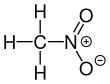
54:
962:
347:
236:
1731:
854:
844:
839:
849:
64:
957:
961:
1224:
960:
1634:
because it provides power even in the absence of atmospheric oxygen. When rich air–fuel mixtures are used, hydrogen and carbon monoxide are two of the combustion products. These gases often ignite, sometimes spectacularly, as the normally very rich mixtures of the still burning fuel exits the exhaust
1564:
The amount of air required to burn 1 kg (2.2 lb) of gasoline is 14.7 kg (32 lb), but only 1.7 kg (3.7 lb) of air is required for 1 kg of nitromethane. Since an engine's cylinder can only contain a limited amount of air on each stroke, 8.6 times as much nitromethane
1927:
Nitromethane reacts with solutions of sodium hydroxide or methoxide in alcohol to produce an insoluble salt of nitromethane. This substance is a sensitive explosive which reverts to nitromethane under acidic conditions and decomposes in water to form another explosive compound, sodium methazonate,
985:
1923:
Nitromethane is used as a model explosive, along with TNT. It has several advantages as a model explosive over TNT, namely its uniform density and lack of solid post-detonation species that complicate the determination of equation of state and further calculations.
1685:
It formerly was used in the explosives industry as a component in a binary explosive formulation with ammonium nitrate and in shaped charges, and it was used as a chemical stabilizer to prevent decomposition of various halogenated hydrocarbons.
1626:
of about 2,400 °C (4,350 °F). The high heat of vaporization of 0.56 MJ/kg together with the high fuel flow provides significant cooling of the incoming charge (about twice that of methanol), resulting in reasonably low temperatures.
1635:
ports. Very rich mixtures are necessary to reduce the temperature of combustion chamber hot parts in order to control pre-ignition and subsequent detonation. Operational details depend on the particular mixture and engine characteristics.
1904:
compression, a hazard common to all liquid explosives. This is when small entrained air bubbles compress and superheat with rapid rises in pressure. It was thought that an operator rapidly snapped shut a valve creating a
1900:. Pure nitromethane is an insensitive explosive with a VoD of approximately 6,400 m/s (21,000 ft/s), but even so inhibitors may be used to reduce the hazards. The tank car explosion was speculated to be due to
1677:)). Even moderate amounts of nitromethane tend to increase the power created by the engine (as the limiting factor is often the air intake), making the engine easier to tune (adjust for the proper air/fuel ratio).
1489:
Although a minor application in terms of volume, nitromethane also is used as a fuel or fuel additive for sports and hobby. For some application, it is mixed with methanol in racing cars, boats, and model engines.
1642:
blended in nitromethane can increase the power output even further. With nitromethane, hydrazine forms an explosive salt that is again a monopropellant. This unstable mixture poses a severe safety hazard. The
862:
1473:= 36 at 20 °C and μ = 3.5 Debye) but aprotic and weakly basic. This combination makes it useful for dissolving positively charged, strongly electrophilic species. It is a solvent for acrylate
819:
978:
2628:
Bordwell, F. G.; Satish, A. V. (1994). "Is
Resonance Important in Determining the Acidities of Weak Acids or the Homolytic Bond Dissociation Enthalpies (BDEs) of Their Acidic H-A Bonds?".
1569:/kg, whereas nitromethane provides only 11.3 MJ/kg. This analysis indicates that nitromethane generates about 2.3 times the power of gasoline when combined with a given amount of oxygen.
1536:
O. Nitric oxide contributes to air pollution, acid rain, and ozone layer depletion. Recent (2020) studies suggest the correct stoichiometric equation for the burning of nitromethane is:
1288:. It is a polar liquid commonly used as a solvent in a variety of industrial applications such as in extractions, as a reaction medium, and as a cleaning solvent. As an intermediate in
1769:. Its acidity allows it to undergo deprotonation, enabling condensation reactions analogous to those of carbonyl compounds. Thus, under base catalysis, nitromethane adds to
963:
2799:
1841:
Nitromethane is a popular solvent in organic and electroanalytical chemistry. It can be purified by cooling below its freezing point, washing the solid with cold
971:
1469:
The major application is as a stabilizer in chlorinated solvents. As an organic solvent, nitromethane has an unusual combination of properties: highly polar (ε
2220:
2083:
1116:
2332:"What is Nitro Methane Fuel: Understanding High-Performance Racing's Power Source - Ran When Parked - Car, Vehicle & Truck Guides and Repair Journals"
1237:
2053:
2825:
1513:. In this context, nitromethane is commonly referred to as "nitro fuel" or simply "nitro", and is the principal ingredient for fuel used in the "
2356:
Shrestha, Krishna Prasad; Vin, Nicolas; Herbinet, Olivier; Seidel, Lars; Battin-Leclerc, Frédérique; Zeuch, Thomas; Mauss, Fabian (2020-02-01).
1577:
3229:
1622:
of approximately 0.5 m/s, somewhat higher than gasoline, thus making it suitable for high-speed engines. It also has a somewhat higher
1292:, it is used widely in the manufacture of pesticides, explosives, fibers, and coatings. Nitromethane is used as a fuel additive in various
396:
53:
2604:
1576:, i.e., a propellant that decomposes to release energy without added oxygen. It was first tested as rocket monopropellant in 1930s by
1524:
content of nitromethane enables it to burn with much less atmospheric oxygen than conventional fuels. During nitromethane combustion,
924:
2420:"Insights into nitromethane combustion from detailed kinetic modeling – Pyrolysis experiments in jet-stirred and flow reactors"
2358:"Insights into nitromethane combustion from detailed kinetic modeling – Pyrolysis experiments in jet-stirred and flow reactors"
1367:. These alkoxy radicals are susceptible to C—C fragmentation reactions, which explains the formation of a mixture of products.
2858:
2528:
2495:
2037:
746:
912:
2925:
2688:
Dauben, H. J. Jr.; Ringold, H. J.; Wade, R. H.; Pearson, D. L.; Anderson, A. G. Jr.; de Boer, T. J.; Backer, H. J. (1963).
1565:
as gasoline can be burned in one stroke. Nitromethane, however, has a lower specific energy: gasoline provides about 42–44
2788:
2603:
SABIC, Cas AardenGraduate
University of Groningen Worked as a chemist in companies such as Wilmar Oleochemicals B. Vand.
