2874:) is that lowering the energy of the oxygen atom's nonbonding hybrid orbitals (by assigning them more s character and less p character) and correspondingly raising the energy of the oxygen atom's hybrid orbitals bonded to the hydrogen atoms (by assigning them more p character and less s character) has the net effect of lowering the energy of the occupied molecular orbitals because the energy of the oxygen atom's nonbonding hybrid orbitals contributes completely to the energy of the oxygen atom's lone pairs while the energy of the oxygen atom's other two hybrid orbitals contributes only partially to the energy of the bonding orbitals (the remainder of the contribution coming from the hydrogen atoms' 1s orbitals).
2537:
2348:
1775:
94:
1720:
2784:
2525:
41:
4339:
64:
1658:
1849:. This denser saltwater sinks by convection and the replacing seawater is subject to the same process. This produces essentially freshwater ice at −1.9 °C on the surface. The increased density of the seawater beneath the forming ice causes it to sink towards the bottom. On a large scale, the process of brine rejection and sinking cold salty water results in ocean currents forming to transport such water away from the Poles, leading to a global system of currents called the
1800:
2018:
54:
1947:
2451:
3713:). In the inner Solar System, planets, asteroids, and moons formed almost entirely of metals and silicates. Water has since been delivered to the inner Solar System via an as-yet unknown mechanism, theorized to be the impacts of asteroids or comets carrying water from the outer Solar System, where bodies contain much more water ice. The difference between planetary bodies located inside and outside the frost line can be stark. Earth's mass is 0.000023% water, while
1123:
9102:
2275:). Because of autoionization, at ambient temperatures pure liquid water has a similar intrinsic charge carrier concentration to the semiconductor germanium and an intrinsic charge carrier concentration three orders of magnitude greater than the semiconductor silicon, hence, based on charge carrier concentration, water can not be considered to be a completely dielectric material or electrical insulator but to be a limited conductor of ionic charge.
9091:
9124:
8819:
2571:
1862:
4948:
9113:
2496:
1296:
7480:
1783:
bottom up, and all life in it would be killed. Furthermore, given that water is a good thermal insulator (due to its heat capacity), some frozen lakes might not completely thaw in summer. As it is, the inversion of the density curve leads to a stable layering for surface temperatures below 4 °C, and with the layer of ice that floats on top insulating the water below, even e.g.,
3598:, other than the simple difference in specific mass, involve properties that are affected by hydrogen bonding, such as freezing and boiling, and other kinetic effects. This is because the nucleus of deuterium is twice as heavy as protium, and this causes noticeable differences in bonding energies. The difference in boiling points allows the isotopologues to be separated. The
1126:
1696:(more commonly known as latent heat) of water is 333.55 kJ/kg at 0 °C: the same amount of energy is required to melt ice as to warm ice from −160 °C up to its melting point or to heat the same amount of water by about 80 °C. Of common substances, only that of ammonia is higher. This property confers resistance to melting on the ice of
7498:
5459:
1128:
1928:. The reverse process accounts for the fog burning off in the morning. If the humidity is increased at room temperature, for example, by running a hot shower or a bath, and the temperature stays about the same, the vapor soon reaches the pressure for phase change and then condenses out as minute water droplets, commonly referred to as steam.
1558:. The molecules of water are constantly moving concerning each other, and the hydrogen bonds are continually breaking and reforming at timescales faster than 200 femtoseconds (2 × 10 seconds). However, these bonds are strong enough to create many of the peculiar properties of water, some of which make it integral to life.
1932:
the air is small, relative humidity, the ratio of the partial pressure due to the water vapor to the saturated partial vapor pressure, is much more useful. Vapor pressure above 100% relative humidity is called supersaturated and can occur if the air is rapidly cooled, for example, by rising suddenly in an updraft.
5445:
7511:
Notice that the bond angles decrease as the number of nonbonding electron pairs increases. A bonding pair of electrons is attracted by both nuclei of the bonded atoms, but a nonbonding pair is attracted primarily by only one nucleus. Because a nonbonding pair experiences less nuclear attraction, its
3657:
may affect biochemical processes—ingestion of large amounts impairs kidney and central nervous system function. Small quantities can be consumed without any ill-effects; humans are generally unaware of taste differences, but sometimes report a burning sensation or sweet flavor. Very large amounts of
1931:
A saturated gas or one with 100% relative humidity is when the vapor pressure of water in the air is at equilibrium with vapor pressure due to (liquid) water; water (or ice, if cool enough) will fail to lose mass through evaporation when exposed to saturated air. Because the amount of water vapor in
4712:
The gases produced bubble to the surface, where they can be collected or ignited with a flame above the water if this was the intention. The required potential for the electrolysis of pure water is 1.23 V at 25 °C. The operating potential is actually 1.48 V or higher in practical electrolysis.
2823:
structures form due to the four hydrogen bonds, thereby forming an open structure and a three-dimensional bonding network, resulting in the anomalous decrease in density when cooled below 4 °C. This repeated, constantly reorganizing unit defines a three-dimensional network extending throughout
2491:
observed a strong repulsive force between hydrophilic surfaces. To dehydrate hydrophilic surfaces—to remove the strongly held layers of water of hydration—requires doing substantial work against these forces, called hydration forces. These forces are very large but decrease rapidly over a nanometer
1748:
0–4 °C allows for a denser molecular packing in which some of the lattice cavities are filled by water molecules. Above 4 °C, however, thermal expansion becomes the dominant effect, and water near the boiling point (100 °C) is about 4% less dense than water at 4 °C (39 °F).
6026:
Ocean warming dominates the global energy change inventory. Warming of the ocean accounts for about 93% of the increase in the Earth's energy inventory between 1971 and 2010 (high confidence), with the warming of the upper (0 to 700 m) ocean accounting for about 64% of the total. Melting ice
1747:
These peculiar effects are due to the highly directional bonding of water molecules via the hydrogen bonds: ice and liquid water at low temperature have comparatively low-density, low-energy open lattice structures. The breaking of hydrogen bonds on melting with increasing temperature in the range
2468:), due to the collective action of hydrogen bonds between water molecules. These hydrogen bonds are constantly breaking, with new bonds being formed with different water molecules; but at any given time in a sample of liquid water, a large portion of the molecules are held together by such bonds.
1731:
of water is about 1 gram per cubic centimetre (62 lb/cu ft): this relationship was originally used to define the gram. The density varies with temperature, but not linearly: as the temperature increases, the density rises to a peak at 3.98 °C (39.16 °F) and then decreases; the
1782:
The unusual density curve and lower density of ice than of water is essential for much of the life on earth—if water were most dense at the freezing point, then in winter the cooling at the surface would lead to convective mixing. Once 0 °C are reached, the water body would freeze from the
2206:
point of about 231 K (−42 °C; −44 °F). The melting point of ordinary hexagonal ice falls slightly under moderately high pressures, by 0.0073 °C (0.0131 °F)/atm or about 0.5 °C (0.90 °F)/70 atm as the stabilization energy of hydrogen bonding is exceeded by
8563:
Weingärtner, Hermann; Teermann, Ilka; Borchers, Ulrich; Balsaa, Peter; Lutze, Holger V.; Schmidt, Torsten C.; Franck, Ernst Ulrich; Wiegand, Gabriele; Dahmen, Nicolaus; Schwedt, Georg; Frimmel, Fritz H.; Gordalla, Birgit C. (2016). "Water, 1. Properties, Analysis, and
Hydrological Cycle".
2002:
The bulk modulus of water ice ranges from 11.3 GPa at 0 K up to 8.6 GPa at 273 K. The large change in the compressibility of ice as a function of temperature is the result of its relatively large thermal expansion coefficient compared to other common solids.
7512:
electron domain is spread out more in space than is the electron domain for a bonding pair (Figure 9.7). Nonbonding electron pairs, therefore, take up more space than bonding pairs; in essence, they act as large and fatter balloons in our analogy of Figure 9.5. As a result,
2301:
and deionized ultra-pure water systems used, for instance, in semiconductor manufacturing plants. A salt or acid contaminant level exceeding even 100 parts per trillion (ppt) in otherwise ultra-pure water begins to noticeably lower its resistivity by up to several kΩ·m.
1810:
The density of saltwater depends on the dissolved salt content as well as the temperature. Ice still floats in the oceans, otherwise, they would freeze from the bottom up. However, the salt content of oceans lowers the freezing point by about 1.9 °C (due to
3213:
2639:
are relatively soluble in water, and nonpolar substances such as fats and oils are not. Nonpolar molecules stay together in water because it is energetically more favorable for the water molecules to hydrogen bond to each other than to engage in
2401:
Although hydrogen bonding is a relatively weak attraction compared to the covalent bonds within the water molecule itself, it is responsible for several of the water's physical properties. These properties include its relatively high
2394:, points from a region between the two hydrogen atoms to the oxygen atom. The charge differences cause water molecules to aggregate (the relatively positive areas being attracted to the relatively negative areas). This attraction,
1648:
and the critical pressure is caused by the weight of the ocean at the extreme depths where the vents are located. This pressure is reached at a depth of about 2200 meters: much less than the mean depth of the ocean (3800 meters).
1392:
in salts and bond to other polar substances such as alcohols and acids, thus dissolving them. Its hydrogen bonding causes its many unique properties, such as having a solid form less dense than its liquid form, a relatively high
5768:
1994:
of water is about 2.2 GPa. The low compressibility of non-gasses, and of water in particular, leads to their often being assumed as incompressible. The low compressibility of water means that even in the deep
2717:. Unlike previously reported tunneling motions in water, this involved the concerted breaking of two hydrogen bonds. Later in the same year, the discovery of the quantum tunneling of water molecules was reported.
4382:) and an acidic proton are added to the two carbon atoms bonded together in the carbon-carbon double bond, resulting in an alcohol. When the addition of water to an organic molecule cleaves the molecule in two,
1819:
blocked by an expansion of water as it becomes colder near the freezing point. The oceans' cold water near the freezing point continues to sink. So creatures that live at the bottom of cold oceans like the
2824:
the liquid. This view is based upon neutron scattering studies and computer simulations, and it makes sense in the light of the unambiguously tetrahedral arrangement of water molecules in ice structures.
2831:
suggested that water molecules in the liquid state typically bind not to four but only two others; thus forming chains and rings. The term "string theory of water" (which is not to be confused with the
5624:
Ramires, Maria L. V.; Castro, Carlos A. Nieto de; Nagasaka, Yuchi; Nagashima, Akira; Assael, Marc J.; Wakeham, William A. (1995-05-01). "Standard
Reference Data for the Thermal Conductivity of Water".
1736:
so that the density only decreases as a function of temperature. The increase observed for water from 0 °C (32 °F) to 3.98 °C (39.16 °F) and for a few other liquids is described as
1897:. Water being a relatively polar compound will tend to be miscible with liquids of high polarity such as ethanol and acetone, whereas compounds with low polarity will tend to be immiscible and poorly
1539:
at wavelengths of around 750 nm which cause it to appear to have a blue color. This can easily be observed in a water-filled bath or wash-basin whose lining is white. Large ice crystals, as in
7202:
Richardson, Jeremy O.; Pérez, Cristóbal; Lobsiger, Simon; Reid, Adam A.; Temelso, Berhane; Shields, George C.; Kisiel, Zbigniew; Wales, David J.; Pate, Brooks H.; Althorpe, Stuart C. (2016-03-18).
4817:, or its equivalent in different languages, although there are other systematic names which can be used to describe the molecule. Oxidane is only intended to be used as the name of the mononuclear
2613:
from the water. Contrary to the common misconception, water and hydrophobic substances do not "repel", and the hydration of a hydrophobic surface is energetically, but not entropically, favorable.
1791:
freezes only to about 1 m thickness in winter. In general, for deep enough lakes, the temperature at the bottom stays constant at about 4 °C (39 °F) throughout the year (see diagram).
7894:
2556:
Because water has strong cohesive and adhesive forces, it exhibits capillary action. Strong cohesion from hydrogen bonding and adhesion allows trees to transport water more than 100 m upward.
5085:(VSMOW), used for calibration, melts at 273.1500089(10) K (0.000089(10) °C, and boils at 373.1339 K (99.9839 °C). Other isotopic compositions melt or boil at slightly different temperatures.
3327:
3759:) donor and a base is a proton acceptor. When reacting with a stronger acid, water acts as a base; when reacting with a stronger base, it acts as an acid. For instance, water receives an
4603:
Water can be split into its constituent elements, hydrogen, and oxygen, by passing an electric current through it. This process is called electrolysis. The cathode half reaction is:
7588:
Hardy, Edme H.; Zygar, Astrid; Zeidler, Manfred D.; Holz, Manfred; Sacher, Frank D. (2001). "Isotope effect on the translational and rotational motion in liquid water and ammonia".
1127:
2609:
that water molecules generate between other water molecules. If a substance has properties that do not allow it to overcome these strong intermolecular forces, the molecules are
1839:, 3.5%) the ice that forms is essentially salt-free, with about the same density as freshwater ice. This ice floats on the surface, and the salt that is "frozen out" adds to the
5760:
3628:. Because water molecules exchange hydrogen atoms with one another, hydrogen deuterium oxide (DOH) is much more common in low-purity heavy water than pure dideuterium monoxide
1815:) and lowers the temperature of the density maximum of water to the former freezing point at 0 °C. This is why, in ocean water, the downward convection of colder water is
3552:
exists in nature only in minute quantities, being produced primarily via cosmic ray-induced nuclear reactions in the atmosphere. Water with one protium and one deuterium atom
2479:
the water may form a thin film because the molecular forces between glass and water molecules (adhesive forces) are stronger than the cohesive forces. In biological cells and
2333:
10 S/cm, but this conductivity is now thought to be almost entirely from surface defects, and without those, ice is an insulator with an immeasurably small conductivity.
6304:; Salzmann, Christoph; Kohl, Ingrid; Mayer, Erwin; Hallbrucker, Andreas (2001-01-01). "A second distinct structural "state" of high-density amorphous ice at 77 K and 1 bar".
2321:
into oxygen and hydrogen gases but in the absence of dissolved ions this is a very slow process, as very little current is conducted. In ice, the primary charge carriers are
3096:
5417:
Tanaka, M; Girard, G; Davis, R; Peuto, A; Bignell, N (August 2001). "Recommended table for the density of water between 0 C and 40 C based on recent experimental reports".
2815:, or structural properties like those observed in water because none of them can form four hydrogen bonds: either they cannot donate or accept hydrogen atoms, or there are
8739:
3047:
1912:
that is thermodynamically stable with the liquid (or solid) at a given temperature is relatively low compared with total atmospheric pressure. For example, if the vapor's
4833:
for the –OH group. The name oxane is explicitly mentioned by the IUPAC as being unsuitable for this purpose, since it is already the name of a cyclic ether also known as
5568:
3705:, where the Sun's radiation is too weak to vaporize solid and liquid water (as well as other elements and chemical compounds with relatively low melting points, such as
1637:. In nature, this only rarely occurs in extremely hostile conditions. A likely example of naturally occurring supercritical water is in the hottest parts of deep water
14950:
3344:, physical processes that convert solid rocks and minerals into soil and sediment, but under some conditions chemical reactions with water occur as well, resulting in
1129:
14959:
14243:
14215:
7778:
5660:
17792:
17465:
14927:
14253:
14234:
14206:
2286:. If water has even a tiny amount of such an impurity, then the ions can carry charges back and forth, allowing the water to conduct electricity far more readily.
17784:
17561:
17529:
16766:
16742:
16730:
15145:
14918:
10931:
5388:
2866:
explanation is that the oxygen atom's lone pairs are physically larger and therefore take up more space than the oxygen atom's bonds to the hydrogen atoms. The
2532:
is under the water level, which has risen gently and smoothly. Surface tension prevents the clip from submerging and the water from overflowing the glass edges.
17776:
17720:
17553:
17521:
17473:
17433:
17210:
17189:
16802:
16790:
16778:
16754:
10343:
8408:
6928:
Hoy, AR; Bunker, PR (1979). "A precise solution of the rotation bending Schrödinger equation for a triatomic molecule with application to the water molecule".
17768:
17744:
17712:
17670:
17633:
17505:
17425:
17417:
17197:
17184:
17168:
17128:
17112:
17104:
8191:
2624:. The partially negative dipole ends of the water are attracted to positively charged components of the solute, and vice versa for the positive dipole ends.
17654:
17625:
17609:
17577:
17545:
17497:
17215:
17120:
17096:
15150:
5280:
2605:("water-fearing") substances. The ability of a substance to dissolve in water is determined by whether or not the substance can match or better the strong
5444:
Lemmon, Eric W.; Bell, Ian H.; Huber, Marcia L.; McLinden, Mark O. "Thermophysical
Properties of Fluid Systems". In Linstrom, P.J.; Mallard, W.G. (eds.).
3658:
heavy water must be consumed for any toxicity to become apparent. Rats, however, are able to avoid heavy water by smell, and it is toxic to many animals.
17646:
17601:
17593:
17569:
17537:
17457:
17202:
10216:
1812:
1137:
2548:
of 71.99 mN/m at 25 °C which is caused by the strength of the hydrogen bonding between water molecules. This allows insects to walk on water.
17585:
17449:
17309:
10505:
6011:
3437:
is the current international standard for water isotopes. Naturally occurring water is almost completely composed of the neutron-less hydrogen isotope
2836:
of physics) was coined. These observations were based upon X-ray absorption spectroscopy that probed the local environment of individual oxygen atoms.
1309:
17662:
17273:
17176:
14501:
10752:
8565:
8366:
7882:
4865:
6027:(including Arctic sea ice, ice sheets, and glaciers) and warming of the continents and atmosphere account for the remainder of the change in energy.
2067:
in Göttingen produced data on several other triple points in the early 20th century. Kamb and others documented further triple points in the 1960s.
17760:
16427:
16333:
16309:
16297:
8012:
6863:
Light, Truman S.; Licht, Stuart; Bevilacqua, Anthony C.; Morash, Kenneth R. (2005-01-01). "The
Fundamental Conductivity and Resistivity of Water".
5301:
Sometimes these compounds have generic or common names (e.g., H2O is "water") and they also have systematic names (e.g., H2O, dihydrogen monoxide).
2436:, despite hydrogen sulfide having nearly twice the molar mass of water. The extra bonding between water molecules also gives liquid water a large
16369:
16357:
16345:
16321:
8041:
2363:
of electrons. One effect usually ascribed to the lone pairs is that the H–O–H gas-phase bend angle is 104.48°, which is smaller than the typical
2202:
well below that temperature without freezing if the liquid is not mechanically disturbed. It can remain in a fluid state down to its homogeneous
1151:
7810:
2687:. The water dipoles make hydrogen bonds with the polar regions of the sugar molecule (OH groups) and allow it to be carried away into solution.
16415:
15931:
15919:
14074:
9015:
6547:
8148:
1916:
is 2% of atmospheric pressure and the air is cooled from 25 °C, starting at about 22 °C, water will start to condense, defining the
15754:
8389:
2063:(forms) of ice, water has other triple points, which have either three polymorphs of ice or two polymorphs of ice and liquid in equilibrium.
6601:
5503:
2492:
or less. They are important in biology, particularly when cells are dehydrated by exposure to dry atmospheres or to extracellular freezing.
2406:
and boiling point temperatures: more energy is required to break the hydrogen bonds between water molecules. In contrast, hydrogen sulfide (
1711:
The specific heat capacity of ice at −10 °C is 2030 J/(kg·K) and the heat capacity of steam at 100 °C is 2080 J/(kg·K).
16381:
16257:
15968:
15907:
15790:
15778:
15766:
15499:
15464:
9173:
7514:
electron domains for nonbonding electron pairs exert greater repulsive forces on adjacent electron domains and tend to compress bond angles
468:
1979:
around 45 °C before increasing again with increasing temperature. As the pressure is increased, the compressibility decreases, being
16142:
16118:
16106:
15054:
6213:
93:
6113:
5589:
Silverstein, Todd P.; Heller, Stephen T. (17 April 2017). "pKa Values in the
Undergraduate Curriculum: What Is the Real pKa of Water?".
3967:, although it can also react with Lewis bases, forming hydrogen bonds between the electron pair donors and the hydrogen atoms of water.
2858:, molecular structure, allowing it to be polar. The hydrogen–oxygen–hydrogen angle is 104.45°, which is less than the 109.47° for ideal
2705:
dynamics in water was reported as early as 1992. At that time it was known that there are motions which destroy and regenerate the weak
16976:
16178:
16166:
16154:
16130:
15879:
10082:
7181:
6903:
6850:
C. S. Fuller "Defect
Interactions in Semiconductors" Chapter 5 pp. 192-221 in "Semiconductors" N. B. Hannay Ed. Reinhold, New York 1959
3405:
Rain is generally mildly acidic, with a pH between 5.2 and 5.8 if not having any acid stronger than carbon dioxide. If high amounts of
2767:
take advantage of water's opacity to microwave radiation to heat the water inside of foods. Water's light blue color is caused by weak
6627:
15845:
4806:
1669:
of 4184 J/(kg·K) at 20 °C (4182 J/(kg·K) at 25 °C) —the second-highest among all the heteroatomic species (after
2371:
to the hydrogens, so they require more space. The increased repulsion of the lone pairs forces the O–H bonds closer to each other.
6522:
6063:
8720:
6899:
6384:
8728:
8666:
Release on the IAPWS Formulation 1995 for the
Thermodynamic Properties of Ordinary Water Substance for General and Scientific Use
6456:
7455:
O, is similar. It has two electron pairs with nothing attached to them. They, too, must be taken into account. Molecules like NH
40:
7056:
6083:, le poids absolu d'un volume d'eau pure égal au cube de la centième partie du mètre, et à la température de la glace fondante.
5321:
2768:
2064:
1304:
5560:
4522:
to emit oxygen gas, but very few oxidants react with water even if their reduction potential is greater than the potential of
3971:
describes water as both a weak hard acid and a weak hard base, meaning that it reacts preferentially with other hard species:
8606:
8553:
8421:
8383:
8356:
8330:
8298:
8277:
8254:
7492:
5851:
4724:
showed that water was composed of oxygen and hydrogen in 1781. The first decomposition of water into hydrogen and oxygen, by
1025:
8476:
6551:
1893:
are immiscible, usually forming layers according to increasing density from the top. This can be predicted by comparing the
14000:
6827:
5732:
4857:
6044:
5890:
Smith, Jared D.; Christopher D. Cappa; Kevin R. Wilson; Ronald C. Cohen; Phillip L. Geissler; Richard J. Saykally (2005).
5857:
2390:
points from each H to the O, making the oxygen partially negative and each hydrogen partially positive. A large molecular
8716:
7763:
6780:
5007:
4977:
3678:
Water is the most abundant substance on Earth's surface and also the third most abundant molecule in the universe, after
2726:
1052:
2620:). The relatively small size of water molecules (~ 3 angstroms) allows many water molecules to surround one molecule of
1963:
of water is a function of pressure and temperature. At 0 °C, at the limit of zero pressure, the compressibility is
8665:
8583:
8523:
8500:
8470:
8304:
8233:
5669:, 8—Concentrative Properties of Aqueous Solutions: Density, Refractive Index, Freezing Point Depression, and Viscosity.
3371:, with a variety of small molecules that can be embedded in its spacious crystal lattice. The most notable of these is
2198:
The melting point of ice is 0 °C (32 °F; 273 K) at standard pressure; however, pure liquid water can be
8491:
Reece, Jane B.; Urry, Lisa A.; Cain, Michael L.; Wasserman, Steven A.; Minorsky, Peter V.; Jackson, Robert B. (2013).
7564:
5349:
5157:. Also, fairly pure silicon has a negative coefficient of thermal expansion for temperatures between about 18 and 120
3229:
8724:
8698:
7444:
6207:
1547:
443:
8435:
6252:
63:
53:
11202:
9166:
8181:
6285:
5082:
4891:, using acid and base names. None of these exotic names are used widely. The polarized form of the water molecule,
3666:
refers to deuterium-depleted water (DDW), water in which the deuterium content has been reduced below the standard
3434:
2799:
because it can accept two bonds using the lone pairs on oxygen and donate two hydrogen atoms. Other molecules like
2512:
2060:
1752:
555:
6636:
1316:
17900:
15047:
8770:
8650:
5564:
1876:
1768:
8672:
Online calculator using the IAPWS Supplementary
Release on Properties of Liquid Water at 0.1 MPa, September 2008
5288:
1677:(40.65 kJ/mol or 2257 kJ/kg at the normal boiling point), both of which are a result of the extensive
14968:
11156:
10075:
7298:
6727:
5697:
2364:
1079:
4777:
scales were, or currently are, defined by the freezing and boiling points of water. The less common scales of
17840:
8990:
8144:
7077:
5529:
4519:
2606:
118:
6004:
17905:
17855:
15063:
10043:
5949:
Deguchi, Shigeru; Tsujii, Kaoru (2007-06-19). "Supercritical water: a fascinating medium for soft matter".
2845:
2713:. On 18 March 2016, it was reported that the hydrogen bond can be broken by quantum tunneling in the water
2289:
It is known that the theoretical maximum electrical resistivity for water is approximately 18.2 MΩ·cm (182
420:
5517:
4354:
crystallizes with one molecule of "lattice" water, which interacts with the sulfate and with the centers.
278:
17875:
17870:
17850:
14761:
9159:
8925:
4729:
8005:
7319:
Pope; Fry (1996). "Absorption spectrum (380-700nm) of pure water. II. Integrating cavity measurements".
6405:
Fine, R.A.; Millero, F.J. (1973). "Compressibility of water as a function of temperature and pressure".
2827:
However, there is an alternative theory for the structure of water. In 2004, a controversial paper from
15040:
11412:
4967:
4873:
3208:{\displaystyle K_{\rm {eq}}={\frac {a_{\rm {H_{3}O^{+}}}\cdot a_{\rm {OH^{-}}}}{a_{\rm {H_{2}O}}^{2}}}}
2696:
1764:
1737:
813:
795:
6817:"Revised Release on the Pressure along the Melting and Sublimation Curves of Ordinary Water Substance"
17915:
17860:
17072:
14840:
14820:
14781:
10068:
8962:
8734:
8033:
7802:
4962:
4822:
3702:
3080:
2855:
2610:
329:
8136:
17885:
14985:
14880:
14771:
14431:
13993:
8947:
8876:
6452:
5474:
4304:
3661:
2961:
2894:
2888:
2867:
2241:
1941:
1850:
1687:
Most of the additional energy stored in the climate system since 1970 has accumulated in the oceans
1485:
1447:
8370:
6032:
2967:
2536:
17845:
17688:
14895:
14810:
14791:
14604:
14549:
14415:
14355:
14272:
14262:
14196:
12642:
11378:
9867:
7436:
6662:
6588:
5499:
5234:). Using the standard definition of atmosphere, 1,013,250 dynes/cm, it works out to 0.0073°C/atm.
5047:
4737:
4505:
2859:
2679:, each being surrounded by water molecules. The ions are then easily transported away from their
2597:
due to its high dielectric constant. Substances that mix well and dissolve in water are known as
2356:
2306:
2049:
2012:
1744:
is also less dense than liquid water—upon freezing, the density of water decreases by about 9%.
17910:
17489:
14870:
14850:
14830:
14801:
14444:
14345:
14224:
13213:
13199:
12387:
12285:
11128:
11114:
9814:
9116:
9058:
9000:
8545:
4972:
4758:
4741:
3087:
2760:
2437:
2391:
2347:
1666:
1001:
9025:
6195:
4516:
is a reaction between iron and oxygen that is dissolved in water, not between iron and water.
3569:
in far lower amounts (0.000003%) and any such molecules are temporary as the atoms recombine.
3413:
oxides are present in the air, they too will dissolve into the cloud and raindrops, producing
2215:, i.e., reaching 355 K (82 °C) at 2.216 GPa (21,870 atm) (triple point of
1999:
at 4 km depth, where pressures are 40 MPa, there is only a 1.8% decrease in volume.
17895:
17617:
17481:
17441:
17152:
16599:
16582:
15135:
15130:
14905:
14860:
14742:
14600:
14590:
14472:
13814:
13550:
12659:
12438:
12370:
11994:
11446:
11142:
11058:
10542:
9946:
9551:
8967:
8348:
8244:
7681:
7654:
6170:
4598:
2756:
2237:
1774:
1645:
9040:
7157:
6640:
2440:. This high heat capacity makes water a good heat storage medium (coolant) and heat shield.
1908:
As a gas, water vapor is completely miscible with air. On the other hand, the maximum water
1476:
is a constant, so their respective concentrations are inversely proportional to each other.
17752:
17696:
17228:
17144:
16881:
15644:
14751:
14325:
14311:
14301:
13763:
13533:
13499:
13297:
13014:
12829:
12591:
12574:
12217:
12166:
11327:
11310:
11276:
11195:
11100:
11044:
10989:
10977:
10891:
10796:
10764:
10461:
10432:
10408:
10134:
9643:
9063:
8896:
8071:
7628:
7597:
7587:
7383:
7328:
7272:
7215:
7012:
6937:
6764:
6701:
6597:
6498:
6414:
6313:
6138:
5958:
5903:
5892:"Unified description of temperature-dependent hydrogen bond rearrangements in liquid water"
5633:
5598:
5364:
4747:
4439:
4415:
4411:
2957:
2828:
2504:
2465:
2044:
of water. Since 1954, this point had been used to define the base unit of temperature, the
1674:
1622:
1614:). Visible steam and clouds are formed from minute droplets of water suspended in the air.
869:
429:
106:
20:
9123:
6690:"Review of the vapour pressures of ice and supercooled water for atmospheric applications"
6629:
Impact of High
Pressure — Low Temperature Processes on Cellular Materials Related to Foods
202:
8:
17865:
17813:
17704:
17389:
17223:
16845:
15865:
15639:
15140:
14732:
14580:
14393:
14321:
14291:
14179:
14129:
14119:
14044:
13986:
13870:
13780:
13626:
13241:
13171:
13115:
12916:
12888:
12812:
12795:
12778:
12744:
12693:
12625:
12557:
12421:
12353:
12319:
12268:
12132:
11974:
11964:
11924:
11727:
11560:
11480:
11463:
11429:
11293:
11086:
11069:
10923:
10903:
10878:
10849:
10566:
10525:
10481:
10420:
10396:
10375:
10268:
10256:
10102:
9721:
9685:
9488:
9231:
9221:
9068:
8763:
7707:
Miller, Inglis J. Jr.; Mooser, Gregory (Jul 1979). "Taste
Responses to Deuterium Oxide".
