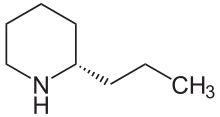
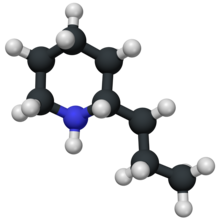
1384:
468:
279:
851:
24:
1475:
1406:
1433:
1098:
2019:"Socrates is guilty, firstly, of denying the gods recognized by the state and introducing new divinities, and, secondly, of corrupting the young." ... Under ordinary circumstances the condemned criminal drank the cup of hemlock on the day after the trial; but in the case of Socrates the rule that during the absence of the sacred ship sent annually to Delos no one should be put to death caused an exceptional
820:
41:
671:
1558:. For example, Pd-catalyzed 1,3-chirality transfer reaction can stereospecifically transform a single enantiomer of an allyl alcohol into a cyclic structure (in this case a piperidine). In this way, starting from (S)-alcohol an (S)-enantiomer of Coniine is obtained and vice versa. Remarkably, the separation of racemic alcohol into different enantiomers is done with the help of
898:. The most famous hemlock poisoning occurred in 399 BCE, when the philosopher Socrates is believed to have consumed a liquid infused with hemlock to carry out his death sentence, his having been convicted of impiety toward the gods, and the corruption of youth. Hemlock juice was often used to execute criminals in
972:
convulsion just prior to death, disguised by the muscular paralysis such that the person may just weakly shudder. Cause of death is lack of oxygen to the brain and heart as a consequence of respiratory paralysis, so that a poisoned person may recover if artificial ventilation can be maintained until
967:
since they are both depolarizing neuromuscular blockers. Symptoms of paralysis generally occur within a half-hour, although death may take several hours. The central nervous system is not affected: the person remains conscious and aware until respiratory paralysis results in cessation of breathing.
1586:
Further elongation of butyryl-CoA using 2 malonyl-CoA forms 5-ketooctanal. Ketooctanal then undergoes transamination using alanine:5-keto-octanal aminotransferase. The amine then spontaneously cyclizes and is dehydrated to form the coniine precursor γ–coniceine. This is then reduced using NADPH
1575:
The biosynthesis of coniine is still being investigated, but much of the pathway has been elucidated. Originally thought to use 4 acetyl groups as feed compounds for the polyketide synthase that forms coniine, it is in fact derived from two malonyl and a butyryl CoA, which are derived in the usual
2018:
SOCRATES, son of the statuary
Sophroniscus and of the midwife Phaenarete, was born at Athens, not earlier than 471 nor later than May or June 469 B.C. ... In 399, four years after the restoration and the amnesty, he was indicted as an offender against public morality. ... The accusation ran thus:
1235:
forms small yellow needles, mp. 75 °C, from hot water. The 2,4-dinitrobenzoyl- and 3,5-dinitrobenzoyl-derivates have mps. 139.0–139.5 °C and 108–9 °C respectively. The precipitate afforded by potassium cadmium iodide solution is crystalline, mp. 118 °C, while that given by
2158:
of: Peschier (1821) "Neue analytische
Untersuchungen über den unter verschiedenen Himmelsstrichen gebauten Mohn; ferner über einige inländische Narcotica, und Entdeckung neuer Pflanzensäuren und Alkälien in denselben" (New analytical investigations into poppies grown in various climates;
885:
The history of coniine is understandably tied to the poison hemlock plant, since the natural product was not synthesizable until the 1880s. Jews in the Middle East were poisoned by coniine after consuming quail in the area that usually ate hemlock seeds, and Greeks on the island of
1591:
1580:
944:)-(−) enantiomer of coniine is the more biologically active, at least in one system (TE-671 cells expressing human fetal nicotinic neuromuscular receptors), and in mouse bioassay, the same enantiomer and the racemic mixture are about two-fold more toxic than the (
2693:"The relationship between the optical rotatory powers and the relative configurations of optically active compounds. The influence of certain inorganic haloids on the optical rotatory powers of α-hydroxy-acids, α-amino-acids, and their derivatives"
1426:
dust and water. Finally, the product of the second step is treated with sodium in ethanol. Note: although the graphic below shows a single enantiomer of coniine, this reaction produces a racemic mixture that is then purified and separated.
2602:
Talapatra, Sunil Kumar; Talapatra, Bani (2015), Talapatra, Sunil Kumar; Talapatra, Bani (eds.), "Coniine, Conhydrine, and
Pseudoconhydrine (The C-Skeleton Derived from a C8-Fatty Acid and N from Transamination)",
838:
amounts of coniine. Its presence on farmland is an issue for livestock farmers because animals will eat it if they are not well fed or the hemlock is mixed in with pasture grass. The coniine is present in
1391:
The scheme proposed by
Ladenburg gave poor yields, so the quest for alternative routes was open. A slightly better yield is observed if 2-methylpyridine and acetaldehyde are heated in a sealed tube with
871:
to the southeastern United States. The plant uses a mixture of sugar and coniine to simultaneously attract and poison insects, which then fall into a digestive tube. Coniine is also found in
740:). Its ingestion and extended exposure are toxic to humans and all classes of livestock; its mechanism of poisoning involves disruption of the central nervous system, with death caused by
2724:
Hande, Sudhir M.; Kawai, Nobuyuki; Uenishi, Jun’ichi (2009-01-02). "An
Efficient Synthesis of 2- and 2,6-Substituted Piperidines Using PdII-Catalyzed 1,3-Chirality Transfer Reaction".
959:. The subsequent depolarization results in nicotinic toxicity; as coniine stays bound to the receptor, the nerve stays depolarized, inactivating it. This results, systemically, in a
1060:-isomer, but the salts have slightly different melting points; the platinichloride has mp. 160 °C (Löffler and Friedrich report 175 °C), the aurichloride mp. 59 °C.
799:
Hemlock poisoning has been a periodic human concern, a regular veterinary concern, and has had significant occurrences in human and cultural history. Notably, in 399 BC,
1672:
Stephen T. Lee; Benedict T. Green; Kevin D. Welch; James A. Pfister; Kip E. Panter (2008). "Stereoselective potencies and relative toxicities of coniine enantiomers".
1191:
in the air. The salts crystallize well and are soluble in water or alcohol. The hydrochloride, B•HCl, crystallizes from water in rhombs, mp. 220 °C,
1227:
O, separates from concentrated solution as an oil, which solidifies to a mass of orange-yellow crystals, mp. 175 °C (dry). The aurichloride, B•HAuCl
1332:
The original synthesis (shown below) of
Coniine was performed by Ladenburg in 1886. Ladenburg heated N-methylpyridine iodide to 250 °C, to obtain
684:
2199:
N. The error in the amount of carbon is due, in part, to his having assumed that the atomic mass of carbon is 6, not 12 — a common error at the time.
