223:
70:
31:
298:
141:
82:
219:
plant, it accounts for about 40% of the total energy consumption. Industrial distillation is typically performed in large, vertical cylindrical columns (as shown in Figure 2) known as "distillation towers" or "distillation columns" with diameters ranging from about 65 centimeters to 6 meters and heights ranging from about 6 meters to 60 meters or more.
258:
Inside the column, the downflowing reflux liquid provides cooling and condensation of upflowing vapors thereby increasing the efficacy of the distillation tower. The more reflux and/or more trays provided, the better is the tower's separation of lower boiling materials from higher boiling materials.
250:
Figure 3 depicts an industrial fractionating column separating a feed stream into one distillate fraction and one bottoms fraction. However, many industrial fractionating columns have outlets at intervals up the column so that multiple products having different boiling ranges may be withdrawn from a
218:
Distillation is one of the most common and energy-intensive separation processes. Effectiveness of separation is dependent upon the height and diameter of the column, the ratio of the column's height to diameter, and the material that comprises the distillation column itself. In a typical chemical
246:
and with the feed must equal the amount heat removed by the overhead condenser and with the products. The heat entering a distillation column is a crucial operating parameter, addition of excess or insufficient heat to the column can lead to foaming, weeping, entrainment, or flooding.
274:
Bubble-cap "trays" or "plates" are one of the types of physical devices, which are used to provide good contact between the upflowing vapor and the downflowing liquid inside an industrial fractionating column. Such trays are shown in
Figures 4 and 5.
254:
Industrial fractionating columns use external reflux to achieve better separation of products. Reflux refers to the portion of the condensed overhead liquid product that returns to the upper part of the fractionating column as shown in Figure 3.
238:
Industrial distillation towers are usually operated at a continuous steady state. Unless disturbed by changes in feed, heat, ambient temperature, or condensing, the amount of feed being added normally equals the amount of product being removed.
199:. In such refineries, the crude oil feedstock is a complex, multicomponent mixture that must be separated. Yields of pure chemical compounds are generally not expected, however, yields of groups of compounds within a relatively small range of
251:
column distilling a multi-component feed stream. The "lightest" products with the lowest boiling points exit from the top of the columns and the "heaviest" products with the highest boiling points exit from the bottom.
262:
The design and operation of a fractionating column depends on the composition of the feed as well as the composition of the desired products. Given a simple, binary component feed, analytical methods such as the
109:-vaporization cycle, the vapors are enriched in a certain component. A larger surface area allows more cycles, improving separation. This is the rationale for a Vigreux column or a packed fractionating column.
116:
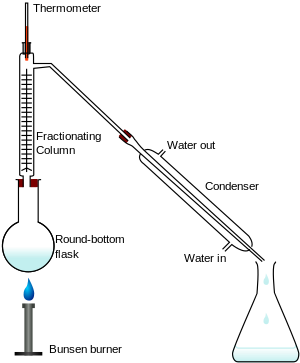
In a typical fractional distillation, a liquid mixture is heated in the distilling flask, and the resulting vapor rises up the fractionating column (see Figure 1). The vapor condenses on glass spurs (known as
231:
629:
290:
137:, which cools the vapor until it condenses into a liquid distillate. The separation may be enhanced by the addition of more trays (to a practical limitation of heat, flow, etc.).
113:
achieves the same outcome by using a rotating band within the column to force the rising vapors and descending condensate into close contact, achieving equilibrium more quickly.
89:
A laboratory fractionating column is a piece of glassware used to separate vaporized mixtures of liquid compounds with close volatility. Most commonly used is either a
325:
takes place. Differently shaped packings have different surface areas and void space between packings. Both of these factors affect packing performance.
164:. Fractionating columns are widely used in chemical process industries where large quantities of liquids have to be distilled. Such industries are
639:
507:
309:
is used in the column instead of trays, especially when low pressure drops across the column are required, as when operating under
532:
645:
571:
654:
61:. Fractionating columns are used in small-scale laboratory distillations as well as large-scale industrial distillations.
282:. Hence, a fractionating column almost always needs more actual, physical plates than the required number of theoretical
17:
669:
611:
516:
482:
457:
427:
402:
133:. Only the most volatile of the vapors stays in gas form all the way to the top, where it may then proceed through a
125:
the rising distillate vapor. The hottest tray is at the bottom of the column and the coolest tray is at the top. At
634:
271:
can be used. For a multi-component feed, simulation models are used both for design, operation, and construction.