7429:
3364:
2863:
2641:
2632:
2508:
1618:
1532:
765:
298:
192:
144:
8075:
7632:
7601:
7387:
7332:
7276:
7219:
7016:
6941:
6768:
6705:
6502:
6478:
6418:
6317:
6142:
6067:
5962:
5907:
5637:
5602:
5368:
4193:
When a salt of a weak acid or of a weak base is dissolved in water, water can partially
1373:
on Earth's surface. It is also the third most abundant molecule in the universe (behind
258:
17890:
17377:
17372:
17281:
17265:
16872:
16706:
16676:
16612:
14654:
14623:
14614:
14388:
14282:
14159:
14083:
14064:
13797:
13696:
13567:
13448:
13409:
13381:
12986:
12846:
12761:
12727:
12676:
12489:
12455:
12234:
12091:
12081:
11874:
11864:
11847:
11817:
11747:
11508:
11395:
11344:
11225:
10915:
10866:
10804:
10711:
10628:
10616:
10595:
10493:
10244:
10204:
9979:
9834:
9747:
9703:
9493:
9182:
8818:
8704:
8538:
8512:
8266:
7732:
7661:. Vol. 126, no. 4. New York: Popular Science Publishing. Apr 1935. p. 17
7409:
7352:
7033:
7000:
6907:
6719:
6514:
6162:
6128:
5926:
5891:
5889:
4997:
4911:
4368:
4351:
4271:
3748:
3368:
2387:
2053:
1693:
1686:
1638:
1491:
1421:
1212:
1102:
948:
933:
751:
238:
6444:
6376:
2601:("water-loving") substances, while those that do not mix well with water are known as
1570:
is the most common and is the form that is generally denoted by the word "water". The
17736:
17728:
17294:
17257:
17160:
17051:
17036:
17031:
16836:
16628:
16055:
15456:
15386:
15246:
15194:
15178:
15170:
15091:
14633:
14531:
14491:
14482:
14462:
14405:
14184:
14169:
14139:
14104:
14039:
13898:
13842:
13710:
13668:
13640:
13612:
13584:
13516:
13482:
13465:
13423:
13325:
13311:
13227:
13101:
13073:
13031:
12874:
12608:
12506:
12404:
12336:
12302:
12183:
12101:
11984:
11938:
11934:
11807:
11677:
11616:
11547:
11361:
11076:
11001:
10965:
10883:
10788:
10776:
10697:
10689:
10677:
10633:
10554:
10517:
10473:
10449:
10384:
10355:
10224:
10158:
10126:
9899:
9881:
9761:
9587:
9405:
9400:
9386:
9298:
9293:
9226:
9090:
9075:
8858:
8798:
8708:
8694:
8602:
8579:
8549:
8519:
8496:
8466:
8427:
8417:
8379:
8352:
8326:
8294:
8273:
8250:
8229:
7724:
7720:
7552:"Guideline on the Use of Fundamental Physical Constants and Basic Constants of Water"
7488:
7440:
7401:
7344:
7290:
7241:
7233:
7173:
7071:
7038:
6949:
6880:
6723:
6518:
6329:
6281:
6203:
6154:
5982:
5974:
5931:
5847:
5649:
5380:
5227:
5209:
5032:
5017:
4845:
4197:
the salt, producing the corresponding base or acid, which gives aqueous solutions of
3771:
3694:. 0.23 ppm of the earth's mass is water and 97.39% of the global water volume of 1.38
3537:
3442:
3372:
3349:
2850:
The repulsive effects of the two lone pairs on the oxygen atom cause water to have a
2800:
2744:
2702:
2578:
2383:
2342:
1894:
1803:
1733:
1642:
1630:
1587:
1451:
1357:. It is by far the most studied chemical compound and is described as the "universal
1342:
1278:
1258:
1222:
1217:
1207:
1179:
1173:
919:
743:
17325:
7736:
7356:
3743:
2524:
1681:
between its molecules. These two unusual properties allow water to moderate Earth's
226:
17513:
17249:
17236:
17136:
17080:
17041:
16967:
16954:
16908:
16697:
15575:
15219:
15096:
14995:
14936:
14659:
14536:
14457:
14378:
14149:
14114:
13856:
13724:
13654:
13367:
13255:
13185:
13157:
13129:
13000:
12972:
12958:
12200:
12061:
12044:
12014:
11944:
11837:
11827:
11767:
11612:
11573:
11534:
11030:
10948:
10837:
10740:
10728:
10702:
10607:
10537:
10444:
10367:
10335:
10322:
10146:
10091:
10032:
9736:
9657:
9533:
9450:
9333:
9258:
9253:
9242:
8995:
8793:
8690:
8686:
8632:
8598:
8571:
8340:
8221:
8079:
7716:
7636:
7623:
Urey, Harold C.; et al. (15 Mar 1935). "Concerning the Taste of Heavy Water".
7605:
7413:
7391:
7336:
7285:
7280:
7223:
7165:
7028:
7020:
6945:
6872:
6772:
6709:
6506:
6422:
6321:
6166:
6146:
5966:
5921:
5911:
5839:
5641:
5606:
5451:
5430:
5426:
5372:
5313:
4849:
4733:
4307:, water can be either a ligand or simply lodged in the framework, or both. Thus,
4267:
3739:
2851:
2783:
2772:
2736:
2636:
2616:
When an ionic or polar compound enters water, it is surrounded by water molecules (
2565:
2433:
2326:
2283:
1913:
1555:
1495:
1417:
1346:
1202:
954:
880:
491:
17398:
17345:
16932:
8646:
8575:
7640:
7204:"Concerted hydrogen-bond breaking by quantum tunneling in the water hexamer prism"
4786:
2763:
utilize the portion of the light spectrum that is transmitted well through water.
2367:
angle of 109.47°. The lone pairs are closer to the oxygen atom than the electrons
17880:
17364:
17244:
17088:
17046:
17022:
17008:
16994:
15660:
15311:
15186:
15101:
15081:
14521:
13969:
13965:
13884:
13682:
13395:
13339:
13269:
13059:
12944:
12902:
12540:
12523:
12472:
12149:
12034:
12024:
11914:
11894:
11797:
11757:
11638:
11586:
11521:
11259:
11188:
11169:
11035:
11018:
10861:
10816:
10723:
10659:
10583:
10305:
10292:
10236:
10199:
10170:
10114:
9928:
9829:
9671:
9415:
9410:
9323:
8957:
8952:
8891:
8881:
8830:
8678:
8533:
8456:
8320:
7688:. Vol. 130, no. 6. New York: Popular Science Publishing. pp. 22–23
7084:
6543:
6301:
5736:
4853:
4834:
4825:. These derivatives commonly have other recommended names. For example, the name
4721:
4504:, are oxidized by water as well, but their oxides adhere to the metal and form a
4427:
4395:
4309:
3701:
Water is far more prevalent in the outer Solar System, beyond a point called the
3533:
3357:
2764:
2680:
2648:
2545:
2500:
2488:
2298:
2294:
1960:
1845:
1832:
1536:
1378:
1361:" and the "solvent of life". It is the most abundant substance on the surface of
962:
769:
747:
731:
348:
17057:
15111:
15071:
8225:
6816:
6112:
Shell, Scott M.; Debenedetti, Pablo G.; Panagiotopoulos, Athanassios Z. (2002).
5729:
5610:
2212:
1719:
393:
17339:
17289:
16560:
15652:
15359:
15335:
15323:
15299:
15202:
15086:
15032:
14335:
13738:
13283:
13143:
13045:
12930:
12710:
12251:
11904:
11884:
11651:
11242:
10578:
10297:
10182:
9990:
9910:
9839:
9503:
9283:
9105:
8756:
8624:
6150:
5831:
4982:
4818:
4387:
4296:
3599:
3222:
O or OH is approximated by its concentration, and the activity of the solvent H
2871:
2808:
2748:
2582:
2379:
2310:
2040:
at which ordinary solid, liquid, and gaseous water coexist in equilibrium is a
1909:
1385:
1354:
1287:
852:
831:
785:
528:
16538:
16012:
6749:
4790:
2329:). Ice was previously thought to have a small but measurable conductivity of 1
2211:) above 209.9 MPa (2,072 atm), the melting point increases markedly
1420:, depending on the pH of the solution that it is in; it readily produces both
1388:
with each other and are strongly polar. This polarity allows it to dissociate
17834:
14511:
14365:
13353:
12071:
11787:
11777:
11625:
11599:
10956:
10828:
10330:
10317:
10004:
9853:
9772:
9562:
9474:
9460:
9440:
9372:
9338:
9303:
9193:
9030:
9010:
7237:
6999:
Chiavazzo, Eliodoro; Fasano, Matteo; Asinari, Pietro; Decuzzi, Paolo (2014).
6884:
6333:
6053:, 6. Properties of Water and Steam as a Function of Temperature and Pressure.
5978:
5653:
5384:
5214:
5175:
5154:
5027:
4778:
4770:
4399:
3714:
3353:
3341:
2833:
2796:
2788:
2732:
2706:
2419:), has much weaker hydrogen bonding due to sulfur's lower electronegativity.
2403:
2395:
2240:, but not even "deionized" water is completely free of ions. Water undergoes
1705:
1678:
1657:
1634:
1603:
1524:
1402:
1394:
982:
773:
761:
651:
641:
527:
Almost colorless or white crystalline solid, almost colorless liquid, with a
15520:
9138:
8431:
8288:
7228:
7203:
5916:
5843:
2499:
Rain water flux from a canopy. Among the forces that govern drop formation:
2398:, explains many of the properties of water, such as its solvent properties.
17805:
17356:
16959:
16664:
16652:
16395:
16285:
16204:
13554:
13217:
13203:
13087:
12646:
12004:
11998:
11954:
11737:
11690:
11664:
11416:
11009:
10943:
10194:
10018:
9964:
9800:
9786:
9629:
9615:
9513:
9343:
9313:
9263:
9207:
9094:
8974:
8940:
8671:
8462:
7551:
7405:
7348:
7294:
7245:
7042:
6158:
6000:
5986:
5935:
5066:
A commonly quoted value of 15.7 used mainly in organic chemistry for the pK
5022:
4953:
4794:
4782:
4725:
4435:
4403:
3479:
or T), which has two neutrons. Oxygen also has three stable isotopes, with
3430:
3345:
2586:
2359:
for the two hydrogens from the oxygen vertex. The oxygen atom also has two
2318:
2041:
1991:
1902:
1824:
generally live in water 4 °C colder than at the bottom of frozen-over
1821:
1799:
1567:
905:
755:
739:
6244:
2293:·m) at 25 °C. This figure agrees well with what is typically seen on
1946:
1751:
Under increasing pressure, ice undergoes a number of transitions to other
1150:
16999:
16814:
16689:
16403:
16273:
15730:
15718:
15617:
15612:
15607:
15584:
15287:
15275:
15263:
13953:
12920:
12391:
12289:
10668:
10650:
9523:
9420:
9273:
9035:
9005:
8930:
8920:
8886:
8848:
8803:
7728:
7627:. Vol. 81, no. 2098. New York: The Science Press. p. 273.
7340:
6133:
5253:
5042:
5037:
4992:
4751:
4744:
showed that water is composed of two parts hydrogen and one part oxygen.
4364:
4332:
4202:
3968:
3541:
3529:
3461:
3336:
The action of water on rock over long periods of time typically leads to
2820:
2602:
2598:
2484:
2450:
2207:
intermolecular repulsion, but as ice transforms into its polymorphs (see
2199:
2033:
2017:
1825:
1784:
1607:
1370:
1163:
727:
715:
685:
7261:"Quantum Tunneling of Water in Beryl: A New State of the Water Molecule"
3460:
or D), a hydrogen isotope with one neutron, and fewer than 20 parts per
1136:
318:
16718:
16640:
16514:
15742:
15347:
14094:
14054:
13948:
13818:
13630:
13537:
13301:
12663:
12629:
12442:
12374:
12111:
11978:
9601:
9430:
7024:
5231:
4987:
4924:
O) when one does not wish to specify whether one is speaking of liquid
4774:
4430:+1 and oxygen in the oxidation state −2. It oxidizes chemicals such as
4383:
4194:
3964:
3960:
3731:
3438:
3337:
2812:
2529:
2458:
2368:
2360:
2355:
An important feature of water is its polar nature. The structure has a
2314:
2244:
in the liquid state when two water molecules form one hydroxide anion (
2203:
1971:. At the zero-pressure limit, the compressibility reaches a minimum of
1554:, which are generally gaseous. This unique property of water is due to
1409:
1398:
1143:
667:
661:
514:
330:
289:
9127:
8162:
8095:
8093:
8083:
8062:
Lewis, G. N.; MacDonald, R. T. (1933). "Concentration of H2 Isotope".
7609:
7427:
Gonick, Larry; Criddle, Craig (2005-05-03). "Chapter 3 Togetherness".
7177:
7001:"Scaling behaviour for the water transport in nanoconfined geometries"
6876:
6776:
6510:
6426:
5376:
4757:
The properties of water have historically been used to define various
1550:, water is primarily a liquid, unlike other analogous hydrides of the
16573:
16265:
16236:
16082:
15952:
15895:
15706:
15622:
15429:
14009:
13923:
13874:
13784:
13700:
13427:
13315:
13245:
13175:
13119:
13105:
13035:
12892:
12878:
12833:
12595:
12578:
12544:
12221:
12170:
12153:
12028:
11968:
11928:
11918:
11878:
11761:
11751:
11731:
11642:
11512:
11382:
9151:
8935:
8901:
8410:
Principles of chemical nomenclature: a guide to IUPAC recommendations
6714:
6325:
5970:
5645:
5183:
4501:
4497:
4391:
3956:
3556:
occur naturally in ordinary water in low concentrations (~0.03%) and
3545:
3446:
3414:
3054:
2902:
2898:
2752:
2617:
2480:
2375:
1917:
1701:
1652:
1583:
1551:
1434:
1097:
899:
857:
836:
735:
671:
7988:
7986:
7396:
7371:
7260:
7169:
6689:
5871:
5707:, National Institute of Standards and Technology, Gaithersburg (MD)
5455:
4338:
2570:
1861:
1286:
Except where otherwise noted, data are given for materials in their
16989:
16568:
16464:
16224:
16094:
15995:
15960:
15853:
15824:
15694:
15491:
15251:
13943:
13933:
13928:
13767:
13714:
13672:
13644:
13616:
13588:
13571:
13503:
13452:
13413:
13385:
13371:
13259:
13189:
13161:
13133:
13018:
12990:
12976:
12816:
12799:
12782:
12748:
12714:
12697:
12561:
12425:
12357:
12323:
12187:
12136:
12095:
12085:
11988:
11958:
11868:
11851:
11821:
11811:
11707:
11681:
11655:
11629:
11564:
11331:
11297:
11280:
11229:
10060:
9508:
8117:
8090:
7908:
7890:
6095:
6093:
6091:
5226:
The source gives it as 0.0072°C/atm. However the author defines an
4826:
4545:
4431:
4331:
consists of centers and one "lattice water". Water is typically a
3406:
3226:
O is approximated by 1, so that we obtain the simple ionic product
2804:
2710:
2472:
2037:
2028:
and water vapor in the lower left portion of a water phase diagram.
1882:
1870:
1840:
1836:
1835:
of saltwater begins to freeze (at −1.9 °C for normal salinity
1579:
1521:
1515:
1511:
1374:
1253:
1109:
1093:
723:
711:
703:
689:
675:
368:
309:
6571:
6569:
6198:. In O'Mara, William C.; Herring, Robert B.; Hunt, Lee P. (eds.).
5802:
5800:
5798:
5447:
NIST Chemistry WebBook, NIST Standard Reference Database Number 69
5186:(melting point of 1,211 K (938 °C; 1,720 °F)), and
2807:
can also form hydrogen bonds. However, they do not show anomalous
2483:, water is in contact with membrane and protein surfaces that are
1708:, ice was and still is in common use for retarding food spoilage.
1661:
Heat of vaporization of water from melting to critical temperature
225:
16828:
16491:
16212:
16074:
15887:
15816:
15802:
15678:
15421:
15394:
15230:
13938:
13902:
13846:
13801:
13728:
13658:
13520:
13486:
13469:
13399:
13343:
13329:
13273:
13231:
13077:
13063:
12906:
12850:
12765:
12731:
12680:
12493:
12459:
12272:
12238:
12204:
12105:
12065:
12018:
11791:
11781:
11771:
11603:
11577:
11538:
11484:
11467:
11450:
8186:
7983:
7973:
7971:
7113:
5538:, Chapter 8: Dissociation Constants of Inorganic Acids and Bases.
5187:
5179:
5171:
4947:
4766:
4509:
4300:
3851:
3710:
3706:
3465:
3426:
3218:
However, for dilute solutions, the activity of a solute such as H
2740:
2714:
2684:
2594:
2575:
2322:
2216:
2110:
1898:
1886:
1843:
and density of the seawater just below it, in a process known as
1788:
1760:
1728:
1697:
1682:
1670:
1540:
1358:
1263:
1248:
1243:
1234:
1144:
777:
719:
707:
693:
546:
380:
8514:
Organic Solvents Physical Properties and Methods of Purification
7841:
7839:
7743:
7125:
6968:
6340:
6088:
5741:
5710:
5541:
3429:
of both hydrogen and oxygen exist, giving rise to several known
2278:
Because water is such a good solvent, it almost always has some
2208:
1599:
16456:
16448:
15686:
15602:
15597:
15592:
15238:
13860:
13686:
13287:
13147:
13049:
13004:
12962:
12948:
12934:
12612:
12510:
12408:
12340:
12306:
12048:
12038:
12008:
11948:
11898:
11841:
11831:
11801:
11741:
11694:
11668:
11590:
11551:
11525:
11433:
11399:
11314:
11263:
8372:
Nomenclature of Inorganic Chemistry: IUPAC Recommendations 2005
7158:"Vibration-Rotation-Tunneling Dynamics in Small Water Clusters"
6566:
6111:
5795:
5158:
4872:, which is a rarely used name of water, and mostly used in the
4762:
4442:
metals. One example of an alkali metal reacting with water is:
4263:
3410:
2816:
2662:
2621:
2290:
2279:
2156:
2138:
2088:
2045:
2022:
1756:
1741:
1626:
1595:
1528:
1350:
697:
269:
8287:
Campbell, Neil A.; Williamson, Brad; Heyden, Robin J. (2006).
8216:
Boyd, Claude E. (2000). "pH, Carbon Dioxide, and Alkalinity".
7968:
7944:
7932:
7920:
5623:
16190:
16066:
13978:
13888:
13742:
12255:
11246:
11211:
8912:
8853:
8838:
8779:
7863:
7851:
7836:
7824:
7559:
7137:
7103:
7101:
7099:
6998:
6956:
6823:
6352:
5767:(website). United States of America: USGS. October 22, 2019.
5700:
in Linstrom, Peter J.; Mallard, William G. (eds.);
5012:
4929:
4925:
2795:
A single water molecule can participate in a maximum of four
2676:
2495:
2476:
2124:
1996:
1611:
1571:
1362:
969:
679:
404:
249:
215:
27:
4335:
ligand, i.e., it forms only one bond with the central atom.
2487:; that is, surfaces that have a strong attraction to water.
2305:
In pure water, sensitive equipment can detect a very slight
13091:
12527:
12476:
12115:
11908:
11888:
11365:
11348:
8743:
7201:
6980:
6202:. Park Ridge, New Jersey: Noyes Publications. p. 431.
6114:"Molecular structural order and anomalies in liquid silica"
4513:
4198:
3735:
2628:
2540:
Temperature dependence of the surface tension of pure water
1591:
1518:
1413:
1389:
536:
359:
7096:
6862:
6479:"Elastic Constants, Bulk Modulus, and Compressibility of H
5812:
5783:
5702:
5526:, Vapor Pressure of Water From 0 to 370 °C in Sec. 6.
3397:, naturally found in large quantities on the ocean floor.
1813:
freezing-point depression of a solvent containing a solute
1641:, in which water is heated to the critical temperature by
13357:
12075:
11180:
8843:
8679:"Structure and Properties of Water in its Various States"
6371:
6369:
6367:
6300:
6280:(2nd ed.). Pearson Prentice-Hall. pp. 162–163.
5002:
4933:
4386:
is said to occur. Notable examples of hydrolysis are the
3352:, a type of chemical alteration of a rock which produces
2475:
properties because of its polar nature. On clean, smooth
2454:
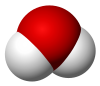
2233:
1925:
1921:
1890:
1732:
initial increase is unusual because most liquids undergo
1575:
1366:
1193:
925:
8748:
8286:
5877:
5190:(melting point of 545 K (272 °C; 521 °F))
3086:
The thermodynamic equilibrium constant is a quotient of
1446:
ions. Related to its amphoteric character, it undergoes
7956:
5483:, Properties of Ice and Supercooled Water in Section 6.
5252:
Both acid and base names exist for water because it is
5182:(melting point of 303 K (30 °C; 86 °F),
4797:
of water is a more commonly used standard point today.
3050:
1778:
Temperature distribution in a lake in summer and winter
7655:"Experimenter Drinks 'Heavy Water' at $ 5,000 a Quart"
6364:
5416:
4299:, which contains two water molecules coordinated to a
3717:, a moon of Saturn, is almost entirely made of water.
1578:
and commonly takes the structure of hard, amalgamated
790:
3.1690 kilopascals or 0.031276 atm at 25 °C
16011:
8407:
Leigh, G. J.; Favre, H. A; Metanomski, W. V. (1998).
8220:. Boston, Massachusetts: Springer. pp. 105–122.
8034:"Joseph Louis Gay-Lussac, French chemist (1778–1850)"
6694:
Quarterly Journal of the Royal Meteorological Society
6669:
6041:, Chapter 6: Properties of Ice and Supercooled Water.
5672:
3232:
3099:
3079:), with a value close to 10 mol L at 25 °C. See
2970:
2021:
The solid/liquid/vapor triple point of liquid water,
1723:
Density of ice and water as a function of temperature
15519:
8406:
8168:
8123:
8105:
8099:
5348:
Braun, Charles L.; Smirnov, Sergei N. (1993-08-01).
4943:
3049:, has a value of about 10 at 25 °C. At neutral
2819:
effects in bulky residues. In water, intermolecular
7532:
7520:
6797:
6383:. National Oceanic and Atmospheric Administration.
5443:
4864:, but this is not among the names published by the
1987:at 0 °C and 100 megapascals (1,000 bar).
8562:
8537:
8511:
8265:
7749:
7428:
6747:
6575:
6276:Housecroft, Catherine E.; Sharpe, Alan G. (2005).
5806:
5547:
5450:. National Institute of Standards and Technology.
4363:As a hard base, water reacts readily with organic
3321:
3207:
3041:
2683:into solution. An example of a nonionic solute is
1653:Heat capacity and heats of vaporization and fusion
8540:Living Ice: Understanding Glaciers and Glaciation
8367:International Union of Pure and Applied Chemistry
8339:
8190:. National Center for Biotechnology Information.
7992:
7478:
7164:. Lawrence Berkeley Lab., CA (United States): 6.
7131:
6346:
6193:
6099:
5747:
5588:
5178:of 1,687 K (1,414 °C; 2,577 °F)),
4866:International Union of Pure and Applied Chemistry
4844:. This is analogous to related compounds such as
17832:
15062:
6598:International Committee for Weights and Measures
6275:
5343:
5341:
5339:
3322:{\displaystyle K_{\rm {eq}}\approx K_{\rm {w}}=}
2336:
1685:by buffering large fluctuations in temperature.
392:
8629:Molecular Theory of Water and Aqueous Solutions
8518:. Techniques of Chemistry. Wiley-Interscience.
8242:
7066:. Archived from the original on August 7, 2007.
6974:
6491:Journal of Physical and Chemical Reference Data
5626:Journal of Physical and Chemical Reference Data
5153:Negative thermal expansion is also observed in
4856:(heavy water). Using chemical nomenclature for
4262:Water's Lewis base character makes it a common
3090:of all products and reactants including water:
2709:by internal rotations of the substituent water
2627:In general, ionic and polar substances such as
1856:
1794:
1755:with higher density than liquid water, such as
1566:Within the Earth's atmosphere and surface, the
1531:atom. Water is a tasteless, odorless liquid at
1412:, meaning that it can exhibit properties of an
1125:
201:
8593:Zumdahl, Steven S.; Zumdahl, Susan A. (2013).
8592:
8567:Ullmann's Encyclopedia of Industrial Chemistry
8061:
7977:
7950:
7938:
7926:
7914:
7869:
7857:
7845:
7830:
7581:
7143:
7119:
6962:
6900:"Lecture 12: Proton Conduction, Stoichiometry"
6548:National Institute of Standards and Technology
6358:
3615:at 25 °C is 23% higher than the value of
3572:The most notable physical differences between
3068:) equals that of the (solvated) hydrogen ion (
656:99.98 °C (211.96 °F; 373.13 K)
16:Physical and chemical properties of pure water
15048:
13994:
11196:
10076:
9167:
9143:
8764:
8263:
8243:Campbell, Mary K.; Farrell, Shawn O. (2007).
7426:
6986:
6858:
6856:
6271:
6269:
5948:
5336:
5256:(able to react both as an acid or an alkali).
5170:Other substances that expand on freezing are
3532:because of its higher density. It is used in
2720:
646:0.00 °C (32.00 °F; 273.15 K)
8685:. Wiley Online Library 2019. pp. 1–19.
8490:
7706:
7369:
7107:
6200:Handbook of semiconductor silicon technology
5999:
5818:
5789:
5705:, NIST Standard Reference Database Number 69
5347:
3963:, or electron-pair donor, in reactions with
2651:; the sodium chloride, NaCl, separates into
2351:Water molecule - structure and dipole moment
1704:. Before and since the advent of mechanical
1365:and the only common substance to exist as a
595:(temperature of maximum density, often 4 °C)
16924:
16688:
16611:
15583:
15123:
6748:Debenedetti, P. G.; Stanley, H. E. (2003).
6404:
5493:
5491:
5489:
4548:. An example of the oxidation of water is:
4421:
1714:
16507:
15055:
15041:
14001:
13987:
11203:
11189:
10083:
10069:
9174:
9160:
8771:
8757:
8264:Campbell, Neil A.; Reece, Jane B. (2009).
7622:
7258:
6904:University of Illinois at Urbana-Champaign
6897:
6853:
6266:
6238:
6236:
6234:
6232:
6230:
5824:
4936:, or a component in a mixture or mineral.
2464:Water molecules stay close to each other (
2227:
1454:, or approximately, the concentrations of
297:
19:"HOH" redirects here. For other uses, see
16530:
16394:
16256:
15163:
8495:(10th ed.). Boston, Mass.: Pearson.
8446:Lewis, William C.M.; Rice, James (1922).
8445:
7682:"Is 'Heavy Water' the Fountain of Youth?"
7395:
7284:
7227:
7057:"Physical Forces Organizing Biomolecules"
7032:
6927:
6713:
6675:
6619:
6132:
5925:
5915:
4879:Other systematic names for water include
4840:The simplest systematic name of water is
4414:reactions; the latter is then known as a
3955:Because the oxygen atom in water has two
3734:: it has the ability to act as either an
2056:, rather than the triple point of water.
2013:Triple point § Triple point of water
428:
15988:
15951:
15484:
15449:
8623:
8570:. Wiley-VCH Verlag GmbH & Co. KGaA.
7761:
7673:
7647:
7435:(1st ed.). HarperResource. p.
7155:
6625:
6476:
5836:IUPAC Compendium of Chemical Terminology
5497:
5486:
5412:
5410:
5408:
5078:
5076:
4821:used for naming derivatives of water by
4337:
3742:in chemical reactions. According to the
2782:
2569:
2535:
2523:
2494:
2449:
2443:
2346:
2222:
2016:
1945:
1860:
1798:
1773:
1718:
1656:
1561:
57:Ball-and-stick model of a water molecule
26:For broader coverage of this topic, see
16865:
16484:
15878:
15414:
8676:
8509:
8318:
7962:
7791:– via Applied Physics Laboratory.
7479:Theodore L. Brown; et al. (2015).
7318:
7259:Kolesnikov, Alexander I. (2016-04-22).
6865:Electrochemical and Solid-State Letters
6656:
6438:
6436:
6227:
5693:
5691:
5689:
5687:
1353:, which is nearly colorless apart from
317:
67:Space filling model of a water molecule
17833:
16901:
16827:
16447:
15844:
15815:
15677:
15229:
14029:
9181:
8544:. Cambridge University Press. p.
7679:
6687:
6056:
6010:. IPCC WGI AR5 (Report). p. 257.
5878:Campbell, Williamson & Heyden 2006
5243:Using the fact that 0.5/0.0073 = 68.5.
5237:
4728:, was done in 1800 by English chemist
4544:. Almost all such reactions require a
4342:Some hydrogen-bonding contacts in FeSO
3725:
2877:
2846:Molecular orbital diagram § Water
2839:
2581:from high concentrations of dissolved
2065:Gustav Heinrich Johann Apollon Tammann
2052:, the kelvin is now defined using the
1479:
16203:
16065:
15379:
15036:
14721:
13982:
11184:
10064:
9155:
9142:
8752:
8532:
8458:CRC Handbook of Chemistry and Physics
8365:
8111:
7616:
7487:(13 ed.). Pearson. p. 351.
6809:
6803:
6487:for the Temperature Range 50 K-273 K"
5771:from the original on December 1, 2021
5405:
5220:
5073:
4270:complexes, examples of which include
2559:
455:Key: XLYOFNOQVPJJNP-UHFFFAOYSA-N
277:
257:
10090:
9112:
8454:
8215:
7538:
7526:
7501:from the original on 4 February 2024
7485:Chemistry : the central science
7093:, Surface Tension of Common Liquids.
7090:
6607:from the original on 27 January 2018
6442:
6433:
6050:
6038:
5716:
5684:
5678:
5666:
5535:
5523:
5480:
5462:from the original on 23 October 2023
4920:is a term used for hydrogen oxide (H
4496:Some other reactive metals, such as
4358:
3877:ion, and is thus acting as an acid:
3400:
2690:
2317:at 25.00 °C. Water can also be
8647:"Water Properties and Measurements"
8182:"Compound Summary for CID 22247451"
7700:
6554:from the original on 20 August 2018
6306:Physical Chemistry Chemical Physics
6242:
5763:. U.S. Department of the Interior.
5324:from the original on 13 August 2017
5199:(1-0.95865/1.00000) × 100% = 4.135%
5008:Optical properties of water and ice
4978:Electromagnetic absorption by water
4257:
2731:Water is relatively transparent to
2727:Electromagnetic absorption by water
2551:
2232:Pure water containing no exogenous
2071:The various triple points of water
1889:in all proportions. Water and most
383:
367:
13:
8817:
8617:
8249:(6th ed.). Cengage Learning.
8169:Leigh, Favre & Metanomski 1998
8124:Leigh, Favre & Metanomski 1998
8100:Leigh, Favre & Metanomski 1998
7771:Johns Hopkins APL Technical Digest
5088:
4750:isolated the first sample of pure
3305:
3301:
3282:
3272:
3257:
3242:
3239:
3192:
3183:
3163:
3159:
3138:
3128:
3109:
3106:
3083:for values at other temperatures.
