246:
125:
33:
1714:
195:
180:
1708:
1720:
446:: The π systems form two parallel rings overlap in a "face-to-face" orientation. Aromatic molecules are also able to interact with each other in an "edge-to-face" orientation: The slight positive charge of the substituents on the ring atoms of one molecule are attracted to the slight negative charge of the aromatic system on another molecule.
462:
was discovered to adopt an asymmetric, rectangular configuration in which single and double bonds indeed alternate; there is no resonance and the single bonds are markedly longer than the double bonds, reducing unfavorable p-orbital overlap. This reduction of symmetry lifts the degeneracy of the two
403:
is not, since the number of π delocalized electrons is 4, which of course is a multiple of 4. The cyclobutadienide (2−) ion, however, is aromatic (6 electrons). An atom in an aromatic system can have other electrons that are not part of the system, and are therefore ignored for the 4n + 2 rule. In
185:
Since they are out of the plane of the atoms, these orbitals can interact with each other freely, and become delocalized. This means that, instead of being tied to one atom of carbon, each electron is shared by all six in the ring. Thus, there are not enough electrons to form double bonds on all the
467:
forces the two unpaired electrons into a new, weakly bonding orbital (and also creates a weakly antibonding orbital). Hence, cyclobutadiene is non-aromatic; the strain of the asymmetric configuration outweighs the anti-aromatic destabilization that would afflict the symmetric, square configuration.
411:
Aromatic molecules typically display enhanced chemical stability, compared to similar non-aromatic molecules. A molecule that can be aromatic will tend to alter its electronic or conformational structure to be in this situation. This extra stability changes the chemistry of the molecule. Aromatic
439:. The NMR signal of protons in the plane of an aromatic ring are shifted substantially further down-field than those on non-aromatic sp² carbons. This is an important way of detecting aromaticity. By the same mechanism, the signals of protons located near the ring axis are shifted up-field.
427:
Many of the earliest-known examples of aromatic compounds, such as benzene and toluene, have distinctive pleasant smells. This property led to the term "aromatic" for this class of compounds, and hence the term "aromaticity" for the eventually discovered electronic property.
876:. A π system with 4n electrons in a flat (non-twisted) ring would be anti-aromatic, and therefore highly unstable, due to the symmetry of the combinations of p atomic orbitals. By twisting the ring, the symmetry of the system changes and becomes allowed (see also
1248:
Alexander Kuhn, Puravankara
Sreeraj, Rainer Pöttgen, Hans-Dieter Wiemhöfer, Martin Wilkening,Paul Heitjans (2011). "Li NMR Spectroscopy on Crystalline Li12Si7: Experimental Evidence for the Aromaticity of the Planar Cyclopentadienyl-Analogous Si56− Rings".
561:. About 35 million tonnes are produced worldwide every year. They are extracted from complex mixtures obtained by the refining of oil or by distillation of coal tar, and are used to produce a range of important chemicals and polymers, including
140:
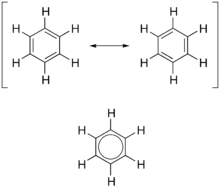
compound, which is best represented by a hybrid (average) of these structures, which can be seen at right. A C=C bond is shorter than a C−C bond, but benzene is perfectly hexagonal—all six carbon-carbon bonds have the same
837:) are structurally analogous to benzene, with the carbon atoms replaced by another element or elements. In borazine, the boron and nitrogen atoms alternate around the ring. Quite recently, the aromaticity of planar Si
115:
forms, which corresponds to the double and single bonds superimposing to give rise to six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization.
453:
and are, in general, destabilized. Molecules that could be antiaromatic will tend to alter their electronic or conformational structure to avoid this situation, thereby becoming non-aromatic. For example,
920:
cation. Guanidinium does not have a ring structure but has six π-electrons which are delocalized over the molecule. However, this concept is controversial and some authors have stressed different effects.
294:) ... and when an additive compound is formed, the inner cycle of affinity suffers disruption, the contiguous carbon-atoms to which nothing has been attached of necessity acquire the ethylenic condition".
408:, the oxygen atom is sp² hybridized. One lone pair is in the π system and the other in the plane of the ring (analogous to C-H bond on the other positions). There are 6 π electrons, so furan is aromatic.
329:, since he recognized that his affinities had direction, not merely being point particles, and collectively having a distribution that could be altered by introducing substituents onto the benzene ring (
99:
to one another. These bonds may be seen as a hybrid of a single bond and a double bond, each bond in the ring identical to every other. This commonly seen model of aromatic rings, namely the idea that
1338:
253:
In the 19th century chemists found it puzzling that benzene could be so unreactive toward addition reactions, given its presumed high degree of unsaturation. The cyclohexatriene structure for
809:
the aromaticity is still retained. Aromaticity also occurs in compounds that are not carbon-based at all. Inorganic 6-membered-ring compounds analogous to benzene have been synthesized.
1474:
R. Caminiti, A. Pieretti, L. Bencivenni, F. Ramondo, N. Sanna (1996). "Amidine N−C(N)−N Skeleton: Its
Structure in Isolated and Hydrogen-Bonded Guanidines from ab Initio Calculations".
3005:
1420:
Alberto Gobbi, Gemot
Frenking (1993). "Y-Conjugated compounds: the equilibrium geometries and electronic structures of guanidine, guanidinium cation, urea, and 1,1-diaminoethylene".
136:, a double-headed arrow is used to indicate that the two structures are not distinct entities, but merely hypothetical possibilities. Neither is an accurate representation of the
233:, which are not aromatic in the chemical sense. But terpenes and benzenoid substances do have a chemical characteristic in common, namely higher unsaturation indices than many
261:
in 1865. Over the next few decades, most chemists readily accepted this structure, since it accounted for most of the known isomeric relationships of aromatic chemistry.
5220:
229:
character to apply to a group of chemical substances only some of which have notable aromas. Also, many of the most odoriferous organic substances known are
2019:
225:
in 1855. If this is indeed the earliest introduction of the term, it is curious that
Hofmann says nothing about why he introduced an adjective indicating
636:), one or more of the atoms in the aromatic ring is of an element other than carbon. This can lessen the ring's aromaticity, and thus (as in the case of
68:
exhibits a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by
4336:
4281:
810:
5049:
1807:
1501:
1371:
1325:
1276:
1339:
Claire Castro, Zhongfang Chen, Chaitanya S. Wannere, Haijun Jiao, William L. Karney, Michael
Mauksch, Ralph Puchta, Nico J. R. van Eikema Hommes,
4391:
4541:
3175:
5270:
5044:
2870:
897:
1247:
4146:
2067:
1963:
4716:
3916:
2660:
896:. As of 2012, there is no proof that a Möbius aromatic molecule was synthesized. Aromatics with two half-twists corresponding to the
4881:
4811:
4791:
4286:
3453:
2915:
104:
3334:
2890:
1925:
1852:
1533:
950:
2103:
1999:
4636:
1343:(2005). "Investigation of a Putative Möbius Aromatic Hydrocarbon. The Effect of Benzannelation on Möbius Annulene Aromaticity".
1139:
Armit, James Wilson; Robinson, Robert (1925). "CCXI.?Polynuclear heterocyclic aromatic types. Part II. Some anhydronium bases".
5114:
5064:
3468:
1050:
A. T. Balaban, P. v. R. Schleyer and H. S. Rzepa (2005). "Crocker, Not Armit and
Robinson, Begat the Six Aromatic Electrons".
4571:
5210:
5019:
4676:
4656:
4616:
3423:
2366:
683:
are molecules containing two or more simple aromatic rings fused together by sharing two neighboring carbon atoms (see also
5205:
5135:
5034:
4691:
4546:
4176:
4021:
3631:
3258:
3035:
164:
above and below the ring. This model more correctly represents the location of electron density within the aromatic ring.
5369:
5285:
5069:
4381:
3871:
3546:
1847:
413:
302:
4091:
103:
was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by
5280:
4994:
4856:
4646:
4611:
417:
5170:
5109:
4641:
4556:
4526:
4506:
4371:
4366:
3741:
3666:
3309:
3263:
3130:
2391:
272:
5275:
5235:
5185:
4861:
4661:
4411:
4341:
2830:
2401:
1772:
297:
Here, Armstrong is describing at least four modern concepts. First, his "affinity" is better known nowadays as the
4476:
3369:
3090:
5029:
4871:
4741:
4736:
4551:
4026:
3936:
3526:
3458:
3349:
2925:
2680:
2605:
2060:
1956:
1596:
945:
680:
4786:
1787:
5315:
5200:
5099:
5039:
4686:
4481:
4441:
4416:
4326:
3786:
2820:
2750:
2386:
2316:
1623:
1584:
1574:
916:
Y-aromaticity is a concept which was developed to explain the extraordinary stability and high basicity of the
877:
458:(COT) distorts itself out of planarity, breaking π overlap between adjacent double bonds. Relatively recently,
3906:
5305:
4891:
4781:
4401:
4126:
3911:
3856:
3701:
3661:
3493:
3248:
2965:
2815:
1579:
124:
5265:
4826:
4271:
1221:
Merino, Gabriel; Heine, Thomas; Seifert, Gotthard (2004). "The
Induced Magnetic Field in Cyclic Molecules".
589:
The overwhelming majority of aromatic compounds are compounds of carbon, but they need not be hydrocarbons.
5300:
5215:
5190:
5165:
5150:
5074:
4989:
4886:
4846:
4711:
4666:
4431:
3976:
3961:
3826:
3616:
3284:
3040:
2720:
2695:
2665:
2256:
1526:
1447:
Kenneth B. Wiberg (1990). "Resonance interactions in acyclic systems. 2. Y-Conjugated anions and cations".
476:
Aromatic compounds play key roles in the biochemistry of all living things. The four aromatic amino acids
268:, the discoverer of the electron, proposed three equivalent electrons between each carbon atom in benzene.
