5427:
4473:
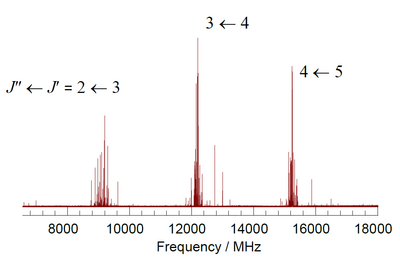
into three states, J = K + 1, K, and K - 1, each J state of this so-called p-type triplet arising from a different orientation of the spin with respect to the rotational motion of the molecule. The energy difference between successive J terms in any of these triplets is about 2 cm (60 GHz), with the single exception of J = 1←0 difference which is about 4 cm. Selection rules for magnetic dipole transitions allow transitions between successive members of the triplet (ΔJ = ±1) so that for each value of the rotational angular momentum quantum number K there are two allowed transitions. The O nucleus has zero nuclear spin angular momentum, so that symmetry considerations demand that K have only odd values.
5906:
frequency in less than a microsecond and (ii) the use of a high speed (>40 GS/s) oscilloscope to digitise and
Fourier transform the molecular free induction decay. The result is an instrument that allows the study of weakly bound molecules but which is able to exploit a measurement bandwidth (12 GHz) that is greatly enhanced compared with the Balle-Flygare FTMW spectrometer. Modified versions of the original CP-FTMW spectrometer have been constructed by a number of groups in the United States, Canada and Europe. The instrument offers a broadband capability that is highly complementary to the high sensitivity and resolution offered by the Balle-Flygare design.
2105:, between the vibrational motion of the nuclei in the rotating (non-inertial) frame. However, as long as the vibrational quantum number does not change (i.e., the molecule is in only one state of vibration), the effect of vibration on rotation is not important, because the time for vibration is much shorter than the time required for rotation. The Coriolis coupling is often negligible, too, if one is interested in low vibrational and rotational quantum numbers only.
3436:
7996:
5402:
20:
8008:
5863:. First, a short (typically 0-3 microsecond duration) microwave pulse is introduced on resonance with a rotational transition. Those molecules that absorb the energy from this pulse are induced to rotate coherently in phase with the incident radiation. De-activation of the polarisation pulse is followed by microwave emission that accompanies decoherence of the molecular ensemble. This
5782:, fundamental physical constraints exist on the operational bandwidth of instrument components. It is often impractical and costly to switch to measurements within an entirely different frequency region. The instruments and operating principals described below are generally appropriate to microwave spectroscopy experiments conducted at frequencies between 6 and 24 GHz.
1989:
2629:θ and φ which describe the direction of the molecular axis, and the quantum state is determined by two quantum numbers J and M. J defines the magnitude of the rotational angular momentum, and M its component about an axis fixed in space, such as an external electric or magnetic field. In the absence of external fields, the energy depends only on J. Under the
5091:
5102:
3286:
5888:
enhancement in the sensitivity and resolution of spectrometers along with a reduction in the complexity of observed spectra; (ii) it became possible to isolate and study molecules that are very weakly bound because there is insufficient energy available for them to undergo fragmentation or chemical reaction at such low temperatures.
4181:
2610:
1484:), which have both a zero dipole moment and isotropic polarizability, would not have a pure rotation spectrum but for the effect of centrifugal distortion; when the molecule rotates about a 3-fold symmetry axis a small dipole moment is created, allowing a weak rotation spectrum to be observed by microwave spectroscopy.
5450:
The water molecule is an important example of an asymmetric top. It has an intense pure rotation spectrum in the far infrared region, below about 200 cm. For this reason far infrared spectrometers have to be freed of atmospheric water vapour either by purging with a dry gas or by evacuation. The
6036:
Such transitions are called electric dipole-allowed transitions. Other transitions involving quadrupoles, octupoles, hexadecapoles etc. may also be allowed but the spectral intensity is very much smaller, so these transitions are difficult to observe. Magnetic-dipole-allowed transitions can occur in
5905:
at the
University of Virginia designed a spectrometer which retains many advantages of the Balle-Flygare FT-MW spectrometer while innovating in (i) the use of a high speed (>4 GS/s) arbitrary waveform generator to generate a "chirped" microwave polarisation pulse that sweeps up to 12 GHz in
5892:
was a pioneer in using this instrument for the exploration of weakly bound interactions. While the Fabry-Perot cavity of a Balle-Flygare FTMW spectrometer can typically be tuned into resonance at any frequency between 6 and 18 GHz, the bandwidth of individual measurements is restricted to about
5745:
which includes all molecules except for spherical tops. This means that rotational transitions of molecules with no permanent dipole moment, which cannot be observed in absorption or emission, can be observed, by scattering, in Raman spectroscopy. Very high resolution Raman spectra can be obtained
2584:
Spherical top molecules have no net dipole moment. A pure rotational spectrum cannot be observed by absorption or emission spectroscopy because there is no permanent dipole moment whose rotation can be accelerated by the electric field of an incident photon. Also the polarizability is isotropic, so
481:
can be deduced. When this is not possible, as with most asymmetric tops, all that can be done is to fit the spectra to three moments of inertia calculated from an assumed molecular structure. By varying the molecular structure the fit can be improved, giving a qualitative estimate of the structure.
4472:
with two unpaired electrons so that there are magnetic-dipole allowed transitions which can be observed by microwave spectroscopy. The unit electron spin has three spatial orientations with respect to the given molecular rotational angular momentum vector, K, so that each rotational level is split
114:
For rotational spectroscopy, molecules are classified according to symmetry into spherical tops, linear molecules, and symmetric tops; analytical expressions can be derived for the rotational energy terms of these molecules. Analytical expressions can be derived for the fourth category, asymmetric
5777:
The great majority of contemporary spectrometers use a mixture of commercially available and bespoke components which users integrate according to their particular needs. Instruments can be broadly categorised according to their general operating principles. Although rotational transitions can be
3455:
The probability of a transition taking place is the most important factor influencing the intensity of an observed rotational line. This probability is proportional to the population of the initial state involved in the transition. The population of a rotational state depends on two factors. The
173:
Rotational spectroscopy has primarily been used to investigate fundamental aspects of molecular physics. It is a uniquely precise tool for the determination of molecular structure in gas-phase molecules. It can be used to establish barriers to internal rotation such as that associated with the
5887:
in the throat of an expanding gas jet. This was a revolutionary development because (i) cooling molecules to low temperatures concentrates the available population in the lowest rotational energy levels. Coupled with benefits conferred by the use of a Fabry-Perot cavity, this brought a great
5798:
can serve as an absorption cell. An important variation of the technique in which an alternating current is applied across electrodes within the absorption cell results in a modulation of the frequencies of rotational transitions. This is referred to as Stark modulation and allows the use of
1555:
and anti-Stokes lines can be observed and they have similar intensities due to the fact that many rotational states are thermally populated. The selection rule for linear molecules is ΔJ = 0, ±2. The reason for the values ±2 is that the polarizability returns to the same value twice during a
6026:
For a symmetric top, the values of the 2 moments of inertia can be used to derive 2 molecular parameters. Values from each additional isotopologue provide the information for one more molecular parameter. For asymmetric tops a single isotopologue provides information for at most 3 molecular
1792:
4686:
5397:{\displaystyle {\tilde {\nu }}_{J^{\prime }\leftrightarrow J^{\prime \prime },K}=F\left(J^{\prime },K\right)-F\left(J^{\prime \prime },K\right)=2\left(B-2D_{JK}K^{2}\right)\left(J^{\prime \prime }+1\right)-4D_{J}\left(J^{\prime \prime }+1\right)^{3}\qquad J^{\prime \prime }=0,1,2,...}
4882:
3099:
320:. Rotation about each unique axis is associated with a set of quantized energy levels dependent on the moment of inertia about that axis and a quantum number. Thus, for linear molecules the energy levels are described by a single moment of inertia and a single quantum number,
5438:
refers to the total angular momentum, as before. Since there are three independent moments of inertia, there are two other independent quantum numbers to consider, but the term values for an asymmetric rotor cannot be derived in closed form. They are obtained by individual
2326:
5656:
components. The extent of splitting depends on the square of the electric field strength and the square of the dipole moment of the molecule. In principle this provides a means to determine the value of the molecular dipole moment with high precision. Examples include
4000:
4440:
is the harmonic vibration frequency, follows. If anharmonicity is to be taken into account, terms in higher powers of J should be added to the expressions for the energy levels and line positions. A striking example concerns the rotational spectrum of
731:(and, hence, of transitions in the rotational spectrum) for a molecule is determined by its symmetry. A convenient way to look at the molecules is to divide them into four different classes, based on the symmetry of their structure. These are
3966:
367:. Analysis of spectroscopic data with the expressions detailed below results in quantitative determination of the value(s) of the moment(s) of inertia. From these precise values of the molecular structure and dimensions may be obtained.
3609:
2072:
4509:
of the molecule. In the absence of an external electrical field, the rotational energy of a symmetric top is a function of only J and K and, in the rigid rotor approximation, the energy of each rotational state is given by
1984:{\displaystyle {\tilde {\nu }}/{\text{cm}}^{-1}={\frac {1}{\lambda /{\text{cm}}}}={\frac {\nu /{\text{s}}^{-1}}{c/\left({\text{cm}}\cdot \mathrm {s} ^{-1}\right)}}={\frac {\nu /{\text{s}}^{-1}}{2.99792458\times 10^{10}}}.}
6083:
This value of J corresponds to the maximum of the population considered as a continuous function of J. However, since only integer values of J are allowed, the maximum line intensity is observed for a neighboring integer
2431:
are the rotational quantum numbers of the upper and lower levels. In reality, this expression has to be modified for the effects of anharmonicity of the vibrations, for centrifugal distortion and for
Coriolis coupling.
234:). When fine or hyperfine structure can be observed, the technique also provides information on the electronic structures of molecules. Much of current understanding of the nature of weak molecular interactions such as
3723:
2913:
5086:{\displaystyle {\tilde {\nu }}_{J^{\prime }\leftrightarrow J^{\prime \prime },K}=F\left(J^{\prime },K\right)-F\left(J^{\prime \prime },K\right)=2B\left(J^{\prime \prime }+1\right)\qquad J^{\prime \prime }=0,1,2,...}
4516:
398:
in the vibrational ground state, to which the rotational states refer, whereas the equilibrium bond length is at the minimum in the potential energy curve. The relation between the rotational constants is given by
2756:
is the rotational constant of the molecule and is related to the moment of inertia of the molecule. In a linear molecule the moment of inertia about an axis perpendicular to the molecular axis is unique, that is,
2731:
3281:{\displaystyle {\tilde {\nu }}_{J^{\prime }\leftrightarrow J^{\prime \prime }}=F\left(J^{\prime }\right)-F\left(J^{\prime \prime }\right)=2B\left(J^{\prime \prime }+1\right)\qquad J^{\prime \prime }=0,1,2,...}
6484:
Jennings, D.A.; Evenson, K.M; Zink, L.R.; Demuynck, C.; Destombes, J.L.; Lemoine, B; Johns, J.W.C. (April 1987). "High-resolution spectroscopy of HF from 40 to 1100 cm: Highly accurate rotational constants".
3091:
465:
3793:
4406:
3417:
1551:
that can be represented as an ellipsoid. The polarizability ellipsoid of spherical top molecules is in fact spherical so those molecules show no rotational Raman spectrum. For all other molecules both
5867:
occurs on a timescale of 1-100 microseconds depending on instrument settings. Following pioneering work by Dicke and co-workers in the 1950s, the first FTMW spectrometer was constructed by Ekkers and
4292:
2090:) decrease. Consequently, the rotation frequencies in each vibration state are different from each other. This can give rise to "satellite" lines in the rotational spectrum. An example is provided by
3003:
2625:
connected by rigid bonds. A linear molecule lies on a single axis and each atom moves on the surface of a sphere around the centre of mass. The two degrees of rotational freedom correspond to the
7160:
Grubbs, G.S.; Dewberry, C.T.; Etchison, K.C.; Kerr, K.E.; Cooke, S.A. (2007). "A search accelerated correct intensity
Fourier transform microwave spectrometer with pulsed laser ablation source".
3830:, when it is calculated using the expression for the rigid rotor. To account for this a centrifugal distortion correction term is added to the rotational energy levels of the diatomic molecule.
343:
For nonlinear molecules which are symmetric rotors (or symmetric tops - see next section), there are two moments of inertia and the energy also depends on a second rotational quantum number,
5623:
4176:{\displaystyle {\tilde {\nu }}_{J^{\prime }\leftrightarrow J^{\prime \prime }}=2B\left(J^{\prime \prime }+1\right)-4D\left(J^{\prime \prime }+1\right)^{3}\qquad J^{\prime \prime }=0,1,2,...}
4867:
4805:
4747:
7125:
Brown, G.G.; Dian, B.C.; Douglass, K.O.; Geyer, S.M.; Pate, B.H. (2006). "The rotational spectrum of epifluorohydrin measured by chirped-pulse
Fourier transform microwave spectroscopy".
1703:
6070:
In Raman spectroscopy the photon energies for Stokes and anti-Stokes scattering are respectively less than and greater than the incident photon energy. See the energy-level diagram at
2188:
678:
2525:
2479:
470:
where v is a vibrational quantum number and α is a vibration-rotation interaction constant which can be calculated if the B values for two different vibrational states can be found.
2086:, so low-frequency vibrational states are appreciably populated even at room temperatures. As the moment of inertia is higher when a vibration is excited, the rotational constants (
1765:
5790:
A microwave spectrometer can be most simply constructed using a source of microwave radiation, an absorption cell into which sample gas can be introduced and a detector such as a
1251:
1191:
944:
2814:
4438:
3319:
791:
6015:
2571:
Rotational constants obtained from infrared measurements are in good accord with those obtained by microwave spectroscopy, while the latter usually offers greater precision.
1656:
2404:
2354:
6524:
5803:
methods offering improved sensitivity. Absorption spectroscopy allows the study of samples that are thermodynamically stable at room temperature. The first study of the
3346:
1127:
1087:
5562:. Thus, observation of nuclear quadrupole splitting permits the magnitude of the nuclear quadrupole moment to be determined. This is an alternative method to the use of
2554:
5839:
was able to prepare a review of the results contained in approximately 100 research papers. Commercial versions of microwave absorption spectrometer were developed by
3372:
2127:, so the energy of rotation is added to the energy of vibration. For example, the rotational energy levels for linear molecules (in the rigid-rotor approximation) are
2429:
2379:
5769:. It shows the effect of nuclear spin, resulting in intensities variation of 3:1 in adjacent lines. A bond length of 109.9985 ± 0.0010 pm was deduced from the data.
3836:
971:
609:
582:
555:
1723:
3484:
7827:
5969:
5949:
5879:
Balle, Campbell, Keenan and
Flygare demonstrated that the FTMW technique can be applied within a "free space cell" comprising an evacuated chamber containing a
4343:
4323:
4211:
3989:
3828:
2754:
718:
698:
528:
361:
338:
2000:
6203:"Quality assessment of ground-based microwave measurements of chlorine monoxide, ozone, and nitrogen dioxide from the NDSC radiometer at the Plateau de Bure"
2199:
5471:
moment. In that case, coupling of nuclear spin angular momentum with rotational angular momentum causes splitting of the rotational energy levels. If the
146:
from which very precise values of molecular bond lengths and angles can be derived in favorable cases. In the presence of an electrostatic field there is
7061:
Balle, T.J.; Campbell, E.J.; Keenan, M.R.; Flygare, W.H. (1980). "A new method for observing the rotational spectra of weak molecular complexes: KrHCl".