57:
of liquid mixtures to separate the mixture into its component parts, or fractions, based on their differences in
283:
130:
313:. This packing material can either be random dumped packing (1–3 in or 2.5–7.6 cm wide) such as
689:
321:. Liquids tend to wet the surface of the packing, and the vapors pass across this wetted surface, where
293:
Figure 5: Section of fractionating tower of Figure 4 showing detail of a pair of trays with bubble caps
110:
134:
264:
642:
by Ivar J. Halvorsen and Sigurd
Skogestad, Norwegian University of Science and Technology, Norway
374:
349:
344:
334:
153:
694:
278:
The efficiency of a tray or plate is typically lower than that of a theoretical 100% efficient
173:
58:
234:
Figure 4: Chemical engineering schematic of typical bubble-cap trays in a fractionating column
684:
222:
354:
161:
97:. Fractionating columns help to separate the mixture by allowing the mixed vapors to cool,
8:
448:
369:
69:
359:
339:
318:
607:
567:
512:
478:
453:
423:
398:
364:
279:
118:
39:
35:
30:
540:
74:
658:
268:
157:
102:
651:
200:
90:
297:
678:
322:
226:
Figure 3: Chemical engineering schematic of a continuous fractionating column
169:
587:
Beychok, Milton (May 1951). "Algebraic
Solution of McCabe-Thiele Diagram".
314:
196:
126:
106:
98:
94:
54:
230:
189:
140:
306:
185:
165:
93:
or a straight column packed with glass beads or metal pieces such as
81:
195:
production. Fractional distillation finds its widest application in
289:
243:
192:
177:
181:
310:
122:
601:
504:
670:
121:) inside the column, and returns to the distilling flask,
664:
397:(5th ed.). Hoboken, New Jersey: Wiley-Interscience.
418:
Smith, Julian; McCabe, Warren; Harriott, Peter (2004).
207:, are expected. This process is the origin of the name
129:
conditions, the vapor and liquid on each tray reach an
561:
635:
More drawings of glassware including
Vigreux columns
73:
Figure 1: Fractional distillation apparatus using a
417:
148:
64:
144:Figure 2: Typical industrial fractionating columns
676:
445:
392:
242:The amount of heat entering the column from the
395:Kirk-Othmer Encyclopedia of Chemical Technology
301:Figure 6: Entire view of a Distillation Column
393:Kroschwitz, Jacqueline; Seidel, Arza (2004).
27:Equipment to separate liquids by distillation
630:Use of distillation columns in Oil & Gas
595:
564:Elementary Principles of Chemical Processes
472:
602:Seader, J. D.; Henley, Ernest J. (1998).
505:Perry, Robert H.; Green, Don W. (1984).
441:
439:
296:
288:
229:
221:
139:
101:, and vaporize again in accordance with
80:
68:
29:
586:
500:
498:
496:
494:
420:Unit Operations of Chemical Engineering
119:theoretical trays or theoretical plates
14:
677:
648:by Ming Tham, Newcastle University, UK
580:
436:
508:Perry's Chemical Engineers' Handbook
491:
85:Vigreux column in a laboratory setup
24:
25:
706:
623:
665:Distillation simulation software
562:Felder, R.; Roussea, W. (2005).
305:In industrial uses, sometimes a
149:Industrial fractionating columns
65:Laboratory fractionating columns
661:by the Distillation Group, USA
555:
525:
466:
411:
386:
34:Giant fractionating column of
13:
1:
646:Distillation, An Introduction
604:Separation Process Principles
589:Chemical Engineering Progress
511:(6th ed.). McGraw-Hill.
477:(2nd ed.). McGraw Hill.
452:(1st ed.). McGraw-Hill.
422:(7th ed.). McGraw Hill.
380:
7:
328:
10:
711:
111:Spinning band distillation
446:Kister, Henry Z. (1992).
53:is equipment used in the
284:vapor–liquid equilibrium
566:(3rd ed.). Wiley.
375:Fractional distillation
350:Extractive distillation
345:Continuous distillation
335:Azeotropic distillation
209:fractional distillation
154:Fractional distillation
40:Machine Sazi Arak (MSA)
533:"Distillation Columns"
319:structured sheet metal
302:
294:
235:
227:
174:natural gas processing
145:
86:
78:
42:
300:
292:
233:
225:
143:
84:
72:
33:
543:on 23 September 2015
475:Separation Processes
355:Laboratory glassware
265:McCabe–Thiele method
197:petroleum refineries
162:chemical engineering
47:fractionating column
640:Distillation Theory
606:. New York: Wiley.