194:
5364:
5250:
5195:
5140:
4851:
4771:
4671:
4386:
4351:
4196:
4086:
3801:
3796:
3621:
3581:
3478:
3289:
3253:
3105:
3095:
2950:
2810:
2670:
2620:
2615:
2590:
2550:
2496:
2261:
2251:
2226:
331:
much as the distribution of the electric charge in a body is altered by bringing it near to another body
286:, who in 1890 wrote "the (six) centric affinities act within a cycle...benzene may be represented by a
5225:
4926:
4731:
4166:
4051:
3731:
3706:
3646:
3503:
3238:
2945:
2725:
2690:
2595:
2286:
2221:
2053:
1949:
186:
carbon atoms, but the "extra" electrons strengthen all of the bonds on the ring equally. The resulting
4516:
2780:
175:
above and below the plane of the ring. The following diagram shows the positions of these p-orbitals:
5325:
5230:
5084:
4964:
4936:
4906:
4821:
4751:
4706:
4681:
4601:
4501:
4461:
4156:
3776:
3766:
3691:
3215:
3075:
3070:
3050:
2735:
2532:
2511:
2471:
2396:
2034:
1842:
1832:
1822:
1797:
1767:
179:
2216:
5290:
5180:
5160:
5024:
4866:
4776:
4746:
4726:
4621:
4576:
4406:
4316:
4246:
4131:
4121:
3951:
3508:
3448:
3413:
3220:
3200:
3160:
2935:
2805:
2770:
2730:
2481:
2321:
2311:
2241:
1613:
245:
4756:
5260:
5119:
4969:
4911:
4836:
4816:
4536:
4486:
4346:
4311:
4251:
4181:
3736:
3483:
3463:
3195:
3115:
3010:
2970:
2940:
2875:
2760:
2745:
2655:
2645:
2306:
2231:
2186:
1874:
1777:
1749:
1519:
1340:
901:
2521:
1290:
D. Ajami, O. Oeckler, A. Simon, R. Herges (2003). "Synthesis of a Möbius aromatic hydrocarbon".
301:, which was to be discovered only seven years later by J. J. Thomson. Second, he is describing
167:
The single bonds are formed with electrons in line between the carbon nuclei — these are called
4999:
4721:
4471:
4451:
4426:
4376:
4291:
4266:
4221:
4191:
4171:
4141:
4106:
4061:
4036:
4011:
3896:
3821:
3601:
3294:
3230:
3030:
2755:
2675:
2361:
2336:
2113:
2108:
1166:
Ernest C. Crocker (1922). "Application Of The Octet Theory To Single-Ring
Aromatic Compounds".
617:
421:
283:
222:
69:
72:
in 1855. There is no general relationship between aromaticity as a chemical property and the
5335:
4921:
4876:
4591:
4561:
4531:
4466:
4446:
4361:
4356:
4321:
4276:
4261:
4256:
4236:
4226:
4161:
4151:
4081:
4031:
3551:
3354:
2930:
2885:
2715:
2705:
2451:
2376:
2171:
2133:
1918:
1879:
1495:
1473:
1365:
1319:
1270:
893:
779:
629:
432:
133:
112:
84:
37:
2381:
1913:
858:
5104:
5054:
5004:
4984:
4974:
4831:
4806:
4521:
4511:
4396:
4211:
4206:
4136:
3921:
3721:
3681:
3611:
3576:
3531:
3498:
3364:
3339:
3319:
3140:
3100:
3060:
3025:
2955:
2710:
2580:
2555:
2093:
1837:
1728:
1591:
1550:
955:
930:
759:
dianion (10e). Aromatic properties have been attributed to non-benzenoid compounds such as
684:
536:
443:
391:. That is, 4n + 2 number of π electrons, where n=0, 1, 2, 3, and so on. This is known as
365:
306:
8:
5310:
5295:
4941:
4916:
4901:
4896:
4626:
4581:
4566:
4456:
4436:
4331:
4216:
4201:
4046:
3991:
3981:
3946:
3711:
3586:
3561:
3473:
3329:
3314:
3299:
3120:
3065:
2835:
2685:
2630:
2501:
2416:
2276:
2201:
1739:
1603:
1569:
344:
in 1931. He was the first to separate the bonding electrons into sigma and pi electrons.
218:
5320:
3971:
3155:
2346:
1903:
392:
271:
An explanation for the exceptional stability of benzene is conventionally attributed to
171:. Double bonds consist of a σ-bond and a π-bond. The π-bonds are formed from overlap of
5059:
5009:
4979:
4841:
4631:
4421:
4306:
4241:
4231:
3996:
3926:
3891:
3886:
3866:
3861:
3806:
3716:
3566:
3428:
3418:
3324:
3110:
3055:
2985:
2905:
2800:
2700:
2635:
2560:
2406:
2271:
2206:
2191:
1658:
337:
234:
4796:
4116:
4001:
3966:
3931:
3876:
3831:
3746:
3726:
3676:
3671:
3641:
3626:
3536:
3443:
3379:
3344:
3170:
3045:
2920:
2845:
2825:
2740:
2575:
2570:
2516:
2426:
2331:
2291:
2246:
2128:
2123:
2088:
2014:
1973:
1889:
1678:
1638:
1628:
1402:
1345:
1307:
1168:
1032:
852:
756:
708:
605:
455:
310:
187:
53:
45:
3791:
5330:
5175:
5145:
5089:
5014:
4946:
4701:
4651:
4496:
4301:
4076:
4071:
4016:
4006:
3781:
3591:
3571:
3541:
3438:
3374:
3359:
3190:
3145:
3135:
3125:
3020:
3000:
2995:
2980:
2975:
2855:
2850:
2790:
2775:
2765:
2610:
2600:
2466:
2456:
2356:
2351:
2326:
2266:
2118:
2077:
2009:
2004:
1994:
1930:
1670:
1643:
1483:
1456:
1429:
1394:
1385:
Rzepa, Henry S. (2005). "A Double-Twist Möbius-Aromatic
Conformation of Annulene".
1353:
1299:
1258:
1230:
1203:
1176:
1148:
1121:
1092:
1061:
1052:
1024:
997:
965:
872:
by 4n (n is an integer) electrons, is given a single half-twist to correspond to a
748:
712:
57:
2446:
282:
In fact, this concept can be traced further back, via Ernest
Crocker in 1922, to
258:
5240:
4931:
4766:
4761:
4056:
4041:
3986:
3941:
3901:
3851:
3816:
3811:
3756:
3751:
3686:
3636:
3556:
3384:
3268:
3243:
3205:
3180:
3165:
3150:
3085:
2960:
2910:
2900:
2880:
2840:
2650:
2640:
2625:
2421:
2341:
2211:
2181:
2166:
2161:
2029:
1908:
1782:
1653:
940:
865:
771:
716:
450:
237:, and Hofmann may not have been making a distinction between the two categories.
1941:
1049:
873:
5245:
5155:
5094:
4186:
4096:
4066:
3841:
3696:
3433:
3210:
3080:
2895:
2865:
2565:
2461:
2236:
2098:
1817:
1618:
960:
935:
794:
492:
each serve as one of the 20 basic building-blocks of proteins. Further, all 5
464:
459:
400:
172:
80:
32:
20:
2045:
5358:
5255:
4956:
4801:
4696:
4491:
3881:
3846:
3836:
3771:
3761:
3651:
3488:
3304:
3015:
2990:
2860:
2506:
2491:
2476:
2371:
2301:
2281:
2196:
1989:
1866:
1826:
1759:
1713:
1688:
1561:
1542:
1125:
905:
806:
798:
763:. Aromatic properties are tested to the limit in a class of compounds called
744:
669:
665:
481:
373:
357:
265:
96:
65:
516:) that make up the sequence of the genetic code in DNA and RNA are aromatic
4296:
3656:
3408:
3185:
2785:
2585:
2436:
2431:
2296:
2151:
2024:
1812:
1406:
1311:
1262:
1234:
1036:
1001:
885:
869:
696:
341:
2795:
2441:
2411:
1289:
1207:
1152:
1112:
August Kekulé (1872). "Ueber einige Condensationsproducte des Aldehyds".
881:
802:
790:
786:
724:
688:
529:
521:
388:
369:
150:
146:
142:
1460:
1433:
1303:
1180:
5079:
4606:
3956:
1194:
Henry Edward Armstrong (1890). "The structure of cycloid hydrocarbon".
764:
692:
493:
485:
322:
168:
40:
forms of benzene (top) combine to produce an average structure (bottom)
1487:
1398:
1357:
1097:
1080:
1065:
1028:
372:
system, most commonly an arrangement of alternating single and double
1633:
1608:
917:
752:
661:
649:
574:
477:
442:
Aromatic molecules are able to interact with each other in so-called
226:
73:
61:
24:
156:
A better representation is that of the circular π bond (Armstrong's
2486:
2156:
1707:
822:
653:
645:
641:
601:
505:
489:
378:
340:
origins of this stability, or aromaticity, were first modelled by
298:
230:
88:
2146:
1511:
861:
occurs when a cyclic system of molecular orbitals, formed from p
760:
720:
657:
597:
570:
562:
544:
540:
517:
509:
501:
497:
449:
Planar monocyclic molecules containing 4n π electrons are called
325:'s notation. It is argued that he also anticipated the nature of
254:
161:
160:), in which the electron density is evenly distributed through a
100:
399:
Whereas benzene is aromatic (6 electrons, from 3 double bonds),
566:
555:
548:
513:
347:
215:
1015:
Schleyer, Paul von Ragué (2001). "Introduction: Aromaticity".
1419:
855:
is believed to exist in certain metal clusters of aluminium.
637:
578:
405:
79:
Aromaticity can also be considered a manifestation of cyclic
19:"Aromatic" redirects here. For meanings related to odor, see
431:
The circulating π electrons in an aromatic molecule produce
381:
structure, with all the contributing atoms in the same plane
908:
the ring bonds are extended with alkyne and allene groups.
525:
353:
326:
92:
1719:
275:, who was apparently the first (in 1925) to coin the term
740:
728:
436:
785:
When carbon in benzene is replaced by other elements in
1193:
424:
reactions as happens with carbon-carbon double bonds.
214:
term — namely, to apply to compounds that contain the
111:
section below). The model for benzene consists of two
279:
as a group of six electrons that resists disruption.
52:
is a chemical property describing the way in which a
5221:
Erlenmeyer–Plöchl azlactone and amino-acid synthesis
849:
was experimentally evidenced by Li solid state NMR.
240:
535:Aromatic compounds are important in industry. Key
471:
1220:
640:) increase its reactivity. Other examples include
463:formerly non-bonding molecular orbitals, which by
91:are free to cycle around circular arrangements of
4282:Divinylcyclopropane-cycloheptadiene rearrangement
1971:
1446:
1165:
528:contains an aromatic system with 22 π electrons.
5356:
384:Contributing atoms arranged in one or more rings
313:of the ring is broken. He introduced the symbol
249:Historic benzene formulae as proposed by Kekulé.