3025:
3021:
3002:
2992:
2977:
2882:
2519:
1954:
1935:
1598:, other crystalline and amorphous
1121:
62:
52:
14:
17927:
8639:
8293:. Boston: Pearson Prentice Hall.
6930:Journal of Molecular Spectroscopy
4914:hydroxide by IUPAC nomenclature.
3746:definition, an acid is a proton (
2647:An example of an ionic solute is
9122:
9111:
9101:
9100:
9089:
8174:
8129:
8055:
8026:
8006:"Enterprise and electrolysis..."
7998:
7803:"Planetologie und Fernerkundung"
6590:Proceedings of the 106th meeting
6064:"Decree on weights and measures"
5500:"Properties of substance: water"
5246:
5144:and more complex ions that form.
5083:Vienna Standard Mean Ocean Water
4946:
4508:protective layer. Note that the
3435:Vienna Standard Mean Ocean Water
3420:
2960:for this reaction, known as the
2193:
1950:Vapor pressure diagrams of water
1596:common hexagonal crystalline ice
1533:ambient temperature and pressure
1294:
519:18.01528(33) g/mol
92:
39:
8740:Why does ice float in my drink?
8651:United States Geological Survey
8479:from the original on 2024-02-04
8395:from the original on 2019-12-12
8307:from the original on 2014-11-02
8208:
8194:from the original on 2014-08-27
8151:from the original on 2016-08-16
8064:The Journal of Chemical Physics
8044:from the original on 2023-05-29
8015:from the original on 2016-03-03
7897:from the original on 2016-06-22
7875:
7813:from the original on 2023-04-11
7795:
7784:from the original on 2023-04-11
7755:
7680:Müller, Grover C. (June 1937).
7570:from the original on 2017-01-28
7544:
7472:
7420:
7363:
7312:
7301:from the original on 2020-11-18
7252:
7195:
7184:from the original on 2020-08-01
7149:
7049:
6992:
6921:
6891:
6844:
6833:from the original on 2014-03-02
6786:from the original on 2018-11-01
6741:
6730:from the original on 2020-08-18
6681:
6650:
6626:Schlüter, Oliver (2003-07-28).
6581:
6544:"Base unit definitions: Kelvin"
6536:
6525:from the original on 2021-11-28
6470:
6459:from the original on 2007-10-28
6398:
6387:from the original on 2020-07-06
6294:
6255:from the original on 2016-06-25
6216:from the original on 2024-02-04
6187:
6105:
6066:. April 7, 1795. Archived from
6017:from the original on 2020-10-16
5993:
5942:
5883:
5860:from the original on 2019-04-29
5753:
5722:
5617:
5582:
5571:from the original on 2016-02-14
5565:University of California, Davis
5553:
5506:from the original on 2014-06-02
5394:from the original on 2019-12-01
5202:
5193:
5164:
5147:
5060:
4800:
4592:
4426:Water contains hydrogen in the
3331:
2006:
1877:List of water-miscible solvents
1769:very-high-density amorphous ice
1290:(at 25 °C , 100 kPa).
14008:
8691:10.1002/9781119300762.wsts0002
8448:A System of Physical Chemistry
8378:. Royal Society of Chemistry.
8322:Qualitative Inorganic Analysis
7431:The cartoon guide to chemistry
7286:10.1103/PhysRevLett.116.167802
6750:"Supercooled and Glassy Water"
6657:Tammann, Gustav H.J.A (1925).
6600:. Sèvres. 16–20 October 2017.
5761:"Water, the Universal Solvent"
5437:
5306:
5273:
3698:10 km is found in the oceans.
3316:
3296:
3293:
3266:
3036:
3016:
3013:
2986:
2893:In liquid water there is some
2791:(1) between molecules of water
1345:inorganic compound that is at
1080:Occupational safety and health
138:Hydrogen hydroxide (HH or HOH)
1:
8991:Extraterrestrial liquid water
8576:10.1002/14356007.a28_001.pub3
8416:. Oxford: Blackwell Science.
8145:National Institutes of Health
7993:Greenwood & Earnshaw 1997
7641:10.1126/science.81.2098.273-a
7132:Greenwood & Earnshaw 1997
6637:Technische Universität Berlin
6347:Greenwood & Earnshaw 1997
6249:The USGS Water Science School
6100:Greenwood & Earnshaw 1997
5748:Greenwood & Earnshaw 1997
5591:Journal of Chemical Education
5357:Journal of Chemical Education
5262:
4858:type I ionic binary compounds
3673:
3644:Consumption of pure isolated
2337:Polarity and hydrogen bonding
2076:Phases in stable equilibrium
2059:Due to the existence of many
1885:with many liquids, including
995:75.385 ± 0.05 J/(mol·K)
15064:Binary compounds of hydrogen
8465:(84th ed.). CRC Press.
7762:Prockter, Louise M. (2005).
7721:10.1016/0031-9384(79)90124-0
6950:10.1016/0022-2852(79)90019-5
5498:Anatolievich, Kiper Ruslan.
5281:"naming molecular compounds"
5054:
4860:, water would take the name
4793:were defined similarly. The
4651:The anode half reaction is:
3720:
3512:in 0.2% of water molecules.
3042:{\displaystyle K_{\rm {w}}=}
2778:
2544:Water has an unusually high
2513:Plateau–Rayleigh instability
2255:) and one hydronium cation (
1857:Miscibility and condensation
1795:Density of saltwater and ice
1019:69.95 ± 0.03 J/(mol·K)
7:
8226:10.1007/978-1-4615-4485-2_7
7372:"Water—an enduring mystery"
7156:Pugliano, N. (1992-11-01).
6975:Campbell & Farrell 2007
6407:Journal of Chemical Physics
5611:10.1021/acs.jchemed.6b00623
4939:
3053:, the concentration of the
2743:light, but it absorbs most
2382:. Due to the difference in
2374:Another consequence of its
2236:is an excellent electronic
2170:ice II, ice III, and ice V
2123:liquid water, ice III, and
1046:−285.83 ± 0.04 kJ/mol
48:
10:
17932:
14723:
11413:Praseodymium(III,IV) oxide
11210:
8009:Royal Society of Chemistry
7978:Zumdahl & Zumdahl 2013
7951:Zumdahl & Zumdahl 2013
7939:Zumdahl & Zumdahl 2013
7927:Zumdahl & Zumdahl 2013
7915:Zumdahl & Zumdahl 2013
7870:Zumdahl & Zumdahl 2013
7858:Zumdahl & Zumdahl 2013
7846:Zumdahl & Zumdahl 2013
7831:Zumdahl & Zumdahl 2013
7144:Zumdahl & Zumdahl 2013
7120:Zumdahl & Zumdahl 2013
6963:Zumdahl & Zumdahl 2013
6359:Zumdahl & Zumdahl 2013
6194:Bullis, W. Murray (1990).
6151:10.1103/PhysRevE.66.011202
5896:Proc. Natl. Acad. Sci. USA
5561:"What is the pKa of Water"
4968:Dihydrogen monoxide parody
4874:dihydrogen monoxide parody
4716:
4596:
2886:
2843:
2724:
2721:Electromagnetic absorption
2697:Quantum tunneling of water
2694:
2644:with non-polar molecules.
2642:van der Waals interactions
2563:
2340:
2181:ice II, ice V, and ice VI
2010:
1939:
1874:
1868:
1765:high-density amorphous ice
1738:negative thermal expansion
1483:
25:
18:
17804:
17645:
17416:
17073:Transition metal hydrides
17071:
17021:
16598:
16054:
15635:
15574:
15218:
15110:
15070:
14031:
14016:
13962:
13916:
13835:
13756:
13605:
13441:
12867:
12125:
11720:
11501:
11218:
10098:
9189:
9149:
9144:Links to related articles
9084:
9051:
8983:
8910:
8869:
8829:
8815:
8786:
8778:
8450:. Longmans, Green and Co.
8345:Chemistry of the Elements
8343:; Earnshaw, Alan (1997).
8272:(8th ed.). Pearson.
8187:Pubchem Compound Database
7809:(in German). 2006-02-28.
7764:"Ice in the Solar System"
7709:Physiology & Behavior
7076:: CS1 maint: unfit URL (
6987:Campbell & Reece 2009
6659:The States Of Aggregation
6445:"Bulk Elastic Properties"
5832:"Autoprotolysis constant"
5230:as 1,000,000 dynes/cm (a
4963:Chemical bonding of water
4868:(IUPAC). Another name is
2282:dissolved in it, often a
2209:crystalline states of ice
2137:liquid water, ice V, and
1865:Red line shows saturation
1586:, or loosely accumulated
1349:a tasteless and odorless
1284:
1277:
1272:
1185:
1077:
1072:
975:
912:
484:
464:
439:
185:
129:
117:
105:
100:
91:
47:
38:
8735:Water Density Calculator
8725:dynamic liquid viscosity
8677:Chaplin, Martin (2019).
6453:Georgia State University
6005:"3: Observations: Ocean"
6003:; Rintoul, S.R. (2013).
5431:10.1088/0026-1394/38/4/3
5267:
4823:substituent nomenclature
4422:Water in redox reactions
3959:, water often acts as a
3088:thermodynamic activities
2889:Self-ionization of water
2868:molecular orbital theory
2100:273.16 K (0.01 °C)
1942:Vapour pressure of water
1851:thermohaline circulation
1715:Density of water and ice
1535:. Liquid water has weak
1486:Water chemistry analysis
1355:an inherent hint of blue
1273:Supplementary data page
760:Partially miscible with
12643:Praseodymium(III) oxide
11379:Manganese(II,III) oxide
8455:Lide, David R. (2003).
8290:Biology: Exploring Life
7750:Weingärtner et al. 2016
7686:Popular Science Monthly
7659:Popular Science Monthly
7265:Physical Review Letters
7229:10.1126/science.aae0012
6576:Weingärtner et al. 2016
6477:Neumeier, J.J. (2018).
6377:"Can the ocean freeze?"
5917:10.1073/pnas.0506899102
5844:10.1351/goldbook.A00532
5807:Weingärtner et al. 2016
5548:Weingärtner et al. 2016
5048:Water thread experiment
4738:Joseph Louis Gay-Lussac
2771:in the red part of the
2761:photosynthetic pigments
2357:bent molecular geometry
2307:electrical conductivity
2228:Electrical conductivity
1397:of 100 °C for its
684:Improved solubility in
17901:Extraterrestrial water
17806:Exotic matter hydrides
13214:Protactinium(IV) oxide
13200:Praseodymium(IV) oxide
12388:Einsteinium(III) oxide
12286:Californium(III) oxide
11704:Water (hydrogen oxide)
11219:Mixed oxidation states
9001:Planetary oceanography
8823:
8510:Riddick, John (1970).
6688:Murphy, D. M. (2005).
6381:National Ocean Service
5703:NIST Chemistry WebBook
5314:"Definition of Hydrol"
5070:of water is incorrect.
5023:Viscosity § Water
4973:Double distilled water
4742:Alexander von Humboldt
4398:. Water can also be a
4355:
3356:. It also occurs when
3323:
3209:
3043:
2792:
2593:Water is an excellent
2590:
2541:
2533:
2516:
2461:
2438:specific heat capacity
2352:
2029:
1951:
1866:
1807:
1779:
1724:
1667:specific heat capacity
1665:Water has a very high
1662:
1132:
68:
58:
13964:Oxides are sorted by
13815:Technetium(VII) oxide
13551:Protactinium(V) oxide
12660:Promethium(III) oxide
12439:Gadolinium(III) oxide
12371:Dysprosium(III) oxide
11995:Protactinium monoxide
11447:Terbium(III,IV) oxide
8821:
8683:Encyclopedia of Water
8668:(simpler formulation)
8534:Sharp, Robert Phillip
8349:Butterworth-Heinemann
7481:"9.2 The Vsepr Model"
7370:Ball, Philip (2008).
7005:Nature Communications
6676:Lewis & Rice 1922
6663:Constable And Company
4599:Electrolysis of water
4520:Water can be oxidized
4341:
3850:In the reaction with
3324:
3210:
3044:
2786:
2573:
2539:
2527:
2498:
2453:
2444:Cohesion and adhesion
2350:
2223:Electrical properties
2020:
1949:
1864:
1802:
1777:
1722:
1673:), as well as a high
1660:
1606:of water is known as
1574:of water is known as
1562:Water, ice, and vapor
1450:. The product of the
1384:Water molecules form
1131:
171:κ-Hydroxylhydrogen(0)
119:Systematic IUPAC name
66:
56:
17841:Amphoteric compounds
13764:Dichlorine heptoxide
13534:Phosphorus pentoxide
13500:Dinitrogen pentoxide
13298:Technetium(IV) oxide
13015:Dinitrogen tetroxide
12830:Ytterbium(III) oxide
12592:Neodymium(III) oxide
12575:Manganese(III) oxide
12218:Berkelium(III) oxide
12167:Americium(III) oxide
11328:Dichlorine pentoxide
11311:Cobalt(II,III) oxide
11277:Chlorine perchlorate
9064:Ocean stratification
8897:Doubly labeled water
8341:Greenwood, Norman N.
8319:Charlot, G. (2007).
7917:, pp. 932, 936.
7807:www.geo.fu-berlin.de
7341:10.1364/ao.36.008710
6070:on February 25, 2013
5350:"Why is water blue?"
5291:on 24 September 2018
4829:is recommended over
4748:Gilbert Newton Lewis
4416:dehydration reaction
4371:, a hydroxyl group (
4272:metal aquo complexes
3230:
3097:
2968:
2958:equilibrium constant
2829:Stockholm University
2505:Cohesion (chemistry)
2471:Water also has high
2457:drops adhering to a
2309:of 0.05501 ± 0.0001
2118:251 K (−22 °C)
1675:heat of vaporization
1623:critical temperature
1543:, also appear blue.
1114:(fire diamond)
1068:−237.24 kJ/mol
894:1.3330 (20 °C)
870:Thermal conductivity
147:(DHMO) (parody name)
107:Preferred IUPAC name
21:HOH (disambiguation)
17906:Transport phenomena
17856:Triatomic molecules
17418:Lanthanide hydrides
17025:(Group 17 hydrides)
16604:(Group 16 hydrides)
16058:(Group 15) hydrides
13871:Ruthenium tetroxide
13781:Manganese heptoxide
13627:Molybdenum trioxide
13242:Ruthenium(IV) oxide
13172:Plutonium(IV) oxide
13116:Neptunium(IV) oxide
12917:Californium dioxide
12889:Berkelium(IV) oxide
12813:Vanadium(III) oxide
12796:Tungsten(III) oxide
12779:Titanium(III) oxide
12745:Thallium(III) oxide
12694:Samarium(III) oxide
12626:Phosphorus trioxide
12558:Lutetium(III) oxide
12422:Europium(III) oxide
12354:Dinitrogen trioxide
12320:Chromium(III) oxide
12269:Caesium sesquioxide
12133:Actinium(III) oxide
11975:Phosphorus monoxide
11965:Palladium(II) oxide
11925:Manganese(II) oxide
11728:Aluminium(II) oxide
11561:Dichlorine monoxide
11481:Triuranium octoxide
11464:Tribromine octoxide
11430:Silver(I,III) oxide
11294:Chloryl perchlorate
9069:Lake stratification
9052:Physical parameters
8076:1933JChPh...1..341L
7633:1935Sci....81..273U
7602:2001JChPh.114.3174H
7388:2008Natur.452..291B
7333:1997ApOpt..36.8710P
7277:2016PhRvL.116p7802K
7220:2016Sci...351.1310R
7214:(6279): 1310–1313.
7162:UNT Digital Library
7122:, pp. 458–459.
7064:Biophysical Society
7017:2014NatCo...5.4565C
6942:1979JMoSp..74....1H
6898:Crofts, A. (1996).
6769:2003PhT....56f..40D
6706:2005QJRMS.131.1539M
6503:2018JPCRD..47c3101N
6419:1973JChPh..59.5529F
6318:2001PCCP....3.5355L
6278:Inorganic Chemistry
6143:2002PhRvE..66a1202S
5963:2007SMat....3..797D
5908:2005PNAS..10214171S
5902:(40): 14171–14174.
5719:, 9—Dipole Moments.
5638:1995JPCRD..24.1377R
5603:2017JChEd..94..690S
5369:1993JChEd..70..612B
5213:resulting from the
4870:dihydrogen monoxide
4367:; for example in a
3726:Acid–base reactions
3528:, is also known as
3490:present in 99.76%,
3365:clathrate compounds
3363:Water ice can form
3202:
2878:Chemical properties
2864:valence bond theory
2840:Molecular structure
2681:crystalline lattice
2585:turns the water of
2509:Van der Waals force
2378:is that water is a
2105:liquid water, ice I
2072:
1619:supercritical fluid
1617:Water also forms a
1548:standard conditions
1480:Physical properties
904:0.890 mPa·s (0.890
766:methyl ethyl ketone
633:0.9167 g/mL at 0 °C
239:Beilstein Reference
145:Dihydrogen monoxide
35:
17876:Neutron moderators
17871:Inorganic solvents
17851:Hydrogen compounds
15074:(Group 1) hydrides
13836:+8 oxidation state
13798:Rhenium(VII) oxide
13757:+7 oxidation state
13697:Tellurium trioxide
13606:+6 oxidation state
13568:Tantalum pentoxide
13449:Antimony pentoxide
13442:+5 oxidation state
13410:Vanadium(IV) oxide
13382:Tungsten(IV) oxide
12987:Chromium(IV) oxide
12868:+4 oxidation state
12847:Yttrium(III) oxide
12762:Thulium(III) oxide
12728:Terbium(III) oxide
12677:Rhodium(III) oxide
12490:Holmium(III) oxide
12456:Gallium(III) oxide
12235:Bismuth(III) oxide
12126:+3 oxidation state
12092:Vanadium(II) oxide
12082:Titanium(II) oxide
11875:Germanium monoxide
11865:Europium(II) oxide
11848:Dinitrogen dioxide
11818:Chromium(II) oxide
11748:Berkelium monoxide
11721:+2 oxidation state
11509:Aluminium(I) oxide
11502:+1 oxidation state
11396:Mellitic anhydride
11345:Iron(II,III) oxide
11226:Antimony tetroxide
9183:Hydrogen compounds
9016:List of Candidates
8877:Deuterium-depleted
8824:
8040:. Footnote 122-1.
7893:. 2 October 2013.
7025:10.1038/ncomms4565
6826:. September 2011.
6700:(608): 1539–1565.
5735:2023-07-28 at the
4998:Hydrogen polyoxide
4889:hydrogen hydroxide
4759:temperature scales
4369:hydration reaction
4356:
4352:metal aquo complex
4303:center. In solid
3866:, water donates a
3770:ion from HCl when
3544:, decaying with a
3369:clathrate hydrates
3319:
3205:
3176:
3039:
2793:
2591:
2560:Water as a solvent
2542:
2534:
2517:
2462:
2388:bond dipole moment
2353:
2094:, and water vapor
2070:
2054:Boltzmann constant
2030:
1952:
1867:
1828:lakes and rivers.
1808:
1780:
1725:
1694:enthalpy of fusion
1663:
1639:hydrothermal vents
1492:chemical substance
1375:molecular hydrogen
1317:Infobox references
1213:Hydrogen telluride
1186:Related compounds
1133:
1103:Water intoxication
752:dimethyl sulfoxide
666:Poorly soluble in
156:Hydrohydroxic acid
69:
59:
33:
17828:
17827:
17822:
17821:
17647:Actinide hydrides
17023:Hydrogen halides
16985:
16950:
16949:
16920:
16919:
16897:
16896:
16861:
16860:
16823:
16822:
16684:
16683:
16590:
16556:
16555:
16526:
16525:
16503:
16502:
16480:
16479:
16443:
16442:
16390:
16389:
16252:
16251:
16199:
16198:
16050:
16049:
16007:
16006:
15984:
15983:
15947:
15946:
15874:
15873:
15840:
15839:
15811:
15810:
15673:
15672:
15631:
15630:
15576:Group 14 hydrides
15570:
15569:
15515:
15514:
15480:
15479:
15445:
15444:
15410:
15409:
15375:
15374:
15214:
15213:
15159:
15158:
15030:
15029:
15024:
15023:
13976:
13975:
13899:Hassium tetroxide
13843:Iridium tetroxide
13711:Tungsten trioxide
13669:Selenium trioxide
13641:Polonium trioxide
13613:Chromium trioxide
13585:Vanadium(V) oxide
13517:Niobium pentoxide
13483:Bismuth pentoxide
13466:Arsenic pentoxide
13424:Zirconium dioxide
13326:Terbium(IV) oxide
13312:Tellurium dioxide
13228:Rhodium(IV) oxide
13102:Manganese dioxide
13074:Hafnium(IV) oxide
13032:Germanium dioxide
12875:Americium dioxide
12609:Nickel(III) oxide
12507:Indium(III) oxide
12405:Erbium(III) oxide
12337:Cobalt(III) oxide
12303:Cerium(III) oxide
12184:Antimony trioxide
12102:Yttrium(II) oxide
11985:Polonium monoxide
11935:Mercury(II) oxide
11808:Chlorine monoxide
11678:Thallium(I) oxide
11548:Dicarbon monoxide
11362:Lead(II,IV) oxide
11178:
11177:
10058:
10057:
9136:
9135:
9076:Ocean temperature
8608:978-1-13-361109-7
8555:978-0-521-33009-1
8423:978-0-86542-685-6
8385:978-0-85404-438-2
8358:978-0-08-037941-8
8332:978-1-4067-4789-8
8300:978-0-13-250882-7
8279:978-0-8053-6844-4
8256:978-0-495-39041-1
8171:, pp. 27–28.
8137:"Tetrahydropyran"
8084:10.1063/1.1749300
8038:1902 Encyclopedia
7610:10.1063/1.1340584
7494:978-0-321-91041-7
7382:(7185): 291–292.
7108:Reece et al. 2013
6977:, pp. 37–38.
6877:10.1149/1.1836121
6777:10.1063/1.1595053
6511:10.1063/1.5030640
6427:10.1063/1.1679903
6312:(24): 5355–5357.
6243:Perlman, Howard.
5853:978-0-9678550-9-7
5819:Reece et al. 2013
5790:Reece et al. 2013
5377:10.1021/ed070p612
5210:Adiabatic cooling
5033:Water (data page)
5018:Superheated water
4910:, is also called
4862:hydrogen monoxide
4846:hydrogen peroxide
4809:name of water is
4730:William Nicholson
4577:→ 4 AgF + 4 HF +
4438:metals, and some
4359:Organic chemistry
3772:hydrochloric acid
3538:neutron moderator
3515:Deuterium oxide,
3401:Acidity in nature
3373:methane clathrate
3350:mineral hydration
3203:
2801:hydrogen fluoride
2745:ultraviolet light
2703:quantum tunneling
2691:Quantum tunneling
2607:attractive forces
2579:calcium carbonate
2384:electronegativity
2343:Chemical polarity
2191:
2190:
1734:thermal expansion
1631:critical pressure
1514:of water has two
1325:Chemical compound
1323:
1322:
1279:Water (data page)
1259:Hydrogen fluoride
1223:Hydrogen peroxide
1218:Hydrogen polonide
1208:Hydrogen selenide
1174:Safety data sheet
1053:Gibbs free energy
920:Crystal structure
744:dimethylformamide
452:InChI=1S/H2O/h1H2
227:Interactive image
168:μ-Oxidodihydrogen
87:
86:
81: Hydrogen, H
17923:
17916:Greenhouse gases
17861:Oxygen compounds
16983:
16922:
16921:
16899:
16898:
16863:
16862:
16825:
16824:
16686:
16685:
16609:
16608:
16588:
16528:
16527:
16505:
16504:
16482:
16481:
16445:
16444:
16392:
16391:
16254:
16253:
16201:
16200:
16063:
16062:
16009:
16008:
15986:
15985:
15949:
15948:
15876:
15875:
15842:
15841:
15813:
15812:
15675:
15674:
15633:
15632:
15581:
15580:
15517:
15516:
15482:
15481:
15447:
15446:
15412:
15411:
15377:
15376:
15227:
15226:
15161:
15160:
15121:
15120:
15057:
15050:
15043:
15034:
15033:
15001:
14991:
14978:
14965:
14956:
14946:
14933:
14924:
14911:
14901:
14886:
14876:
14866:
14856:
14846:
14836:
14826:
14816:
14807:
14797:
14787:
14777:
14767:
14757:
14748:
14738:
14665:
14639:
14629:
14620:
14610:
14596:
14586:
14555:
14542:
14517:
14507:
14497:
14488:
14478:
14468:
14450:
14437:
14421:
14411:
14399:
14371:
14361:
14351:
14341:
14331:
14317:
14307:
14297:
14288:
14278:
14268:
14259:
14249:
14240:
14230:
14221:
14212:
14202:
14190:
14165:
14155:
14145:
14135:
14125:
14100:
14090:
14080:
14070:
14060:
14050:
14020:
14019:
14003:
13996:
13989:
13980:
13979:
13857:Osmium tetroxide
13725:Uranium trioxide
13655:Rhenium trioxide
13368:Titanium dioxide
13256:Selenium dioxide
13186:Polonium dioxide
13158:Platinum dioxide
13130:Nitrogen dioxide
13001:Curium(IV) oxide
12973:Chlorine dioxide
12959:Cerium(IV) oxide
12201:Arsenic trioxide
12062:Thorium monoxide
12045:Disulfur dioxide
12015:Silicon monoxide
11945:Nickel(II) oxide
11838:Copper(II) oxide
11828:Cobalt(II) oxide
11768:Bromine monoxide
11613:Mercury(I) oxide
11574:Gallium(I) oxide
11535:Caesium monoxide
11205:
11198:
11191:
11182:
11181:
11166:
11152:
11138:
11124:
11110:
11096:
11082:
11072:
11065:
11054:
10288:
10287:
10286:
10278:
10277:
10092:Oxygen compounds
10085:
10078:
10071:
10062:
10061:
10050:
10039:
10028:
10014:
10000:
9986:
9975:
9960:
9942:
9924:
9906:
9895:
9877:
9863:
9849:
9825:
9810:
9796:
9782:
9768:
9757:
9743:
9732:
9717:
9699:
9681:
9667:
9653:
9639:
9625:
9611:
9597:
9583:
9572:
9558:
9547:
9529:
9519:
9499:
9484:
9470:
9456:
9446:
9436:
9426:
9396:
9382:
9368:
9353:
9329:
9319:
9309:
9289:
9279:
9269:
9249:
9238:
9217:
9203:
9176:
9169:
9162:
9153:
9152:
9140:
9139:
9126:
9115:
9114:
9104:
9103:
9093:
8996:Asteroidal water
8984:Extraterrestrial
8773:
8766:
8759:
8750:
8749:
8712:
8662:
8660:
8658:
8635:
8633:World Scientific
8612:
8599:Cengage Learning
8597:(9th ed.).
8589:
8559:
8543:
8529:
8517:
8506:
8493:Campbell Biology
8487:
8485:
8484:
8451:
8442:
8440:
8434:. Archived from
8415:
8403:
8401:
8400:
8394:
8377:
8362:
8347:(2nd ed.).
8336:
8315:
8313:
8312:
8283:
8271:
8260:
8239:
8203:
8202:
8200:
8199:
8178:
8172:
8166:
8160:
8159:
8157:
8156:
8133:
8127:
8121:
8115:
8109:
8103:
8097:
8088:
8087:
8059:
8053:
8052:
8050:
8049:
8030:
8024:
8023:
8021:
8020:
8002:
7996:
7990:
7981:
7975:
7966:
7960:
7954:
7948:
7942:
7936:
7930:
7924:
7918:
7912:
7906:
7905:
7903:
7902:
7879:
7873:
7867:
7861:
7855:
7849:
7843:
7834:
7828:
7822:
7821:
7819:
7818:
7799:
7793:
7792:
7790:
7789:
7783:
7768:
7759:
7753:
7747:
7741:
7740:
7704:
7698:
7697:
7695:
7693:
7677:
7671:
7670:
7668:
7666:
7651:
7645:
7644:
7620:
7614:
7613:
7596:(7): 3174–3181.
7585:
7579:
7578:
7576:
7575:
7569:
7556:
7548:
7542:
7536:
7530:
7524:
7518:
7517:
7508:
7506:
7476:
7470:
7469:
7434:
7424:
7418:
7417:
7399:
7367:
7361:
7360:
7316:
7310:
7309:
7307:
7306:
7288:
7256:
7250:
7249:
7231:
7199:
7193:
7192:
7190:
7189:
7153:
7147:
7141:
7135:
7129:
7123:
7117:
7111:
7105:
7094:
7088:
7082:
7081:
7075:
7067:
7061:
7053:
7047:
7046:
7036:
6996:
6990:
6984:
6978:
6972:
6966:
6960:
6954:
6953:
6925:
6919:
6918:
6916:
6915:
6906:. Archived from
6895:
6889:
6888:
6860:
6851:
6848:
6842:
6841:
6839:
6838:
6832:
6821:
6813:
6807:
6801:
6795:
6794:
6792:
6791:
6785:
6754:
6745:
6739:
6738:
6736:
6735:
6717:
6715:10.1256/qj.04.94
6685:
6679:
6673:
6667:
6666:
6654:
6648:
6647:
6645:
6639:. Archived from
6634:
6623:
6617:
6616:
6614:
6612:
6606:
6595:
6585:
6579:
6573:
6564:
6563:
6561:
6559:
6540:
6534:
6533:
6531:
6530:
6474:
6468:
6467:
6465:
6464:
6440:
6431:
6430:
6402:
6396:
6395:
6393:
6392:
6373:
6362:
6356:
6350:
6344:
6338:
6337:
6326:10.1039/b108676f
6302:Loerting, Thomas
6298:
6292:
6291:
6273:
6264:
6263:
6261:
6260:
6240:
6225:
6224:
6222:
6221:
6191:
6185:
6184:
6182:
6181:
6175:
6169:. Archived from
6136:
6134:cond-mat/0203383
6118:
6109:
6103:
6097:
6086:
6085:
6077:
6075:
6060:
6054:
6048:
6042:
6036:
6030:
6029:
6023:
6022:
6016:
6009:
5997:
5991:
5990:
5971:10.1039/b611584e
5946:
5940:
5939:
5929:
5919:
5887:
5881:
5875:
5869:
5868:
5866:
5865:
5828:
5822:
5816:
5810:
5804:
5793:
5787:
5781:
5780:
5778:
5776:
5757:
5751:
5745:
5739:
5726:
5720:
5714:
5708:
5695:
5682:
5676:
5670:
5664:
5658:
5657:
5646:10.1063/1.555963
5632:(3): 1377–1381.
