167:
137:
284:
299:
132:
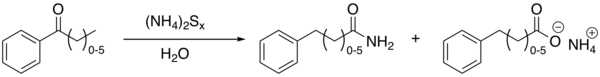
chain (n typically 0 to 5), multiple reactions take place with the amide group always ending up at the terminal end. The net effect is thus migration of the carbonyl group to the end of the chain and oxidation.
437:
Carmack, Marvin; F. DeTar, DeLos (1946). "The
Willgerodt and Kindler Reactions. III. Amides from Acetylenes and Olefins; Studies Relating to the Reaction Mechanisms".
157:
534:
271:
233:
67:
166:
283:
17:
298:
136:
537:(1923). "Studien über den Mechanismus chemischer Reaktionen. Erste Abhandlung. Reduktion von Amiden und Oxydation von Aminen".
600:
485:
218:
618:
307:
226:
60:
408:"Ueberführung von Ketonen und Aldehyden in Säuren und Säureamide mittelst gelben Schwefelammoniums"
331:
623:
501:
The
Willgerodt Reaction. II. A Study of Reaction Conditions with Acetophenone and Other Ketones
335:
202:
48:
343:
8:
292:
145:
596:
565:
505:
481:
190:
161:
113:
38:
577:
548:
517:
473:
446:
419:
388:
89:
539:
355:
276:
117:
477:
612:
552:
423:
392:
351:
327:
121:
339:
267:
153:
377:"Ueber die Einwirkung von gelbem Schwefelammonium auf Ketone und Chinone"
323:
109:
521:
450:
270:
which can again be hydrolyzed to the amide. The reaction is named after
259:
125:
347:
263:
129:
464:
Carmack, Marvin; Spielman, M. A. (1946). "The
Willgerodt Reaction".
407:
376:
149:
319:
311:
251:
101:
97:
93:
315:
255:
105:
592:
Bradford P. Mundy, Michael G. Ellerd, Frank G. Jr. Favaloro
302:
The likely reaction mechanism for the
Kindler modification.
287:
The
Kindler modification of the Willgerodt rearrangement
144:
An example with modified reagents (sulfur, concentrated
610:
590:Name Reactions and Reagents in Organic Synthesis
463:
436:
170:The Willgerodt rearrangement using acetophenone
140:General scheme for the Willgerodt rearrangement
412:Berichte der Deutschen Chemischen Gesellschaft
381:Berichte der Deutschen Chemischen Gesellschaft
174:
338:takes place when the amine group attacks the
295:for the Kindler variation is depicted below:
533:
405:
374:
306:The first stage of the reaction is basic
128:of the amide. When the alkyl group is an
439:Journal of the American Chemical Society
14:
611:
116:. The formation of the corresponding
24:
503:DeLos F. DeTar and Marvin Carmack
297:
282:
165:
135:
25:
635:
318:group of morpholine to give an
583:
559:
527:
494:
457:
430:
399:
368:
13:
1:
361:
182:Willgerodt–Kindler reaction
7:
478:10.1002/0471264180.or003.02
406:Willgerodt, Conrad (1888).
375:Willgerodt, Conrad (1887).
262:. The initial product is a
250:takes place with elemental
248:Willgerodt–Kindler reaction
219:willgerodt-kindler-reaction
175:Willgerodt–Kindler reaction
18:Willgerodt-Kindler reaction
10:
640:
240:
214:Organic Chemistry Portal
208:
181:
92:converting an aryl alkyl
74:
54:
30:Willgerodt rearrangement
29:
553:10.1002/jlac.19234310111
424:10.1002/cber.18870200278
393:10.1002/cber.18870200278
332:Stork enamine alkylation
82:Willgerodt rearrangement
619:Rearrangement reactions
346:temporarily forming an
152:) is the conversion of
568:, Coll. Vol. 9, p.99 (
336:rearrangement reaction
330:sulfur, similar to an
303:
288:
203:Rearrangement reaction
171:
141:
49:Rearrangement reaction
344:nucleophilic addition
334:reaction. The actual
301:
286:
169:
139:
104:to the corresponding
266:for example that of
110:ammonium polysulfide
572:); Vol. 74, p.257 (
522:10.1021/ja01214a047
451:10.1021/ja01214a048
322:. This reacts as a
86:Willgerodt reaction
304:
293:reaction mechanism
289:
172:
146:ammonium hydroxide
142:
566:Organic Syntheses
506:J. Am. Chem. Soc.