773:
1232:
3224:
2872:
2262:
1698:. Other mixtures include ANNM and ANNMAl – explosive mixtures of ammonium nitrate, nitromethane and aluminium powder.
2672:
2655:
Kramarz, K. W.; Norton, J. R. (2007). "Slow Proton-Transfer
Reactions in Organometallic and Bioinorganic Chemistry".
361:
17:
1694:
It can be used as an explosive, when gelled with several percent of gelling agent. This type of mixture is called
1244:
3049:
2893:
1454:
496:
153:
2415:
1829:, nitromethane serves as a Michael donor, adding to α,β-unsaturated carbonyl compounds via 1,4-addition in the
800:
2419:
2357:
3209:
3120:
3054:
2307:
1648:
1644:
1316:
906:
304:
231:
1970:
1860:
Nitromethane is "reasonably anticipated to be a human carcinogen" according to a U.S. government report.
1623:
325:
193:
920:
853:
3204:
3044:
3016:
2836:
2753:
2021:
1140:
1127:
562:
2971:
1727:
of about 11. It is so acidic because the anion admits an alternate, stabilizing resonance structure:
1308:
3029:
2918:
2018:
Nomenclature of
Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book)
1304:
1206:
1008:
936:
342:
3214:
3189:
3135:
3079:
3059:
1880:. After much testing, it was realized that nitromethane was a more energetic high explosive than
594:
1587:
fom
Italian Rocket Society. There is a renewed interest in nitromethane as safer replacement of
843:
838:
2986:
2745:
722:
2520:
3199:
3184:
2746:"Recommended Methods for the Purification of Solvents and Tests for Impurities: Nitromethane"
1953:
1892:. Both of these explosives are oxygen-poor, and some benefits are gained from mixing with an
1032:
876:
831:
83:
3219:
2574:
2434:
2372:
848:
677:
606:
313:
93:
63:
928:
213:
8:
2911:
1885:
1774:
1619:
687:
119:
2687:
2438:
2376:
2331:
346:
235:
173:
129:
3039:
2770:
2513:
2458:
2396:
2047:
1669:
with some nitromethane (0% to 65%, but rarely over 30%, and 10–20% lubricants (usually
1416:
1430:
The dominant use of the nitromethane is as a precursor reagent. A major derivative is
3094:
3074:
3034:
2868:
2722:
2694:
2668:
2524:
2491:
2462:
2450:
2400:
2388:
2284:
2258:
2033:
1991:
1901:
1826:
1790:
1762:
1376:
1375:
It can be prepared in other methods that are of instructional value. The reaction of
1289:
667:
2774:
1351:. The reaction involves free radicals, including the alkoxyl radicals of the type CH
3158:
2976:
2762:
2660:
2637:
2554:
2483:
2442:
2380:
2250:
2025:
1913:
1897:
1830:
1498:
1260:
617:
516:
419:
2888:
2254:
3163:
3145:
3069:
2134:
1986:
1881:
1822:
1582:
1412:
1172:
1021:
651:
277:
2717:
2689:
2279:
2029:
1339:
reaction produces the four industrially significant nitroalkanes: nitromethane,
3194:
3089:
3006:
2547:
Nitromethane as a Green
Propellant: First Results of a Combustion Test Campaign
2545:
2446:
2384:
1976:
1738:
1730:
1655:
1631:
1573:
1450:
1380:
1348:
1344:
1285:
1215:
1194:
1190:
916:
552:
36:
32:
2898:
2664:
2477:
2079:
1098:
1078:
1058:
3178:
2934:
2454:
2392:
1842:
1674:
1478:
703:
533:
486:
476:
224:
28:
2766:
2559:
2212:
3024:
3001:
1981:
1906:
1778:
1525:
1446:
1431:
1312:
642:
984:
932:
3125:
2966:
2956:
2414:
Shrestha, Krishna Prasad; Vin, Nicolas; Herbinet, Olivier; Seidel, Lars;
1712:
1510:
1494:
1458:
1340:
1332:
1300:
1293:
1178:
997:
882:
2796:
National
Toxicology Program U.S. Department of Health and Human Services
2641:
970:
3115:
2487:
1916:, which is used as an oxidizer, it forms an explosive mixture known as
1805:). Reduction of the latter gives tris(hydroxymethyl)aminomethane, (HOCH
1670:
1336:
977:
527:
438:
204:
1973:, a thermodynamic calculation of the flame temperature of nitromethane
3153:
3110:
2961:
2864:
1996:
1869:
1662:
1659:
1639:
1588:
1566:
636:
1214:
Except where otherwise noted, data are given for materials in their
3130:
2991:
2981:
2951:
2605:"Nitromethane: An Ultimate Guide to Properties, Uses and Synthesis"
1965:
1893:
1889:
1873:
1770:
1666:
1514:
1506:
1297:
943:
545:
252:
2077:
152:
3064:
2996:
1766:
1750:
1474:
1384:
1328:
541:
537:
466:
264:
1917:
2544:
Kurilov, Maxim; Werling, Lukas; Kirchberger, Christoph (2023).
1591:
monopropellant. The following equation describes this process:
1521:
184:
2903:
2482:. 44th AIAA Aerospace Sciences Meeting and Exhibit. Reno, NV.
2277:
2584:. Academy of Model Aeronautics. February 15, 2016. p. 24
2217:
Immediately
Dangerous to Life or Health Concentrations (IDLH)
1493:
Nitromethane is used as a fuel in motor racing, particularly
1364:
288:
164:
142:
2627:
1335:
in the gas phase at 350–450 °C (662–842 °F). This
330:
2937:
2654:
2479:
1854:
1818:
1720:
1502:
1153:
456:
243:
2823:
2743:
2515:
Ignition! an informal history of liquid rocket propellants
1363:
O, which arise via homolysis of the corresponding nitrite
2543:
2413:
2355:
1695:
898:
886:
2789:"National Toxicology Program 15th Report on Carcinogens"
1528:(NO) is one of the major emission products along with CO
2245:
Markofsky, S. B. (2000). "Nitro
Compounds, Aliphatic".