5621:
5615:
5614:
5586:
5580:
5579:
5577:
5576:
5557:
5551:
5545:
5539:
5533:
5527:
5521:
5515:
5514:
5512:
5511:
5495:
5484:
5478:
5472:
5471:
5469:
5467:
5441:
5435:
5434:
5414:
5403:
5402:
5400:
5399:
5393:
5354:
5345:
5334:
5333:
5331:
5329:
5310:
5304:
5303:
5298:
5296:
5287:. Archived from
5277:
5257:
5250:
5244:
5241:
5235:
5224:
5218:
5206:
5200:
5197:
5191:
5168:
5162:
5151:
5145:
5143:
5142:
5141:
5131:
5130:
5122:
5121:
5114:
5113:
5103:
5102:
5101:
5092:
5086:
5080:
5071:
5064:
4956:
4951:
4950:
4909:
4908:
4907:
4900:
4899:
4850:hydrogen sulfide
4734:Anthony Carlisle
4708:
4707:
4706:
4699:
4698:
4690:
4689:
4688:
4679:
4678:
4677:
4667:
4665:
4664:
4647:
4646:
4645:
4635:
4634:
4633:
4626:
4625:
4617:
4616:
4615:
4588:
4587:
4586:
4576:
4574:
4573:
4563:
4562:
4561:
4543:
4541:
4540:
4532:
4531:
4492:
4491:
4490:
4481:
4480:
4479:
4470:
4469:
4468:
4458:
4456:
4455:
4394:of proteins and
4390:of fats and the
4381:
4380:
4379:
4330:
4327:
4326:
4318:
4317:
4294:
4293:
4292:
4284:
4283:
4268:transition metal
4258:Ligand chemistry
4253:
4252:
4251:
4241:
4239:
4238:
4228:
4227:
4226:
4218:
4217:
4205:their basic pH:
4189:
4188:
4187:
4179:
4177:
4176:
4164:
4163:
4162:
4161:
4156:
4155:
4153:
4152:
4139:
4138:
4137:
4136:
4131:
4130:
4129:
4128:
4115:
4114:
4113:
4105:
4103:
4102:
4090:
4089:
4088:
4087:
4082:
4081:
4079:
4078:
4065:
4064:
4063:
4062:
4057:
4056:
4055:
4054:
4041:
4040:
4039:
4032:
4031:
4021:
4020:
4019:
4018:
4013:
4012:
4010:
4009:
3996:
3995:
3994:
3993:
3988:
3987:
3986:
3985:
3951:
3950:
3949:
3940:
3939:
3938:
3928:
3927:
3926:
3925:
3920:
3919:
3917:
3916:
3903:
3902:
3901:
3900:
3895:
3894:
3893:
3892:
3876:
3875:
3874:
3865:
3864:
3863:
3846:
3845:
3844:
3835:
3834:
3833:
3826:
3825:
3815:
3814:
3813:
3812:
3807:
3806:
3804:
3803:
3790:
3789:
3788:
3787:
3782:
3769:
3768:
3767:
3758:
3756:
3755:
3697:
3693:
3689:
3688:
3687:
3669:
3656:
3654:
3653:
3640:
3638:
3637:
3627:
3625:
3624:
3614:
3612:
3611:
3597:
3595:
3594:
3584:
3582:
3581:
3568:
3566:
3565:
3555:
3551:
3534:nuclear reactors
3527:
3525:
3524:
3511:
3509:
3508:
3500:
3498:
3497:
3489:
3487:
3486:
3478:
3476:
3475:
3459:
3457:
3456:
3396:
3394:
3393:
3385:
3384:
3328:
3326:
3325:
3320:
3315:
3314:
3313:
3312:
3292:
3291:
3290:
3289:
3280:
3279:
3262:
3261:
3260:
3247:
3246:
3245:
3214:
3212:
3211:
3206:
3204:
3201:
3196:
3195:
3191:
3190:
3175:
3174:
3173:
3172:
3171:
3170:
3149:
3148:
3147:
3146:
3145:
3136:
3135:
3119:
3114:
3113:
3112:
3078:
3077:
3076:
3067:
3066:
3065:
3048:
3046:
3045:
3040:
3035:
3034:
3033:
3032:
3012:
3011:
3010:
3009:
3000:
2999:
2982:
2981:
2980:
2952:
2951:
2950:
2941:
2940:
2939:
2932:
2931:
2921:
2919:
2918:
2860:sp hybridization
2773:visible spectrum
2737:near ultraviolet
2675:
2674:
2673:
2661:
2660:
2659:
2611:precipitated out
2566:Aqueous solution
2552:Capillary action
2434:room temperature
2431:
2429:
2428:
2418:
2416:
2415:
2396:hydrogen bonding
2332:
2327:proton conductor
2274:
2273:
2272:
2265:
2264:
2254:
2253:
2252:
2073:
2069:
2050:starting in 2019
1986:
1984:
1978:
1976:
1970:
1968:
1914:partial pressure
1679:hydrogen bonding
1556:hydrogen bonding
1537:absorption bands
1509:
1507:
1506:
1496:chemical formula
1475:
1474:
1473:
1464:
1463:
1462:
1444:
1443:
1442:
1431:
1430:
1429:
1347:room temperature
1339:
1307:
1301:
1298:
1297:
1203:Hydrogen sulfide
1153:
1146:
1139:
1124:
1064:
1042:
1015:
991:
976:Thermochemistry
881:Refractive index
627:
623:
621:
618:
611:
607:
605:
602:
594:
592:
586:
584:
581:
574:
570:
568:
565:
531:, colorless gas
508:
506:
505:
492:Chemical formula
432:
396:
385:
371:
349:Gmelin Reference
332:
321:
301:
281:
261:
229:
205:
150:Dihydrogen oxide
96:
80:
74:
49:
43:
36:
32:
17931:
17930:
17926:
17925:
17924:
17922:
17921:
17920:
17886:Water chemistry
17831:
17830:
17829:
17824:
17823:
17818:
17800:
17796:
17788:
17780:
17772:
17764:
17756:
17748:
17740:
17732:
17724:
17716:
17708:
17700:
17692:
17685:
17681:
17674:
17666:
17658:
17641:
17637:
17629:
17621:
17613:
17605:
17597:
17589:
17581:
17573:
17565:
17557:
17549:
17541:
17533:
17525:
17517:
17509:
17501:
17493:
17485:
17477:
17469:
17461:
17453:
17445:
17437:
17429:
17412:
17402:
17393:
17385:
17381:
17368:
17360:
17329:
17315:
17300:
17285:
17277:
17269:
17261:
17253:
17240:
17232:
17219:
17206:
17193:
17180:
17172:
17164:
17156:
17148:
17140:
17132:
17124:
17116:
17108:
17100:
17092:
17084:
17067:
17024:
17017:
17012:
17003:
16980:
16971:
16963:
16946:
16936:
16916:
16912:
16893:
16889:
16885:
16876:
16857:
16853:
16849:
16840:
16819:
16810:
16806:
16798:
16794:
16786:
16782:
16774:
16770:
16762:
16758:
16750:
16746:
16738:
16734:
16726:
16722:
16714:
16710:
16701:
16680:
16672:
16668:
16660:
16656:
16648:
16644:
16636:
16632:
16623:
16603:
16601:
16594:
16586:
16577:
16564:
16552:
16542:
16522:
16518:
16499:
16495:
16476:
16472:
16468:
16460:
16439:
16435:
16431:
16423:
16419:
16411:
16407:
16386:
16377:
16373:
16365:
16361:
16353:
16349:
16341:
16337:
16329:
16325:
16317:
16313:
16305:
16301:
16293:
16289:
16281:
16277:
16269:
16248:
16244:
16240:
16232:
16228:
16220:
16216:
16195:
16186:
16182:
16174:
16170:
16162:
16158:
16150:
16146:
16138:
16134:
16126:
16122:
16114:
16110:
16102:
16098:
16090:
16086:
16078:
16057:
16046:
16042:
16034:
16003:
15999:
15980:
15976:
15972:
15964:
15943:
15939:
15935:
15927:
15923:
15915:
15911:
15903:
15899:
15891:
15870:
15861:
15857:
15836:
15832:
15828:
15807:
15798:
15794:
15786:
15782:
15774:
15770:
15762:
15758:
15750:
15746:
15738:
15734:
15726:
15722:
15714:
15710:
15702:
15698:
15690:
15669:
15664:
15656:
15648:
15627:
15566:
15562:
15554:
15550:
15542:
15511:
15507:
15503:
15495:
15476:
15472:
15468:
15460:
15441:
15437:
15433:
15425:
15406:
15402:
15398:
15390:
15371:
15367:
15363:
15355:
15351:
15343:
15339:
15331:
15327:
15319:
15315:
15307:
15303:
15295:
15291:
15283:
15279:
15271:
15267:
15259:
15255:
15242:
15221:
15210:
15206:
15198:
15190:
15182:
15174:
15155:
15115:
15113:
15106:
15073:
15066:
15061:
15031:
15026:
15025:
15000:
14996:
14990:
14986:
14977:
14973:
14969:
14967:
14964:
14960:
14958:
14955:
14951:
14945:
14941:
14937:
14935:
14932:
14928:
14926:
14923:
14919:
14910:
14906:
14900:
14896:
14885:
14881:
14875:
14871:
14865:
14861:
14855:
14851:
14845:
14841:
14835:
14831:
14825:
14821:
14815:
14811:
14809:
14806:
14802:
14796:
14792:
14786:
14782:
14776:
14772:
14766:
14762:
14756:
14752:
14750:
14747:
14743:
14737:
14733:
14664:
14660:
14638:
14634:
14628:
14624:
14622:
14619:
14615:
14609:
14605:
14603:
14595:
14591:
14585:
14581:
14554:
14550:
14541:
14537:
14516:
14512:
14506:
14502:
14496:
14492:
14490:
14487:
14483:
14477:
14473:
14467:
14463:
14449:
14445:
14436:
14432:
14420:
14416:
14410:
14406:
14398:
14394:
14370:
14366:
14360:
14356:
14350:
14346:
14340:
14336:
14330:
14326:
14324:
14316:
14312:
14306:
14302:
14296:
14292:
14290:
14287:
14283:
14277:
14273:
14267:
14263:
14261:
14258:
14254:
14248:
14244:
14242:
14239:
14235:
14229:
14225:
14223:
14220:
14216:
14214:
14211:
14207:
14201:
14197:
14189:
14185:
14164:
14160:
14154:
14150:
14144:
14140:
14134:
14130:
14124:
14120:
14099:
14095:
14088:
14084:
14082:
14079:
14075:
14069:
14065:
14059:
14055:
14049:
14045:
14012:
14007:
13977:
13972:
13970:Category:Oxides
13966:oxidation state
13958:
13912:
13908:
13894:
13885:Xenon tetroxide
13880:
13866:
13852:
13831:
13827:
13823:
13810:
13806:
13793:
13789:
13776:
13772:
13752:
13748:
13734:
13720:
13706:
13692:
13683:Sulfur trioxide
13678:
13664:
13650:
13636:
13622:
13601:
13597:
13593:
13580:
13576:
13563:
13559:
13546:
13542:
13529:
13525:
13512:
13508:
13495:
13491:
13478:
13474:
13461:
13457:
13437:
13433:
13419:
13405:
13396:Uranium dioxide
13391:
13377:
13363:
13349:
13340:Thorium dioxide
13335:
13321:
13307:
13293:
13279:
13270:Silicon dioxide
13265:
13251:
13237:
13223:
13209:
13195:
13181:
13167:
13153:
13139:
13125:
13111:
13097:
13083:
13069:
13060:Iridium dioxide
13055:
13041:
13027:
13023:
13010:
12996:
12982:
12968:
12954:
12945:Carbon trioxide
12940:
12926:
12912:
12903:Bromine dioxide
12898:
12884:
12863:
12859:
12855:
12842:
12838:
12825:
12821:
12808:
12804:
12791:
12787:
12774:
12770:
12757:
12753:
12740:
12736:
12723:
12719:
12706:
12702:
12689:
12685:
12672:
12668:
12655:
12651:
12638:
12634:
12621:
12617:
12604:
12600:
12587:
12583:
12570:
12566:
12553:
12549:
12541:Lanthanum oxide
12536:
12532:
12524:Iron(III) oxide
12519:
12515:
12502:
12498:
12485:
12481:
12473:Gold(III) oxide
12468:
12464:
12451:
12447:
12434:
12430:
12417:
12413:
12400:
12396:
12383:
12379:
12366:
12362:
12349:
12345:
12332:
12328:
12315:
12311:
12298:
12294:
12281:
12277:
12264:
12260:
12247:
12243:
12230:
12226:
12213:
12209:
12196:
12192:
12179:
12175:
12162:
12158:
12150:Aluminium oxide
12145:
12141:
12121:
12057:
12053:
12035:Sulfur monoxide
12025:Strontium oxide
11915:Magnesium oxide
11895:Iodine monoxide
11860:
11856:
11798:Carbon monoxide
11758:Beryllium oxide
11716:
11712:
11699:
11686:
11673:
11660:
11647:
11639:Potassium oxide
11634:
11621:
11608:
11595:
11587:Iodine(I) oxide
11582:
11569:
11556:
11543:
11530:
11522:Copper(I) oxide
11517:
11497:
11493:
11489:
11476:
11472:
11459:
11455:
11442:
11438:
11425:
11421:
11408:
11404:
11391:
11387:
11374:
11370:
11357:
11353:
11340:
11336:
11323:
11319:
11306:
11302:
11289:
11285:
11272:
11268:
11260:Carbon suboxide
11255:
11251:
11238:
11234:
11214:
11209:
11179:
11174:
11164:
11160:
11155:
11150:
11146:
11141:
11136:
11132:
11127:
11122:
11118:
11113:
11108:
11104:
11099:
11094:
11090:
11085:
11080:
11075:
11068:
11062:
11057:
11052:
11048:
11043:
11039:
11026:
11022:
11013:
11005:
10997:
10993:
10985:
10981:
10973:
10969:
10960:
10952:
10939:
10935:
10927:
10919:
10911:
10907:
10899:
10895:
10887:
10874:
10870:
10857:
10853:
10845:
10841:
10832:
10824:
10820:
10812:
10808:
10800:
10792:
10784:
10780:
10772:
10768:
10760:
10756:
10748:
10744:
10736:
10732:
10719:
10715:
10706:
10693:
10685:
10681:
10672:
10663:
10654:
10645:
10637:
10624:
10620:
10611:
10603:
10599:
10591:
10587:
10574:
10570:
10562:
10558:
10550:
10546:
10533:
10529:
10521:
10513:
10509:
10501:
10497:
10489:
10485:
10477:
10469:
10465:
10457:
10453:
10440:
10436:
10428:
10424:
10416:
10412:
10404:
10400:
10392:
10388:
10379:
10371:
10363:
10359:
10351:
10347:
10339:
10326:
10313:
10309:
10301:
10285:
10282:
10281:
10280:
10276:
10273:
10272:
10271:
10269:
10264:
10260:
10252:
10248:
10240:
10232:
10228:
10220:
10212:
10208:
10190:
10186:
10178:
10174:
10166:
10162:
10154:
10150:
10142:
10138:
10130:
10122:
10118:
10110:
10106:
10094:
10089:
10059:
10054:
10048:
10044:
10037:
10033:
10027:
10023:
10019:
10013:
10009:
10005:
9999:
9995:
9991:
9984:
9980:
9973:
9969:
9965:
9959:
9955:
9951:
9947:
9941:
9937:
9933:
9929:
9923:
9919:
9915:
9911:
9904:
9900:
9894:
9890:
9886:
9882:
9876:
9872:
9868:
9862:
9858:
9854:
9848:
9844:
9840:
9823:
9819:
9815:
9809:
9805:
9801:
9795:
9791:
9787:
9781:
9777:
9773:
9766:
9762:
9756:
9752:
9748:
9741:
9737:
9730:
9726:
9722:
9716:
9712:
9708:
9704:
9698:
9694:
9690:
9686:
9680:
9676:
9672:
9666:
9662:
9658:
9652:
9648:
9644:
9638:
9634:
9630:
9624:
9620:
9616:
9610:
9606:
9602:
9596:
9592:
9588:
9581:
9577:
9571:
9567:
9563:
9556:
9552:
9546:
9542:
9538:
9534:
9528:
9524:
9518:
9514:
9498:
9494:
9483:
9479:
9475:
9469:
9465:
9461:
9455:
9451:
9445:
9441:
9435:
9431:
9425:
9421:
9395:
9391:
9387:
9381:
9377:
9373:
9367:
9363:
9359:
9355:
9352:
9348:
9344:
9328:
9324:
9318:
9314:
9308:
9304:
9288:
9284:
9278:
9274:
9268:
9264:
9247:
9243:
9236:
9232:
9216:
9212:
9208:
9202:
9198:
9194:
9185:
9180:
9145:
9137:
9132:
9080:
9047:
8979:
8906:
8865:
8825:
8813:
8782:
8777:
8729:surface tension
8715:Calculation of
8701:
8656:
8654:
8645:
8642:
8620:
8618:Further reading
8615:
8609:
8586:
8556:
8526:
8503:
8482:
8480:
8473:
8438:
8424:
8413:
8398:
8396:
8392:
8386:
8375:
8359:
8333:
8310:
8308:
8301:
8280:
8257:
8236:
8211:
8206:
8197:
8195:
8180:
8179:
8175:
8167:
8163:
8154:
8152:
8135:
8134:
8130:
8122:
8118:
8110:
8106:
8098:
8091:
8060:
8056:
8047:
8045:
8032:
8031:
8027:
8018:
8016:
8011:. August 2003.
8004:
8003:
7999:
7991:
7984:
7976:
7969:
7961:
7957:
7949:
7945:
7937:
7933:
7925:
7921:
7913:
7909:
7900:
7898:
7881:
7880:
7876:
7868:
7864:
7856:
7852:
7844:
7837:
7829:
7825:
7816:
7814:
7801:
7800:
7796:
7787:
7785:
7781:
7766:
7760:
7756:
7748:
7744:
7705:
7701:
7691:
7689:
7678:
7674:
7664:
7662:
7653:
7652:
7648:
7621:
7617:
7586:
7582:
7573:
7571:
7567:
7554:
7550:
7549:
7545:
7537:
7533:
7525:
7521:
7504:
7502:
7495:
7477:
7473:
7462:
7458:
7454:
7447:
7425:
7421:
7397:10.1038/452291a
7368:
7364:
7327:(33): 8710–23.
7317:
7313:
7304:
7302:
7257:
7253:
7200:
7196:
7187:
7185:
7170:10.2172/6642535
7154:
7150:
7142:
7138:
7130:
7126:
7118:
7114:
7106:
7097:
7089:
7085:
7069:
7068:
7059:
7055:
7054:
7050:
6997:
6993:
6985:
6981:
6973:
6969:
6961:
6957:
6926:
6922:
6913:
6911:
6896:
6892:
6861:
6854:
6849:
6845:
6836:
6834:
6830:
6819:
6815:
6814:
6810:
6802:
6798:
6789:
6787:
6783:
6752:
6746:
6742:
6733:
6731:
6686:
6682:
6674:
6670:
6655:
6651:
6643:
6632:
6624:
6620:
6610:
6608:
6604:
6593:
6587:
6586:
6582:
6574:
6567:
6557:
6555:
6542:
6541:
6537:
6528:
6526:
6482:
6475:
6471:
6462:
6460:
6441:
6434:
6403:
6399:
6390:
6388:
6375:
6374:
6365:
6357:
6353:
6345:
6341:
6299:
6295:
6288:
6274:
6267:
6258:
6256:
6245:"Water Density"
6241:
6228:
6219:
6217:
6210:
6192:
6188:
6179:
6177:
6173:
6116:
6110:
6106:
6098:
6089:
6073:
6071:
6062:
6061:
6057:
6049:
6045:
6037:
6033:
6020:
6018:
6014:
6007:
5998:
5994:
5947:
5943:
5888:
5884:
5876:
5872:
5863:
5861:
5854:
5838:. IUPAC. 2009.
5830:
5829:
5825:
5817:
5813:
5805:
5796:
5788:
5784:
5774:
5772:
5759:
5758:
5754:
5746:
5742:
5737:Wayback Machine
5727:
5723:
5715:
5711:
5696:
5685:
5677:
5673:
5665:
5661:
5622:
5618:
5587:
5583:
5574:
5572:
5559:
5558:
5554:
5546:
5542:
5534:
5530:
5522:
5518:
5509:
5507:
5496:
5487:
5479:
5475:
5465:
5463:
5456:10.18434/T4D303
5442:
5438:
5415:
5406:
5397:
5395:
5391:
5352:
5346:
5337:
5327:
5325:
5318:Merriam-Webster
5312:
5311:
5307:
5294:
5292:
5279:
5278:
5274:
5270:
5265:
5260:
5251:
5247:
5242:
5238:
5225:
5221:
5207:
5203:
5198:
5194:
5169:
5165:
5152:
5148:
5140:
5135:
5134:
5133:
5129:
5126:
5125:
5124:
5120:
5118:
5117:
5116:
5112:
5109:
5108:
5107:
5105:
5100:
5098:
5097:
5096:
5094:
5093:
5089:
5081:
5074:
5069:
5065:
5061:
5057:
5052:
4952:
4945:
4942:
4932:, some form of
4923:
4918:Water substance
4906:
4904:
4903:
4902:
4898:
4896:
4895:
4894:
4892:
4885:hydroxylic acid
4854:deuterium oxide
4835:tetrahydropyran
4803:
4761:. Notably, the
4722:Henry Cavendish
4719:
4705:
4703:
4702:
4701:
4697:
4695:
4694:
4693:
4692:
4687:
4685:
4684:
4683:
4681:
4676:
4673:
4672:
4671:
4669:
4663:
4660:
4659:
4658:
4656:
4644:
4641:
4640:
4639:
4637:
4632:
4630:
4629:
4628:
4624:
4622:
4621:
4620:
4619:
4614:
4612:
4611:
4610:
4608:
4601:
4595:
4585:
4582:
4581:
4580:
4578:
4572:
4569:
4568:
4567:
4565:
4560:
4557:
4556:
4555:
4553:
4539:
4536:
4535:
4534:
4530:
4527:
4526:
4525:
4523:
4489:
4487:
4486:
4485:
4483:
4478:
4476:
4475:
4474:
4472:
4467:
4464:
4463:
4462:
4460:
4454:
4451:
4450:
4449:
4447:
4428:oxidation state
4424:
4407:
4396:polysaccharides
4378:
4376:
4375:
4374:
4372:
4361:
4349:
4345:
4325:
4322:
4321:
4320:
4316:
4313:
4312:
4311:
4308:
4291:
4288:
4287:
4286:
4282:
4279:
4278:
4277:
4275:
4260:
4250:
4247:
4246:
4245:
4243:
4237:
4234:
4233:
4232:
4230:
4225:
4222:
4221:
4220:
4216:
4213:
4212:
4211:
4209:
4186:
4183:
4182:
4181:
4175:
4172:
4171:
4170:
4168:
4166:
4159:
4158:
4157:
4151:
4148:
4147:
4146:
4144:
4143:
4142:
4141:
4134:
4133:
4132:
4127:
4125:
4124:
4123:
4121:
4120:
4119:
4118:
4112:
4109:
4108:
4107:
4101:
4098:
4097:
4096:
4094:
4092:
4085:
4084:
4083:
4077:
4074:
4073:
4072:
4070:
4069:
4068:
4067:
4060:
4059:
4058:
4053:
4051:
4050:
4049:
4047:
4046:
4045:
4044:
4038:
4036:
4035:
4034:
4030:
4027:
4026:
4025:
4023:
4016:
4015:
4014:
4008:
4005:
4004:
4003:
4001:
4000:
3999:
3998:
3991:
3990:
3989:
3984:
3982:
3981:
3980:
3978:
3977:
3976:
3975:
3948:
3946:
3945:
3944:
3942:
3937:
3934:
3933:
3932:
3930:
3923:
3922:
3921:
3915:
3912:
3911:
3910:
3908:
3907:
3906:
3905:
3898:
3897:
3896:
3891:
3888:
3887:
3886:
3884:
3883:
3882:
3881:
3873:
3871:
3870:
3869:
3867:
3862:
3859:
3858:
3857:
3855:
3843:
3841:
3840:
3839:
3837:
3832:
3830:
3829:
3828:
3824:
3821:
3820:
3819:
3817:
3810:
3809:
3808:
3802:
3799:
3798:
3797:
3795:
3794:
3793:
3792:
3785:
3784:
3783:
3780:
3779:
3778:
3766:
3764:
3763:
3762:
3760:
3754:
3752:
3751:
3750:
3747:
3728:
3723:
3695:
3691:
3686:
3683:
3682:
3681:
3679:
3676:
3667:
3652:
3649:
3648:
3647:
3645:
3636:
3633:
3632:
3631:
3629:
3623:
3620:
3619:
3618:
3616:
3610:
3607:
3606:
3605:
3603:
3602:coefficient of
3593:
3590:
3589:
3588:
3586:
3580:
3577:
3576:
3575:
3573:
3564:
3561:
3560:
3559:
3557:
3553:
3549:
3523:
3520:
3519:
3518:
3516:
3507:
3505:
3504:
3503:
3502:
3496:
3494:
3493:
3492:
3491:
3485:
3483:
3482:
3481:
3480:
3474:
3472:
3471:
3470:
3469:
3455:
3453:
3452:
3451:
3450:
3423:
3403:
3392:
3389:
3388:
3387:
3383:
3380:
3379:
3378:
3376:
3358:Portland cement
3334:
3308:
3304:
3300:
3299:
3285:
3281:
3275:
3271:
3270:
3269:
3256:
3255:
3251:
3238:
3237:
3233:
3231:
3228:
3227:
3225:
3221:
3197:
3186:
3182:
3181:
3180:
3166:
3162:
3158:
3157:
3153:
3141:
3137:
3131:
3127:
3126:
3125:
3121:
3120:
3118:
3105:
3104:
3100:
3098:
3095:
3094:
3075:
3073:
3072:
3071:
3069:
3064:
3062:
3061:
3060:
3058:
3028:
3024:
3020:
3019:
3005:
3001:
2995:
2991:
2990:
2989:
2976:
2975:
2971:
2969:
2966:
2965:
2949:
2947:
2946:
2945:
2943:
2938:
2936:
2935:
2934:
2930:
2927:
2926:
2925:
2923:
2917:
2914:
2913:
2912:
2910:
2895:self-ionization
2891:
2885:
2883:Self-ionization
2880:
2848:
2842:
2803:, ammonia, and
2781:
2765:Microwave ovens
2729:
2723:
2699:
2693:
2672:
2670:
2669:
2668:
2666:
2658:
2656:
2655:
2654:
2652:
2568:
2562:
2554:
2546:surface tension
2522:
2520:Surface tension
2501:Surface tension
2489:Irving Langmuir
2446:
2427:
2424:
2423:
2422:
2420:
2414:
2411:
2410:
2409:
2407:
2345:
2339:
2330:
2295:reverse osmosis
2271:
2269:
2268:
2267:
2263:
2260:
2259:
2258:
2256:
2251:
2249:
2248:
2247:
2245:
2230:
2225:
2196:
2154:
2108:
2092:
2026:
2015:
2009:
1982:
1980:
1974:
1972:
1966:
1964:
1961:compressibility
1957:
1955:Compressibility
1944:
1938:
1936:Vapour pressure
1920:, and creating
1879:
1873:
1859:
1846:brine rejection
1806:surface density
1797:
1717:
1655:
1602:are known. The
1590:crystals, like
1564:
1505:
1502:
1501:
1500:
1498:
1488:
1482:
1472:
1470:
1469:
1468:
1466:
1461:
1459:
1458:
1457:
1455:
1448:self-ionization
1441:
1439:
1438:
1437:
1435:
1428:
1426:
1425:
1424:
1422:
1379:carbon monoxide
1337:
1333:
1326:
1319:
1314:
1313:
1312: ?)
