519:. Consequently, one can have recordings of the entire cell, as in whole-cell patch clamping, while retaining most intracellular signaling mechanisms, as in cell-attached recordings. As a result, there is reduced current rundown, and stable perforated patch recordings can last longer than one hour. Disadvantages include a higher access resistance, relative to whole-cell, due to the partial membrane occupying the tip of the electrode. This may decrease current resolution and increase recording noise. It can also take a significant amount of time for the antibiotic to perforate the membrane (about 15 minutes for amphothericin-B, and even longer for gramicidin and nystatin). The membrane under the electrode tip is weakened by the perforations formed by the antibiotic and can rupture. If the patch ruptures, the recording is then in whole-cell mode, with antibiotic contaminating the inside of the cell.
544:
This flexibility has been especially useful to researchers for studying muscle cells as they contract under real physiological conditions, obtaining recordings quickly, and doing so without resorting to drastic measures to stop the muscle fibers from contracting. A major disadvantage is that the resistance between the pipette and the membrane is greatly reduced, allowing current to leak through the seal, and significantly reducing the resolution of small currents. This leakage can be partially corrected for, however, which offers the opportunity to compare and contrast recordings made from different areas on the cell of interest. Given this, it has been estimated that the loose patch technique can resolve currents smaller than 1 mA/cm.
406:
511:, which diffuses into the membrane patch and forms small pores in the membrane, providing electrical access to the cell interior. When comparing the whole-cell and perforated patch methods, one can think of the whole-cell patch as an open door, in which there is complete exchange between molecules in the pipette solution and the cytoplasm. The perforated patch can be likened to a screen door that only allows the exchange of certain molecules from the pipette solution to the cytoplasm of the cell.
540:
the greater the resistance of the pipette tip becomes, but if too close a seal is formed, and it could become difficult to remove the pipette without damaging the cell. For the loose patch technique, the pipette does not get close enough to the membrane to form a gigaseal or a permanent connection, nor to pierce the cell membrane. The cell membrane stays intact, and the lack of a tight seal creates a small gap through which ions can pass outside the cell without entering the pipette.
319:
20:
445:
198:
168:
231:
487:
364:
160:
327:
of membrane captured by the pipette. By only attaching to the exterior of the cell membrane, there is very little disturbance of the cell structure. Also, by not disrupting the interior of the cell, any intracellular mechanisms normally influencing the channel will still be able to function as they would physiologically. Using this method it is also relatively easy to obtain the right configuration, and once obtained it is fairly stable.
528:
32:
294:
462:
right shows, this means that the fluid inside the pipette will be simulating the intracellular fluid, while a researcher is free to move the pipette and the bleb with its channels to another bath of solution. While multiple channels can exist in a bleb of membrane, single channel recordings are also possible in this conformation if the bleb of detached membrane is small and only contains one channel.
590:, Patch-seq allows for neurons to be characterized in multiple ways simultaneously. It currently suffers from low throughput relative to other sequencing methods mainly due to the manual labor involved in achieving a successful patch-clamp recording on a neuron. Investigations are currently underway to automate patch-clamp technology which will improve the throughput of patch-seq as well.
179:, for whole-cell recording. The solution in the bath solution may match the physiological extracellular solution, the cytoplasm, or be entirely non-physiological, depending on the experiment to be performed. The researcher can also change the content of the bath solution (or less commonly the pipette solution) by adding ions or drugs to study the ion channels under different conditions.
617:
and the membrane is now in the inside-out conformation, at the tip of the pipette. In a completely automated system, the pipette and the membrane patch can then be rapidly moved through a series of different test solutions, allowing different test compounds to be applied to the intracellular side of the membrane during recording.
419:
on the type of cell and size of the pipette. The other method requires a large current pulse to be sent through the pipette. How much current is applied and the duration of the pulse also depend on the type of cell. For some types of cells, it is convenient to apply both methods simultaneously to break the patch.