210:The first known use of the word "aromatic" as a
205:
2075:
4542:Thermal rearrangement of aromatic hydrocarbons
3176:Thermal rearrangement of aromatic hydrocarbons
734:
584:
5271:Lectka enantioselective beta-lactam synthesis
2531:
2061:
1957:
1527:
1141:Journal of the Chemical Society, Transactions
1138:
1111:
987:
983:
981:
774:where conjugation is interrupted by a single
5050:Inverse electron-demand Diels–Alder reaction
2871:Heterogeneous metal catalyzed cross-coupling
1500:: CS1 maint: multiple names: authors list (
1370:: CS1 maint: multiple names: authors list (
1324:: CS1 maint: multiple names: authors list (
1275:: CS1 maint: multiple names: authors list (
1196:Proceedings of the Chemical Society (London)
1105:
616:where n ≥ 4 and is an even number, such as
387:A number of π delocalized electrons that is
348:Characteristics of aromatic (aryl) compounds
317:centered on the ring as a shorthand for the
4392:Lobry de Bruyn–Van Ekenstein transformation
1081:"Introduction: Delocalization Pi and Sigma"
988:A. W. Hofmann (1855). "On Insolinic Acid".
87:. This is usually considered to be because
23:. For the lack of romantic attraction, see
2068:
2054:
1964:
1950:
1534:
1520:
978:
770:A special case of aromaticity is found in
435:that oppose the applied magnetic field in
4882:Petrenko-Kritschenko piperidone synthesis
4337:Fritsch–Buttenberg–Wiechell rearrangement
1096:
5045:Intramolecular Diels–Alder cycloaddition
1926:Polyhedral skeletal electron pair theory
1449:Journal of the American Chemical Society
1422:Journal of the American Chemical Society
1078:
1014:
702:
244:
95:that are alternately single- and double-
31:
880:for details). Because the twist can be
5357:
5065:Metal-centered cycloaddition reactions
4717:Debus–Radziszewski imidazole synthesis
2661:Bodroux–Chichibabin aldehyde synthesis
888:, the resulting Möbius aromatics are
592:
5211:Diazoalkane 1,3-dipolar cycloaddition
5115:Vinylcyclopropane (5+2) cycloaddition
5020:Diazoalkane 1,3-dipolar cycloaddition
4792:Hurd–Mori 1,2,3-thiadiazole synthesis
4287:Dowd–Beckwith ring-expansion reaction
3454:Hurd–Mori 1,2,3-thiadiazole synthesis
2530:
2367:LFER solvent coefficients (data page)
2049:
1945:
1515:
1384:
841:rings occurring in the Zintl phase Li
360:atoms with specific characteristics:
4022:Sharpless asymmetric dihydroxylation
3259:Methoxymethylenetriphenylphosphorane
900:topologies were first suggested by
532:also has a similar aromatic system.
4147:Allen–Millar–Trippett rearrangement
414:electrophilic aromatic substitution
303:electrophilic aromatic substitution
13:
5286:Nitrone-olefin (3+2) cycloaddition
5281:Niementowski quinazoline synthesis
5070:Nitrone-olefin (3+2) cycloaddition
4995:Azide-alkyne Huisgen cycloaddition
4857:Niementowski quinazoline synthesis
4612:Azide-alkyne Huisgen cycloaddition
3917:Meerwein–Ponndorf–Verley reduction
3469:Leimgruber–Batcho indole synthesis
1541:
418:nucleophilic aromatic substitution
123:
14:
5381:
5110:Trimethylenemethane cycloaddition
4812:Johnson–Corey–Chaykovsky reaction
4677:Cadogan–Sundberg indole synthesis
4657:Bohlmann–Rahtz pyridine synthesis
4617:Baeyer–Emmerling indole synthesis
3424:Cadogan–Sundberg indole synthesis
2916:Johnson–Corey–Chaykovsky reaction
1476:The Journal of Physical Chemistry
1079:Schleyer, Paul von Ragué (2005).
305:, proceeding (third) through a
241:The structure of the benzene ring
145:, intermediate between that of a
5206:Cook–Heilbron thiazole synthesis
5035:Hexadehydro Diels–Alder reaction
4862:Niementowski quinoline synthesis
4692:Cook–Heilbron thiazole synthesis
4637:Bischler–Möhlau indole synthesis
4547:Tiffeneau–Demjanov rearrangement
4177:Baker–Venkataraman rearrangement
3335:Horner–Wadsworth–Emmons reaction
3006:Mizoroki-Heck vs. Reductive Heck
2891:Horner–Wadsworth–Emmons reaction
2402:Neighbouring group participation
1718:
1712:
1706:
990:Proceedings of the Royal Society
911:
681:Polycyclic aromatic hydrocarbons
623:
472:Importance of aromatic compounds
193:
178:
4742:Fiesselmann thiophene synthesis
4572:Westphalen–Lettré rearrangement
4552:Vinylcyclopropane rearrangement
4382:Kornblum–DeLaMare rearrangement
4027:Epoxidation of allylic alcohols
3937:Noyori asymmetric hydrogenation
3872:Kornblum–DeLaMare rearrangement
3547:Gallagher–Hollander degradation
1467:
1440:
1413:
1378:
1332:
1283:
1241:
5201:Chichibabin pyridine synthesis
4687:Chichibabin pyridine synthesis
4647:Blum–Ittah aziridine synthesis
4482:Ring expansion and contraction
2751:Cross dehydrogenative coupling
1223:Chemistry – A European Journal
1214:
1187:
1159:
1132:
1072:
1043:
1008:
711:are aromatic rings with other
675:
76:properties of such compounds.
1:
5171:Bischler–Napieralski reaction
5129:Heterocycle forming reactions
4782:Hemetsberger indole synthesis
4642:Bischler–Napieralski reaction
4557:Wagner–Meerwein rearrangement
4527:Sommelet–Hauser rearrangement
4507:Seyferth–Gilbert homologation
4372:Ireland–Claisen rearrangement
4367:Hofmann–Martius rearrangement
4127:2,3-sigmatropic rearrangement
3742:Corey–Winter olefin synthesis
3667:Barton–McCombie deoxygenation
3310:Corey–Winter olefin synthesis
3264:Seyferth–Gilbert homologation
3131:Seyferth–Gilbert homologation
971:
389:even, but not a multiple of 4
356:) compound contains a set of
5276:Lehmstedt–Tanasescu reaction
5236:Gabriel–Colman rearrangement
5191:Bucherer carbazole synthesis
5186:Borsche–Drechsel cyclization
5166:Bernthsen acridine synthesis
5151:Bamberger triazine synthesis
5136:Algar–Flynn–Oyamada reaction
4847:Nazarov cyclization reaction
4712:De Kimpe aziridine synthesis
4667:Bucherer carbazole synthesis
4662:Borsche–Drechsel cyclization
4432:Nazarov cyclization reaction
4412:Meyer–Schuster rearrangement
4342:Gabriel–Colman rearrangement
4092:Wolffenstein–Böters reaction
3977:Reduction of nitro compounds
3827:Grundmann aldehyde synthesis
3632:Algar–Flynn–Oyamada reaction
3041:Olefin conversion technology
3036:Nozaki–Hiyama–Kishi reaction
2831:Gabriel–Colman rearrangement
2721:Claisen-Schmidt condensation
2666:Bouveault aldehyde synthesis
715:attached. Examples include
608:excepted) with the formula C
108:
7:
5251:Hantzsch pyridine synthesis
5030:Enone–alkene cycloadditions
4852:Nenitzescu indole synthesis
4772:Hantzsch pyridine synthesis
4737:Ferrario–Ackermann reaction
4387:Kowalski ester homologation
4352:Halogen dance rearrangement
4197:Benzilic acid rearrangement
3622:Akabori amino-acid reaction
3582:Von Braun amide degradation
3527:Barbier–Wieland degradation
3479:Nenitzescu indole synthesis
3459:Kharasch–Sosnovsky reaction
3350:Julia–Kocienski olefination
3254:Kowalski ester homologation
2951:Kowalski ester homologation
2926:Julia–Kocienski olefination
2681:Cadiot–Chodkiewicz coupling
2606:Aza-Baylis–Hillman reaction
2551:Acetoacetic ester synthesis
2262:Dynamic binding (chemistry)
2252:Conrotatory and disrotatory
2227:Charge remote fragmentation
924:
735:Atypical aromatic compounds
585:Types of aromatic compounds
539:of commercial interest are
128:Modern depiction of benzene
10:
5386:
5370:Physical organic chemistry
5316:Robinson–Gabriel synthesis
5266:Kröhnke pyridine synthesis
5100:Retro-Diels–Alder reaction
5040:Imine Diels–Alder reaction
4827:Kröhnke pyridine synthesis
4442:Newman–Kwart rearrangement
4417:Mislow–Evans rearrangement
4327:Fischer–Hepp rearrangement
4272:Di-π-methane rearrangement
4052:Stephen aldehyde synthesis
3787:Eschweiler–Clarke reaction
3504:Williamson ether synthesis
2821:Fujiwara–Moritani reaction
2726:Combes quinoline synthesis
2691:Carbonyl olefin metathesis
2392:More O'Ferrall–Jencks plot
2317:Grunwald–Winstein equation
2287:Electron-withdrawing group
2222:Catalytic resonance theory
1624:Metal–ligand multiple bond
221:— occurs in an article by
200:
18:
5326:Urech hydantoin synthesis
5306:Pomeranz–Fritsch reaction
5231:Fischer oxazole synthesis
5128:
4965:1,3-Dipolar cycloaddition
4955:
4937:Urech hydantoin synthesis
4907:Reissert indole synthesis
4892:Pomeranz–Fritsch reaction
4822:Knorr quinoline synthesis
4752:Fischer oxazole synthesis
4682:Camps quinoline synthesis
4602:1,3-Dipolar cycloaddition
4590:
4502:Semipinacol rearrangement
4477:Ramberg–Bäcklund reaction
4462:Piancatelli rearrangement
4402:McFadyen–Stevens reaction
4157:Alpha-ketol rearrangement
4105:
3912:McFadyen–Stevens reaction
3857:Kiliani–Fischer synthesis
3777:Elbs persulfate oxidation
3702:Bouveault–Blanc reduction
3662:Baeyer–Villiger oxidation
3600:
3517:
3494:Schotten–Baumann reaction
3397:
3370:Ramberg–Bäcklund reaction
3277:
3249:Kiliani–Fischer synthesis
3229:
3091:Ramberg–Bäcklund reaction
3076:Pinacol coupling reaction
3071:Piancatelli rearrangement
2966:Liebeskind–Srogl coupling
2816:Fujimoto–Belleau reaction
2539:
2533:List of organic reactions
2397:Negative hyperconjugation
2142:
2084:
2035:List of organic compounds
1980:
1888:
1865:
1796:
1758:
1738:
1727:
1704:
1687:
1669:
1560:
1549:
727:, and the nucleotides of
309:, in which (fourth) the
119:
5301:Pictet–Spengler reaction
5216:Einhorn–Brunner reaction
5181:Boger pyridine synthesis
5075:Oxo-Diels–Alder reaction
4990:Aza-Diels–Alder reaction
4887:Pictet–Spengler reaction
4787:Hofmann–Löffler reaction
4777:Hegedus indole synthesis
4747:Fischer indole synthesis
4622:Bartoli indole synthesis
4577:Willgerodt rearrangement
