248:
155:
1022:
1015:
1043:
50:
1036:
1029:
41:
844:
for chlorite in drinking water after scientists in the state reported that exposure to higher levels of chlorite affect sperm and thyroid function, cause stomach ulcers, and caused red blood cell damage in laboratory animals. Some studies have indicated that at certain levels chlorite may also be
803:), used in the bleaching of textiles, pulp, and paper. However, despite its strongly oxidizing nature, it is often not used directly, being instead used to generate the neutral species
848:
The federal legal limit in the United States allows chlorite up to levels of 1,000 parts per billion in drinking water, 20 times as much chlorite as
California’s public health goal.
837:
1425:
1347:
1559:
515:
on the chlorine atom, with an O–Cl–O bond angle of 111° and Cl–O bond lengths of 156 pm. Chlorite is the strongest oxidiser of the chlorine
499:
is produced by reducing sodium chlorate with a suitable reducing agent such as methanol, hydrogen peroxide, hydrochloric acid or sulfur dioxide.
900:, known commonly and respectively as chloride, hypochlorite, chlorite, chlorate, and perchlorate. These are part of a greater family of other
287:
1251:
1301:
1223:
1139:
262:
1154:
359:
1107:
112:
205:
150:
468:) being the only commercially important chlorite. Heavy metal chlorites (Ag, Hg, Tl, Pb, and also Cu and
226:
1279:
162:
1179:
1808:
1021:
243:
1294:
1042:
508:
1014:
1209:
62:
452:
at low concentrations. Since it cannot be concentrated, it is not a commercial product. The
132:
1369:
1057:
1035:
1028:
457:
214:
88:
49:
8:
78:
247:
154:
1803:
1415:
1287:
1127:
841:
431:
40:
1197:
1135:
1103:
383:
1123:
804:
492:
449:
415:
387:
307:
194:
27:
1523:
1382:
861:
796:
484:
461:
411:
901:
840:, or OEHHA, released a public health goal of maintaining amounts lower than 50
353:
339:
23:
1797:
1326:
437:
419:
344:
143:
938:
552:
453:
953:
721:
329:
163:
123:
1309:
1252:"Stage 1 and Stage 2 Disinfectants and Disinfection Byproducts Rules"
520:
516:
512:
1122:
352:
Except where otherwise noted, data are given for materials in their
948:
933:
857:
653:
407:
864:
of −1, +1, +3, +5, or +7 within the corresponding anions Cl, ClO,
111:
1063:
445:
181:
480:) are unstable and decompose explosively with heat or shock.
379:
101:
1102:(2nd ed.). Oxford: Butterworth-Heinemann. p. 861.
231:
1276:, Martin Grayson, Editor, John Wiley & Sons, Inc., 1985
1134:(5th ed.), New York: Wiley-Interscience, p. 564,
838:
375:
406:(compound) is a compound that contains this group, with
1180:"EWG's Tap Water Database: Contaminants in Your Water"
1224:"Public Health Goal for Chlorite in Drinking Water"
1097:
460:compounds are all colorless or pale yellow, with
1795:
193:
87:
1295:
1274:Kirk-Othmer Concise Encyclopedia of Chemistry
448:of chlorine and has only been observed as an
1093:
1091:
502:
483:Sodium chlorite is derived indirectly from
1302:
1288:
860:of chlorine exist, in which it can assume
246:
153:
131:
213:
26:. For the neutral chemical compound, see
1088:
1155:"Final Public Health Goal for Chlorite"
242:
1796:
1337:
1310:Salts and covalent derivatives of the
1249:
1098:Greenwood, N.N.; Earnshaw, A. (2006).
491:. First, the explosively unstable gas
144:
1684:
1283:
811:), normally via a reaction with HCl:
274:Key: QBWCMBCROVPCKQ-UHFFFAOYSA-M
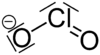
16:Oxyanion with as chemical formula ClO
414:of +3. Chlorites are also known as
184:
13:
851:
271:InChI=1S/ClHO2/c2-1-3/h(H,2,3)/p-1
14:
1820:
1177:
1041:
1034:
1027:
1020:
1013:
48:
39:
831:
795:The most important chlorite is
356:(at 25 °C , 100 kPa).
1243:
1216:
1178:Group, Environmental Working.
