
1539:
1496:
1455:
1398:
1349:
1314:
1265:
1230:
1173:
1130:
1066:
1017:
979:
975:
936:
878:
831:
782:
698:
647:
612:
571:
479:
474:
457:
431:
401:
383:
224:
1857:
1841:
1761:
1551:
626:
160:, a Hungarian physiologist, turned his focus on muscle physiology after winning the
1837:
1804:
1784:
1741:
1696:
1686:
1651:
1631:
1586:
1578:
1531:
1486:
1445:
1437:
1410:
1388:
1341:
1304:
1296:
1257:
1220:
1212:
1185:
1165:
1120:
1112:
1056:
1009:
971:
948:
928:
862:
821:
813:
772:
745:
733:
716:
688:
680:
604:
583:
563:
523:
515:
469:
309:
267:
259:
85:
1745:
1034:
274:
was not suitable for fine structures of muscle fibres, and thus developed his own
850:
641:
598:
279:
66:
1261:
379:
365:
184:
62:
608:
1870:
1116:
263:
251:
81:
74:
1216:
1849:
1818:
Fitts, R. H. (2007). "The cross-bridge cycle and skeletal muscle fatigue".
1796:
1710:
1691:
1543:
1459:
1353:
1318:
1269:
1234:
1177:
1070:
1000:
Huxley, H. E. (1996). "A personal view of muscle and motility mechanisms".
983:
940:
882:
835:
786:
702:
575:
483:
255:
169:
1753:
1643:
1600:
1500:
1441:
1402:
1134:
1021:
817:
519:
119:
1369:"Constancy of axial spacings in frog sartorius muscle during contraction"
216:
204:
145:
97:
93:
31:
1300:
1035:
Goldman, Yale E.; Franzini-Armstrong, Clara; Armstrong, Clay M. (2012).
874:
684:
1582:
1393:
1368:
1788:
1635:
1169:
932:
737:
567:
228:
165:
144:
The first muscle protein discovered was myosin by a German scientist
113:
42:
1775:
Spudich, James A. (2001). "The myosin swinging cross-bridge model".
1535:
1061:
1036:
866:
1724:
Huxley, H. E. (1969). "The
Mechanism of Muscular Contraction".
149:
58:
1514:
Geeves, Michael A. (2002). "Stretching the lever-arm theory".
430:(7th ed.). San Francisco, CA: Pearson. pp. 377–416.
399:
1960s".) He published his theory in the 20 June 1969 issue of
1475:"Birth of the sliding filament concept in muscle contraction"
802:"The early history of the biochemistry of muscle contraction"
603:. New York: Springer Science+Business Media. pp. 21–23.
70:
1101:"Discoveries on muscle: observation, theory, and experiment"
299:
195:
37:
30:"Crossbridge" redirects here. For Adobe cross-compiler, see
458:"Fifty years of muscle and the sliding filament hypothesis"
219:
in
January 1953. Hanson had a PhD in muscle structure from
1285:"The double array of filaments in cross-striated muscle"
303:
Diagrammatic explanation of sliding filament hypothesis
231:(a segment of muscle fibre). Their main aim was to use
851:"Free-energy relations and contraction of actomyosin"
545:
386:, in 1965. It was only after a conference in 1972 at
1613:
1289:
The
Journal of Biophysical and Biochemical Cytology
1250:
Biochemical and Biophysical Research Communications
715:
45:
in relaxed (above) and contracted (below) positions
643:Physiology, Biophysics, and Biomedical Engineering
1366:
640:Wood, A.W. (2012). "Skeletal muscle biophysics".
1868:
1367:Huxley, H. E.; Brown, W.; Holmes, K. C. (1965).
1147:
316:
1671:Proceedings of the National Academy of Sciences
419:
666:
294:
848:
799:
758:
372:
910:
426:Silverthorn, Dee Unglaub (2016). "Muscles".
995:
993:
425:
333:
646:. Taylor & Francis. pp. 158–162.