4186:
In consequence, the spacing between lines is not constant, as in the rigid rotor approximation, but decreases with increasing rotational quantum number.
7500:
5922:
The spectrum was measured over a couple of hours with the aid of a chirped-pulse
Fourier transform microwave spectrometer at the University of Bristol.
6799:
Burkhalter, James H.; Roy S. Anderson; William V. Smith; Walter Gordy (1950). "The Fine
Structure of the Microwave Absorption Spectrum of Oxygen".
292:. Current projects in astrochemistry involve both laboratory microwave spectroscopy and observations made using modern radiotelescopes such as the
5096:
which is the same as in the case of a linear molecule. With a first order correction for centrifugal distortion the transition wavenumbers become
5794:. A spectrum can be obtained by sweeping the frequency of the source while detecting the intensity of transmitted radiation. A simple section of
4681:{\displaystyle F\left(J,K\right)=BJ\left(J+1\right)+\left(A-B\right)K^{2}\qquad J=0,1,2,\ldots \quad {\mbox{and}}\quad K=+J,\ldots ,0,\ldots ,-J}
6393:
Alexander, A. J.; Kroto, H. W.; Walton, D. R. M. (1967). "The microwave spectrum, substitution structure and dipole moment of cyanobutadiyne".
5893:
1 MHz. An animation illustrates the operation of this instrument which is currently the most widely used tool for microwave spectroscopy.
3653:
2822:
7718:
6119:
Nair, K.P.R.; Demaison, J.; Wlodarczak, G.; Merke, I. (236). "Millimeterwave rotational spectrum and internal rotation in o-chlorotoluene".
2123:, which was used before microwave spectroscopy had become practical. To a first approximation, the rotation and vibration can be treated as
7651:
7596:
7565:
2643:
2119:
Historically, the theory of rotational energy levels was developed to account for observations of vibration-rotation spectra of gases in
2585:
that pure rotational transitions cannot be observed by Raman spectroscopy either. Nevertheless, rotational constants can be obtained by
7560:
107:
where changes in rotational energy occur together with changes in vibrational energy, and also from ro-vibronic spectroscopy (or just
7933:
7751:
7613:
5668:. However, because the splitting depends on μ, the orientation of the dipole must be deduced from quantum mechanical considerations.
382:
this process is straightforward. For linear molecules with more than two atoms it is necessary to measure the spectra of two or more
95:
spectroscopy. The rotational spectra of non-polar molecules cannot be observed by those methods, but can be observed and measured by
119:
need to be determined using numerical methods. The rotational energies are derived theoretically by considering the molecules to be
7882:
7701:
3035:
2593:
is a spherical top but the asymmetric C-H stretching band shows rotational fine structure in the infrared spectrum, illustrated in
2527:
so that a quantum of rotational energy is lost while a quantum of vibrational energy is gained. The purely vibrational transition,
405:
7822:
7624:
7545:
7525:
6724:
Simmons, James W.; Anderson, Wallace E.; Gordy, Walter (1950). "Microwave
Spectrum and Molecular Constants of Hydrogen Cyanide".
5901:
Noting that digitisers and related electronics technology had significantly progressed since the inception of FTMW spectroscopy,
5747:
3734:
2124:
2114:
4350:
3377:
3374:=1 selection rule. The dashed lines show how these transitions map onto features that can be observed experimentally. Adjacent
7768:
7746:
7493:
4219:
1662:, written as cm, which is literally the number of waves in one centimeter, or the reciprocal of the wavelength in centimeters (
1617:
series. Since Raman transitions involve two photons, it is possible for the molecular angular momentum to change by two units.
2924:
7834:
7756:
7406:
7378:
6869:
Cleeton, C.E.; Williams, N.H. (1934). "Electromagnetic waves of 1.1 cm wave-length and the absorption spectrum of ammonia".
7691:
7636:
7586:
84:
7203:
Wilcox, D.S.; Hotopp, K.M.; Dian, B.C. (2011). "Two-Dimensional
Chirped-Pulse Fourier Transform Microwave Spectroscopy".
2594:
2586:
139:
104:
2621:
is a good starting point from which to construct a model of a rotating molecule. It is assumed that component atoms are
1448:. A consequence of this rule is that no microwave spectrum can be observed for centrosymmetric linear molecules such as
394:). A bond length obtained in this way is slightly different from the equilibrium bond length. This is because there is
7918:
7670:
7486:
7357:
7314:
7289:
5843:
in the 1970s and were once widely used for fundamental research. Most research laboratories now exploit either Balle-
5572:
794:
7923:
7741:
4814:
4752:
4694:
1504:
3810:
pulls the atoms apart. As a result, the moment of inertia of the molecule increases, thus decreasing the value of
8044:
7938:
7908:
7839:
7773:
1393:
The three moments of inertia have different values. Examples of small molecules that are asymmetric tops include
1665:
1385:
axis. Since the unique moment of inertia is larger than the other two, the molecule is an oblate symmetric top.
7867:
7658:
7555:
7446:
7425:
7335:
7270:
5883:. This technique allows a sample to be probed only milliseconds after it undergoes rapid cooling to only a few
5426:
2133:
630:
371:
293:
316:
of the molecule. Free rotation is not possible for molecules in liquid or solid phases due to the presence of
8012:
7665:
7570:
6553:
5440:
495:
5517:) in HCN, all levels with J > 0 are split into 3. The energies of the sub-levels are proportional to the
7799:
7646:
7535:
5563:
1735:
45:
1424:
whose symmetry axis of highest order is a 2-fold rotation axis. Most large molecules are asymmetric tops.
1203:
1143:
896:
482:
Isotopic substitution is invaluable when using this approach to the determination of molecular structure.
7955:
7794:
7763:
7696:
2760:
1507:
implies that the molecular angular momentum can change by at most one unit. Moreover, the quantum number
157:
An important application of rotational spectroscopy is in exploration of the chemical composition of the
5819:) was performed by Cleeton & Williams in 1934. Subsequent experiments exploited powerful sources of
4414:
3294:
1625:
The units used for rotational constants depend on the type of measurement. With infrared spectra in the
1539:
photon is emitted. The general selection rule for such a transition to be allowed is that the molecular
1253:. The spectra look rather different, and are instantly recognizable. Examples of symmetric tops include
374:
and the moment of inertia of the molecule, and, knowing the atomic masses, can be used to determine the
55:
is concerned with the measurement of the energies of transitions between quantized rotational states of
7945:
7887:
7736:
7608:
3032:
dictate that during emission or absorption the rotational quantum number has to change by unity; i.e.,
743:
6373:
6371:
7971:
7950:
7591:
5974:
2589:. This occurs when a molecule is polar in the vibrationally excited state. For example, the molecule
1632:
1503:. Since these transitions are due to absorption (or emission) of a single photon with a spin of one,
7713:
3798:
The diagram at the right shows an intensity pattern roughly corresponding to the spectrum above it.
6368:
5791:
5779:
4485:
is associated with the total angular momentum of the molecule. For a given value of J, there is a 2
3636:
1133:. As a matter of convenience, spectroscopists divide molecules into two classes of symmetric tops,
7468:
5931:
This article uses the molecular spectroscopist's convention of expressing the rotational constant
7844:
7392:
5835:. The number of experiments in microwave spectroscopy surged immediately after the war. By 1948,
5447:
value. Formulae are available for molecules whose shape approximates to that of a symmetric top.
3324:
1487:
With symmetric tops, the selection rule for electric-dipole-allowed pure rotation transitions is
1092:
1052:
2530:
1130:
721:
7631:
7262:
6154:
Cheung, A.C.; Rank, D.M.; Townes, C.H.; Thornton, D.D. & Welch, W.J. (1968). "Detection of
5855:
The theoretical framework underpinning FTMW spectroscopy is analogous to that used to describe
5472:
5414:
has the effect of removing degeneracy present in the rigid rotor approximation, with different
4190:
3961:{\displaystyle F\left(J\right)=BJ\left(J+1\right)-DJ^{2}\left(J+1\right)^{2}\qquad J=0,1,2,...}
3475:
2083:
1445:
387:
151:
68:
3354:
2488:
2442:
8034:
8000:
7872:
7603:
7517:
6939:
2626:
2120:
874:
627:
of the system. The general convention, used in this article, is to define the axes such that
473:
For other molecules, if the spectra can be resolved and individual transitions assigned both
289:
108:
7254:
3604:{\displaystyle {\frac {N_{J}}{N_{0}}}=e^{-{\frac {E_{J}}{kT}}}=e^{-{\frac {BhcJ(J+1)}{kT}}}}
7212:
7169:
7134:
7070:
7035:
7000:
6965:
6913:
6878:
6808:
6733:
6696:
6665:
6581:
6536:
6494:
6402:
6214:
6175:
6128:
5864:
3628:
1547:, which means that it is not the same in all directions. Polarizability is a 3-dimensional
949:
810:
587:
560:
533:
317:
142:. Fitting the spectra to the theoretical expressions gives numerical values of the angular
72:
24:
5430:
Pure rotation spectrum of atmospheric water vapour measured at Mauna Kea (33 cm to 100 cm)
1708:
8:
7928:
7641:
7550:
7388:
5507:
5460:
2384:
2334:
1557:
1300:
858:
281:
255:
254:. Microwave transitions are measured in the laboratory and matched to emissions from the
251:
235:
158:
132:
80:
7216:
7173:
7138:
7074:
7039:
7004:
6977:
6969:
6917:
6882:
6812:
6737:
6700:
6669:
6656:
Hall, Richard T.; Dowling, Jerome M. (1967). "Pure Rotational Spectrum of Water Vapor".
6585:
6540:
6498:
6406:
6218:
6179:
6132:
2409:
2359:
2067:{\displaystyle {\tilde {\nu }}/{\text{cm}}^{-1}\approx {\frac {\nu /{\text{GHz}}}{30}}.}
7976:
7913:
7892:
7708:
7686:
7619:
7530:
7112:"Web page of B.H. Pate Research Group, Department of Chemistry, University of Virginia"
6071:
5954:
5934:
5735:
4506:
4328:
4308:
4196:
3974:
3813:
3620:
2739:
2560:
branch of the spectrum. Because of the thermal population of the rotational states the
2381:
are rotational constants for the upper and lower vibrational state respectively, while
1659:
1556:
rotation. The value ΔJ = 0 does not correspond to a molecular transition but rather to
1528:
703:
683:
513:
478:
364:
346:
323:
96:
2321:{\displaystyle {\tilde {\nu }}={\tilde {\nu }}_{\text{vib}}+B''J''(J''+1)-B'J'(J'+1),}
8039:
7877:
7804:
7778:
7442:
7421:
7402:
7374:
7353:
7331:
7310:
7285:
7266:
7255:
7228:
7185:
7026:
Ekkers, J.; Flygare, W.H. (1976). "Pulsed microwave Fourier transform spectrometer".
6798:
6506:
6414:
6242:
5889:
5868:
5844:
5800:
5518:
4442:
3807:
1783:
507:
499:
491:
395:
285:
143:
124:
76:
7097:
1444:
Transitions between rotational states can be observed in molecules with a permanent
111:) where rotational, vibrational and electronic energy changes occur simultaneously.
7220:
7177:
7142:
7078:
7043:
7008:
6973:
6921:
6886:
6816:
6741:
6704:
6673:
6589:
6544:
6502:
6410:
6222:
6183:
6136:
5856:
5708:
5688:
5658:
4189:
An assumption underlying these expressions is that the molecular vibration follows
3435:
2098:
2091:
1410:
1394:
1026:
1022:
1010:
700:
corresponding to the smallest moment of inertia. Some authors, however, define the
503:
370:
For a linear molecule, analysis of the rotational spectrum provides values for the
7436:
7396:
7368:
7325:
7304:
7300:
7253:; de Paula, J. (2006). "Molecular Spectroscopy: Section: Pure rotation spectra".
6856:
5860:
5840:
3423:
in the observed spectrum. Frequency or wavenumber units can also be used for the
3029:
2598:
2481:
so that there is simultaneous excitation of both vibration and rotation. For the
2102:
1434:
1337:
1256:
1135:
1006:
259:
250:, the technique has a key role in exploration of the chemical composition of the
247:
162:
6566:
6187:
7473:
6991:
Dicke, R.H.; Romer, R.H. (1955). "Pulse Techniques in Microwave Spectroscopy".
6227:
6202:
5880:
5742:
5704:
5634:
4302:
2622:
1540:
1014:
624:
313:
246:
bonds has been established through rotational spectroscopy. In connection with
136:
128:
7146:
6925:
6140:
2609:
363:, which defines the vector component of rotational angular momentum along the
44:, of the final and initial states, and is extensively split by the effects of
8028:
5676:
3093:. Thus, the locations of the lines in a rotational spectrum will be given by
2193:
In this approximation, the vibration-rotation wavenumbers of transitions are
1321:
239:
209:
7159:
7060:
6548:
2613:
Energy levels and line positions calculated in the rigid rotor approximation
1049:
A symmetric top is a molecule in which two moments of inertia are the same,
7509:
7250:
7232:
7189:
6890:
6820:
6777:, p. 102 gives the equations for diatomic molecules and symmetric tops
6166:
molecules in the interstellar medium by their microwave emission spectra".
6058:
6038:
5836:
5832:
5684:
5672:
5664:
5646:
4469:
728:
383:
243:
147:
116:
92:
6956:
Schwendemann, R.H. (1978). "Transient Effects in Microwave Spectroscopy".
6745:
6483:
6153:
1729:. The relationship between these two units is derived from the expression
7345:
7111:
6511:
5902:
5739:
5680:
3718:{\displaystyle {\text{population}}\propto (2J+1)e^{-{\frac {E_{J}}{kT}}}}
2908:{\displaystyle B={h \over {8\pi ^{2}cI_{B}}}={h \over {8\pi ^{2}cI_{C}}}}
2630:
2618:
1552:
1544:
530:, of the molecule. For any molecule, there are three moments of inertia:
474:
391:
375:
120:
7124:
6723:
5645:
degeneracy of each rotational state is partly removed, an instance of a
5468:
2597:. This spectrum is also interesting because it shows clear evidence of
1775:
1626:
1461:
309:
277:
7224:
7181:
7047:
7012:
6709:
6684:
6677:
6593:
6118:
7082:
6522:
6392:
5820:
5804:
5795:
5738:. Rotational transitions are Raman-allowed for any molecule with an
5566:
spectroscopy. The selection rule for rotational transitions becomes
1994:
As 1 GHz = 10 Hz, the numerical conversion can be expressed as
1771:
1726:
1030:
305:
88:
60:
2726:{\displaystyle F\left(J\right)=BJ\left(J+1\right)\qquad J=0,1,2,...}
1705:). On the other hand, for microwave spectra in the frequency scale (
506:
can take only certain fixed values, which are related simply to the
6042:
5847:
or chirped-pulse Fourier transform microwave (FTMW) spectrometers.