342:
or drug being studied is usually included in the pipette solution, where it can interact with what used to be the external surface of the membrane. The resulting channel activity can be attributed to the drug being used, although it is usually not possible to then change the drug concentration inside
494:
This variation of the patch clamp method is very similar to the whole-cell configuration. The main difference lies in the fact that when the experimenter forms the gigaohm seal, suction is not used to rupture the patch membrane. Instead, the electrode solution contains small amounts of an antifungal
426:
recording is that the larger opening at the tip of the patch clamp electrode provides lower resistance and thus better electrical access to the inside of the cell. A disadvantage of this technique is that because the volume of the electrode is larger than the volume of the cell, the soluble contents
418:
of the cell. This provides a means to administer and study how treatments (e.g. drugs) can affect cells in real time. Once the pipette is attached to the cell membrane, there are two methods of breaking the patch. The first is by applying more suction. The amount and duration of this suction depends
384:
of membrane in the pipette tip, because the ends of the patch membrane fuse together quickly after excision. The outer face of the vesicle must then be broken open to enter into inside-out mode; this may be done by briefly taking the membrane through the bath solution/air interface, by exposure to a
543:
A significant advantage of the loose seal is that the pipette that is used can be repeatedly removed from the membrane after recording, and the membrane will remain intact. This allows repeated measurements in a variety of locations on the same cell without destroying the integrity of the membrane.
539:
To achieve a loose patch clamp on a cell membrane, the pipette is moved slowly towards the cell, until the electrical resistance of the contact between the cell and the pipette increases to a few times greater resistance than that of the electrode alone. The closer the pipette gets to the membrane,
375:
of the membrane is exposed to the external media, or bath. One advantage of this method is that the experimenter has access to the intracellular surface of the membrane via the bath and can change the chemical composition of what the inside surface of the membrane is exposed to. This is useful when
326:
For this method, the pipette is sealed onto the cell membrane to obtain a gigaseal (a seal with electrical resistance on the order of a gigaohm), while ensuring that the cell membrane remains intact. This allows the recording of currents through single, or a few, ion channels contained in the patch
616:
In one form of such an automated system, a pressure differential is used to force the cells being studied to be drawn towards the pipette opening until they form a gigaseal. Then, by briefly exposing the pipette tip to the atmosphere, the portion of the membrane protruding from the pipette bursts,
477:
curve can then be obtained. This ability to measure current through exactly the same piece of membrane in different solutions is the distinct advantage of the outside-out patch relative to the cell-attached method. On the other hand, it is more difficult to accomplish. The longer formation process
461:
out from the cell. When the electrode is pulled far enough away, this bleb will detach from the cell and reform as a convex membrane on the end of the electrode (like a ball open at the electrode tip), with the original outside of the membrane facing outward from the electrode. As the image at the
413:
Whole-cell recordings involve recording currents through multiple channels simultaneously, over a large region of the cell membrane. The electrode is left in place on the cell, as in cell-attached recordings, but more suction is applied to rupture the membrane patch, thus providing access from the
276:
or microtomes is essential, in addition to patch clamp methods. By supplying thin, uniform tissue slices, these devices provide optimal electrode implantation. To prepare tissues for patch clamp studies in a way that ensures accurate and dependable recordings, researchers can select between using
535:
A loose patch clamp is different from the other techniques discussed here in that it employs a loose seal (low electrical resistance) rather than the tight gigaseal used in the conventional technique. This technique was used as early as the year 1961, as described in a paper by
Strickholm on the
514:
Advantages of the perforated patch method, relative to whole-cell recordings, include the properties of the antibiotic pores, that allow equilibration only of small monovalent ions between the patch pipette and the cytosol, but not of larger molecules that cannot permeate through the pores. This
301:
Several variations of the basic technique can be applied, depending on what the researcher wants to study. The inside-out and outside-out techniques are called "excised patch" techniques, because the patch is excised (removed) from the main body of the cell. Cell-attached and both excised patch
107:
developed the patch clamp in the late 1970s and early 1980s. This discovery made it possible to record the currents of single ion channel molecules for the first time, which improved understanding of the involvement of channels in fundamental cell processes such as action potentials and nerve
379:
To achieve the inside-out configuration, the pipette is attached to the cell membrane as in the cell-attached mode, forming a gigaseal, and is then retracted to break off a patch of membrane from the rest of the cell. Pulling off a membrane patch often results initially in the formation of a
250:. To make these recordings, the patch pipette is compared to the ground electrode. Current is then injected into the system to maintain a constant, set voltage. The current that is needed to clamp the voltage is opposite in sign and equal in magnitude to the current through the membrane.
452:
The name "outside-out" emphasizes both this technique's complementarity to the inside-out technique, and the fact that it places the external rather than intracellular surface of the cell membrane on the outside of the patch of membrane, in relation to the patch electrode.
305:
Whole-cell patch and perforated patch allow the researcher to study the electrical behavior of the entire cell, instead of single channel currents. The whole-cell patch, which enables low-resistance electrical access to the inside of a cell, has now largely replaced
536:
impedance of a muscle cell's surface, but received little attention until being brought up again and given a name by Almers, Stanfield, and Stühmer in 1982, after patch clamp had been established as a major tool of electrophysiology.