1952:
Nitromethane's reaction with solid sodium hydroxide is
1737:
The acid deprotonates only slowly. Protonation of the
2575:"AMA Competition Regulations 2015–2016 Part 7. Fuels"
2221:
National Institute for Occupational Safety and Health
2084:
National Institute for Occupational Safety and Health
1777:. Some important derivatives include the pesticides
1327:Nitromethane is produced industrially by combining
2856:
2512:
2166:
2164:
1457:("tris"), a widely used buffer and ingredient in
3176:
2244:
890:
276:
2161:
1259:, sometimes shortened to simply "nitro", is an
959:
128:
2247:Ullmann's Encyclopedia of Industrial Chemistry
1445:), a widely used pesticide. It condenses with
894:
2919:
2715:
2418:; Zeuch, Thomas; Mauss, Fabian (2020-02-01).
2052:: CS1 maint: DOI inactive as of April 2024 (
1793:, and tris(hydroxymethyl)nitromethane, ((HOCH
2899:CDC – NIOSH Pocket Guide to Chemical Hazards
2240:
2238:
2236:
2234:
2232:
2230:
2139:University of Wisconsin Chemistry Department
1729:
1723:solution. This value indicates an aqueous pK
491:101.2 °C (214.2 °F; 374.3 K)
481:−28.7 °C (−19.7 °F; 244.5 K)
2510:
2926:
2912:
1853:Nitromethane has a modest acute toxicity.
345:
234:
212:
2558:
2278:Whitmore, F. C.; Whitmore, M. G. (1941).
2227:
2126:
1765:nitromethane is employed as a one carbon
1464:
312:
2630:Journal of the American Chemical Society
2504:
2475:
2078:NIOSH Pocket Guide to Chemical Hazards.
2009:
1868:Nitromethane was not known to be a high
1481:(more commonly known as "super-glues").
2207:
2205:
2203:
1863:
1706:
1651:do not permit its use in competitions.
341:
14:
3177:
2305:
2184:
2182:
2180:
2178:
2176:
2073:
2071:
2069:
2067:
2065:
2063:
1665:, the primary ingredient is generally
1016:418 °C (784 °F; 691 K)
225:
2907:
2860:CRC Handbook of Chemistry and Physics
2744:Coetzee, J. F.; Chang, T.-H. (1986).
2519:. Rutgers University Press. pp.
2101:
2099:
2097:
2095:
2093:
1857:(oral, rats) is 1210±322 mg/kg.
1370:
373:Key: LYGJENNIWJXYER-UHFFFAOYSA-N
192:
172:
3230:Organic compounds with 1 carbon atom
2805:from the original on October 2, 2023
2553:. Aerospace Europe Conference 2023.
2511:Clark, J. D.; Asimov, Isaac (1972).
2200:
2135:"Bordwell pKa table: "Nitroalkanes""
1756:
1711:Nitromethane is a relatively acidic
1002:35 °C (95 °F; 308 K)
2476:Boyer, E.; Kuo, K. (January 2006).
2173:
2060:
1572:Nitromethane can also be used as a
383:Key: LYGJENNIWJXYER-UHFFFAOYAW
267:
251:
24:
2090:
1877:
1630:Nitromethane is usually used with
955:
62:
57:Structural formula of nitromethane
52:
25:
3241:
2882:
2602:
2308:"HPBG: The Power of Racing Fuels"
2132:
1928:which has a reddish-brown color:
1749:, which is nearly isosteric with
1387:solution produces this compound:
2857:Haynes, William M., ed. (2011).
2850:
2826:"Accident Near Mt. Pulaski, ILL"
2824:Interstate Commerce Commission.
1222:
852:
847:
842:
837:
3050:2,4-Dimethyl-6-tert-butylphenol
2933:
2817:
2781:
2737:
2709:
2681:
2657:Progress in Inorganic Chemistry
2648:
2621:
2596:
2567:
2537:
2469:
2407:
2349:
2324:
2299:
2271:
2191:
1836:
1455:tris(hydroxymethyl)aminomethane
1218:(at 25 °C , 100 kPa).
2152:
2117:
2108:
2022:The Royal Society of Chemistry
1753:, occurs initially at oxygen.
1680:
1322:
801:Occupational safety and health
13:
1:
2889:WebBook page for nitromethane
2255:10.1002/14356007.a17_401.pub2
2002:
1284:. It is the simplest organic
1121:(US health exposure limits):
370:InChI=1S/CH3NO2/c1-2(3)4/h1H3
3121:Automatic transmission fluid
3055:Dinonylnaphthylsulfonic acid
3007:Racing fuel (Tetraethyllead)
2306:Carley, Larry (2013-01-06).
1884:, although TNT has a higher
1845:, followed by distillation.
1701:
1649:Academy of Model Aeronautics
1645:National Hot Rod Association
380:InChI=1/CH3NO2/c1-2(3)4/h1H3
7:
2030:10.1039/9781849733069-FP001
1971:Adiabatic flame temperature
1959:
1876:loaded with it exploded on
1620:laminar combustion velocity
1517:" category of drag racing.
1305:internal combustion engines
1047:or concentration (LD, LC):
48:
10:
3246:
3045:Dimethyl methylphosphonate
2754:Pure and Applied Chemistry
2732:, vol. 4, p. 833
2704:, vol. 4, p. 221
2447:10.1016/j.fuel.2019.116349
2416:Battin-Leclerc, Frédérique
2385:10.1016/j.fuel.2019.116349
2294:, vol. 1, p. 401
1263:with the chemical formula
26:
3225:IARC Group 2B carcinogens
3144:
3103:
3015:
2944:
2665:10.1002/9780470166437.ch1
1848:
1212:
1205:
1200:
1164:
1115:
1043:
818:
814:Flammable, health hazard
798:
793:
696:
661:
443:61.04 g/mol
412:
392:
357:
112:
104:
92:
82:
77:
47:
3030:Butylated hydroxytoluene
1689:
1207:Nitromethane (data page)
1201:Supplementary data page
1085:750 mg/kg (rabbit, oral)
1067:950 mg/kg (oral, mouse)
907:Precautionary statements
27:Not to be confused with
3136:Windshield washer fluid
3080:Methyl tert-butyl ether
3060:2,6-Di-tert-butylphenol
2972:Lead Replacement Petrol
2894:History of Nitromethane
2767:10.1351/pac198658111541
2560:10.13009/EUCASS2023-372
2312:Engine Builder Magazine
2249:. Weinheim: Wiley-VCH.