1276:
composition of "coniine" is a matter of some importance, since its two enantiomers do not have identical biological properties, and many of the older
1400:-coniine. Note: although the scheme below shows a single enantiomer of coniine, the final reaction produces a racemic mixture that is then separated
1000:
753:
1843:
1508:
2968:
2697:
1396:
for 10 hours. A mixture of 2-propenylpyridine and 2-chloropropylpyridine is formed and is subsequently reduced by sodium in ethanol to give
724:), where its presence has been a source of significant economic, medical, and historico-cultural interest; coniine is also produced by the
525:
1441:
A number of other syntheses of coniine have been effected, of which that of Diels and Alder is of special interest. The initial adduct of
2941:
2012:
1868:
1296:). These authors note that Ladenburg's value, +15°, is for a "neat", i.e. undiluted, sample. A similarly high value of +16° for the
932:
While the yellow pitcher plant and fool's parsley also contain coniine, there are no reports of traditional uses for these plants.
1108:
780:)-(−)-coniine, depending on the direction taken by the chain that branches from the ring. Both enantiomers are toxic, with the (
1369:
2620:
2067:
905:
Hemlock has had a limited medical use throughout history. The Greeks used it not just as capital punishment, but also as an
1709:
N. V. Mody; R. Henson; P. A. Hedin; U. Kokpol; D. H. Miles (1976). "Isolation of the insect paralyzing agent coniine from
2057:
1017:)-Coniine has since been determined to be a colorless alkaline liquid, with a penetrating odour and a burning taste; has
922:
1166:
2961:
482:
1138:
3282:
1260:
gives a deep red color, which disappears on warming, but reappears on cooling, and is changed to blue or violet by
968:
The flaccid, muscular paralysis is an ascending paralysis, lower limbs being first affected. The person may have a
679:
2171:(An illustration of the crystalline acid, see Fig. 1 ; the sodium salt of conium acid is depicted in Fig. 2.)
691:
2563:
1674:
1446:
1068:
Coniine is slightly soluble (1 in 90) in cold water, less so in hot water, so that a clear cold solution becomes
917:
it was believed that hemlock could be used to cure rabies; in later
European times it came to be associated with
161:
2091:
1543:(piperidine-2-carboxylic acid) and some of its derivatives under varying conditions, that it must belong to the
1352:, a cyclic trimer of acetaldehyde that readily forms acetaldehyde upon heating. Finally, 2-propenylpyridine was
3272:
1145:
1123:
2181:
173:
3267:
2977:
2209:
1883:
991:-(+) enantiomers, and the racemate, are approximately 7 and 12, and 8 milligrams per kilogram, respectively.
1802:
2954:
2353:
1414:
In 1907, another route with better yield was proposed. First, 2-(2'-hydroxypropyl)pyridine is reduced with
796:
in 1886, and it has been synthesized in the laboratory in a number of unique ways through to modern times.
383:
274:
2842:
1453:
is converted into trimethyl indolizine-tricarboxylate. This, on hydrolysis and decarboxylation, furnishes
784:)-enantiomer being the more biologically active and toxic of the two in general. Coniine holds a place in
227:
1909:
1152:
1072:
when warmed. On the other hand, the base dissolves about 25% of water at room temperature. It mixes with
437:
2155:
2843:"Separation of the formation of γ-coniceine and aliphatic amines from got activity in Conium maculatum"
2131:
1997:
1555:
1004:
2769:"Biosynthesis of the Hemlock Alkaloids. The Incorporation of Acetate-1-C into Coniine and Conhydrine"
286:
2605:
Chemistry of Plant
Natural Products: Stereochemistry, Conformation, Synthesis, Biology, and Medicine
2159:
furthermore, on some domestic narcotics, and discovery of new plant acids and alkalis in the same),
1134:
2799:
2150:(2) : 97–111. On p. 99, Giseke credits the Swiss apothecary Peschier with coining the name
1337:
463:
2169:"Eine Abbildung der krystallisirten Säure s. Fig. 1 das coniumsaure Natron ist Fig. 2 abgebildet."
3226:
1617:
1615:
The R and S 2-Propylpiperidine stereoisomers are a neurotoxin present in a slug-like lifeform in
3201:
3067:
2798:
Hotti, Hannu; Seppänen-Laakso, Tuulikki; Arvas, Mikko; Teeri, Teemu H.; Rischer, Heiko (2015).
2512:
1539:
and alcohol at 180–230 °C. Leithe has shown by observation of the optical rotation of (+)-
956:
2882:
2238:
149:
3277:
2936:
1671:
1638:
741:
53:
256:
3262:
3206:
3155:
2854:
1962:
1257:
425:
413:
401:
2663:
2409:
1457:, the octahydro-derivate of which, also known as octahydropyrrocoline is converted by the
1312:) for synthetic R-(−)-coniine is given by other chemists. The hydrochloride salts of the (
104:
92:
8:
3124:
3105:
895:
116:
73:
2858:
2002:
467:
278:
202:
2942:
Mitch Tucker student work, Hemlock and Death of
Socrates, at the University of Oklahoma
1979:
1942:
1926:
1837:
1778:
1751:
1732:
1560:
977:
952:
2866:
1862:
3000:
2907:
2823:
2749:
2741:
2616:
2493:
2474:"Noch ein Wort über das Isoconlin. 14. Mitteilung über den asymmetryschen Stickstoff"
2454:
2063:
1983:
1934:
1783:
1691:
1393:
1159:
1037:
969:
960:
865:
804:
785:
707:
1946:
1736:
1621:. The toxin is shown as causing almost instant death upon skin contact in the show.
1511:
was the aldehyde (β-2-piperidyl-propaldehyde) corresponding to coniine, and yielded
3231:
3221:
3054:
3012:
2932:
2899:
2862:
2815:
2780:
2733:
2706:
2608:
2568:
2524:
2485:
2446:
2362:
1971:
1918:
1773:
1763:
1724:
1683:
1608:
1536:
1458:
1449:
is tetramethylquinolizine-1,2,3,4-tetracarboxylate, which on oxidation with dilute
1333:
1081:
873:
860:
808:
793:
736:
730:
725:
720:
641:
574:
372:
2191: : 345–363. Blyth found the empirical formula of coniine to be (p. 351): C
2031:
1003:
by
Giesecke, but the formula was suggested by Blyth and definitely established by
951:
Coniine, as racemate or as pure enantiomer, begins by binding and stimulating the
3241:
3007:
2612:
2396:
2351:
Craig J. Cymerman; A. R. Pinder (1971). "Improved method resolution of coniine".
2229:
Panter, K. E. and Keeler, R. F., Ch. 5: Piperidine alkaloids of poison hemlock (
1603:
1520:
1419:
1383:
1119:
964:
918:
761:
360:
2884:
913:. Books from the 10th century attest to medical use by the Anglo-Saxons. In the
3170:
3120:
3038:
2768:
1540:
1353:
1273:
899:
827:
662:
2903:
2489:
2450:
2016:. Vol. 25 (11th ed.). Cambridge University Press. pp. 331–338.