376:
an experimenter wishes to manipulate the environment at the intracellular surface of single ion channels. For example, channels that are activated by intracellular ligands can then be studied through a range of ligand concentrations.
1239:
von
Beckerath, N; Adelsberger, H; Parzefall, F; Franke, C; Dudel, J (Apr 1995). "GABAergic inhibition of crayfish deep extensor abdominal muscle exhibits a steep dose-response relationship and a high degree of cooperativity".
209:
seal with the cell membrane. To obtain this high resistance seal, the micropipette is pressed against a cell membrane and suction is applied. A portion of the cell membrane is suctioned into the pipette, creating an
456:
The formation of an outside-out patch begins with a whole-cell recording configuration. After the whole-cell configuration is formed, the electrode is slowly withdrawn from the cell, allowing a bulb of membrane to
435:
environment of the interior of the cell to minimize any changes this may cause. There is often a period at the beginning of a whole-cell recording when one can take measurements before the cell has been dialyzed.
932:
Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ.; Marty; Neher; Sakmann; Sigworth (1981). "Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches".
355:
can be established in only one patch. Another potential drawback of this technique is that, just as the intracellular pathways of the cell are not disturbed, they cannot be directly modified either.
465:
Outside-out patching gives the experimenter the opportunity to examine the properties of an ion channel when it is isolated from the cell and exposed successively to different solutions on the
218:
range, called a "gigaohm seal" or "gigaseal". The high resistance of this seal makes it possible to isolate electronically the currents measured across the membrane patch with little competing
190:
surface area or "patch" that often contains just one or a few ion channel molecules. This type of electrode is distinct from the "sharp microelectrode" used to puncture cells in traditional
126:
93:
technique can be used. In this case, the current passing across the membrane is controlled by the experimenter and the resulting changes in voltage are recorded, generally in the form of
1591:
431:
the cell's contents. After a while, any properties of the cell that depend on soluble intracellular contents will be altered. The pipette solution used usually approximates the high-
469:
surface of the membrane. The experimenter can perfuse the same patch with a variety of solutions in a relatively short amount of time, and if the channel is activated by a
1155:
Staley, K.J.; Otis, T. S.; Mody, I (May 1, 1992). "Membrane properties of dentate gyrus granule cells: comparison of sharp microelectrode and whole-cell recordings".
89:
technique. In this case, the voltage across the cell membrane is controlled by the experimenter and the resulting currents are recorded. Alternatively, the
601:
systems have been developed in order to collect large amounts of data inexpensively in a shorter period of time. Such systems typically include a single-use
351:
can be clamped successively at different membrane potentials in a single patch. This results in channel activation as a function of voltage, and a complete
175:
The solution filling the patch pipette might match the ionic composition of the bath solution, as in the case of cell-attached recording, or match the
246:
that use the bath electrode to set the zero current (ground) level. This allows a researcher to keep the voltage constant while observing changes in
35:
A patch clamp recording of current reveals transitions between two conductance states of a single ion channel: closed (at top) and open (at bottom).
986:
1604:
234:
Patch clamp of a nerve cell within a slice of brain tissue. The pipette in the photograph has been marked with a slight blue color.
156:
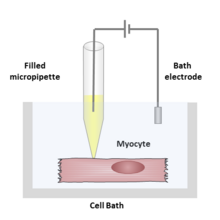
electrode. An electrical circuit can be formed between the recording and reference electrode with the cell of interest in between.
371:
In the inside-out method, a patch of the membrane is attached to the patch pipette, detached from the rest of the cell, and the
1435:"Effect of Agrin on the Distribution of Acetylcholine Receptors and Sodium Channels on Adult Skeletal Muscle Fibers in Culture"
205:
In some experiments, the micropipette tip is heated in a microforge to produce a smooth surface that assists in forming a high
182:
Depending on what the researcher is trying to measure, the diameter of the pipette tip used may vary, but it is usually in the
1305:
860:
109:
302:
techniques are used to study the behavior of individual ion channels in the section of membrane attached to the electrode.
55:, tissue sections, or patches of cell membrane. The technique is especially useful in the study of excitable cells such as
1482:
Tripathy, Shreejoy J.; Toker, Lilah; Bomkamp, Claire; Mancarci, B. Ogan; Belmadani, Manuel; Pavlidis, Paul (2018-10-08).