541:
539:
497:
495:
493:
451:
449:
447:
1831:
1700:
1690:
1590:
1490:
1449:
1392:
1308:
1224:
1124:
1060:
825:
776:
692:
527:
504:"50-Year Anniversary of Sliding Filament"
473:
393:
1472:
1201:"Memories of Hugh E. Huxley (1924-2013)"
1141:
990:
501:
428:Human Physiology: An Integrated Approach
298:
194:
125:
118:
36:
1774:
1667:"Molecular model of muscle contraction"
1564:
1426:"The sliding filament model: 1972-2004"
1198:
667:Hartman, M. A.; Spudich, J. A. (2012).
536:
490:
444:
14:
1869:
1723:
1513:
1331:
1282:
1247:
1098:
999:
961:
455:
1817:
1777:Nature Reviews Molecular Cell Biology
1492:10.1093/oxfordjournals.jbchem.a124692
1423:
213:Massachusetts Institute of Technology
162:Nobel Prize in Physiology or Medicine
102:Massachusetts Institute of Technology
1664:
1083:
1037:"Andrew Fielding Huxley (1917–2012)"
895:
669:"The myosin superfamily at a glance"
639:
596:
1014:10.1146/annurev.ph.58.030196.000245
778:10.1146/annurev.bi.32.070163.000245
633:
24:
286:). He spent the summer of 1953 at
25:
1898:
1430:The Journal of General Physiology
1086:Mechanism of Muscular Contraction
898:Mechanism of Muscular Contraction
806:The Journal of General Physiology
600:Mechanism of Muscular Contraction
508:The Journal of General Physiology
27:Explanation of muscle contraction
475:10.1111/j.1432-1033.2004.04044.x
462:European Journal of Biochemistry
1842:10.1152/japplphysiol.01200.2007
1811:
1768:
1717:
1658:
1607:
1567:"In pursuit of myosin function"
1558:
1507:
1466:
1417:
1360:
1325:
1276:
1241:
1192:
1092:
1077:
1028:
955:
904:
889:
842:
793:
761:"Lost in the twentieth century"
759:Szent-Györgyi, Albert (1963).
752:
709:
660:
590:
152:) property that can breakdown
139:
92:, and the other consisting of
13:
1:
1820:Journal of Applied Physiology
1746:10.1126/science.164.3886.1356
1346:10.1016/s0022-2836(63)80008-x
1205:Molecular Biology of the Cell
964:Biochimica et Biophysica Acta
800:Szent-Gyorgyi, A. G. (2004).
765:Annual Review of Biochemistry
413:
388:Cold Spring Harbor Laboratory
317:Huxley-Niedergerke hypothesis
1334:Journal of Molecular Biology
976:10.1016/0006-3002(53)90156-5
288:Marine Biological Laboratory
7:
1002:Annual Review of Physiology
295:The sliding filament theory
282:(later winner of the 1964
41:Sliding filament theory: A
10:
1903:
1262:10.1016/j.bbrc.2007.11.130
373:Reception and consequences
134:
53:explains the mechanism of
29:
18:Sliding filament mechanism
849:Szent-Györgyi, A (1949).
609:10.1007/978-1-4939-2007-5
272:phase-contrast microscope
223:in 1951. Huxley had used
190:
164:in 1937 for his works on
1117:10.1136/bmj.293.6539.115
502:Andersen, O. S. (2004).
456:Huxley, Hugh E. (2004).
334:Huxley-Hanson hypothesis
284:Nobel Prize in Chemistry
1665:Duke, T. A. J. (1999).
1479:Journal of Biochemistry
1217:10.1091/mbc.E13-08-0454
1105:British Medical Journal
855:The Biological Bulletin
673:Journal of Cell Science
276:interference microscope
90:University of Cambridge
51:sliding filament theory
1692:10.1073/pnas.96.6.2770
1084:Rall, Jack A. (2014).
896:Rall, Jack A. (2014).
597:Rall, Jack A. (2014).
394:Cross-bridge mechanism
304:
291:similar conclusions".