1975:
1768:
3256:
2883:
Green, Benedict T.; Lee, Stephen T.; Panter, Kip E.; Brown, David R. (2012).
2745:
2497:
2458:
2007:
1857:
1373:
1345:
1249:
906:
891:
630:
620:
267:
1752:"The killer of Socrates: Coniine and Related Alkaloids in the Plant Kingdom"
1495:-propenylpiperidine) by Löffler and Friedrich provides means for converting
3236:
3046:
2911:
2827:
2753:
1938:
1787:
1695:
1341:
1277:
1077:
926:
769:
745:
2529:
3216:
3160:
3061:
2710:
2572:
1715:
1450:
1349:
1301:
1253:
1187:
Coniine solidifies into a soft crystalline mass at −2 °C. It slowly
914:
850:
757:
2784:
2366:
1280:
studies on this compound were carried out using the naturally-occurring
3186:
2819:
1872:. Vol. 6 (11th ed.). Cambridge University Press. p. 942.
1728:
1708:
1548:
1496:
1466:
1454:
1415:
1377:
844:
599:
287:
238:
2946:
2737:
1930:
1687:
760:
differing from coniine only by its carbon-nitrogen double bond in the
3130:
3080:
3019:
2120:(11th ed.). New York: McGraw-Hill. pp. Chapter 118: Plants.
1516:
1069:
910:
509:): InChI=1/C8H17N/c1-2-5-8-6-3-4-7-9-8/h8-9H,2-7H2,1H3/t8-/m0/s1
2692:
2650:
Pelletierine is now known to be 1-(2-piperidinyl)-2-propanone; see:
2473:
1215:
O, in rhombic crystals, mp. 54 °C. The platinichloride, (B•HCl)
1097:
661:
Except where otherwise noted, data are given for materials in their
3196:
3191:
3165:
3150:
3135:
2992:
2182:"On the composition of coniine, and its products of decomposition,"
1922:
1442:
1365:
1300:
of "coniine" is given, without explicit citation of the source, in
1281:
1261:
1237:
868:
800:
789:
765:
711:
326:
23:
1474:
1432:
1405:
890:
who also consumed quail suffered from the same poisoning, causing
172:
160:
148:
3140:
1554:
Currently, Coniine, and many other alkaloids, can be synthesized
1535:)-coniine is rendered almost optically inactive when heated with
1524:
1361:
1232:
1188:
1073:
338:
2437:
Ladenburg, A. (1886-01-01). "Versuche zur Synthese des Coniin".
2062:(2nd ed.). Mineola, N.Y.: Dover Publications. p. 392.
3087:
3030:
2662:, O'Neil: The Royal Society of Chemistry. Available online at:
2517:
Facta universitatis - series: Physics, Chemistry and Technology
2513:"Synthetic approaches to coniine and other 2-alkyl piperidines"
2001:
1564:
1357:
1195:+10.1°; the hydrobromide, in needles, mp. 211 °C, and the
887:
749:
715:
213:
2797:
2161:
Neues Journal der Pharmacie für Aerzte, Apotheker und Chemiker
764:. This pathway results in natural coniine that is a mixture—a
491:
InChI=1S/C8H17N/c1-2-5-8-6-3-4-7-9-8/h8-9H,2-7H2,1H3/t8-/m0/s1
3211:
3074:
2350:
1861:
835:
184:
129:
40:
442:
3101:
3024:
2511:
Denić, Marija; Blagojević, Polina; Radulović, Niko (2013).
1590:
1579:
1461:
method successively into the bromocyanamide, cyanamide and
1423:
635:
166 to 167 °C (331 to 333 °F; 439 to 440 K)
308:
2561:
G. R. Clemo; G. R. Ramage (1932). "Octahydropyrrocoline".
864:, the yellow pitcher plant. The yellow pitcher plant is a
2237:, vol. 1 (Boca Raton, Florida: CRC Press, Inc., 1989),
1827:
1324:
of +4.6° and -5.2°, respectively (c = 0.5, in methanol).
819:
2885:"Piperidine Alkaloids: Human and Food Animal Teratogens"
2510:
2210:"Einwirkung der Wärme auf die Ammoniumbasen: 2. Coniin"
1809:. United States Department of Agriculture Forest Service
1115:
2664:
http://www.rsc.org/Merck-Index/monograph/mono1500007181
2560:
2410:
http://www.rsc.org/Merck-Index/monograph/mono1500002489
1507:)-coniine. Hess and Eichel reported, incorrectly, that
1308:) for synthetic S-(+)-coniine and -7.9° (c = 0.5, CHCl
2690:
1884:"Massachusetts Medical Society: Don't Eat the Quails"
1465:-coniine. A synthesis of the alkaloid, starting from
1348:
to yield 2-propenylpyridine. In fact, Ladenburg used
788:
history as being the first of the important class of
1422:
at 125 °C. Second, the product is treated with
748:
of coniine contains as its penultimate step the non-
2185:
Quarterly Journal of the Chemical Society of London
1960:R. G. Frey (1978). "Did Socrates commit suicide?".
2601:
2723:
2212:(Effect of heat on ammonium bases: 2. Coniine),
1587:dependent y-coniceine reductase to form coniine.
3254:
1527:at 156–170 °C. According to these authors,
1469:(pyrrocoline) is described by Ochiai and Tsuda.
1231:, crystallizes on standing, mp. 77 °C. The
1080:and most organic solvents. Coniine dissolves in
371:
359:
973:the toxin is removed from the victim's system.
115:
103:
91:
2478:Berichte der Deutschen Chemischen Gesellschaft
2439:Berichte der Deutschen Chemischen Gesellschaft
2214:Berichte der deutschen chemischen Gesellschaft
935:
880:
2962:
2698:Journal of the Chemical Society, Transactions
2132:"Ueber das wirksame Princip des Schierlings,
983:values (in mouse, i.v. administered) for the
2800:"Polyketide synthases from poison hemlock (
1906:
1842:: CS1 maint: multiple names: authors list (
1749:
1320:)-(−) enantiomers of coniine have values of
1124:introducing citations to additional sources
807:by drinking a coniine-containing mixture of
1491:)-coniine by the reduction of β-coniceine (
2969:
2955:
2408:, O'Neil: The Royal Society of Chemistry.
2265:
2263:
2115:
1959:
1076:in all proportions, is readily soluble in
466:
277:
255:
2528:
2471:
2436:
2430:
2419:
2417:
1907:James Warren (2001). "Socratic suicide".
1777:
1767:
424:
412:
400:
2773:Journal of the American Chemical Society
1856:
1636:
1382:
1114:Relevant discussion may be found on the
1056:15° and in other respects resembles its
849:
818:
2976:
2840:
2260:
1996:
1807:United States Department of Agriculture
462:
3255:
2414:
2089:
2083:
2055:
1288:-(+)-Coniine has a specific rotation,
1041:
994:
268:
2950:
2766:
2138:(On the active component of hemlock,
2097:. San Francisco Peaks Weed Management
1750:Hotti, Hannu; Rischer, Heiko (2017).