798:
Sigworth, Fredrick J.; Neher, E. (October 2, 1980). "Single Na+ channel currents observed in cultured rat muscle cells".
335:
1383:"Lateral distribution of sodium and potassium channels in frog skeletal muscle: measurements with a patch clamp method"
427:
of the cell's interior will slowly be replaced by the contents of the electrode. This is referred to as the electrode
1103:
1026:
730:
381:
163:
Schematic depiction of a pipette puller device used to prepare micropipettes for patch clamp and other recordings
1633:
516:
423:
307:
1192:"Currents through single glutamate receptor channels in outside-out patches from rat cerebellar granule cells"
191:
753:
Sakmann, B.; Neher, E. (1984). "Patch clamp techniques for studying ionic channels in excitable membranes".
1043:
558:
is used to fully characterize neurons across multiple modalities. As neural tissues are one of the most
1070:
662:
1628:
575:
474:
344:
931:
348:
331:
1623:
947:
687:
347:
per patch. Therefore, the dose response is accomplished using several cells and patches. However,
254:
1596:
570:
in order to understand the circuits they form is a major challenge for neuroscientists. Combining
515:
property maintains endogenous levels of divalent ions such as Ca and signaling molecules such as
1292:. Methods in Molecular Biology. Vol. 998 (Second ed.). Humana Press. pp. 149–157.
559:
428:
1601:
942:
583:
448:
Outside-out patch formation technique. In order: top-left, top-right, bottom-left, bottom-right
243:
194:, in that it is sealed onto the surface of the cell membrane, rather than inserted through it.
1288:
Linley, John (2013). "Perforated Whole-Cell Patch-Clamp
Recording". In Gamper, Nikita (ed.).
980:
878:"Patch clamp techniques used for studying synaptic transmission in slices of mammalian brain"
850:
598:
206:
578:
post-hoc has proved to be difficult and slow. By combining multiple data modalities such as
807:
657:
610:
153:
44:
904:
8:
458:
415:
811:
766:
715:
Essential Guide to
Reading Biomedical Papers: Recognizing and Interpreting Best Practice
478:
involves more steps that could fail and results in a lower frequency of usable patches.
1518:
1483:
1459:
1434:
1407:
1382:
1355:
1330:
1265:
1216:
1191:
1132:
968:
831:
258:
214:-shaped area of membrane which, if formed properly, creates a resistance in the 10–100
152:. Another electrode is placed in a bath surrounding the cell or tissue as a reference
1571:
1523:
1505:
1464:
1412:
1360:
1311:
1301:
1257:
1221:
1172:
1137:
1119:
1022:
960:
909:
894:
877:
856:
823:
780:
775:
726:
667:
647:
606:
579:
90:
48:
972:
141:
1561:
1513:
1495:
1454:
1446:
1402:
1398:
1394:
1350:
1342:
1293:
1269:
1249:
1211:
1207:
1203:
1164:
1127:
1111:
1014:
952:
899:
889:
835:
815:
770:
762:
718:
652:
555:
548:
470:
339:
278:
247:
219:
134:
94:
1608:
1376:
1374:
1297:
500:
257:
in whole-cell mode, keeping current constant while observing changes in membrane
1183:
627:
563:
405:
149:
52:
1371:
1168:
571:
1617:
1566:
1545:
1509:
1500:
1123:
602:
466:
282:
239:
187:
86:
60:
1331:"Impedance of a Small Electrically Isolated Area of the Muscle Cell Surface"
318:
1575:
1527:
1364:
1315:
1141:
642:
632:
613:(PDMS) cast chip, to capture a cell or cells, and an integrated electrode.
390:
352:
104:
79:
64:
1468:
1450:
1416:
1346:
1261:
1225:
1176:
964:
913:
827:
784:
722:
444:
197:
167:
148:
connected to an amplifier is brought into contact with the membrane of an
125:
19:
486:
363:
310:
recording techniques to record currents across the entire cell membrane.
230:
100:
24:
1592:
The Axon Guide - Electrophysiology and
Biophysics Laboratory Techniques
1253:
1238:
1018:
956:
637:
587:
508:
496:
215:
183:
130:
1011:
Patch
Clamping: An Introductory Guide To Patch Clamp Electrophysiology
1546:"Development of a Novel Automated Ion Channel Recording Method Using
819:
567:
432:
277:
vibratomes for softer tissues and microtomes for tougher structures.