1303:
1299:
1295:
1291:
1268:
1237:
1227:
1196:
1158:
1157:
1156:
1155:
1148:
1141:
1134:
1130:
1122:
1101:
1096:
1090:
1065:
1059:
1055:
1043:
1040:
1034:
1030:
1027:
1026:Std enthalpy of
1016:
1013:
1006:
1003:
992:
985:
965:
951:
949:Molecular shape
942:
936:
922:
891:
889:
874:0.6065 W/(m·K)
863:
846:
842:
822:
804:
770:dichloromethane
759:
748:dimethoxyethane
732:tetrahydrofuran
701:
683:
636:
625:
619:
616:
614:
609:
603:
600:
598:
590:
588:
582:
579:
577:
572:
566:
563:
561:
554:Liquid (1 atm,
504:
501:
500:
499:
497:
494:
480:
477:
472:
471:
460:
457:
456:
453:
447:
446:
435:
415:
399:
386:
374:
351:
342:
324:
304:
284:
264:
241:
232:
219:
208:
195:
181:
180:
141:Hydroxylic acid
125:
124:
113:
112:
83:
82:
78:
76:
75: Oxygen, O
72:
31:
24:
17:
12:
11:
5:
17929:
17919:
17918:
17913:
17908:
17903:
17898:
17893:
17888:
17883:
17878:
17873:
17868:
17863:
17858:
17853:
17848:
17846:Forms of water
17843:
17826:
17825:
17820:
17819:
17817:
17816:
17810:
17808:
17802:
17801:
17799:
17798:
17794:
17790:
17786:
17782:
17778:
17774:
17770:
17766:
17762:
17758:
17754:
17750:
17746:
17742:
17738:
17734:
17730:
17726:
17722:
17718:
17714:
17710:
17706:
17702:
17698:
17694:
17690:
17686:
17683:
17679:
17676:
17672:
17668:
17664:
17660:
17656:
17651:
17649:
17643:
17642:
17640:
17639:
17635:
17631:
17627:
17623:
17619:
17615:
17611:
17607:
17603:
17599:
17595:
17591:
17587:
17583:
17579:
17575:
17571:
17567:
17563:
17559:
17555:
17551:
17547:
17543:
17539:
17535:
17531:
17527:
17523:
17519:
17515:
17511:
17507:
17503:
17499:
17495:
17491:
17487:
17483:
17479:
17475:
17471:
17467:
17463:
17459:
17455:
17451:
17447:
17443:
17439:
17435:
17431:
17427:
17422:
17420:
17414:
17413:
17411:
17410:
17400:
17395:
17391:
17387:
17383:
17379:
17375:
17370:
17366:
17362:
17358:
17354:
17342:
17337:
17327:
17322:
17311:
17307:
17296:
17292:
17287:
17283:
17279:
17275:
17271:
17267:
17263:
17259:
17255:
17251:
17247:
17242:
17238:
17234:
17230:
17226:
17221:
17217:
17213:
17208:
17204:
17200:
17195:
17191:
17187:
17182:
17178:
17174:
17170:
17166:
17162:
17158:
17154:
17150:
17146:
17142:
17138:
17134:
17130:
17126:
17122:
17118:
17114:
17110:
17106:
17102:
17098:
17094:
17090:
17086:
17082:
17077:
17075:
17069:
17068:
17066:
17054:
17049:
17044:
17039:
17034:
17029:
17027:
17019:
17018:
17016:
17015:
17010:
17006:
17001:
16997:
16992:
16987:
16978:
16973:
16969:
16965:
16961:
16957:
16951:
16948:
16947:
16945:
16944:
16934:
16928:
16926:
16918:
16917:
16915:
16914:
16910:
16905:
16903:
16895:
16894:
16892:
16891:
16887:
16883:
16879:
16874:
16869:
16867:
16859:
16858:
16856:
16855:
16851:
16847:
16843:
16838:
16833:
16831:
16821:
16820:
16818:
16817:
16812:
16808:
16804:
16800:
16796:
16792:
16788:
16784:
16780:
16776:
16772:
16768:
16764:
16760:
16756:
16752:
16748:
16744:
16740:
16736:
16732:
16728:
16724:
16720:
16716:
16712:
16708:
16704:
16699:
16694:
16692:
16682:
16681:
16679:
16674:
16670:
16666:
16662:
16658:
16654:
16650:
16646:
16642:
16638:
16634:
16630:
16626:
16621:
16617:
16615:
16606:
16602:chalcogenides
16596:
16595:
16593:
16592:
16584:
16579:
16575:
16571:
16566:
16562:
16557:
16554:
16553:
16551:
16550:
16540:
16534:
16532:
16524:
16523:
16521:
16520:
16516:
16511:
16509:
16501:
16500:
16498:
16497:
16493:
16488:
16486:
16478:
16477:
16475:
16474:
16470:
16466:
16462:
16458:
16453:
16451:
16441:
16440:
16438:
16437:
16433:
16429:
16425:
16421:
16417:
16413:
16409:
16405:
16400:
16398:
16388:
16387:
16385:
16384:
16379:
16375:
16371:
16367:
16363:
16359:
16355:
16351:
16347:
16343:
16339:
16335:
16331:
16327:
16323:
16319:
16315:
16311:
16307:
16303:
16299:
16295:
16291:
16287:
16283:
16279:
16275:
16271:
16267:
16262:
16260:
16250:
16249:
16247:
16246:
16242:
16238:
16234:
16230:
16226:
16222:
16218:
16214:
16209:
16207:
16197:
16196:
16194:
16193:
16188:
16184:
16180:
16176:
16172:
16168:
16164:
16160:
16156:
16152:
16148:
16144:
16140:
16136:
16132:
16128:
16124:
16120:
16116:
16112:
16108:
16104:
16100:
16096:
16092:
16088:
16084:
16080:
16076:
16071:
16069:
16060:
16052:
16051:
16048:
16047:
16045:
16044:
16040:
16036:
16032:
16028:
16022:
16020:
16005:
16004:
16002:
16001:
15997:
15992:
15990:
15982:
15981:
15979:
15978:
15974:
15970:
15966:
15962:
15957:
15955:
15945:
15944:
15942:
15941:
15937:
15933:
15929:
15925:
15921:
15917:
15913:
15909:
15905:
15901:
15897:
15893:
15889:
15884:
15882:
15872:
15871:
15869:
15868:
15863:
15859:
15855:
15850:
15848:
15838:
15837:
15835:
15834:
15830:
15826:
15821:
15819:
15809:
15808:
15806:
15805:
15800:
15796:
15792:
15788:
15784:
15780:
15776:
15772:
15768:
15764:
15760:
15756:
15752:
15748:
15744:
15740:
15736:
15732:
15728:
15724:
15720:
15716:
15712:
15708:
15704:
15700:
15696:
15692:
15688:
15683:
15681:
15671:
15670:
15668:
15667:
15662:
15658:
15654:
15650:
15646:
15642:
15636:
15629:
15628:
15626:
15625:
15620:
15615:
15610:
15605:
15600:
15595:
15589:
15587:
15578:
15572:
15571:
15568:
15567:
15565:
15564:
15560:
15556:
15552:
15548:
15544:
15540:
15536:
15530:
15528:
15513:
15512:
15510:
15509:
15505:
15501:
15497:
15493:
15488:
15486:
15478:
15477:
15475:
15474:
15470:
15466:
15462:
15458:
15453:
15451:
15443:
15442:
15440:
15439:
15435:
15431:
15427:
15423:
15418:
15416:
15408:
15407:
15405:
15404:
15400:
15396:
15392:
15388:
15383:
15381:
15373:
15372:
15370:
15369:
15365:
15361:
15357:
15353:
15349:
15345:
15341:
15337:
15333:
15329:
15325:
15321:
15317:
15313:
15309:
15305:
15301:
15297:
15293:
15289:
15285:
15281:
15277:
15273:
15269:
15265:
15261:
15257:
15253:
15249:
15244:
15240:
15235:
15233:
15224:
15216:
15215:
15212:
15211:
15209:
15208:
15204:
15200:
15196:
15192:
15188:
15184:
15180:
15176:
15172:
15167:
15165:
15157:
15156:
15154:
15153:
15148:
15143:
15138:
15133:
15127:
15125:
15118:
15116:earth hydrides
15108:
15107:
15105:
15104:
15099:
15094:
15089:
15084:
15078:
15076:
15068:
15067:
15060:
15059:
15052:
15045:
15037:
15028:
15027:
15022:
15021:
15018:
15015:
15012:
15009:
15006:
15003:
14998:
14993:
14988:
14983:
14980:
14975:
14971:
14962:
14953:
14948:
14943:
14939:
14930:
14921:
14916:
14913:
14908:
14903:
14898:
14893:
14889:
14888:
14883:
14878:
14873:
14868:
14863:
14858:
14853:
14848:
14843:
14838:
14833:
14828:
14823:
14818:
14813:
14804:
14799:
14794:
14789:
14784:
14779:
14774:
14769:
14764:
14759:
14754:
14745:
14740:
14735:
14730:
14726:
14725:
14722:
14719:
14718:
14715:
14712:
14709:
14706:
14703:
14700:
14697:
14694:
14691:
14688:
14685:
14682:
14679:
14676:
14673:
14670:
14667:
14662:
14657:
14651:
14650:
14647:
14644:
14641:
14636:
14631:
14626:
14617:
14612:
14607:
14598:
14593:
14588:
14583:
14578:
14575:
14572:
14569:
14566:
14563:
14560:
14557:
14552:
14547:
14544:
14539:
14534:
14528:
14527:
14524:
14519:
14514:
14509:
14504:
14499:
14494:
14485:
14480:
14475:
14470:
14465:
14460:
14455:
14452:
14447:
14442:
14439:
14434:
14429:
14426:
14423:
14418:
14413:
14408:
14403:
14401:
14396:
14391:
14385:
14384:
14381:
14376:
14373:
14368:
14363:
14358:
14353:
14348:
14343:
14338:
14333:
14328:
14319:
14314:
14309:
14304:
14299:
14294:
14285:
14280:
14275:
14270:
14265:
14256:
14251:
14246:
14237:
14232:
14227:
14218:
14209:
14204:
14199:
14194:
14192:
14187:
14182:
14176:
14175:
14172:
14167:
14162:
14157:
14152:
14147:
14142:
14137:
14132:
14127:
14122:
14117:
14111:
14110:
14107:
14102:
14097:
14092:
14086:
14077:
14072:
14067:
14062:
14057:
14052:
14047:
14042:
14036:
14035:
14032:
14030:
14028:
14026:
14018:
14017:
14014:
14013:
14006:
14005:
13998:
13991:
13983:
13974:
13973:
13963:
13960:
13959:
13957:
13956:
13951:
13946:
13941:
13936:
13931:
13926:
13920:
13918:
13914:
13913:
13911:
13910:
13906:
13896:
13892:
13882:
13878:
13868:
13864:
13854:
13850:
13839:
13837:
13833:
13832:
13830:
13829:
13825:
13821:
13812:
13808:
13804:
13795:
13791:
13787:
13778:
13774:
13770:
13760:
13758:
13754:
13753:
13751:
13750:
13746:
13739:Xenon trioxide
13736:
13732:
13722:
13718:
13708:
13704:
13694:
13690:
13680:
13676:
13666:
13662:
13652:
13648:
13638:
13634:
13624:
13620:
13609:
13607:
13603:
13602:
13600:
13599:
13595:
13591:
13582:
13578:
13574:
13565:
13561:
13557:
13548:
13544:
13540:
13531:
13527:
13523:
13514:
13510:
13506:
13497:
13493:
13489:
13480:
13476:
13472:
13463:
13459:
13455:
13445:
13443:
13439:
13438:
13436:
13435:
13431:
13421:
13417:
13407:
13403:
13393:
13389:
13379:
13375:
13365:
13361:
13351:
13347:
13337:
13333:
13323:
13319:
13309:
13305:
13295:
13291:
13284:Sulfur dioxide
13281:
13277:
13267:
13263:
13253:
13249:
13239:
13235:
13225:
13221:
13211:
13207:
13197:
13193:
13183:
13179:
13169:
13165:
13155:
13151:
13144:Osmium dioxide
13141:
13137:
13127:
13123:
13113:
13109:
13099:
13095:
13085:
13081:
13071:
13067:
13057:
13053:
13046:Iodine dioxide
13043:
13039:
13029:
13025:
13021:
13012:
13008:
12998:
12994:
12984:
12980:
12970:
12966:
12956:
12952:
12942:
12938:
12931:Carbon dioxide
12928:
12924:
12914:
12910:
12900:
12896:
12886:
12882:
12871:
12869:
12865:
12864:
12862:
12861:
12857:
12853:
12844:
12840:
12836:
12827:
12823:
12819:
12810:
12806:
12802:
12793:
12789:
12785:
12776:
12772:
12768:
12759:
12755:
12751:
12742:
12738:
12734:
12725:
12721:
12717:
12711:Scandium oxide
12708:
12704:
12700:
12691:
12687:
12683:
12674:
12670:
12666:
12657:
12653:
12649:
12640:
12636:
12632:
12623:
12619:
12615:
12606:
12602:
12598:
12589:
12585:
12581:
12572:
12568:
12564:
12555:
12551:
12547:
12538:
12534:
12530:
12521:
12517:
12513:
12504:
12500:
12496:
12487:
12483:
12479:
12470:
12466:
12462:
12453:
12449:
12445:
12436:
12432:
12428:
12419:
12415:
12411:
12402:
12398:
12394:
12385:
12381:
12377:
12368:
12364:
12360:
12351:
12347:
12343:
12334:
12330:
12326:
12317:
12313:
12309:
12300:
12296:
12292:
12283:
12279:
12275:
12266:
12262:
12258:
12252:Boron trioxide
12249:
12245:
12241:
12232:
12228:
12224:
12215:
12211:
12207:
12198:
12194:
12190:
12181:
12177:
12173:
12164:
12160:
12156:
12147:
12143:
12139:
12129:
12127:
12123:
12122:
12120:
12119:
12109:
12099:
12089:
12079:
12069:
12059:
12055:
12051:
12042:
12032:
12022:
12012:
12002:
11992:
11982:
11972:
11962:
11952:
11942:
11932:
11922:
11912:
11905:Lead(II) oxide
11902:
11892:
11885:Iron(II) oxide
11882:
11872:
11862:
11858:
11854:
11845:
11835:
11825:
11815:
11805:
11795:
11785:
11775:
11765:
11755:
11745:
11735:
11724:
11722:
11718:
11717:
11715:
11714:
11710:
11701:
11697:
11688:
11684:
11675:
11671:
11662:
11658:
11652:Rubidium oxide
11649:
11645:
11636:
11632:
11623:
11619:
11610:
11606:
11597:
11593:
11584:
11580:
11571:
11567:
11558:
11554:
11545:
11541:
11532:
11528:
11519:
11515:
11505:
11503:
11499:
11498:
11496:
11495:
11491:
11487:
11478:
11474:
11470:
11461:
11457:
11453:
11444:
11440:
11436:
11427:
11423:
11419:
11410:
11406:
11402:
11393:
11389:
11385:
11376:
11372:
11368:
11359:
11355:
11351:
11342:
11338:
11334:
11325:
11321:
11317:
11308:
11304:
11300:
11291:
11287:
11283:
11274:
11270:
11266:
11257:
11253:
11249:
11243:Boron suboxide
11240:
11236:
11232:
11222:
11220:
11216:
11215:
11208:
11207:
11200:
11193:
11185:
11176:
11175:
11173:
11172:
11167:
11162:
11158:
11153:
11148:
11144:
11139:
11134:
11130:
11125:
11120:
11116:
11111:
11106:
11102:
11097:
11092:
11088:
11083:
11078:
11073:
11066:
11060:
11055:
11050:
11046:
11041:
11037:
11033:
11028:
11024:
11020:
11016:
11011:
11007:
11003:
10999:
10995:
10991:
10987:
10983:
10979:
10975:
10971:
10967:
10963:
10958:
10954:
10950:
10946:
10941:
10937:
10933:
10929:
10925:
10921:
10917:
10913:
10909:
10905:
10901:
10897:
10893:
10889:
10885:
10881:
10876:
10872:
10868:
10864:
10859:
10855:
10851:
10847:
10843:
10839:
10835:
10830:
10826:
10822:
10818:
10814:
10810:
10806:
10802:
10798:
10794:
10790:
10786:
10782:
10778:
10774:
10770:
10766:
10762:
10758:
10754:
10750:
10746:
10742:
10738:
10734:
10730:
10726:
10721:
10717:
10713:
10709:
10704:
10700:
10695:
10691:
10687:
10683:
10679:
10675:
10670:
10666:
10661:
10657:
10652:
10648:
10643:
10639:
10635:
10631:
10626:
10622:
10618:
10614:
10609:
10605:
10601:
10597:
10593:
10589:
10585:
10581:
10576:
10572:
10568:
10564:
10560:
10556:
10552:
10548:
10544:
10540:
10535:
10531:
10527:
10523:
10519:
10515:
10511:
10507:
10503:
10499:
10495:
10491:
10487:
10483:
10479:
10475:
10471:
10467:
10463:
10459:
10455:
10451:
10447:
10442:
10438:
10434:
10430:
10426:
10422:
10418:
10414:
10410:
10406:
10402:
10398:
10394:
10390:
10386:
10382:
10377:
10373:
10369:
10365:
10361:
10357:
10353:
10349:
10345:
10341:
10337:
10333:
10328:
10324:
10320:
10315:
10311:
10307:
10303:
10299:
10295:
10290:
10283:
10274:
10266:
10262:
10258:
10254:
10250:
10246:
10242:
10238:
10234:
10230:
10226:
10222:
10218:
10214:
10210:
10206:
10202:
10197:
10192:
10188:
10184:
10180:
10176:
10172:
10168:
10164:
10160:
10156:
10152:
10148:
10144:
10140:
10136:
10132:
10128:
10124:
10120:
10116:
10112:
10108:
10104:
10099:
10096:
10095:
10088:
10087:
10080:
10073:
10065:
10056:
10055:
10053:
10052:
10046:
10041:
10035:
10030:
10025:
10021:
10016:
10011:
10007:
10002:
9997:
9993:
9988:
9982:
9977:
9971:
9967:
9962:
9957:
9953:
9949:
9944:
9939:
9935:
9931:
9926:
9921:
9917:
9913:
9908:
9902:
9897:
9892:
9888:
9884:
9879:
9874:
9870:
9865:
9860:
9856:
9851:
9846:
9842:
9837:
9832:
9827:
9821:
9817:
9812:
9807:
9803:
9798:
9793:
9789:
9784:
9779:
9775:
9770:
9764:
9759:
9754:
9750:
9745:
9739:
9734:
9728:
9724:
9719:
9714:
9710:
9706:
9701:
9696:
9692:
9688:
9683:
9678:
9674:
9669:
9664:
9660:
9655:
9650:
9646:
9641:
9636:
9632:
9627:
9622:
9618:
9613:
9608:
9604:
9599:
9594:
9590:
9585:
9579:
9574:
9569:
9565:
9560:
9554:
9549:
9544:
9540:
9536:
9531:
9526:
9521:
9516:
9511:
9506:
9501:
9496:
9491:
9486:
9481:
9477:
9472:
9467:
9463:
9458:
9453:
9448:
9443:
9438:
9433:
9428:
9423:
9418:
9413:
9408:
9403:
9398:
9393:
9389:
9384:
9379:
9375:
9370:
9365:
9361:
9357:
9350:
9346:
9341:
9336:
9331:
9326:
9321:
9316:
9311:
9306:
9301:
9296:
9291:
9286:
9281:
9276:
9271:
9266:
9261:
9256:
9251:
9245:
9240:
9234:
9229:
9224:
9219:
9214:
9210:
9205:
9200:
9196:
9190:
9187:
9186:
9179:
9178:
9171:
9164:
9156:
9150:
9147:
9146:
9134:
9133:
9131:
9130:
9119:
9108:
9097:
9085:
9082:
9081:
9079:
9078:
9073:
9072:
9071:
9066:
9059:Stratification
9055:
9053:
9049:
9048:
9046:
9045:
9044:
9043:
9038:
9033:
9028:
9020:
9019:
9018:
9013:
9008:
9003:
8998:
8987:
8985:
8981:
8980:
8978:
8977:
8972:
8971:
8970:
8965:
8955:
8950:
8945:
8944:
8943:
8938:
8928:
8923:
8917:
8915:
8908:
8907:
8905:
8904:
8899:
8894:
8889:
8884:
8879:
8873:
8871:
8867:
8866:
8864:
8863:
8862:
8861:
8851:
8846:
8841:
8835:
8833:
8827:
8826:
8816:
8814:
8812:
8811:
8806:
8801:
8796:
8790:
8788:
8784:
8783:
8776:
8775:
8768:
8761:
8753:
8747:
8746:
8737:
8732:
8721:liquid density
8717:vapor pressure
8713:
8699:
8674:
8669:
8663:
8641:
8640:External links
8638:
8637:
8636:
8619:
8616:
8614:
8613:
8607:
8590:
8585:978-3527306732
8584:
8560:
8554:
8530:
8525:978-0471927266
8524:
8507:
8502:978-0321775658
8501:
8488:
8472:978-0849304842
8471:
8452:
8443:
8441:on 2011-07-26.
8422:
8404:
8384:
8363:
8357:
8337:
8331:
8325:. Read Books.
8316:
8299:
8284:
8278:
8261:
8255:
8240:
8235:978-1461544852
8234:
8212:
8210:
8207:
8205:
8204:
8173:
8161:
8128:
8116:
8104:
8089:
8054:
8025:
7997:
7995:, p. 601.
7982:
7980:, p. 866.
7967:
7965:, p. 275.
7955:
7953:, p. 981.
7943:
7941:, p. 862.
7931:
7929:, p. 338.
7919:
7907:
7874:
7872:, p. 171.
7862:
7860:, p. 984.
7850:
7848:, p. 654.
7835:
7833:, p. 659.
7823:
7794:
7777:(2): 175–188.
7754:
7742:
7699:
7672:
7646:
7615:
7580:
7543:
7541:, p. 106.
7531:
7529:, p. 105.
7519:
7493:
7471:
7460:
7456:
7452:
7445:
7419:
7362:
7321:Applied Optics
7311:
7271:(16): 167802.
7251:
7194:
7148:
7146:, p. 518.
7136:
7134:, p. 627.
7124:
7112:
7095:
7083:
7048:
6991:
6979:
6967:
6965:, p. 393.
6955:
6920:
6890:
6871:(1): E16–E19.
6852:
6843:
6808:
6796:
6740:
6680:
6668:
6649:
6646:on 2008-03-09.
6618:
6580:
6565:
6535:
6480:
6469:
6432:
6397:
6363:
6361:, p. 493.
6351:
6349:, p. 624.
6339:
6293:
6286:
6265:
6226:
6208:
6186:
6104:
6102:, p. 625.
6087:
6055:
6043:
6031:
5992:
5957:(7): 797–803.
5941:
5882:
5870:
5852:
5823:
5811:
5794:
5782:
5752:
5750:, p. 620.
5740:
5721:
5709:
5683:
5671:
5659:
5616:
5597:(6): 690–695.
5581:
5567:. 2015-08-09.
5552:
5540:
5528:
5516:
5485:
5473:
5436:
5425:(4): 301–309.
5404:
5335:
5305:
5271:
5269:
5266:
5264:
5261:
5259:
5258:
5245:
5236:
5219:
5201:
5192:
5163:
5146:
5136:
5127:
5119:
5110:
5099:
5087:
5072:
5067:
5058:
5056:
5053:
5051:
5050:
5045:
5040:
5035:
5030:
5025:
5020:
5015:
5010:
5005:
5000:
4995:
4990:
4985:
4983:Fluid dynamics
4980:
4975:
4970:
4965:
4959:
4958:
4957:
4941:
4938:
4921:
4905:
4897:
4842:hydrogen oxide
4819:parent hydride
4802:
4799:
4718:
4715:
4710:
4709:
4704:
4696:
4686:
4674:
4661:
4649:
4648:
4642:
4631:
4623:
4613:
4597:Main article:
4594:
4591:
4590:
4589:
4583:
4570:
4558:
4537:
4528:
4494:
4493:
4488:
4477:
4465:
4452:
4440:alkaline earth
4423:
4420:
4412:E2 elimination
4408:2 substitution
4405:
4388:saponification
4377:
4360:
4357:
4347:
4343:
4323:
4314:
4297:perrhenic acid
4289:
4280:
4259:
4256:
4255:
4254:
4248:
4235:
4223:
4214:
4191:
4190:
4184:
4173:
4149:
4126:
4116:
4110:
4099:
4075:
4052:
4042:
4037:
4028:
4006:
3983:
3953:
3952:
3947:
3935:
3913:
3889:
3872:
3860:
3848:
3847:
3842:
3831:
3822:
3800:
3765:
3753:
3744:Brønsted-Lowry
3727:
3724:
3722:
3719:
3684:
3675:
3672:
3650:
3634:
3621:
3608:
3600:self-diffusion
3591:
3578:
3562:
3548:of 4500 days;
3521:
3506:
3501:in 0.04%, and
3495:
3484:
3473:
3454:
3422:
3419:
3402:
3399:
3390:
3381:
3333:
3330:
3318:
3311:
3307:
3303:
3298:
3295:
3288:
3284:
3278:
3274:
3268:
3265:
3259:
3254:
3250:
3244:
3241:
3236:
3223:
3219:
3216:
3215:
3200:
3194:
3189:
3185:
3179:
3169:
3165:
3161:
3156:
3152:
3144:
3140:
3134:
3130:
3124:
3117:
3111:
3108:
3103:
3074:
3063:
3038:
3031:
3027:
3023:
3018:
3015:
3008:
3004:
2998:
2994:
2988:
2985:
2979:
2974:
2954:
2953:
2948:
2937:
2928:
2915:
2887:Main article:
2884:
2881:
2879:
2876:
2841:
2838:
2797:hydrogen bonds
2789:hydrogen bonds
2780:
2777:
2757:photoreceptors
2749:infrared light
2725:Main article:
2722:
2719:
2695:Main article:
2692:
2689:
2671:
2657:
2564:Main article:
2561:
2558:
2553:
2550:
2521:
2518:
2445:
2442:
2425:
2412:
2380:polar molecule
2338:
2335:
2299:ultra-filtered
2270:
2261:
2250:
2242:autoionization
2229:
2226:
2224:
2221:
2195:
2192:
2189:
2188:
2185:
2182:
2178:
2177:
2174:
2171:
2167:
2166:
2163:
2160:
2159:, and ice III
2152:
2148:
2147:
2144:
2141:
2134:
2133:
2132:−17.0 °C
2130:
2127:
2120:
2119:
2116:
2113:
2106:
2102:
2101:
2098:
2095:
2090:
2087:liquid water,
2084:
2083:
2080:
2077:
2024:
2011:Main article:
2008:
2005:
1956:
1953:
1940:Main article:
1937:
1934:
1910:vapor pressure
1869:Main article:
1858:
1855:
1796:
1793:
1716:
1713:
1654:
1651:
1563:
1560:
1503:
1481:
1478:
1471:
1460:
1440:
1427:
1386:hydrogen bonds
1369:, liquid, and
1335:
1324:
1321:
1320:
1315:
1293:
1292:
1288:standard state
1285:
1282:
1281:
1275:
1274:
1270:
1269:
1267:
1266:
1261:
1256:
1251:
1246:
1240:
1238:
1232:
1229:
1228:
1226:
1225:
1220:
1215:
1210:
1205:
1199:
1197:
1191:
1188:
1187:
1183:
1182:
1177:
1170:
1169:
1168:Non-flammable
1166:
1160:
1159:
1149:
1142:
1135:
1120:
1119:
1118:
1117:
1115:
1106:
1105:
1091:
1088:
1085:
1084:
1075:
1074:
1070:
1069:
1066:
1057:
1051:
1048:
1047:
1044:
1038:
1032:
1024:
1021:
1020:
1017:
1011:
1000:
997:
996:
993:
981:
978:
977:
973:
972:
966:
961:
958:
957:
952:
947:
944:
943:
940:
937:
932:
929:
928:
923:
918:
915:
914:
910:
909:
902:
896:
895:
892:
887:
879:
876:
875:
872:
866:
865:
861:
855:
853:Conjugate base
849:
848:
844:
840:
834:
832:Conjugate acid
828:
827:
824:
820:
810:
809:
806:
802:
792:
791:
788:
786:Vapor pressure
782:
781:
702:Miscible with
678:hydrocarbons,
664:
658:
657:
654:
648:
647:
644:
638:
637:
635:
634:
631:
628:
622:(96) g/mL
612:
606:(83) g/mL
596:
585:(84) g/mL
575:
569:(84) g/mL
559:
551:
549:
543:
542:
539:
533:
532:
525:
521:
520:
517:
511:
510:
502:
495:
490:
487:
486:
482:
481:
479:
478:
475:
467:
466:
465:
462:
461:
459:
458:
454:
451:
450:
442:
441:
440:
437:
436:
434:
433:
425:
423:
417:
416:
414:
413:
409:
407:
401:
400:
398:
397:
389:
387:
379:
376:
375:
373:
372:
364:
362:
356:
355:
352:
347:
344:
343:
341:
340:
336:
334:
326:
325:
323:
322:
314:
312:
306:
305:
303:
302:
294:
292:
286:
285:
283:
282:
274:
272:
266:
265:
263:
262:
254:
252:
246:
245:
242:
237:
234:
233:
231:
230:
222:
220:
213:
210:
209:
207:
206:
198:
196:
191:
188:
187:
183:
182:
179:
178:
177:Neutral liquid
175:
172:
169:
166:
163:
162:Hydroxoic acid
160:
157:
154:
151:
148:
142:
139:
136:
135:Hydrogen oxide
132:
131:
127:
126:
122:
121:
115:
114:
110:
109:
103:
102:
98:
97:
89:
88:
85:
84:
77:
71:
70:
60:
45:
44:
15:
9:
6:
4:
3:
2:
17928:
17917:
17914:
17912:
17911:Heat transfer
17909:
17907:
17904:
17902:
17899:
17897:
17894:
17892:
17889:
17887:
17884:
17882:
17879:
17877:
17874:
17872:
17869:
17867:
17864:
17862:
17859:
17857:
17854:
17852:
17849:
17847:
17844:
17842:
17839:
17838:
17836:
17815:
17812:
17811:
17809:
17807:
17803:
17797:
17791:
17789:
17783:
17781:
17775:
17773:
17767:
17765:
17759:
17757:
17751:
17749:
17743:
17741:
17735:
17733:
17727:
17725:
17719:
17717:
17711:
17709:
17703:
17701:
17695:
17693:
17687:
17677:
17675:
17669:
17667:
17661:
17659:
17653:
17652:
17650:
17648:
17644:
17638:
17632:
17630:
17624:
17622:
17616:
17614:
17608:
17606:
17600:
17598:
17592:
17590:
17584:
17582:
17576:
17574:
17568:
17566:
17560:
17558:
17552:
17550:
17544:
17542:
17536:
17534:
17528:
17526:
17520:
17518:
17512:
17510:
17504:
17502:
17496:
17494:
17488:
17486:
17480:
17478:
17472:
17470:
17464:
17462:
17456:
17454:
17448:
17446:
17440:
17438:
17432:
17430:
17424:
17423:
17421:
17419:
17415:
17408:
17404:
17403:
17396:
17394:
17388:
17386:
17376:
17374:
17371:
17369:
17363:
17361:
17355:
17352:
17348:
17347:
17343:
17341:
17338:
17335:
17331:
17330:
17323:
17321:
17319:
17314:
17308:
17306:
17304:
17299:
17293:
17291:
17288:
17286:
17280:
17278:
17272:
17270:
17264:
17262:
17256:
17254:
17248:
17246:
17243:
17241:
17235:
17233:
17227:
17225:
17222:
17220:
17214:
17212:
17209:
17207:
17201:
17199:
17196:
17194:
17188:
17186:
17183:
17181:
17175:
17173:
17167:
17165:
17159:
17157:
17151:
17149:
17143:
17141:
17135:
17133:
17127:
17125:
17119:
17117:
17111:
17109:
17103:
17101:
17095:
17093:
17087:
17085:
17079:
17078:
17076:
17074:
17070:
17064:
17060:
17059:
17055:
17053:
17050:
17048:
17045:
17043:
17040:
17038:
17035:
17033:
17030:
17028:
17026:
17020:
17014:
17007:
17005:
16998:
16996:
16993:
16991:
16988:
16986:
16982:
16974:
16972:
16966:
16964:
16958:
16956:
16953:
16952:
16942:
16938:
16937:
16930:
16929:
16927:
16923:
16913:
16907:
16906:
16904:
16900:
16890:
16880:
16878:
16871:
16870:
16868:
16864:
16854:
16844:
16842:
16835:
16834:
16832:
16830:
16826:
16816:
16813:
16811:
16801:
16799:
16789:
16787:
16777:
16775:
16765:
16763:
16753:
16751:
16741:
16739:
16729:
16727:
16717:
16715:
16705:
16703:
16696:
16695:
16693:
16691:
16687:
16678:
16675:
16673:
16663:
16661:
16651:
16649:
16639:
16637:
16627:
16625:
16618:
16616:
16614:
16610:
16607:
16605:
16597:
16591:
16587:
16580:
16578:
16572:
16570:
16567:
16565:
16559:
16558:
16548:
16544:
16543:
16536:
16535:
16533:
16529:
16519:
16513:
16512:
16510:
16506:
16496:
16490:
16489:
16487:
16483:
16473:
16463:
16461:
16455:
16454:
16452:
16450:
16446:
16436:
16426:
16424:
16414:
16412:
16402:
16401:
16399:
16397:
16393:
16383:
16380:
16378:
16368:
16366:
16356:
16354:
16344:
16342:
16332:
16330:
16320:
16318:
16308:
16306:
16296:
16294:
16284:
16282:
16272:
16270:
16264:
16263:
16261:
16259:
16255:
16245:
16235:
16233:
16223:
16221:
16211:
16210:
16208:
16206:
16202:
16192:
16189:
16187:
16177:
16175:
16165:
16163:
16153:
16151:
16141:
16139:
16129:
16127:
16117:
16115:
16105:
16103:
16093:
16091:
16081:
16079:
16073:
16072:
16070:
16068:
16064:
16061:
16059:
16053:
16043:
16037:
16035:
16029:
16027:
16024:
16023:
16021:
16018:
16014:
16010:
16000:
15994:
15993:
15991:
15987:
15977:
15967:
15965:
15959:
15958:
15956:
15954:
15950:
15940:
15930:
15928:
15918:
15916:
15906:
15904:
15894:
15892:
15886:
15885:
15883:
15881:
15877:
15867:
15864:
15862:
15852:
15851:
15849:
15847:
15843:
15833:
15823:
15822:
15820:
15818:
15814:
15804:
15801:
15799:
15789:
15787:
15777:
15775:
15765:
15763:
15753:
15751:
15741:
15739:
15729:
15727:
15717:
15715:
15705:
15703:
15693:
15691:
15685:
15684:
15682:
15680:
15676:
15666:
15659:
15657:
15651:
15649:
15643:
15641:
15638:
15637:
15634:
15624:
15621:
15619:
15616:
15614:
15611:
15609:
15606:
15604:
15601:
15599:
15596:
15594:
15591:
15590:
15588:
15586:
15582:
15579:
15577:
15573:
15563:
15557:
15555:
15545:
15543:
15537:
15535:
15532:
15531:
15529:
15526:
15522:
15518:
15508:
15498:
15496:
15490:
15489:
15487:
15483:
15473:
15463:
15461:
15455:
15454:
15452:
15448:
15438:
15428:
15426:
15420:
15419:
15417:
15413:
15403:
15393:
15391:
15385:
15384:
15382:
15378:
15368:
15358:
15356:
15346:
15344:
15334:
15332:
15322:
15320:
15310:
15308:
15298:
15296:
15286:
15284:
15274:
15272:
15262:
15260:
15250:
15248:
15245:
15243:
15237:
15236:
15234:
15232:
15228:
15225:
15223:
15217:
15207:
15201:
15199:
15193:
15191:
15185:
15183:
15177:
15175:
15169:
15168:
15166:
15162:
15152:
15149:
15147:
15144:
15142:
15139:
15137:
15134:
15132:
15129:
15128:
15126:
15122:
15119:
15117:
15109:
15103:
15100:
15098:
15095:
15093:
15090:
15088:
15085:
15083:
15080:
15079:
15077:
15075:
15072:Alkali metal
15069:
15065:
15058:
15053:
15051:
15046:
15044:
15039:
15038:
15035:
15019:
15016:
15013:
15010:
15007:
15004:
15002:
14994:
14992:
14984:
14981:
14979:
14966:
14957:
14949:
14947:
14934:
14925:
14917:
14914:
14912:
14904:
14902:
14894:
14891:
14890:
14887:
14879:
14877:
14869:
14867:
14859:
14857:
14849:
14847:
14839:
14837:
14829:
14827:
14819:
14817:
14808:
14800:
14798:
14790:
14788:
14780:
14778:
14770:
14768:
14760:
14758:
14749:
14741:
14739:
14731:
14728:
14727:
14720:
14716:
14713:
14710:
14707:
14704:
14701:
14698:
14695:
14692:
14689:
14686:
14683:
14680:
14677:
14674:
14671:
14668:
14666:
14658:
14656:
14653:
14652:
14648:
14645:
14642:
14640:
14632:
14630:
14621:
14613:
14611:
14602:
14599:
14597:
14589:
14587:
14579:
14576:
14573:
14570:
14567:
14564:
14561:
14558:
14556:
14548:
14545:
14543:
14535:
14533:
14530:
14529:
14525:
14523:
14520:
14518:
14510:
14508:
14500:
14498:
14489:
14481:
14479:
14471:
14469:
14461:
14459:
14456:
14453:
14451:
14443:
14440:
14438:
14430:
14427:
14424:
14422:
14414:
14412:
14404:
14402:
14400:
14392:
14390:
14387:
14386:
14382:
14380:
14377:
14374:
14372:
14364:
14362:
14354:
14352:
14344:
14342:
14334:
14332:
14323:
14320:
14318:
14310:
14308:
14300:
14298:
14289:
14281:
14279:
14271:
14269:
14260:
14252:
14250:
14241:
14233:
14231:
14222:
14213:
14205:
14203:
14195:
14193:
14191:
14183:
14181:
14178:
14177:
14173:
14171:
14168:
14166:
14158:
14156:
14148:
14146:
14138:
14136:
14128:
14126:
14118:
14116:
14113:
14112:
14108:
14106:
14103:
14101:
14093:
14091:
14081:
14073:
14071:
14063:
14061:
14053:
14051:
14043:
14041:
14038:
14037:
14033:
14027:
14025:
14022:
14021:
14015:
14011:
14004:
13999:
13997:
13992:
13990:
13985:
13984:
13981:
13971:
13967:
13961:
13955:
13952:
13950:
13947:
13945:
13942:
13940:
13937:
13935:
13932:
13930:
13927:
13925:
13922:
13921:
13919:
13915:
13904:
13900:
13897:
13890:
13886:
13883:
13876:
13872:
13869:
13862:
13858:
13855:
13848:
13844:
13841:
13840:
13838:
13834:
13820:
13816:
13813:
13803:
13799:
13796:
13786:
13782:
13779:
13769:
13765:
13762:
13761:
13759:
13755:
13744:
13740:
13737:
13730:
13726:
13723:
13716:
13712:
13709:
13702:
13698:
13695:
13688:
13684:
13681:
13674:
13670:
13667:
13660:
13656:
13653:
13646:
13642:
13639:
13632:
13628:
13625:
13618:
13614:
13611:
13610:
13608:
13604:
13590:
13586:
13583:
13573:
13569:
13566:
13556:
13552:
13549:
13539:
13535:
13532:
13522:
13518:
13515:
13505:
13501:
13498:
13488:
13484:
13481:
13471:
13467:
13464:
13454:
13450:
13447:
13446:
13444:
13440:
13429:
13425:
13422:
13415:
13411:
13408:
13401:
13397:
13394:
13387:
13383:
13380:
13373:
13369:
13366:
13359:
13355:
13352:
13345:
13341:
13338:
13331:
13327:
13324:
13317:
13313:
13310:
13303:
13299:
13296:
13289:
13285:
13282:
13275:
13271:
13268:
13261:
13257:
13254:
13247:
13243:
13240:
13233:
13229:
13226:
13219:
13215:
13212:
13205:
13201:
13198:
13191:
13187:
13184:
13177:
13173:
13170:
13163:
13159:
13156:
13149:
13145:
13142:
13135:
13131:
13128:
13121:
13117:
13114:
13107:
13103:
13100:
13093:
13089:
13086:
13079:
13075:
13072:
13065:
13061:
13058:
13051:
13047:
13044:
13037:
13033:
13030:
13020:
13016:
13013:
13006:
13002:
12999:
12992:
12988:
12985:
12978:
12974:
12971:
12964:
12960:
12957:
12950:
12946:
12943:
12936:
12932:
12929:
12922:
12918:
12915:
12908:
12904:
12901:
12894:
12890:
12887:
12880:
12876:
12873:
12872:
12870:
12866:
12852:
12848:
12845:
12835:
12831:
12828:
12818:
12814:
12811:
12801:
12797:
12794:
12784:
12780:
12777:
12767:
12763:
12760:
12750:
12746:
12743:
12733:
12729:
12726:
12716:
12712:
12709:
12699:
12695:
12692:
12682:
12678:
12675:
12665:
12661:
12658:
12648:
12644:
12641:
12631:
12627:
12624:
12614:
12610:
12607:
12597:
12593:
12590:
12580:
12576:
12573:
12563:
12559:
12556:
12546:
12542:
12539:
12529:
12525:
12522:
12512:
12508:
12505:
12495:
12491:
12488:
12478:
12474:
12471:
12461:
12457:
12454:
12444:
12440:
12437:
12427:
12423:
12420:
12410:
12406:
12403:
12393:
12389:
12386:
12376:
12372:
12369:
12359:
12355:
12352:
12342:
12338:
12335:
12325:
12321:
12318:
12308:
12304:
12301:
12291:
12287:
12284:
12274:
12270:
12267:
12257:
12253:
12250:
12240:
12236:
12233:
12223:
12219:
12216:
12206:
12202:
12199:
12189:
12185:
12182:
12172:
12168:
12165:
12155:
12151:
12148:
12138:
12134:
12131:
12130:
12128:
12124:
12117:
12113:
12110:
12107:
12103:
12100:
12097:
12093:
12090:
12087:
12083:
12080:
12077:
12073:
12072:Tin(II) oxide
12070:
12067:
12063:
12060:
12050:
12046:
12043:
12040:
12036:
12033:
12030:
12026:
12023:
12020:
12016:
12013:
12010:
12006:
12003:
12000:
11996:
11993:
11990:
11986:
11983:
11980:
11976:
11973:
11970:
11966:
11963:
11960:
11956:
11953:
11950:
11946:
11943:
11940:
11936:
11933:
11930:
11926:
11923:
11920:
11916:
11913:
11910:
11906:
11903:
11900:
11896:
11893:
11890:
11886:
11883:
11880:
11876:
11873:
11870:
11866:
11863:
11853:
11849:
11846:
11843:
11839:
11836:
11833:
11829:
11826:
11823:
11819:
11816:
11813:
11809:
11806:
11803:
11799:
11796:
11793:
11789:
11788:Calcium oxide
11786:
11783:
11779:
11778:Cadmium oxide
11776:
11773:
11769:
11766:
11763:
11759:
11756:
11753:
11749:
11746:
11743:
11739:
11736:
11733:
11729:
11726:
11725:
11723:
11719:
11709:
11705:
11702:
11696:
11692:
11689:
11683:
11679:
11676:
11670:
11666:
11663:
11657:
11653:
11650:
11644:
11640:
11637:
11631:
11627:
11626:Nitrous oxide
11624:
11618:
11614:
11611:
11605:
11601:
11600:Lithium oxide
11598:
11592:
11588:
11585:
11579:
11575:
11572:
11566:
11562:
11559:
11553:
11549:
11546:
11540:
11536:
11533:
11527:
11523:
11520:
11514:
11510:
11507:
11506:
11504:
11500:
11486:
11482:
11479:
11469:
11465:
11462:
11452:
11448:
11445:
11435:
11431:
11428:
11418:
11414:
11411:
11401:
11397:
11394:
11384:
11380:
11377:
11367:
11363:
11360:
11350:
11346:
11343:
11333:
11329:
11326:
11316:
11312:
11309:
11299:
11295:
11292:
11282:
11278:
11275:
11265:
11261:
11258:
11248:
11244:
11241:
11231:
11227:
11224:
11223:
11221:
11217:
11213:
11206:
11201:
11199:
11194:
11192:
11187:
11186:
11183:
11171:
11168:
11165:
11154:
11151:
11140:
11137:
11126:
11123:
11112:
11109:
11098:
11095:
11084:
11081:
11074:
11071:
11067:
11064:
11056:
11053:
11042:
11040:
11034:
11032:
11029:
11027:
11017:
11015:
11008:
11006:
11000:
10998:
10988:
10986:
10976:
10974:
10964:
10962:
10955:
10953:
10947:
10945:
10942:
10940:
10930:
10928:
10922:
10920:
10914:
10912:
10902:
10900:
10890:
10888:
10882:
10880:
10877:
10875:
10865:
10863:
10860:
10858:
10848:
10846:
10836:
10834:
10827:
10825:
10815:
10813:
10803:
10801:
10795:
10793:
10787:
10785:
10775:
10773:
10763:
10761:
10751:
10749:
10739:
10737:
10727:
10725:
10722:
10720:
10710:
10708:
10701:
10699:
10696:
10694:
10688:
10686:
10676:
10674:
10667:
10665:
10658:
10656:
10649:
10647:
10640:
10638:
10632:
10630:
10627:
10625:
10615:
10613:
10606:
10604:
10594:
10592:
10582:
10580:
10577:
10575:
10565:
10563:
10553:
10551:
10541:
10539:
10536:
10534:
10524:
10522:
10516:
10514:
10504:
10502:
10492:
10490:
10480:
10478:
10472:
10470:
10460:
10458:
10448:
10446:
10443:
10441:
10431:
10429:
10419:
10417:
10407:
10405:
10395:
10393:
10383:
10381:
10374:
10372:
10366:
10364:
10354:
10352:
10342:
10340:
10334:
10332:
10329:
10327:
10321:
10319:
10316:
10314:
10304:
10302:
10296:
10294:
10291:
10289:
10267:
10265:
10255:
10253:
10243:
10241:
10235:
10233:
10223:
10221:
10215:
10213:
10203:
10201:
10198:
10196:
10193:
10191:
10181:
10179:
10169:
10167:
10157:
10155:
10145:
10143:
10133:
10131:
10125:
10123:
10113:
10111:
10101:
10100:
10097:
10093:
10086:
10081:
10079:
10074:
10072:
10067:
10066:
10063:
10051:
10042:
10040:
10031:
10029:
10017:
10015:
10003:
10001:
9989:
9987:
9978:
9976:
9963:
9961:
9945:
9943:
9927:
9925:
9909:
9907:
9898:
9896:
9880:
9878:
9866:
9864:
9852:
9850:
9838:
9836:
9833:
9831:
9828:
9826:
9813:
9811:
9799:
9797:
9785:
9783:
9771:
9769:
9760:
9758:
9746:
9744:
9735:
9733:
9720:
9718:
9702:
9700:
9684:
9682:
9670:
9668:
9656:
9654:
9642:
9640:
9628:
9626:
9614:
9612:
9600:
9598:
9586:
9584:
9575:
9573:
9561:
9559:
9550:
9548:
9532:
9530:
9522:
9520:
9512:
9510:
9507:
9505:
9502:
9500:
9492:
9490:
9487:
9485:
9473:
9471:
9459:
9457:
9449:
9447:
9439:
9437:
9429:
9427:
9419:
9417:
9414:
9412:
9409:
9407:
9404:
9402:
9399:
9397:
9385:
9383:
9371:
9369:
9342:
9340:
9337:
9335:
9332:
9330:
9322:
9320:
9312:
9310:
9302:
9300:
9297:
9295:
9292:
9290:
9282:
9280:
9272:
9270:
9262:
9260:
9257:
9255:
9252:
9250:
9241:
9239:
9230:
9228:
9225:
9223:
9220:
9218:
9206:
9204:
9192:
9191:
9188:
9184:
9177:
9172:
9170:
9165:
9163:
9158:
9157:
9154:
9148:
9141:
9129:
9125:
9120:
9118:
9109:
9107:
9098:
9096:
9092:
9087:
9086:
9083:
9077:
9074:
9070:
9067:
9065:
9062:
9061:
9060:
9057:
9056:
9054:
9050:
9042:
9039:
9037:
9034:
9032:
9029:
9027:
9024:
9023:
9021:
9017:
9014:
9012:
9011:Hycean planet
9009:
9007:
9004:
9002:
8999:
8997:
8994:
8993:
8992:
8989:
8988:
8986:
8982:
8976:
8973:
8969:
8966:
8964:
8961:
8960:
8959:
8956:
8954:
8951:
8949:
8946:
8942:
8939:
8937:
8934:
8933:
8932:
8929:
8927:
8924:
8922:
8919:
8918:
8916:
8914:
8909:
8903:
8900:
8898:
8895:
8893:
8890:
8888:
8885:
8883:
8880:
8878:
8875:
8874:
8872:
8868:
8860:
8857:
8856:
8855:
8852:
8850:
8847:
8845:
8842:
8840:
8837:
8836:
8834:
8832:
8828:
8822:Water droplet
8820:
8810:
8807:
8805:
8802:
8800:
8797:
8795:
8792:
8791:
8789:
8785:
8781:
8774:
8769:
8767:
8762:
8760:
8755:
8754:
8751:
8745:
8741:
8738:
8736:
8733:
8730:
8726:
8722:
8718:
8714:
8710:
8706:
8702:
8700:9781119300755
8696:
8692:
8688:
8684:
8680:
8675:
8673:
8670:
8667:
8664:
8653:. May 2, 2016
8652:
8648:
8644:
8643:
8634:
8630:
8626:
8622:
8621:
8610:
8604:
8600:
8596:
8591:
8587:
8581:
8577:
8573:
8569:
8568:
8561:
8557:
8551:
8547:
8542:
8541:
8535:
8531:
8527:
8521:
8516:
8515:
8508:
8504:
8498:
8494:
8489:
8478:
8474:
8468:
8464:
8460:
8459:
8453:
8449:
8444:
8437:
8433:
8429:
8425:
8419:
8412:
8411:
8405:
8391:
8387:
8381:
8374:
8373:
8368:
8364:
8360:
8354:
8350:
8346:
8342:
8338:
8334:
8328:
8324:
8323:
8317:
8306:
8302:
8296:
8292:
8291:
8285:
8281:
8275:
8270:
8269:
8262:
8258:
8252:
8248:
8247:
8241:
8237:
8231:
8227:
8223:
8219:
8218:Water Quality
8214:
8213:
8193:
8189:
8188:
8183:
8177:
8170:
8165:
8150:
8146:
8142:
8138:
8132:
8126:, p. 99.
8125:
8120:
8114:, p. 85.
8113:
8108:
8102:, p. 34.
8101:
8096:
8094:
8085:
8081:
8077:
8073:
8069:
8065:
8058:
8043:
8039:
8035:
8029:
8014:
8010:
8007:
8001:
7994:
7989:
7987:
7979:
7974:
7972:
7964:
7959:
7952:
7947:
7940:
7935:
7928:
7923:
7916:
7911:
7896:
7892:
7888:
7884:
7878:
7871:
7866:
7859:
7854:
7847:
7842:
7840:
7832:
7827:
7812:
7808:
7804:
7798:
7780:
7776:
7772:
7765:
7758:
7752:, p. 29.
7751:
7746:
7738:
7734:
7730:
7726:
7722:
7718:
7714:
7710:
7703:
7687:
7683:
7676:
7660:
7656:
7650:
7642:
7638:
7634:
7630:
7626:
7619:
7611:
7607:
7603:
7599:
7595:
7591:
7590:J. Chem. Phys
7584:
7566:
7562:
7561:
7553:
7547:
7540:
7535:
7528:
7523:
7516:
7515:
7500:
7496:
7490:
7486:
7482:
7475:
7468:
7466:
7463:O are called
7448:
7446:9780060936778
7442:
7438:
7433:
7432:
7423:
7415:
7411:
7407:
7403:
7398:
7393:
7389:
7385:
7381:
7377:
7373:
7366:
7358:
7354:
7350:
7346:
7342:
7338:
7334:
7330:
7326:
7322:
7315:
7300:
7296:
7292:
7287:
7282:
7278:
7274:
7270:
7266:
7262:
7255:
7247:
7243:
7239:
7235:
7230:
7225:
7221:
7217:
7213:
7209:
7205:
7198:
7183:
7179:
7175:
7171:
7167:
7163:
7159:
7152:
7145:
7140:
7133:
7128:
7121:
7116:
7110:, p. 46.
7109:
7104:
7102:
7100:
7092:
7087:
7079:
7073:
7065:
7058:
7052:
7044:
7040:
7035:
7030:
7026:
7022:
7018:
7014:
7010:
7006:
7002:
6995:
6989:, p. 47.
6988:
6983:
6976:
6971:
6964:
6959:
6951:
6947:
6943:
6939:
6935:
6931:
6924:
6910:on 2009-05-10
6909:
6905:
6901:
6894:
6886:
6882:
6878:
6874:
6870:
6866:
6859:
6857:
6847:
6829:
6825:
6818:
6812:
6806:, p. 27.
6805:
6800:
6782:
6778:
6774:
6770:
6766:
6762:
6758:
6757:Physics Today
6751:
6744:
6729:
6725:
6721:
6716:
6711:
6707:
6703:
6699:
6695:
6691:
6684:
6677:
6672:
6664:
6660:
6653:
6642:
6638:
6631:
6630:
6622:
6603:
6599:
6592:
6591:
6584:
6577:
6572:
6570:
6553:
6549:
6545:
6539:
6524:
6520:
6516:
6512:
6508:
6504:
6500:
6497:(3): 033101.
6496:
6492:
6488:
6486:
6473:
6458:
6454:
6450:
6446:
6439:
6437:
6428:
6424:
6420:
6416:
6412:
6408:
6401:
6386:
6382:
6378:
6372:
6370:
6368:
6360:
6355:
6348:
6343:
6335:
6331:
6327:
6323:
6319:
6315:
6311:
6307:
6303:
6297:
6289:
6283:
6279:
6272:
6270:
6254:
6250:
6246:
6239:
6237:
6235:
6233:
6231:
6215:
6211:
6209:0-8155-1237-6
6205:
6201:
6197:
6190:
6176:on 2016-06-04
6172:
6168:
6164:
6160:
6156:
6152:
6148:
6144:
6140:
6135:
6130:
6127:(1): 011202.
6126:
6122:
6115:
6108:
6101:
6096:
6094:
6092:
6084:
6082:
6069:
6065:
6059:
6052:
6047:
6040:
6035:
6028:
6013:
6006:
6002:
5996:
5988:
5984:
5980:
5976:
5972:
5968:
5964:
5960:
5956:
5952:
5945:
5937:
5933:
5928:
5923:
5918:
5913:
5909:
5905:
5901:
5897:
5893:
5886:
5879:
5874:
5859:
5855:
5849:
5845:
5841:
5837:
5833:
5827:
5821:, p. 44.
5820:
5815:
5808:
5803:
5801:
5799:
5792:, p. 48.
5791:
5786:
5770:
5766:
5762:
5756:
5749:
5744:
5738:
5734:
5731:
5725:
5718:
5713:
5706:
5704:
5699:
5694:
5692:
5690:
5688:
5680:
5675:
5668:
5663:
5655:
5651:
5647:
5643:
5639:
5635:
5631:
5627:
5620:
5612:
5608:
5604:
5600:
5596:
5592:
5585:
5570:
5566:
5562:
5556:
5550:, p. 13.
5549:
5544:
5537:
5532:
5525:
5520:
5505:
5501:
5494:
5492:
5490:
5482:
5477:
5461:
5457:
5453:
5449:
5448:
5440:
5432:
5428:
5424:
5420:
5413:
5411:
5409:
5390:
5386:
5382:
5378:
5374:
5370:
5366:
5362:
5358:
5351:
5344:
5342:
5340:
5323:
5319:
5315:
5309:
5302:
5290:
5286:
5282:
5276:
5272:
5255:
5249:
5240:
5233:
5229:
5223:
5216:
5215:ideal gas law
5212:
5211:
5205:
5196:
5189:
5185:
5181:
5177:
5176:melting point
5173:
5167:
5160:
5156:
5155:molten silica
5150:
5139:
5091:
5084:
5079:
5077:
5063:
5059:
5049:
5046:
5044:
5041:
5039:
5036:
5034:
5031:
5029:
5028:Water cluster
5026:
5024:
5021:
5019:
5016:
5014:
5011:
5009:
5006:
5004:
5001:
4999:
4996:
4994:
4991:
4989:
4986:
4984:
4981:
4979:
4976:
4974:
4971:
4969:
4966:
4964:
4961:
4960:
4955:
4949:
4944:
4937:
4935:
4931:
4927:
4919:
4915:
4913:
4890:
4886:
4882:
4881:hydroxic acid
4877:
4875:
4871:
4867:
4863:
4859:
4855:
4851:
4847:
4843:
4838:
4836:
4832:
4828:
4824:
4820:
4816:
4812:
4808:
4805:The accepted
4798:
4796:
4792:
4788:
4784:
4780:
4776:
4772:
4768:
4764:
4760:
4755:
4753:
4749:
4745:
4743:
4739:
4735:
4731:
4727:
4723:
4714:
4654:
4653:
4652:
4606:
4605:
4604:
4600:
4551:
4550:
4549:
4547:
4521:
4517:
4515:
4511:
4507:
4503:
4499:
4445:
4444:
4443:
4441:
4437:
4433:
4429:
4419:
4417:
4413:
4409:
4401:
4400:leaving group
4397:
4393:
4389:
4385:
4370:
4366:
4353:
4340:
4336:
4334:
4329:
4306:
4302:
4298:
4273:
4269:
4265:
4208:
4207:
4206:
4204:
4200:
4196:
4117:
4043:
3974:
3973:
3972:
3970:
3966:
3962:
3958:
3880:
3879:
3878:
3853:
3777:
3776:
3775:
3773:
3757:
3745:
3741:
3737:
3733:
3718:
3716:
3712:
3708:
3704:
3699:
3671:
3665:
3664:
3659:
3642:
3601:
3570:
3547:
3543:
3540:. Tritium is
3539:
3535:
3531:
3513:
3467:
3463:
3448:
3444:
3440:
3436:
3432:
3431:isotopologues
3428:
3421:Isotopologues
3418:
3416:
3412:
3408:
3398:
3374:
3370:
3366:
3361:
3359:
3355:
3354:clay minerals
3351:
3347:
3343:
3342:water erosion
3339:
3329:
3309:
3286:
3276:
3263:
3252:
3248:
3234:
3198:
3187:
3177:
3167:
3154:
3150:
3142:
3132:
3122:
3115:
3101:
3093:
3092:
3091:
3089:
3084:
3082:
3056:
3052:
3029:
3006:
2996:
2983:
2972:
2963:
2962:ionic product
2959:
2908:
2907:
2906:
2904:
2900:
2896:
2890:
2875:
2873:
2870:explanation (
2869:
2865:
2861:
2857:
2853:
2847:
2837:
2835:
2834:string theory
2830:
2825:
2822:
2818:
2814:
2810:
2809:thermodynamic
2806:
2802:
2798:
2790:
2785:
2776:
2774:
2770:
2766:
2762:
2758:
2754:
2750:
2746:
2742:
2738:
2734:
2733:visible light
2728:
2718:
2716:
2712:
2708:
2707:hydrogen bond
2704:
2698:
2688:
2686:
2682:
2678:
2664:
2650:
2645:
2643:
2638:
2634:
2630:
2625:
2623:
2619:
2614:
2612:
2608:
2604:
2600:
2596:
2588:
2584:
2580:
2577:
2572:
2567:
2557:
2549:
2547:
2538:
2531:
2526:
2514:
2510:
2506:
2502:
2497:
2493:
2490:
2486:
2482:
2478:
2474:
2469:
2467:
2460:
2456:
2452:
2448:
2441:
2439:
2435:
2405:
2399:
2397:
2393:
2389:
2385:
2381:
2377:
2372:
2370:
2366:
2362:
2358:
2349:
2344:
2334:
2328:
2324:
2320:
2316:
2312:
2308:
2303:
2300:
2296:
2292:
2287:
2285:
2281:
2276:
2243:
2239:
2235:
2220:
2218:
2214:
2213:with pressure
2210:
2205:
2201:
2194:Melting point
2186:
2183:
2180:
2179:
2175:
2172:
2169:
2168:
2164:
2161:
2158:
2150:
2149:
2146:0.16 °C
2145:
2142:
2140:
2136:
2135:
2131:
2128:
2126:
2122:
2121:
2117:
2114:
2112:
2104:
2103:
2099:
2096:
2093:
2086:
2085:
2081:
2078:
2075:
2074:
2068:
2066:
2062:
2057:
2055:
2051:
2047:
2043:
2039:
2035:
2027:
2019:
2014:
2004:
2000:
1998:
1993:
1988:
1962:
1948:
1943:
1933:
1929:
1927:
1923:
1919:
1915:
1911:
1906:
1904:
1901:such as with
1900:
1896:
1892:
1888:
1884:
1878:
1872:
1863:
1854:
1852:
1848:
1847:
1842:
1838:
1834:
1829:
1827:
1823:
1818:
1814:
1805:
1801:
1792:
1790:
1786:
1776:
1772:
1770:
1766:
1762:
1758:
1754:
1749:
1745:
1743:
1742:hexagonal ice
1739:
1735:
1730:
1721:
1712:
1709:
1707:
1706:refrigeration
1703:
1699:
1695:
1692:The specific
1690:
1688:
1684:
1680:
1676:
1672:
1668:
1659:
1650:
1647:
1644:
1640:
1636:
1632:
1628:
1624:
1620:
1615:
1613:
1609:
1605:
1604:gaseous phase
1601:
1600:phases of ice
1597:
1594:. Aside from
1593:
1589:
1585:
1581:
1577:
1573:
1569:
1559:
1557:
1553:
1552:oxygen family
1549:
1544:
1542:
1538:
1534:
1530:
1526:
1523:
1520:
1517:
1513:
1497:
1493:
1490:Water is the
1487:
1477:
1453:
1449:
1445:
1432:
1419:
1415:
1411:
1406:
1404:
1403:heat capacity
1401:, and a high
1400:
1396:
1395:boiling point
1391:
1387:
1382:
1380:
1376:
1372:
1368:
1364:
1360:
1356:
1352:
1348:
1344:
1340:
1330:
1318:
1311:
1306:
1289:
1283:
1280:
1276:
1271:
1265:
1262:
1260:
1257:
1255:
1252:
1250:
1247:
1245:
1242:
1241:
1239:
1236:
1231:
1230:
1224:
1221:
1219:
1216:
1214:
1211:
1209:
1206:
1204:
1201:
1200:
1198:
1195:
1190:
1189:
1184:
1181:
1178:
1175:
1172:
1171:
1167:
1165:
1162:
1161:
1154:
1147:
1140:
1116:
1113:
1112:
1108:
1107:
1104:
1099:
1095:
1092:
1087:
1086:
1082:
1081:
1076:
1071:
1067:
1062:
1054:
1050:
1049:
1045:
1037:
1029:
1023:
1022:
1018:
1010:
1005:
999:
998:
994:
989:
984:
983:Heat capacity
980:
979:
974:
971:
967:
964:
963:Dipole moment
960:
959:
956:
953:
950:
946:
945:
938:
935:
931:
930:
927:
924:
921:
917:
916:
911:
907:
903:
901:
898:
897:
893:
886:
882:
878:
877:
873:
871:
868:
867:
859:
856:
854:
851:
850:
838:
835:
833:
830:
829:
825:
819:
815:
812:
811:
807:
801:
797:
794:
793:
789:
787:
784:
783:
779:
775:
774:ethyl acetate
771:
767:
763:
762:diethyl ether
757:
753:
749:
745:
741:
737:
733:
729:
725:
721:
717:
713:
709:
705:
699:
695:
691:
687:
681:
677:
673:
669:
665:
663:
660:
659:
655:
653:
652:Boiling point
650:
649:
645:
643:
642:Melting point
640:
639:
632:
629:
613:
597:
593:(670) °C
576:
560:
557:
553:
552:
550:
548:
545:
544:
540:
538:
535:
534:
530:
526:
523:
522:
518:
516:
513:
512:
496:
493:
489:
488:
483:
474:
473:
470:
463:
449:
448:
445:
438:
431:
427:
426:
424:
422:
419:
418:
411:
410:
408:
406:
403:
402:
395:
391:
390:
388:
382:
378:
377:
370:
366:
365:
363:
361:
358:
357:
353:
350:
346:
345:
338:
337:
335:
333:
328:
327:
320:
316:
315:
313:
311:
308:
307:
300:
296:
295:
293:
291:
288:
287:
280:
279:ChEMBL1098659
276:
275:
273:
271:
268:
267:
260:
256:
255:
253:
251:
248:
247:
243:
240:
236:
235:
228:
224:
223:
221:
217:
212:
211:
204:
200:
199:
197:
194:
190:
189:
184:
176:
173:
170:
167:
164:
161:
159:Hydroxic acid
158:
155:
152:
149:
146:
143:
140:
137:
134:
133:
128:
120:
116:
108:
104:
99:
95:
90:
65:
61:
55:
51:
50:
46:
42:
37:
29:
22:
17896:Oceanography
17406:
17397:
17350:
17344:
17333:
17324:
17317:
17312:
17302:
17297:
17062:
17056:
16975:
16940:
16931:
16925:Livermoranes
16690:Polysulfanes
16619:
16613:Polyoxidanes
16581:
16546:
16537:
16038:
16030:
16025:
16016:
15618:Cycloalkynes
15613:Cycloalkenes
15608:Cycloalkanes
15585:Hydrocarbons
15558:
15546:
15538:
15533:
15524:
15124:Monohydrides
14023:
13088:Lead dioxide
12005:Radium oxide
11955:Nitric oxide
11738:Barium oxide
11703:
11691:Sodium oxide
11665:Silver oxide
10641:
10045:H[Co(CO)
9576:
8941:Hydrobiology
8926:Distribution
8808:
8682:
8655:. Retrieved
8628:
8625:Ben-Naim, A.