1667:
1665:
1663:
1661:
1659:
1597:
955:on the post-synaptic membrane of the
843:as a mixture of the R-(−)- and S-(+)-
494:Key: NDNUANOUGZGEPO-QMMMGPOBSA-N
226:
201:
2235:Toxicants of Plant Origin: Alkaloids
1743:
1267:
1091:
877:, commonly known as fool's parsley.
625:−2 °C (28 °F; 271 K)
1304:. The value of +7.7° (c = 4.0, CHCl
1084:, forming a complex thiocarbamate.
1036:+15.7° (see related comments under
512:Key: NDNUANOUGZGEPO-QMMMGPOBBO
341:
325:
13:
2876:
2167:(1) : 76–101. From p. 87:
1803:"Yellow Pitcher Plant or Trumpets"
1800:
1656:
1589:
1578:
1473:
1431:
1404:
1087:
814:
768:—composed of two enantiomers, the
14:
3294:
2926:
1248:Coniine gives no coloration with
2726:The Journal of Organic Chemistry
2116:Goldrank, Lewis; Nelson, Lewis.
1602:Coniine is the murder weapon in
1243:
1240:with this reagent is amorphous.
1107:relies largely or entirely on a
1096:
669:
592:
586:
39:
22:
2834:
2791:
2760:
2717:
2684:
2668:
2644:
2628:
2595:
2579:
2564:Journal of the Chemical Society
2554:
2537:
2504:
2465:
2423:D. Enders and J. Tiebes (1993)
2389:
2373:
2344:
2331:
2320:Späth, Kuffner and Ensfellner,
2314:
2297:
2280:
2243:
2223:
2202:
2174:
2124:
2109:
2049:
2024:
1990:
1953:
1828:Clapham, Tutin, & Warburg.
1675:Chemical Research in Toxicology
1570:
1447:dimethyl acetylenedicarboxylate
1387:Original Synthesis by Ladenburg
1032:1.4505, and is dextrorotatory,
1025: 0.8438, refractive index
963:, an action similar to that of
665:(at 25 °C , 100 kPa).
2691:George William Clough (1918).
2607:, Springer, pp. 733–748,
2233:) in: Cheeke, Peter R., ed.,
1900:
1876:
1850:
1821:
1794:
1702:
1630:
948:)-(+) enantiomer (see below).
580:
1:
2867:10.1016/S0031-9422(00)89689-2
2841:Roberts, Margaret F. (1978).
1637:Peters, Amy; Bouska, Cassie.
1624:
1063:
714:present in and isolable from
2892:Food and Chemical Toxicology
2613:10.1007/978-3-642-45410-3_17
2472:Ladenburg, A. (1907-06-01).
2354:Journal of Organic Chemistry
1832:(2nd ed.). p. 524.
1372:of racemic coniine with (+)-
1327:
1292:, of +8.4° (c = 4.0, in CHCl
7:
2658:(2013), p. 1314, Monograph
1910:Journal of Hellenic Studies
936:Pharmacology and toxicology
881:History of natural isolates
10:
3299:
2404:(2013), p. 446, Monograph
2381:Justus Liebig's Ann. Chem.
1830:Flora of the British Isles
1370:Fractional crystallisation
3179:
3113:
3096:
2984:
2904:10.1016/j.fct.2012.03.049
2490:10.1002/cber.190704003170
2451:10.1002/cber.188601901108
2130:Giseke, Aug. Lud. (1827)
2032:"The Suicide of Socrates"
1976:10.1017/S0031819100016375
1769:10.3390/molecules22111962
858:Coniine is also found in
823:The poison hemlock plant.
659:
567:
521:
478:
66:
52:
47:
38:
21:
1338:Knoevenagel condensation
999:(+/–)-Coniine was first
925:used hemlock extract as
854:The yellow pitcher plant
3283:2-Piperidinyl compounds
2767:Leete, Edward. (1964).
2269:Löffler and Friedrich,
2118:Toxicologic Emergencies
2013:Encyclopædia Britannica
1869:Encyclopædia Britannica
734:), and fool's parsley (
615:Colorless, oily liquid
3068:Mandragora officinarum
2933:Information on hemlock
2208:Hofmann, A. W. (1881)
1594:
1583:
1478:
1436:
1409:
1388:
1336:. He then performed a
957:neuromuscular junction
855:
824:
792:to be synthesized, by
3273:Nicotinic antagonists
2937:University of Bristol
2530:10.2298/FUPCT1301001D
2036:EyeWitness to History
1643:Osu Extension Service
1593:
1582:
1576:way from acetyl-CoA.
1477:
1435:
1408:
1386:
853:
822:
742:respiratory paralysis
3268:Piperidine alkaloids
2711:10.1039/CT9181300526
2573:10.1039/JR9320002969
2379:A. Ladenburg (1888)
2144:Archiv der Pharmazie
2090:Moser, L; Crisp, D.
1258:Sodium nitroprusside
1120:improve this article
726:yellow pitcher plant
559:): N1(CCC)CCCC1
550:): N1(CCC)CCCC1
61:)-2-Propylpiperidine
2978:Ancient anaesthesia
2859:1978PChem..17..107R
2785:10.1021/ja01066a039
2425:Liebig's Ann. Chem.
2367:10.1021/jo00822a051
2056:Grieve, M. (1971).
1483:The preparation of
1199:-acid tartrate, B•C
995:Chemical properties
896:acute kidney injury
776:)-(+)-coniine and (
607: g·mol
541:): CCCC1CCCCN1
18:
2820:10.1111/febs.13410
1729:10.1007/BF02003710
1598:In popular culture
1595:
1584:
1561:Candida antarctica
1479:
1437:
1410:
1389:
953:nicotinic receptor
909:and treatment for
856:
834:) contains highly
825:
805:sentenced to death
756:to γ-coniceine, a
692:Infobox references
16:
3250:
3249:
3050:(devil's trumpet)
3001:Atropa belladonna
2814:(21): 4141–4156.
2779:(12): 2509–2513.
2738:10.1021/jo801926g
2622:978-3-642-45410-3
2543:Diels and Alder,
2361:(23): 3648–3649.
2339:Chem. Soc. Abstr.
2220: : 705–713.
2180:Blyth, J. (1849)
2156:footnote on p. 87
2069:978-0-486-22798-6
1688:10.1021/tx800229w
1682:(10): 2061–2064.
1606:'s mystery novel
1556:stereoselectively
1546:
1530:
1515:coniine when its
1502:
1494:
1486:
1394:hydrochloric acid
1268:Specific rotation
1198:
1185:
1184:
1170:
1059:
1047:
1038:Specific rotation
1024:
1021: 0.8626 and
1020:
1012:
961:flaccid paralysis
866:carnivorous plant
786:organic chemistry
708:chemical compound
700:Chemical compound
698:
697:
438:CompTox Dashboard
300:): 207-282-6
174:Interactive image
162:Interactive image
150:Interactive image
3290:
3055:Hyoscyamus niger
2985:Plants / animals
2971:
2964:
2957:
2948:
2947:
2922:
2920:
2918:
2898:(6): 2049–2055.