5824:
5712:
5649:. For example, in linear molecules each energy level is split into
1129:. By definition a symmetric top must have a 3-fold or higher order
990:
379:
56:
5850:
340:, which defines the magnitude of the rotational angular momentum.
40:. Each rotational transition is labeled with the quantum numbers,
7474:
A list of microwave spectroscopy research groups around the world
2590:
1481:
1316:
1285:
1261:
1195:
826:
264:
19:
6314:, p. 104 shows part of the observed rotational spectrum of
3994:
Therefore, the line positions for the rotational mode change to
7478:
7463:
6315:
5884:
5859:. The behaviour of the evolving system is described by optical
1548:
1465:
1033:
and dihaloethynes. These molecules belong to the point groups C
974:
842:
5679:. Most species which can be observed in the gaseous state are
973:
can be taken to be zero. Examples of linear molecules include
6106:
Microwave Molecular Spectra in Technique of Organic Chemistry
5828:
3351:
The diagram illustrates rotational transitions that obey the
3086:{\displaystyle \Delta J=J^{\prime }-J^{\prime \prime }=\pm 1}
873:
and other hexahalides. The molecules all belong to the cubic
7202:
6621:, p. 102 shows the effect on the microwave spectrum of
6523:
Strandberg, M. W. P.; Meng, C. Y.; Ingersoll, J. G. (1949).
3456:
number of molecules in an excited state with quantum number
2108:
893:
For a linear molecule the moments of inertia are related by
460:{\displaystyle B_{v}=B-\alpha \left(v+{\frac {1}{2}}\right)}
6200:
5675:
molecule is placed in a magnetic field, an instance of the
3788:{\displaystyle J={\sqrt {\frac {kT}{2hcB}}}-{\frac {1}{2}}}
3460:, relative to the number of molecules in the ground state,
4401:{\displaystyle D={\frac {4B^{3}}{{\tilde {\omega }}^{2}}}}
3412:{\displaystyle J^{\prime \prime }{\leftarrow }J^{\prime }}
7418:
Rotational Structure in the Spectra of Diatomic Molecules
5734:
Molecular rotational transitions can also be observed by
4287:{\displaystyle D={\frac {h^{3}}{32\pi ^{4}I^{2}r^{2}kc}}}
4193:. In the harmonic approximation the centrifugal constant
2082:
The population of vibrationally excited states follows a
5785:
2998:{\displaystyle I={\frac {m_{1}m_{2}}{m_{1}+m_{2}}}d^{2}}
1560:
in which the incident photon merely changes direction.
1354:
As a detailed example, ammonia has a moment of inertia
6682:
6655:
5359:
5328:
5282:
5205:
5141:
5048:
5023:
4985:
4921:
4632:
4138:
4107:
4068:
4039:
3389:
3306:
3243:
3218:
3187:
3138:
3069:
1468:), which are non-polar. Tetrahedral molecules such as
1387:
740:
All three moments of inertia are equal to each other:
99:. Rotational spectroscopy is sometimes referred to as
6868:
5977:
5957:
5937:
5575:
5105:
4885:
4817:
4755:
4697:
4519:
4456:
The electric dipole moment of the dioxygen molecule,
4417:
4353:
4331:
4311:
4222:
4199:
4003:
3977:
3839:
3816:
3737:
3656:
3487:
3380:
3357:
3327:
3297:
3102:
3038:
2927:
2825:
2763:
2742:
2646:
2533:
2491:
2445:
2412:
2387:
2362:
2337:
2202:
2136:
2003:
1795:
1738:
1711:
1668:
1635:
1439:
1206:
1146:
1095:
1055:
952:
899:
746:
706:
686:
633:
590:
563:
536:
516:
485:
408:
349:
326:
3451:
is the quantum number of the lower rotational state.
3348:
denotes the upper level involved in the transition.
1043:
734:
312:
axes of fixed orientation in space, centered on the
16:
Spectroscopy of quantized rotational states of gases
7025:
7261:(8th ed.). Oxford University Press. pp.
6685:"Erratum: Pure Rotational Spectrum of Water Vapor"
6009:
5963:
5943:
5896:
5874:
5671:A similar removal of degeneracy will occur when a
5617:
5396:
5085:
4861:
4799:
4741:
4680:
4432:
4400:
4337:
4317:
4286:
4205:
4175:
3983:
3960:
3822:
3787:
3717:
3603:
3411:
3366:
3340:
3313:
3280:
3085:
2997:
2907:
2808:
2748:
2725:
2574:
2548:
2519:
2473:
2423:
2398:
2373:
2348:
2320:
2182:
2077:
2066:
1983:
1759:
1717:
1697:
1650:
1563:The selection rule for symmetric top molecules is
1245:
1185:
1121:
1081:
965:
938:
785:
712:
692:
672:
603:
576:
549:
522:
459:
355:
332:
1511:is limited to have values between and including +
115:top, for rotational levels up to J=3, but higher
8026:
6990:
5729:
5683:. Exceptions are odd-electron molecules such as
308:is free to rotate relative to a set of mutually
7298:
6574:Journal of Physical and Chemical Reference Data
5851:Fourier transform microwave (FTMW) spectroscopy
5618:{\displaystyle \Delta J=\pm 1,\Delta F=0,\pm 1}
103:rotational spectroscopy to distinguish it from
7387:
7249:
6456:
6362:
6326:
6287:
6262:
3430:
1531:the molecules undergo transitions in which an
7494:
7280:Banwell, Colin N.; McCash, Elaine M. (1994).
7279:
6683:Hall, Richard T.; Dowling, Jerome M. (1971).
6525:"The Microwave Absorption Spectrum of Oxygen"
6471:
6444:
6432:
6377:
6338:
6274:
4862:{\displaystyle A={h \over {8\pi ^{2}cI_{C}}}}
4800:{\displaystyle A={h \over {8\pi ^{2}cI_{A}}}}
4742:{\displaystyle B={h \over {8\pi ^{2}cI_{B}}}}
4489:+1- fold degeneracy with the quantum number,
123:and then applying extra terms to account for
7566:Vibrational spectroscopy of linear molecules
7306:Rotational spectroscopy of diatomic molecule
6955:
6904:Gordy, W. (1948). "Microwave spectroscopy".
6201:Ricaud, P.; Baron, P; de La Noë, J. (2004).
1364:about the 3-fold rotation axis, and moments
386:, such as OCS and OCS. This allows a set of
294:Atacama Large Millimeter/submillimeter Array
7469:Hyperphysics article on Rotational Spectrum
7098:"Balle-Flygare FTMW spectrometer animation"
6836:, p. 113, illustrates the spectrum of
5711:. The Zeeman effect has been observed with
5628:
3639:of the rotational state, which is equal to
7561:Nuclear resonance vibrational spectroscopy
7501:
7487:
7438:Rotational spectra and molecular structure
6855:obtained using 476.5 nm radiation from an
5772:
2637:(J), of the molecule can be expressed as,
1698:{\displaystyle {\tilde {\nu }}=1/\lambda }
7934:Inelastic electron tunneling spectroscopy
7614:Resonance-enhanced multiphoton ionization
7366:
6708:
6226:
5506:levels result. The effect is one type of
4876:This gives the transition wavenumbers as
3801:
3728:The maximum relative intensity occurs at
2601:in the asymmetric structure of the band.
2564:branch is slightly less intense than the
2183:{\displaystyle E_{\text{rot}}=hcBJ(J+1).}
2109:Effect of rotation on vibrational spectra
673:{\displaystyle I_{A}\leq I_{B}\leq I_{C}}
7702:Extended X-ray absorption fine structure
6564:
6467:
6465:
6104:Gordy, W. (1970). A. Weissberger (ed.).
5778:found across a very broad region of the
5425:
3991:is the centrifugal distortion constant.
3434:
2608:
18:
7434:
6862:
6108:. Vol. IX. New York: Interscience.
5748:Fourier Transform Infrared Spectrometer
5454:
1200:(rugby football, or cigar shaped) with
8027:
7415:
7323:
7282:Fundamentals of Molecular Spectroscopy
6833:
6786:
6774:
6643:
6618:
6606:
6420:
6350:
6311:
6299:
5478:of a rotational level is greater than
5451:spectrum has been analyzed in detail.
4505:is associated with rotation about the
4481:For symmetric rotors a quantum number
7482:
7344:
6903:
6758:
6462:
6103:
5786:Absorption cells and Stark modulation
5633:In the presence of a static external
3647:increases. Combining the two factors
2633:model, the rotational energy levels,
1760:{\displaystyle \nu \cdot \lambda =c,}
1381:about any axis perpendicular to the C
793:. Examples of spherical tops include
611:about three mutually orthogonal axes
8007:
6940:"June 1971, Hewlett-Packard Journal"
3635:increases. The second factor is the
1246:{\displaystyle I_{A}<I_{B}=I_{C}}
1186:{\displaystyle I_{A}=I_{B}<I_{C}}
939:{\displaystyle I_{A}\ll I_{B}=I_{C}}
6978:10.1146/annurev.pc.29.100178.002541
5827:, many of which were developed for
2809:{\displaystyle I_{B}=I_{C},I_{A}=0}
2604:
2115:Rotational–vibrational spectroscopy
1389:Asymmetric tops (asymmetric rotors)
887:
494:the free rotation of a molecule is
105:rotational-vibrational spectroscopy
23:Part of the rotational spectrum of
13:
6567:"The Spectrum of Molecular Oxygen"
5594:
5576:
5356:
5325:
5279:
5202:
5170:
5138:
5125:
5045:
5020:
4982:
4950:
4918:
4905:
4433:{\displaystyle {\tilde {\omega }}}
4135:
4104:
4065:
4036:
4023:
3439:Rotational level populations with
3404:
3386:
3358:
3333:
3314:{\displaystyle J^{\prime \prime }}
3303:
3240:
3215:
3184:
3160:
3135:
3122:
3066:
3053:
3039:
2534:
1907:
1440:Microwave and far-infrared spectra
1428:
486:Classification of molecular rotors
14:
8056:
7919:Deep-level transient spectroscopy
7671:Saturated absorption spectroscopy
7457:
7095:
6487:Journal of Molecular Spectroscopy
6121:Journal of Molecular Spectroscopy
5421:
1605:series, whereas transitions with
1045:Symmetric tops (symmetric rotors)
786:{\displaystyle I_{A}=I_{B}=I_{C}}
736:Spherical tops (spherical rotors)
280:molecule to be identified in the
8006:
7995:
7994:
7924:Dual-polarization interferometry
7508:
6761:Basic Principles of Spectroscopy
5750:. An example is the spectrum of
4476:
4445:which was fitted to terms up to
3022:are the masses of the atoms and
2579:
1522:
1505:conservation of angular momentum
390:to be set up and solved for the
7939:Scanning tunneling spectroscopy
7914:Circular dichroism spectroscopy
7909:Acoustic resonance spectroscopy
7350:Molecular Rotation Spectroscopy
7242:
7196:
7153:
7118:
7104:
7089:
7054:
7019:
6984:
6949:
6932:
6897:
6827:
6792:
6780:
6768:
6752:
6717:
6649:
6637:
6612:
6600:
6558:
6516:
6477:
6450:
6438:
6426:
6386:
6356:
6344:
6332:
6320:
6305:
6293:
6280:
6268:
6077:
6064:
6030:
6010:{\displaystyle {\bar {B}}=B/hc}
5971:in this article corresponds to
5897:Chirped-Pulse FTMW spectrometer
5875:Balle–Flygare FTMW spectrometer
5350:
5039:
4638:
4630:
4602:
4129:
3924:
3234:
2689:
2575:Structure of rotational spectra
2078:Effect of vibration on rotation
1651:{\displaystyle {\tilde {\nu }}}
1601:= +1 are said to belong to the
1535:photon is absorbed and another
168:
7868:Fourier-transform spectroscopy
7556:Vibrational circular dichroism
7464:infrared gas spectra simulator
7309:. Cambridge University Press.
6286:Moment of inertia values from
6256:
6235:
6194:
6147:
6112:
6097:
6020:
5984:
5925:
5916:
5130:
5113:
4910:
4893:
4424:
4384:
4028:
4011:
3680:
3665:
3585:
3573:
3419:transitions are separated by 2
3395:
3127:
3110:
3026:is the distance between them.
2312:
2295:
2273:
2256:
2225:
2209:
2174:
2162:
2010:
1802:
1675:
1642:
1:
7666:Cavity ring-down spectroscopy
7571:Thermal infrared spectroscopy
7367:McQuarrie, Donald A. (2008).
7284:(4th ed.). McGraw-Hill.
6382:Pure Rotational Raman Spectra
6091:
5730:Rotational Raman spectroscopy
4468:is zero, but the molecule is
3806:When a molecule rotates, the
1140:(saucer or disc shaped) with
79:molecules can be measured in
7800:Inelastic neutron scattering
7373:. University Science Books.
6507:10.1016/0022-2852(87)90021-X
6415:10.1016/0022-2852(76)90347-7
6243:"Astrochemistry in Virginia"
5564:nuclear quadrupole resonance
5490:levels are produced; but if
5467:, greater than 1/2 it has a
4501:. The third quantum number,
3321:denotes the lower level and
7:
7861:Data collection, processing
7737:Photoelectron/photoemission
6188:10.1103/PhysRevLett.21.1701
6017:in the Rigid rotor article.
4305:. The relationship between
3643:. This factor increases as
3631:. This factor decreases as
3431:Rotational line intensities
3341:{\displaystyle J^{\prime }}
2587:ro–vibrational spectroscopy
1725:), the unit is usually the
1658:), the unit is usually the
1122:{\displaystyle I_{B}=I_{C}}
1082:{\displaystyle I_{A}=I_{B}}
299:
46:nuclear quadrupole coupling
10:
8061:
7946:Photoacoustic spectroscopy
7888:Time-resolved spectroscopy
7435:Wollrab, James E. (1967).
6565:Krupenie, Paul H. (1972).
6457:Atkins & de Paula 2006
6363:Atkins & de Paula 2006
6327:Atkins & de Paula 2006
6288:Atkins & de Paula 2006
6263:Atkins & de Paula 2006
6228:10.5194/angeo-22-1903-2004
4811:symmetric top molecule or
2549:{\displaystyle \Delta J=0}
2112:
1432:
727:The particular pattern of
7990:
7972:Astronomical spectroscopy
7964:
7951:Photothermal spectroscopy
7901:
7860:
7853:
7815:
7787:
7729:
7679:
7579:
7516:
7147:10.1016/j.jms.2006.05.003
6926:10.1103/RevModPhys.20.668
6906:Reviews of Modern Physics
6472:Banwell & McCash 1994
6445:Banwell & McCash 1994
6433:Banwell & McCash 1994
6378:Banwell & McCash 1994
6339:Banwell & McCash 1994
6275:Banwell & McCash 1994
6141:10.1016/j.jms.2006.03.011
5801:phase-sensitive detection
5519:nuclear quadrupole moment
4451:
1031:acetylene (ethyne (HC≡CH)
5909:
5807:spectrum of a molecule (
5792:superheterodyne receiver
5780:electromagnetic spectrum
5629:Stark and Zeeman effects
3367:{\displaystyle \Delta J}
2918:For a diatomic molecule
2520:{\displaystyle J'=J''-1}
2474:{\displaystyle J'=J''+1}
2439:branch of the spectrum,
1620:
825:and other tetrahalides,
7956:Pump–probe spectroscopy
7845:Ferromagnetic resonance
7637:Laser-induced breakdown
7416:Kovács, István (1969).
