756:
322:. This is the ratio of the number of methyl groups to the number of benzene rings in all of the substrates. For example, in the disproportionation of toluene, the M/R ratio is 1. Side reactions in which alkanes are produced reduce the number of methyl groups available which decreases the M/R ratio. This can be mitigated by adding compounds with higher numbers of methyl groups, such as trimethylbenzene. The ratio of products produced depends only on the M/R ratio so different starting materials can produce the same compounds via transalkylation.
886:
103:
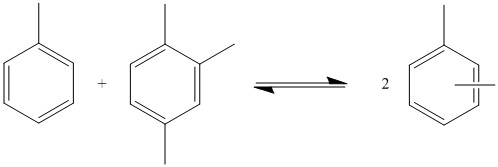
reaction of toluene in which one toluene molecule transfers its methyl group to another one. The reaction is not selective, and the xylene produced can be ortho, meta, or para. There is a higher demand for para xylene, so it is often separated, and the mixture is allowed to reequilibrate to give more
359:
because of their channel openings are often between 0.4 and 1.5 nanometers, just enough for the molecules to pass through. Aromatics molecules enter and exit these channels at different rates, also called diffusion. In addition to their molecular sieving effect, zeolites have weakly bonded protons
330:
Transalkylation reactions of six to ten carbon methylated aromatics are often performed with the cofeed of hydrogen gas, over a zeolite based solid catalyst. Industrial processes operate the transalkylation reactor at elevated temperature and pressure to achieve desired process economics. Zeolites
112:
Diethylbenzenes arise as side-products of the alkylation of benzene with ethylene, which is conducted on a very large scale. Since there is only a limited market for diethylbenzene, much of it is recycled by transalkylation to give ethylbenzene:
927:
Al-Khattaf, Sulaiman; Ali, Syed A.; Aitani, Abdullah M.; Žilková, Naděžda; Kubička, David; Čejka, Jiří (2014). "Recent
Advances in Reactions of Alkylbenzenes over Novel Zeolites: The Effects of Zeolite Structure and Morphology".
317:
to produce xylene. The reaction occurs via equilibrium, so the product is not pure xylene. Many products are produced with varying numbers of methyl groups. The quantities in which each product is produced depends on the
363:
Zeolites of varying sizes are used to perform transalkylation on different substrates. For example, zeolites with a pore size of 5.5Å are suitable for benzene, toluene, xylenes and trimethylbenzenes transalkylations.
735:
884:, Goncalvez De Almeida; Jose Luis & Berna Tejero et al., "Catalytic transalkylation of dialkyl benzenes", published 2010-01-28, assigned to Petroquimica Espanola, S.A. Petresa
963:
Fiege, Helmut; Voges, Heinz-Werner; Hamamoto, Toshikazu; Umemura, Sumio; Iwata, Tadao; Miki, Hisaya; Fujita, Yasuhiro; Buysch, Hans-Josef; Garbe, Dorothea; Paulus, Wilfried (2000). "Phenol
Derivatives".
291:
524:
92:
1037:
849:
302:
812:
Griesbaum, Karl; Behr, Arno; Biedenkapp, Dieter; Voges, Heinz-Werner; Garbe, Dorothea; Paetz, Christian; Collin, Gerd; Mayer, Dieter; Höke, Hartmut (2000). "Hydrocarbons".
1057:
1052:
1001:
966:
814:
99:
Transalkylation, as used by the petrochemical industry, is often used to convert toluene into benzene and xylenes. This is achieved through a
846:
547:
1084:
355:
building blocks. These crystals are porous in nature with characteristic micropore channels, cavities. Zeolite is known as one class of
319:
2003:
2008:
119:
390:
871:
Tsai, Tseng-Chang "Disproportionation and
Transalkylation of Alkylbenzenes over Zeolite Catalysts". Elsevier Science, 1999
360:
originated from its chemical composition. These are chemical active centers for acid-catalyzed transalkylation reaction.