2889:
2871:
2870:
2838:
2832:
2831:
2808:The FEBS Journal
2802:Conium maculatum
2795:
2789:
2788:
2764:
2758:
2757:
2721:
2715:
2714:
2688:
2682:
2672:
2666:
2648:
2642:
2632:
2626:
2625:
2599:
2593:
2583:
2577:
2576:
2558:
2552:
2541:
2535:
2534:
2532:
2508:
2502:
2501:
2484:(3): 3734–3736.
2469:
2463:
2462:
2434:
2428:
2421:
2412:
2393:
2387:
2377:
2371:
2370:
2348:
2342:
2335:
2329:
2318:
2312:
2301:
2295:
2284:
2278:
2267:
2258:
2247:
2241:
2231:Conium maculatum
2227:
2221:
2206:
2200:
2178:
2172:
2154:(coniine). See
2140:Conium maculatum
2134:Conium maculatum
2128:
2122:
2121:
2113:
2107:
2106:
2104:
2102:
2096:
2092:"Poison Hemlock"
2087:
2081:
2080:
2078:
2076:
2053:
2047:
2046:
2044:
2042:
2028:
2022:
2021:
2005:
2003:"Socrates"
1994:
1988:
1987:
1970:(203): 106–108.
1957:
1951:
1950:
1904:
1898:
1897:
1895:
1894:
1880:
1874:
1873:
1865:
1854:
1848:
1847:
1841:
1833:
1825:
1819:
1818:
1816:
1814:
1798:
1792:
1791:
1781:
1771:
1747:
1741:
1740:
1711:Sarracenia flava
1706:
1700:
1699:
1669:
1654:
1653:
1651:
1649:
1639:"Poison Hemlock"
1634:
1609:Five Little Pigs
1544:
1537:barium hydroxide
1528:
1519:was heated with
1500:
1492:
1484:
1459:cyanogen bromide
1334:2-methylpyridine
1196:
1180:
1177:
1171:
1169:
1128:
1100:
1092:
1082:carbon disulfide
1057:
1045:
1022:
1018:
1010:
923:Native Americans
919:flying ointments
874:Aethusa cynapium
861:Sarracenia flava
841:Conium maculatum
832:Conium maculatum
794:Albert Ladenburg
737:Aethusa cynapium
731:Sarracenia flava
721:Conium maculatum
682:
676:
673:
672:
642:Refractive index
606:
594:
588:
582:
575:Chemical formula
471:
470:
446:
444:
428:
416:
404:
375:
363:
343:
329:
289:
281:
270:
259:
230:
205:
176:
164:
152:
119:
107:
95:
43:
26:
19:
15:
3298:
3297:
3293:
3292:
3291:
3289:
3288:
3287:
3253:
3252:
3251:
3246:
3175:
3109:
3092:
2980:
2975:
2929:
2916:
2914:
2887:
2879:
2877:Further reading
2874:
2839:
2835:
2796:
2792:
2765:
2761:
2722:
2718:
2689:
2685:
2673:
2669:
2652:The Merck Index
2649:
2645:
2633:
2629:
2623:
2600:
2596:
2584:
2580:
2559:
2555:
2542:
2538:
2509:
2505:
2470:
2466:
2435:
2431:
2422:
2415:
2397:The Merck Index
2394:
2390:
2378:
2374:
2349:
2345:
2336:
2332:
2319:
2315:
2302:
2298:
2285:
2281:
2268:
2261:
2248:
2244:
2228:
2224:
2207:
2203:
2198:
2194:
2179:
2175:
2129:
2125:
2114:
2110:
2100:
2098:
2094:
2088:
2084:
2074:
2072:
2070:
2059:A Modern Herbal
2054:
2050:
2040:
2038:
2030:
2029:
2025:
1995:
1991:
1958:
1954:
1905:
1901:
1892:
1890:
1888:www.massmed.org
1882:
1881:
1877:
1855:
1851:
1835:
1834:
1826:
1822:
1812:
1810:
1801:Mackie, Robin.
1799:
1795:
1748:
1744:
1707:
1703:
1670:
1657:
1647:
1645:
1635:
1631:
1627:
1604:Agatha Christie
1600:
1573:
1521:sodium ethoxide
1420:hydroiodic acid
1330:
1323:
1311:
1307:
1302:The Merck Index
1299:
1295:
1291:
1278:pharmacological
1270:
1246:
1230:
1226:
1222:
1218:
1214:
1210:
1206:
1202:
1194:
1181:
1175:
1172:
1129:
1127:
1113:
1105:This section
1101:
1090:
1088:Crystallization
1066:
1055:
1035:
1031:
997:
981:
965:succinylcholine
938:
921:in witchcraft.
883:
817:
815:Natural origins
754:5-oxooctylamine
752:cyclisation of
706:is a poisonous
701:
694:
689:
688:
687: ?)
678:
674:
670:
666:
652:
650:
604:
591:
585:
577:
563:
560:
551:
542:
529:
528:
517:
514:
513:
510:
496:
495:
492:
486:
485:
474:
447:
440:
431:
378:
344:
332:
303:
262:
233:
208:
179:
133:
122:
76:
62:
34:
27:
12:
11:
5:
3296:
3286:
3285:
3280:
3275:
3270:
3265:
3248:
3247:
3245:
3244:
3239:
3234:
3229:
3224:
3219:
3214:
3209:
3204:
3199:
3194:
3189:
3183:
3181:
3177:
3176:
3174:
3173:
3168:
3163:
3158:
3153:
3148:
3143:
3138:
3133:
3128:
3117:
3115:
3111:
3110:
3100:
3098:
3094:
3093:
3091:
3090:
3085:
3077:
3072:
3064:
3059:
3051:
3043:
3039:Datura innoxia
3035:
3027:
3022:
3017:
3016:
3015:
3005:
2997:
2988:
2986:
2982:
2981:
2974:
2973:
2966:
2959:
2951:
2945:
2944:
2939:
2928:
2927:External links
2925:
2924:
2923:
2878:
2875:
2873:
2872:
2847:Phytochemistry
2833:
2790:
2759:
2732:(1): 244–253.
2716:
2683:
2667:
2643:
2627:
2621:
2594:
2578:
2553:
2536:
2503:
2464:
2445:(1): 439–441.
2429:
2413:
2388:
2372:
2343:
2341:, 1906, , 711.
2330:
2313:
2311:, 34, 70, 102.
2296:
2279:
2259:
2242:
2222:
2201:
2196:
2192:
2173:
2123:
2108:
2082:
2068:
2048:
2023:
2008:Chisholm, Hugh
1998:Jackson, Henry
1989:
1952:
1923:10.2307/631830
1899:
1875:
1863:"Conine"
1860:, ed. (1911).
1858:Chisholm, Hugh
1849:
1820:
1793:
1742:
1723:(7): 829–830.