7330:(3rd ed.). Wiley.
6759:Chang, Raymond (1971).
6549:10.1103/PhysRev.75.1524
6380:, Section 4.2, p. 105,
6168:Physical Review Letters
5773:Instruments and methods
5510:. For example, with N (
4507:principal rotation axis
623:with the origin at the
365:principal symmetry axis
152:electric dipole moments
150:which allows molecular
53:Rotational spectroscopy
8045:Rigid bodies mechanics
7652:Glow-discharge optical
7632:Raman optical activity
7546:Rotational–vibrational
7398:Microwave Spectroscopy
7324:Hollas, M. J. (1996).
7168:(9): 096106–096106–3.
6891:10.1103/PhysRev.45.234
6821:10.1103/PhysRev.79.651
6011:
5965:
5945:
5663:μ = 0.71521 ± 0.00020
5619:
5441:matrix diagonalization
5431:
5398:
5087:
4863:
4801:
4743:
4682:
4434:
4402:
4339:
4319:
4288:
4207:
4191:simple harmonic motion
4177:
3985:
3962:
3824:
3802:Centrifugal distortion
3789:
3719:
3605:
3476:Boltzmann distribution
3452:
3413:
3368:
3342:
3315:
3282:
3087:
2999:
2909:
2810:
2750:
2727:
2614:
2595:rovibrational coupling
2550:
2521:
2475:
2425:
2400:
2375:
2350:
2322:
2184:
2084:Boltzmann distribution
2068:
1985:
1761:
1719:
1699:
1652:
1446:electric dipole moment
1247:
1187:
1123:
1083:
1027:carbonyl sulfide (OCS)
1023:hydrogen cyanide (HCN)
967:
940:
811:carbon tetrachloride (
787:
720:axis as the molecular
714:
694:
674:
605:
578:
551:
524:
461:
388:simultaneous equations
357:
334:
186:group relative to the
125:centrifugal distortion
69:power spectral density
49:
7873:Hyperspectral imaging
6958:Annu. Rev. Phys. Chem
6746:10.1103/PhysRev.77.77
6012:
5966:
5946:
5620:
5459:When a nucleus has a
5429:
5399:
5088:
4864:
4802:
4744:
4683:
4435:
4403:
4340:
4320:
4289:
4208:
4178:
3986:
3963:
3825:
3790:
3720:
3606:
3438:
3414:
3369:
3343:
3316:
3283:
3088:
3000:
2910:
2811:
2751:
2728:
2627:spherical coordinates
2612:
2551:
2522:
2476:
2426:
2401:
2376:
2351:
2323:
2185:
2121:infrared spectroscopy
2069:
1986:
1762:
1720:
1700:
1653:
1301:xenon tetrafluoride,
1248:
1188:
1124:
1084:
968:
966:{\displaystyle I_{A}}
946:. For most purposes,
941:
859:sulfur hexafluoride (
795:phosphorus tetramer (
788:
715:
695:
675:
606:
604:{\displaystyle I_{C}}
579:
577:{\displaystyle I_{B}}
552:
550:{\displaystyle I_{A}}
525:
462:
358:
335:
318:intermolecular forces
290:atmospheric chemistry
284:. The measurement of
276:was the first stable
109:vibronic spectroscopy
22:
7625:Coherent anti-Stokes
7580:UV–Vis–NIR "Optical"
5975:
5955:
5935:
5865:free induction decay
5573:
5455:Quadrupole splitting
5103:
4883:
4815:
4753:
4695:
4517:
4415:
4351:
4329:
4309:
4220:
4197:
4001:
3975:
3837:
3814:
3735:
3654:
3629:absolute temperature
3485:
3378:
3355:
3325:
3295:
3100:
3036:
2925:
2823:
2761:
2740:
2644:
2556:, gives rise to the
2531:
2489:
2443:
2410:
2385:
2360:
2335:
2200:
2134:
2097:Further, there is a
2001:
1793:
1736:
1718:{\displaystyle \nu }
1709:
1666:
1633:
1204:
1144:
1093:
1053:
1011:hydroxy radical (OH)
1007:carbon monoxide (CO)
950:
897:
744:
704:
684:
631:
588:
561:
534:
514:
406:
347:
324:
73:rotational frequency
25:trifluoroiodomethane
7929:Hadron spectroscopy
7719:Conversion electron
7680:X-ray and Gamma ray
7587:Ultraviolet–visible
7393:Schawlow, Arthur L.
7327:Modern Spectroscopy
7217:2011JPCA..115.8895W
7174:2007RScI...78i6106G
7139:2006JMoSp.238..200B
7075:1980JChPh..72..922B
7040:1976RScI...47..448E
7005:1955RScI...26..915D
6970:1978ARPC...29..537S
6918:1948RvMP...20..668G
6883:1934PhRv...45..234C
6813:1950PhRv...79..651B
6738:1950PhRv...77...77S
6701:1971JChPh..54.4968H
6670:1967JChPh..47.2454H
6586:1972JPCRD...1..423K
6541:1949PhRv...75.1524S
6499:1987JMoSp.122..477J
6407:1976JMoSp..62..175A
6219:2004AnGeo..22.1903R
6180:1968PhRvL..21.1701C
6133:2006JMoSp.237..137N
5857:FT-NMR spectroscopy
5508:hyperfine splitting
5461:spin quantum number
5434:The quantum number
4493:taking the values +
4301:is the vibrational
3427:axis of this plot.
2399:{\displaystyle J''}
2349:{\displaystyle B''}
1786:. It follows that
1558:Rayleigh scattering
372:rotational constant
282:interstellar medium
256:interstellar medium
252:interstellar medium
159:interstellar medium
133:hyperfine structure
91:spectroscopy or by
65:rotational spectrum
48:with the I nucleus.
7977:Force spectroscopy
7902:Measured phenomena
7893:Video spectroscopy
7597:Cold vapour atomic
7441:. Academic Press.
7389:Townes, Charles H.
7257:Physical Chemistry
6072:Raman spectroscopy
6041:molecules such as
6007:
5961:
5941:
5881:Fabry-Perot cavity
5736:Raman spectroscopy
5615:
5521:and a function of
5432:
5394:
5083:
4859:
4797:
4739:
4678:
4636:
4430:
4398:
4335:
4315:
4284:
4213:can be derived as
4203:
4173:
3981:
3958:
3820:
3785:
3715:
3621:Boltzmann constant
3601:
3453:
3409:
3364:
3338:
3311:
3278:
3083:
2995:
2905:
2806:
2746:
2723:
2615:
2546:
2517:
2471:
2435:For the so-called
2424:{\displaystyle J'}
2421:
2396:
2374:{\displaystyle B'}
2371:
2346:
2318:
2180:
2064:
1981:
1757:
1715:
1695:
1660:inverse centimeter
1648:
1597:Transitions with Δ
1411:nitrogen dioxide,
1379:= 2.8059 × 10 kg m
1362:= 4.4128 × 10 kg m
1243:
1183:
1119:
1079:
1015:carbon dioxide (CO
963:
936:
783:
724:of highest order.
710:
690:
670:
601:
574:
547:
520:
457:
380:diatomic molecules
353:
330:
304:A molecule in the
154:to be determined.
144:moments of inertia
97:Raman spectroscopy
50:
8022:
8021:
7986:
7985:
7878:Spectrophotometry
7805:Neutron spin echo
7779:Beta spectroscopy
7692:Energy-dispersive
7408:978-0-486-61798-5
7380:978-1-891389-50-4
7370:Quantum Chemistry
7225:10.1021/jp2043202
7211:(32): 8895–8905.
7182:10.1063/1.2786022
7162:Rev. Sci. Instrum
7127:J. Mol. Spectrosc
7048:10.1063/1.1134647
7028:Rev. Sci. Instrum
7013:10.1063/1.1715156
6993:Rev. Sci. Instrum
6710:10.1063/1.1674785
6678:10.1063/1.1703330
6594:10.1063/1.3253101
6395:J. Mol. Spectrosc
5987:
5964:{\displaystyle B}
5951:in cm. Therefore
5944:{\displaystyle B}
5890:William Klemperer
5116:
4896:
4857:
4795:
4737:
4635:
4443:hydrogen fluoride
4427:
4396:
4387:
4338:{\displaystyle D}
4318:{\displaystyle B}
4282:
4206:{\displaystyle D}
4014:
3984:{\displaystyle D}
3823:{\displaystyle B}
3808:centrifugal force
3783:
3770:
3769:
3711:
3660:
3597:
3543:
3510:
3113:
2983:
2903:
2865:
2749:{\displaystyle B}
2599:Coriolis coupling
2234:
2228:
2212:
2144:
2103:Coriolis coupling
2094:, H−C≡C−C≡C−C≡N.
2059:
2053:
2025:
2013:
1976:
1946:
1926:
1900:
1871:
1851:
1848:
1817:
1805:
1784:velocity of light
1678:
1645:
713:{\displaystyle A}
693:{\displaystyle A}
523:{\displaystyle I}
508:moment of inertia
500:rotational energy
492:quantum mechanics
450:
396:zero-point energy
356:{\displaystyle K}
333:{\displaystyle J}
288:is important for
286:chlorine monoxide
8052:
8010:
8009:
7998:
7997:
7858:
7857:
7769:phenomenological
7518:Vibrational (IR)
7503:
7496:
7489:
7480:
7479:
7452:
7431:
7412:
7384:
7363:
7341:
7320:
7301:Carrington, Alan
7299:Brown, John M.;
7295:
7276:
7260:
7237:
7236:
7205:J. Phys. Chem. A
7200:
7194:
7193:
7157:
7151:
7150:
7122:
7116:
7115:
7108:
7102:
7101:
7093:
7087:
7086:
7083:10.1063/1.439210
7058:
7052:
7051:
7023:
7017:
7016:
6988:
6982:
6981:
6953:
6947:
6946:
6944:
6936:
6930:
6929:
6901:
6895:
6894:
6866:
6860:
6854:
6853:
6852:
6844:
6843:
6831:
6825:
6824:
6796:
6790:
6784:
6778:
6772:
6766:
6764:
6756:
6750:
6749:
6721:
6715:
6714:
6712:
6681:
6653:
6647:
6641:
6635:
6633:
6631:
6630:
6616:
6610:
6604:
6598:
6597:
6571:
6562:
6556:
6552:
6520:
6514:
6510:
6481:
6475:
6469:
6460:
6454:
6448:
6442:
6436:
6430:
6424:
6418:
6390:
6384:
6375:
6366:
6365:, pp. 474–5
6360:
6354:
6348:
6342:
6336:
6330:
6324:
6318:
6309:
6303:
6297:
6291:
6284:
6278:
6272:
6266:
6260:
6254:
6253:
6251:
6249:
6239:
6233:
6232:
6230:
6198:
6192:
6191:
6165:
6164:
6163:
6151:
6145:
6144:
6116:
6110:
6109:
6101:
6085:
6081:
6075:
6068:
6062:
6056:
6055:
6054:
6034:
6028:
6024:
6018:
6016:
6014:
6013:
6008:
6000:
5989:
5988:
5980:
5970:
5968:
5967:
5962:
5950:
5948:
5947:
5942:
5929:
5923:
5920:
5833:Second World War
5818:
5817:
5816:
5768:
5767:
5766:
5758:
5757:
5726:
5725:
5724:
5709:hydroxyl radical
5702:
5701:
5700:
5689:nitrogen dioxide
5667:
5659:carbonyl sulfide
5655:
5644:
5624:
5622:
5621:
5616:
5561:
5542:
5516:
5505:
5489:
5403:
5401:
5400:
5395:
5363:
5362:
5349:
5348:
5343:
5339:
5332:
5331:
5313:
5312:
5297:
5293:
5286:
5285:
5268:
5264:
5263:
5262:
5253:
5252:
5220:
5216:
5209:
5208:
5185:
5181:
5174:
5173:
5153:
5152:
5145:
5144:
5129:
5128:
5118:
5117:
5109:
5092:
5090:
5089:
5084:
5052:
5051:
5038:
5034:
5027:
5026:
5000:
4996:
4989:
4988:
4965:
4961:
4954:
4953:
4933:
4932:
4925:
4924:
4909:
4908:
4898:
4897:
4889:
4868:
4866:
4865:
4860:
4858:
4856:
4855:
4854:
4842:
4841:
4825:
4806:
4804:
4803:
4798:
4796:
4794:
4793:
4792:
4780:
4779:
4763:
4748:
4746:
4745:
4740:
4738:
4736:
4735:
4734:
4722:
4721:
4705:
4687:
4685:
4684:
4679:
4637:
4633:
4601:
4600:
4591:
4587:
4569:
4565:
4541:
4537:
4467:
4466:
4465:
4439:
4437:
4436:
4431:
4429:
4428:
4420:
4407:
4405:
4404:
4399:
4397:
4395:
4394:
4389:
4388:
4380:
4376:
4375:
4374:
4361:
4344:
4342:
4341:
4336:
4324:
4322:
4321:
4316:
4293:
4291:
4290:
4285:
4283:
4281:
4274:
4273:
4264:
4263:
4254:
4253:
4240:
4239:
4230:
4212:
4210:
4209:
4204:
4182:
4180:
4179:
4174:
4142:
4141:
4128:
4127:
4122:
4118:
4111:
4110:
4083:
4079:
4072:
4071:
4045:
4044:
4043:
4042:
4027:
4026:
4016:
4015:
4007:
3990:
3988:
3987:
3982:
3967:
3965:
3964:
3959:
3923:
3922:
3917:
3913:
3897:
3896:
3881:
3877:
3853:
3829:
3827:
3826:
3821:
3794:
3792:
3791:
3786:
3784:
3776:
3771:
3768:
3754:
3746:
3745:
3724:
3722:
3721:
3716:
3714:
3713:
3712:
3710:
3702:
3701:
3692:
3661:
3658:
3642:
3610:
3608:
3607:
3602:
3600:
3599:
3598:
3596:
3588:
3559:
3546:
3545:
3544:
3542:
3534:
3533:
3524:
3511:
3509:
3508:
3499:
3498:
3489:
3474:is given by the
3418:
3416:
3415:
3410:
3408:
3407:
3398:
3393:
3392:
3373:
3371:
3370:
3365:
3347:
3345:
3344:
3339:
3337:
3336:
3320:
3318:
3317:
3312:
3310:
3309:
3287:
3285:
3284:
3279:
3247:
3246:
3233:
3229:
3222:
3221:
3195:
3191:
3190:
3168:
3164:
3163:
3144:
3143:
3142:
3141:
3126:
3125:
3115:
3114:
3106:
3092:
3090:
3089:
3084:
3073:
3072:
3057:
3056:
3004:
3002:
3001:
2996:
2994:
2993:
2984:
2982:
2981:
2980:
2968:
2967:
2957:
2956:
2955:
2946:
2945:
2935:
2914:
2912:
2911:
2906:
2904:
2902:
2901:
2900:
2888:
2887:
2871:
2866:
2864:
2863:
2862:
2850:
2849:
2833:
2815:
2813:
2812:
2807:
2799:
2798:
2786:
2785:
2773:
2772:
2755:
2753:
2752:
2747:
2732:
2730:
2729:
2724:
2688:
2684:
2660:
2605:Linear molecules
2555:
2553:
2552:
2547:
2526:
2524:
2523:
2518:
2510:
2499:
2480:
2478:
2477:
2472:
2464:
2453:
2430:
2428:
2427:
2422:
2420:
2405:
2403:
2402:
2397:
2395:
2380:
2378:
2377:
2372:
2370:
2355:
2353:
2352:
2347:
2345:
2327:
2325:
2324:
2319:
2305:
2294:
2286:
2266:
2255:
2247:
2236:
2235:
2232:
2230:
2229:
2221:
2214:
2213:
2205:
2189:
2187:
2186:
2181:
2146:
2145:
2142:
2099:fictitious force
2092:cyanodiacetylene
2073:
2071:
2070:
2065:
2060:
2055:
2054:
2051:
2049:
2040:
2035:
2034:
2026:
2023:
2020:
2015:
2014:
2006:
1990:
1988:
1987:
1982:
1977:
1975:
1974:
1973:
1957:
1956:
1955:
1947:
1944:
1941:
1932:
1927:
1925:
1924:
1920:
1919:
1918:
1910:
1901:
1898:
1891:
1882:
1881:
1880:
1872:
1869:
1866:
1857:
1852:
1850:
1849:
1846:
1844:
1832:
1827:
1826:
1818:
1815:
1812:
1807:
1806:
1798:
1766:
1764:
1763:
1758:
1724:
1722:
1721:
1716:
1704:
1702:
1701:
1696:
1691:
1680:
1679:
1671:
1657:
1655:
1654:
1649:
1647:
1646:
1638:
1612:
1502:
1494:
1479:
1478:
1477:
1459:
1458:
1457:
1422:
1421:
1420:
1407:
1405:
1404:
1380:
1363:
1350:
1348:
1347:
1334:
1332:
1331:
1312:
1311:
1310:
1297:
1296:
1295:
1282:
1281:
1280:
1272:
1271:
1252:
1250:
1249:
1244:
1242:
1241:
1229:
1228:
1216:
1215:
1192:
1190:
1189:
1184:
1182:
1181:
1169:
1168:
1156:
1155:
1128:
1126:
1125:
1120:
1118:
1117:
1105:
1104:
1088:
1086:
1085:
1080:
1078:
1077:
1065:
1064:
1002:
1001:
1000:
986:
985:
984:
972:
970:
969:
964:
962:
961:
945:
943:
942:
937:
935:
934:
922:
921:
909:
908:
889:Linear molecules
870:
869:
868:
854:
853:
852:
838:
837:
836:
822:
821:
820:
806:
805:
804:
792:
790:
789:
784:
782:
781:
769:
768:
756:
755:
719:
717:
716:
711:
699:
697:
696:
691:
679:
677:
676:
671:
669:
668:
656:
655:
643:
642:
610:
608:
607:
602:
600:
599:
583:
581:
580:
575:
573:
572:
556:
554:
553:
548:
546:
545:
529:
527:
526:
521:
504:angular momentum
466:
464:
463:
458:
456:
452:
451:
443:
418:
417:
362:
360:
359:
354:
339:
337:
336:
331:
275:
273:
272:
233:
231:
230:
222:
221:
207:
205:
204:
196:
195:
185:
184:
183:
174:rotation of the
163:radio telescopes
39:
37:
36:
8060:
8059:
8055:
8054:
8053:
8051:
8050:
8049:
8025:
8024:
8023:
8018:
7982:
7960:
7897:
7849:
7811:
7783:
7725:
7675:
7575:
7536:Resonance Raman
7512:
7507:
7460:
7455:
7449:
7428:
7420:. Adam Hilger.
