1200:
resistance to poisonous species, etc.), low cost, ability to be processed into thin-films, and overall compatibility with other cell components. While polymeric materials are currently the preferred choice of proton-conducting membrane, they require humidification for adequate performance and can sometimes physically degrade due to hydrations effects, thereby causing losses of efficiency. As mentioned, Nafion is also limited by a dehydration temperature of < 100 °C, which can lead to slower reaction kinetics, poor cost efficiency, and CO poisoning of Pt electrode catalysts. Conversely, MOFs have shown encouraging proton conductivities in both low and high temperature regimes as well as over a wide range of humidity conditions. Below 100 °C and under hydration, the presence of hydrogen bonding and solvent water molecules aid in proton transport, whereas anhydrous conditions are suitable for temperatures above 100 °C. MOFs also have the distinct advantage of exhibiting proton conductivity by the framework itself in addition to the inclusion of charge carries (i.e., water, acids, etc.) into their pores.
1049:
the electrode to minimize potential flooding. In order to enable the electrochemical reactions at the electrodes, protons, electrons and the reactant gases (hydrogen or oxygen) must gain access to the surface of the catalyst in the electrodes, while the product water, which can be in either liquid or gaseous phase, or both phases, must be able to permeate from the catalyst to the gas outlet. These properties are typically realized by porous composites of polymer electrolyte binder (ionomer) and catalyst nanoparticles supported on carbon particles. Typically platinum is used as the catalyst for the electrochemical reactions at the anode and cathode, while nanoparticles realize high surface to weight ratios (as further described below) reducing the amount of the costly platinum. The polymer electrolyte binder provides the ionic conductivity, while the carbon support of the catalyst improves the electric conductivity and enables low platinum metal loading. The electric conductivity in the composite electrodes is typically more than 40 times higher as the proton conductivity.
1058:
PTFE. The carbon particles used in the GDL can be larger than those employed in the catalyst because surface area is not the most important variable in this layer. GDL should be around 15–35 μm thick to balance needed porosity with mechanical strength. Often, an intermediate porous layer is added between the GDL and catalyst layer to ease the transitions between the large pores in the GDL and small porosity in the catalyst layer. Since a primary function of the GDL is to help remove water, a product, flooding can occur when water effectively blocks the GDL. This limits the reactants ability to access the catalyst and significantly decreases performance. Teflon can be coated onto the GDL to limit the possibility of flooding. Several microscopic variables are analyzed in the GDLS such as: porosity, tortuosity and permeability. These variables have incidence over the behavior of the fuel cells.
1388:
stack cost. Although the Pt loading of PEM fuel cells has been reduced by two orders of magnitude over the past decade, further reduction is necessary to make the technology economically viable for commercialization. Whereas some research efforts aim to address this issue by improving the electrocatalytic activity of Pt-based catalysts, an alternative is to eliminate the use of Pt altogether by developing a non-platinum-group-metal (non-PGM) cathode catalyst whose performance rivals that of Pt-based technologies. The U.S. Department of Energy has been setting milestones for the development of fuel cells, targeting a durability of 5000 hours and a non-PGM catalyst ORR volumetric activity of 300 A cm.
85:(MEA) which include the electrodes, electrolyte, catalyst, and gas diffusion layers. An ink of catalyst, carbon, and electrode are sprayed or painted onto the solid electrolyte and carbon paper is hot pressed on either side to protect the inside of the cell and also act as electrodes. The pivotal part of the cell is the triple phase boundary (TPB) where the electrolyte, catalyst, and reactants mix and thus where the cell reactions actually occur. Importantly, the membrane must not be electrically conductive so the half reactions do not mix. Operating temperatures above 100 °C are desired so the water byproduct becomes steam and water management becomes less critical in cell design.