1701:
1655:
1628:
1626:
1623:
1599:
1596:
1572:
1569:
1541:pipecolic acid
1481:
1480:
1439:
1438:
1412:
1411:
1356:with metallic
1329:
1326:
1321:
1309:
1305:
1297:
1293:
1289:
1274:stereochemical
1269:
1266:
1245:
1242:
1228:
1224:
1220:
1216:
1212:
1208:
1204:
1200:
1192:
1183:
1182:
1118:. Please help
1104:
1102:
1095:
1089:
1086:
1065:
1062:
1053:
1052:)-Coniine has
1033:
1029:
996:
993:
979:
937:
934:
900:ancient Greece
882:
879:
828:Poison hemlock
816:
813:
809:poison hemlock
716:poison hemlock
699:
696:
695:
690:
668:
667:
663:standard state
660:
657:
656:
653:
648:
640:
637:
636:
633:
627:
626:
623:
617:
616:
613:
609:
608:
602:
596:
595:
589:
583:
578:
573:
570:
569:
565:
564:
562:
561:
554:
552:
545:
543:
532:
524:
523:
522:
519:
518:
516:
515:
511:
500:
499:
497:
493:
490:
489:
481:
480:
479:
476:
475:
473:
472:
450:
448:
436:
433:
432:
430:
429:
417:
405:
388:
386:
380:
379:
377:
376:
364:
347:
345:
337:
334:
333:
331:
330:
313:
311:
305:
304:
302:
301:
293:
291:
283:
282:
272:
264:
263:
261:
260:
243:
241:
235:
234:
232:
231:
218:
216:
210:
209:
207:
206:
189:
187:
181:
180:
178:
177:
165:
153:
136:
134:
127:
124:
123:
121:
120:
108:
96:
79:
77:
72:
69:
68:
64:
63:
56:
50:
49:
45:
44:
36:
35:
28:
9:
6:
4:
3:
2:
3295:
3284:
3281:
3279:
3276:
3274:
3271:
3269:
3266:
3264:
3261:
3260:
3258:
3243:
3240:
3238:
3235:
3233:
3230:
3228:
3225:
3223:
3220:
3218:
3215:
3213:
3210:
3208:
3205:
3203:
3200:
3198:
3195:
3193:
3190:
3188:
3185:
3184:
3182:
3178:
3172:
3169:
3167:
3164:
3162:
3159:
3157:
3154:
3152:
3149:
3147:
3144:
3142:
3139:
3137:
3134:
3132:
3129:
3126:
3122:
3119:
3118:
3116:
3112:
3107:
3103:
3099:
3095:
3089:
3086:
3083:
3082:
3078:
3076:
3073:
3070:
3069:
3065:
3063:
3060:
3057:
3056:
3052:
3049:
3048:
3044:
3042:(thorn-apple)
3041:
3040:
3036:
3033:
3032:
3028:
3026:
3023:
3021:
3018:
3014:
3011:
3010:
3009:
3006:
3003:
3002:
2998:
2995:
2994:
2990:
2989:
2987:
2983:
2979:
2972:
2967:
2965:
2960:
2958:
2953:
2952:
2949:
2943:
2940:
2938:
2934:
2931:
2930:
2913:
2909:
2905:
2901:
2897:
2893:
2886:
2881:
2880:
2868:
2864:
2860:
2856:
2852:
2848:
2844:
2837:
2829:
2825:
2821:
2817:
2813:
2809:
2805:
2803:
2794:
2786:
2782:
2778:
2774:
2770:
2763:
2755:
2751:
2747:
2743:
2739:
2735:
2731:
2727:
2720:
2712:
2708:
2704:
2700:
2699:
2694:
2687:
2680:
2676:
2671:
2665:
2661:
2657:
2653:
2647:
2641:, 1192, 1386.
2640:
2636:
2631:
2624:
2618:
2614:
2610:
2606:
2598:
2591:
2587:
2582:
2574:
2570:
2567:: 2969–2973.
2566:
2565:
2557:
2550:
2546:
2540:
2531:
2526:
2522:
2518:
2514:
2507:
2499:
2495:
2491:
2487:
2483:
2479:
2475:
2468:
2460:
2456:
2452:
2448:
2444:
2440:
2433:
2426:
2420:
2418:
2411:
2407:
2403:
2399:
2398:
2392:
2385:
2382:
2376:
2368:
2364:
2360:
2356:
2355:
2347:
2340:
2334:
2327:
2323:
2317:
2310:
2306:
2303:cf. Dilling,
2300:
2293:
2289:
2283:
2276:
2272:
2266:
2264:
2256:
2252:
2246:
2240:
2236:
2232:
2226:
2219:
2215:
2211:
2205:
2190:
2186:
2183:
2177:
2170:
2166:
2162:
2157:
2153:
2149:
2145:
2141:
2137:
2135:
2127:
2119:
2112:
2093:
2086:
2071:
2065:
2061:
2060:
2052:
2037:
2033:
2027:
2020:
2015:
2014:
2009:
2004:
1999:
1993:
1985:
1981:
1977:
1973:
1969:
1965:
1964:
1956:
1948:
1944:
1940:
1936:
1932:
1928:
1924:
1920:
1916:
1912:
1911:
1903:
1889:
1885:
1879:
1871:
1870:
1864:
1859:
1853:
1845:
1839:
1831:
1824:
1808:
1804:
1797:
1789:
1785:
1780:
1775:
1770:
1765:
1761:
1757:
1753:
1746:
1738:
1734:
1730:
1726:
1722:
1718:
1717:
1712:
1705:
1697:
1693:
1689:
1685:
1681:
1677:
1676:
1668:
1666:
1664:
1662:
1660:
1644:
1640:
1633:
1629:
1622:
1620:
1619:
1613:
1611:
1610:
1605:
1592:
1588:
1581:
1577:
1568:
1566:
1563:
1562:
1557:
1552:
1550:
1542:
1538:
1534:
1526:
1522:
1518:
1514:
1510:
1506:
1498:
1490:
1476:
1472:
1471:
1470:
1468:
1464:
1460:
1456:
1452:
1448:
1444:
1434:
1430:
1429:
1428:
1425:
1421:
1417:
1407:
1403:
1402:
1401:
1399:
1395:
1385:
1381:
1379:
1375:
1374:tartaric acid
1371:
1368:(±) coniine.