246:
221:King's College, London
200:
131:
123:
46:
1442:10.1085/jgp.200409089
1088:. pp. 30–33, 41.
818:10.1085/jgp.200409091
520:10.1085/jgp.200409079
382:, who was trained by
302:
238:
211:'s laboratory at the
198:
129:
122:
40:
1565:Spudich, JA (1989).
1473:Maruyama, K (1995).
1199:Spudich, J. (2013).
158:Albert Szent-Györgyi
1738:1969Sci...164.1356H
1732:(3886): 1356–1366.
1683:1999PNAS...96.2770D
1628:1985Natur.316..366Y
1528:2002Natur.415..129G
1385:1965Natur.206.1358H
1301:10.1083/jcb.3.5.631
1283:Huxley, HE (1957).
1162:1954Natur.173..973H
1099:Huxley, AF (1986).
1053:2012Natur.486..474G
925:1953Natur.172..530H
730:1939Natur.144..668E
560:1954Natur.173..971H
233:electron microscopy
181:Otto Fritz Meyerhof
156:to release energy.
1424:Cooke, R. (2004).
685:10.1242/jcs.094300
408:cross-bridge cycle
305:
209:Francis O. Schmitt
201:
132:
124:
110:cross-bridge cycle
55:muscle contraction
47:
1622:(6026): 366–369.
1583:10.1091/mbc.1.1.1
1522:(6868): 129–131.
1394:10.1038/2061358a0
1211:(18): 2769–2771.
1156:(4412): 973–976.
1111:(6539): 115–117.
919:(4377): 530–532.
724:(3650): 668–669.
653:978-1-46-655279-1
618:978-1-4939-2006-8
554:(4412): 971–973.
437:978-0-321-98122-6
384:Rosalind Franklin
225:X-ray diffraction
16:(Redirected from
1894:
1862:
1861:
1835:
1815:
1809:
1808:
1789:10.1038/35073086
1772:
1766:
1765:
1721:
1715:
1714:
1704:
1694:
1677:(6): 2770–2775.
1662:
1656:
1655:
1636:10.1038/316366a0
1611:
1605:
1604:
1594:
1562:
1556:
1555:
1511:
1505:
1504:
1494:
1470:
1464:
1463:
1453:
1421:
1415:
1414:
1396:
1364:
1358:
1357:
1329:
1323:
1322:
1312:
1280:
1274:
1273:
1245:
1239:
1238:
1228:
1196:
1190:
1189:
1170:10.1038/173973a0
1145:
1139:
1138:
1128:
1096:
1090:
1089:
1081:
1075:
1074:
1064:
1032:
1026:
1025:
997:
988:
987:
959:
953:
952:
933:10.1038/172530b0
908:
902:
901:
893:
887:
886:
846:
840:
839:
829:
797:
791:
790:
780:
756:
750:
749:
738:10.1038/144668b0
713:
707:
706:
696:
679:(7): 1627–1632.
664:
658:
657:
637:
631:
630:
594:
588:
587:
568:10.1038/173971a0
543:
534:
533:
531:
499:
488:
487:
477:
468:(8): 1403–1415.
453:
442:
441:
423:
268:Rolf Niedergerke
260:action potential
86:Rolf Niedergerke
21:
1902:
1901:
1897:
1896:
1895:
1893:
1892:
1891:
1877:Muscular system
1867:
1866:
1865:
1833:10.1.1.569.8211
1816:
1812:
1773:
1769:
1722:
1718:
1663:
1659:
1612:
1608:
1571:Cell Regulation
1563:
1559:
1536:10.1038/415129a
1512:
1508:
1471:
1467:
1422:
1418:
1365:
1361:
1330:
1326:
1281:
1277:
1246:
1242:
1197:
1193:
1146:
1142:
1097:
1093:
1082:
1078:
1062:10.1038/486474a
1033:
1029:
998:
991:
960:
956:
909:
905:
894:
890:
867:10.2307/1538196
847:
843:
798:
794:
757:
753:
714:
710:
665:
661:
654:
638:
634:
619:
595:
591:
544:
537:
500:
491:
454:
445:
438:
424:
420:
416:
396:
375:
336:
319:
297:
280:Dorothy Hodgkin
242:filaments slide
193:
142:
137:
69:slide past the
63:thick filaments
35:
28:
23:
22:
15:
12:
11:
5:
1900:
1890:
1889:
1884:
1879:
1864:
1863:
1826:(2): 551–558.
