241:
392:
127:
334:
135:
366:
377:
302:
269:
51:(TEMPO), the most common subunit used in ORBs, is a stable oxygen-centered molecular radical. Here, the radical is stabilized by delocalization of electrons from the nitrogen onto the oxygen. TEMPO radicals can be attached to polymer backbones to form poly(2,2,6,6-tetramethyl- piperidenyloxyl-4-yl methacrylate) (PTMA). PTMA-based ORBs have a charge-density slightly higher than that of conventional
277:
PTMPM-RAFT. Direct oxidation of PTMPM-RAFT to PTMA is not practical, as direct oxidation causes side reactions involving the thiocaronylthiol end group of PTMPM-RAFT to react to form insoluble gel-like product. Rather, excess AIBN is used to remove the reactive terminus to form PTMPM, which can then be oxidized by meta-chloroperbenzoic acid to the desired PTMA.
194:, an organic radical battery consists of a cathode and an anode that are separated by a porous film and submerged in an electrolyte. In a pure organic radical battery, both terminals are made of organic radical polymers (a p-type and an n-type polymer), while a metal/ORB hybrid battery usually has a radical polymer cathode and a Li-ion/graphite anode.
249:
decreased number of nitroxide groups negatively impacts the charge capacity of the polymer and limits its efficacy in organic radical batteries. Not only are there fewer nitroxide groups present, but also side reactions between non-oxidized groups and oxammonium cations diminishes the redox reversibility of the compound.
448:
Polymerization reactions of the stable radical-containing monomer have also proved to be an area of difficulty in development. The stable organic radicals that are crucial to the functioning of the battery are sometimes consumed in side-reactions of various polymerization reactions. A research group
352:
of nitroxyl-containing monomers has also been used to synthesis PTMA. Anionic polymerization is not ideal because it must be carried using very strict procedures to avoid side reactions. Using 1,1-diphenylhexylllithium as an initiator of the reaction eliminates some side reactions by steric effects,
165:
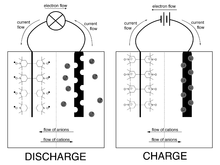
The negative electrode uses the nitroxide - hydroxylamine anion redox pair to create an electrochemical potential, i.e. when the battery discharges the nitroxide radical is reduced to the hydroxylamine anion and when the battery charges the hydroxylamine anion is oxidized back to the nitroxide. This
424:
with 140 mA h g. ORBs also show comparable charge times and retain of charge-discharge capacity well, matching lithium-ion batteries at 75% of their initial charge after 500 cycles. Additionally, radical concentration in ORBs are stable enough at ambient conditions to remain unchanged for over a
276:
RAFT-mediated polymerization of PTMA utilizes the same starting monomer as free-radical polymerization. Using the RAFT-mediated approach to polymerize 2,2,6,6-tetramethyl-4-piperidinyl methacrylate (TMPM), the starting monomer, generates poly(2,2,6,6-tetramethyl-4-piperidnyl methacrylate) or
248:
Free-radical polymerization as a synthetic approach has several drawbacks. The most relevant limitation is the fact that precursor polymer oxidation never proceeds to 100%. As a result, the synthesized PTMA has between 65% and 81% of the theoretically possible amount of nitroxide groups. The
20:(ORB) is a type of battery first developed in 2005. As of 2011, this type of battery was generally not available for the consumer, although their development at that time was considered to be approaching practical use. ORBs are potentially more environmentally friendly than conventional
98:
chemistry of nitroxide radicals, ORBs have been shown useful in keeping a computer running momentarily following a power outage. Although the amount of additional time provided is short, it is adequate to allow a computer to backup any crucial data before completely shutting down.
252:
The difficulties of free-radical polymerization of PTMA could be avoided if the oxidation step were not necessary. However, because nitroxide radicals would react with any carbon radicals formed during polymerization, use of a monomer with a nitroxide radical isn't practical.