1367:
1363:
1359:
1355:
1351:
1347:
1346:zinc chloride
1344:in anhydrous
1343:
1339:
1335:
1325:
1319:
1315:
1303:
1287:
1283:
1279:
1275:
1265:
1263:
1259:
1255:
1251:
1244:Color changes
1241:
1239:
1234:
1190:
1179:
1168:
1165:
1161:
1158:
1154:
1151:
1147:
1144:
1140:
1137: –
1136:
1132:
1131:Find sources:
1125:
1121:
1117:
1111:
1110:
1109:single source
1103:
1099:
1094:
1093:
1085:
1083:
1079:
1075:
1071:
1061:
1051:
1043:
1039:
1028:
1016:
1008:
1006:
1002:
992:
990:
986:
982:
974:
971:
966:
962:
958:
954:
949:
947:
943:
933:
930:
928:
924:
920:
916:
912:
908:
907:antispasmodic
903:
901:
897:
893:
892:myoglobinuria
889:
878:
876:
875:
870:
867:
863:
862:
852:
848:
846:
842:
837:
833:
829:
821:
812:
810:
806:
802:
797:
795:
791:
787:
783:
779:
775:
771:
770:stereoisomers
767:
763:
759:
755:
751:
747:
743:
739:
738:
733:
732:
727:
723:
722:
717:
713:
709:
705:
693:
686:
681:
664:
658:
654:
647:
643:
639:
638:
634:
632:
631:Boiling point
629:
628:
624:
622:
621:Melting point
619:
618:
614:
611:
610:
603:
601:
598:
597:
579:
576:
572:
571:
566:
558:
553:
549:
544:
540:
536:
531:
530:
527:
520:
508:
504:
498:
488:
487:
484:
477:
469:
465:
464:DTXSID8041795
460:
456:
452:
451:
449:
439:
435:
434:
427:
422:
418:
415:
410:
406:
403:
398:
394:
390:
389:
387:
385:
382:
381:
374:
369:
365:
362:
357:
353:
349:
348:
346:
340:
336:
335:
328:
323:
319:
315:
314:
312:
310:
307:
306:
299:
295:
294:
292:
290:
285:
284:
280:
276:
273:
271:
269:ECHA InfoCard
266:
265:
258:
253:
249:
245:
244:
242:
240:
237:
236:
229:
228:ChEMBL2287063
224:
220:
219:
217:
215:
212:
211:
204:
199:
195:
191:
190:
188:
186:
183:
182:
175:
170:
166:
163:
158:
154:
151:
146:
142:
138:
137:
135:
131:
126:
125:
118:
113:
109:
106:
101:
97:
94:
89:
85:
81:
80:
78:
75:
71:
70:
65:
60:
55:
51:
46:
42:
37:
32:
25:
20:
3278:Plant toxins
3237:Theophrastus
3145:
3097:Preparations
3079:
3066:
3053:
3047:Datura metel
3045:
3037:
3029:
3004:(belladonna)
2999:
2991:
2915:. Retrieved
2895:
2891:
2850:
2846:
2836:
2811:
2807:
2801:
2793:
2776:
2772:
2762:
2729:
2725:
2719:
2702:
2696:
2686:
2678:
2674:
2670:
2659:
2655:
2651:
2646:
2638:
2634:
2630:
2604:
2597:
2589:
2585:
2581:
2562:
2556:
2548:
2544:
2539:
2520:
2516:
2506:
2481:
2477:
2467:
2442:
2438:
2432:
2424:
2405:
2401:
2395:
2391:
2383:
2380:
2375:
2358:
2352:
2346:
2338:
2333:
2325:
2321:
2316:
2308:
2304:
2299:
2291:
2288:Arch. Pharm.
2287:
2282:
2274:
2270:
2254:
2250:
2245:
2234:
2230:
2225:
2217:
2213:
2204:
2188:
2184:
2176:
2168:
2164:
2160:
2151:
2147:
2143:
2139:
2133:
2126:
2117:
2111:
2099:. Retrieved
2085:
2073:. Retrieved
2058:
2051:
2039:. Retrieved
2035:
2026:
2017:
2011:
1992:
1967:
1961:
1955:
1914:
1908:
1902:
1891:. Retrieved
1887:
1878:
1867:
1852:
1829:
1823:
1811:. Retrieved
1806:
1796:
1762:(11): 1962.
1759:
1755:
1745:
1720:
1714:
1710:
1704:
1679:
1673:
1646:. Retrieved
1642:
1632:
1616:
1614:
1607:
1601:
1585:
1574:
1571:Biosynthesis
1559:
1553:
1532:
1512:
1509:pelletierine
1504:
1488:
1482:
1462:
1440:
1413:
1397:
1390:
1342:acetaldehyde
1331:
1317:
1313:
1285:
1271:
1247:
1186:
1176:January 2017
1173:
1163:
1156:
1149:
1142:
1130:
1106:
1067:
1049:
1026:
1014:
1009:
998:
988:
984:
975:
950:
945:
941:
939:
931:
927:arrow poison
904:
884:
872:
859:
857:
840:
831:
826:
798:
781:
777:
773:
746:biosynthesis
735:
729:
719:
703:
702:
645:
556:
547:
538:
534:
506:
502:
458:
454:
420:
408:
396:
392:
367:
355:
351:
321:
317:
297:
251:
247:
222:
197:
193:
168:
156:
144:
140:
111:
99:
87:
83:
67:Identifiers
58:
30:
3263:Neurotoxins
3227:Sabuncuoğlu
3217:Hippocrates
3207:Dioscorides
3161:Hyoscyamine
3125:medical use
3106:medical use
3062:Lactucarium
3013:medical use
2917:January 23,
2705:: 526–554.
2523:(1): 1–26.
1716:Experientia
1618:The Expanse
1549:amino acids
1547:-series of
1451:nitric acid
1418:and fuming
1378:enantiopure
1364:to provide
1350:paraldehyde
1316:)-(+) and (
1254:nitric acid
915:Middle Ages
845:enantiomers
758:Schiff base
612:Appearance
568:Properties
275:100.006.621
203:CHEBI:28322
3257:Categories
3171:Salicylate
3084:(saw-wort)
3071:(mandrake)
2853:(1): 107.
2307:, 1909, ,
1963:Philosophy
1917:: 91–106.
1893:2021-07-30
1625:References
1497:conhydrine
1467:indolizine
1455:indolizine
1416:phosphorus
1146:newspapers
1064:Solubility
600:Molar mass
426:QKK1SI92BR
414:04R53ZF48T
402:C479P32L2D
239:ChemSpider
128:3D model (
74:CAS Number
54:IUPAC name
3232:Sushrutha
3187:Abulcasis
3131:Aconitine
3114:Compounds
3081:Saussurea
3058:(henbane)
3034:(hemlock)
3020:Castoreum
2996:(aconite)
2935:from the
2746:0022-3263
2498:0365-9496
2459:0365-9496
2337:Gabutti,
2305:Pharm. J.
1984:170399183
1838:cite book
1756:Molecules
1517:hydrazone
1380:coniine.
1328:Synthesis
1284:mixture.
1262:aldehydes
1211:•2 H
1135:"Coniine"
1116:talk page
987:-(−) and
911:arthritis
790:alkaloids
750:enzymatic
288:EC Number
105:5985-99-9
93:3238-60-6
33:)-Coniine
3197:Avicenna
3192:Avenzoar
3166:Morphine
3151:Hyoscine
3136:Atropine
3008:Cannabis
2993:Aconitum
2912:22449544
2828:26260860
2754:19012434
2677:, 1932,
2656:15th Ed.
