202:
237:
293:
279:
265:
186:
221:
249:
17:
172:
381:
Schwarzenbach, Gerold; Bürgi, Hans-Beat; Jensen, William P.; Lawrance, Geoffrey A.; Mønsted, Lene; Sargeson, Alan M. (1983). "Acid
Cleavage of Nickel(Ii) Complexes Containing cis,cis-1,3,5-Cyclohexanetriamine (TACH), Crystal Structure of
236:
201:
319:
Aimee J. Gamble; Jason M. Lynam; Robert J. Thatcher; Paul H. Walton; Adrian C. Whitwood (2013). "cis-1,3,5-Triaminocyclohexane as a
Facially Capping Ligand for Ruthenium(II)".
292:
220:
466:
74:
185:
192:
141:
89:
positions available on the octahedral metal center. When bound to four- and five-coordinate metal centres, these ligands impose C
278:
507:
Parkin, G. (2000). "The
Bioinorganic Chemistry of Zinc: Synthetic Analogues of Zinc Enzymes that Feature Tripodal Ligands".
242:
NTA is a commercially important tripodal ligand. Its three carboxylic acid groups undergo deprotonation upon complexation.
46:. Because the ligands are polydentate, they do not readily dissociate from the metal centre. Many tripodal ligands have C
248:
156:
are also tripodal. Many kinds of donor groups have been incorporated into the arms of tripodal ligands, including amido
120:
because it is cheaply produced and has a high affinity for divalent metal ions. Other tripodal triamine ligands include
480:
Blackman, A. G. (2008). "Tripodal
Tetraamine Ligands Containing Three Pyridine Units: the other polypyridyl ligands".
264:
284:
270:
66:
207:
535:
97:
splitting patterns. Tripodal ligands are often able to coordinately saturate metal ions with lower
121:
211:
165:
62:
39:
460:
318:
105:
43:
419:"M-E and M=E Complexes of Iron and Cobalt that Emphasize Three-Fold Symmetry (E = O, N, NR)"
8:
390:, and a Correlation Between the Structure and Reactivity of Nickel-Polyamine Complexes".
227:
98:
443:
418:
448:
336:
512:
489:
438:
430:
399:
363:
328:
255:
50:
434:
175:
Structure of (color code: blue = nitrogen, red = oxygen, dark blue = nickel).
529:
493:
452:
354:
Verkade, J. G. (1993). "Atranes: New
Examples with Unexpected Properties".
340:
94:
82:
32:
298:
403:
367:
70:
28:
332:
516:
16:
20:
Motifs for complexation of tri- and tetradentate tripodal ligands
380:
301:, when triply deprotonated, is an unsymmetrical tripodal ligand.
35:
416:
171:
85:
tripodal ligands occupy four contiguous sites, leaving two
73:tripod ligands occupy one face, leading to a fixed
104:One tripodal ligand of commercial significance is
527:
479:
38:. They are popular in research in the areas of
353:
140:) and cis-1,3,5-triaminocyclohexane. Certain
465:: CS1 maint: multiple names: authors list (
210:, a tetradentate tripodal ligand popular in
506:
442:
56:
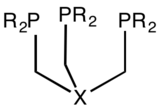
195:, X can be CR, SiR, Si, BR, B, N, P etc.
170:
15:
528:
230:, an anionic tridentate tripod ligand.
417:Saouma, C. T., Peters, J. C. (2011).
93:symmetry, which can lead to uncommon
258:, a dianionic organometallic ligand.
13:
14:
547:
285:cis,cis-1,3,5-triaminocyclohexane
291:
277:
263:
247:
235:
226:Structure of a metal complex of
219:
200:
184:
500:
473:
410:
374:
347:
312:
1:
306:
271:1,1,1-Tris(aminomethyl)ethane
67:octahedral molecular geometry
7:
10:
552:
208:Tris(2-pyridylmethyl)amine
435:10.1016/j.ccr.2011.01.009
180:Tripodal Ligands Examples
509:Chemical Communications
166:N-heterocyclic carbenes
494:10.1002/ejic.200800115
212:bioinorganic chemistry
176:
63:coordination complexes
57:Coordination chemistry
40:coordination chemistry
21:
174:
44:homogeneous catalysis
19:
99:coordination numbers
482:Eur. J. Inorg. Chem
404:10.1021/ic00168a042
392:Inorganic Chemistry
368:10.1021/ar00033a005
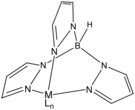
228:trispyrazolylborate
177:
22:
511:(20): 1971–1985.