1171:
1147:
1116:
602:3 H + HOClO + 3 e →
1:
1081:
781:O + 8 e → Cl + 8 OH
713:O + 6 e → Cl + 6 OH
645:O + 4 e → Cl + 4 OH
589:O + 2 e → Cl + 2 OH
1132:Advanced Inorganic Chemistry
783:
762:
720:
715:
694:
652:
647:
626:
596:
591:
581:
551:
511:, due to the effects of the
425:
7:
1051:
10:
1825:
1686:
507:The chlorite ion adopts a
429:
22:For the clay mineral, see
21:
1339:
1319:
1250:US EPA, OW (2015-10-13).
1100:Chemistry of the elements
519:on the basis of standard
350:
300:
283:
258:
71:
61:
56:
47:
38:
819:+ 4 HCl → 5 NaCl + 4 ClO
503:Structure and properties
1060:, a chlorite-based drug
790:
509:bent molecular geometry
541:Neutral/basic reaction
444:, is the least stable
378:, or chlorine dioxide
1058:Tetrachlorodecaoxide
458:alkaline earth metal
334:67.452
1128:Wilkinson, Geoffrey
35:
1208:has generic name (
432:Category:Chlorites
360:Infobox references
33:
1791:
1790:
1785:
1784:
1124:Cotton, F. Albert
1049:
1048:
842:parts per billion
788:
787:
368:Chemical compound
366:
365:
227:CompTox Dashboard
113:Interactive image
1816:
1569:
1529:
1435:
1421:
1388:
1353:
1332:
1323:
1322:
1304:
1297:
1290:
1281:
1280:
1266:
1265:
1263:
1262:
1247:
1241:
1240:
1238:
1237:
1228:
1220:
1214:
1213:
1207:
1203:
1201:
1193:
1191:
1190:
1175:
1169:
1168:
1166:
1165:
1151:
1145:
1144:
1120:
1114:
1113:
1095:
1077:
1076:
1075:
1045:
1038:
1031:
1024:
1017:
1005:
1004:
1003:
992:
991:
990:
979:
978:
977:
910:oxidation state
907:
906:
899:
898:
897:
887:
886:
885:
875:
874:
873:
862:oxidation states
845:carcinogenic.
805:chlorine dioxide
776:
775:
774:
747:
746:
742:
737:
736:
735:
708:
707:
706:
679:
678:
674:
669:
668:
667:
640:
639:
638:
611:
610:
606:
566:
565:
561:
526:
525:
493:chlorine dioxide
479:
478:
477:
450:aqueous solution
401:
400:
399:
388:chemical formula
323:
322:
321:
308:Chemical formula
251:
250:
235:
233:
217:
197:
186:
165:
157:
146:
135:
115:
91:
52:
43:
36:
32:
28:Chlorine dioxide
1824:
1823:
1819:
1818:
1817:
1815:
1814:
1813:
1809:Chlorine oxides
1794:
1793:
1792:
1787:
1786:
1568:
1564:
1560:
1528:
1524:
1434:
1430:
1426:
1420:
1416:
1387:
1383:
1352:
1348:
1331:
1327:
1315:
1308:
1270:
1269:
1260:
1258:
1248:
1244:
1235:
1233:
1226:
1222:
1221:
1217:
1205:
1204:
1195:
1194:
1188:
1186:
1176:
1172:
1163:
1161:
1153:
1152:
1148:
1142:
1121:
1117:
1110:
1096:
1089:
1084:
1074:
1071:
1070:
1069:
1067:
1054:
1002:
999:
998:
997:
995:
989:
986:
985:
984:
982:
976:
973:
972:
971:
969:
902:chlorine oxides
896:
893:
892:
891:
889:
884:
881:
880:
879:
877:
872:
869:
868:
867:
865:
854:
852:Other oxyanions
834:
826:
822:
818:
810:
802:
797:sodium chlorite
793:
780:
773:
770:
769:
768:
766:
759:
751:
744:
740:
739:
734:
731:
730:
729:
727:
712:
705:
702:
701:
700:
698:
691:
683:
676:
672:
671:
666:
663:
662:
661:
659:
644:
637:
634:
633:
632:
630:
623:
615:
608:
604:
603:
588:
578:
570:
563:
559:
558:
557:H + HOCl + e →
532:Acidic reaction
505:
498:
490:
485:sodium chlorate
476:
473:
472:
471:
469:
467:
462:sodium chlorite
443:
436:The free acid,
434:
428:
412:oxidation state
398:
395:
394:
393:
391:
369:
362:
357:
320:
317:
316:
315:
313:
310:
296:
291:
290:
279:
276:
275:
272:
266:
265:
254:
236:
229:
220:
200:
187:
175:
138:
118:
105:
94:
81:
67:
31:
20:
19:
12:
11:
5:
1822:
1812:
1811:
1806:
1789:
1788:
1783:
1782:
1779:
1776:
1773:
1770:
1767:
1764:
1761:
1758:
1755:
1752:
1749:
1746:
1743:
1740:
1736:
1735:
1732:
1729:
1726:
1723:
1720:
1717:
1714:
1711:
1708:
1705:
1702:
1699:
1696:
1693:
1689:
1688:
1685:
1682:
1681:
1678:
1675:
1672:
1669:
1666:
1663:
1660:
1657:
1654:
1651:
1648:
1645:
1642:
1639:
1636:
1633:
1630:
1627:
1623:
1622:
1619:
1616:
1613:
1610:
1607:
1604:
1601:
1598:
1595:
1592:
1589:
1586:
1583:
1580:
1577:
1574:
1571:
1566:
1562:
1557:
1553:
1552:
1549:
1546:
1543:
1540:
1537:
1534:
1531:
1526:
1521:
1518:
1515:
1512:
1509:
1506:
1503:
1500:
1497:
1495:
1492:
1488:
1487:
1484:
1481:
1478:
1475:
1472:
1469:
1466:
1463:
1460:
1457:
1454:
1451:
1448:
1445:
1442:
1439:
1437:
1432:
1428:
1423:
1418:
1412:
1411:
1408:
1405:
1402:
1399:
1396:
1393:
1390:
1385:
1379:
1378:
1375:
1372:
1367:
1364:
1361:
1358:
1355:
1350:
1344:
1343:
1340:
1338:
1336:
1334:
1329:
1321:
1320:
1317:
1316:
1307:
1306:
1299:
1292:
1284:
1278:
1277:
1268:
1267:
1242:
1215:
1170:
1146:
1140:
1115:
1108:
1086:
1085:
1083:
1080:
1079:
1078:
1072:
1061:
1053:
1050:
1047:
1046:
1039:
1032:
1025:
1018:
1011:
1007:
1006:
1000:
993:
987:
980:
974:
967:
964:
961:
957:
956:
951:
946:
941:
936:
931:
927:
926:
923:
920:
917:
914:
911:
894:
882:
870:
853:
850:
833:
830:
829:
828:
824:
820:
816:
808:
800:
792:
789:
786:
785:
782:
778:
771:
764:
761:
757:
749:
732:
724:
718:
717:
714:
710:
703:
696:
693:
689:
681:
664:
656:
650:
649:
646:
642:
635:
628:
625:
621:
613:
600:
594:
593:
590:
586:
583:
580:
576:
568:
555:
549:
548:
542:
539:
533:
530:
504:
501:
496:
488:
474:
465:
441:
427:
424:
396:
367:
364:
363:
358:
354:standard state
351:
348:
347:
342:
340:Conjugate acid
336:
335:
332:
326:
325:
318:
311:
306:
303:
302:
298:
297:
295:
294:
286:
285:
284:
281:
280:
278:
277:
273:
270:
269:
261:
260:
259:
256:
255:
253:
252:
239:
237:
225:
222:
221:
219:
218:
210:
208:
202:
201:
199:
198:
190:
188:
180:
177:
176:
174:
173:
169:
167:
159:
158:
148:
140:
139:
137:
136:
128:
126:
120:
119:
117:
116:
108:
106:
99:
96:
95:
93:
92:
84:
82:
77:
74:
73:
69:
68:
65:
59:
58:
54:
53:
45:
44:
24:Chlorite group
17:
15:
9:
6:
4:
3:
2:
1821:
1810:
1807:
1805:
1802:
1801:
1799:
1780:
1777:
1774:
1771:
1768:
1765:
1762:
1759:
1756:
1753:
1750:
1747:
1744:
1741:
1738:
1737:
1733:
1730:
1727:
1724:
1721:
1718:
1715:
1712:
1709:
1706:
1703:
1700:
1697:
1694:
1691:
1690:
1683:
1679:
1676:
1673:
1670:
1667:
1664:
1661:
1658:
1655:
1652:
1649:
1646:
1643:
1640:
1637:
1634:
1631:
1628:
1625:
1624:
1620:
1617:
1614:
1611:
1608:
1605:
1602:
1599:
1596:
1593:
1590:
1587:
1584:
1581:
1578:
1575:
1572:
1570:
1558:
1555:
1554:
1550:
1547:
1544:
1541:
1538:
1535:
1532:
1530:
1522:
1519:
1516:
1513:
1510:
1507:
1504:
1501:
1498:
1496:
1493:
1490:
1489:
1485:
1482:
1479:
1476:
1473:
1470:
1467:
1464:
1461:
1458:
1455:
1452:
1449:
1446:
1443:
1440:
1438:
1436:
1424:
1422:
1414:
1413:
1409:
1406:
1403:
1400:
1397:
1394:
1391:
1389:
1381:
1380:
1376:
1373:
1371:
1368:
1365:
1362:
1359:
1356:
1354:
1346:
1345:
1341:
1335:
1333:
1325:
1324:
1318:
1313:
1305:
1300:
1298:
1293:
1291:
1286:
1285:
1282:
1275:
1272:
1271:
1257:
1253:
1246:
1232:
1225:
1219:
1211:
1199:
1185:
1181:
1174:
1160:
1156:
1150:
1143:
1141:0-471-84997-9
1137:
1133:
1129:
1125:
1119:
1111:
1105:
1101:
1094:
1092:
1087:
1065:
1062:
1059:
1056:
1055:
1044:
1040:
1037:
1033:
1030:
1026:
1023:
1019:
1016:
1012:
1009:
1008:
994:
981:
968:
965:
962:
959:
958:
955:
952:
950:
947:
945:
942:
940:
937:
935:
932:
929:
928:
924:
921:
918:
915:
912:
909:
908:
905:
903:
863:
859:
849:
846:
843:
839:
836:In 2009, the
814:
813:
812:
806:
798:
765:
755:
738:+ 7 e →
725:
723:
719:
697:
687:
670:+ 5 e →
657:
655:
651:
629:
619:
601:
599:
595:
584:
574:
556:
554:
550:
546:
543:
540:
537:
534:
531:
528:
527:
524:
522:
518:
514:
510:
500:
494:
486:
481:
463:
459:
455:
451:
447:
439:
438:chlorous acid
433:
423:
421:
420:chlorous acid
417:
413:
409:
405:
389:
385:
381:
377:
374:
361:
355:
349:
346:
345:Chlorous acid
343:
341:
338:
337:
333:
331:
328:
327:
312:
309:
305:
304:
299:
293:
292:
289:
282:
268:
267:
264:
257:
249:
245:
244:DTXSID7021522
241:
240:
238:
228:
224:
223:
216:
212:
211:
209:
207:
204:
203:
196:
192:
191:
189:
183:
179:
178:
171:
170:
168:
166:
161:
160:
156:
152:
149:
147:
145:ECHA InfoCard
142:
141:
134:
130:
129:
127:
125:
122:
121:
114:
110:
109:
107:
103:
98:
97:
90:
86:
85:
83:
80:
76:
75:
70:
64:
60:
55:
51:
46:
42:
37:
29:
25:
1311:
1273:
1259:. Retrieved
1255:
1245:
1234:. Retrieved
1231:oehha.ca.gov
1230:
1218:
1187:. Retrieved
1183:
1173:
1162:. Retrieved
1159:oehha.ca.gov
1158:
1149:
1131:
1118:
1099:
943:
939:hypochlorite
930:anion named
855:
847:
835:
832:Health risks
794:
756:) + 4 H
753:
688:) + 3 H
685:
620:) + 2 H
617:
597:
572:
553:Hypochlorite
544:
535:
523:potentials.