1810:
1783:(5): 387–392.
1767:
1716:
1657:
1606:
1557:
1506:
1465:
1436:(6): 643–656.
1416:
1379:(4991): 1358.
1359:
1340:(3): 281–308.
1324:
1295:(5): 631–648.
1275:
1240:
1191:
1140:
1091:
1076:
1027:
989:
970:(3): 387–394.
954:
903:
888:
861:(2): 140–161.
841:
812:(6): 631–641.
792:
751:
708:
659:
652:
632:
617:
589:
535:
489:
443:
436:
417:
415:
412:
395:
392:
380:Kenneth Holmes
374:
371:
370:
369:
368:by the myosin.
366:ATP hydrolysis
362:
359:
356:
353:
350:
347:
344:
335:
332:
331:
330:
327:
318:
315:
296:
293:
192:
189:
185:Archibald Hill
141:
138:
136:
133:
75:thin filaments
26:
9:
6:
4:
3:
2:
1899:
1888:
1887:Cell movement
1885:
1883:
1880:
1878:
1875:
1874:
1872:
1859:
1855:
1851:
1847:
1843:
1839:
1834:
1829:
1825:
1821:
1814:
1806:
1802:
1798:
1794:
1790:
1786:
1782:
1778:
1771:
1763:
1759:
1755:
1751:
1747:
1743:
1739:
1735:
1731:
1727:
1720:
1712:
1708:
1703:
1698:
1693:
1688:
1684:
1680:
1676:
1672:
1668:
1661:
1653:
1649:
1645:
1641:
1637:
1633:
1629:
1625:
1621:
1617:
1610:
1602:
1598:
1593:
1588:
1584:
1580:
1576:
1572:
1568:
1561:
1553:
1549:
1545:
1541:
1537:
1533:
1529:
1525:
1521:
1517:
1510:
1502:
1498:
1493:
1488:
1484:
1480:
1476:
1469:
1461:
1457:
1452:
1447:
1443:
1439:
1435:
1431:
1427:
1420:
1412:
1408:
1404:
1400:
1395:
1390:
1386:
1382:
1378:
1374:
1370:
1363:
1355:
1351:
1347:
1343:
1339:
1335:
1328:
1320:
1316:
1311:
1306:
1302:
1298:
1294:
1290:
1286:
1279:
1271:
1267:
1263:
1259:
1255:
1251:
1244:
1236:
1232:
1227:
1222:
1218:
1214:
1210:
1206:
1202:
1195:
1187:
1183:
1179:
1175:
1171:
1167:
1163:
1159:
1155:
1151:
1144:
1136:
1132:
1127:
1122:
1118:
1114:
1110:
1106:
1102:
1095:
1087:
1080:
1072:
1068:
1063:
1058:
1054:
1050:
1047:(7404): 474.
1046:
1042:
1038:
1031:
1023:
1019:
1015:
1011:
1007:
1003:
996:
994:
985:
981:
977:
973:
969:
965:
958:
950:
946:
942:
938:
934:
930:
926:
922:
918:
914:
907:
900:. p. 23.