7409:
7381:
7360:
7338:
7317:
7292:
7273:
7245:
7240:
7201:
7197:
7158:
7154:
7123:
7119:
7110:
7109:
7105:
7094:
7090:
7059:
7055:
7024:
7020:
6999:(10): 915–928.
6989:
6985:
6954:
6950:
6942:
6938:
6937:
6933:
6902:
6898:
6871:Physical Review
6867:
6863:
6857:argon ion laser
6851:
6848:
6847:
6846:
6842:
6840:
6839:
6838:
6837:
6832:
6828:
6797:
6793:
6785:
6781:
6773:
6769:
6757:
6753:
6722:
6718:
6654:
6650:
6642:
6638:
6629:
6626:
6625:
6624:
6622:
6617:
6613:
6605:
6601:
6569:
6563:
6559:
6521:
6517:
6482:
6478:
6470:
6463:
6455:
6451:
6443:
6439:
6431:
6427:
6419:Illustrated in
6391:
6387:
6376:
6369:
6361:
6357:
6349:
6345:
6337:
6333:
6325:
6321:
6310:
6306:
6298:
6294:
6285:
6281:
6273:
6269:
6261:
6257:
6247:
6245:
6241:
6240:
6236:
6199:
6195:
6162:
6159:
6158:
6157:
6155:
6152:
6148:
6117:
6113:
6102:
6098:
6094:
6089:
6088:
6082:
6078:
6069:
6065:
6053:
6050:
6049:
6048:
6046:
6035:
6031:
6025:
6021:
5996:
5979:
5978:
5976:
5973:
5972:
5956:
5953:
5952:
5936:
5933:
5932:
5930:
5926:
5921:
5917:
5912:
5899:
5877:
5861:Bloch equations
5853:
5841:Hewlett-Packard
5815:
5812:
5811:
5810:
5808:
5788:
5775:
5765:
5762:
5761:
5760:
5756:
5754:
5753:
5752:
5751:
5732:
5723:
5720:
5719:
5718:
5716:
5705:chlorine oxides
5699:
5696:
5695:
5694:
5692:
5662:
5650:
5638:
5631:
5574:
5571:
5570:
5544:
5530:
5511:
5499:
5483:
5473:quantum number
5457:
5424:
5412:
5355:
5351:
5344:
5324:
5320:
5319:
5315:
5314:
5308:
5304:
5278:
5274:
5273:
5269:
5258:
5254:
5245:
5241:
5231:
5227:
5201:
5197:
5196:
5192:
5169:
5165:
5164:
5160:
5137:
5133:
5124:
5120:
5119:
5108:
5107:
5106:
5104:
5101:
5100:
5044:
5040:
5019:
5015:
5014:
5010:
4981:
4977:
4976:
4972:
4949:
4945:
4944:
4940:
4917:
4913:
4904:
4900:
4899:
4888:
4887:
4886:
4884:
4881:
4880:
4850:
4846:
4837:
4833:
4829:
4824:
4816:
4813:
4812:
4788:
4784:
4775:
4771:
4767:
4762:
4754:
4751:
4750:
4730:
4726:
4717:
4713:
4709:
4704:
4696:
4693:
4692:
4631:
4596:
4592:
4577:
4573:
4555:
4551:
4527:
4523:
4518:
4515:
4514:
4479:
4464:
4461:
4460:
4459:
4457:
4454:
4419:
4418:
4416:
4413:
4412:
4390:
4379:
4378:
4377:
4370:
4366:
4362:
4360:
4352:
4349:
4348:
4330:
4327:
4326:
4310:
4307:
4306:
4269:
4265:
4259:
4255:
4249:
4245:
4241:
4235:
4231:
4229:
4221:
4218:
4217:
4198:
4195:
4194:
4134:
4130:
4123:
4103:
4099:
4098:
4094:
4093:
4064:
4060:
4059:
4055:
4035:
4031:
4022:
4018:
4017:
4006:
4005:
4004:
4002:
3999:
3998:
3976:
3973:
3972:
3918:
3903:
3899:
3898:
3892:
3888:
3867:
3863:
3843:
3838:
3835:
3834:
3815:
3812:
3811:
3804:
3775:
3755:
3747:
3744:
3736:
3733:
3732:
3703:
3697:
3693:
3691:
3687:
3683:
3657:
3655:
3652:
3651:
3640:
3589:
3560:
3558:
3554:
3550:
3535:
3529:
3525:
3523:
3519:
3515:
3504:
3500:
3494:
3490:
3488:
3486:
3483:
3482:
3473:
3465:
3433:
3403:
3399:
3394:
3385:
3381:
3379:
3376:
3375:
3356:
3353:
3352:
3332:
3328:
3326:
3323:
3322:
3302:
3298:
3296:
3293:
3292:
3239:
3235:
3214:
3210:
3209:
3205:
3183:
3179:
3175:
3159:
3155:
3151:
3134:
3130:
3121:
3117:
3116:
3105:
3104:
3103:
3101:
3098:
3097:
3065:
3061:
3052:
3048:
3037:
3034:
3033:
3030:Selection rules
3021:
3014:
2989:
2985:
2976:
2972:
2963:
2959:
2958:
2951:
2947:
2941:
2937:
2936:
2934:
2926:
2923:
2922:
2896:
2892:
2883:
2879:
2875:
2870:
2858:
2854:
2845:
2841:
2837:
2832:
2824:
2821:
2820:
2794:
2790:
2781:
2777:
2768:
2764:
2762:
2759:
2758:
2741:
2738:
2737:
2674:
2670:
2650:
2645:
2642:
2641:
2607:
2582:
2577:
2532:
2529:
2528:
2503:
2492:
2490:
2487:
2486:
2457:
2446:
2444:
2441:
2440:
2413:
2411:
2408:
2407:
2388:
2386:
2383:
2382:
2363:
2361:
2358:
2357:
2338:
2336:
2333:
2332:
2298:
2287:
2279:
2259:
2248:
2240:
2231:
2220:
2219:
2218:
2204:
2203:
2201:
2198:
2197:
2141:
2137:
2135:
2132:
2131:
2117:
2111:
2080:
2050:
2045:
2041:
2039:
2027:
2022:
2021:
2016:
2005:
2004:
2002:
1999:
1998:
1969:
1965:
1958:
1948:
1943:
1942:
1937:
1933:
1931:
1911:
1906:
1905:
1897:
1896:
1892:
1887:
1883:
1873:
1868:
1867:
1862:
1858:
1856:
1845:
1840:
1836:
1831:
1819:
1814:
1813:
1808:
1797:
1796:
1794:
1791:
1790:
1737:
1734:
1733:
1710:
1707:
1706:
1687:
1670:
1669:
1667:
1664:
1663:
1637:
1636:
1634:
1631:
1630:
1623:
1606:
1525:
1496:
1488:
1476:
1473:
1472:
1471:
1469:
1456:
1453:
1452:
1451:
1449:
1442:
1437:
1435:selection rules
1431:
1429:Selection rules
1419:
1416:
1415:
1414:
1412:
1403:
1400:
1399:
1398:
1396:
1390:
1384:
1378:
1371:
1365:
1361:
1355:
1346:
1343:
1342:
1341:
1339:
1330:
1327:
1326:
1325:
1323:
1322:Chloromethane,
1309:
1306:
1305:
1304:
1302:
1294:
1291:
1290:
1289:
1287:
1279:
1276:
1275:
1274:
1270:
1267:
1266:
1265:
1263:
1237:
1233:
1224:
1220:
1211:
1207:
1205:
1202:
1201:
1177:
1173:
1164:
1160:
1151:
1147:
1145:
1142:
1141:
1113:
1109:
1100:
1096:
1094:
1091:
1090:
1073:
1069:
1060:
1056:
1054:
1051:
1050:
1046:
1040:
1036:
1018:
999:
996:
995:
994:
992:
983:
980:
979:
978:
976:
957:
953:
951:
948:
947:
930:
926:
917:
913:
904:
900:
898:
895:
894:
890:
884:
880:
867:
864:
863:
862:
860:
851:
848:
847:
846:
844:
835:
832:
831:
830:
828:
819:
816:
815:
814:
812:
803:
800:
799:
798:
796:
777:
773:
764:
760:
751:
747:
745:
742:
741:
737:
705:
702:
701:
685:
682:
681:
664:
660:
651:
647:
638:
634:
632:
629:
628:
595:
591:
589:
586:
585:
568:
564:
562:
559:
558:
541:
537:
535:
532:
531:
515:
512:
511:
488:
442:
435:
431:
413:
409:
407:
404:
403:
348:
345:
344:
325:
322:
321:
302:
271:
268:
267:
266:
263:
260:radio telescope
248:radio astronomy
229:
226:
225:
224:
220:
217:
216:
215:
213:
203:
200:
199:
198:
194:
191:
190:
189:
187:
182:
179:
178:
177:
175:
171:
148:Stark splitting
35:
32:
31:
30:
28:
17:
12:
11:
5:
8058:
8048:
8047:
8042:
8037:
8020:
8019:
8017:
8016:
8004:
7991:
7988:
7987:
7984:
7983:
7981:
7980:
7974:
7968:
7966:
7962:
7961:
7959:
7958:
7953:
7948:
7943:
7942:
7941:
7931:
7926:
7921:
7916:
7911:
7905:
7903:
7899:
7898:
7896:
7895:
7890:
7885:
7880:
7875:
7870:
7864:
7862:
7855:
7851:
7850:
7848:
7847:
7842:
7837:
7832:
7831:
7830:
7819:
7817:
7813:
7812:
7810:
7809:
7808:
7807:
7797:
7791:
7789:
7785:
7784:
7782:
7781:
7776:
7771:
7766:
7761:
7760:
7759:
7754:
7752:Angle-resolved
7749:
7744:
7733:
7731:
7727:
7726:
7724:
7723:
7722:
7721:
7711:
7706:
7705:
7704:
7699:
7694:
7683:
7681:
7677:
7676:
7674:
7673:
7668:
7663:
7662:
7661:
7656:
7655:
7654:
7639:
7634:
7629:
7628:
7627:
7617:
7611:
7606:
7601:
7600:
7599:
7589:
7583:
7581:
7577:
7576:
7574:
7573:
7568:
7563:
7558:
7553:
7548:
7543:
7538:
7533:
7528:
7522:
7520:
7514:
7513:
7506:
7505:
7498:
7491:
7483:
7477:
7476:
7471:
7466:
7459:
7458:External links
7456:
7454:
7453:
7447:
7432:
7426:
7413:
7407:
7385:
7379:
7364:
7358:
7342:
7336:
7321:
7315:
7296:
7290:
7277:
7271:
7246:
7244:
7241:
7239:
7238:
7195:
7152:
7133:(2): 200–212.
7117:
7103:
7088:
7069:(2): 922–932.
7053:
7034:(4): 448–454.
7018:
6983:
6948:
6931:
6912:(4): 668–717.
6896:
6861:
6849:
6841:
6826:
6791:
6779:
6767:
6763:. McGraw-Hill.
6751:
6716:
6664:(7): 2454–61.
6648:
6636:
6627:
6611:
6599:
6580:(2): 423–534.