473:King, C.J. (1980).
449:Distillation Design
370:Vacuum distillation
18:Distillation column
690:Chemical equipment
657:2014-07-13 at the
360:Steam distillation
340:Batch distillation
303:
295:
236:
228:
146:
87:
79:
43:
573:978-0-471-68757-3
365:Theoretical plate
280:equilibrium stage
51:fractional column
36:Arak Oil Refinery
16:(Redirected from
702:
618:
617:
599:
593:
592:
584:
578:
577:
559:
553:
552:
550:
548:
539:. Archived from
529:
523:
522:
502:
489:
488:
470:
464:
463:
443:
434:
433:
415:
409:
408:
390:
307:packing material
188:separation, and
75:Liebig condenser
38:manufactured by
21:
710:
709:
705:
704:
703:
701:
700:
699:
675:
674:
659:Wayback Machine
626:
621:
614:
600:
596:
585:
581:
574:
560:
556:
546:
544:
531:
530:
526:
519:
503:
492:
485:
471:
467:
460:
444:
437:
430:
416:
412:
405:
391:
387:
383:
331:
269:Fenske equation
158:unit operations
151:
67:
28:
23:
22:
15:
12:
11:
5:
708:
698:
697:
692:
687:
673:
672:
667:
662:
649:
643:
637:
632:
625:
624:External links
622:
620:
619:
612:
594:
579:
572:
554:
524:
517:
490:
483:
465:
458:
435:
428:
410:
403:
384:
382:
379:
378:
377:
372:
367:
362:
357:
352:
347:
342:
337:
330:
327:
203:, also called
201:boiling points
156:is one of the
150:
147:
91:Vigreux column
66:
63:
26:
9:
6:
4:
3:
2:
707:
696:
695:Fractionation
693:
691:
688:
686:
683:
682:
680:
671:
668:
666:
663:
660:
656:
653:
650:
647:
644:
641:
638:
636:
633:
631:
628:
627:
615:
613:0-471-58626-9
609:
605:
598:
590:
583:
575:
569:
565:
558:
542:
538:
534:
528:
520:
518:0-07-049479-7
514:
510:
509:
501:
499:
497:
495:
486:
484:0-07-034612-7
480:
476:
469:
461:
459:0-07-034909-6
455:
451:
450:
442:
440:
431:
429:0-07-284823-5
425:
421:
414:
406:
404:0-471-48810-0
400:
396:
389:
385:
376:
373:
371:
368:
366:
363:
361:
358:
356:
353:
351:
348:
346:
343:
341:
338:
336:
333:
332:
326:
324:
323:mass transfer
320:
316:
315:Raschig rings
312:
308:
299:
291:
287:
285:
281:
276:
272:
270:
266:
260:
256:
252:
248:
245:
240:
232:
224:
220:
216:
214:
213:fractionation
210:
206:
202:
198:
194:
191:
187:
186:liquefied air
183:
179:
175:
171:
170:petrochemical
167:
163:
159:
155:
142:
138:
136:
132:
128:
124:
120:
114:
112:
108:
104:
100:
96:
95:Raschig rings
92:
83:
76:
71:
62:
60:
56:
52:
48:
41:
37:
32:
19:
685:Distillation
652:Distillation
603:
597:
588:
582:
563:
557:
545:. Retrieved
541:the original
536:
527:
506:
474:
468:
447:
419:
413:
394:
388:
304:
277:
273:
261:
257:
253:
249:
241:
237:
217:
212:
208:
204:
180:processing,
172:production,
168:processing,
152:
127:steady-state
115:
107:condensation
105:. With each
103:Raoult's law
88:
55:distillation
50:
46:
44:
190:hydrocarbon
131:equilibrium
679:Categories
381:References
59:volatility
205:fractions
166:petroleum
135:condenser
123:refluxing
655:Archived
547:4 August
537:Brewhaus
329:See also
286:stages.
244:reboiler
193:solvents
178:coal tar
99:condense
267:or the
182:brewing
610:
570:
515:
481:
456:
426:
401:
311:vacuum
608:ISBN
568:ISBN
549:2015
513:ISBN
479:ISBN
454:ISBN
424:ISBN
399:ISBN
317:or
211:or
160:of
49:or
681::
535:.
493:^
438:^
215:.
184:,
176:,
45:A
616:.
591:.
576:.
551:.
521:.
487:.
462:.
432:.
407:.
77:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.