1421:
work solely on PEM fuel cells due to their high power density and excellent dynamic characteristics as compared with other types of fuel cells. Due to their light weight, PEMFCs are most suited for transportation applications. PEMFCs for buses, which use compressed hydrogen for fuel, can operate at up to 40% efficiency. Generally PEMFCs are implemented on buses over smaller cars because of the available volume to house the system and store the fuel. Technical issues for transportation involve incorporation of PEMs into current vehicle technology and updating energy systems. Full fuel cell vehicles are not advantageous if hydrogen is sourced from
1192:
combinations, which is attractive from a design standpoint. MOFs exhibit many unique properties due to their tunable pore sizes, thermal stability, high volume capacities, large surface areas, and desirable electrochemical characteristics. Among their many diverse uses, MOFs are promising candidates for clean energy applications such as hydrogen storage, gas separations, supercapacitors, Li-ion batteries, solar cells, and fuel cells. Within the field of fuel cell research, MOFs are being studied as potential electrolyte materials and electrode catalysts that could someday replace traditional polymer membranes and Pt catalysts, respectively.
968:
diffusion layer assembly (MEGA) and promotes O2 diffusion to the catalyst layer. Unlike conventional flow fields, the 3D micro-lattices in the complex field, which act as baffles and induce frequent micro-scale interfacial flux between the GDL and flow-fields. Due to this repeating micro-scale convective flow, oxygen transport to catalyst layer (CL) and liquid water removal from GDL is significantly enhanced. The generated water is quickly drawn out through the flow field, preventing accumulation within the pores. As a result, the power generation from this flow field is uniform across the cross-section and self-humidification is enabled.
964:
fields.The flow field is a structure made up of a rib and channels. However, the rib partially covers the gas diffusion layer (GDL) and the resultant gas-transport distance is longer than the inter-channel distance. Furthermore, the contact pressure between the GDL and the rib also compresses the GDL, making its thickness non-uniform across the rib and channel. The large width and non-uniform thickness of the rib will increase potential for water vapor to accumulate and the oxygen will be compromised. As a result, oxygen will be impeded to diffuse into catalyst layer, leading to nonuniform power generation in the FC.
864:
31:
1175:
PEM and catalyst layers, the backplate needs to be structurally tough and leakage-resistant in case of structural failure due to fuel cell vibration and temperature cycling. As fuel cells operate in wide ranges of temperatures and in highly reductive/oxidative environments, plates must have high surface tolerances over the wide ranges of temperatures and should be chemically stable. Since the backplate accounts for more than three-quarters of the fuel cell mass, the material must also be lightweight to maximize the energy density.
1404:
content conducive to ORR activity. The power density of the FeAc/Phen/ZIF-8-catalyst was found to be 0.75 W cm at 0.6 V. This value is a significant improvement over the maximal 0.37 W cm power density of previous M/N/C-catalysts and is much closer to matching the typical value of 1.0–1.2 W cm for Pt-based catalysts with a Pt loading of 0.3 mg cm. The catalyst also demonstrated a volumetric activity of 230 A·cm, the highest value for non-PGM catalysts to date, approaching the U.S. Department of Energy milestone.
1278:
connection between the catalyst and the rest of the cell. Platinum is so effective because it has high activity and bonds to the hydrogen just strongly enough to facilitate electron transfer but not inhibit the hydrogen from continuing to move around the cell. However, platinum is less active in the cathode oxygen reduction reaction. This necessitates the use of more platinum, increasing the cell's expense and thus feasibility. Many potential catalyst choices are ruled out because of the extreme acidity of the cell.
1433:. Whereas PEMFCs generally require high purity hydrogen for operation, other fuel cell types can run on methane and are thus more flexible systems. Therefore, PEMFCs are best for small scale systems until economically scalable pure hydrogen is available. Furthermore, PEMFCs have the possibility of replacing batteries for portable electronics, though integration of the hydrogen supply is a technical challenge particularly without a convenient location to store it within the device.
1400:, protons, and electrons have greater difficulty in migrating through the catalytic layer, decreasing the voltage output of the cell. While high microporosity of the M/N/C catalytic network results in high volumetric activity, improved mass transport properties are instead associated to macroporosity of the network. These M/N/C materials are synthesized using high temperature pyrolysis and other high temperature treatments of precursors containing the metal, nitrogen, and carbon.
872:
1220:
reaction at the anode, and thus non-PGM and metal-free catalysts are being investigated as alternatives. The high volumetric density, large pore surface areas, and openness of metal-ion sites in MOFs make them ideal candidates for catalyst precursors. Despite promising catalytic abilities, the durability of these proposed MOF-based catalysts is currently less than desirable and the ORR mechanism in this context is still not completely understood.