1018:
983:
831:
372:
Transalkylation is employed in the commercial production of aromatics beyond the usual BTX feedstocks. For example,
1077:
75:. Transalkylation can convert toluene, which is overproduced, into benzene and xylene, which are under-produced.
67:. Motivation for using transalkylation reactions is based on a difference in production and demand for benzene,
1907:
881:
2031:
779:
1480:
1070:
1517:
1990:
1890:
745:
52:
1997:
1885:
755:
1966:
1411:
8:
1272:
789:
945:
741:
373:
100:
64:
913:
1956:
1926:
1684:
1306:
1014:
979:
827:
784:
28:
20:
949:
900:
Harmer, Mark A.; Sun, Qun (2001). "Solid acid catalysis using ion-exchange resins".
1661:
1155:
1093:
1006:
971:
937:
909:
819:
314:
306:
36:
1042:
1010:
880:
1880:
1639:
1634:
1617:
1600:
1401:
1150:
941:
853:
774:
356:
1047:
91:
1951:
1946:
1822:
1817:
1812:
1605:
1572:
1356:
1338:
1328:
1038:
Process and apparatus for ethylbenzene production and transalkylation to xylene
2025:
1971:
1919:
1850:
1736:
1726:
1721:
1711:
1706:
1656:
1651:
1567:
1562:
1552:
1406:
1361:
1323:
1311:
1282:
1160:
975:
823:
1902:
1789:
1784:
1761:
1512:
1351:
1277:
1214:
1209:
1187:
1143:
1128:
1118:
40:
1961:
1914:
1875:
1756:
1644:
1629:
1624:
1612:
1177:
1172:
1138:
1133:
1123:
1101:
44:
32:
380:
is produced in part via two transalkylation reactions. In one example,
1870:
1861:
1741:
1696:
1592:
1557:
1547:
1487:
1423:
1346:
1294:
769:
749:
730:{\displaystyle {\ce {HOC6H3(C(CH3)3)2 + HOC6H5 -> 2 HOC6H4C(CH3)3}}}
1837:
1751:
1716:
1701:
1689:
1532:
1507:
1316:
1062:
1845:
1799:
1766:
1462:
1368:
1242:
1197:
1182:
80:
56:
301:
16:
Chemical reaction which transfers an alkyl group between molecules
1807:
1731:
1582:
1577:
1542:
1527:
1522:
1492:
1475:
1299:
1226:
1192:
76:
68:
60:
48:
1895:
1827:
1671:
1380:
1373:
1267:
1248:
1237:
1221:
1167:
538:
72:
1776:
1746:
1679:
1537:
1502:
1497:
1470:
1418:
1385:
1289:
1113:
313:
This type of reaction can also be performed with toluene and
811:
1204:
286:{\displaystyle {\ce {C6H4(C2H5)2 + C6H6 -> 2 C6H5C2H5}}}
926:
962:
723:
710:
689:
676:
656:
643:
627:
614:
601:
575:
562:
512:
499:
478:
465:
449:
436:
423:
410:
279:
266:
253:
240:
220:
207:
191:
178:
165:
147:
134:
998:
519:{\displaystyle {\ce {(CH3)3COC6H5 -> HOC6H4C(CH3)3}}}
759:
1,3-Diisopropylbenzene is produced via transalkylation.
309:
to produce xylene. In this example, the M/R ratio is 2.
847:
Styrene process with recycle from dehydrogenation zone
550:
393:
331:
are micro-crystalline solids composed of tetrahedral
122:
39:
to another. The reaction is used for the transfer of
729:
518:
285:
999:K. W. Schmiedel; D. Decker (2012). "Resorcinol".