2032:(inactive 2024-04-14).
1940:+ NaOH → HON=CHCH=NO
1773:in 1,2-addition in the
1484:
1425:
1134:TWA 100 ppm (250 mg/m)
1111:5000 ppm (rabbit, 6 h)
1109:2500 ppm (rabbit, 12 h)
611:0.204 W/(m·K) at 25 °C
595:Magnetic susceptibility
451:colorless, oily liquid
2987:Compressed natural gas
2716:Noland, W. E. (1963).
1886:velocity of detonation
1825:. In more specialized
1734:
1632:rich air–fuel mixtures
1465:Solvent and stabilizer
1453:) to eventually give
1087:125 mg/kg (dog, oral)
966:
68:
58:
2798:. December 21, 2021.
2582:www.modelaircraft.org
2024:. 2014. p. 662.
1733:
1105:7087 ppm (mouse, 2 h)
1065:940 mg/kg (oral, rat)
1033:Threshold limit value
965:
66:
56:
1864:Explosive properties
1707:Acid-base properties
1377:sodium chloroacetate
948:(fire diamond)
678:Friction sensitivity
607:Thermal conductivity
471:1.1371 g/cm (20 °C)
94:Preferred IUPAC name
3210:Explosive chemicals
2842:on 1 November 2020.
2642:10.1021/ja00099a004
2439:2020Fuel..26116349S
2377:2020Fuel..26116349S
1775:nitroaldol reaction
1618:Nitromethane has a
1501:model power boats,
1296:and hobbies, e.g.
688:Detonation velocity
517:Solubility in water
497:Critical point
44:
3040:1,2-Dichloroethane
2488:10.2514/6.2006-361
1909:" pressure surge.
1817:, better known as
1735:
1638:A small amount of
1371:Laboratory methods
1245:Infobox references
1165:Related compounds
1156:(Immediate danger)
967:
69:
59:
42:
3205:Liquid explosives
3172:
3171:
3095:Tetranitromethane
3075:Metal deactivator
3035:1,2-Dibromoethane
2863:(92nd ed.).
2761:(11): 1541–1545.
2730:Collected Volumes
2723:Organic Syntheses
2702:Collected Volumes
2695:Organic Syntheses
2659:. pp. 1–65.
2636:(20): 8885–8889.
2530:978-0-8135-0725-5
2497:978-1-62410-039-0
2336:ranwhenparked.net
2292:Collected Volumes
2285:Organic Syntheses
2039:978-0-85404-182-4
1992:Tetranitromethane
1872:until a railroad
1827:organic synthesis
1821:, a widely used
1791:beta-nitrostyrene
1763:organic synthesis
1757:Organic reactions
1624:flame temperature
1497:, as well as for
1290:organic synthesis
1253:Chemical compound
1251:
1250:
1186:Related compounds
1107:1000 ppm (monkey)
877:Hazard statements
774:Gibbs free energy
668:Shock sensitivity
326:CompTox Dashboard
154:Interactive image
73:
72:
18:Nitromethane fuel
16:(Redirected from
3237:
3159:MTBE controversy
2928:
2921:
2914:
2905:
2904:
2878:
2844:
2843:
2841:
2835:. Archived from
2830:
2821:
2815:
2814:
2812:
2810:
2804:
2793:
2785:
2779:
2778:
2750:
2741:
2735:
2733:
2726:
2718:"2-Nitroethanol"
2713:
2707:
2705:
2698:
2690:"Cycloheptanone"
2685:
2679:
2678:
2652:
2646:
2645:
2625:
2619:
2618:
2616:
2615:
2600:
2594:
2593:
2591:
2589:
2579:
2571:
2565:
2564:
2562:
2552:
2541:
2535:
2534:
2518:
2508:
2502:
2501:
2500:. AIAA 2006-361.
2473:
2467:
2466:
2424:
2411:
2405:
2404:
2362:
2353:
2347:
2346:
2344:
2343:
2328:
2322:
2321:
2319:
2318:
2303:
2297:
2295:
2288:
2275:
2269:
2268:
2242:
2225:
2224:
2209:
2198:
2195:
2189:
2188:Haynes, p. 15.19
2186:
2171:
2170:Haynes, p. 6.231
2168:
2159:
2158:Haynes, p. 3.576
2156:
2150:
2149:
2147:
2145:
2130:
2124:
2121:
2115:
2112:
2106:
2105:Haynes, p. 3.414
2103:
2088:
2087:
2075:
2058:
2057:
2051:
2043:
2016:"Front Matter".
2013:
1914:ammonium nitrate
1898:ammonium nitrate
1879:
1831:Michael reaction
1586:
1499:radio-controlled
1444:
1319:model aircraft.