899:
892:
884:
880:
876:
872:
868:
864:
860:
856:
852:
845:
837:
833:
828:
823:
819:
815:
811:
807:
803:
796:
788:
784:
779:
774:
770:
766:
762:
755:
747:
743:
739:
735:
731:
727:
723:
719:
712:
704:
700:
695:
690:
686:
682:
678:
674:
670:
663:
655:
649:
645:
644:
636:
628:
624:
620:
614:
610:
606:
602:
601:
593:
585:
581:
577:
573:
569:
565:
561:
557:
553:
549:
542:
540:
530:
525:
521:
517:
513:
509:
505:
498:
496:
494:
485:
481:
476:
471:
467:
463:
459:
452:
450:
448:
439:
433:
429:
422:
418:
411:
409:
404:
403:
391:
389:
385:
381:
367:
363:
360:
357:
354:
351:
348:
345:
342:
341:
340:
328:
325:
324:
323:
314:
312:
311:
301:
292:
289:
285:
281:
277:
273:
269:
265:
264:voltage clamp
261:
257:
253:
252:Andrew Huxley
249:
245:
243:
237:
234:
230:
226:
222:
218:
214:
210:
206:
197:
188:
186:
182:
178:
176:
171:
167:
163:
159:
155:
151:
147:
128:
121:
117:
115:
111:
105:
103:
99:
95:
91:
87:
83:
82:Andrew Huxley
78:
76:
72:
68:
67:muscle fibers
64:
60:
56:
52:
44:
39:
33:
19:
1823:
1819:
1813:
1780:
1776:
1770:
1729:
1725:
1719:
1674:
1670:
1660:
1619:
1615:
1609:
1574:
1570:
1560:
1519:
1515:
1509:
1482:
1478:
1468:
1433:
1429:
1419:
1376:
1372:
1362:
1337:
1333:
1327:
1292:
1288:
1278:
1256:(1): 34–42.
1253:
1249:
1243:
1208:
1204:
1194:
1153:
1149:
1143:
1108:
1104:
1094:
1085:
1079:
1044:
1040:
1030:
1005:
1001:
967:
963:
957:
916:
912:
906:
897:
891:
858:
854:
844:
809:
805:
795:
768:
764:
754:
721:
717:
711:
676:
672:
662:
642:
635:
599:
592:
551:
547:
511:
507:
465:
461:
427:
421:
400:
397:
376:
337:
320:
308:
306:
256:Alan Hodgkin
250:
247:
241:
239:
203:By the time
202:
175:
174:Brunó Ferenc
170:fumaric acid
143:
106:
79:
50:
48:
1577:(1): 1–11.
1008:(1): 1–19.
771:(1): 1–15.
217:Jean Hanson
205:Hugh Huxley
146:Willy Kühne
140:Early works
98:Jean Hanson
94:Hugh Huxley
32:CrossBridge
1882:Physiology
1871:Categories
1485:(1): 1–6.
514:(6): 629.
414:References
114:actomyosin
1828:CiteSeerX
229:sarcomere
166:vitamin C
100:from the
88:from the
43:sarcomere
1858:21949216
1850:18162480
1797:11331913
1762:43434748
1711:10077586
1552:30618615
1544:11805818
1460:15173218
1354:14064165
1319:13475381
1270:18070595
1235:24030511
1178:13165698
1071:22739307
984:13115446
941:13099257
883:18120626
836:15173217
787:14140702
703:22566666
627:26766432
576:13165697
484:15066167
1805:6214339
1754:4181952
1734:Bibcode
1726:Science
1679:Bibcode
1652:4352361
1644:4022127
1624:Bibcode
1601:2519609
1524:Bibcode
1501:7775372
1451:2234572
1411:4199846
1403:5838248
1381:Bibcode
1310:2224118
1226:3771940
1186:4180166
1158:Bibcode
1135:3089413
1126:1340847
1049:Bibcode
1022:8815787
949:4220823
921:Bibcode
875:1538196
827:2234565
746:4084186
726:Bibcode
694:3346823
584:4275495
556:Bibcode
529:2234570
402:Science
254:, whom