95:Reaction of toluene to produce benzene and xylene
2023:
384:-butylphenyl ether is isomerized to the phenol:
1002:Ullmann's Encyclopedia of Industrial Chemistry
967:Ullmann's Encyclopedia of Industrial Chemistry
815:Ullmann's Encyclopedia of Industrial Chemistry
1078:
1058:Transalkylation in the Petrochemical Industry
1085:
1071:
807:
805:
51:rings. This is of particular value in the
899:
665:
229:
754:
740:Transalkylation in conjunction with the
300:
90:
802:
2024:
867:
865:
863:
861:
1092:
1066:
86:
325:
1053:Exxon Mobil Transalkylation Process
858:
13:
992:
107:
14:
2043:
1048:Transalkylation of dialkylbenzene
1031:
744:contributes to the production of
537:-butylphenol by treatment with
533:-butylphenol is converted to 4-
305:Transalkylation of toluene and
956:
920:
893:
882:US application 20100022814
874:
840:
713:
697:
659:
617:
604:
588:
580:
502:
486:
452:
413:
397:
223:
181:
152:
83:in transalkylation reactions.
1:
1011:10.1002/14356007.a23_111.pub2
914:10.1016/S0926-860X(01)00794-3
795:
31:involving the transfer of an
942:10.1080/01614940.2014.946846
902:Applied Catalysis A: General
296:
7:
763:
10:
2048:
367:
1980:
1939:
1859:
1836:
1798:
1775:
1670:
1591:
1461:
1438:
1394:
1337:
1260:
1235:
1100:
976:10.1002/14356007.a19_313
824:10.1002/14356007.a13_227
1991:chemical classification
1043:Transalkylation process
1005:. Weinheim: Wiley-VCH.
970:. Weinheim: Wiley-VCH.
818:. Weinheim: Wiley-VCH.
780:Friedel–Crafts reaction
760:
746:1,3-diisopropylbenzene
731:
520:
310:
287:
96:
53:petrochemical industry
1998:chemical nomenclature
758:
732:
529:Additionally, 2,4-di-
521:
304:
288:
94:
548:
541:by transalkylation:
391:
120:
1454:not C, H or O)
790:Transesterification
725:
712:
691:
678:
658:
645:
629:
616:
603:
577:
564:
514:
501:
480:
467:
451:
438:
425:
412:
281:
268:
255:
242:
222:
209:
193:
180:
167:
149:
136:
2032:Addition reactions
1896:Hypervalent iodine
852:2012-03-20 at the
761:
742:Hock rearrangement
727:
700:
695:
679:
666:
646:
633:
591:
586:
578:
565:
552:
516:
489:
484:
468:
455:
439:
426:
400:
395:
311:
283:
269:
256:
243:
230:
210:
197:
168:
155:
150:
137:
124:
101:disproportionation
97:
87:Disproportionation
79:are often used as
65:aromatic compounds
2019:
2018:
1957:Sulfenyl chloride
1935:
1934:
1434:
1433:
1253:(only C, H and O)
1094:Functional groups
930:Catalysis Reviews
785:Hydrodealkylation
748:, a precursor to
703:
694:
682:
669:
649:
636:
594:
585:
568:
555:
492:
483:
471:
458:
442:
429:
403:
326:Zeolite catalysts
272:
259:
246:
233:
213:
200:
171:
158:
140:
127:
29:chemical reaction
21:organic chemistry
2039:
1986:
1891:Trifluoromethoxy
1459:
1458:
1455:
1258:
1257:
1254:
1107:
1087:
1080:
1073:
1064:
1063:
1025:
1024:
996:
990:
989:
960:
954:
953:
924:
918:
917:
897:
891:
890:
889:
885:
878:
872:
869:
856:
844:
838:
837:
809:
736:
734:
733:
728:
726:
724:
721:
716:
711:
708:
701:
692:
690:
687:
680:
677:
674:
667:
657:
654:
647:
644:
641:
634:
628:
625:
620:
615:
612:
607:
602:
599:
592:
583:
576:
573:
566:
563:
560:
553:
525:
523:
522:
517:
515:
513:
510:
505:
500:
497:
490:
481:
479:
476:
469:
466:
463:
456:
450:
447:
440:
437:
434:
427:
424:
421:
416:
411:
408:
401:
354:
353:
352:
342:
341:
340:
315:trimethylbenzene
307:trimethylbenzene
292:
290:
289:
284:
282:
280:
277:
270:
267:
264:
257:
254:
251:
244:
241:
238:
231:
221:
218:
211:
208:
205:
198:
192:
189:
184:
179:
176:
169:
166:
163:
156:
148:
145:
138:
135:
132:
125:
37:organic compound
2047:
2046:
2042:
2041:
2040:
2038:
2037:
2036:
2022:
2021:
2020:
2015:
1984:
1976:
1931:
1886:Trichloromethyl
1881:Trifluoromethyl
1855:
1832:
1794:
1771:
1666:
1635:Phosphine oxide
1587:
1453:
1451:
1450:
1448:
1446:
1444:
1442:
1440:
1430:
1390:
1333:
1252:
1251:
1246:
1241:
1231:
1105:
1104:
1096:
1091:
1034:
1029:
1028:
1021:
997:
993:
986:
961:
957:
925:
921:
898:
894:
887:
879:
875:
870:
859:
854:Wayback Machine
845:
841:
834:
810:
803:
798:
775:BTX (chemistry)
766:
722:
717:
709:
704:
696:
688:
683:
675:
670:
655:
650:
642:
637:
626:
621:
613:
608:
600:
595:
587:
579:
574:
569:
561:
556:
551:
549:
546:
545:
511:
506:
498:
493:
485:
477:
472:
464:
459:
448:
443:
435:
430:
422:
417:
409:
404:
396:
394:
392:
389:
388:
370:
357:molecular sieve
351:
348:
347:
346:
344:
339:
336:
335:
334:
332:
328:
299:
278:
273:
265:
260:
252:
247:
239:
234:
219:
214:
206:
201:
190:
185:
177:
172:
164:
159:
151:
146:
141:
133:
128:
123:
121:
118:
117:
110:
108:Diethylbenzenes
89:
55:to manufacture
25:transalkylation
17:
12:
11:
5:
2045:
2035:
2034:
2017:
2016:
2014:
2013:
2012:
2011:
2006:
1994:
1987:
1981:
1978:
1977:
1975:
1974:
1972:Sulfinylamines
1969:
1964:
1959:
1954:
1952:Phosphoramides
1949:
1947:Isothiocyanate
1943:
1941:
1937:
1936:
1933:
1932:
1930:
1929:
1924:
1923:
1922:
1912:
1911:
1910:
1900:
1899:
1898:
1893:
1888:
1883:
1878:
1867:
1865:
1857:
1856:
1854:
1853:
1848:
1842:
1840:
1834:
1833:
1831:
1830:
1825:
1823:Selenenic acid
1820:
1818:Seleninic acid
1815:
1813:Selenonic acid
1810:
1804:
1802:
1796:
1795:
1793:
1792:
1787:
1781:
1779:
1773:
1772:
1770:
1769:
1764:
1759:
1754:
1749:
1744:
1739:
1734:
1729:
1724:
1719:
1714:
1709:
1704:
1699:
1694:
1693:
1692:
1682:
1676:
1674:
1668:
1667:
1665:
1664:
1659:
1654:
1649:
1648:
1647:
1637:
1632:
1627:
1622:
1621:
1620:
1610:
1609:
1608:
1606:Phosphodiester
1597:
1595:
1589:
1588:
1586:
1585:
1580:
1575:
1570:
1565:
1560:
1555:
1550:
1545:
1540:
1535:
1530:
1525:
1520:
1515:
1510:
1505:
1500:
1495:
1490:
1485:
1484:
1483:
1478:
1467:
1465:
1456:
1452:(one element,
1436:
1435:
1432:
1431:
1429:
1428:
1427:
1426:
1416:
1415:
1414:
1409:
1398:
1396:
1392:
1391:
1389:
1388:
1383:
1378:
1377:
1376:
1366:
1365:
1364:
1359:
1354:
1343:
1341:
1335:
1334:
1332:
1331:
1329:Methylenedioxy
1326:
1321:
1320:
1319:
1314:
1304:
1303:
1302:
1297:
1287:
1286:
1285:
1275:
1270:
1264:
1262:
1255:
1233:
1232:
1230:
1229:
1224:
1219:
1218:
1217:
1212:
1202:
1201:
1200:
1195:
1190:
1185:
1180:
1175:
1165:
1164:
1163:
1158:
1148:
1147:
1146:
1141:
1136:
1131:
1126:
1121:
1110:
1108:
1106:(only C and H)
1098:
1097:
1090:
1089:
1082:
1075:
1067:
1061:
1060:
1055:
1050:
1045:
1040:
1033:
1032:External links
1030:
1027:
1026:
1020:978-3527306732
1019:
991:
985:978-3527306732
984:
955:
936:(4): 333–402.