8594:
8566:
8539:
8513:
8492:
8481:. Retrieved
8463:CRC Handbook
8457:
8447:
8436:the original
8409:
8397:. Retrieved
8371:
8344:
8321:
8309:. Retrieved
8289:
8267:
8246:Biochemistry
8245:
8217:
8209:Bibliography
8196:. Retrieved
8185:
8176:
8164:
8153:. Retrieved
8140:
8131:
8119:
8107:
8067:
8063:
8057:
8046:. Retrieved
8037:
8028:
8017:. Retrieved
8008:
8000:
7963:Charlot 2007
7958:
7946:
7934:
7922:
7910:
7899:. Retrieved
7886:
7877:
7865:
7853:
7826:
7815:. Retrieved
7806:
7797:
7786:. Retrieved
7774:
7770:
7757:
7745:
7715:(1): 69–74.
7712:
7708:
7702:
7690:. Retrieved
7685:
7675:
7663:. Retrieved
7658:
7649:
7624:
7618:
7593:
7589:
7583:
7572:. Retrieved
7558:
7546:
7534:
7522:
7513:
7510:
7503:. Retrieved
7484:
7474:
7464:
7450:
7430:
7422:
7379:
7375:
7365:
7324:
7320:
7314:
7303:. Retrieved
7268:
7264:
7254:
7211:
7207:
7197:
7186:. Retrieved
7161:
7151:
7139:
7127:
7115:
7086:
7063:
7051:
7008:
7004:
6994:
6982:
6970:
6958:
6933:
6929:
6923:
6912:. Retrieved
6908:the original
6893:
6868:
6864:
6846:
6835:. Retrieved
6811:
6799:
6788:. Retrieved
6763:(6): 40–46.
6760:
6756:
6743:
6732:. Retrieved
6697:
6693:
6683:
6671:
6658:
6652:
6641:the original
6628:
6621:
6609:. Retrieved
6589:
6583:
6578:, p. 5.
6556:. Retrieved
6538:
6527:. Retrieved
6494:
6490:
6484:
6472:
6461:. Retrieved
6449:HyperPhysics
6448:
6413:(10): 5529.
6410:
6406:
6400:
6389:. Retrieved
6380:
6354:
6342:
6309:
6305:
6296:
6287:0130-39913-2
6277:
6257:. Retrieved
6248:
6218:. Retrieved
6199:
6189:
6178:. Retrieved
6171:the original
6124:
6121:Phys. Rev. E
6120:
6107:
6080:
6079:
6072:. Retrieved
6068:the original
6058:
6046:
6034:
6025:
6019:. Retrieved
5995:
5954:
5950:
5944:
5899:
5895:
5885:
5873:
5862:. Retrieved
5835:
5826:
5814:
5809:, p. 2.
5785:
5775:December 15,
5773:. Retrieved
5764:
5755:
5743:
5724:
5712:
5701:
5674:
5662:
5629:
5625:
5619:
5594:
5590:
5584:
5573:. Retrieved
5555:
5543:
5531:
5519:
5508:. Retrieved
5476:
5464:. Retrieved
5446:
5439:
5422:
5418:
5396:. Retrieved
5360:
5356:
5326:. Retrieved
5317:
5308:
5300:
5293:. Retrieved
5289:the original
5284:
5275:
5248:
5239:
5222:
5208:
5204:
5195:
5166:
5149:
5137:
5090:
5062:
4954:Water portal
4917:
4916:
4888:
4884:
4880:
4878:
4869:
4861:
4841:
4839:
4830:
4814:
4810:
4804:
4801:Nomenclature
4795:triple point
4756:
4746:
4726:electrolysis
4720:
4711:
4650:
4602:
4593:Electrolysis
4518:
4495:
4425:
4365:carbocations
4362:
4261:
4192:
4160:(Lewis acid)
4135:(Lewis base)
4086:(Lewis base)
4061:(Lewis acid)
4017:(Lewis base)
3992:(Lewis acid)
3954:
3849:
3729:
3700:
3677:
3662:
3660:
3643:
3571:
3514:
3424:
3404:
3362:
3346:metasomatism
3335:
3332:Geochemistry
3217:
3085:
2955:
2892:
2849:
2826:
2794:
2730:
2700:
2646:
2626:
2615:
2592:
2587:Havasu Falls
2574:Presence of
2555:
2543:
2470:
2463:
2447:
2432:is a gas at
2400:
2373:
2369:sigma bonded
2354:
2319:electrolyzed
2304:
2288:
2277:
2231:
2197:
2187:−70 °C
2176:−24 °C
2165:−35 °C
2082:Temperature
2058:
2042:triple point
2031:
2007:Triple point
2001:
1992:bulk modulus
1989:
1958:
1930:
1907:
1903:hydrocarbons
1880:
1844:
1830:
1822:Arctic Ocean
1816:
1809:
1781:
1750:
1746:
1726:
1710:
1691:
1664:
1616:
1568:liquid phase
1565:
1545:
1527:to a single
1489:
1407:
1383:
1332:
1328:
1327:
1110:
1089:Main hazards
1078:
1060:
1035:
1008:
987:
884:
817:
799:
756:acetonitrile
740:acetaldehyde
686:carboxylates
529:hint of blue
405:RTECS number
186:Identifiers
130:Other names
16508:Bismuthanes
13954:Oxypnictide
13354:Tin dioxide
9006:Ocean world
8931:Hydrosphere
8859:superheated
6611:19 November
6196:"Chapter 6"
5951:Soft Matter
5730:PubChem 962
5285:www.iun.edu
5104:represents
5043:Water model
5038:Water dimer
4993:Heavy water
4752:heavy water
4736:. In 1805,
4333:monodentate
4203:baking soda
3969:HSAB theory
3965:Lewis acids
3774:is formed:
3663:Light water
3542:radioactive
3530:heavy water
3462:quintillion
3441:. Only 155
3367:, known as
2872:Bent's rule
2821:tetrahedral
2739:light, and
2685:table sugar
2603:hydrophobic
2599:hydrophilic
2485:hydrophilic
2365:tetrahedral
2200:supercooled
2097:611.657 Pa
2034:temperature
1826:fresh water
1787:in central
1785:Lake Baikal
1767:(HDA), and
1740:. Regular,
1608:water vapor
1572:solid phase
1164:Flash point
1083:(OHS/OSH):
934:Point group
728:1,4-dioxane
716:isopropanol
668:haloalkanes
524:Appearance
485:Properties
259:CHEBI:15377
153:Hydric acid
17866:Hydroxides
17835:Categories
16531:Moscovanes
16396:Phosphenes
16258:Phosphanes
16056:Pnictogen
16013:Flerovanes
15164:Dihydrides
15114:(Group 2)
14010:Hydroxides
13949:Superoxide
12112:Zinc oxide
9128:Wiktionary
8963:management
8809:Properties
8657:August 31,
8483:2016-05-29
8399:2016-07-31
8311:2008-11-19
8198:2017-09-08
8155:2016-07-31
8112:IUPAC 2005
8070:(6): 341.
8048:2016-05-26
8019:2016-06-24
7901:2016-06-25
7883:"Hydrides"
7817:2023-04-11
7788:2023-04-11
7574:2008-03-21
7305:2019-09-08
7188:2019-07-05
6936:(1): 1–8.
6914:2009-12-06
6837:2013-02-19
6804:Sharp 1988
6790:2011-11-22
6734:2020-08-31
6635:(Thesis).
6529:2021-08-03
6463:2007-10-26
6391:2016-06-09
6259:2016-06-03
6220:2010-07-11
6180:2009-07-07
6021:2017-12-22
5864:2018-08-09
5575:2016-04-09
5510:2014-06-01
5466:17 October
5419:Metrologia
5398:2018-08-09
5363:(8): 612.
5263:References
5254:amphoteric
5228:atmosphere
4988:Hard water
4813:or simply
4775:Fahrenheit
4384:hydrolysis
3961:Lewis base
3957:lone pairs
3732:amphoteric
3703:frost line
3674:Occurrence
3433:of water.
3338:weathering
2964:of water,
2844:See also:
2769:absorption
2753:microwaves
2649:table salt
2589:turquoise.
2530:paper clip
2481:organelles
2459:spider web
2361:lone pairs
2341:See also:
2204:nucleation
2143:632.4 MPa
2129:350.1 MPa
2115:209.9 MPa
2061:polymorphs
1985:10 Pa
1977:10 Pa
1969:10 Pa
1875:See also:
1753:polymorphs
1633:is 22.064
1582:, such as
1522:covalently
1484:See also:
1452:activities
1410:amphoteric
1399:molar mass
913:Structure
662:Solubility
626:95 °C
610:25 °C
515:Molar mass
430:059QF0KO0R
290:ChemSpider
214:3D model (
193:CAS Number
17891:Limnology
17407:predicted
17351:predicted
17334:predicted
17063:predicted
16941:predicted
16600:Hydrogen
16547:predicted
16017:predicted
15989:Plumbanes
15953:Stannanes
15623:Annulenes
15525:predicted
15521:Nihonanes
15485:Thallanes
15450:Indiganes
15220:Group 13
15112:Alkaline
13924:Oxocarbon
9727:[PtCl
9041:Enceladus
9022:Specific
8958:Resources
8953:Pollution
8936:Hydrology
8902:Hydronium
8892:Tritiated
8882:Semiheavy
8787:Overviews
8709:213738895
8595:Chemistry
7539:Boyd 2000
7527:Boyd 2000
7238:0036-8075
7091:Lide 2003
6885:1099-0062
6724:122365938
6519:105357042
6443:Nave, R.
6334:1463-9084
6074:August 3,
6051:Lide 2003
6039:Lide 2003
6001:Rhein, M.
5979:1744-6848
5717:Lide 2003
5679:Lide 2003
5667:Lide 2003
5654:0047-2689
5536:Lide 2003
5524:Lide 2003
5481:Lide 2003
5385:0021-9584
5295:1 October
5184:germanium
5055:Footnotes
4754:in 1933.
4502:beryllium
4498:aluminium
4446:2 Na + 2
4392:digestion
4242:⇌ NaOH +
4195:hydrolyze
3730:Water is
3721:Reactions
3546:half-life
3447:deuterium
3415:acid rain
3360:hardens.
3310:−
3249:≈
3168:−
3151:⋅
3081:data page
3055:hydroxide
3030:−
2903:hydroxide
2901:ions and
2899:hydronium
2787:Model of
2779:Structure
2618:hydration
2576:colloidal
2376:structure
2238:insulator
2079:Pressure
1918:dew point
1881:Water is
1702:drift ice
1584:ice cubes
1408:Water is
1100:(as snow)
1098:Avalanche
1028:formation
1002:Std molar
926:Hexagonal
900:Viscosity
858:Hydroxide
837:Hydronium
736:sulfolane
672:aliphatic
573:0 °C
541:Odorless
412:ZC0110000
339:231-791-2
331:EC Number
203:7732-18-5
17320:< 1)
16866:Tellanes
16485:Stibanes
15880:Germanes
15415:Gallanes
15222:hydrides
13944:Peroxide
13934:Oxyanion
13929:Suboxide
9820:[SiF
9244:H[BF
9106:Category
8731:of water
8627:(2011),
8536:(1988).
8477:Archived
8432:37341352
8390:Archived
8369:(2005).
8305:Archived
8192:Archived
8149:Archived
8042:Archived
8013:Archived
7895:Archived
7891:UC Davis
7887:Chemwiki
7811:Archived
7779:Archived
7737:39474797
7565:Archived
7563:. 2001.
7505:21 April
7499:Archived
7451:Water, H
7406:18354466
7357:11061625
7349:18264420
7299:Archived
7295:27152824
7246:26989250
7182:Archived
7072:cite web
7043:24699509
7011:: 4565.
6828:Archived
6781:Archived
6728:Archived
6602:Archived
6558:9 August
6552:Archived
6523:Archived
6457:Archived
6385:Archived
6253:Archived
6214:Archived
6159:12241346
6012:Archived
5987:32900070
5936:16179387
5858:Archived
5769:Archived
5765:usgs.gov
5733:Archived
5681:, 6.186.
5569:Archived
5504:Archived
5460:Archived
5389:Archived
5328:21 April
5322:Archived
4940:See also
4831:oxidanyl
4827:hydroxyl
4546:catalyst
4432:hydrides
4350:O. This
4305:hydrates
4274:such as
3464:include
3445:include
3427:isotopes
3425:Several
3407:nitrogen
2805:methanol
2711:monomers
2633:alcohols
2473:adhesion
2466:cohesion
2184:626 MPa
2173:344 MPa
2162:213 MPa
2038:pressure
1895:polarity
1883:miscible
1871:Humidity
1841:salinity
1837:seawater
1771:(VHDA).
1698:glaciers
1643:volcanic
1629:and the
1588:granular
1580:crystals
1541:glaciers
1516:hydrogen
1512:molecule
1254:Methanol
1235:solvents
1233:Related
1111:NFPA 704
1094:Drowning
1073:Hazards
814:Basicity
724:glycerol
712:propanol
704:methanol
690:alcohols
676:aromatic
310:DrugBank
244:3587155
17305:< 1)
16902:Polanes
16829:Selanes
16815:more...
16677:more...
16449:Arsanes
16382:more...
16191:more...
15846:Silynes
15817:Silenes
15803:more...
15679:Silanes
15603:alkynes
15598:alkenes
15593:alkanes
15231:Boranes
14724:
14085:[NH
13939:Ozonide
13917:Related
11170:more...
9117:Commons
8794:Outline
8268:Biology
8141:Pubchem
8072:Bibcode
7629:Bibcode
7625:Science
7598:Bibcode
7414:4365814
7384:Bibcode
7329:Bibcode
7273:Bibcode
7216:Bibcode
7208:Science
7178:6642535
7034:3988813
7013:Bibcode
6938:Bibcode
6765:Bibcode
6702:Bibcode
6499:Bibcode
6483:O Ice I
6415:Bibcode
6314:Bibcode
6167:6109212
6139:Bibcode
5959:Bibcode
5927:1242322
5904:Bibcode
5634:Bibcode
5599:Bibcode
5365:Bibcode
5188:bismuth
5180:gallium
5172:silicon
5159:kelvins
4811:oxidane
4787:Réaumur
4779:Delisle
4771:Rankine
4767:Celsius
4717:History
4510:rusting
4506:passive
4301:rhenium
3852:ammonia
3711:ammonia
3707:methane
3670:level.
3668:155 ppm
3466:tritium
3439:protium
2897:giving
2813:kinetic
2755:. Most
2741:far-red
2715:hexamer
2663:cations
2595:solvent
2404:melting
2323:protons
2217:Ice VII
2111:ice III
2048:, but,
1899:soluble
1887:ethanol
1833:surface
1831:As the
1789:Siberia
1761:ice III
1729:density
1683:climate
1671:ammonia
1625:is 647
1359:solvent
1341:) is a
1310:what is
1308: (
1264:Ammonia
1249:Ethanol
1244:Acetone
1056:(Δ
1004:entropy
968:1.8546
826:13.995
808:13.995
796:Acidity
778:bromine
720:acetone
708:ethanol
694:ketones
547:Density
509:
381:PubChem
319:DB09145
123:Oxidane
17881:Oxides
16205:Azenes
16067:Azanes
15380:Alanes
14997:Cm(OH)
14987:Am(OH)
14961:Np(OH)
14952:Np(OH)
14907:Th(OH)
14897:Ac(OH)
14882:Yb(OH)
14872:Tm(OH)
14862:Er(OH)
14852:Ho(OH)
14842:Dy(OH)
14832:Tb(OH)
14822:Gd(OH)
14812:Eu(OH)
14803:Eu(OH)
14793:Sm(OH)
14783:Pm(OH)
14773:Nd(OH)
14763:Pr(OH)
14753:Ce(OH)
14744:Ce(OH)
14734:La(OH)
14661:Ra(OH)
14635:Bi(OH)
14625:Pb(OH)
14616:Pb(OH)
14606:Tl(OH)
14592:Hg(OH)
14582:Au(OH)
14551:Lu(OH)
14538:Ba(OH)
14513:Te(OH)
14503:Sb(OH)
14493:Sn(OH)
14484:Sn(OH)
14474:In(OH)
14464:Cd(OH)
14446:Rh(OH)
14433:Tc(OH)
14417:Zr(OH)
14395:Sr(OH)
14367:As(OH)
14357:Ge(OH)
14347:Ga(OH)
14337:Zn(OH)
14327:Cu(OH)
14313:Ni(OH)
14303:Co(OH)
14293:Fe(OH)
14284:Fe(OH)
14274:Mn(OH)
14264:Cr(OH)
14255:Cr(OH)
14226:Ti(OH)
14217:Ti(OH)
14208:Ti(OH)
14198:Sc(OH)
14186:Ca(OH)
14141:Si(OH)
14131:Al(OH)
14121:Mg(OH)
14046:Be(OH)
11212:Oxides
9121:
9110:
9099:
9095:Portal
9088:
9026:Europa
8975:Supply
8968:policy
8948:Origin
8839:Liquid
8831:States
8727:, and
8707:
8697:
8605:
8582:
8552:
8522:
8499:
8469:
8430:
8420:
8382:
8355:
8329:
8297:
8276:
8253:
8232:
7735:
7729:515218
7727:
7491:
7443:
7412:
7404:
7376:Nature
7355:
7347:
7293:
7244:
7236:
7176:
7041:
7031:
6883:
6722:
6517:
6332:
6284:
6206:
6165:
6157:
6081:Gramme
5985:
5977:
5934:
5924:
5850:
5652:
5383:
4912:hydron
4887:, and
4852:, and
4789:, and
4783:Newton
4773:, and
4763:Kelvin
4436:alkali
4264:ligand
3924:(acid)
3899:(base)
3811:(base)
3786:(acid)
3715:Tethys
3411:sulfur
2905:ions.
2862:. The
2856:linear
2854:, not
2817:steric
2751:, and
2677:anions
2635:, and
2622:solute
2392:dipole
2280:solute
2157:Ice II
2139:ice VI
2109:, and
2046:kelvin
1997:oceans
1757:ice II
1646:plumes
1621:. The
1546:Under
1529:oxygen
1525:bonded
1510:; one
1351:liquid
1305:verify
1302:
1194:anions
1192:Other
1176:(SDS)
860:OH (pK
698:amines
680:ethers
630:Solid:
469:SMILES
369:C00001
270:ChEMBL
165:Hydrol
101:Names
79:
73:
34:Water
14929:U(OH)
14920:U(OH)
14407:Y(OH)
14245:V(OH)
14236:V(OH)
14161:S(OH)
14151:P(OH)
14096:O(OH)
14076:N(OH)
14066:C(OH)
14056:B(OH)
9495:NaHCO
8921:Cycle
8913:Earth
8887:Heavy
8870:Forms
8854:Steam
8849:Vapor
8804:Model
8780:Water
8705:S2CID
8439:(PDF)
8414:(PDF)
8393:(PDF)
8376:(PDF)
7782:(PDF)
7767:(PDF)
7733:S2CID
7692:7 Jan
7665:7 Jan
7568:(PDF)
7560:IAPWS
7555:(PDF)
7459:and H
7410:S2CID
7353:S2CID
7060:(PDF)
6831:(PDF)
6824:IAPWS
6820:(PDF)
6784:(PDF)
6753:(PDF)
6720:S2CID
6644:(PDF)
6633:(PDF)
6605:(PDF)
6594:(PDF)
6515:S2CID
6174:(PDF)
6163:S2CID
6129:arXiv
6117:(PDF)
6015:(PDF)
6008:(PDF)
5728:GHS:
5698:Water
5392:(PDF)
5353:(PDF)
5268:Notes
5013:Steam
4930:steam
4926:water
4815:water
4807:IUPAC
4791:Rømer
4244:NaHCO
3738:or a
3536:as a
3057:ion (
2637:salts
2629:acids
2528:This
2477:glass
2325:(see
2151:ice I
2125:ice V
2089:ice I
2023:ice I
1612:steam
1519:atoms
1494:with
1416:or a
1367:solid
1363:Earth
1343:polar
1329:Water
864:= 0)
847:= 0)
843:O (pK
615:0.961
599:0.997
589:3.983
578:0.999
562:0.999
556:VSMOW
444:InChI
250:ChEBI
216:JSmol
111:Water
28:Water
14974:(OH)
14942:(OH)
14655:FrOH
14601:TlOH
14532:CsOH
14458:AgOH
14389:RbOH
14379:BrOH
14322:CuOH
14170:ClOH
14115:NaOH
14040:LiOH
9835:HNCS
9830:HSCN
9504:HNCO
9452:HMnO
9339:HCNO
9325:HClO
9315:HClO
9305:HClO
9299:HClO
9285:HBrO
9275:HBrO
9265:HBrO
9259:HBrO
9222:HArF
9036:Moon
9031:Mars
8799:Data
8744:NASA
8695:ISBN
8659:2016
8603:ISBN
8580:ISBN
8550:ISBN
8520:ISBN
8497:ISBN
8467:ISBN
8428:OCLC
8418:ISBN
8380:ISBN
8353:ISBN
8327:ISBN
8295:ISBN
8274:ISBN
8251:ISBN
8230:ISBN
7725:PMID
7694:2011
7667:2011
7507:2019
7489:ISBN
7465:bent
7441:ISBN
7402:PMID
7345:PMID
7291:PMID
7242:PMID
7234:ISSN
7174:OSTI
7078:link
7039:PMID
6881:ISSN
6613:2018
6560:2018
6330:ISSN
6282:ISBN
6204:ISBN
6155:PMID
6076:2016
5983:PMID
5975:ISSN
5932:PMID
5848:ISBN
5777:2020
5650:ISSN
5468:2023
5381:ISSN
5330:2019
5297:2018
4740:and
4732:and
4691:+ 4
4680:+ 4
4618:+ 2
4564:+ 2
4514:iron
4500:and
4482:+ 2
4471:+ 2
4410:and
4310:FeSO
4276:Fe(H
4201:and
4199:soap
3740:base
3736:acid
3709:and
3690:and
3585:and
3409:and
3386:·23H
3375:, 4
3340:and
2956:The
2852:bent
2759:and
2701:The
2665:and
2583:lime
2386:, a
2284:salt
2234:ions
2036:and
2032:The
1990:The
1959:The
1891:oils
1727:The
1700:and
1610:(or
1592:snow
1465:and
1433:and
1418:base
1414:acid
1390:ions
1377:and
955:Bent
674:and
537:Odor
421:UNII
360:KEGG
354:117
174:Aqua
17814:PsH
17793:CfH
17785:CfH
17777:BkH
17769:BkH
17761:CmH
17753:AmH
17745:AmH
17737:PuH
17729:PuH
17721:NpH
17713:NpH
17689:PaH
17671:ThH
17663:ThH
17655:AcH
17634:LuH
17626:LuH
17618:YbH
17610:TmH
17602:TmH
17594:ErH
17586:ErH
17578:HoH
17570:HoH
17562:DyH
17554:DyH
17546:TbH
17538:TbH
17530:GdH
17522:GdH
17514:EuH
17506:SmH
17498:SmH
17490:NdH
17482:NdH
17474:PrH
17466:PrH
17458:CeH
17450:CeH
17442:LaH
17434:LaH
17426:LaH
17399:CnH
17390:HgH
17373:HgH
17365:CdH
17357:ZnH
17346:RgH
17340:CuH
17326:DsH
17310:PtH
17295:PdH
17290:NiH
17282:IrH
17274:RhH
17266:CoH
17258:FeH
17250:FeH
17245:FeH
17237:CrH
17229:CrH
17224:CrH
17216:TaH
17211:TaH
17203:NbH
17198:NbH
17177:HfH
17169:HfH
17161:ZrH
17153:ZrH
17145:TiH
17137:TiH
17129:LuH
17121:LuH
17081:ScH
17058:HTs
17052:HAt
17042:HBr
17037:HCl
16995:HDO
16984:(?)
16981:O–O
16933:LvH
16909:PoH
16589:(?)
16539:McH
16515:BiH
16492:SbH
16457:AsH
16039:FlH
16031:FlH
16026:FlH
15996:PbH
15961:SnH
15888:GeH
15866:SiH
15687:SiH
15559:NhH
15539:NhH
15534:NhH
15492:TlH
15457:InH
15422:GaH
15387:AlH
15203:BaH
15195:SrH
15187:CaH
15179:MgH
15171:BeH
15151:BaH
15146:SrH
15141:CaH
15136:MgH
15131:BeH
15102:CsH
15097:RbH
15087:NaH
15082:LiH
15020:No
15017:Md
15014:Fm
15011:Es
15008:Cf
15005:Bk
14982:Pu
14970:NpO
14915:Pa
14892:**
14717:Og
14714:Ts
14711:Lv
14708:Mc
14705:Fl
14702:Nh
14699:Cn
14696:Rg
14693:Ds
14690:Mt
14687:Hs
14684:Bh
14681:Sg
14678:Db
14675:Rf
14672:Lr
14669:**
14649:Rn
14646:At
14643:Po
14577:Pt
14574:Ir
14571:Os
14568:Re
14562:Ta
14559:Hf
14526:Xe
14522:IOH
14454:Pd
14441:Ru
14428:Mo
14425:Nb
14383:Kr
14375:Se
14180:KOH
14174:Ar
14109:Ne
14105:FOH
14089:]OH
14034:He
14024:HOH
11161:PtF
11036:NbO
11031:NbO
11002:NaO
10924:MoO
10916:MoO
10884:MnO
10879:MnO
10862:MgO
10789:IrO
10698:HgO
10690:HfO
10634:GeO
10629:GeO
10579:FeO
10538:CuO
10518:CsO
10474:CrO
10445:CoO
10368:ClO
10336:CeO
10331:CdO
10323:CaO
10318:CaO
10237:BrO
10217:BiO
10200:BeO
10195:BaO
10127:AmO
10024:TiO
10010:TeO
9996:TeO
9806:SiO
9792:SeO
9778:SeO
9568:NSO
9553:HNO
9525:HNO
9515:HNO
9509:HNO
9489:HNC
9480:MoO
9466:MnO
9442:HIO
9432:HIO
9422:HIO
9416:HIO
9406:HFO
9349:CrO
9334:HCN
9294:HCl
9254:HBr
9233:HSO
9227:HAt
9213:AsO
9199:AsO
8911:On
8844:Ice
8687:doi
8572:doi
8222:doi
8080:doi
7717:doi
7637:doi
7606:doi
7594:114
7392:doi
7380:452
7337:doi
7281:doi
7269:116
7224:doi
7212:351
7166:doi
7029:PMC
7021:doi
6946:doi
6873:doi
6773:doi
6710:doi
6698:131
6507:doi
6423:doi
6322:doi
6147:doi
5967:doi
5922:PMC
5912:doi
5900:102
5840:doi
5642:doi
5607:doi
5452:doi
5427:doi
5373:doi
5232:bar
5003:Ice
4934:ice
4554:AgF
4512:of
4402:in
4319:·7H
4295:to
4266:in
4167:Cl(
4093:Fe(
3781:HCl
3554:HDO
3550:THO
3443:ppm
3348:or
2455:Dew
2219:).
1981:3.9
1973:4.4
1965:5.1
1926:dew
1924:or
1922:fog
1817:not
1804:WOA
1635:MPa
1576:ice
1381:).
1371:gas
1180:SDS
1039:298
1012:298
624:at
617:887
608:at
601:047
591:035
587:at
580:974
571:at
564:842
394:962
384:CID
299:937
17837::
17705:UH
17697:UH
17684:15
17678:Th
17444:10
17378:Hg
17190:VH
17185:VH
17113:YH
17105:YH
17097:YH
17089:YH
17047:HI
17032:HF
16990:HS
16968:HO
16960:HO
16955:HO
16886:Te
16877:Te
16850:Se
16841:Se
16809:10
16583:NH
16574:HN
16569:NH
16561:HN
16465:As
16376:12
16372:10
16364:11
16352:10
16266:PH
16185:12
16181:10
16173:11
16161:10
16075:NH
15969:Sn
15938:12
15932:Ge
15926:10
15920:Ge
15908:Ge
15896:Ge
15854:Si
15825:Si
15797:22
15793:10
15791:Si
15785:20
15779:Si
15773:18
15767:Si
15761:16
15755:Si
15749:14
15743:Si
15737:12
15731:Si
15725:10
15719:Si
15707:Si
15695:Si
15653:CH
15645:CH
15640:CH
15547:Nh
15500:Tl
15465:In
15430:Ga
15395:Al
15366:22
15362:18
15354:14
15350:10
15342:12
15330:10
15318:11
15294:10
15247:BH
15239:BH
15092:KH
14938:UO
14729:*
14565:W
14546:*
13968:.