135:History
59:myosin
1856:
1848:
1830:
1803:
1795:
1760:
1752:
1709:
1699:
1650:
1642:
1616:Nature
1599:
1592:361420
1589:
1550:
1542:
1516:Nature
1499:
1458:
1448:
1409:
1401:
1373:Nature
1352:
1317:
1307:
1268:
1233:
1223:
1184:
1176:
1150:Nature
1133:
1123:
1069:
1041:Nature
1020:
982:
947:
939:
913:Nature
881:
873:
834:
824:
785:
744:
718:Nature
701:
691:
650:
625:
615:
582:
574:
548:Nature
526:
482:
434:
339:them:
310:Nature
191:Origin
177:Straub
150:ATPase
1854:S2CID
1801:S2CID
1758:S2CID
1702:15844
1648:S2CID
1548:S2CID
1407:S2CID
1182:S2CID
945:S2CID
871:JSTOR
742:S2CID
623:S2CID
580:S2CID
71:actin
65:) of
1846:PMID
1793:PMID
1750:PMID
1707:PMID
1640:PMID
1597:PMID
1540:PMID
1497:PMID
1456:PMID
1399:PMID
1350:PMID
1315:PMID
1266:PMID
1231:PMID
1174:PMID
1131:PMID
1067:PMID
1018:PMID
980:PMID
937:PMID
879:PMID
832:PMID
783:PMID
699:PMID
648:ISBN
613:ISBN
572:PMID
480:PMID
432:ISBN
183:and
168:and
96:and
84:and
49:The
1838:doi
1824:104
1785:doi
1742:doi
1730:164
1697:PMC
1687:doi
1632:doi
1620:316
1587:PMC
1579:doi
1532:doi
1520:415
1487:doi
1483:117
1446:PMC
1438:doi
1434:123
1389:doi
1377:206
1342:doi
1305:PMC
1297:doi
1258:doi
1254:369
1221:PMC
1213:doi
1166:doi
1154:173
1121:PMC
1113:doi
1109:293
1057:doi
1045:486
1010:doi
972:doi
929:doi
917:172
863:doi
822:PMC
814:doi
810:123
773:doi
734:doi
722:144
689:PMC
681:doi
677:125
605:doi
564:doi
552:173
524:PMC
516:doi
512:123
470:doi
466:271
154:ATP
1873::
1852:.
1844:.
1836:.
1822:.
1799:.
1791:.
1779:.
1756:.
1748:.
1740:.
1728:.
1705:.
1695:.
1685:.
1675:96
1673:.
1669:.
1646:.
1638:.
1630:.
1618:.
1595:.
1585:.
1573:.
1569:.
1546:.
1538:.
1530:.
1518:.
1495:.
1481:.
1477:.
1454:.
1444:.
1432:.
1428:.
1405:.
1397:.
1387:.
1375:.
1371:.
1348:.
1336:.
1313:.
1303:.
1291:.
1287:.
1264:.
1252:.
1229:.
1219:.
1209:24
1207:.
1203:.
1180:.
1172:.
1164:.
1152:.
1129:.
1119:.
1107:.
1103:.
1065:.
1055:.
1043:.
1039:.
1016:.
1006:58
1004:.
992:^
978:.
968:12
966:.
943:.
935:.
927:.
915:.
877:.
869:.
859:96
857:.
853:.
830:.
820:.
808:.
804:.
781:.
769:32
767:.
763:.
740:.
732:.
720:.
697:.
687:.
675:.
671:.
621:.
611:.
578:.
570:.
562:.
550:.
538:^
522:.
510:.
506:.
492:^
478:.
464:.
460:.
446:^
410:.
1860:.
1840::
1807:.
1787::
1781:2
1764:.
1744::
1736::
1713:.
1689::
1681::
1654:.
1634::
1626::
1603:.
1581::
1575:1
1554:.
1534::
1526::
1503:.
1489::
1462:.
1440::
1413:.
1391::
1383::
1356:.
1344::
1338:7
1321:.
1299::
1293:3
1272:.
1260::
1237:.
1215::
1188:.
1168::
1160::
1137:.
1115::
1073:.
1059::
1051::
1024:.
1012::
986:.
974::
951:.
931::
923::
885:.
865::
838:.
816::
789:.
775::
748:.
736::
728::
705:.
683::
656:.
629:.
607::
586:.
566::
558::
532:.
518::
486:.
472::
440:.
73:(
61:(
34:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.