6557:
6535:(10): 1524–8.
6515:
6493:(2): 477–480.
6476:
6461:
6449:
6437:
6425:
6401:(2): 175–180.
6385:
6367:
6355:
6343:
6331:
6319:
6304:
6292:
6279:
6267:
6255:
6234:
6213:(6): 1903–15.
6193:
6174:(25): 1701–5.
6160:
6146:
6127:(2): 137–142.
6111:
6095:
6093:
6090:
6087:
6086:
6076:
6063:
6051:
6029:
6019:
6006:
6003:
5999:
5995:
5992:
5986:
5983:
5960:
5940:
5924:
5914:
5913:
5911:
5908:
5898:
5895:
5876:
5873:
5852:
5849:
5813:
5787:
5784:
5774:
5771:
5763:
5755:
5746:by adapting a
5743:polarizability
5731:
5728:
5721:
5697:
5635:electric field
5630:
5627:
5626:
5625:
5614:
5611:
5608:
5605:
5602:
5599:
5596:
5593:
5590:
5587:
5584:
5581:
5578:
5456:
5453:
5423:
5422:Asymmetric top
5420:
5410:
5405:
5404:
5393:
5390:
5387:
5384:
5381:
5378:
5375:
5372:
5369:
5366:
5361:
5358:
5354:
5347:
5342:
5338:
5335:
5330:
5327:
5323:
5318:
5311:
5307:
5303:
5300:
5296:
5292:
5289:
5284:
5281:
5277:
5272:
5267:
5261:
5257:
5251:
5248:
5244:
5240:
5237:
5234:
5230:
5226:
5223:
5219:
5215:
5212:
5207:
5204:
5200:
5195:
5191:
5188:
5184:
5180:
5177:
5172:
5168:
5163:
5159:
5156:
5151:
5148:
5143:
5140:
5136:
5132:
5127:
5123:
5115:
5112:
5094:
5093:
5082:
5079:
5076:
5073:
5070:
5067:
5064:
5061:
5058:
5055:
5050:
5047:
5043:
5037:
5033:
5030:
5025:
5022:
5018:
5013:
5009:
5006:
5003:
4999:
4995:
4992:
4987:
4984:
4980:
4975:
4971:
4968:
4964:
4960:
4957:
4952:
4948:
4943:
4939:
4936:
4931:
4928:
4923:
4920:
4916:
4912:
4907:
4903:
4895:
4892:
4853:
4849:
4845:
4840:
4836:
4832:
4828:
4823:
4820:
4791:
4787:
4783:
4778:
4774:
4770:
4766:
4761:
4758:
4733:
4729:
4725:
4720:
4716:
4712:
4708:
4703:
4700:
4689:
4688:
4677:
4674:
4671:
4668:
4665:
4662:
4659:
4656:
4653:
4650:
4647:
4644:
4641:
4629:
4626:
4623:
4620:
4617:
4614:
4611:
4608:
4605:
4599:
4595:
4590:
4586:
4583:
4580:
4576:
4572:
4568:
4564:
4561:
4558:
4554:
4550:
4547:
4544:
4540:
4536:
4533:
4530:
4526:
4522:
4478:
4475:
4462:
4453:
4450:
4426:
4423:
4409:
4408:
4393:
4386:
4383:
4373:
4369:
4365:
4359:
4356:
4334:
4314:
4303:force constant
4295:
4294:
4280:
4277:
4272:
4268:
4262:
4258:
4252:
4248:
4244:
4238:
4234:
4228:
4225:
4202:
4184:
4183:
4172:
4169:
4166:
4163:
4160:
4157:
4154:
4151:
4148:
4145:
4140:
4137:
4133:
4126:
4121:
4117:
4114:
4109:
4106:
4102:
4097:
4092:
4089:
4086:
4082:
4078:
4075:
4070:
4067:
4063:
4058:
4054:
4051:
4048:
4041:
4038:
4034:
4030:
4025:
4021:
4013:
4010:
3980:
3969:
3968:
3957:
3954:
3951:
3948:
3945:
3942:
3939:
3936:
3933:
3930:
3927:
3921:
3916:
3912:
3909:
3906:
3902:
3895:
3891:
3887:
3884:
3880:
3876:
3873:
3870:
3866:
3862:
3859:
3856:
3852:
3849:
3846:
3842:
3819:
3803:
3800:
3796:
3795:
3782:
3779:
3774:
3767:
3764:
3761:
3758:
3753:
3750:
3743:
3740:
3726:
3725:
3709:
3706:
3700:
3696:
3690:
3686:
3682:
3679:
3676:
3673:
3670:
3667:
3664:
3613:
3612:
3595:
3592:
3587:
3584:
3581:
3578:
3575:
3572:
3569:
3566:
3563:
3557:
3553:
3549:
3541:
3538:
3532:
3528:
3522:
3518:
3514:
3507:
3503:
3497:
3493:
3471:
3463:
3432:
3429:
3406:
3402:
3397:
3391:
3388:
3384:
3363:
3360:
3335:
3331:
3308:
3305:
3301:
3289:
3288:
3277:
3274:
3271:
3268:
3265:
3262:
3259:
3256:
3253:
3250:
3245:
3242:
3238:
3232:
3228:
3225:
3220:
3217:
3213:
3208:
3204:
3201:
3198:
3194:
3189:
3186:
3182:
3178:
3174:
3171:
3167:
3162:
3158:
3154:
3150:
3147:
3140:
3137:
3133:
3129:
3124:
3120:
3112:
3109:
3082:
3079:
3076:
3071:
3068:
3064:
3060:
3055:
3051:
3047:
3044:
3041:
3019:
3012:
3006:
3005:
2992:
2988:
2979:
2975:
2971:
2966:
2962:
2954:
2950:
2944:
2940:
2933:
2930:
2916:
2915:
2899:
2895:
2891:
2886:
2882:
2878:
2874:
2869:
2861:
2857:
2853:
2848:
2844:
2840:
2836:
2831:
2828:
2805:
2802:
2797:
2793:
2789:
2784:
2780:
2776:
2771:
2767:
2745:
2734:
2733:
2722:
2719:
2716:
2713:
2710:
2707:
2704:
2701:
2698:
2695:
2692:
2687:
2683:
2680:
2677:
2673:
2669:
2666:
2663:
2659:
2656:
2653:
2649:
2606:
2603:
2581:
2578:
2576:
2573:
2545:
2542:
2539:
2536:
2516:
2513:
2509:
2506:
2502:
2498:
2495:
2470:
2467:
2463:
2460:
2456:
2452:
2449:
2419:
2416:
2394:
2391:
2369:
2366:
2344:
2341:
2329:
2328:
2317:
2314:
2311:
2308:
2304:
2301:
2297:
2293:
2290:
2285:
2282:
2278:
2275:
2272:
2269:
2265:
2262:
2258:
2254:
2251:
2246:
2243:
2239:
2227:
2224:
2217:
2211:
2208:
2191:
2190:
2179:
2176:
2173:
2170:
2167:
2164:
2161:
2158:
2155:
2152:
2149:
2140:
2113:Main article:
2110:
2107:
2079:
2076:
2075:
2074:
2063:
2058:
2048:
2044:
2038:
2033:
2030:
2019:
2012:
2009:
1992:
1991:
1980:
1972:
1968:
1964:
1961:
1954:
1951:
1940:
1936:
1930:
1923:
1917:
1914:
1909:
1904:
1895:
1890:
1886:
1879:
1876:
1865:
1861:
1855:
1843:
1839:
1835:
1830:
1825:
1822:
1811:
1804:
1801:
1768:
1767:
1756:
1753:
1750:
1747:
1744:
1741:
1714:
1694:
1690:
1686:
1683:
1677:
1674:
1644:
1641:
1622:
1619:
1595:
1594:
1583:
1572:
1541:polarizability
1524:
1521:
1474:
1454:
1441:
1438:
1433:Main article:
1430:
1427:
1426:
1425:
1417:
1401:
1391:
1388:
1386:
1382:
1376:
1369:
1359:
1353:
1352:
1344:
1328:
1319:
1314:
1307:
1292:
1277:
1268:
1259:
1240:
1236:
1232:
1227:
1223:
1219:
1214:
1210:
1198:symmetric tops
1180:
1176:
1172:
1167:
1163:
1159:
1154:
1150:
1138:symmetric tops
1116:
1112:
1108:
1103:
1099:
1076:
1072:
1068:
1063:
1059:
1047:
1044:
1042:
1038:
1034:
1016:
997:
981:
960:
956:
933:
929:
925:
920:
916:
912:
907:
903:
891:
888:
886:
882:
878:
865:
849:
833:
817:
801:
780:
776:
772:
767:
763:
759:
754:
750:
738:
735:
709:
689:
667:
663:
659:
654:
650:
646:
641:
637:
625:center of mass
598:
594:
571:
567:
544:
540:
519:
498:, so that the
487:
484:
468:
467:
455:
449:
446:
441:
438:
434:
430:
427:
424:
421:
416:
412:
378:directly. For
352:
329:
314:center of mass
301:
298:
269:
227:
218:
201:
192:
180:
170:
167:
129:fine structure
33:
15:
9:
6:
4:
3:
2:
8057:
8046:
8043:
8041:
8038:
8036:
8033:
8032:
8030:
8015:
8014:
8005:
8003:
8002:
7993:
7992:
7989:
7978:
7975:
7973:
7970:
7969:
7967:
7963:
7957:
7954:
7952:
7949:
7947:
7944:
7940:
7937:
7936:
7935:
7932:
7930:
7927:
7925:
7922:
7920:
7917:
7915:
7912:
7910:
7907:
7906:
7904:
7900:
7894:
7891:
7889:
7886:
7884:
7881:
7879:
7876:
7874:
7871:
7869:
7866:
7865:
7863:
7859:
7856:
7852:
7846:
7843:
7841:
7838:
7836:
7833:
7829:
7826:
7825:
7824:
7821:
7820:
7818:
7814:
7806:
7803:
7802:
7801:
7798:
7796:
7793:
7792:
7790:
7786:
7780:
7777:
7775:
7772:
7770:
7767:
7765:
7762:
7758:
7755:
7753:
7750:
7748:
7745:
7743:
7740:
7739:
7738:
7735:
7734:
7732:
7728:
7720:
7717:
7716:
7715:
7712:
7710:
7707:
7703:
7700:
7698:
7695:
7693:
7690:
7689:
7688:
7685:
7684:
7682:
7678:
7672:
7669:
7667:
7664:
7660:
7657:
7653:
7650:
7649:
7648:
7645:
7644:
7643:
7640:
7638:
7635:
7633:
7630:
7626:
7623:
7622:
7621:
7618:
7615:
7612:
7610:
7609:Near-infrared
7607:
7605:
7602:
7598:
7595:
7594:
7593:
7590:
7588:
7585:
7584:
7582:
7578:
7572:
7569:
7567:
7564:
7562:
7559:
7557:
7554:
7552:
7549:
7547:
7544:
7542:
7539:
7537:
7534:
7532:
7529:
7527:
7524:
7523:
7521:
7519:
7515:
7511:
7504:
7499:
7497:
7492:
7490:
7485:
7484:
7481:
7475:
7472:
7470:
7467:
7465:
7462:
7461:
7450:
7444:
7440:
7439:
7433:
7429:
7423:
7419:
7414:
7410:
7404:
7400:
7399:
7394:
7390:
7386:
7382:
7376:
7372:
7371:
7365:
7361:
7359:0-486-49540-X
7355:
7351:
7347:
7343:
7339:
7333:
7329:
7328:
7322:
7318:
7316:0-521-53078-4
7312:
7308:
7307:
7302:
7297:
7293:
7291:0-07-707976-0
7287:
7283:
7278:
7274:
7268:
7264:
7259:
7258:
7252:
7251:Atkins, P. W.
7248:
7247:
7234:
7230:
7226:
7222:
7218:
7214:
7210:
7206:
7199:
7191:
7187:
7183:
7179:
7175:
7171:
7167:
7163:
7156:
7148:
7144:
7140:
7136:
7132:
7128:
7121:
7113:
7107:
7099:
7092:
7084:
7080:
7076:
7072:
7068:
7064:
7063:J. Chem. Phys
7057:
7049:
7045:
7041:
7037:
7033:
7029:
7022:
7014:
7010:
7006:
7002:
6998:
6994:
6987:
6979:
6975:
6971:
6967:
6963:
6959:
6952:
6941:
6935:
6927:
6923:
6919:
6915:
6911:
6907:
6900:
6892:
6888:
6884:
6880:
6876:
6872:
6865:
6858:
6835:
6830:
6822:
6818:
6814:
6810:
6806:
6802:
6795:
6789:, p. 102
6788:
6783:
6776:
6771:
6762:
6755:
6747:
6743:
6739:
6735:
6731:
6727:
6720:
6711:
6706:
6702:
6698:
6694:
6690:
6689:J. Chem. Phys
6686:
6679:
6675:
6671:
6667:
6663:
6659:
6658:J. Chem. Phys
6652:
6646:, p. 103
6645:
6640:
6620:
6615:
6609:, p. 101
6608:
6603:
6595:
6591:
6587:
6583:
6579:
6575:
6568:
6561:
6555:
6550:
6546:
6542:
6538:
6534:
6530:
6526:
6519:
6513:
6508:
6504:
6500:
6496:
6492:
6488:
6480:
6473:
6468:
6466:
6459:, p. 449
6458:
6453:
6446:
6441:
6435:, p. 63.