4407:McLafferty rearrangement
4317:Ferrier carbocyclization
4132:2,3-Wittig rearrangement
4122:1,2-Wittig rearrangement
3962:Parikh–Doering oxidation
3952:Oxygen rebound mechanism
3617:Adkins–Peterson reaction
3509:Yamaguchi esterification
3449:Hegedus indole synthesis
3414:Bartoli indole synthesis
3285:Bamford–Stevens reaction
3201:Weinreb ketone synthesis
3161:Stork enamine alkylation
2936:Knoevenagel condensation
2806:Ferrier carbocyclization
2696:Castro–Stephens coupling
2322:Hammett acidity function
2312:Free-energy relationship
2257:Curtin–Hammett principle
2242:Conformational isomerism
1126:10.1002/jlac.18721620110
747:cation (2e system), the
739:Aromaticity is found in
600:, as well as most other
5261:Knorr pyrrole synthesis
5196:Bucherer–Bergs reaction
5141:Allan–Robinson reaction
5120:Wagner-Jauregg reaction
4912:Ring-closing metathesis
4837:Larock indole synthesis
4817:Knorr pyrrole synthesis
4672:Bucherer–Bergs reaction
4537:Stieglitz rearrangement
4517:Skattebøl rearrangement
4487:Ring-closing metathesis
4347:Group transfer reaction
4312:Favorskii rearrangement
4252:Cornforth rearrangement
4182:Bamberger rearrangement
4087:Wolff–Kishner reduction
3907:Markó–Lam deoxygenation
3802:Fleming–Tamao oxidation
3797:Fischer–Tropsch process
3484:Oxymercuration reaction
3464:Knorr pyrrole synthesis
3290:Barton–Kellogg reaction
3196:Wagner-Jauregg reaction
3116:Ring-closing metathesis
3106:Reimer–Tiemann reaction
3096:Rauhut–Currier reaction
3011:Nef isocyanide reaction
2971:Malonic ester synthesis
2941:Knorr pyrrole synthesis
2876:High dilution principle
2811:Friedel–Crafts reaction
2746:Cross-coupling reaction
2671:Bucherer–Bergs reaction
2656:Blanc chloromethylation
2646:Blaise ketone synthesis
2621:Baylis–Hillman reaction
2616:Barton–Kellogg reaction
2591:Allan–Robinson reaction
2497:Woodward–Hoffmann rules
2232:Charge-transfer complex
751:anion (6e system), the
264:Between 1897 and 1906,
5226:Feist–Benary synthesis
5000:Bradsher cycloaddition
4970:4+4 Photocycloaddition
4927:Simmons–Smith reaction
4872:Paternò–Büchi reaction
4732:Feist–Benary synthesis
4722:Dieckmann condensation
4472:Pummerer rearrangement
4452:Oxy-Cope rearrangement
4427:Myers allene synthesis
4377:Jacobsen rearrangement
4292:Electrocyclic reaction
4267:Demjanov rearrangement
4222:Buchner ring expansion
4192:Beckmann rearrangement
4172:Aza-Cope rearrangement
4167:Arndt–Eistert reaction
4142:Alkyne zipper reaction
4062:Transfer hydrogenation
4037:Sharpless oxyamination
4012:Selenoxide elimination
3897:Lombardo methylenation
3822:Griesbaum coozonolysis
3732:Corey–Itsuno reduction
3707:Boyland–Sims oxidation
3647:Angeli–Rimini reaction
3295:Boord olefin synthesis
3239:Arndt–Eistert reaction
3231:Homologation reactions
3031:Nitro-Mannich reaction
2946:Kolbe–Schmitt reaction
2756:Cross-coupling partner
2676:Buchner ring expansion
2596:Arndt–Eistert reaction
2362:Kinetic isotope effect
2109:Rearrangement reaction
1263:10.1002/anie.201105081
1235:10.1002/chem.200400457
1002:10.1098/rspl.1856.0002
618:cyclotetradecaheptaene
422:electrophilic addition
284:Henry Edward Armstrong
257:was first proposed by
250:
223:August Wilhelm Hofmann
129:
70:August Wilhelm Hofmann
41:
5085:Pauson–Khand reaction
4922:Sharpless epoxidation
4877:Pechmann condensation
4757:Friedländer synthesis
4707:Davis–Beirut reaction
4562:Wallach rearrangement
4532:Stevens rearrangement
4467:Pinacol rearrangement
4447:Overman rearrangement
4362:Hofmann rearrangement
4357:Hayashi rearrangement
4322:Ferrier rearrangement
4277:Dimroth rearrangement
4262:Curtius rearrangement
4257:Criegee rearrangement
4237:Claisen rearrangement
4227:Carroll rearrangement
4162:Amadori rearrangement
4152:Allylic rearrangement
4032:Sharpless epoxidation
3767:Dess–Martin oxidation
3692:Bohn–Schmidt reaction
3552:Hofmann rearrangement
3355:Kauffmann olefination
3278:Olefination reactions
3216:Wurtz–Fittig reaction
3051:Palladium–NHC complex
2931:Kauffmann olefination
2886:Homologation reaction
2736:Corey–House synthesis
2716:Claisen rearrangement
2512:Yukawa–Tsuno equation
2472:Swain–Lupton equation
2452:Spherical aromaticity
2387:Möbius–Hückel concept
2172:Aromatic ring current
2134:Substitution reaction
878:Möbius–Hückel concept
703:Substituted aromatics
685:simple aromatic rings
537:aromatic hydrocarbons
248:
127:
35:
5291:Paal–Knorr synthesis
5161:Barton–Zard reaction
5105:Staudinger synthesis
5055:Ketene cycloaddition
5025:Diels–Alder reaction
5005:Cheletropic reaction
4985:Alkyne trimerisation
4867:Paal–Knorr synthesis
4832:Kulinkovich reaction
4807:Jacobsen epoxidation
4727:Diels–Alder reaction
4522:Smiles rearrangement
4512:Sigmatropic reaction
4397:Lossen rearrangement
4247:Corey–Fuchs reaction
4212:Boekelheide reaction
4207:Bergmann degradation
4137:Achmatowicz reaction
3922:Methionine sulfoxide
3722:Clemmensen reduction
3682:Bergmann degradation
3612:Acyloin condensation
3577:Strecker degradation
3532:Bergmann degradation
3499:Ullmann condensation
3365:Peterson olefination
3340:Hydrazone iodination
3320:Elimination reaction
3221:Zincke–Suhl reaction
3141:Sonogashira coupling
3101:Reformatsky reaction
3061:Peterson olefination
3026:Nierenstein reaction
2956:Kulinkovich reaction
2771:Diels–Alder reaction
2731:Corey–Fuchs reaction
2711:Claisen condensation
2581:Alkyne trimerisation
2556:Acyloin condensation
2522:Σ-bishomoaromaticity
2482:Thorpe–Ingold effect
2094:Elimination reaction
1614:Coordinate (dipolar)
1341:Paul von R. Schleyer
1251:Angew. Chem. Int. Ed
1208:10.1039/PL8900600095
1153:10.1039/CT9252701604
956:Simple aromatic ring
931:Aromatic hydrocarbon
721:acetylsalicylic acid
321:, thus anticipating
307:Wheland intermediate
5311:Prilezhaev reaction
5296:Pellizzari reaction
4975:(4+3) cycloaddition
4942:Van Leusen reaction
4917:Robinson annulation
4902:Pschorr cyclization
4897:Prilezhaev reaction
4627:Bergman cyclization
4582:Wolff rearrangement
4567:Weerman degradation
4457:Pericyclic reaction
4437:Neber rearrangement
4332:Fries rearrangement
4217:Brook rearrangement
4202:Bergman cyclization
4047:Staudinger reaction
3992:Rosenmund reduction
3982:Reductive amination
3947:Oppenauer oxidation
3737:Corey–Kim oxidation
3712:Cannizzaro reaction
3587:Weerman degradation
3562:Isosaccharinic acid
3474:Mukaiyama hydration
3330:Hofmann elimination
3315:Dehydrohalogenation
3300:Chugaev elimination
3121:Robinson annulation
3066:Pfitzinger reaction
2836:Gattermann reaction
2781:Wulff–Dötz reaction
2761:Dakin–West reaction
2686:Carbonyl allylation
2631:Bergman cyclization
2417:Kennedy J. P. Orton
2337:Hammond's postulate
2307:Flippin–Lodge angle
2277:Electromeric effect
2202:Beta-silicon effect
2187:Baker–Nathan effect
1788:C–H···O interaction
1570:Electron deficiency
1461:10.1021/ja00167a011
1434:10.1021/ja00059a035
1304:10.1038/nature02224
1181:10.1021/ja01429a002
868:and populated in a
593:Neutral homocyclics
420:reactions, but not
273:Sir Robert Robinson
235:aliphatic compounds
206:The term "aromatic"
132:As is standard for
5365:Aromatic compounds
5060:McCormack reaction
5010:Conia-ene reaction
4842:Madelung synthesis
4632:Biginelli reaction
4422:Mumm rearrangement
4307:Favorskii reaction
4242:Cope rearrangement
4232:Chan rearrangement
3997:Rubottom oxidation
3927:Miyaura borylation
3892:Lipid peroxidation
3887:Lindgren oxidation
3867:Kornblum oxidation
3862:Kolbe electrolysis
3807:Fukuyama reduction
3717:Carbonyl reduction
3567:Marker degradation
3429:Diazonium compound
3419:Boudouard reaction
3398:Carbon-heteroatom
3325:Grieco elimination
3111:Rieche formylation
3056:Passerini reaction
2986:Meerwein arylation
2906:Hydroxymethylation
2801:Favorskii reaction
2701:Chan rearrangement
2636:Biginelli reaction
2561:Aldol condensation
2407:2-Norbornyl cation
2382:Möbius aromaticity
2377:Markovnikov's rule
2272:Effective molarity
2217:Bürgi–Dunitz angle
2207:Bicycloaromaticity
1773:Resonance-assisted
859:Möbius aromaticity
755:ion (6e), and the
709:chemical compounds
412:compounds undergo
338:quantum mechanical
251:
134:resonance diagrams
130:
42:
5352:
5351:
5348:
5347:
5344:
5343:
5336:Wohl–Aue reaction
4980:6+4 Cycloaddition
4797:Iodolactonization
4117:1,2-rearrangement
4082:Wohl–Aue reaction
4002:Sabatier reaction
3967:Pinnick oxidation
3932:Mozingo reduction
3877:Leuckart reaction
3832:Haloform reaction
3747:Criegee oxidation
3727:Collins oxidation
3677:Benkeser reaction
3672:Bechamp reduction
3642:Andrussow process
3627:Alcohol oxidation
3537:Edman degradation
3444:Haloform reaction
3393:
3392:
3380:Takai olefination
3345:Julia olefination
3171:Takai olefination
3046:Olefin metathesis
2921:Julia olefination
2846:Grignard reaction
2826:Fukuyama coupling
2741:Coupling reaction
2706:Chan–Lam coupling
2576:Alkyne metathesis
2571:Alkane metathesis
2427:Phosphaethynolate
2332:George S. Hammond
2292:Electronic effect
2247:Conjugated system
2129:Stereospecificity
2124:Stereoselectivity
2089:Addition reaction
2078:organic reactions
2043:
2042:
2015:Organic synthesis
2010:Organic reactions
2005:Organic compounds
1995:Functional groups
1974:organic chemistry
1939:
1938:
1890:Electron counting
1861:
1860:
1750:London dispersion
1702:
1701:
1679:Metal aromaticity
1488:10.1021/jp960311p
1399:10.1021/ol0518333
1358:10.1021/ja0458165
1346:J. Am. Chem. Soc.