1479:. General Electric continued working on PEM cells and in the mid-1970s developed PEM water electrolysis technology for undersea life support, leading to the US Navy Oxygen Generating Plant. The British Royal Navy adopted this technology in early 1980s for their submarine fleet. In the late 1980s and early 1990s, Los Alamos National Lab and Texas A&M University experimented with ways to reduce the amount of platinum required for PEM cells.
1208:
pores. The maximum temperature achieved was 150 °C with an optimum conductivity of 5 × 10 S/cm, which is lower than other current electrolyte membranes. However, this model holds promise for its temperature regime, anhydrous conditions, and ability to control the quantity of guest molecules within the pores, all of which allowed for the tunability of proton conductivity. Additionally, the triazole-loaded PCMOF2 was incorporated into a H
1561:
1547:
1282:
this catalyst layer, which limits the lower cost limit. Below 4 nm, Pt will form islands on the paper, limiting its activity. Above this thickness, the Pt will coat the carbon and be an effective catalyst. To further complicate things, Nafion cannot be infiltrated beyond 10 um, so using more Pt than this is an unnecessary expense. Thus the amount and shape of the catalyst is limited by the constraints of other materials.
1204:"superprotonic" conductivity (8 × 10 S/cm) at 25 °C and 98% relative humidity (RH). They later found that increasing the hydrophilic nature of the cations introduced into the pores could enhance proton conductivity even more. In this low temperature regime that is dependent on degree of hydration, it has also been shown that proton conductivity is heavily dependent on humidity levels.
1212:/air membrane-electrode assembly and achieved an open circuit voltage of 1.18 V at 100 °C that was stable for 72 hours and managed to remain gas tight throughout testing. This was the first instance that proved MOFs could actually be implemented into functioning fuel cells, and the moderate potential difference showed that fuel crossover due to porosity was not an issue.
1030:, HT-PEMFC): higher temperature allow for better efficiencies, power densities, ease of cooling (because of larger allowable temperature differences), reduced sensitivity to carbon monoxide poisoning and better controllability (because of absence of water management issues in the membrane); however, these recent types are not as common. PBI can be doped with phosphoric or
1392:
properties. While M/N/C-catalysts still demonstrate poorer volumetric activities than Pt-based catalysts, the reduced costs of such catalysts allows for greater loading to compensate. However, increasing the loading of M/N/C-catalysts also renders the catalytic layer thicker, impairing its mass transport properties. In other words, H
1445:
were only applied in extreme conditions. Such fuel cells also required very expensive materials and could only be used for stationary applications due to their size. These issues were addressed by the PEM fuel cell. The PEM fuel cell was invented in the early 1960s by
Willard Thomas Grubb and Leonard
1324:
were found to exhibit an enhanced activity and most importantly, an extended durability compared to many previous designs. While the observed enhancement in the activities is ascribed to a strained lattice, the authors report that their findings on the degradation kinetics establish that the extended
1178:
Materials that fulfill all of these requirements are often very expensive. Gold has been shown to fulfill these criteria well, but is only used for small production volumes due to its high cost. Titanium nitride (TiN) is a cheaper material that is used in fuel cell backplates due to its high chemical
1253:
As mentioned above, platinum is by far the most effective element used for PEM fuel cell catalysts, and nearly all current PEM fuel cells use platinum particles on porous carbon supports to catalyze both hydrogen oxidation and oxygen reduction. However, due to their high cost, current Pt/C catalysts
1219:
MOFs have also been targeted as potential replacements of platinum group metal (PGM) materials for electrode catalysts, although this research is still in the early stages of development. In PEMFCs, the oxygen reduction reaction (ORR) at the Pt cathode is significantly slower than the fuel oxidation
1207:
A high temperature anhydrous example is PCMOF2, which consists of sodium ions coordinated to a trisulfonated benzene derivative. To improve performance and allow for higher operating temperatures, water can be replaced as the proton carrier by less volatile imidazole or triazole molecules within the
1174:
The external electrodes, often referred to as bipolar plates or backplates, serve to distribute fuel and oxygen uniformly to the catalysts, to remove water, to collect and transmit electric current. Thus, they need to be in close contact with the catalyst. Because the plates are in contact with both