6434:
6429:
6422:
6416:
6412:
6408:
6404:
6400:
6396:
6389:
6383:
6379:
6374:
6372:
6364:
6359:
6353:, p. 111
6352:
6347:
6340:
6335:
6329:, p. 447
6328:
6323:
6317:
6313:
6308:
6301:
6296:
6290:, p. 445
6289:
6283:
6276:
6271:
6265:, p. 444
6264:
6259:
6244:
6238:
6229:
6224:
6220:
6216:
6212:
6208:
6204:
6197:
6189:
6185:
6181:
6177:
6173:
6169:
6150:
6142:
6138:
6134:
6130:
6126:
6122:
6115:
6107:
6100:
6096:
6080:
6073:
6067:
6060:
6044:
6040:
6033:
6023:
6004:
6001:
5997:
5993:
5990:
5981:
5958:
5938:
5928:
5919:
5915:
5907:
5904:
5894:
5891:
5886:
5882:
5872:
5870:
5866:
5862:
5858:
5848:
5846:
5842:
5838:
5834:
5830:
5826:
5822:
5806:
5802:
5797:
5793:
5783:
5781:
5770:
5749:
5744:
5741:
5737:
5727:
5714:
5710:
5706:
5690:
5686:
5682:
5678:
5677:Zeeman effect
5674:
5669:
5666:
5660:
5653:
5648:
5642:
5636:
5612:
5609:
5606:
5603:
5600:
5597:
5591:
5588:
5585:
5582:
5579:
5569:
5568:
5567:
5565:
5559:
5555:
5551:
5547:
5541:
5537:
5533:
5528:
5524:
5520:
5514:
5509:
5503:
5497:
5494:is less than
5493:
5487:
5481:
5477:
5476:
5470:
5466:
5462:
5452:
5448:
5446:
5442:
5437:
5428:
5419:
5417:
5413:
5391:
5388:
5385:
5382:
5379:
5376:
5373:
5370:
5367:
5364:
5352:
5345:
5340:
5336:
5333:
5321:
5316:
5309:
5305:
5301:
5298:
5294:
5290:
5287:
5275:
5270:
5265:
5259:
5255:
5249:
5246:
5242:
5238:
5235:
5232:
5228:
5224:
5221:
5217:
5213:
5210:
5198:
5193:
5189:
5186:
5182:
5178:
5175:
5166:
5161:
5157:
5154:
5149:
5146:
5134:
5121:
5110:
5099:
5098:
5097:
5080:
5077:
5074:
5071:
5068:
5065:
5062:
5059:
5056:
5053:
5041:
5035:
5031:
5028:
5016:
5011:
5007:
5004:
5001:
4997:
4993:
4990:
4978:
4973:
4969:
4966:
4962:
4958:
4955:
4946:
4941:
4937:
4934:
4929:
4926:
4914:
4901:
4890:
4879:
4878:
4877:
4874:
4872:
4851:
4847:
4843:
4838:
4834:
4830:
4826:
4821:
4818:
4810:
4789:
4785:
4781:
4776:
4772:
4768:
4764:
4759:
4756:
4731:
4727:
4723:
4718:
4714:
4710:
4706:
4701:
4698:
4675:
4672:
4669:
4666:
4663:
4660:
4657:
4654:
4651:
4648:
4645:
4642:
4639:
4627:
4624:
4621:
4618:
4615:
4612:
4609:
4606:
4603:
4597:
4593:
4588:
4584:
4581:
4578:
4574:
4570:
4566:
4562:
4559:
4556:
4552:
4548:
4545:
4542:
4538:
4534:
4531:
4528:
4524:
4520:
4513:
4512:
4511:
4508:
4504:
4500:
4496:
4492:
4488:
4484:
4477:Symmetric top
4474:
4471:
4449:
4447:
4444:
4421:
4391:
4381:
4371:
4367:
4363:
4357:
4354:
4347:
4346:
4345:
4332:
4312:
4304:
4300:
4278:
4275:
4270:
4266:
4260:
4256:
4250:
4246:
4242:
4236:
4232:
4226:
4223:
4216:
4215:
4214:
4200:
4192:
4187:
4170:
4167:
4164:
4161:
4158:
4155:
4152:
4149:
4146:
4143:
4131:
4124:
4119:
4115:
4112:
4100:
4095:
4090:
4087:
4084:
4080:
4076:
4073:
4061:
4056:
4052:
4049:
4046:
4032:
4019:
4008:
3997:
3996:
3995:
3992:
3978:
3955:
3952:
3949:
3946:
3943:
3940:
3937:
3934:
3931:
3928:
3925:
3919:
3914:
3910:
3907:
3904:
3900:
3893:
3889:
3885:
3882:
3878:
3874:
3871:
3868:
3864:
3860:
3857:
3854:
3850:
3847:
3844:
3840:
3833:
3832:
3831:
3817:
3809:
3799:
3780:
3777:
3772:
3765:
3762:
3759:
3756:
3751:
3748:
3741:
3738:
3731:
3730:
3729:
3707:
3704:
3698:
3694:
3688:
3684:
3677:
3674:
3671:
3668:
3662:
3650:
3649:
3648:
3646:
3638:
3634:
3630:
3626:
3622:
3618:
3593:
3590:
3582:
3579:
3576:
3570:
3567:
3564:
3561:
3555:
3551:
3547:
3539:
3536:
3530:
3526:
3520:
3516:
3512:
3505:
3501:
3495:
3491:
3481:
3480:
3479:
3477:
3470:
3466:
3459:
3450:
3446:
3442:
3437:
3428:
3426:
3422:
3400:
3382:
3361:
3349:
3329:
3299:
3275:
3272:
3269:
3266:
3263:
3260:
3257:
3254:
3251:
3248:
3236:
3230:
3226:
3223:
3211:
3206:
3202:
3199:
3196:
3192:
3180:
3176:
3172:
3169:
3165:
3156:
3152:
3148:
3145:
3131:
3118:
3107:
3096:
3095:
3094:
3080:
3077:
3074:
3062:
3058:
3049:
3045:
3042:
3031:
3027:
3025:
3018:
3011:
2990:
2986:
2977:
2973:
2969:
2964:
2960:
2952:
2948:
2942:
2938:
2931:
2928:
2921:
2920:
2919:
2897:
2893:
2889:
2884:
2880:
2876:
2872:
2867:
2859:
2855:
2851:
2846:
2842:
2838:
2834:
2829:
2826:
2819:
2818:
2817:
2803:
2800:
2795:
2791:
2787:
2782:
2778:
2774:
2769:
2765:
2743:
2720:
2717:
2714:
2711:
2708:
2705:
2702:
2699:
2696:
2693:
2690:
2685:
2681:
2678:
2675:
2671:
2667:
2664:
2661:
2657:
2654:
2651:
2647:
2640:
2639:
2638:
2636:
2632:
2628:
2624:
2620:
2611:
2602:
2600:
2596:
2592:
2588:
2580:Spherical top
2572:
2569:
2567:
2563:
2559:
2543:
2540:
2537:
2514:
2511:
2507:
2504:
2500:
2496:
2493:
2484:
2468:
2465:
2461:
2458:
2454:
2450:
2447:
2438:
2433:
2417:
2414:
2392:
2389:
2367:
2364:
2342:
2339:
2315:
2309:
2306:
2302:
2299:
2291:
2288:
2283:
2280:
2276:
2270:
2267:
2263:
2260:
2252:
2249:
2244:
2241:
2237:
2222:
2215:
2206:
2196:
2195:
2194:
2177:
2171:
2168:
2165:
2159:
2156:
2153:
2150:
2147:
2138:
2130:
2129:
2128:
2126:
2122:
2116:
2106:
2104:
2100:
2095:
2093:
2089:
2085:
2061:
2056:
2046:
2042:
2036:
2031:
2028:
2017:
2007:
1997:
1996:
1995:
1978:
1970:
1966:
1962:
1959:
1952:
1949:
1938:
1934:
1928:
1921:
1915:
1912:
1902:
1893:
1888:
1884:
1877:
1874:
1863:
1859:
1853:
1841:
1837:
1833:
1828:
1823:
1820:
1809:
1799:
1789:
1788:
1787:
1785:
1781:
1777:
1773:
1770:where ν is a
1754:
1751:
1748:
1745:
1742:
1739:
1732:
1731:
1730:
1728:
1712:
1692:
1688:
1684:
1681:
1672:
1661:
1639:
1628:
1618:
1616:
1613:belong to an
1610:
1604:
1600:
1592:
1588:
1584:
1581:
1577:
1573:
1570:
1566:
1565:
1564:
1561:
1559:
1554:
1550:
1546:
1542:
1538:
1534:
1530:
1529:Raman spectra
1523:Raman spectra
1520:
1518:
1514:
1510:
1506:
1500:
1492:
1485:
1483:
1467:
1463:
1447:
1436:
1423:
1408:
1392:
1375:
1368:
1358:
1351:
1335:
1320:
1318:
1315:
1313:
1298:
1283:
1260:
1258:
1255:
1254:
1238:
1234:
1230:
1225:
1221:
1217:
1212:
1208:
1199:
1197:
1178:
1174:
1170:
1165:
1161:
1157:
1152:
1148:
1139:
1137:
1132:
1131:rotation axis
1114:
1110:
1106:
1101:
1097:
1074:
1070:
1066:
1061:
1057:
1048:
1032:
1028:
1024:
1020:
1012:
1008:
1004:
988:
958:
954:
931:
927:
923:
918:
914:
910:
905:
901:
892:
876:
872:
856:
840:
824:
808:
778:
774:
770:
765:
761:
757:
752:
748:
739:
733:
732:
730:
729:energy levels
725:
723:
722:rotation axis
707:
687:
665:
661:
657:
652:
648:
644:
639:
635:
626:
622:
618:
614:
596:
592:
569:
565:
542:
538:
517:
509:
505:
501:
497:
493:
483:
480:
476:
471:
453:
447:
444:
439:
436:
432:
428:
425:
422:
419:
414:
410:
402:
401:
400:
397:
393:
389:
385:
384:isotopologues
381:
377:
373:
368:
366:
350:
341:
327:
319:
315:
311:
307:
297:
295:
291:
287:
283:
279:
274:
261:
257:
253:
249:
245:
241:
237:
236:van der Waals
211:
210:chlorotoluene
166:
164:
160:
155:
153:
149:
145:
141:
138:
134:
130:
126:
122:
118:
117:energy levels
112:
110:
106:
102:
98:
94:
90:
86:
82:
78:
74:
70:
66:
62:
58:
54:
47:
43:
26:
21:
8035:Spectroscopy
8011:
7999:
7979:(a misnomer)
7965:Applications
7883:Time-stretch
7774:paramagnetic
7592:Fluorescence
7540:
7510:Spectroscopy
7437:
7417:
7397:
7369:
7349:
7346:Kroto, H. W.
7326:
7305:
7281:
7256:
7243:Bibliography
7208:
7204:
7198:
7165:
7161:
7155:
7130:
7126:
7120:
7106:
7091:
7066:
7062:
7056:
7031:
7027:
7021:
6996:
6992:
6986:
6961:
6957:
6951:
6934:
6909:
6905:
6899:
6877:(4): 234–7.
6874:
6870:
6864:
6829:
6807:(4): 651–5.
6804:
6800:
6794:
6782:
6770:
6760:
6754:
6732:(1): 77–79.
6729:
6725:
6719:
6695:(11): 4968.
6692:
6688:
6661:
6657:
6651:
6639:
6614:
6602:
6577:
6573:
6560:
6532:
6528:
6518:
6490:
6486:
6479:
6474:, p. 45
6452:
6447:, p. 40
6440:
6428:
6423:, p. 97
6398:
6394:
6388:
6381:
6358:
6346:
6341:, p. 49
6334:
6322:
6307:
6302:, p. 95
6295:
6282:
6277:, p. 99
6270:
6258:
6246:. Retrieved
6237:
6210:
6207:Ann. Geophys
6206:
6196:
6171:
6167:
6149:
6124:
6120:
6114:
6105:
6099:
6079:
6066:
6059:nitric oxide
6039:paramagnetic
6032:
6022:
5927:
5918:
5900:
5878:
5854:
5837:Walter Gordy
5823:such as the
5789:
5776:
5733:
5685:nitric oxide
5673:paramagnetic
5670:
5661:, OCS, with
5651:
5647:Stark effect
5640:
5632:
5557:
5553:
5549:
5545:
5539:
5535:
5531:
5526:
5522:
5512:
5501:
5495:
5491:
5485:
5479:
5474:
5464:
5458:
5449:
5444:
5435:
5433:
5415:
5408:
5407:The term in
5406:
5095:
4875:
4870:
4808:
4690:
4502:
4498:
4494:
4490:
4486:
4482:
4480:
4470:paramagnetic
4455:
4446:
4410:
4298:
4296:
4188:
4185:
3993:
3970:
3805:
3797:
3727:
3644:
3632:
3624:
3616:
3614:
3468:
3461:
3457:
3454:
3448:
3444:
3440:
3424:
3420:
3350:
3290:
3028:
3023:
3016:
3009:
3007:
2917:
2735:
2634:
2623:point masses
2616:
2583:
2570:
2565:
2561:
2557:
2482:
2436:
2434:
2330:
2192:
2118:
2096:
2087:
2081:
1993:
1779:
1769:
1624:
1614:
1608:
1602:
1598:
1596:
1590:
1586:
1579:
1575:
1568:
1562:
1536:
1532:
1526:
1516:
1512:
1508:
1498:
1490:
1486:
1443:
1373:
1366:
1356:
1194:
1134:
991:dinitrogen (
875:point groups
726:
680:, with axis
620:
616:
612:
489:
475:bond lengths
472:
469:
392:bond lengths
369:
342:
303:
172:
169:Applications
156:
121:rigid rotors
113:
100:
93:far infrared
64:
52:
51:
41:
7551:Vibrational
6964:: 537–558.
6834:Hollas 1996
6787:Hollas 1996
6775:Hollas 1996
6644:Hollas 1996
6619:Hollas 1996
6607:Hollas 1996
6421:Hollas 1996
6351:Hollas 1996
6312:Hollas 1996
6300:Hollas 1996
6027:parameters.
5831:during the
5740:anisotropic
5681:diamagnetic
2631:rigid rotor
2619:rigid rotor
1593:= 0, ±1, ±2
1589:≠ 0, then Δ
1578:= 0, then Δ
1545:anisotropic
1464:) or HCCH (
479:bond angles
376:bond length
8029:Categories
7757:Two-photon
7659:absorption
7541:Rotational
7448:148319485X
7427:0852741421
7337:0471965227
7272:0198700725
7096:Jager, W.
6248:2 December
6092:References
5821:microwaves
5469:quadrupole
4873:molecule.
4497:...0 ... -
3659:population
3637:degeneracy
1960:2.99792458
1776:wavelength
1627:wavenumber
1462:dinitrogen
975:dioxygen (
310:orthogonal
278:polyatomic
81:absorption
7835:Terahertz
7816:Radiowave
7714:Mössbauer
7401:. Dover.
7352:. Dover.
6801:Phys. Rev
6726:Phys. Rev
6529:Phys. Rev
5985:¯
5903:B.H. Pate
5871:in 1975.
5805:microwave
5796:waveguide
5610:±
5595:Δ
5586:±
5577:Δ
5552:− 1, …, |
5443:for each
5360:′
5357:′
5329:′
5326:′
5299:−
5283:′
5280:′
5236:−
5206:′
5203:′
5187:−
5171:′
5142:′
5139:′
5131:↔
5126:′
5114:~
5111:ν
5049:′
5046:′
5024:′
5021:′
4986:′
4983:′
4967:−
4951:′
4922:′
4919:′
4911:↔
4906:′
4894:~
4891:ν
4835:π
4773:π
4715:π
4673:−
4667:…
4655:…
4628:…
4582:−
4425:~
4422:ω
4385:~
4382:ω
4247:π
4139:′
4136:′
4108:′
4105:′
4085:−
4069:′
4066:′
4040:′
4037:′
4029:↔
4024:′
4012:~
4009:ν
3883:−
3773:−
3689:−
3663:∝
3556:−
3521:−
3405:′
3396:←
3390:′
3387:′
3359:Δ
3334:′
3307:′
3304:′
3244:′
3241:′
3219:′
3216:′
3188:′
3185:′
3170:−
3161:′
3139:′
3136:′
3128:↔
3123:′
3111:~
3108:ν
3078:±
3070:′
3067:′
3059:−
3054:′
3040:Δ
2881:π
2843:π
2535:Δ
2512:−
2277:−
2226:~
2223:ν
2210:~
2207:ν
2125:separable
2043:ν
2037:≈
2029:−
2011:~
2008:ν
1963:×
1950:−
1935:ν
1913:−
1903:⋅
1875:−
1860:ν
1838:λ
1821:−
1803:~
1800:ν
1774:, λ is a
1772:frequency
1746:λ
1743:⋅
1740:ν
1727:gigahertz
1713:ν
1693:λ
1676:~
1673:ν
1643:~
1640:ν
1537:scattered
1338:propyne,
1286:ammonia,
1262:Benzene,
911:≪
843:silane, (
827:methane (
658:≤
645:≤
496:quantized
429:α
426:−
306:gas phase
208:group in
89:microwave
61:gas phase
57:molecules
8040:Rotation
8001:Category
7730:Electron
7697:Emission
7647:emission
7604:Vibronic
7395:(1975).