1283:
1282:
1281:
1273:
1272:
1261:organic compound
1235:
1229:
1226:
1225:
1099:lowest published
1079:lowest published
1022:Explosive limits
987:
980:
973:
958:
938:
934:
930:
926:
922:
918:
914:
900:
896:
892:
888:
884:
856:
851:
846:
841:
785:
763:
740:171.8 J/(mol·K)
736:
716:106.6 J/(mol·K)
712:
697:Thermochemistry
618:Refractive index
601:-21.0·10 cm/mol
557:28 mmHg (20 °C)
522:ca. 10 g/100 mL
420:Chemical formula
350:
349:
334:
332:
316:
280:
269:
255:
238:
227:
216:
196:
176:
156:
132:
49:
45:
41:
21:
3245:
3244:
3240:
3239:
3238:
3236:
3235:
3234:
3175:
3174:
3173:
3168:
3164:Pay at the pump
3140:
3099:
3070:Ethylenediamine
3011:
2952:Gasoline/petrol
2940:
2932:
2885:
2875:
2853:
2848:
2847:
2839:
2833:Ex Parte No 213
2828:
2822:
2818:
2808:
2806:
2802:
2791:
2787:
2786:
2782:
2748:
2742:
2738:
2728:
2714:
2710:
2700:
2686:
2682:
2675:
2653:
2649:
2626:
2622:
2613:
2611:
2601:
2597:
2587:
2585:
2577:
2573:
2572:
2568:
2550:
2542:
2538:
2531:
2509:
2505:
2498:
2474:
2470:
2422:
2412:
2408:
2360:
2354:
2350:
2341:
2339:
2330:
2329:
2325:
2316:
2314:
2304:
2300:
2290:
2276:
2272:
2265:
2243:
2228:
2211:
2210:
2201:
2197:Haynes, p. 5.20
2196:
2192:
2187:
2174:
2169:
2162:
2157:
2153:
2143:
2141:
2131:
2127:
2123:Haynes, p. 5.94
2122:
2118:
2114:Haynes, p. 6.69
2113:
2109:
2104:
2091:
2076:
2061:
2045:
2044:
2040:
2015:
2014:
2010:
2005:
1987:Trinitromethane
1962:
1947:
1943:
1939:
1935:
1866:
1851:
1839:
1816:
1812:
1808:
1804:
1800:
1796:
1788:
1784:
1759:
1748:
1744:
1726:
1718:
1709:
1704:
1692:
1683:
1614:
1610:
1606:
1602:
1598:
1580:
1559:
1555:
1551:
1547:
1543:
1535:
1531:
1487:
1472:
1467:
1443:
1439:
1435:
1428:
1420:
1410:
1406:
1402:
1398:
1394:
1373:
1362:
1358:
1354:
1325:
1280:
1277:
1276:
1275:
1271:
1268:
1267:
1266:
1264:
1254:
1247:
1242:
1241:
1240: ?)
1231:
1227:
1223:
1219:
1193:
1187:
1175:
1173:nitro compounds
1157:
1144:
1131:
1110:
1108:
1106:
1102:
1096:
1086:
1082:
1076:
1066:
1062:
1056:
1036:
1013:
1010:
992:
991:
990:
989:
982:
975:
968:
964:
956:
909:
879:
865:
834:
811:
786:
780:
776:
764:
761:
755:
751:
748:
747:Std enthalpy of
737:
734:
727:
724:
713:
706:
662:Explosive data
654:
631:1.3817 (20 °C)
628:
626:
598:
588:
581:
571:
519:
510:588 K, 6.0 MPa
432:
428:
422:
408:
405:
400:
399:
388:
385:
384:
381:
375:
374:
371:
365:
364:
353:
335:
328:
319:
299:
283:
270:
258:
219:
199:
179:
159:
146:
135:
122:
108:
100:
99:
88:
40:
23:
22:
15:
12:
11:
5:
3243:
3233:
3232:
3227:
3222:
3217:
3215:Fuel additives
3212:
3207:
3202:
3197:
3192:
3190:Nitro solvents
3187:
3170:
3169:
3167:
3166:
3161:
3156:
3150:
3148:
3142:
3141:
3139:
3138:
3133:
3128:
3123:
3118:
3113:
3107:
3105:
3101:
3100:
3098:
3097:
3092:
3090:Tetraethyllead
3087:
3082:
3077:
3072:
3067:
3062:
3057:
3052:
3047:
3042:
3037:
3032:
3027:
3021:
3019:
3017:Fuel additives
3013:
3012:
3010:
3009:
3004:
2999:
2994:
2989:
2984:
2979:
2974:
2969:
2964:
2959:
2954:
2948:
2946:
2942:
2941:
2931:
2930:
2923:
2916:
2908:
2902:
2901:
2896:
2891:
2884:
2883:External links
2881:
2880:
2879:
2874:978-1439855119
2873:
2852:
2849:
2846:
2845:
2816:
2780:
2736:
2708:
2680:
2673:
2647:
2620:
2595:
2566:
2536:
2529:
2503:
2496:
2468:
2406:
2348:
2323:
2298:
2280:"Nitromethane"
2270:
2264:978-3527306732
2263:
2226:
2213:"Nitromethane"
2199:
2190:
2172:
2160:
2151:
2125:
2116:
2107:
2089:
2059:
2038:
2007:
2006:
2004:
2001:
2000:
1999:
1994:
1989:
1984:
1979:
1977:Dinitromethane
1974:
1968:
1961:
1958:
1950:
1949:
1945:
1941:
1937:
1933:
1912:If mixed with
1865:
1862:
1850:
1847:
1838:
1835:
1814:
1810:
1806:
1802:
1798:
1794:
1786:
1782:
1767:building block
1758:
1755:
1746:
1742:
1739:conjugate base
1724:
1716:
1708:
1705:
1703:
1700:
1691:
1688:
1682:
1679:
1656:model aircraft
1616:
1615:
1612:
1608:
1604:
1603:→ 2 CO + 2 H