202:
Several synthetic approaches have been utilized in the synthesis of polyradical species for use in organic radical batteries. The following methods have been used to synthesize poly(2,2,6,6- tetramethylpiperidinyloxy-4-yl methacrylate) (PTMA) and other nitroxide polymers.
408:
because ORBs do not contain any metals that pose the problem of proper disposal. ORBs are non-toxic and non-flammable and do not require additional care when handling. Burning nitroxide radical polymers yields carbon dioxide, water, and nitrogen oxide without ash or odor.
82:
Organic radical batteries were first researched and developed by NEC in 2005 with the intent of being widely used to power tiny gadgets in the near future. They began with a size of 0.3 mm and an extremely quick charge time. Since the beginning of development,
361:
Group-transfer polymerization, like rhodium-catalyzed polymerization of PTMA, allows for polymerization of nitroxyl radical monomers. Unlike rhodium-catalyzed monomers, group-transfer polymerization utilizes silicon to catalyze the polymerization.
154:, i.e. when the battery discharges the nitroxide radical is oxidized to the oxammonium cation and when the battery charges the oxammonium cation is reduced back to the nitroxide. The redox potentials for nitroxide show some variation and for the
178:
at the positive electrode, several research groups have steered away from using pure organic radical batteries and instead use metal/ORB hybrid batteries usually consist of a radical polymer cathode and the same anode found in rechargeable
329:
derivatives and various TEMPO derivatives. Polymerization of the monomers is completed using a
Rhodium catalyst (nbd)Rh. Rhodium catalyzed synthesis of TEMPO containing polymers has been performed with high quantitative yield.
384:
Polymerization using 1-methoxy-2-methyl-1trimethylsilyloxy-propene (MTS) as a catalyst proceeds rapidly at room temperature to form PTMA. Tetrabutylammonium fluoride (TBAF) is used as an additional catalyst.
62:
As of 2007, ORB research was being directed mostly towards Hybrid ORB/Li-ion batteries because organic radical polymers with appropriate electrical properties for the anode are difficult to synthesize.
341:
While use of a rhodium catalyst may be advantageous due to its high yield, use of a metal catalyst provides the additional challenge of having to separate the catalyst from the final product.
237:(mCPBA). Similar synthetic approaches have been proposed using 4-methacryloyloxy-N-hydroxy-2,2,6,6-tetramethylpiperidine as a monomer rather than 2,2,6,6- tetramethylpiperidine methacrylate.
262:
650:
Nakahara, K.; Iwasa, S.; Satoh, M.; Morioka, Y.; Iriyama, J.; Suguro, M.; Hasegawa, E. Chemical
Physics Letters 2002, 359 (5–6), 351–354. doi: 10.1016/S0009-2614(02)00705-4
24:, because they use organic radical polymers (flexible plastics) to provide electrical power instead of metals. ORBs are considered to be a high-power alternative to the
240:
91:
tags were the main targets for ORB usage. NEC has also worked on a larger 0.7 mm battery which is thicker, but also has a high charge capacity of 5 mAh.
158:
nitroxide for this redox pair has an oxidation potential of +0.87 V. The positive electrode often takes the shape of a gel made of organic radical solids and
441:
reaction of the negative electrode is not fully reversible. Hybrid ORB/Li-ion batteries, in which the negative electrode is replaced by the one found in a
28:. Functional prototypes of the battery have been researched and developed by different research groups and corporations including the Japanese corporation
449:
has, however, successfully synthesized a cross-linked organic radical polymer while only losing 0.4% of the organic radicals in synthesis of the polymer.
632:
Katsumata, T.; Satoh, M.; Wada, J.; Shiotsuki, M.; Sanda, F.; Masuda, T. Macromol. Rapid Commun. 2006, 27 (15), 1206–1211. doi: 10.1002/marc.200600286
620:
Nishide, H.; Iwasa, S.; Pu, Y.-J.; Suga, T.; Nakahara, K.; Satoh, M. Electrochimica Acta 2004, 50 (2–3), 827–831. doi: 10.1016/j.electacta.2004.02.052
437:
A major difficulty in the development of ORBs is difficulty of synthesizing an appropriate negative electrode. This disadvantage arises because the
295:-bearing monomers avoids some of the challenges free-radical polymerization poses because an oxidation step to generate the radical is not needed.