1048:
An electrode typically consists of carbon support, Pt particles, Nafion ionomer, and/or Teflon binder. The carbon support functions as an electrical conductor; the Pt particles are reaction sites; the ionomer provides paths for proton conduction, and the Teflon binder increases the hydrophobicity of
1513:
Despite their success in space programs, fuel cell systems were limited to space missions and other special applications, where high cost could be tolerated. It was not until the late 1980s and early 1990s that fuel cells became a real option for wider application base. Several pivotal innovations,
1370:
In addition, researchers have been investigating ways of reducing the CO content of hydrogen fuel before it enters a fuel cell as a possible way to avoid poisoning the catalysts. One recent study revealed that ruthenium-platinum core–shell nanoparticles are particularly effective at oxidizing CO to
1366:
ions than the (100) facets, reducing the number of catalytic sites open to oxygen molecules. The nanocubes they synthesized, in contrast, had almost exclusively (100) facets, which are known to interact with sulfate more weakly. As a result, a greater fraction of the surface area of those particles
1215:
To date, the highest proton conductivity achieved for a MOF electrolyte is 4.2 × 10 S/cm at 25 °C under humid conditions (98% RH), which is competitive with Nafion. Some recent experiments have even successfully produced thin-film MOF membranes instead of the traditional bulk samples or single
1195:
As electrolyte materials, the inclusion of MOFs seems at first counter-intuitive. Fuel cell membranes generally have low porosity to prevent fuel crossover and loss of voltage between the anode and cathode. Additionally, membranes tend to have low crystallinity because the transport of ions is more
1420:
The major application of PEM fuel cells focuses on transportation primarily because of their potential impact on the environment, e.g. the control of emission of the green house gases (GHG). Other applications include distributed/stationary and portable power generation. Most major motor companies
1403:
Recently, researchers have developed a Fe/N/C catalyst derived from iron (II) acetate (FeAc), phenanthroline (Phen), and a metal-organic-framework (MOF) host. The MOF is a Zn(II) zeolitic imidazolate framework (ZIF) called ZIF-8, which demonstrates a high microporous surface area and high nitrogen
1281:
The most effective ways of achieving the nanoscale Pt on carbon powder, which is currently the best option, are through vacuum deposition, sputtering, and electrodeposition. The platinum particles are deposited onto carbon paper that is permeated with PTFE. However, there is an optimal thinness to
1203:
A low temperature example is work by
Kitagawa, et al. who used a two-dimensional oxalate-bridged anionic layer framework as the host and introduced ammonium cations and adipic acid molecules into the pores to increase proton concentration. The result was one of the first instances of a MOF showing
1057:
The GDL electrically connects the catalyst and current collector. It must be porous, electrically conductive, and thin. The reactants must be able to reach the catalyst, but conductivity and porosity can act as opposing forces. Optimally, the GDL should be composed of about one third Nafion or 15%
952:
Water management is crucial to performance: if water is evaporated too slowly, it will flood the membrane and the accumulation of water inside of field flow plate will impede the flow of oxygen into the fuel cell, but if water evaporates too fast, the membrane will dry and the resistance across it
910:
PEMFCs have a thin, polymeric membrane as the electrolyte. This membrane is located in between the anode and cathode catalysts and allows the passage of protons to pass to the cathode while restricting the passage of electrons. Compared to liquid electrolytes, a polymeric membrane has a much lower
1509:
Extremely expensive materials were used and the fuel cells required very pure hydrogen and oxygen. Early fuel cells tended to require inconveniently high operating temperatures that were a problem in many applications. However, fuel cells were seen to be desirable due to the large amounts of fuel
1387:
The challenge for the viability of PEM fuel cells today still remains in their cost and stability. The high cost can in large part be attributed to the use of the precious metal of platinum in the catalyst layer of PEM cells. The electrocatalyst currently accounts for nearly half of the fuel cell
1277:
Since the most common and effective catalyst, platinum, is extremely expensive, alternative processing is necessary to maximize surface area and minimize loading. Deposition of nanosized Pt particles onto carbon powder (Pt/C) provides a large Pt surface area while the carbon allows for electrical
1039:
and backbone of Nafion) and their polar character leads to hydration that is less temperature dependent than Nafion. However, PEEK is far less ionically conductive than Nafion and thus is a less favorable electrolyte choice. Recently, protic ionic liquids and protic organic ionic plastic crystals
364:
output of the fuel cell. Meanwhile, a stream of oxygen is delivered to the cathode side of the MEA. At the cathode side oxygen molecules react with the protons permeating through the polymer electrolyte membrane and the electrons arriving through the external circuit to form water molecules. This
1312:
under atmospheric conditions resulting in high efficiency spray. Studies have shown that due to the uniform size of the droplets created by this type of spray, due to the high transfer efficiency of the technology, due to the non-clogging nature of the nozzle and finally due to the fact that the
1265:
One method of increasing the performance of platinum catalysts is to optimize the size and shape of the platinum particles. Decreasing the particles' size alone increases the total surface area of catalyst available to participate in reactions per volume of platinum used, but recent studies have
1199:
The general requirements of a good electrolyte for PEMFCs are: high proton conductivity (>10 S/cm for practical applications) to enable proton transport between electrodes, good chemical and thermal stability under fuel cell operating conditions (environmental humidity, variable temperatures,
1034:
and the conductivity scales with amount of doping and temperature. At high temperatures, it is difficult to keep Nafion hydrated, but this acid doped material does not use water as a medium for proton conduction. It also exhibits better mechanical properties, higher strength, than Nafion and is
919:
and
Aquivion), which minimize gas crossover and short circuiting of the fuel cell. A disadvantage of fluor containing polymers is the fact that during production (and disposal) PFAS products are formed. PFAS, the so-called forever chemicals, are highly toxic. Newer polymers such as the recently
1182:
To perform their main function of distributing gas and fuel, these plates often have straight, parallel channels across its surface. However, this simple approach has led to issues such as uneven pressure distribution, water droplets blocking gas flow, and output power oscillations. Innovative
967:
This new design enabled the first FC stack functions without a humidifying system meanwhile overcoming water recirculation issues and achieving high power output stability. The 3D micro lattice allows more pathways for gas flow; therefore, it promotes airflow toward membrane electrode and gas
963:
Another innovative method to resolve the water recirculation problem is the 3D fine mesh flow field design used in the Toyota Mirai, 2014. Conventional design of FC stack recirculates water from the air outlet to the air inlet through a humidifier with a straight channel and porous metal flow
1191:
Metal-organic frameworks (MOFs) are a relatively new class of porous, highly crystalline materials that consist of metal nodes connected by organic linkers. Due to the simplicity of manipulating or substituting the metal centers and ligands, there are a virtually limitless number of possible
1391:
Promising alternatives to Pt-based catalysts are Metal/Nitrogen/ Carbon-catalysts (M/N/C-catalysts). To achieve high power density, or output of power over surface area of the cell, a volumetric activity of at least 1/10 that of Pt-based catalysts must be met, along with good mass transport
979:
to eliminate CO from product gases and form more hydrogen. Additionally, the membrane is sensitive to the presences of metal ions, which may impair proton conduction mechanisms and can be introduced by corrosion of metallic bipolar plates, metallic components in the fuel cell system or from
926:
Under extreme sub-freezing conditions, the water produced by fuel cells can freeze in porous layers and flow channels. This freezing water can block gas and fuel transport as well as cover catalyst reaction sites, resulting in a loss of output power and a start-up failure of the fuel cell.
2083:
Jiangshui Luo; Annemette H. Jensen; Neil R. Brooks; Jeroen
Sniekers; Martin Knipper; David Aili; Qingfeng Li; Bram Vanroy; Michael Wübbenhorst; Feng Yan; Luc Van Meervelt; Zhigang Shao; Jianhua Fang; Zheng-Hong Luo; Dirk E. De Vos; Koen Binnemans; Jan Fransaer (2015). "1,2,4-Triazolium
857:
1313:
ultrasonic energy de-agglomerates the suspension just before atomization, fuel cells MEA's manufactured this way have a greater homogeneity in the final MEA, and the gas flow through the cell is more uniform, maximizing the efficiency of the platinum in the MEA. Recent studies using
1216:
crystals, which is crucial for their industrial applicability. Once MOFs are able to consistently achieve sufficient conductivity levels, mechanical strength, water stability, and simple processing, they have the potential to play an important role in PEMFCs in the near future.