1600:
1596:
1574:monopropellant
1562:
1561:
1557:
1553:
1549:
1545:
1541:
1533:
1529:
1486:
1483:
1479:cyanoacrylates
1470:
1466:
1463:
1451:Henry reaction
1441:
1437:
1427:
1424:
1423:
1422:
1418:
1408:
1404:
1400:
1396:
1392:
1381:sodium nitrite
1372:
1369:
1360:
1356:
1352:
1349:2-nitropropane
1345:1-nitropropane
1324:
1321:
1303:and miniature
1286:nitro compound
1278:
1269:
1252:
1249:
1248:
1243:
1221:
1220:
1216:standard state
1213:
1210:
1209:
1203:
1202:
1198:
1197:
1195:methyl nitrate
1191:methyl nitrite
1188:
1185:
1182:
1181:
1176:
1170:
1167:
1166:
1162:
1161:
1158:
1152:
1149:
1148:
1145:
1139:
1136:
1135:
1132:
1126:
1123:
1122:
1113:
1112:
1103:
1094:
1092:
1089:
1088:
1083:
1074:
1072:
1069:
1068:
1063:
1054:
1052:
1049:
1048:
1041:
1040:
1037:
1031:
1028:
1027:
1024:
1018:
1017:
1014:
1007:
1004:
1003:
1000:
994:
993:
983:
976:
969:
954:
953:
952:
951:
949:
940:
939:
910:
905:
902:
901:
880:
875:
872:
871:
866:
861:
858:
857:
835:
830:
827:
826:
816:
815:
812:
809:
806:
805:
796:
795:
791:
790:
787:
778:
772:
769:
768:
767:-112.6 kJ/mol
765:
759:
753:
745:
742:
741:
738:
732:
721:
718:
717:
714:
702:
699:
698:
694:
693:
690:
684:
683:
680:
674:
673:
670:
664:
663:
659:
658:
655:
650:
647:
646:
639:
633:
632:
629:
624:
616:
613:
612:
609:
603:
602:
599:
593:
590:
589:
587:
586:
583:
579:
575:
573:
569:
559:
558:
555:
553:Vapor pressure
549:
548:
530:
524:
523:
520:
515:
512:
511:
508:
493:
492:
489:
483:
482:
479:
473:
472:
469:
463:
462:
461:Light, fruity
459:
453:
452:
449:
445:
444:
441:
435:
434:
430:
426:
423:
418:
415:
414:
410:
409:
407:
406:
403:
395:
394:
393:
390:
389:
387:
386:
382:
379:
378:
376:
372:
369:
368:
360:
359:
358:
355:
354:
352:
351:
338:
336:
324:
321:
320:
318:
317:
309:
307:
301:
300:
298:
297:
293:
291:
285:
284:
282:
281:
273:
271:
263:
260:
259:
257:
256:
248:
246:
240:
239:
229:
221:
220:
218:
217:
209:
207:
201:
200:
198:
197:
189:
187:
181:
180:
178:
177:
169:
167:
161:
160:
158:
157:
149:
147:
140:
137:
136:
134:
133:
125:
123:
118:
115:
114:
110:
109:
106:
102:
101:
97:
96:
90:
89:
86:
80:
79:
75:
74:
71:
70:
60:
37:methyl nitrite
33:methyl nitrate
9:
6:
4:
3:
2:
3242:
3231:
3228:
3226:
3223:
3221:
3218:
3216:
3213:
3211:
3208:
3206:
3203:
3201:
3198:
3196:
3193:
3191:
3188:
3186:
3183:
3182:
3180:
3165:
3162:
3160:
3157:
3155:
3152:
3151:
3149:
3147:
3143:
3137:
3134:
3132:
3129:
3127:
3124:
3122:
3119:
3117:
3114:
3112:
3109:
3108:
3106:
3102:
3096:
3093:
3091:
3088:
3086:
3083:
3081:
3078:
3076:
3073:
3071:
3068:
3066:
3063:
3061:
3058:
3056:
3053:
3051:
3048:
3046:
3043:
3041:
3038:
3036:
3033:
3031:
3028:
3026:
3023:
3022:
3020:
3018:
3014:
3008:
3005:
3003:
3000:
2998:
2995:
2993:
2990:
2988:
2985:
2983:
2980:
2978:
2975:
2973:
2970:
2968:
2965:
2963:
2960:
2958:
2955:
2953:
2950:
2949:
2947:
2943:
2939:
2936:
2929:
2924:
2922:
2917:
2915:
2910:
2909:
2906:
2900:
2897:
2895:
2892:
2890:
2887:
2886:
2876:
2870:
2866:
2862:
2861:
2855:
2854:
2851:Cited sources
2838:
2834:
2827:
2820:
2801:
2797:
2790:
2784:
2776:
2772:
2768:
2764:
2760:
2756:
2755:
2747:
2740:
2731:
2725:
2724:
2719:
2712:
2703:
2697:
2696:
2691:
2684:
2676:
2674:9780470166437
2670:
2666:
2662:
2658:
2651:
2643:
2639:
2635:
2631:
2624:
2610:
2606:
2599:
2583:
2576:
2570:
2561:
2556:
2549:
2548:
2540:
2532:
2526:
2522:
2517:
2516:
2507:
2499:
2493:
2489:
2485:
2481:
2480:
2472:
2464:
2460:
2456:
2452:
2448:
2444:
2440:
2436:
2432:
2428:
2421:
2417:
2410:
2402:
2398:
2394:
2390:
2386:
2382:
2378:
2374:
2370:
2366:
2359:
2352:
2337:
2333:
2327:
2313:
2309:
2302:
2293:
2287:
2286:
2281:
2274:
2266:
2260:
2256:
2252:
2248:
2241:
2239:
2237:
2235:
2233:
2231:
2222:
2218:
2214:
2208:
2206:
2204:
2194:
2185:
2183:
2181:
2179:
2177:
2167:
2165:
2155:
2140:
2136:
2133:Reich, Hans.