273:
176:
145:
144:
or patch pipette filled with an electrolyte solution and a recording
71:
1428:
1426:
527:
159:
1102:
Segev, Amir; Garcia-Oscos, Francisco; Kourrich, Saïd (2016-06-15).
504:
394:
222:, as well as providing some mechanical stability to the recording.
75:
68:
1232:
1115:
554:
A combination of cellular imaging, RNA sequencing and patch clamp
1423:
386:
372:
31:
875:
140:
During a patch clamp recording, a hollow glass tube known as a
56:
876:
Sakmann, B.; Edwards, F.; Konnerth, A.; Takahashi, T. (1989).
927:
925:
923:
389:
solution, or by momentarily making contact with a droplet of
343:
the pipette. The technique is thus limited to one point in a
293:
211:
201:
Typical equipment used during classical patch clamp recording
1481:
400:
920:
171:
Circuit formed during whole-cell or perforated patch clamp
1101:
713:
Bannister, Niel (November 1, 2012). Langton, Phil (ed.).
1544:
Bowlby, Mark; Merrill, Thomas; Vasilyev, Dmitry (2005).
1322:
1484:"Assessing Transcriptome Quality in Patch-Seq Datasets"
1189:
422:
The advantage of whole-cell patch clamp recording over
297:
Diagram showing variations of the patch clamp technique
1380:
848:
1543:
1190:Howe, JR; Cull-Candy, SG; Colquhoun, D (Jan 1991).
1104:"Whole-cell Patch-clamp Recordings in Brain Slices"
1615:
688:"The Nobel Prize in Physiology or Medicine 1991"
935:Pflügers Archiv: European Journal of Physiology
1154:
842:
1597:Alternative images for patch clamp variations
797:
1432:
985:: CS1 maint: multiple names: authors list (
882:Quarterly Journal of Experimental Physiology
752:
238:Many patch clamp amplifiers do not use true
186:range. This small size is used to enclose a
593:
285:are the notable producer of these devices.
51:used to study ionic currents in individual
1381:Almers W, Stanfield PR, Stühmer W (1983).
1328:
1068:
1004:
1002:
1000:
998:
996:
748:
746:
744:
742:
85:Patch clamping can be performed using the
74:, and can also be applied to the study of
1565:
1539:
1537:
1517:
1499:
1458:
1406:
1354:
1283:
1281:
1279:
1215:
1131:
1041:
946:
903:
893:
855:. Oxford University Press. pp. 22–.
774:
712:
108:activity. Neher and Sakmann received the
78:ion channels in specially prepared giant
16:Laboratory technique in electrophysiology
1035:
1008:
706:
526:
485:
443:
404:
401:Whole-cell recording or whole-cell patch
362:
317:
292:
229:
196:
166:
158:
124:
30:
18:
993:
739:
473:or drug from the extracellular face, a
1616:
1534:
1287:
1276:
1148:
313:
1097:
1095:
1062:
110:Nobel Prize in Physiology or Medicine
680:
439:
267:
1602:Animation of the Patch Clamp Method
1488:Frontiers in Molecular Neuroscience
1433:Lupa, MT; Caldwell, JH (Nov 1991).
767:10.1146/annurev.ph.46.030184.002323
481:
358:
13:
1092:
1009:Molleman, Areles (March 6, 2003).
852:Basic Electrophysiological Methods
334:or channels that are modulated by
129:Classical patch clamp setup, with
115:
14:
1645:
1585:
1554:Journal of Biomolecular Screening
1108:Journal of Visualized Experiments
849:Ellen Covey; Matt Carter (2015).
322:Cell-attached patch configuration
272:Accurate tissue sectioning with
1069:Ogden, David; Stanfield, Peter.
1042:Veitinger, Sophie (2011-11-09).
895:10.1113/expphysiol.1989.sp003336
572:classical classification methods
1475:
414:interior of the pipette to the
253:Alternatively, the cell can be
1399:10.1113/jphysiol.1983.sp014580
1242:European Journal of Physiology
1208:10.1113/jphysiol.1991.sp018381
905:11858/00-001M-0000-002C-270A-9
869:
791:
522:
409:Whole-cell patch configuration
367:Inside-out patch configuration
308:high-resistance microelectrode
1:
1335:Journal of General Physiology
674:
288:
1329:Strickholm, A (1 Jul 1961).