119:. The most studied example of such an organic radical redox reaction is that of nitroxide radicals, such as the one found on a molecule called
333:
391:
126:
373:
Preparation of the monomer, 4-methacryloxyloxy-TEMPO can be accomplished by acylation of 4-hydroxy-TEMPO with methacryloyl chloride.
659:
Kurosaki, T.; Takahashi, O.; Okawara, M. J. Polym. Sci. Polym. Chem. Ed. 1974, 12 (7), 1407–1420. doi: 10.1002/pol.1974.170120705
641:
Kurosaki, T.; Lee, K. W.; Okawara, M. J. Polym. Sci. A-1 Polym. Chem. 1972, 10 (11), 3295–3310. doi: 10.1002/pol.1972.170101116
551:
Bugnon, L.; Morton, C. J. H.; Novak, P.; Vetter, J.; Nesvadba, P. Chem. Mater. 2007, 19 (11), 2910–2914. doi: 10.1021/cm063052h
675:
Rostro, L.; Baradwaj, A. G.; Boudouris, B. W. ACS Appl. Mater. Interfaces 2013, 5 (20), 9896–9901. doi: 10.1021/am403223s
301:
365:
123:, also known as TEMPO. A nitroxide radical can be oxidized to an oxammonium cation or reduced to a hydroxylamine anion.
585:
138:
Discharge and charge of a hybrid ORB/Li-ion battery. The positive terminal is an organic radical polymer carrying the
417:
76:
718:
684:
Allgaier, J.; Finkelmann, H. Makromol. Chem., Rapid Commun. 1993, 14 (5), 267–271. doi: 10.1002/marc.1993.030140502
746:
349:
261:
One of the more recent techniques identified to synthesis PTMA is a type of free radical polymerization known as
88:
234:
219:
71:
As of 2015, ORBs were still under development and not in commercial use. Theoretically, ORBs could replace
280:
Despite the promise of the RAFT-mediated polymerization, reported radical concentration was only 69 ± 4%.
211:
Initial attempts to synthesize PTMA involved synthesizing the polymer without radical functionality via
376:
268:
525:
Nakahara, K.; Oyaizu, K.; Nishide, H. Chemistry
Letters 2011, 40 (3), 222–227. doi:10.1246/cl.2011.222
79:
and similar or shorter charge time. This would make ORBs well-suited for handheld electronic devices.
696:"NEC Develops New Ultra-Thin, Flexible, Rechargeable Battery Boasting Super-Fast Charging Capability"
563:"NEC Develops New Ultra-Thin, Flexible, Rechargeable Battery Boasting Super-Fast Charging Capability"
155:
151:
139:
120:
116:
48:
489:
229:) as a radical initiator. The monomer was prepared via 2,2,6,6-tetramethyl-4-piperidinol (1) and
429:, which would make them more adaptable to different design constraints, such as curved devices.
162:, permeated with electrolytes. Graphite is mixed with the polymer to increase the conductivity.
226:
212:
695:
458:
310:
230:
44:
412:
While being environmentally friendly, they have properties that are otherwise comparable to
244:
Free-radical polymerization of 4-methacryloyloxy-2,2,6,6-tetramethylpiperidine to form PTMA
218:
Several groups have described synthesis of PTMA (4) using free radical polymerization of
215:. Once the polymer is synthesized, the nitroxide function can be introduced by oxidation.
8:
112:
36:
55:, which should theoretically make it possible for an ORB to provide more charge than a
233:. The precursor neutral polymer (3) was oxidized to the stable radical polymer (4) by
150:
The positive electrode uses the nitroxide - oxammonium cation redox pair to create an
611:
Nishide, H.; Suga, T. The
Electrochemical Society Interface 2005, No. Winter, 32–36
426:
298:
The structure of (2,2,6,6-Tetramethylpiperidine-1-yl)oxyl or TEMPO is shown below.