2129:
2120:
2111:
2102:
2100:
2098:
2096:
2094:
2085:
2081:
2074:
2072:
2070:
2068:
2066:
2064:
2055:
2049:
2041:
2035:
2031:
2027:
2023:
2020:. Cambridge:
2019:
2012:
2008:
1998:
1995:
1993:
1990:
1988:
1985:
1983:
1980:
1978:
1975:
1972:
1969:
1967:
1964:
1963:
1957:
1955:
1931:
1930:
1929:
1925:
1921:
1919:
1915:
1910:
1908:
1903:
1899:
1895:
1891:
1887:
1883:
1875:
1871:
1861:
1858:
1856:
1846:
1844:
1843:diethyl ether
1834:
1832:
1828:
1824:
1820:
1792:
1780:
1776:
1772:
1768:
1764:
1754:
1752:
1740:
1732:
1728:
1722:
1715:. It has a pK
1714:
1699:
1697:
1687:
1678:
1676:
1675:synthetic oil
1672:
1668:
1664:
1661:
1657:
1652:
1650:
1646:
1641:
1636:
1633:
1628:
1625:
1621:
1594:
1593:
1592:
1590:
1584:
1579:
1575:
1570:
1568:
1539:
1538:
1537:
1527:
1523:
1518:
1516:
1512:
1508:
1504:
1500:
1496:
1491:
1482:
1480:
1476:
1462:
1460:
1456:
1452:
1448:
1433:
1421:
1414:
1390:
1389:
1388:
1386:
1382:
1378:
1368:
1366:
1350:
1346:
1342:
1338:
1334:
1330:
1320:
1318:
1314:
1310:
1309:radio control
1306:
1302:
1299:
1295:
1291:
1287:
1262:
1258:
1246:
1239:
1234:
1217:
1211:
1208:
1204:
1199:
1196:
1192:
1189:
1184:
1183:
1180:
1177:
1174:
1169:
1168:
1163:
1159:
1155:
1151:
1150:
1146:
1143:(Recommended)
1142:
1138:
1137:
1133:
1130:(Permissible)
1129:
1125:
1124:
1120:
1119:
1114:
1104:
1100:
1091:
1090:
1084:
1080:
1071:
1070:
1064:
1060:
1051:
1050:
1046:
1042:
1038:
1034:
1030:
1029:
1025:
1023:
1020:
1019:
1015:
1012:
1006:
1005:
1001:
999:
996:
995:
988:
981:
974:
950:
947:
946:
942:
941:
911:
908:
904:
903:
881:
878:
874:
873:
870:
867:
864:
860:
859:
855:
850:
845:
840:
836:
833:
829:
828:
824:
822:
817:
813:
808:
807:
803:
802:
797:
792:
789:-14.4 kJ/mol
788:
783:
775:
771:
770:
766:
758:
750:
744:
743:
739:
731:
726:
720:
719:
715:
710:
705:
704:Heat capacity
701:
700:
695:
691:
689:
686:
685:
681:
679:
676:
675:
671:
669:
666:
665:
660:
656:
653:
652:Dipole moment
649:
648:
644:
640:
638:
635:
634:
630:
623:
619:
615:
614:
610:
608:
605:
604:
600:
596:
592:
591:
584:
577:
576:
574:
568:
564:
561:
560:
556:
554:
551:
550:
547:
543:
539:
535:
534:diethyl ether
531:
529:
526:
525:
521:
518:
514:
513:
509:
506:
502:
498:
495:
494:
490:
488:
487:Boiling point
485:
484:
480:
478:
477:Melting point
475:
474:
470:
468:
465:
464:
460:
458:
455:
454:
450:
447:
446:
442:
440:
437:
436:
424:
421:
417:
416:
411:
402:
401:
398:
391:
377:
367:
366:
363:
356:
348:
344:
343:DTXSID2020977
340:
339:
337:
327:
323:
322:
315:
311:
310:
308:
306:
303:
302:
295:
294:
292:
290:
287:
286:
279:
275:
274:
272:
266:
262:
261:
254:
250:
249:
247:
245:
242:
241:
237:
233:
230:
228:
226:ECHA InfoCard
223:
222:
215:
211:
210:
208:
206:
203:
202:
195:
191:
190:
188:
186:
183:
182:
175:
171:
170:
168:
166:
163:
162:
155:
151:
150:
148:
144:
139:
138:
131:
127:
126:
124:
121:
117:
116:
111:
103:
95:
91:
85:
81:
76:
65:
61:
55:
51:
50:
46:
43:Nitromethane
38:
34:
30:
29:nitrous oxide
19:
3200:Rocket fuels
3185:Nitroalkanes
3085:Nitromethane
3084:
3025:Butyl rubber
3002:Butanol fuel
2859:
2837:the original
2832:
2819:
2807:. Retrieved
2795:
2783:
2758:
2752:
2739:
2729:
2721:
2711:
2701:
2693:
2683:
2656:
2650:
2633:
2629:
2623:
2612:. Retrieved
2608:
2598:
2586:. Retrieved
2581:
2569:
2546:
2539:
2514:
2506:
2478:
2471:
2430:
2426:
2409:
2368:
2364:
2351:
2340:. Retrieved
2338:. 2024-03-05
2335:
2326:
2315:. Retrieved
2311:
2301:
2291:
2283:
2273:
2246:
2216:
2193:
2154:
2142:. Retrieved
2138:
2128:
2119:
2110:
2017:
2011:
1982:Model engine
1951:
1926:
1922:
1911:
1878:June 1, 1958
1867:
1859:
1852:
1840:
1837:Purification
1779:chloropicrin
1760:
1736:
1710:
1693:
1684:
1653:
1637:
1629:
1617:
1578:Luigi Crocco
1571:
1563:
1526:nitric oxide
1519:
1492:
1488:
1468:
1459:alkyd resins
1447:formaldehyde
1432:chloropicrin
1429:
1395:COONa + NaNO
1374:
1326:
1313:control line
1257:Nitromethane
1256:
1255:
1117:
1044:
1009:Autoignition
944:
868:
820:
810:Main hazards
799:
781:
756:
729:
708:
621:
585:17.2 (DMSO)
566:
532:miscible in
504:
500:
289:RTECS number
194:ChEMBL276924
113:Identifiers
105:Other names
98:Nitromethane
87:Nitromethane
67:Nitromethane
3220:Drag racing
3126:Brake fluid
2977:Electricity
2967:Biogasoline
1907:hammer-lock
1719:of 17.2 in
1713:carbon acid
1681:Former uses
1581: [
1511:helicopters
1495:drag racing
1341:nitroethane
1333:nitric acid
1323:Preparation
1317:free flight
1301:drag racing
1294:motorsports
1179:nitroethane
1059:median dose
1045:Lethal dose
1011:temperature
998:Flash point
863:Signal word
804:(OHS/OSH):
448:Appearance
413:Properties
232:100.000.797
174:CHEBI:77701
107:Nitrocarbol
3179:Categories
3116:Antifreeze
2945:Fuel types
2614:2024-05-31
2433:: 116349.
2371:: 116349.
2342:2024-05-31
2317:2024-05-31
2144:27 January
2003:References
1954:hypergolic
1896:, such as
1888:(VoD) and
1671:castor oil
1477:, such as
1337:exothermic
832:Pictograms
528:Solubility
439:Molar mass
314:RU5WG8C3F4
205:ChemSpider
141:3D model (
120:CAS Number
84:IUPAC name
3154:Fuel card
3111:Motor oil
2962:Biodiesel
2865:CRC Press
2588:April 18,
2463:208755285
2455:0016-2361
2401:208755285
2393:0016-2361
2048:cite book
1997:RE factor
1902:adiabatic
1870:explosive
1771:aldehydes
1702:Reactions
1663:glow fuel
1640:hydrazine
1589:hydrazine
937:P403+P233
933:P370+P378
925:P304+P340
823:labelling
749:formation
723:Std molar
692:6400 m/s
645:at 25 °C
637:Viscosity
296:PA9800000
3131:Gear oil
2992:Hydrogen
2982:Kerosene
2800:Archived
2775:95631774
2223:(NIOSH).