1169:J. Am. Chem. Soc.
1114:Liebigs Ann. Chem
1098:10.1021/cr030095y
1066:10.1021/cr0300946
1060:(10): 3436–3447.
1029:10.1021/cr0103221
853:Metal aromaticity
757:cyclooctatetraene
713:functional groups
606:cyclodecapentaene
456:cyclooctatetraene
188:molecular orbital
173:atomic p-orbitals
58:unsaturated bonds
46:organic chemistry
16:Chemical property
5377:
5331:Wenker synthesis
5321:Stollé synthesis
5176:Bobbitt reaction
5146:Auwers synthesis
5090:Povarov reaction
5015:Cyclopropanation
4953:
4952:
4947:Wenker synthesis
4702:Darzens reaction
4652:Bobbitt reaction
4497:Schmidt reaction
4302:Enyne metathesis
4077:Whiting reaction
4072:Wharton reaction
4017:Shapiro reaction
4007:Sarett oxidation
3972:Prévost reaction
3782:Emde degradation
3592:Wohl degradation
3572:Ruff degradation
3542:Emde degradation
3439:Grignard reagent
3375:Shapiro reaction
3360:McMurry reaction
3227:
3226:
3191:Ullmann reaction
3156:Stollé synthesis
3146:Stetter reaction
3136:Shapiro reaction
3126:Sakurai reaction
3021:Negishi coupling
3001:Minisci reaction
2996:Michael reaction
2981:McMurry reaction
2976:Mannich reaction
2856:Hammick reaction
2851:Grignard reagent
2791:Enyne metathesis
2776:Doebner reaction
2766:Darzens reaction
2611:Barbier reaction
2601:Auwers synthesis
2528:
2527:
2502:Woodward's rules
2467:Superaromaticity
2457:Spiroaromaticity
2357:Inductive effect
2352:Hyperconjugation
2327:Hammett equation
2267:Edwards equation
2119:Regioselectivity
2070:
2063:
2056:
2047:
2046:
1990:Covalent bonding
1966:
1959:
1952:
1943:
1942:
1931:Jemmis mno rules
1783:Dihydrogen bonds
1736:
1735:
1722:
1716:
1710:
1644:Hyperconjugation
1558:
1557:
1536:
1529:
1522:
1513:
1512:
1506:
1505:
1499:
1491:
1471:
1465:
1464:
1444:
1438:
1437:
1417:
1411:
1410:
1382:
1376:
1375:
1369:
1361:
1352:(8): 2425–2432.
1336:
1330:
1329:
1323:
1315:
1298:(6968): 819–21.
1287:
1281:
1280:
1274:
1266:
1245:
1239:
1238:
1218:
1212:
1211:
1191:
1185:
1184:
1175:(8): 1618–1630.
1163:
1157:
1156:
1136:
1130:
1129:
1109:
1103:
1102:
1100:
1085:Chemical Reviews
1076:
1070:
1069:
1053:Chemical Reviews
1047:
1041:
1040:
1017:Chemical Reviews
1012:
1006:
1005:
985:
966:Avoided crossing
749:cyclopentadienyl
687:). Examples are
672:, for example).
524:. The molecule
358:covalently bound
352:An aromatic (or
197:
190:has π symmetry.
182:
5385:
5384:
5380:
5379:
5378:
5376:
5375:
5374:
5355:
5354:
5353:
5340:
5241:Gewald reaction
5124:
4951:
4932:Skraup reaction
4767:Graham reaction
4762:Gewald reaction
4593:
4586:
4108:
4101:
4057:Swern oxidation
4042:Stahl oxidation
3987:Riley oxidation
3942:Omega oxidation
3902:Luche reduction
3852:Jones oxidation
3817:Glycol cleavage
3812:Ganem oxidation
3757:Davis oxidation
3752:Dakin oxidation
3687:Birch reduction
3637:Amide reduction
3603:
3596:
3557:Hooker reaction
3519:
3513:
3401:
3399:
3389:
3385:Wittig reaction
3273:
3269:Wittig reaction
3244:Hooker reaction
3225:
3206:Wittig reaction
3181:Thorpe reaction
3166:Suzuki reaction
3151:Stille reaction
3086:Quelet reaction
2961:Kumada coupling
2911:Ivanov reaction
2901:Hydrovinylation
2881:Hiyama coupling
2841:Glaser coupling
2651:Blaise reaction
2641:Bingel reaction
2626:Benary reaction
2543:
2541:
2535:
2526:
2422:Passive binding
2342:Homoaromaticity
2192:Baldwin's rules
2167:Antiaromaticity
2162:Anomeric effect
2138:
2080:
2074:
2044:
2039:
2030:Stereochemistry
1976:
1970:
1940:
1935:
1884:
1857:
1800:
1792:
1754:
1741:
1731:
1723:
1717:
1711:
1698:
1683:
1665:
1553:
1545:
1540:
1510:
1509:
1493:
1492:
1482:: 10928–10935.
1472:
1468:
1445:
1441:
1418:
1414:
1387:Organic Letters
1383:
1379:
1363:
1362:
1337:
1333:
1317:
1316:
1288:
1284:
1268:
1267:
1246:
1242:
1219:
1215:
1192:
1188:
1164:
1160:
1137:
1133:
1110:
1106:
1077:
1073:
1048:
1044:
1013:
1009:
986:
979:
974:
941:BTX (chemistry)
927:
914:
866:atomic orbitals
864:
848:
844:
840:
836:
832:
828:
820:
816:
811:Hexasilabenzene
772:homoaromaticity
737:
717:trinitrotoluene
705:
678:
626:
615:
611:
595:
587:
474:
350:
277:aromatic sextet
243:
208:
203:
122:
28:
17:
12:
11:
5:
5383:
5373:
5372:
5367:
5350:
5349:
5346:
5345:
5342:
5341:
5339:
5338:
5333:
5328:
5323:
5318:
5313:
5308:
5303:
5298:
5293:
5288:
5283:
5278:
5273:
5268:
5263:
5258:
5253:
5248:
5246:Hantzsch ester
5243:
5238:
5233:
5228:
5223:
5218:
5213:
5208:
5203:
5198:
5193:
5188:
5183:
5178:
5173:
5168:
5163:
5158:
5156:Banert cascade
5153:
5148:
5143:
5138:
5132:
5130:
5126:
5125:
5123:
5122:
5117:
5112:
5107:
5102:
5097:
5095:Prato reaction
5092:
5087:
5082:
5077:
5072:
5067:
5062:
5057:
5052:
5047:
5042:
5037:
5032:
5027:
5022:
5017:
5012:
5007:
5002:
4997:
4992:
4987:
4982:
4977:
4972:
4967:
4961:
4959:
4950:
4949:
4944:
4939:
4934:
4929:
4924:
4919:
4914:
4909:
4904:
4899:
4894:
4889:
4884:
4879:
4874:
4869:
4864:
4859:
4854:
4849:
4844:
4839:
4834:
4829:
4824:
4819:
4814:
4809:
4804:
4799:
4794:
4789:
4784:
4779:
4774:
4769:
4764:
4759:
4754:
4749:
4744:
4739:
4734:
4729:
4724:
4719:
4714:
4709:
4704:
4699:
4694:
4689:
4684:
4679:
4674:
4669:
4664:
4659:
4654:
4649:
4644:
4639:
4634:
4629:
4624:
4619:
4614:
4609:
4604:
4598:
4596:
4588:
4587:
4585:
4584:
4579:
4574:
4569:
4564:
4559:
4554:
4549:
4544:
4539:
4534:
4529:
4524:
4519:
4514:
4509:
4504:
4499:
4494:
4489:
4484:
4479:
4474:
4469:
4464:
4459:
4454:
4449:
4444:
4439:
4434:
4429:
4424:
4419:
4414:
4409:
4404:
4399:
4394:
4389:
4384:
4379:
4374:
4369:
4364:
4359:
4354:
4349:
4344:
4339:
4334:
4329:
4324:
4319:
4314:
4309:
4304:
4299:
4294:
4289:
4284:
4279:
4274:
4269:
4264:
4259:
4254:
4249:
4244:
4239:
4234:
4229:
4224:
4219:
4214:
4209:
4204:
4199:
4194:
4189:
4187:Banert cascade
4184:
4179:
4174:
4169:
4164:
4159:
4154:
4149:
4144:
4139:
4134:
4129:
4124:
4119:
4113:
4111:
4107:Rearrangement
4103:
4102:
4100:
4099:
4097:Zinin reaction
4094:
4089:
4084:
4079:
4074:
4069:
4067:Wacker process
4064:
4059:
4054:
4049:
4044:
4039:
4034:
4029:
4024:
4019:
4014:
4009:
4004:
3999:
3994:
3989:
3984:
3979:
3974:
3969:
3964:
3959:
3954:
3949:
3944:
3939:
3934:
3929:
3924:
3919:
3914:
3909:
3904:
3899:
3894:
3889:
3884:
3879:
3874:
3869:
3864:
3859:
3854:
3849:
3844:
3842:Hydrogenolysis
3839:
3834:
3829:
3824:
3819:
3814:
3809:
3804:
3799:
3794:
3792:Étard reaction
3789:
3784:
3779:
3774:
3769:
3764:
3759:
3754:
3749:
3744:
3739:
3734:
3729:
3724:
3719:
3714:
3709:
3704:
3699:
3697:Bosch reaction
3694:
3689:
3684:
3679:
3674:
3669:
3664:
3659:
3654:
3649:
3644:
3639:
3634:
3629:
3624:
3619:
3614:
3608:
3606:
3602:Organic redox
3598:
3597:
3595:
3594:
3589:
3584:
3579:
3574:
3569:
3564:
3559:
3554:
3549:
3544:
3539:
3534:
3529:
3523:
3521:
3515:
3514:
3512:
3511:
3506:
3501:
3496:
3491:
3486:
3481:
3476:
3471:
3466:
3461:
3456:
3451:
3446:
3441:
3436:
3434:Esterification
3431:
3426:
3421:
3416:
3411:
3405:
3403:
3395:
3394:
3391:
3390:
3388:
3387:
3382:
3377:
3372:
3367:
3362:
3357:
3352:
3347:
3342:
3337:
3332:
3327:
3322:
3317:
3312:
3307:
3302:
3297:
3292:
3287:
3281:
3279:
3275:
3274:
3272:
3271:
3266:
3261:
3256:
3251:
3246:
3241:
3235:
3233:
3224:
3223:
3218:
3213:
3211:Wurtz reaction
3208:
3203:
3198:
3193:
3188:
3183:
3178:
3173:
3168:
3163:
3158:
3153:
3148:
3143:
3138:
3133:
3128:
3123:
3118:
3113:
3108:
3103:
3098:
3093:
3088:
3083:
3081:Prins reaction
3078:
3073:
3068:
3063:
3058:
3053:
3048:
3043:
3038:
3033:
3028:
3023:
3018:
3013:
3008:
3003:
2998:
2993:
2988:
2983:
2978:
2973:
2968:
2963:
2958:
2953:
2948:
2943:
2938:
2933:
2928:
2923:
2918:
2913:
2908:
2903:
2898:
2896:Hydrocyanation
2893:
2888:
2883:
2878:
2873:
2868:
2866:Henry reaction
2863:
2858:
2853:
2848:
2843:
2838:
2833:
2828:
2823:
2818:
2813:
2808:
2803:
2798:
2793:
2788:
2783:
2778:
2773:
2768:
2763:
2758:
2753:
2748:
2743:
2738:
2733:
2728:
2723:
2718:
2713:
2708:
2703:
2698:
2693:
2688:
2683:
2678:
2673:
2668:
2663:
2658:
2653:
2648:
2643:
2638:
2633:
2628:
2623:
2618:
2613:
2608:
2603:
2598:
2593:
2588:
2583:
2578:
2573:
2568:
2566:Aldol reaction
2563:
2558:
2553:
2547:
2545:
2540:Carbon-carbon
2537:
2536:
2525:
2524:
2519:
2517:Zaitsev's rule
2514:
2509:
2504:
2499:
2494:
2489:
2484:
2479:
2474:
2469:
2464:
2462:Steric effects
2459:
2454:
2449:
2444:
2439:
2434:
2429:
2424:
2419:
2414:
2409:
2404:
2399:
2394:
2389:
2384:
2379:
2374:
2369:
2364:
2359:
2354:
2349:
2344:
2339:
2334:
2329:
2324:
2319:
2314:
2309:
2304:
2299:
2294:
2289:
2284:
2279:
2274:
2269:
2264:
2259:
2254:
2249:
2244:
2239:
2234:
2229:
2224:
2219:
2214:
2209:
2204:
2199:
2194:
2189:
2184:
2179:
2174:
2169:
2164:
2159:
2154:
2149:
2143:
2140:
2139:
2137:
2136:
2131:
2126:
2121:
2116:
2114:Redox reaction
2111:
2106:
2101:
2099:Polymerization
2096:
2091:
2085:
2082:
2081:
2073:
2072:
2065:
2058:
2050:
2041:
2040:
2038:
2037:
2032:
2027:
2022:
2017:
2012:
2007:
2002:
1997:
1992:
1987:
1981:
1978:
1977:
1969:
1968:
1961:
1954:
1946:
1937:
1936:
1934:
1933:
1928:
1923:
1922:
1921:
1916:
1911:
1906:
1895:
1893:
1886:
1885:
1883:
1882:
1877:
1871:
1869:
1863:
1862:
1859:
1858:
1856:
1855:
1850:
1845:
1840:
1835:
1830:
1820:
1815:
1810:
1804:
1802:
1794:
1793:
1791:
1790:
1785:
1780:
1775:
1770:
1764:
1762:
1756:
1755:
1753:
1752:
1746:
1744:
1733:
1729:Intermolecular
1725:
1724:
1705:
1703:
1700:
1699:
1697:
1696:
1693:
1691:
1685:
1684:
1682:
1681:
1675:
1673:
1667:
1666:
1664:
1663:
1662:
1661:
1656:
1646:
1641:
1636:
1631:
1626:
1621:
1616:
1611:
1606:
1601:
1600:
1599:
1589:
1588:
1587:
1582:
1577:
1566:
1564:
1555:
1551:Intramolecular
1547:
1546:
1543:Chemical bonds
1539:
1538:
1531:
1524:
1516:
1508:
1507:
1466:
1439:
1412:
1393:(21): 4637–9.
1377:
1331:
1282:
1240:
1213:
1202:(85): 95–106.
1186:
1158:
1131:
1104:
1071:
1042:
1007:
976:
975:
973:
970:
969:
968:
963:
961:Pi interaction
958:
953:
948:
943:
938:
936:Aromatic amine
933:
926:
923:
913:
910:
902:Johann Listing
862:
846:
842:
838:
834:
830:
826:
818:
814:
807:pyrylium salts
795:germanabenzene
736:
733:
704:
701:
677:
674:
625:
622:
613:
609:
594:
591:
586:
583:
473:
470:
460:cyclobutadiene
401:cyclobutadiene
397:
396:
385:
382:
376:
349:
346:
327:wave mechanics
242:
239:
207:
204:
202:
199:
149:and that of a
121:
118:
81:delocalization
66:empty orbitals
36:Two different
21:aroma compound
15:
9:
6:
4:
3:
2:
5382:
5371:
5368:
5366:
5363:
5362:
5360:
5337:
5334:
5332:
5329:
5327:
5324:
5322:
5319:
5317:
5314:
5312:
5309:
5307:
5304:
5302:
5299:
5297:
5294:
5292:
5289:
5287:
5284:
5282:
5279:
5277:
5274:
5272:
5269:
5267:
5264:
5262:
5259:
5257:
5256:Herz reaction
5254:
5252:
5249:
5247:
5244:
5242:
5239:
5237:
5234:
5232:
5229:
5227:
5224:
5222:
5219:
5217:
5214:
5212:
5209:
5207:
5204:
5202:
5199:
5197:
5194:
5192:
5189:
5187:
5184:
5182:
5179:
5177:
5174:
5172:
5169:
5167:
5164:
5162:
5159:
5157:
5154:
5152:
5149:
5147:
5144:
5142:
5139:
5137:
5134:
5133:
5131:
5127:
5121:
5118:
5116:
5113:
5111:
5108:
5106:
5103:
5101:
5098:
5096:
5093:
5091:
5088:
5086:
5083:
5081:
5078:
5076:
5073:
5071:
5068:
5066:
5063:
5061:
5058:
5056:
5053:
5051:
5048:
5046:
5043:
5041:
5038:
5036:
5033:
5031:
5028:
5026:
5023:
5021:
5018:
5016:
5013:
5011:
5008:
5006:
5003:
5001:
4998:
4996:
4993:
4991:
4988:
4986:
4983:
4981:
4978:
4976:
4973:
4971:
4968:
4966:
4963:
4962:
4960:
4958:
4957:Cycloaddition
4954:
4948:
4945:
4943:
4940:
4938:
4935:
4933:
4930:
4928:
4925:
4923:
4920:
4918:
4915:
4913:
4910:
4908:
4905:
4903:
4900:
4898:
4895:
4893:
4890:
4888:
4885:
4883:
4880:
4878:
4875:
4873:
4870:
4868:
4865:
4863:
4860:
4858:
4855:
4853:
4850:
4848:
4845:
4843:
4840:
4838:
4835:
4833:
4830:
4828:
4825:
4823:
4820:
4818:
4815:
4813:
4810:
4808:
4805:
4803:
4802:Isay reaction
4800:
4798:
4795:
4793:
4790:
4788:
4785:
4783:
4780:
4778:
4775:
4773:
4770:
4768:
4765:
4763:
4760:
4758:
4755:
4753:
4750:
4748:
4745:
4743:
4740:
4738:
4735:
4733:
4730:
4728:
4725:
4723:
4720:
4718:
4715:
4713:
4710:
4708:
4705:
4703:
4700:
4698:
4697:Cycloaddition
4695:
4693:
4690:
4688:
4685:
4683:
4680:
4678:
4675:
4673:
4670:
4668:
4665:
4663:
4660:
4658:
4655:
4653:
4650:
4648:
4645:
4643:
4640:
4638:
4635:
4633:
4630:
4628:
4625:
4623:
4620:
4618:
4615:
4613:
4610:
4608:
4605:
4603:
4600:
4599:
4597:
4595:
4592:Ring forming
4589:
4583:
4580:
4578:
4575:
4573:
4570:
4568:
4565:
4563:
4560:
4558:
4555:
4553:
4550:
4548:
4545:
4543:
4540:
4538:
4535:
4533:
4530:
4528:
4525:
4523:
4520:
4518:
4515:
4513:
4510:
4508:
4505:
4503:
4500:
4498:
4495:
4493:
4492:Rupe reaction
4490:
4488:
4485:
4483:
4480:
4478:
4475:
4473:
4470:
4468:
4465:
4463:
4460:
4458:
4455:
4453:
4450:
4448:
4445:
4443:
4440:
4438:
4435:
4433:
4430:
4428:
4425:
4423:
4420:
4418:
4415:
4413:
4410:
4408:
4405:
4403:
4400:
4398:
4395:
4393:
4390:
4388:
4385:
4383:
4380:
4378:
4375:
4373:
4370:
4368:
4365:
4363:
4360:
4358:
4355:
4353:
4350:
4348:
4345:
4343:
4340:
4338:
4335:
4333:
4330:
4328:
4325:
4323:
4320:
4318:
4315:
4313:
4310:
4308:
4305:
4303:
4300:
4298:
4295:
4293:
4290:
4288:
4285:
4283:
4280:
4278:
4275:
4273:
4270:
4268:
4265:
4263:
4260:
4258:
4255:
4253:
4250:
4248:
4245:
4243:
4240:
4238:
4235:
4233:
4230:
4228:
4225:
4223:
4220:
4218:
4215:
4213:
4210:
4208:
4205:
4203:
4200:
4198:
4195:
4193:
4190:
4188:
4185:
4183:
4180:
4178:
4175:
4173:
4170:
4168:
4165:
4163:
4160:
4158:
4155:
4153:
4150:
4148:
4145:
4143:
4140:
4138:
4135:
4133:
4130:
4128:
4125:
4123:
4120:
4118:
4115:
4114:
4112:
4110:
4104:
4098:
4095:
4093:
4090:
4088:
4085:
4083:
4080:
4078:
4075:
4073:
4070:
4068:
4065:
4063:
4060:
4058:
4055:
4053:
4050:
4048:
4045:
4043:
4040:
4038:
4035:
4033:
4030:
4028:
4025:
4023:
4020:
4018:
4015:
4013:
4010:
4008:
4005:
4003:
4000:
3998:
3995:
3993:
3990:
3988:
3985:
3983:
3980:
3978:
3975:
3973:
3970:
3968:
3965:
3963:
3960:
3958:
3955:
3953:
3950:
3948:
3945:
3943:
3940:
3938:
3935:
3933:
3930:
3928:
3925:
3923:
3920:
3918:
3915:
3913:
3910:
3908:
3905:
3903:
3900:
3898:
3895:
3893:
3890:
3888:
3885:
3883:
3882:Ley oxidation
3880:
3878:
3875:
3873:
3870:
3868:
3865:
3863:
3860:
3858:
3855:
3853:
3850:
3848:
3847:Hydroxylation
3845:
3843:
3840:
3838:
3837:Hydrogenation
3835:
3833:
3830:
3828:
3825:
3823:
3820:
3818:
3815:
3813:
3810:
3808:
3805:
3803:
3800:
3798:
3795:
3793:
3790:
3788:
3785:
3783:
3780:
3778:
3775:
3773:
3772:DNA oxidation
3770:
3768:
3765:
3763:
3762:Deoxygenation
3760:
3758:
3755:
3753:
3750:
3748:
3745:
3743:
3740:
3738:
3735:
3733:
3730:
3728:
3725:
3723:
3720:
3718:
3715:
3713:
3710:
3708:
3705:
3703:
3700:
3698:
3695:
3693:
3690:
3688:
3685:
3683:
3680:
3678:
3675:
3673:
3670:
3668:
3665:
3663:
3660:
3658:
3655:
3653:
3652:Aromatization
3650:
3648:
3645:
3643:
3640:
3638:
3635:
3633:
3630:
3628:
3625:
3623:
3620:
3618:
3615:
3613:
3610:
3609:
3607:
3605:
3599:
3593:
3590:
3588:
3585:
3583:
3580:
3578:
3575:
3573:
3570:
3568:
3565:
3563:
3560:
3558:
3555:
3553:
3550:
3548:
3545:
3543:
3540:
3538:
3535:
3533:
3530:
3528:
3525:
3524:
3522:
3516:
3510:
3507:
3505:
3502:
3500:
3497:
3495:
3492:
3490:
3489:Reed reaction
3487:
3485:
3482:
3480:
3477:
3475:
3472:
3470:
3467:
3465:
3462:
3460:
3457:
3455:
3452:
3450:
3447:
3445:
3442:
3440:
3437:
3435:
3432:
3430:
3427:
3425:
3422:
3420:
3417:
3415:
3412:
3410:
3407:
3406:
3404:
3400:bond forming
3396:
3386:
3383:
3381:
3378:
3376:
3373:
3371:
3368:
3366:
3363:
3361:
3358:
3356:
3353:
3351:
3348:
3346:
3343:
3341:
3338:
3336:
3333:
3331:
3328:
3326:
3323:
3321:
3318:
3316:
3313:
3311:
3308:
3306:
3305:Cope reaction
3303:
3301:
3298:
3296:
3293:
3291:
3288:
3286:
3283:
3282:
3280:
3276:
3270:
3267:
3265:
3262:
3260:
3257:
3255:
3252:
3250:
3247:
3245:
3242:
3240:
3237:
3236:
3234:
3232:
3228:
3222:
3219:
3217:
3214:
3212:
3209:
3207:
3204:
3202:
3199:
3197:
3194:
3192:
3189:
3187:
3184:
3182:
3179:
3177:
3174:
3172:
3169:
3167:
3164:
3162:
3159:
3157:
3154:
3152:
3149:
3147:
3144:
3142:
3139:
3137:
3134:
3132:
3129:
3127:
3124:
3122:
3119:
3117:
3114:
3112:
3109:
3107:
3104:
3102:
3099:
3097:
3094:
3092:
3089:
3087:
3084:
3082:
3079:
3077:
3074:
3072:
3069:
3067:
3064:
3062:
3059:
3057:
3054:
3052:
3049:
3047:
3044:
3042:
3039:
3037:
3034:
3032:
3029:
3027:
3024:
3022:
3019:
3017:
3016:Nef synthesis
3014:
3012:
3009:
3007:
3004:
3002:
2999:
2997:
2994:
2992:
2991:Methylenation
2989:
2987:
2984:
2982:
2979:
2977:
2974:
2972:
2969:
2967:
2964:
2962:
2959:
2957:
2954:
2952:
2949:
2947:
2944:
2942:
2939:
2937:
2934:
2932:
2929:
2927:
2924:
2922:
2919:
2917:
2914:
2912:
2909:
2907:
2904:
2902:
2899:
2897:
2894:
2892:
2889:
2887:
2884:
2882:
2879:
2877:
2874:
2872:
2869:
2867:
2864:
2862:
2861:Heck reaction
2859:
2857:
2854:
2852:
2849:
2847:
2844:
2842:
2839:
2837:
2834:
2832:
2829:
2827:
2824:
2822:
2819:
2817:
2814:
2812:
2809:
2807:
2804:
2802:
2799:
2797:
2794:
2792:
2789:
2787:
2784:
2782:
2779:
2777:
2774:
2772:
2769:
2767:
2764:
2762:
2759:
2757:
2754:
2752:
2749:
2747:
2744:
2742:
2739:
2737:
2734:
2732:
2729:
2727:
2724:
2722:
2719:
2717:
2714:
2712:
2709:
2707:
2704:
2702:
2699:
2697:
2694:
2692:
2689:
2687:
2684:
2682:
2679:
2677:
2674:
2672:
2669:
2667:
2664:
2662:
2659:
2657:
2654:
2652:
2649:
2647:
2644:
2642:
2639:
2637:
2634:
2632:
2629:
2627:
2624:
2622:
2619:
2617:
2614:
2612:
2609:
2607:
2604:
2602:
2599:
2597:
2594:
2592:
2589:
2587:
2584:
2582:
2579:
2577:
2574:
2572:
2569:
2567:
2564:
2562:
2559:
2557:
2554:
2552:
2549:
2548:
2546:
2542:bond forming
2538:
2534:
2529:
2523:
2520:
2518:
2515:
2513:
2510:
2508:
2507:Y-aromaticity
2505:
2503:
2500:
2498:
2495:
2493:
2492:Walsh diagram
2490:
2488:
2485:
2483:
2480:
2478:
2477:Taft equation
2475:
2473:
2470:
2468:
2465:
2463:
2460:
2458:
2455:
2453:
2450:
2448:
2447:Σ-aromaticity
2445:
2443:
2440:
2438:
2435:
2433:
2430:
2428:
2425:
2423:
2420:
2418:
2415:
2413:
2410:
2408:
2405:
2403:
2400:
2398:
2395:
2393:
2390:
2388:
2385:
2383:
2380:
2378:
2375:
2373:
2372:Marcus theory
2370:
2368:
2365:
2363:
2360:
2358:
2355:
2353:
2350:
2348:
2347:Hückel's rule
2345:
2343:
2340:
2338:
2335:
2333:
2330:
2328:
2325:
2323:
2320:
2318:
2315:
2313:
2310:
2308:
2305:
2303:
2302:Evelyn effect
2300:
2298:
2295:
2293:
2290:
2288:
2285:
2283:
2282:Electron-rich
2280:
2278:
2275:
2273:
2270:
2268:
2265:
2263:
2260:
2258:
2255:
2253:
2250:
2248:
2245:
2243:
2240:
2238:
2235:
2233:
2230:
2228:
2225:
2223:
2220:
2218:
2215:
2213:
2210:
2208:
2205:
2203:
2200:
2198:
2197:Bema Hapothle
2195:
2193:
2190:
2188:
2185:
2183:
2180:
2178:
2175:
2173:
2170:
2168:
2165:
2163:
2160:
2158:
2155:
2153:
2150:
2148:
2145:
2144:
2141:
2135:
2132:
2130:
2127:
2125:
2122:
2120:
2117:
2115:
2112:
2110:
2107:
2105:
2102:
2100:
2097:
2095:
2092:
2090:
2087:
2086:
2083:
2079:
2071:
2066:
2064:
2059:
2057:
2052:
2051:
2048:
2036:
2033:
2031:
2028:
2026:
2023:
2021:
2018:
2016:
2013:
2011:
2008:
2006:
2003:
2001:
1998:
1996:
1993:
1991:
1988:
1986:
1983:
1982:
1979:
1975:
1967:
1962:
1960:
1955:
1953:
1948:
1947:
1944:
1932:
1929:
1927:
1924:
1920:
1917:
1915:
1912:
1910:
1907:
1905:
1904:Hückel's rule
1902:
1901:
1900:
1897:
1896:
1894:
1891:
1887:
1881:
1878:
1876:
1873:
1872:
1870:
1868:
1867:Bond cleavage
1864:
1854:
1851:
1849:
1846:
1844:
1841:
1839:
1836:
1834:
1833:Intercalation
1831:
1828:
1824:
1823:Metallophilic
1821:
1819:
1816:
1814:
1811:
1809:
1806:
1805:
1803:
1799:
1795:
1789:
1786:
1784:
1781:
1779:
1776:
1774:
1771:
1769:
1766:
1765:
1763:
1761:
1757:
1751:
1748:
1747:
1745:
1743:
1740:Van der Waals
1737:
1734:
1730:
1726:
1721:
1715:
1709:
1695:
1694:
1692:
1690:
1686:
1680:
1677:
1676:
1674:
1672:
1668:
1660:
1657:
1655:
1652:
1651:
1650:
1647:
1645:
1642:
1640:
1637:
1635:
1632:
1630:
1627:
1625:
1622:
1620:
1617:
1615:
1612:
1610:
1607:
1605:
1602:
1598:
1595:
1594:
1593:
1590:
1586:
1583:
1581:
1578:
1576:
1573:
1572:
1571:
1568:
1567:
1565:
1563:
1559:
1556:
1552:
1548:
1544:
1537:
1532:
1530:
1525:
1523:
1518:
1517:
1514:
1503:
1497:
1489:
1485:
1481:
1477:
1470:
1462:
1458:
1455:: 4177–4182.