506:
482:
454:alkali metal
435:
403:
372:
370:
72:Identifiers
1256:www.epa.gov
1206:|last=
1184:www.ewg.org
954:perchlorate
726:8 H +
722:Perchlorate
658:6 H +
301:Properties
151:100.123.477
1798:Categories
1261:2023-08-08
1236:2023-08-08
1189:2023-08-08
1164:2023-08-08
1109:0750633654
1082:References
1010:structure
777:+ 4 H
709:+ 3 H
641:+ 2 H
513:lone pairs
430:See also:
330:Molar mass
215:Z63H374SB6
124:ChemSpider
100:3D model (
89:14998-27-7
79:CAS Number
63:IUPAC name
1804:Chlorites
858:oxyanions
521:half cell
517:oxyanions
426:Compounds
386:with the
382:, is the
172:215-285-9
164:EC Number
34:Chlorite
1312:chlorite
1198:cite web
1130:(1988),
1052:See also
960:formula
949:chlorate
944:chlorite
934:chloride
856:Several
748: Cl
680: Cl
654:Chlorate
612: Cl
598:Chlorite
567: Cl
408:chlorine
404:chlorite
373:chlorite
66:Chlorite
1687:
1064:Chloryl
815:5 NaClO
743:⁄
675:⁄
607:⁄
585:ClO + H
562:⁄
487:, NaClO
446:oxoacid
410:in the
324:
182:PubChem
1561:Ba(ClO
1427:Ca(ClO
1138:
1106:
799:(NaClO
547:° (V)
464:(NaClO
384:halite
288:SMILES
195:197148
133:170734
57:Names
1525:AgClO
1384:NaClO
1349:LiClO
1227:(PDF)
888:, or
823:+ 2 H
784:0.56
716:0.63
648:0.78
592:0.89
575:) + H
538:° (V)
495:, ClO
416:salts
380:anion
263:InChI
102:JSmol
1417:KClO
1370:TCDO
1328:HClO
1210:help
1136:ISBN
1104:ISBN
966:ClO
807:(ClO
791:Uses
763:1.42
695:1.47
627:1.64
582:1.63
456:and
440:HClO
402:. A
371:The
206:UNII
1781:No
1778:Md
1775:Fm
1772:Es
1769:Cf
1766:Bk
1763:Cm
1760:Am
1757:Pu
1754:Np
1748:Pa
1745:Th
1742:Ac
1739:**
1734:Yb
1731:Tm
1728:Er
1725:Ho
1722:Dy
1719:Tb
1716:Gd
1713:Eu
1710:Sm
1707:Pm
1704:Nd
1701:Pr
1698:Ce
1695:La
1680:Og
1677:Ts
1674:Lv
1671:Mc
1668:Fl
1665:Nh
1662:Cn
1659:Rg
1656:Ds
1653:Mt
1650:Hs
1647:Bh
1644:Sg
1641:Db
1638:Rf
1635:Lr
1632:**
1629:Ra
1626:Fr
1621:Rn
1618:At
1615:Po
1612:Bi
1609:Pb
1606:Tl
1603:Hg
1600:Au
1597:Pt
1594:Ir
1591:Os
1588:Re
1582:Ta
1579:Hf
1576:Lu
1556:Cs
1551:Xe
1545:Te
1542:Sb
1539:Sn
1536:In
1533:Cd
1520:Pd
1517:Rh
1514:Ru
1511:Tc
1508:Mo
1505:Nb
1502:Zr
1494:Sr
1491:Rb
1486:Kr
1483:Br
1480:Se
1477:As
1474:Ge
1471:Ga
1468:Zn
1465:Cu
1462:Ni
1459:Co
1456:Fe
1453:Mn
1450:Cr
1444:Ti
1441:Sc
1410:Ar
1407:Cl
1398:Si
1395:Al
1392:Mg
1377:Ne
1357:Be
1342:He
1314:ion
1068:ClO
996:ClO
983:ClO
970:ClO
963:Cl
925:+7
922:+5
919:+3
916:+1
913:−1
890:ClO
878:ClO
866:ClO
767:ClO
728:ClO
699:ClO
660:ClO
631:ClO
529:Ion
418:of
392:ClO
390:of
376:ion
314:ClO
232:EPA
185:CID
1800::
1751:U
1692:*
1585:W
1573:*
1548:I
1499:Y
1447:V
1404:S
1401:P
1374:F
1366:N
1363:C
1360:B
1254:.
1229:.
1202::
1200:}}
1196:{{
1182:.
1157:.
1126:;
1090:^
1066:,
904:.
876:,
470:NH
422:.
1567:2
1565:)
1563:2
1527:2
1433:2
1431:)
1429:2
1419:2
1386:2
1351:2
1330:2
1303:e
1296:t
1289:v
1264:.
1239:.
1212:)
1192:.
1167:.
1112:.
1073:2
1001:4
988:3
975:2
895:4
883:3
871:2
827:O
825:2
821:2
817:2
809:2
801:2
779:2
772:4
760:O
758:2
754:g
752:(
750:2
745:2
741:1
733:4
711:2
704:3
692:O
690:2
686:g
684:(
682:2
677:2
673:1
665:3
643:2
636:2
624:O
622:2
618:g
616:(
614:2
609:2
605:1
587:2
579:O
577:2
573:g
571:(
569:2
564:2
560:1
545:E
536:E
497:2
489:3
475:4
466:2
442:2
397:2
319:2
234:)
230:(
104:)
30:.
18:2
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.