1298:10.1007/978-1-62703-351-0_11
547:
225:
27:patched with a glass pipette
7:
1044:"The Patch-Clamp Technique"
755:Annual Review of Physiology
620:
566:, classifying neurons into
531:Loose patch clamp technique
353:I-V (current-voltage) curve
242:circuitry, but instead are
133:, antivibration table, and
10:
1650:
1157:Journal of Neurophysiology
576:single cell RNA-sequencing
490:Perforated patch technique
349:voltage-gated ion channels
1169:10.1152/jn.1992.67.5.1346
776:21.11116/0000-0000-D552-3
424:sharp electrode technique
332:ligand-gated ion channels
120:
1567:10.1177/1087057105279481
1501:10.3389/fnmol.2018.00363
1071:"Patch Clamp Techniques"
594:Automatic patch clamping
192:intracellular recordings
1439:Journal of Cell Biology
562:diverse populations of
244:differential amplifiers
112:in 1991 for this work.
532:
491:
449:
410:
368:
336:metabotropic receptors
323:
298:
274:compresstome vibratome
235:
202:
172:
164:
137:
36:
28:
1634:Laboratory techniques
1550:Whole-Cell Membranes"
1451:10.1083/jcb.115.3.765
1387:Journal of Physiology
1347:10.1085/jgp.44.6.1073
1196:Journal of Physiology
723:10.1002/9781118402184
599:Automated patch clamp
530:
489:
447:
408:
366:
321:
296:
233:
200:
170:
162:
128:
53:isolated living cells
41:patch clamp technique
34:
22:
1050:. Leica Microsystems
658:Microelectrode array
611:polydimethylsiloxane
393:or a piece of cured
45:laboratory technique
812:1980Natur.287..447S
717:. Wiley-Blackwell.
416:intracellular space
345:dose response curve
314:Cell-attached patch
1607:2017-01-30 at the
1254:10.1007/bf00374801
1019:10.1002/0470856521
957:10.1007/BF00656997
663:Planar patch clamp
605:device, either an
560:transcriptomically
533:
492:
450:
411:
369:
324:
299:
236:
203:
173:
165:
138:
37:
29:
1629:Electrophysiology
1307:978-1-62703-351-0
862:978-0-19-993980-0
806:(5781): 447–449.
668:Slice preparation
648:GHK flux equation
580:electrophysiology
440:Outside-out patch
373:cytosolic surface
268:Tissue sectioning
135:micromanipulators
95:action potentials
49:electrophysiology
1641:
1580:
1579:
1569:
1541:
1532:
1531:
1521:
1503:
1479:
1473:
1472:
1462:
1430:
1421:
1420:
1410:
1378:
1369:
1368:
1358:
1326:
1320:
1319:
1285:
1274:
1273:
1236:
1230:
1229:
1219:
1187:
1181:
1180:
1163:(5): 1346–1358.
1152:
1146:
1145:
1135:
1099:
1090:
1089:
1087:
1085:
1080:. pp. 53–78
1075:
1066:
1060:
1059:
1057:
1055:
1039:
1033:
1032:
1006:
991:
990:
984:
976:
950:
929:
918:
917:
907:
897:
888:(7): 1107–1118.
873:
867:
866:
846:
840:
839:
820:10.1038/287447a0
795:
789:
788:
778:
750:
737:
736:
710:
704:
703:
701:
699:
694:. Nobel Media AB
684:
653:Goldman equation
607:injection molded
482:Perforated patch
471:neurotransmitter
359:Inside-out patch
340:neurotransmitter
279:Leica Biosystems
1649:
1648:
1644:
1643:
1642:
1640:
1639:
1638:
1624:Neurophysiology
1614:
1613:
1609:Wayback Machine
1588:
1583:
1542:
1535:
1480:
1476:
1431:
1424:
1393:(10): 261–284.
1379:
1372:
1327:
1323:
1308:
1286:
1277:
1237:
1233:
1188:
1184:
1153:
1149:
1110:(112): e54024.
1100:
1093:
1083:
1081:
1073:
1067:
1063:
1053:
1051:
1040:
1036:
1029:
1007:
994:
978:
977:
930:
921:
874:
870:
863:
847:
843:
796:
792:
751:
740:
733:
711:
707:
697:
695:
686:
685:
681:
677:
672:
623:
596:
552:
525:
501:amphothericin-B
499:agent, such as
484:
442:
403:
361:
316:
291:
270:
264:
255:current clamped
228:
123:
118:
116:Basic technique
17:
12:
11:
5:
1647:
1637:
1636:
1631:
1626:
1612:
1611:
1599:
1594:
1587:
1586:External links
1584:
1582:
1581:
1560:(8): 806–813.
1533:
1474:
1445:(3): 765–778.