1454:
1450:
1443:
1435:
1431:
1428:: 2362–2372.
1427:
1423:
1416:
1408:
1404:
1400:
1396:
1392:
1388:
1381:
1373:
1367:
1359:
1355:
1351:
1348:
1347:
1342:
1335:
1327:
1321:
1313:
1309:
1305:
1301:
1297:
1293:
1286:
1278:
1272:
1264:
1260:
1257:(50): 12099.
1256:
1252:
1244:
1236:
1232:
1228:
1224:
1217:
1209:
1205:
1201:
1197:
1190:
1182:
1178:
1174:
1171:
1170:
1162:
1154:
1150:
1146:
1142:
1135:
1127:
1123:
1120:(1): 77–124.
1119:
1115:
1108:
1099:
1094:
1090:
1086:
1082:
1075:
1067:
1063:
1059:
1055:
1054:
1046:
1038:
1034:
1030:
1026:
1023:(5): 1115–8.
1022:
1018:
1011:
1003:
999:
995:
991:
984:
982:
977:
967:
964:
962:
959:
957:
954:
952:
949:
947:
944:
942:
939:
937:
934:
932:
929:
928:
922:
919:
912:Y-aromaticity
909:
907:
906:carbo-benzene
903:
899:
895:
891:
887:
883:
879:
875:
871:
867:
860:
856:
854:
850:
824:
812:
808:
804:
800:
799:stannabenzene
796:
792:
788:
783:
782:carbon atom.
781:
777:
773:
768:
766:
762:
758:
754:
750:
746:
745:cyclopropenyl
743:as well: the
742:
732:
730:
726:
722:
718:
714:
710:
700:
698:
694:
690:
686:
682:
673:
671:
670:benzimidazole
667:
666:benzannulated
663:
659:
655:
651:
647:
643:
639:
635:
634:heteroaromats
631:
624:Heterocyclics
621:
619:
607:
603:
599:
590:
582:
580:
576:
572:
568:
564:
560:
558:
553:
551:
546:
542:
538:
533:
531:
527:
523:
519:
515:
511:
507:
503:
499:
495:
491:
487:
483:
482:phenylalanine
479:
469:
466:
461:
457:
452:
447:
445:
440:
438:
434:
433:ring currents
429:
425:
423:
419:
415:
409:
407:
402:
394:
393:Hückel's Rule
390:
386:
383:
380:
377:
375:
371:
367:
363:
362:
361:
359:
355:
345:
343:
339:
334:
332:
328:
324:
320:
316:
312:
308:
304:
300:
295:
293:
289:
285:
280:
278:
274:
269:
267:
266:J. J. Thomson
262:
260:
259:August Kekulé
256:
247:
238:
236:
232:
228:
224:
220:
217:
213:
198:
196:
191:
189:
183:
181:
176:
174:
170:
165:
163:
159:
154:
152:
148:
144:
139:
135:
126:
117:
114:
110:
106:
102:
98:
94:
90:
86:
82:
77:
75:
71:
67:
63:
59:
55:
51:
47:
39:
34:
30:
26:
22:
4297:Ene reaction
3657:Autoxidation
3518:Degradation
3409:Azo coupling
3186:Ugi reaction
2786:Ene reaction
2586:Alkynylation
2437:Polyfluorene
2432:Polar effect
2297:Electrophile
2212:Bredt's rule
2182:Baird's rule
2176:
2152:Alpha effect
2025:Spectroscopy
2020:Publications
2000:Nomenclature
1984:
1972:Concepts in
1909:Baird's rule
1898:
1648:
1629:Charge-shift
1592:Hypervalence
1496:cite journal
1479:
1475:
1469:
1452:
1448:
1442:
1425:
1421:
1415:
1390:
1386:
1380:
1366:cite journal
1349:
1344:
1334:
1320:cite journal
1295:
1291:
1285:
1271:cite journal
1254:
1250:
1243:
1229:(17): 4367.
1226:
1222:
1216:
1199:
1195:
1189:
1172:
1167:
1161:
1144:
1140:
1134:
1117:
1113:
1107:
1091:(10): 3433.
1088:
1084:
1074:
1057:
1051:
1045:
1020:
1016:
1010:
993:
989:
915:
890:dissymmetric
889:
886:right-handed
874:Möbius strip
870:closed shell
857:
851:
784:
775:
769:
738:
706:
697:phenanthrene
679:
664:, and their
633:
630:heterocyclic
627:
596:
588:
556:
549:
534:
475:
451:antiaromatic
448:
444:π-π stacking
441:
430:
426:
410:
398:
351:
335:
330:
318:
314:
296:
291:
287:
281:
276:
270:
263:
252:
211:
209:
192:
184:
177:
166:
157:
155:
137:
131:
78:
49:
43:
29:
2796:Ethenolysis
2442:Ring strain
2412:Nucleophile
2237:Clar's rule
2177:Aromaticity
1985:Aromaticity
1899:Aromaticity
1875:Heterolysis
1853:Salt bridge
1798:Noncovalent
1768:Low-barrier
1649:Aromaticity
1639:Conjugation
1619:Pi backbond
918:guanidinium
882:left-handed
803:phosphorine
791:silabenzene
787:borabenzene
765:cyclophanes
725:paracetamol
723:(aspirin),
689:naphthalene
676:Polycyclics
632:aromatics (
530:Chlorophyll
522:pyrimidines
494:nucleotides
465:Hund's rule
368:conjugated
366:delocalized
319:inner cycle
311:conjugation
288:double ring
158:inner cycle
151:double bond
50:aromaticity
5359:Categories
5080:Ozonolysis
4607:Annulation
3957:Ozonolysis
2076:Topics in
1827:aurophilic
1808:Mechanical
972:References
898:paradromic
780:hybridized
693:anthracene
486:tryptophan
323:Erich Clar
62:lone pairs
54:conjugated
4594:reactions
4109:reactions
3604:reactions
3520:reactions
3402:reactions
2544:reactions
1919:spherical
1880:Homolysis
1843:Cation–pi
1818:Chalcogen
1778:Symmetric
1634:Hapticity
753:tropylium
668:analogs (
662:thiophene
650:imidazole
602:annulenes
575:polyester
478:histidine
227:olfactory
113:resonance
89:electrons
85:resonance
74:olfactory
38:resonance
25:aromantic
2487:Vinylogy
2157:Annulene
2104:Reagents
1848:Anion–pi
1838:Stacking
1760:Hydrogen
1671:Metallic
1562:Covalent
1554:(strong)
1407:16209498
1312:14685233
1147:: 1604.
1037:11749368
925:See also
823:borazine
654:pyrazole
646:pyrazine
642:pyridine
506:cytosine
490:tyrosine
379:Coplanar
299:electron
231:terpenes
212:chemical
56:ring of
2147:A value
1813:Halogen
1659:bicyclo
1604:Agostic
996:: 1–3.
761:tropone
719:(TNT),
658:oxazole
598:Benzene
571:aniline
563:styrene
559:-xylene
552:-xylene
545:toluene
541:benzene
518:purines
510:guanine
502:thymine
498:adenine
255:benzene
219:radical
201:History
169:σ-bonds
109:History
101:benzene
83:and of
1914:Möbius
1742:forces
1732:(weak)
1405:
1310:
1292:Nature
1035:
894:chiral
821:) and
695:, and
567:phenol
514:uracil
512:, and
488:, and
342:Hückel
216:phenyl
162:π-bond
147:single
143:length
138:actual
120:Theory
105:Kekulé
97:bonded
1892:rules
1801:other
1689:Ionic
1597:3c–4e
1585:8c–2e
1580:4c–2e
1575:3c–2e
904:. In
707:Many
638:furan
579:nylon
550:ortho
406:furan
374:bonds
107:(see
93:atoms
64:, or
1654:homo
1609:Bent
1502:link
1403:PMID
1372:link
1326:link
1308:PMID
1277:link
1033:PMID
951:SARA
884:or
741:ions
577:and
557:para
554:and
526:heme
416:and
354:aryl
336:The
1484:doi
1480:100
1457:doi
1453:112
1430:doi
1426:115
1395:doi
1354:doi
1350:127
1300:doi
1296:426
1259:doi
1231:doi
1204:doi
1177:doi
1149:doi
1145:127
1122:doi
1118:162
1093:doi
1089:105
1062:doi
1058:105
1025:doi
1021:101
998:doi
946:PAH
892:or
813:(Si
805:or
729:DNA
628:In
520:or
437:NMR
333:).
292:sic
44:In
5361::
1498:}}
1494:{{
1478:.
1451:.
1424:.
1401:.
1389:.
1368:}}
1364:{{
1322:}}
1318:{{
1306:.
1294:.
1273:}}
1269:{{
1255:50
1253:.
1227:10
1225:.
1198:.
1173:44
1143:.
1116:.
1087:.
1083:.
1056:.
1031:.
1019:.
992:.
980:^
845:Si
843:12
825:(B
801:,
797:,
793:,
789:,
778:³
776:sp
767:.
731:.
699:.
691:,
660:,
656:,
652:,
648:,
644:,
620:.
581:.
573:,
569:,
565:,
547:,
543:,
508:,
504:,
500:,
484:,
480:,
364:A
153:.
60:,
48:,
2069:e
2062:t
2055:v
1965:e
1958:t
1951:v
1829:)
1825:(
1535:e
1528:t
1521:v
1504:)
1490:.
1486::
1463:.
1459::
1436:.
1432::
1409:.
1397::
1391:7
1374:)
1360:.
1356::
1328:)
1314:.
1302::
1279:)
1265:.
1261::
1237:.
1233::
1210:.
1206::
1200:6
1183:.
1179::
1155:.
1151::
1128:.
1124::
1101:.
1095::
1068:.
1064::
1039:.
1027::
1004:.
1000::
994:8
863:π
847:7
839:5
835:6
833:H
831:3
829:N
827:3
819:6
817:H
815:6
614:n
612:H
610:n
604:(
496:(
395:.
370:π
315:C
290:(
27:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.