398:(26): 4029–4038.
333:10.1021/ic302819j
106:nitrilotriacetate
81:) geometry. The
543:
536:Tripodal ligands
521:
520:
517:10.1039/B004816J
504:
498:
497:
477:
471:
470:
464:
456:
446:
429:(7–8): 920–937.
423:Coord. Chem. Rev
414:
408:
407:
378:
372:
371:
351:
345:
344:
327:(8): 4517–4527.
316:
295:
281:
267:
251:
239:
223:
204:
188:
163:
25:Tripodal ligands
551:
550:
546:
545:
544:
542:
541:
540:
526:
525:
524:
505:
501:
478:
474:
458:
457:
415:
411:
389:
385:
379:
375:
352:
348:
317:
313:
309:
302:
296:
287:
282:
273:
268:
259:
252:
243:
240:
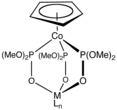
231:
224:
215:
205:
196:
189:
161:
157:
155:
151:
147:
139:
135:
131:
127:
119:
115:
111:
92:
59:
49:
12:
11:
5:
549:
539:
538:
523:
522:
499:
472:
409:
387:
383:
373:
356:Acc. Chem. Res
346:
310:
308:
305:
304:
303:
297:
290:
288:
283:
276:
274:
269:
262:
260:
253:
246:
244:
241:
234:
232:
225:
218:
216:
206:
199:
197:
190:
183:
181:
159:
153:
149:
145:
137:
133:
129:
125:
117:
113:
109:
90:
58:
55:
47:
9:
6:
4:
3:
2:
548:
537:
534:
533:
531:
518:
514:
510:
503:
495:
491:
487:
483:
476:
468:
462:
454:
450:
445:
440:
436:
432:
428:
424:
420:
413:
405:
401:
397:
393:
377:
369:
365:
361:
357:
350:
342:
338:
334:
330:
326:
322:
315:
311:
300:
294:
289:
286:
280:
275:
272:
266:
261:
257:
250:
245:
238:
233:
229:
222:
217:
213:
209:
203:
198:
194:
193:triphosphines
187:
182:
179:
178:
173:
169:
167:
144:such as RC(CH
143:
142:triphosphines
123:
107:
102:
100:
96:
88:
84:
80:
76:
72:
68:
64:
54:
52:
45:
41:
37:
34:
30:
26:
18:
508:
502:
488:(17): 2633.
485:
481:
475:
461:cite journal
426:
422:
412:
395:
391:
376:
359:
355:
349:
324:
320:
314:
256:Kläui ligand
103:
95:ligand field
86:
83:tetradentate
78:
60:
33:tetradentate
24:
23:
321:Inorg. Chem
299:citric acid
191:A tripodal
362:(9): 483.
307:References
71:tridentate
61:In their
530:Category
453:21625302
341:23517123
65:with an
51:symmetry
444:3103469
36:ligands
451:
441:
339:
164:, and
108:, N(CH
75:facial
124:(N(CH
486:2008
467:link
449:PMID
337:PMID
254:The
122:tren
77:(or
69:the
42:and
31:and
29:tri-
27:are
513:doi
490:doi
439:PMC
431:doi
427:255
400:doi
382:(NO
364:doi
329:doi
148:PPh
87:cis
79:fac
532::
484:.
463:}}
459:{{
447:.
437:.
425:.
421:.
396:22
394:.
360:26
358:.
335:.
325:52
323:.
168:.
162:N)
158:(R
132:NH
128:CH
112:CO
101:.
53:.
519:.
515::
496:.
492::
469:)
455:.
433::
406:.
402::
388:2
386:)
384:3
370:.
366::
343:.
331::
214:.
160:2
154:3
152:)
150:2
146:2
138:3
136:)
134:2
130:2
126:2
118:3
116:)
114:2
110:2
91:3
48:3
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.