466:Organic Reactions
445:(10): 2029–2033.
244:
243:
191:Conrad Willgerodt
162:phenylacetic acid
158:2-phenylacetamide
114:Conrad Willgerodt
108:by reaction with
78:
77:
39:Conrad Willgerodt
16:(Redirected from
631:
603:
587:
581:
563:
557:
556:
531:
525:
516:, 2025 - 2029. (
498:
492:
491:
461:
455:
454:
434:
428:
427:
403:
397:
396:
387:(2): 2467–2470.
372:
280:
236:
221:
179:
178:
90:organic reaction
70:
27:
26:
21:
639:
638:
634:
633:
632:
630:
629:
628:
609:
608:
607:
606:
588:
584:
564:
560:
540:Liebigs Annalen
532:
528:
499:
495:
488:
462:
458:
435:
431:
404:
400:
373:
369:
364:
356:tautomerization
308:imine formation
274:
232:
217:
193:
177:
124:resulting from
118:carboxylic acid
66:
23:
22:
15:
12:
11:
5:
637:
627:
626:
624:Name reactions
621:
605:
604:
582:
558:
547:(1): 187–230.
526:
493:
486:
456:
429:
418:(1): 534–536.
398:
366:
365:
363:
360:
314:group and the
242:
241:
238:
237:
230:
223:
222:
215:
211:
210:
206:
205:
200:
199:Reaction type
196:
195:
188:
184:
183:
176:
173:
112:, named after
76:
75:
72:
71:
64:
57:
56:
52:
51:
46:
45:Reaction type
42:
41:
36:
32:
31:
9:
6:
4:
3:
2:
636:
625:
622:
620:
617:
616:
614:
602:
601:0-471-22854-0
598:
595:
591:
586:
579:
575:
571:
567:
562:
554:
550:
546:
542:
541:
536:
530:
523:
519:
515:
511:
508:
507:
502:
497:
489:
487:9780471005285
483:
479:
475:
471:
467:
460:
452:
448:
444:
440:
433:
425:
421:
417:
413:
409:
402:
394:
390:
386:
382:
378:
371:
367:
359:
357:
353:
352:thioacetamide
349:
345:
341:
337:
333:
329:
328:electrophilic
325:
321:
317:
313:
309:
300:
296:
294:
285:
281:
278:
273:
269:
265:
261:
257:
253:
249:
239:
235:
231:
228:
225:
224:
220:
216:
213:
212:
207:
204:
201:
198:
197:
194:Karl Kindler
192:
189:
186:
185:
180:
168:
164:
163:
159:
155:
151:
147:
138:
134:
131:
127:
123:
122:side reaction
119:
115:
111:
107:
103:
99:
95:
91:
87:
83:
73:
69:
65:
62:
59:
58:
53:
50:
47:
44:
43:
40:
37:
34:
33:
28:
19:
593:
589:
585:
573:
569:
561:
544:
538:
535:Karl Kindler
529:
513:
509:
504:
500:
496:
469:
465:
459:
442:
438:
432:
415:
411:
401:
384:
380:
370:
340:thiocarbonyl
305:
290:
272:Karl Kindler
268:acetophenone
247:
246:The related
245:
234:RXNO:0000186
229:ontology ID
209:Identifiers
187:Named after
154:acetophenone
143:
85:
81:
79:
68:RXNO:0000185
63:ontology ID
55:Identifiers
35:Named after
324:nucleophile
291:A possible
275: [
613:Categories
472:: 83–107.
362:References
260:morpholine
126:hydrolysis
348:aziridine
264:thioamide
130:aliphatic
350:and the
150:pyridine
578:Article
320:enamine
310:by the
254:and an
599:
514:68(10)
484:
312:ketone
252:sulfur
102:alkene
98:alkyne
94:ketone
88:is an
342:in a
326:with
316:amine
279:]
258:like
256:amine
120:is a
106:amide
100:, or
597:ISBN
594:2005
576:). (
574:1997
570:1998
510:1946
482:ISBN
160:and
148:and
80:The
549:doi
545:431
518:doi
474:doi
447:doi
420:doi
389:doi
354:by
227:RSC
156:to
84:or
61:RSC
615::
543:.
512:,
480:.
468:.
443:68
441:.
416:21
414:.
410:.
385:20
383:.
379:.
358:.
277:de
96:,
580:)
555:.
551::
524:)
520::
490:.
476::
470:3
453:.
449::
426:.
422::
395:.
391::
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.