919:
908:(1–2): 45–62.
892:
873:
857:
839:
833:978-3527306732
832:
800:
799:
797:
794:
793:
792:
787:
782:
777:
772:
765:
762:
738:
737:
720:
715:
707:
699:
686:
673:
664:
661:
653:
640:
632:
624:
619:
611:
606:
598:
590:
582:
572:
559:
527:
526:
509:
504:
496:
488:
475:
462:
454:
446:
433:
420:
415:
407:
399:
369:
366:
349:
337:
327:
324:
298:
295:
294:
293:
276:
263:
250:
237:
228:
225:
217:
204:
196:
188:
183:
175:
162:
154:
144:
131:
109:
106:
104:para product.
88:
85:
15:
9:
6:
4:
3:
2:
2044:
2033:
2030:
2029:
2027:
2010:
2007:
2005:
2002:
2001:
2000:
1999:
1995:
1993:
1992:
1988:
1983:
1982:
1979:
1973:
1970:
1968:
1965:
1963:
1960:
1958:
1955:
1953:
1950:
1948:
1945:
1944:
1942:
1938:
1928:
1925:
1921:
1918:
1917:
1916:
1913:
1909:
1906:
1905:
1904:
1901:
1897:
1894:
1892:
1889:
1887:
1884:
1882:
1879:
1877:
1874:
1873:
1872:
1869:
1868:
1866:
1864:
1863:
1858:
1852:
1851:Telluroketone
1849:
1847:
1844:
1843:
1841:
1839:
1835:
1829:
1826:
1824:
1821:
1819:
1816:
1814:
1811:
1809:
1806:
1805:
1803:
1801:
1797:
1791:
1788:
1786:
1783:
1782:
1780:
1778:
1774:
1768:
1765:
1763:
1760:
1758:
1755:
1753:
1750:
1748:
1745:
1743:
1740:
1738:
1737:Sulfonic acid
1735:
1733:
1730:
1728:
1727:Sulfinic acid
1725:
1723:
1722:Thiosulfonate
1720:
1718:
1715:
1713:
1712:Thiosulfinate
1710:
1708:
1707:Sulfenic acid
1705:
1703:
1700:
1698:
1695:
1691:
1688:
1687:
1686:
1683:
1681:
1678:
1677:
1675:
1673:
1669:
1663:
1662:Phosphaallene
1660:
1658:
1657:Phosphaalkyne
1655:
1653:
1652:Phosphaalkene
1650:
1646:
1643:
1642:
1641:
1638:
1636:
1633:
1631:
1628:
1626:
1623:
1619:
1616:
1615:
1614:
1611:
1607:
1604:
1603:
1602:
1599:
1598:
1596:
1594:
1590:
1584:
1581:
1579:
1576:
1574:
1571:
1569:
1566:
1564:
1561:
1559:
1556:
1554:
1551:
1549:
1546:
1544:
1541:
1539:
1536:
1534:
1531:
1529:
1526:
1524:
1521:
1519:
1516:
1514:
1511:
1509:
1506:
1504:
1501:
1499:
1496:
1494:
1491:
1489:
1486:
1482:
1479:
1477:
1474:
1473:
1472:
1469:
1468:
1466:
1464:
1460:
1457:
1437:
1425:
1422:
1421:
1420:
1417:
1413:
1410:
1408:
1405:
1404:
1403:
1400:
1399:
1397:
1393:
1387:
1384:
1382:
1379:
1375:
1372:
1371:
1370:
1367:
1363:
1360:
1358:
1355:
1353:
1350:
1349:
1348:
1345:
1344:
1342:
1340:
1336:
1330:
1327:
1325:
1324:Ethylenedioxy
1322:
1318:
1315:
1313:
1310:
1309:
1308:
1305:
1301:
1298:
1296:
1293:
1292:
1291:
1288:
1284:
1281:
1280:
1279:
1276:
1274:
1271:
1269:
1266:
1265:
1263:
1259:
1256:
1250:
1244:
1239:
1234:
1228:
1225:
1223:
1220:
1216:
1213:
1211:
1208:
1207:
1206:
1203:
1199:
1196:
1194:
1191:
1189:
1186:
1184:
1181:
1179:
1176:
1174:
1171:
1170:
1169:
1166:
1162:
1159:
1157:
1154:
1153:
1152:
1149:
1145:
1142:
1140:
1137:
1135:
1132:
1130:
1127:
1125:
1122:
1120:
1117:
1116:
1115:
1112:
1111:
1109:
1103:
1099:
1095:
1088:
1083:
1081:
1076:
1074:
1069:
1068:
1065:
1059:
1056:
1054:
1051:
1049:
1046:
1044:
1041:
1039:
1036:
1035:
1022:
1016:
1012:
1008:
1004:
1003:
995:
987:
981:
977:
973:
969:
968:
959:
951:
947:
943:
939:
935:
931:
923:
915:
911:
907:
903:
896:
883:
877:
868:
866:
864:
862:
855:
851:
848:
843:
835:
829:
825:
821:
817:
816:
808:
806:
801:
791:
788:
786:
783:
781:
778:
776:
773:
771:
768:
767:
757:
753:
751:
747:
743:
718:
705:
684:
671:
662:
651:
638:
630:
622:
609:
596:
570:
557:
544:
543:
542:
540:
536:
532:
507:
494:
473:
460:
444:
431:
418:
405:
387:
386:
385:
383:
379:
377:
365:
361:
358:
323:
321:
316:
308:
303:
274:
261:
248:
235:
226:
215:
202:
194:
186:
173:
160:
142:
129:
116:
115:
114:
105:
102:
93:
84:
82:
78:
74:
70:
66:
62:
58:
54:
50:
46:
42:
38:
34:
30:
26:
22:
1996:
1989:
1903:Vinyl halide
1860:
1790:Borinic acid
1785:Boronic acid
1762:Thioxanthate
1102:Hydrocarbons
1000:
994:
965:
958:
933:
929:
922:
905:
901:
895:
876:
842:
813:
739:
534:
530:
528:
381:
378:-butylphenol
375:
371:
362:
329:
312:
111:
98:
63:, and other
45:ethyl groups
24:
18:
1967:Thiocyanate
1962:Sulfonamide
1927:Perchlorate
1915:Acyl halide
1876:Fluoroethyl
1757:Thionoester
1645:Phosphonium
1630:Phosphinate
1625:Phosphonous
1613:Phosphonate
1312:Hydroperoxy
1134:Cyclopropyl
33:alkyl group
1871:Haloalkane
1742:Thioketone
1697:Persulfide
1593:Phosphorus
1558:Isocyanate
1548:Isonitrile
1449:or oxygen
1447:hydrogen,
1443:not being
1424:Orthoester
1317:Dioxiranes
1295:Enol ether
1183:1-Propenyl
796:References
770:Alkylation
750:resorcinol
2004:inorganic
1838:Tellurium
1752:Thioester
1717:Sulfoxide
1702:Disulfide
1690:Sulfonium
1640:Phosphine
1618:Phosphite
1601:Phosphate
1533:Carbamate