2637:, 1917,
2588:, 1934,
2547:, 1932,
2427:173-177.
2402:15th Ed.
2324:, 1933,
2290:, 1898,
2286:Melzer,
2273:, 1909,
2253:, 1902,
2249:Ahrens,
2000:(1911).
1947:24221544
1939:19681231
1788:29135964
1737:38319708
1696:18763813
1443:pyridine
1376:yielded
1282:isomeric
1250:sulfuric
1238:nicotine
1189:oxidizes
1040:section
1001:isolated
801:Socrates
766:racemate
712:alkaloid
461:):
423:):
411:):
399:):
370:):
358:):
324:):
254:):
225:):
200:):
171:):
159:):
147:):
117:458-88-8
114:):
102:):
90:):
17:Coniine
3146:Coniine
3141:Cocaine
3121:Ethanol
2855:Bibcode
2592:, 1011.
2545:Annalen
2239:p. 116.
2010:(ed.).
1779:6150177
1525:ethanol
1366:racemic
1362:ethanol
1354:reduced
1233:picrate
1160:scholar
1074:alcohol
1005:Hofmann
970:hypoxic
869:endemic
704:Coniine
685:what is
683: (
655:1.4505
605:127.231
339:PubChem
3222:Rhazes
3202:Celsus
3180:People
3156:Δ9-THC
3088:Willow
3031:Conium
2910:
2826:
2752:
2744:
2681:, 927.
2619:
2496:
2457:
2328:, 596.
2277:, 107.
2257:, 1330
2152:coniin
2066:
1982:
1945:
1937:
1931:631830
1929:
1786:
1776:
1735:
1694:
1565:lipase
1358:sodium
1162:
1155:
1148:
1141:
1133:
1070:turbid
888:Lesbos
744:. The
680:verify
677:
526:SMILES
373:441072
327:C06523
257:389878
214:ChEMBL
48:Names
3242:Zhang
3212:Galen
3075:Opium
2888:(PDF)
2551:, 16.
2386:1-98.
2294:, 701
2101:3 May
2095:(PDF)
2075:3 May
2041:3 May
2006:. In
1980:S2CID
1943:S2CID
1927:JSTOR
1813:3 May
1733:S2CID
1648:3 May
1340:with
1219:•PtCl
1167:JSTOR
1153:books
1078:ether
1042:below
940:The (
836:toxic
710:, an
483:InChI
185:ChEBI
130:JSmol
3102:Beer
3025:Coca
2919:2017
2908:PMID
2824:PMID
2804:L.)"
2750:PMID
2742:ISSN
2675:Ber.
2660:7181
2635:Ber.
2617:ISBN
2586:Ber.
2494:ISSN
2455:ISSN
2406:2489
2322:Ber.
2271:Ber.
2251:Ber.
2103:2015
2077:2015
2064:ISBN
2043:2015
1935:PMID
1844:link
1815:2015
1784:PMID
1692:PMID
1650:2015
1513:rac-
1463:rac.
1445:and
1424:zinc
1272:The
1139:news
976:The
894:and
803:was
762:ring
384:UNII
361:9985
309:KEGG
2900:doi
2863:doi
2816:doi
2812:282
2781:doi
2734:doi
2707:doi
2703:113
2609:doi
2569:doi
2549:498
2525:doi
2486:doi
2447:doi
2384:247
2363:doi
2292:236
2142:),
1972:doi
1919:doi
1915:121
1774:PMC
1764:doi
1725:doi
1713:".
1684:doi
1523:in
1499:to
1398:rac
1360:in
1252:or
1122:by
1044:).
847:.
443:EPA
342:CID
3259::
2906:.
2896:50
2894:.
2890:.
2861:.
2851:17
2849:.
2845:.
2822:.
2810:.
2806:.
2777:86
2775:.
2771:.
2748:.
2740:.
2730:74
2728:.
2701:.
2695:.
2679:65
2654:,
2639:50
2615:,
2590:67
2521:11
2519:.
2515:.
2492:.
2482:40
2480:.
2476:.
2453:.
2443:19
2441:.
2416:^
2400:,
2359:36
2357:.
2326:66
2309:29
2275:42
2262:^
2255:35
2218:14
2216:,
2197:17
2193:17
2187:,
2163:,
2148:20
2146:,
2034:.
1978:.
1968:53
1966:.
1941:.
1933:.
1925:.
1913:.
1886:.
1866:.
1840:}}
1836:{{
1805:.
1782:.
1772:.
1760:22
1758:.
1754:.
1731:.
1721:32
1719:.
1690:.
1680:21
1678:.
1658:^
1641:.
1612:.
1567:.
1551:.
1531:-(
1503:-(
1487:-(
1264:.
1256:.
1223:•H
1048:-(
1013:-(
1007:.
980:50
978:LD
929:.
902:.
811:.
590:17
59:2S
3127:)
3123:(
3108:)
3104:(
2970:e
2963:t
2956:v
2921:.
2902::
2869:.
2865::
2857::
2830:.
2818::
2787:.
2783::
2756:.
2736::
2713:.
2709::
2611::
2575:.
2571::
2533:.
2527::
2500:.
2488::
2461:.
2449::
2369:.
2365::
2195:H
2189:1
2165:5
2136:"
2105:.
2079:.
2045:.
1986:.
1974::
1949:.
1921::
1896:.
1846:)
1817:.
1790:.
1766::
1739:.
1727::
1698:.
1686::
1652:.
1545:D
1533:S
1529:D
1505:R
1501:L
1493:L
1489:R
1485:L
1322:D
1318:R
1314:S
1310:3
1306:3
1298:D
1294:3
1290:D
1286:S
1229:4
1225:2
1221:4
1217:2
1213:2
1209:6
1207:O
1205:6
1203:H
1201:4
1197:D
1193:D
1178:)
1174:(
1164:·
1157:·
1150:·
1143:·
1126:.
1112:.
1058:D
1054:D
1050:R
1046:L
1034:D
1030:D
1027:n
1023:D
1019:D
1015:S
1011:D
989:S
985:R
946:S
942:R
830:(
782:R
778:R
774:S
772:(
728:(
718:(
675:N
651:)
649:D
646:n
644:(
593:N
587:H
584:8
581:C
557:S
555:(
548:R
546:(
539:S
537:/
535:R
533:(
507:S
505:/
503:R
501:(
459:S
457:/
455:R
453:(
445:)
441:(
421:S
419:(
409:R
407:(
397:S
395:/
393:R
391:(
368:S
366:(
356:S
354:/
352:R
350:(
322:S
320:/
318:R
316:(
298:S
296:(
252:S
250:/
248:R
246:(
223:S
221:(
198:S
196:/
194:R
192:(
169:S
167:(
157:R
155:(
145:S
143:/
141:R
139:(
132:)
112:S
110:(
100:R
98:(
88:S
86:/
84:R
82:(
57:(
31:S
29:(
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.