1422:
1370:
1341:(6): 1073–88.
1321:
1306:
1275:
1248:(6): 781–788.
1231:
1202:(1): 143–202.
1182:
1147:
1091:
1061:
1034:
1027:
992:
948:10.1.1.456.107
919:
868:
861:
841:
790:
738:
731:
705:
692:nobelprize.org
678:
676:
673:
671:
670:
665:
660:
655:
650:
645:
640:
635:
630:
628:Bioelectronics
624:
622:
619:
595:
592:
551:
546:
524:
521:
483:
480:
441:
438:
402:
399:
360:
357:
315:
312:
290:
287:
269:
266:
227:
224:
122:
119:
117:
114:
61:cardiomyocytes
15:
9:
6:
4:
3:
2:
1646:
1635:
1632:
1630:
1627:
1625:
1622:
1621:
1619:
1610:
1606:
1603:
1600:
1598:
1595:
1593:
1590:
1589:
1577:
1573:
1568:
1563:
1559:
1555:
1551:
1549:
1540:
1538:
1529:
1525:
1520:
1515:
1511:
1507:
1502:
1497:
1493:
1489:
1485:
1478:
1470:
1466:
1461:
1456:
1452:
1448:
1444:
1440:
1436:
1429:
1427:
1418:
1414:
1409:
1404:
1400:
1396:
1392:
1388:
1384:
1377:
1375:
1366:
1362:
1357:
1352:
1348:
1344:
1340:
1336:
1332:
1325:
1317:
1313:
1309:
1303:
1299:
1295:
1291:
1284:
1282:
1280:
1271:
1267:
1263:
1259:
1255:
1251:
1247:
1243:
1235:
1227:
1223:
1218:
1213:
1209:
1205:
1201:
1197:
1193:
1186:
1178:
1174:
1170:
1166:
1162:
1158:
1151:
1143:
1139:
1134:
1129:
1125:
1121:
1117:
1116:10.3791/54024
1113:
1109:
1105:
1098:
1096:
1079:
1072:
1065:
1049:
1045:
1038:
1030:
1028:9780470856529
1024:
1020:
1016:
1012:
1005:
1003:
1001:
999:
997:
988:
982:
974:
970:
966:
962:
958:
954:
949:
944:
941:(2): 85–100.
940:
936:
928:
926:
924:
915:
911:
906:
901:
896:
891:
887:
883:
879:
872:
864:
858:
854:
853:
845:
837:
833:
829:
825:
821:
817:
813:
809:
805:
801:
794:
786:
782:
777:
772:
768:
764:
760:
756:
749:
747:
745:
743:
734:
732:9781118402184
728:
724:
720:
716:
709:
693:
689:
683:
679:
669:
666:
664:
661:
659:
656:
654:
651:
649:
646:
644:
641:
639:
636:
634:
631:
629:
626:
625:
618:
614:
612:
608:
604:
600:
591:
589:
585:
581:
577:
573:
569:
565:
561:
557:
550:
545:
541:
537:
529:
520:
518:
512:
510:
506:
502:
498:
488:
479:
476:
475:dose-response
472:
468:
467:extracellular
463:
460:
454:
446:
437:
434:
430:
425:
420:
417:
407:
398:
396:
392:
388:
383:
377:
374:
365:
356:
354:
350:
346:
341:
337:
333:
328:
320:
311:
309:
303:
295:
286:
284:
283:Carl Zeiss AG
280:
275:
265:
262:
260:
256:
251:
249:
245:
241:
240:voltage clamp
232:
223:
221:
217:
213:
208:
199:
195:
193:
189:
188:cell membrane
185:
180:
178:
169:
161:
157:
155:
151:
150:isolated cell
147:
143:
136:
132:
127:
113:
111:
106:
102:
98:
96:
92:
91:current clamp
88:
87:voltage clamp
83:
81:
77:
73:
70:
66:
65:muscle fibers
62:
58:
54:
50:
46:
42:
33:
26:
21:
1557:
1553:
1547:
1491:
1487:
1477:
1442:
1438:
1390:
1386:
1338:
1334:
1324:
1290:Ion Channels
1289:
1245:
1241:
1234:
1199:
1195:
1185:
1160:
1156:
1150:
1107:
1084:November 11,
1082:. Retrieved
1078:utdallas.edu
1077:
1064:
1054:November 10,
1052:. Retrieved
1047:
1037:
1010:
981:cite journal
938:
934:
885:
881:
871:
851:
844:
803:
799:
793:
758:
754:
714:
708:
696:. Retrieved
691:
682:
643:Channelomics
633:Cable theory
615:
603:microfluidic
597:
553:
542:
538:
534:
513:
493:
464:
455:
451:
421:
412:
378:
370:
329:
325:
304:
300:
271:
263:
252:
237:
204:
181:
174:
142:micropipette
139:
105:Bert Sakmann
99:
84:
80:spheroplasts
40:
38:
23:A bacterial
1048:Science Lab
761:: 455–472.