314:
29:
562:
442:
421:
413:
405:
191:
180:
143:
72:
56:
52:
40:
25:
740:
353:
however, the procedures necessary are not amenable to large-scale synthesis.
187:
175:
171:
167:
21:
222:
288:
134:
84:
432:
404:
Organic radical batteries are much more environmentally friendly than
326:
337:
Rhodium-Catalyzed polymerization of TEMPO-bearing acetylene monomers
322:
159:
445:, have been proposed as a compromise to overcome this difficulty.
35:
The organic radical polymers used in ORBs are examples of stable
388:
The following is a rationale for group-transfer polymerization.
75:
as more environmentally friendly batteries of similar or higher
395:
Mechanistic rationale for group transfer polymerization of PTMA
130:
Redox chemistry of the TEMPO-group, which contains a nitroxide.
438:
318:
292:
108:
95:
142:-unit and the negative terminal is the same as found in a
586:"NEC Develops Organic Radical Battery for Practical Use"
305:
Structure of 2,2,6,6-Tetramethylpiperidineoxyl (TEMPO)
420:
of 147 mA h g, which is slightly higher than that of
433:
Disadvantages and difficulties faced in development
309:The following monomers (1-3) can be synthesized by
283:
170:has an oxidation potential of -0.11 V. Since this
738:
521:
519:
517:
515:
513:
511:
509:
507:
356:
263:reversibly addition-fragmentation chain transfer
197:
256:
47:effects. For example, the nitroxide radical in
607:
605:
603:
369:Synthesis of 4-Methacryloxyloxy-TEMPO Monomers
206:
504:
390:
375:
364:
332:
300:
267:
239:
125:
628:
626:
600:
653:
671:
669:
667:
665:
644:
344:
678:
635:
623:
547:
545:
543:
541:
539:
537:
535:
533:
531:
133:
425:year. ORBs are also more flexible than
121:(2,2,6,6-Tetramethylpiperidin-1-yl)oxyl
49:(2,2,6,6-tetramethylpiperidin-1-yl)oxyl
739:
662:
614:
484:
482:
480:
478:
476:
474:
528:
490:"What is an Organic Radical Battery?"
272:RAFT mediated polymerization of TEMPO
225:(2) with 2,2'-azobisiobutryonitrile (
716:
710:
687:
107:Radical polymer batteries rely on a
577:
554:
471:
13:
583:
14:
758:
693:
560:
174:is not readily reversible as the
284:Rhodium catalyzed polymerization
265:(RAFT) mediated polymerization.
66:
1:
464:
399:
357:Group-transfer polymerization
350:Direct anionic polymerization
291:-catalyzed polymerization of
220:2,2,6,6-tetramethylpiperidine
198:Synthesis of radical polymers
257:RAFT-mediated polymerization
59:of similar size and weight.
7:
452:
213:free radical polymerization
207:Free-radical polymerization
102:
10:
763:
416:: ORBs have a theoretical
380:GTP polymerization of PTMA
235:3-chloroperoxybenzoic acid
39:, which are stabilized by
152:electrochemical potential
117:electrochemical potential
719:"Flexible battery power"
492:. Conjecture Corporation
18:organic radical battery
747:Rechargeable batteries
396:
381:
370:
345:Anionic polymerization
338:
306:
273:
245:
147:
131:
459:List of battery types
394:
379:
368:
336:
311:condensation reaction
304:
271:
243:
231:methacryloyl chloride
137:
129:
22:metal-based batteries
188:traditional battery
717:Stoddart, Alison.
397:
382:
371:
339:
307:
274:
246:
148:
132:
698:. NEC Corporation
588:. NEC Corporation
565:. NEC Corporation
754:
731:
730:
728:
726:
721:. RSC Publishing
714:
708:
707:
705:
703:
691:
685:
682:
676:
673:
660:
657:
651:
648:
642:
639:
633:
630:
621:
618:
612:
609:
598:
597:
595:
593:
584:Jasper, Joseph.