1508:Hydrazone
1441:element,
1439:Only one
1412:Anhydride
1151:Methylene
660:⟶
453:⟶
320:M/R ratio
297:M/R Ratio
224:⟶
81:catalysts
35:from one
2026:Category
1985:See also
1920:Chloride
1846:Tellurol
1800:Selenium
1767:Xanthate
1481:Ammonium
1463:Nitrogen
1445:carbon,
1402:Carboxyl
1369:Aldehyde
1357:Acryloyl
1339:carbonyl
1243:hydrogen
1198:Cumulene
950:97415930
850:Archived
764:See also
77:Zeolites
57:p-xylene
47:between
2009:organic
1808:Selenol
1732:Sulfone
1685:Sulfide
1583:NONOate
1578:Nitroso
1568:Nitrite
1563:Nitrate
1553:Cyanate
1543:Nitrile
1528:Amidine
1523:Imidate
1493:Nitrene
1488:Hydrazo
1476:Enamine
1407:Acetoxy
1395:carboxy
1362:Benzoyl
1300:Epoxide
1283:Methoxy
1273:Alcohol
1227:Carbene
1161:Methine
368:Phenols
73:xylenes
69:toluene
61:styrene
49:benzene
1908:Iodide
1828:Selone
1672:Sulfur
1381:Ketone
1374:Ketene
1352:Acetyl
1307:Peroxy
1278:Alkoxy
1268:Acetal
1249:oxygen
1238:carbon
1222:Alkyne
1215:Benzyl
1210:Phenyl
1193:Allene
1188:Crotyl
1168:Alkene
1156:Bridge
1144:Pentyl
1129:Propyl
1119:Methyl
1017:
982:
948:
888:
830:
539:phenol
71:, and
41:methyl
1940:Other
1777:Boron
1747:Thial
1680:Thiol
1573:Nitro
1538:Imide
1518:Amide
1503:Oxime
1498:Imine
1471:Amine
1419:Ester
1386:Ynone
1290:Ether
1261:R-O-R
1236:Only
1178:Allyl
1173:Vinyl
1139:Butyl
1124:Ethyl
1114:Alkyl
946:S2CID
27:is a
1862:Halo
1347:Acyl
1247:and
1205:Aryl
1015:ISBN
980:ISBN
828:ISBN
535:tert
531:tert
382:tert
376:tert
343:and
43:and
1513:Azo
1007:doi
972:doi
938:doi
910:doi
906:221
820:doi
668:HOC
635:HOC
554:HOC
457:HOC
428:COC
345:SiO
333:AlO
19:In
2028::
1245:,
1240:,
1013:.
978:.
944:.
934:56
932:.
904:.
860:^
826:.
804:^
752:.
702:CH
593:CH
491:CH
402:CH
374:4-
59:,
23:,
1086:e
1079:t
1072:v
1023:.
1009::
988:.
974::
952:.
940::
916:.
912::
836:.
822::
719:3
714:)
706:3
698:(
693:C
685:4
681:H
672:6
663:2
652:5
648:H
639:6
631:+
623:2
618:)
610:3
605:)
597:3
589:(
584:C
581:(
571:3
567:H
558:6
508:3
503:)
495:3
487:(
482:C
474:4
470:H
461:6
445:5
441:H
432:6
419:3
414:)
406:3
398:(
350:4
338:4
275:5
271:H
262:2
258:C
249:5
245:H
236:6
232:C
227:2
216:6
212:H
203:6
199:C
195:+
187:2
182:)
174:5
170:H
161:2
157:C
153:(
143:4
139:H
130:6
126:C
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.