698:November 8,
556:this method
523:Loose patch
429:"dialyzing"
101:Erwin Neher
25:spheroplast
1618:Categories
1548:Inside-Out
675:References
638:Channelome
588:microscopy
584:sequencing
568:cell types
509:gramicidin
497:antibiotic
289:Variations
207:resistance
184:micrometer
131:microscope
72:beta cells
69:pancreatic
1510:1662-5099
1124:1940-087X
1013:. Wiley.
943:CiteSeerX
549:Patch-Seq
433:potassium
397:polymer.
226:Recording
177:cytoplasm
146:electrode
76:bacterial
1605:Archived
1576:16234349
1528:30349457
1365:19873540
1316:23529427
1142:27341060
973:12014433
621:See also
505:nystatin
395:silicone
391:paraffin
216:gigaohms
1519:6187980
1494:: 363.
1469:1655812
1460:2289169
1417:6308223
1408:1198969
1356:2195146
1270:7824699
1262:7541524
1226:1715916
1217:1181322
1177:1597717
1133:4927800
965:6270629
914:2560557
836:4238010
828:6253802
808:Bibcode
785:6143532
382:vesicle
259:voltage
248:current
57:neurons
1574:
1526:
1516:
1508:
1467:
1457:
1415:
1405:
1363:
1353:
1314:
1304:
1268:
1260:
1224:
1214:
1175:
1140:
1130:
1122:
1025:
971:
963:
945:
912:
859:
834:
826:
800:Nature
783:
729:
338:, the
154:ground
121:Set-up
67:, and
1266:S2CID
1074:(PDF)
969:S2CID
832:S2CID
609:or a
574:with
564:cells
507:, or
220:noise
212:omega
43:is a
1572:PMID
1524:PMID
1506:ISSN
1465:PMID
1413:PMID
1361:PMID
1312:PMID
1302:ISBN
1258:PMID
1222:PMID
1173:PMID
1138:PMID
1120:ISSN
1086:2014
1056:2014
1023:ISBN
987:link
961:PMID
910:PMID
857:ISBN
824:PMID
781:PMID
727:ISBN
700:2014
586:and
517:cAMP
459:bleb
385:low
330:For
103:and
39:The
1562:doi
1514:PMC
1496:doi
1455:PMC
1447:doi
1443:115
1403:PMC
1395:doi
1391:336
1351:PMC
1343:doi
1294:doi
1250:doi
1246:429
1212:PMC
1204:doi
1200:432
1165:doi
1128:PMC
1112:doi
1015:doi
953:doi
939:391
900:hdl
890:doi
816:doi
804:287
771:hdl
763:doi
719:doi
495:or
47:in
1620::
1570:.
1558:10
1556:.
1552:.
1536:^
1522:.
1512:.
1504:.
1492:11
1490:.
1486:.
1463:.
1453:.
1441:.
1437:.
1425:^
1411:.
1401:.
1389:.
1385:.
1373:^
1359:.
1349:.
1339:44
1337:.
1333:.
1310:.
1300:.
1278:^
1264:.
1256:.
1244:.
1220:.
1210:.
1198:.
1194:.
1171:.
1161:67
1159:.
1136:.
1126:.
1118:.
1106:.
1094:^
1076:.
1046:.
1021:.
995:^
983:}}
979:{{
967:.
959:.
951:.
937:.
922:^
908:.
898:.
886:74
884:.
880:.
830:.
822:.
814:.
802:.
779:.
769:.
759:46
757:.
741:^
725:.
690:.
582:,
503:,
387:Ca
281:,
261:.
97:.
82:.
63:,
59:,
1578:.
1564::
1530:.
1498::
1471:.
1449::
1419:.
1397::
1367:.
1345::
1318:.
1296::
1272:.
1252::
1228:.
1206::
1179:.
1167::
1144:.
1114::
1088:.
1058:.
1031:.
1017::
989:)
975:.
955::
916:.
902::
892::
865:.
838:.
818::
810::
787:.
773::
765::
735:.
721::
702:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.