13903:Hs
13889:Xe
13875:Ru
13861:Os
13847:Ir
13819:Tc
13802:Re
13785:Mn
13768:Cl
13743:Xe
13701:Te
13673:Se
13659:Re
13645:Po
13631:Mo
13617:Cr
13572:Ta
13555:Pa
13521:Nb
13487:Bi
13470:As
13453:Sb
13428:Zr
13372:Ti
13358:Sn
13344:Th
13330:Tb
13316:Te
13302:Tc
13274:Si
13260:Se
13246:Ru
13232:Rh
13218:Pa
13204:Pr
13190:Po
13176:Pu
13162:Pt
13148:Os
13120:Np
13106:Mn
13092:Pb
13078:Hf
13064:Ir
13036:Ge
13005:Cm
12991:Cr
12977:Cl
12963:Ce
12921:Cf
12907:Br
12893:Bk
12879:Am
12834:Yb
12783:Ti
12766:Tm
12749:Tl
12732:Tb
12715:Sc
12698:Sm
12681:Rh
12664:Pm
12647:Pr
12613:Ni
12596:Nd
12579:Mn
12562:Lu
12545:La
12528:Fe
12511:In
12494:Ho
12477:Au
12460:Ga
12443:Gd
12426:Eu
12409:Er
12392:Es
12375:Dy
12341:Co
12324:Cr
12307:Ce
12290:Cf
12273:Cs
12239:Bi
12222:Bk
12205:As
12188:Sb
12171:Am
12154:Al
12137:Ac
12118:O)
12116:Zn
12108:O)
12098:O)
12088:O)
12086:Ti
12078:O)
12076:Sn
12068:O)
12066:Th
12041:O)
12031:O)
12029:Sr
12021:O)
12019:Si
12011:O)
12009:Ra
12001:O)
11999:Pa
11991:O)
11989:Po
11981:O)
11971:O)
11969:Pd
11961:O)
11951:O)
11949:Ni
11941:O)
11939:Hg
11931:O)
11929:Mn
11921:O)
11919:Mg
11911:O)
11909:Pb
11901:O)
11891:O)
11889:Fe
11881:O)
11879:Ge
11871:O)
11869:Eu
11844:O)
11842:Cu
11834:O)
11832:Co
11824:O)
11822:Cr
11814:O)
11812:Cl
11804:O)
11794:O)
11792:Ca
11784:O)
11782:Cd
11774:O)
11772:Br
11764:O)
11762:Be
11754:O)
11752:Bk
11744:O)
11742:Ba
11734:O)
11732:Al
11713:O)
11700:O)
11695:Na
11687:O)
11682:Tl
11674:O)
11669:Ag
11661:O)
11656:Rb
11648:O)
11635:O)
11622:O)
11617:Hg
11609:O)
11604:Li
11596:O)
11583:O)
11578:Ga
11570:O)
11565:Cl
11557:O)
11544:O)
11539:Cs
11531:O)
11526:Cu
11518:O)
11513:Al
11468:Br
11451:Tb
11434:Ag
11424:11
11417:Pr
11403:12
11383:Mn
11366:Pb
11349:Fe
11332:Cl
11315:Co
11298:Cl
11281:Cl
11250:12
11230:Sb
11077:OF
11070:OF
11045:Nd
11019:Na
11010:Na
10949:NO
10944:NO
10932:Mo
10904:Mn
10892:Mn
10867:Mg
10850:Lu
10838:Li
10829:Li
10817:La
10797:KO
10777:In
10724:IO
10712:Ho
10703:Hg
10617:Ga
10608:Ga
10596:Fe
10584:Fe
10567:Eu
10555:Er
10543:Dy
10526:Cs
10512:12
10506:Cr
10494:Cr
10482:Cr
10462:Co
10450:Co
10433:Cl
10421:Cl
10409:Cl
10397:Cl
10385:Cl
10376:Cl
10356:Ce
10344:Ce
10298:CO
10293:CO
10270:Br
10257:Br
10245:Br
10225:Bi
10205:Bi
10171:Au
10159:As
10147:As
10135:Am
10115:Al
10103:Ag
10038:Po
9985:Te
9970:SO
9966:CF
9873:SO
9859:SO
9845:SO
9767:Se
9715:10
9677:PO
9663:PO
9649:PO
9411:HI
9401:HF
9392:CS
9378:CO
9360:Cr
8742:,
8723:,
8719:,
8703:.
8693:.
8681:.
8649:.
8631:,
8601:.
8578:.
8548:.
8546:27
8475:.
8461:.
8426:.
8388:.
8351:.
8303:.
8228:.
8184:.
8147:.
8143:.
8139:.
8092:^
8078:.
8066:.
8036:.
7985:^
7970:^
7889:.
7885:.
7838:^
7805:.
7775:26
7773:.
7769:.
7731:.
7723:.
7713:23
7711:.
7684:.
7657:.
7635:.
7604:.
7592:.
7557:.
7509:.
7497:.
7483:.
7449:.
7439:.
7437:59
7408:.
7400:.
7390:.
7378:.
7374:.
7351:.
7343:.
7335:.
7325:36
7323:.
7297:.
7289:.
7279:.
7267:.
7263:.
7240:.
7232:.
7222:.
7210:.
7206:.
7180:.
7172:.
7160:.
7098:^
7074:}}
7070:{{
7062:.
7037:.
7027:.
7019:.
7007:.
7003:.
6944:.
6934:74
6932:.
6902:.
6879:.
6867:.
6855:^
6822:.
6779:.
6771:.
6761:56
6759:.
6755:.
6726:.
6718:.
6708:.
6696:.
6692:.
6661:.
6596:.
6568:^
6550:.
6546:.
6521:.
6513:.
6505:.
6495:47
6493:.
6489:.
6455:.
6451:.
6447:.
6435:^
6421:.
6411:59
6409:.
6379:.
6366:^
6328:.
6320:.
6308:.
6268:^
6251:.
6247:.
6229:^
6212:.
6161:.
6153:.
6145:.
6137:.
6125:66
6123:.
6119:.
6090:^
6078:.
6024:.
5981:.
5973:.
5965:.
5953:.
5930:.
5920:.
5910:.
5898:.
5894:.
5856:.
5846:.
5834:.
5797:^
5686:^
5648:.
5640:.
5630:24
5628:.
5605:.
5595:94
5593:.
5563:.
5502:.
5488:^
5458:.
5423:38
5421:.
5407:^
5387:.
5379:.
5371:.
5361:70
5359:.
5355:.
5338:^
5320:.
5316:.
5299:.
5283:.
5132:O)
5123:(H
5075:^
4928:,
4901:OH
4883:,
4876:.
4848:,
4837:.
4785:,
4781:,
4769:,
4765:,
4668:→
4655:2
4636:→
4607:2
4552:4
4533:/H
4484:OH
4473:Na
4459:→
4434:,
4418:.
4373:OH
4346:7H
4285:O)
4229:+
4219:CO
4210:Na
4165:→
4140:+
4122:Cl
4091:→
4066:+
4048:Fe
4022:→
3997:+
3943:OH
3941:+
3931:NH
3929:⇌
3904:+
3885:NH
3856:NH
3854:,
3838:Cl
3836:+
3816:⇌
3791:+
3692:CO
3641:.
3417:.
3377:CH
3059:OH
3051:pH
2944:OH
2942:+
2922:⇌
2909:2
2811:,
2775:.
2747:,
2735:,
2667:Cl
2653:Na
2631:,
2511:,
2507:,
2503:,
2315:cm
2311:μS
2297:,
2291:kΩ
2246:OH
2155:,
1905:.
1853:.
1763:,
1759:,
1689:.
1467:OH
1436:OH
1405:.
1031:(Δ
941:2v
908:)
906:cP
823:)
816:(p
805:)
798:(p
780:.
776:,
772:,
768:,
764:,
758:.
754:,
750:,
746:,
742:,
738:,
734:,
730:,
726:,
722:,
718:,
714:,
710:,
706:,
700:.
696:,
692:,
688:,
682:.
670:,
620:91
604:02
583:95
567:83
558:):
17795:3
17787:2
17779:3
17771:2
17763:2
17755:3
17747:2
17739:3
17731:2
17723:3
17715:2
17707:4
17699:3
17691:3
17682:H
17680:4
17673:4
17665:2
17657:2
17636:3
17628:2
17620:2
17612:3
17604:2
17596:3
17588:2
17580:3
17572:2
17564:3
17556:2
17548:3
17540:2
17532:3
17524:2
17516:2
17508:3
17500:2
17492:3
17484:2
17476:3
17468:2
17460:3
17452:2
17436:3
17428:2
17409:)
17405:(
17401:2
17392:2
17384:2
17382:H
17380:2
17367:2
17359:2
17353:)
17349:(
17336:)
17332:(
17328:2
17318:x
17316:(
17313:x
17303:x
17301:(
17298:x
17284:3
17276:2
17268:2
17260:5
17252:2
17239:x
17231:2
17218:2
17205:2
17192:2
17179:4
17171:2
17163:4
17155:2
17147:4
17139:2
17131:3
17123:2
17115:9
17107:6
17099:3
17091:2
17083:2
17065:)
17061:(
17013:O
17011:2
17009:T
17004:O
17002:2
17000:D
16979:2
16977:H
16970:3
16962:2
16943:)
16939:(
16935:2
16911:2
16888:2
16884:2
16882:H
16875:2
16873:H
16852:2
16848:2
16846:H
16839:2
16837:H
16807:S
16805:2
16803:H
16797:9
16795:S
16793:2
16791:H
16785:8
16783:S
16781:2
16779:H
16773:7
16771:S
16769:2
16767:H
16761:6
16759:S
16757:2
16755:H
16749:5
16747:S
16745:2
16743:H
16737:4
16735:S
16733:2
16731:H
16725:3
16723:S
16721:2
16719:H
16713:2
16711:S
16709:2
16707:H
16702:S
16700:2
16698:H
16671:5
16669:O
16667:2
16665:H
16659:4
16657:O
16655:2
16653:H
16647:3
16645:O
16643:2
16641:H
16635:2
16633:O
16631:2
16629:H
16624:O
16622:2
16620:H
16585:5
16576:5
16563:3
16549:)
16545:(
16541:3
16517:3
16494:3
16471:4
16469:H
16467:2
16459:3
16434:4
16432:H
16430:4
16428:P
16422:3
16420:H
16418:3
16416:P
16410:2
16408:H
16406:2
16404:P
16374:H
16370:P
16362:H
16360:9
16358:P
16350:H
16348:8
16346:P
16340:9
16338:H
16336:7
16334:P
16328:8
16326:H
16324:6
16322:P
16316:7
16314:H
16312:5
16310:P
16304:6
16302:H
16300:4
16298:P
16292:5
16290:H
16288:3
16286:P
16280:4
16278:H
16276:2
16274:P
16268:3
16243:4
16241:H
16239:4
16237:N
16231:3
16229:H
16227:3
16225:N
16219:2
16217:H
16215:2
16213:N
16183:H
16179:N
16171:H
16169:9
16167:N
16159:H
16157:8
16155:N
16149:9
16147:H
16145:7
16143:N
16137:8
16135:H
16133:6
16131:N
16125:7
16123:H
16121:5
16119:N
16113:6
16111:H
16109:4
16107:N
16101:5
16099:H
16097:3
16095:N
16089:4
16087:H
16085:2
16083:N
16077:3
16041:4
16033:2
16019:)
16015:(
15998:4
15975:6
15973:H
15971:2
15963:4
15936:H
15934:5
15924:H
15922:4
15914:8
15912:H
15910:3
15902:6
15900:H
15898:2
15890:4
15860:2
15858:H
15856:2
15831:4
15829:H
15827:2
15795:H
15783:H
15781:9
15771:H
15769:8
15759:H
15757:7
15747:H
15745:6
15735:H
15733:5
15723:H
15721:4
15713:8
15711:H
15709:3
15701:6
15699:H
15697:2
15689:4
15665:H
15663:2
15661:C
15655:3
15647:2
15561:5
15553:6
15551:H
15549:2
15541:3
15527:)
15523:(
15506:6
15504:H
15502:2
15494:3
15471:6
15469:H
15467:2
15459:3
15436:6
15434:H
15432:2
15424:3
15401:6
15399:H
15397:2
15389:3
15364:H
15360:B
15352:H
15348:B
15340:H
15338:6
15336:B
15328:H
15326:6
15324:B
15316:H
15314:5
15312:B
15306:9
15304:H
15302:5
15300:B
15292:H
15290:4
15288:B
15282:4
15280:H
15278:2
15276:B
15270:2
15268:H
15266:2
15264:B
15258:6
15256:H
15254:2
15252:B
15241:3
15205:2
15197:2
15189:2
15181:2
15173:2
15056:e
15049:t
15042:v
14999:3
14989:3
14976:3
14972:2
14963:4
14954:3
14944:2
14940:2
14931:3
14922:2
14909:4
14899:3
14884:3
14874:3
14864:3
14854:3
14844:3
14834:3
14824:3
14814:3
14805:2
14795:3
14785:3
14775:3
14765:3
14755:4
14746:3
14736:3
14663:2
14637:3
14627:4
14618:2
14608:3
14594:2
14584:3
14553:3
14540:2
14515:6
14505:3
14495:4
14486:2
14476:3
14466:2
14448:3
14435:4
14419:4
14409:3
14397:2
14369:3
14359:2
14349:3
14339:2
14329:2
14315:2
14305:2
14295:3
14286:2
14276:2
14266:3
14257:2
14247:3
14238:2
14228:4
14219:3
14210:2
14200:3
14188:2
14163:2
14153:3
14143:4
14133:3
14123:2
14098:2
14087:4
14078:3
14068:4
14058:3
14048:2
14002:e
13995:t
13988:v
13909:)
13907:4
13905:O
13901:(
13895:)
13893:4
13891:O
13887:(
13881:)
13879:4
13877:O
13873:(
13867:)
13865:4
13863:O
13859:(
13853:)
13851:4
13849:O
13845:(
13828:)
13826:7
13824:O
13822:2
13817:(
13811:)
13809:7
13807:O
13805:2
13800:(
13794:)
13792:7
13790:O
13788:2
13783:(
13777:)
13775:7
13773:O
13771:2
13766:(
13749:)
13747:3
13745:O
13741:(
13735:)
13733:3
13731:O
13729:U
13727:(
13721:)
13719:3
13717:O
13715:W
13713:(
13707:)
13705:3
13703:O
13699:(
13693:)
13691:3
13689:O
13687:S
13685:(
13679:)
13677:3
13675:O
13671:(
13665:)
13663:3
13661:O
13657:(
13651:)
13649:3
13647:O
13643:(
13637:)
13635:3
13633:O
13629:(
13623:)
13621:3
13619:O
13615:(
13598:)
13596:5
13594:O
13592:2
13589:V
13587:(
13581:)
13579:5
13577:O
13575:2
13570:(
13564:)
13562:5
13560:O
13558:2
13553:(
13547:)
13545:5
13543:O
13541:2
13538:P
13536:(
13530:)
13528:5
13526:O
13524:2
13519:(
13513:)
13511:5
13509:O
13507:2
13504:N
13502:(
13496:)
13494:5
13492:O
13490:2
13485:(
13479:)
13477:5
13475:O
13473:2
13468:(
13462:)
13460:5
13458:O
13456:2
13451:(
13434:)
13432:2
13430:O
13426:(
13420:)
13418:2
13416:O
13414:V
13412:(
13406:)
13404:2
13402:O
13400:U
13398:(
13392:)
13390:2
13388:O
13386:W
13384:(
13378:)
13376:2
13374:O
13370:(
13364:)
13362:2
13360:O
13356:(
13350:)
13348:2
13346:O
13342:(
13336:)
13334:2
13332:O
13328:(
13322:)
13320:2
13318:O
13314:(
13308:)
13306:2
13304:O
13300:(
13294:)
13292:2
13290:O
13288:S
13286:(
13280:)
13278:2
13276:O
13272:(
13266:)
13264:2
13262:O
13258:(
13252:)
13250:2
13248:O
13244:(
13238:)
13236:2
13234:O
13230:(
13224:)
13222:2
13220:O
13216:(
13210:)
13208:2
13206:O
13202:(
13196:)
13194:2
13192:O
13188:(
13182:)
13180:2
13178:O
13174:(
13168:)
13166:2
13164:O
13160:(
13154:)
13152:2
13150:O
13146:(
13140:)
13138:2
13136:O
13134:N
13132:(
13126:)
13124:2
13122:O
13118:(
13112:)
13110:2
13108:O
13104:(
13098:)
13096:2
13094:O
13090:(
13084:)
13082:2
13080:O
13076:(
13070:)
13068:2
13066:O
13062:(
13056:)
13054:2
13052:O
13050:I
13048:(
13042:)
13040:2
13038:O
13034:(
13028:)
13026:4
13024:O
13022:2
13019:N
13017:(
13011:)
13009:2
13007:O
13003:(
12997:)
12995:2
12993:O
12989:(
12983:)
12981:2
12979:O
12975:(
12969:)
12967:2
12965:O
12961:(
12955:)
12953:3
12951:O
12949:C
12947:(
12941:)
12939:2
12937:O
12935:C
12933:(
12927:)
12925:2
12923:O
12919:(
12913:)
12911:2
12909:O
12905:(
12899:)
12897:2
12895:O
12891:(
12885:)
12883:2
12881:O
12877:(
12860:)
12858:3
12856:O
12854:2
12851:Y
12849:(
12843:)
12841:3
12839:O
12837:2
12832:(
12826:)
12824:3
12822:O
12820:2
12817:V
12815:(
12809:)
12807:3
12805:O
12803:2
12800:W
12798:(
12792:)
12790:3
12788:O
12786:2
12781:(
12775:)
12773:3
12771:O
12769:2
12764:(
12758:)
12756:3
12754:O
12752:2
12747:(
12741:)
12739:3
12737:O
12735:2
12730:(
12724:)
12722:3
12720:O
12718:2
12713:(
12707:)
12705:3
12703:O
12701:2
12696:(
12690:)
12688:3
12686:O
12684:2
12679:(
12673:)
12671:3
12669:O
12667:2
12662:(
12656:)
12654:3
12652:O
12650:2
12645:(
12639:)
12637:6
12635:O
12633:4
12630:P
12628:(
12622:)
12620:3
12618:O
12616:2
12611:(
12605:)
12603:3
12601:O
12599:2
12594:(
12588:)
12586:3
12584:O
12582:2
12577:(
12571:)
12569:3
12567:O
12565:2
12560:(
12554:)
12552:3
12550:O
12548:2
12543:(
12537:)
12535:3
12533:O
12531:2
12526:(
12520:)
12518:3
12516:O
12514:2
12509:(
12503:)
12501:3
12499:O
12497:2
12492:(
12486:)
12484:3
12482:O
12480:2
12475:(
12469:)
12467:3
12465:O
12463:2
12458:(
12452:)
12450:3
12448:O
12446:2
12441:(
12435:)
12433:3
12431:O
12429:2
12424:(
12418:)
12416:3
12414:O
12412:2
12407:(
12401:)
12399:3
12397:O
12395:2
12390:(
12384:)
12382:3
12380:O
12378:2
12373:(
12367:)
12365:3
12363:O
12361:2
12358:N
12356:(
12350:)
12348:3
12346:O
12344:2
12339:(
12333:)
12331:3
12329:O
12327:2
12322:(
12316:)
12314:3
12312:O
12310:2
12305:(
12299:)
12297:3
12295:O
12293:2
12288:(
12282:)
12280:3
12278:O
12276:2
12271:(
12265:)
12263:3
12261:O
12259:2
12256:B
12254:(
12248:)
12246:3
12244:O
12242:2
12237:(
12231:)
12229:3
12227:O
12225:2
12220:(
12214:)
12212:3
12210:O
12208:2
12203:(
12197:)
12195:3
12193:O
12191:2
12186:(
12180:)
12178:3
12176:O
12174:2
12169:(
12163:)
12161:3
12159:O
12157:2
12152:(
12146:)
12144:3
12142:O
12140:2
12135:(
12114:(
12106:Y
12104:(
12096:V
12094:(
12084:(
12074:(
12064:(
12058:)
12056:2
12054:O
12052:2
12049:S
12047:(
12039:S
12037:(
12027:(
12017:(
12007:(
11997:(
11987:(
11979:P
11977:(
11967:(
11959:N
11957:(
11947:(
11937:(
11927:(
11917:(
11907:(
11899:I
11897:(
11887:(
11877:(
11867:(
11861:)
11859:2
11857:O
11855:2
11852:N
11850:(
11840:(
11830:(
11820:(
11810:(
11802:C
11800:(
11790:(
11780:(
11770:(
11760:(
11750:(
11740:(
11730:(
11711:2
11708:H
11706:(
11698:2
11693:(
11685:2
11680:(
11672:2
11667:(
11659:2
11654:(
11646:2
11643:K
11641:(
11633:2
11630:N
11628:(
11620:2
11615:(
11607:2
11602:(
11594:2
11591:I
11589:(
11581:2
11576:(
11568:2
11563:(
11555:2
11552:C
11550:(
11542:2
11537:(
11529:2
11524:(
11516:2
11511:(
11494:)
11492:8
11490:O
11488:3
11485:U
11483:(
11477:)
11475:8
11473:O
11471:3
11466:(
11460:)
11458:7
11456:O
11454:4
11449:(
11443:)
11441:2
11439:O
11437:2
11432:(
11426:)
11422:O
11420:6
11415:(
11409:)
11407:9
11405:O
11400:C
11398:(
11392:)
11390:4
11388:O
11386:3
11381:(
11375:)
11373:4
11371:O
11369:3
11364:(
11358:)
11356:4
11354:O
11352:3
11347:(
11341:)
11339:5
11337:O
11335:2
11330:(
11324:)
11322:4
11320:O
11318:3
11313:(
11307:)
11305:6
11303:O
11301:2
11296:(
11290:)
11288:4
11286:O
11284:2
11279:(
11273:)
11271:2
11269:O
11267:3
11264:C
11262:(
11256:)
11254:2
11252:O
11247:B
11245:(
11239:)
11237:4
11235:O
11233:2
11228:(
11204:e
11197:t
11190:v
11163:6
11159:2
11157:O
11149:2
11147:F
11145:6
11143:O
11135:2
11133:F
11131:5
11129:O
11121:2
11119:F
11117:4
11115:O
11107:2
11105:F
11103:3
11101:O
11093:2
11091:F
11089:2
11087:O
11079:2
11063:F
11061:2
11059:O
11051:3
11049:O
11047:2
11038:2
11025:2
11023:O
11021:2
11014:O
11012:2
11004:2
10996:5
10994:O
10992:2
10990:N
10984:4
10982:O
10980:2
10978:N
10972:3
10970:O
10968:2
10966:N
10961:O
10959:2
10957:N
10951:2
10938:3
10936:O
10934:2
10926:3
10918:2
10910:7
10908:O
10906:2
10898:3
10896:O
10894:2
10886:2
10873:3
10871:O
10869:2
10856:3
10854:O
10852:2
10844:2
10842:O
10840:2
10833:O
10831:2
10823:3
10821:O
10819:2
10811:2
10809:O
10807:2
10805:K
10799:2
10791:2
10783:3
10781:O
10779:2
10771:9
10769:O
10767:4
10765:I
10759:6
10757:O
10755:2
10753:I
10747:5
10745:O
10743:2
10741:I
10735:4
10733:O
10731:2
10729:I
10718:3
10716:O
10714:2
10707:O
10705:2
10692:2
10684:2
10682:O
10680:2
10678:H
10673:O
10671:2
10669:H
10664:O
10662:2
10660:H
10655:O
10653:2
10651:H
10646:O
10644:2
10642:H
10636:2
10623:3
10621:O
10619:2
10612:O
10610:2
10602:4
10600:O
10598:3
10590:3
10588:O
10586:2
10573:3
10571:O
10569:2
10561:3
10559:O
10557:2
10549:3
10547:O
10545:2
10532:3
10530:O
10528:2
10520:2
10510:O
10508:5
10500:5
10498:O
10496:2
10488:3
10486:O
10484:2
10476:3
10468:4
10466:O
10464:3
10456:3
10454:O
10452:2
10439:7
10437:O
10435:2
10427:6
10425:O
10423:2
10415:4
10413:O
10411:2
10403:3
10401:O
10399:2
10391:2
10389:O
10387:2
10380:O
10378:2
10370:2
10362:3
10360:O
10358:2
10350:4
10348:O
10346:3
10338:2
10325:2
10312:2
10310:O
10308:3
10306:C
10300:2
10284:8
10279:O
10275:3
10263:5
10261:O
10259:2
10251:3
10249:O
10247:2
10239:2
10231:5
10229:O
10227:2
10219:2
10211:3
10209:O
10207:2
10189:3
10187:O
10185:2
10183:B
10177:3
10175:O
10173:2
10165:5
10163:O
10161:2
10153:3
10151:O
10149:2
10141:3
10139:O
10137:2
10129:2
10121:3
10119:O
10117:2
10109:4
10107:O
10105:4
10084:e
10077:t
10070:v
10049:]
10047:4
10036:2
10034:H
10026:4
10022:4
10020:H
10012:6
10008:6
10006:H
9998:3
9994:2
9992:H
9983:2
9981:H
9974:H
9972:3
9968:3
9958:8
9956:O
9954:2
9952:S
9950:2
9948:H
9940:7
9938:O
9936:2
9934:S
9932:2
9930:H
9922:6
9920:O
9918:2
9916:S
9914:2
9912:H
9905:O
9903:3
9901:H
9893:3
9891:O
9889:2
9887:S
9885:2
9883:H
9875:5
9871:2
9869:H
9861:4
9857:2
9855:H
9847:3
9843:2
9841:H
9824:]
9822:6
9818:2
9816:H
9808:4
9804:4
9802:H
9794:4
9790:2
9788:H
9780:3
9776:2
9774:H
9765:2
9763:H
9755:2
9753:S
9751:2
9749:H
9742:S
9740:2
9738:H
9731:]
9729:6
9725:2
9723:H
9713:O
9711:3
9709:P
9707:5
9705:H
9697:7
9695:O
9693:2
9691:P
9689:4
9687:H
9679:4
9675:3
9673:H
9665:3
9661:3
9659:H
9651:2
9647:3
9645:H
9637:5
9635:O
9633:2
9631:H
9623:4
9621:O
9619:2
9617:H
9609:3
9607:O
9605:2
9603:H
9595:2
9593:O
9591:2
9589:H
9582:O
9580:2
9578:H
9570:3
9566:3
9564:H
9557:S
9555:5
9545:2
9543:O
9541:2
9539:N
9537:2
9535:H
9527:3
9517:2
9497:3
9482:4
9478:2
9476:H
9468:4
9464:2
9462:H
9454:4
9444:4
9434:3
9424:2
9394:3
9390:2
9388:H
9380:3
9376:2
9374:H
9366:7
9364:O
9362:2
9358:2
9356:H
9354:/
9351:4
9347:2
9345:H
9327:4
9317:3
9307:2
9287:4
9277:3
9267:2
9248:]
9246:4
9237:F
9235:3
9215:4
9211:3
9209:H
9201:3
9197:3
9195:H
9175:e
9168:t
9161:v
8772:e
8765:t
8758:v
8711:.
8689::
8661:.
8611:.
8588:.
8574::
8558:.
8528:.
8505:.
8486:.
8402:.
8361:.
8335:.
8314:.
8282:.
8259:.
8238:.
8224::
8201:.
8158:.
8086:.
8082::
8074::
8068:1
8051:.
8022:.
7904:.
7820:.
7739:.
7719::
7696:.
7669:.
7643:.
7639::
7631::
7612:.
7608::
7600::
7577:.
7467:.
7461:2
7457:3
7453:2
7416:.
7394::
7386::
7359:.
7339::
7331::
7308:.
7283::
7275::
7248:.
7226::
7218::
7191:.
7168::
7080:)
7045:.
7023::
7015::
7009:5
6952:.
6948::
6940::
6917:.
6887:.
6875::
6869:8
6840:.
6793:.
6775::
6767::
6737:.
6712::
6704::
6678:.
6665:.
6615:.
6562:.
6532:.
6509::
6501::
6485:h
6481:2
6466:.
6429:.
6425::
6417::
6394:.
6336:.
6324::
6316::
6310:3
6290:.
6262:.
6223:.
6183:.
6149::
6141::
6131::
5989:.
5969::
5961::
5955:3
5938:.
5914::
5906::
5880:.
5867:.
5842::
5779:.
5656:.
5644::
5636::
5613:.
5609::
5601::
5578:.
5513:.
5470:.
5454::
5433:.
5429::
5401:.
5375::
5367::
5332:.
5217:.
5174:(
5161:.
5138:n
5128:2
5115:O
5111:3
5106:H
5095:H
5068:a
4922:2
4893:H
4700:e
4682:H
4675:2
4670:O
4666:O
4662:2
4657:H
4643:2
4638:H
4627:e
4609:H
4584:2
4579:O
4575:O
4571:2
4566:H
4559:2
4542:O
4538:2
4529:2
4524:O
4466:2
4461:H
4457:O
4453:2
4448:H
4406:N
4404:S
4348:2
4344:4
4328:O
4324:2
4315:4
4290:6
4281:2
4249:3
4240:O
4236:2
4231:H
4224:3
4215:2
4185:6
4180:)
4178:O
4174:2
4169:H
4154:O
4150:2
4145:H
4111:6
4106:)
4104:O
4100:2
4095:H
4080:O
4076:2
4071:H
4033:O
4029:3
4024:H
4011:O
4007:2
4002:H
3979:H
3936:4
3918:O
3914:2
3909:H
3890:3
3868:H
3861:3
3827:O
3823:3
3818:H
3805:O
3801:2
3796:H
3761:H
3749:H
3696:×
3685:2
3680:H
3655:O
3651:2
3646:D
3639:O
3635:2
3630:D
3626:O
3622:2
3617:D
3613:O
3609:2
3604:H
3596:O
3592:2
3587:D
3583:O
3579:2
3574:H
3567:O
3563:2
3558:D
3526:O
3522:2
3517:D
3510:O
3499:O
3488:O
3477:H
3468:(
3458:H
3449:(
3395:O
3391:2
3382:4
3317:]
3306:H
3302:O
3297:[
3294:]
3287:+
3283:O
3277:3
3273:H
3267:[
3264:=
3258:w
3253:K
3243:q
3240:e
3235:K
3224:2
3220:3
3199:2
3193:O
3188:2
3184:H
3178:a
3164:H
3160:O
3155:a
3143:+
3139:O
3133:3
3129:H
3123:a
3116:=
3110:q
3107:e
3102:K
3070:H
3037:]
3026:H
3022:O
3017:[
3014:]
3007:+
3003:O
2997:3
2993:H
2987:[
2984:=
2978:w
2973:K
2933:O
2929:3
2924:H
2920:O
2916:2
2911:H
2515:.
2430:S
2426:2
2421:H
2417:S
2413:2
2408:H
2331:×
2313:/
2266:O
2262:3
2257:H
2153:h
2107:h
2091:h
2025:h
1983:×
1975:×
1967:×
1627:K
1508:O
1504:2
1499:H
1456:H
1423:H
1338:O
1336:2
1334:H
1331:(
1300:Y
1152:0
1145:0
1138:0
1063:)
1061:G
1058:f
1041:)
1036:H
1033:f
1014:)
1009:S
1007:(
990:)
988:C
986:(
970:D
939:C
890:)
888:D
885:n
883:(
862:b
845:a
841:3
839:H
821:b
818:K
803:a
800:K
507:O
503:2
498:H
476:O
218:)
30:.
23:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.