581:
575:
574:
572:
570:
558:
552:
549:
526:
523:
502:
501:
499:
497:
486:
427:Li-ion batteries
422:Li-ion batteries
414:Li-ion batteries
406:Li-ion batteries
181:Li-ion batteries
73:Li-ion batteries
53:Li-ion batteries
762:
761:
757:
756:
755:
753:
752:
751:
737:
736:
735:
734:
724:
722:
715:
711:
701:
699:
692:
688:
683:
679:
674:
663:
658:
654:
649:
645:
640:
636:
631:
624:
619:
615:
610:
601:
591:
589:
582:
578:
568:
566:
559:
555:
550:
529:
524:
505:
495:
493:
488:
487:
472:
467:
455:
435:
402:
359:
347:
315:carboxyl groups
286:
259:
209:
200:
115:to generate an
105:
94:Given the fast
77:charge capacity
69:
12:
11:
5:
760:
750:
749:
733:
732:
709:
694:Foley, Diane.
686:
677:
661:
652:
643:
634:
622:
613:
599:
576:
561:Foley, Diane.
553:
527:
503:
469:
468:
466:
463:
462:
461:
454:
451:
443:Li-ion battery
434:
431:
401:
398:
358:
355:
346:
343:
323:hydroxyl group
285:
282:
258:
255:
208:
205:
199:
196:
192:Li-ion battery
144:Li-ion battery
111:of an organic
109:redox reaction
104:
101:
68:
65:
57:Li-ion battery
26:Li-ion battery
9:
6:
4:
3:
2:
759:
748:
745:
744:
742:
720:
713:
697:
690:
681:
672:
670:
668:
666:
656:
647:
638:
629:
627:
617:
608:
606:
604:
587:
580:
564:
557:
548:
546:
544:
542:
540:
538:
536:
534:
532:
522:
520:
518:
516:
514:
512:
510:
508:
491:
485:
483:
481:
479:
477:
475:
470:
460:
457:
456:
450:
446:
444:
440:
430:
428:
423:
419:
415:
410:
407:
393:
389:
386:
378:
374:
367:
363:
354:
351:
342:
335:
331:
328:
324:
320:
316:
312:
303:
299:
296:
294:
290:
281:
278:
270:
266:
264:
254:
250:
242:
238:
236:
232:
228:
224:
221:
216:
214:
204:
195:
193:
189:
184:
182:
177:
176:half-reaction
173:
172:half-reaction
169:
168:half-reaction
163:
161:
157:
153:
145:
141:
136:
128:
124:
122:
118:
114:
110:
100:
97:
92:
90:
86:
80:
78:
74:
64:
60:
58:
54:
50:
46:
42:
38:
33:
31:
27:
23:
19:
723:. Retrieved
712:
700:. Retrieved
689:
680:
655:
646:
637:
616:
590:. Retrieved
579:
567:. Retrieved
556:
494:. Retrieved
447:
436:
411:
403:
387:
383:
372:
360:
348:
340:
308:
297:
287:
279:
275:
260:
251:
247:
223:methacrylate
217:
210:
201:
186:Much like a
185:
164:
149:
106:
93:
81:
70:
67:Applications
61:
34:
17:
15:
85:smart cards
725:30 October
702:30 October
592:6 November
569:5 November
496:8 November
465:References
400:Advantages
190:such as a
327:acetylene
317:with the
45:resonance
741:Category
453:See also
418:capacity
313:between
160:graphite
103:Function
37:radicals
289:Rhodium
113:radical
43:and/or
41:steric
439:redox
319:amino
293:TEMPO
156:TEMPO
140:TEMPO
96:redox
727:2012
704:2012
594:2012
571:2012
498:2012
227:AIBN
89:RFID
87:and
325:of
321:or
30:NEC
16:An
743::
664:^
625:^
602:^
530:^
506:^
473:^
183:.
32:.
729:.
706:.
596:.
573:.
500:.
146:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.