2086:(NIOSH).
1966:Top Fuel
1960:See also
1944:Na + 2 H
1894:oxidizer
1890:brisance
1874:tank car
1667:methanol
1560:O + 4 NO
1515:Top Fuel
1475:monomers
1298:Top Fuel
1171:Related
1160:750 ppm
945:NFPA 704
794:Hazards
597:(χ)
578:10.21 (H
546:methanol
3065:Ecalene
2997:Ethanol
2809:May 30,
2609:Safrole
2435:Bibcode
2373:Bibcode
2080:"#0457"
1751:nitrate
1673:and/or
1607:O + H
1385:aqueous
1329:propane
1238:what is
1236: (
1039:20 ppm
777:(Δ
725:entropy
563:Acidity
542:ethanol
538:acetone
467:Density
433:
265:PubChem
130:75-52-5
3146:Retail
3104:Fluids
2957:Diesel
2871:
2773:
2671:
2527:
2494:
2461:
2453:
2399:
2391:
2261:
2036:
1849:Safety
1823:buffer
1552:→ 4 CO
1548:+ 5 O
1522:oxygen
1507:planes
1403:O → CH
1347:, and
1233:verify
1230:
1026:7–22%
869:Danger
397:SMILES
253:C19275
185:ChEMBL
78:Names
3195:Fuels
2938:fuels
2935:Motor
2840:(PDF)
2829:(PDF)
2803:(PDF)
2792:(PDF)
2771:S2CID
2749:(PDF)
2578:(PDF)
2551:(PDF)
2523:-10.
2459:S2CID
2423:(PDF)
2397:S2CID
2361:(PDF)
1690:Other
1585:]
1556:+ 6 H
1532:and H
1417:NaHCO
1379:with
1365:ester
1147:none
1118:NIOSH
1035:(TLV)
657:3.46
641:0.63
404:C(=O)
362:InChI
165:ChEBI
143:JSmol
35:, or
2869:ISBN
2811:2024
2669:ISBN
2590:2014
2525:ISBN
2492:ISBN
2451:ISSN
2427:Fuel
2389:ISSN
2365:Fuel
2259:ISBN
2146:2022
2054:link
2034:ISBN
1932:2 CH
1918:ANNM
1855:LD50
1819:tris
1721:DMSO
1658:and
1647:and
1611:+ N
1595:2 CH
1540:4 CH
1520:The
1509:and
1503:cars
1485:Fuel
1426:Uses
1413:NaCl
1391:ClCH
1331:and
1315:and
1154:IDLH
929:P312
921:P280
917:P261
913:P210
899:H351
895:H331
891:H301
887:H226
883:H203
682:Low
672:Low
457:Odor
305:UNII
278:6375
244:KEGG
214:6135
2763:doi
2661:doi
2638:doi
2634:116
2555:doi
2484:doi
2443:doi
2431:261
2381:doi
2369:261
2251:doi
2026:doi
1882:TNT
1813:CNH
1801:CNO
1789:),
1785:CNO
1781:(Cl
1761:In
1745:NCH
1696:PLX
1660:car
1654:In
1436:CCl
1399:+ H
1383:in
1307:in
1141:REL
1128:PEL
821:GHS
760:298
733:298
331:EPA
268:CID
3181::
2867:.
2831:.
2794:.
2769:.
2759:58
2757:.
2751:.
2727:;
2720:.
2699:;
2692:.
2667:.
2632:.
2607:.
2580:.
2490:.
2457:.
2449:.
2441:.
2429:.
2425:.
2395:.
2387:.
2379:.
2367:.
2363:.
2334:.
2310:.
2289:;
2282:.
2257:.
2229:^
2219:.
2215:.
2202:^
2175:^
2163:^
2137:.
2092:^
2082:.
2062:^
2050:}}
2046:{{
1956:.
1936:NO
1920:.
1833:.
1599:NO
1583:it
1567:MJ
1544:NO
1505:,
1461:.
1440:NO
1415:+
1411:+
1407:NO
1359:CH
1355:CH
1343:,
1311:,
1274:NO
1265:CH
1095:Lo
1093:LC
1075:Lo
1073:LD
1055:50
1053:LD
935:,
931:,
927:,
923:,
919:,
915:,
897:,
893:,
889:,
885:,
825::
752:(Δ
643:cP
582:O)
572:)
565:(p
544:,
540:,
536:,
507:)
503:,
429:NO
425:CH
31:,
2927:e
2920:t
2913:v
2877:.
2813:.
2777:.
2765::
2734:.
2706:.
2677:.
2663::
2644:.
2640::
2617:.
2592:.
2563:.
2557::
2533:.
2521:9
2486::
2465:.
2445::
2437::
2403:.
2383::
2375::
2345:.
2320:.
2296:.
2267:.
2253::
2148:.
2056:)
2042:.
2028::
1948:O
1946:2
1942:2
1938:2
1934:3
1905:"
1815:2
1811:3
1809:)
1807:2
1803:2
1799:3
1797:)
1795:2
1787:2
1783:3
1747:2
1743:2
1741:O
1725:a
1717:a
1613:2
1609:2
1605:2
1601:2
1597:3
1558:2
1554:2
1550:2
1546:2
1542:3
1534:2
1530:2
1471:r
1449:(
1442:2
1438:3
1434:(
1419:3
1409:2
1405:3
1401:2
1397:2
1393:2
1361:2
1357:2
1353:3
1279:2
1270:3
1228:N
1101:)
1097:(
1081:)
1077:(
1061:)
1057:(
986:3
979:3
972:2
784:)
782:G
779:f
762:)
757:H
754:f
735:)
730:S
728:(
711:)
709:C
707:(
627:)
625:D
622:n
620:(
580:2
570:a
567:K
505:P
501:T
499:(
431:2
427:3
333:)
329:(
145:)
39:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.