7348:(2003).
7303:(2003).
7233:21728367
7190:17902981
6043:dioxygen
5825:klystron
5713:dioxygen
5707:and the
5529:. where
5418:values.
3447:= 0.05.
2568:branch.
2508:″
2497:′
2485:branch,
2462:″
2451:′
2418:′
2393:″
2368:′
2343:″
2303:′
2292:′
2284:′
2264:″
2253:″
2245:″
1543:must be
1533:incident
502:and the
300:Overview
296:(ALMA).
258:using a
240:hydrogen
140:coupling
137:Coriolis
85:emission
8013:Commons
7840:ESR/EPR
7788:Nucleon
7616:(REMPI)
7213:Bibcode
7170:Bibcode
7135:Bibcode
7071:Bibcode
7036:Bibcode
7001:Bibcode
6966:Bibcode
6914:Bibcode
6879:Bibcode
6809:Bibcode
6734:Bibcode
6697:Bibcode
6666:Bibcode
6582:Bibcode
6537:Bibcode
6495:Bibcode
6403:Bibcode
6215:Bibcode
6176:Bibcode
6129:Bibcode
5885:kelvins
5869:Flygare
5845:Flygare
5703:, some
4869:for an
4809:prolate
3619:is the
2591:methane
1782:is the
1629:scale (
1482:methane
1395:water,
1317:Prolate
1196:Prolate
244:halogen
59:in the
7854:Others
7642:Atomic
7445:
7424:
7405:
7377:
7356:
7334:
7313:
7288:
7269:
7265:–469.
7231:
7188:
6316:silane
5687:, NO,
4871:oblate
4807:for a
4691:where
4452:Oxygen
4411:where
4297:where
3971:where
3641:2J + 1
3615:where
3291:where
3008:where
2816:, so
2736:where
2331:where
1553:Stokes
1549:tensor
1466:ethyne
1257:Oblate
1136:Oblate
619:, and
161:using
63:. The
7795:Alpha
7764:Auger
7742:X-ray
7709:Gamma
7687:X-ray
7620:Raman
7531:Raman
7526:FT-IR
6943:(PDF)
6632:SiNCS
6570:(PDF)
5910:Notes
5829:radar
5665:debye
2406:and
1621:Units
77:polar
75:) of
7443:ISBN
7422:ISBN
7403:ISBN
7375:ISBN
7354:ISBN
7332:ISBN
7311:ISBN
7286:ISBN
7267:ISBN
7229:PMID
7186:PMID
6765:p139
6250:2012
6061:, NO
6057:and
5637:the
5525:and
4749:and
4325:and
3627:the
3623:and
3015:and
2617:The
2356:and
1778:and
1611:= +2
1582:= ±2
1527:For
1515:to -
1501:= ±1
1409:and
1349:C≡CH
1218:<
1193:and
1171:<
1037:or D
881:or O
584:and
477:and
242:and
135:and
101:pure
71:vs.
7823:NMR
7263:431
7221:doi
7209:115
7178:doi
7143:doi
7131:238
7079:doi
7044:doi
7009:doi
6974:doi
6922:doi
6887:doi
6817:doi
6742:doi
6705:doi
6674:doi
6590:doi
6554:pdf
6545:doi
6512:pdf
6503:doi
6491:122
6411:doi
6223:doi
6184:doi
6137:doi
6125:237
5654:+ 1
5643:+ 1
5515:= 1
5504:+ 1
5488:+ 1
4634:and
3478:as
3441:Bhc
2233:vib
2143:rot
2052:GHz
1585:If
1574:If
1571:= 0
1493:= 0
1303:XeF
1089:or
845:SiH
813:CCl
490:In
87:by
83:or
8031::
7828:2D
7747:UV
7391:;
7227:.
7219:.
7207:.
7184:.
7176:.
7166:78
7164:.
7141:.
7129:.
7077:.
7067:72
7065:.
7042:.
7032:47
7030:.
7007:.
6997:26
6995:.
6972:.
6962:29
6960:.
6920:.
6910:20
6908:.
6885:.
6875:45
6873:.
6815:.
6805:79
6803:.
6740:.
6730:77
6728:.
6703:.
6693:54
6691:.
6687:.
6672:.
6662:47
6660:.
6588:.
6576:.
6572:.
6543:.
6533:75
6531:.
6527:.
6501:.
6489:.
6464:^
6409:.
6399:62
6397:.
6370:^
6221:.
6211:22
6209:.
6205:.
6182:.
6172:21
6170:.
6156:NH
6135:.
6123:.
6084:J.
6045:,
5809:NH
5715:,
5693:NO
5691:,
5556:−
5548:+
5543:,
5538:+
5534:=
5498:,
5482:,
5463:,
5411:JK
4448:.
4243:32
3445:kT
2101:,
2057:30
2024:cm
1971:10
1967:10
1899:cm
1847:cm
1816:cm
1519:.
1495:,
1470:CH
1413:NO
1372:=
1340:CH
1336:,
1333:Cl
1324:CH
1299:;
1288:NH
1284:;
1041:.
1039:∞h
1035:∞v
1029:,
1025:,
1021:,
1013:,
1009:,
1005:,
989:,
885:.
861:SF
857:,
841:,
829:CH
809:,
615:,
557:,
510:,
265:NH
262:.
238:,
232:Cl
206:Cl
176:CH
165:.
131:,
127:,
29:CF
27:,
7502:e
7495:t
7488:v
7451:.
7430:.
7411:.
7383:.
7362:.
7340:.
7319:.
7294:.
7275:.
7235:.
7223::
7215::
7192:.
7180::
7172::
7149:.
7145::
7137::
7114:.
7100:.
7085:.
7081::
7073::
7050:.
7046::
7038::
7015:.
7011::
7003::
6980:.
6976::
6968::
6945:.
6928:.
6924::
6916::
6893:.
6889::
6881::
6859:.
6850:2
6845:N
6823:.
6819::
6811::
6748:.
6744::
6736::
6713:.
6707::
6699::
6680:.
6676::
6668::
6634:.
6628:3
6623:H
6596:.
6592::
6584::
6578:1
6551:.
6547::
6539::
6509:.
6505::
6497::
6417:.
6413::
6405::
6252:.
6231:.
6225::
6217::
6190:.
6186::
6178::
6161:3
6143:.
6139::
6131::
6074:.
6052:2
6047:O
6005:c
6002:h
5998:/
5994:B
5991:=
5982:B
5959:B
5939:B
5814:3
5764:2
5759:N
5722:2
5717:O
5698:2
5652:J
5641:J
5639:2
5613:1
5607:,
5604:0
5601:=
5598:F
5592:,
5589:1
5583:=
5580:J
5560:|
5558:I
5554:J
5550:I
5546:J
5540:I
5536:J
5532:F
5527:J
5523:F
5513:I
5502:J
5500:2
5496:I
5492:J
5486:I
5484:2
5480:I
5475:J
5465:I
5445:J
5436:J
5416:K
5409:D
5392:.
5389:.
5386:.
5383:,
5380:2
5377:,
5374:1
5371:,
5368:0
5365:=
5353:J
5346:3
5341:)
5337:1
5334:+
5322:J
5317:(
5310:J
5306:D
5302:4
5295:)
5291:1
5288:+
5276:J
5271:(
5266:)
5260:2
5256:K
5250:K
5247:J
5243:D
5239:2
5233:B
5229:(
5225:2
5222:=
5218:)
5214:K
5211:,
5199:J
5194:(
5190:F
5183:)
5179:K
5176:,
5167:J
5162:(
5158:F
5155:=
5150:K
5147:,
5135:J
5122:J
5081:.
5078:.
5075:.
5072:,
5069:2
5066:,
5063:1
5060:,
5057:0
5054:=
5042:J
5036:)
5032:1
5029:+
5017:J
5012:(
5008:B
5005:2
5002:=
4998:)
4994:K
4991:,
4979:J
4974:(
4970:F
4963:)
4959:K
4956:,
4947:J
4942:(
4938:F
4935:=
4930:K
4927:,
4915:J
4902:J
4852:C
4848:I
4844:c
4839:2
4831:8
4827:h
4822:=
4819:A
4790:A
4786:I
4782:c
4777:2
4769:8
4765:h
4760:=
4757:A
4732:B
4728:I
4724:c
4719:2
4711:8
4707:h
4702:=
4699:B
4676:J
4670:,
4664:,
4661:0
4658:,
4652:,
4649:J
4646:+
4643:=
4640:K
4625:,
4622:2
4619:,
4616:1
4613:,
4610:0
4607:=
4604:J
4598:2
4594:K
4589:)
4585:B
4579:A
4575:(
4571:+
4567:)
4563:1
4560:+
4557:J
4553:(
4549:J
4546:B
4543:=
4539:)
4535:K
4532:,
4529:J
4525:(
4521:F
4503:K
4499:J
4495:J
4491:M
4487:J
4483:J
4463:2
4458:O
4392:2
4372:3
4368:B
4364:4
4358:=
4355:D
4333:D
4313:B
4299:k
4279:c
4276:k
4271:2
4267:r
4261:2
4257:I
4251:4
4237:3
4233:h
4227:=
4224:D
4201:D
4171:.
4168:.
4165:.
4162:,
4159:2
4156:,
4153:1
4150:,
4147:0
4144:=
4132:J
4125:3
4120:)
4116:1
4113:+
4101:J
4096:(
4091:D
4088:4
4081:)
4077:1
4074:+
4062:J
4057:(
4053:B
4050:2
4047:=
4033:J
4020:J
3979:D
3956:.
3953:.
3950:.
3947:,
3944:2
3941:,
3938:1
3935:,
3932:0
3929:=
3926:J
3920:2
3915:)
3911:1
3908:+
3905:J
3901:(
3894:2
3890:J
3886:D
3879:)
3875:1
3872:+
3869:J
3865:(
3861:J
3858:B
3855:=
3851:)
3848:J
3845:(
3841:F
3818:B
3781:2
3778:1
3766:B
3763:c
3760:h
3757:2
3752:T
3749:k
3742:=
3739:J
3708:T
3705:k
3699:J
3695:E
3685:e
3681:)
3678:1
3675:+
3672:J
3669:2
3666:(
3645:J
3633:J
3625:T
3617:k
3611:,
3594:T
3591:k
3586:)
3583:1
3580:+
3577:J
3574:(
3571:J
3568:c
3565:h
3562:B
3552:e
3548:=
3540:T
3537:k
3531:J
3527:E
3517:e
3513:=
3506:0
3502:N
3496:J
3492:N
3472:0
3469:N
3467:/
3464:J
3462:N
3458:J
3449:J
3443:/
3425:x
3421:B
3401:J
3383:J
3362:J
3330:J
3300:J
3276:.
3273:.
3270:.
3267:,
3264:2
3261:,
3258:1
3255:,
3252:0
3249:=
3237:J
3231:)
3227:1
3224:+
3212:J
3207:(
3203:B
3200:2
3197:=
3193:)
3181:J
3177:(
3173:F
3166:)
3157:J
3153:(
3149:F
3146:=
3132:J
3119:J
3081:1
3075:=
3063:J
3050:J
3046:=
3043:J
3024:d
3020:2
3017:m
3013:1
3010:m
2991:2
2987:d
2978:2
2974:m
2970:+
2965:1
2961:m
2953:2
2949:m
2943:1
2939:m
2932:=
2929:I
2898:C
2894:I
2890:c
2885:2
2877:8
2873:h
2868:=
2860:B
2856:I
2852:c
2847:2
2839:8
2835:h
2830:=
2827:B
2804:0
2801:=
2796:A
2792:I
2788:,
2783:C
2779:I
2775:=
2770:B
2766:I
2744:B
2721:.
2718:.
2715:.
2712:,
2709:2
2706:,
2703:1
2700:,
2697:0
2694:=
2691:J
2686:)
2682:1
2679:+
2676:J
2672:(
2668:J
2665:B
2662:=
2658:)
2655:J
2652:(
2648:F
2635:F
2566:R
2562:P
2558:Q
2544:0
2541:=
2538:J
2515:1
2505:J
2501:=
2494:J
2483:P
2469:1
2466:+
2459:J
2455:=
2448:J
2437:R
2415:J
2390:J
2365:B
2340:B
2316:,
2313:)
2310:1
2307:+
2300:J
2296:(
2289:J
2281:B
2274:)
2271:1
2268:+
2261:J
2257:(
2250:J
2242:B
2238:+
2216:=
2178:.
2175:)
2172:1
2169:+
2166:J
2163:(
2160:J
2157:B
2154:c
2151:h
2148:=
2139:E
2088:B
2062:.
2047:/
2032:1
2018:/
1979:.
1953:1
1945:s
1939:/
1929:=
1922:)
1916:1
1908:s
1894:(
1889:/
1885:c
1878:1
1870:s
1864:/
1854:=
1842:/
1834:1
1829:=
1824:1
1810:/
1780:c
1755:,
1752:c
1749:=
1689:/
1685:1
1682:=
1615:S
1609:J
1607:Δ
1603:R
1599:J
1591:J
1587:K
1580:J
1576:K
1569:K
1567:Δ
1517:J
1513:J
1509:K
1499:J
1497:Δ
1491:K
1489:Δ
1480:(
1475:4
1460:(
1455:2
1450:N
1418:2
1406:O
1402:2
1397:H
1383:3
1377:B
1374:I
1370:A
1367:I
1360:C
1357:I
1345:3
1329:3
1308:4
1293:3
1278:6
1273:H
1269:6
1264:C
1239:C
1235:I
1231:=
1226:B
1222:I
1213:A
1209:I
1179:C
1175:I
1166:B
1162:I
1158:=
1153:A
1149:I
1115:C
1111:I
1107:=
1102:B
1098:I
1075:B
1071:I
1067:=
1062:A
1058:I
1019:)
1017:2
1003:)
998:2
993:N
987:)
982:2
977:O
959:A
955:I
932:C
928:I
924:=
919:B
915:I
906:A
902:I
883:h
879:d
877:T
871:)
866:6
855:)
850:4
839:)
834:4
823:)
818:4
807:)
802:4
797:P
779:C
775:I
771:=
766:B
762:I
758:=
753:A
749:I
708:A
688:A
666:C
662:I
653:B
649:I
640:A
636:I
621:C
617:B
613:A
597:C
593:I
570:B
566:I
543:A
539:I
518:I
454:)
448:2
445:1
440:+
437:v
433:(
423:B
420:=
415:v
411:B
351:K
328:J
270:3
228:7
223:H
219:7
214:C
212:(
202:4
197:H
193:6
188:C
181:3
67:(
42:J
38:I
34:3
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.