801:
582:
937:
PEM fuel cells have been shown to be capable of high power densities up to 39.7 kW/kg, compared to 2.5 kW/kg for solid oxide fuel cells. Due to this high power density, much research is being done on potential applications in transportation as well as wearable technology.
1407:
While the power density achieved by the novel FeAc/Phen/ZIF-8-catalyst is promising, its durability remains inadequate for commercial application. It is reported that the best durability exhibited by this catalyst still had a 15% drop in current density over 100 hours in
476:
316:
1425:; however, they become beneficial when implemented as hybrids. There is potential for PEMFCs to be used for stationary power generation, where they provide 5 kW at 30% efficiency; however, they run into competition with other types of fuel cells, mainly
930:
However, the low operating temperature of a PEM fuel cell allows it to reach a suitable temperature with less heating compared to other types of fuel cells. With this approach, PEM fuel cells have been shown to be capable of cold start processes from −20°C.
837:
The reversible reaction is expressed in the equation and shows the reincorporation of the hydrogen protons and electrons together with the oxygen molecule and the formation of one water molecule. The potentials in each case are given with respect to the
974:
The platinum catalyst on the membrane is easily poisoned by carbon monoxide, which is often present in product gases formed by methane reforming (no more than one part per million is usually acceptable). This generally necessitates the use of the
695:
1018:, which relies on liquid water humidification of the membrane to transport protons. This implies that it is not feasible to use temperatures above 80 to 90 °C, since the membrane would dry. Other, more recent membrane types, based on
953:
increases. Both cases will cause damage to stability and power output. Water management is a very difficult subject in PEM systems, primarily because water in the membrane is attracted toward the cathode of the cell through polarization.
1262:. Consequently, one main goal of catalyst design for PEM fuel cells is to increase the catalytic activity of platinum by a factor of four so that only one-fourth as much of the precious metal is necessary to achieve similar performance.
898:
catalyst. Unfortunately however, splitting the oxygen molecule is more difficult, and this causes significant electric losses. An appropriate catalyst material for this process has not been discovered, and platinum is the best option.
1518:
loading and thin film electrodes, drove the cost of fuel cells down, making development of PEMFC systems more realistic. However, there is significant debate as to whether hydrogen fuel cells will be a realistic technology for use in
1361:
displayed a fourfold increase in oxygen reduction activity compared to randomly faceted platinum nanoparticles of similar size. The authors concluded that the (111) facets of the randomly shaped nanoparticles bonded more strongly to
987:
were proposed, as in
Daimler Chrysler Necar 5; reforming methanol, i.e. making it react to obtain hydrogen, is however a very complicated process, that also requires purification from the carbon monoxide the reaction produces. A
1333:
The other popular approach to improving catalyst performance is to reduce its sensitivity to impurities in the fuel source, especially carbon monoxide (CO). Presently, pure hydrogen gas is becoming economical to mass-produce by
1196:
favorable in disordered materials. On the other hand, pores can be filled with additional ion carriers that ultimately enhance the ionic conductivity of the system and high crystallinity makes the design process less complex.
1150:
61:. Their distinguishing features include lower temperature/pressure ranges (50 to 100 °C) and a special proton-conducting polymer electrolyte membrane. PEMFCs generate electricity and operate on the opposite principle to
1412:/air. Hence while the Fe-based non-PGM catalysts rival Pt-based catalysts in their electrocatalytic activity, there is still much work to be done in understanding their degradation mechanisms and improving their durability.
3347:
213:
3349:
Y. Wang, Ken S. Chen, Jeffrey
Mishler, Sung Chan Cho, Xavier Cordobes Adroher, A Review of Polymer Electrolyte Membrane Fuel Cells: Technology, Applications, and Needs on Fundamental Research, Applied Energy 88 (2011)
1628:
Loyselle, Patricia; Prokopius, Kevin (August 2011). Teledyne Energy
Systems, Inc., Proton Exchange Member (PEM) Fuel Cell Engineering Model Powerplant. Test Report: Initial Benchmark Tests in the Original Orientation.
1956:
Coletta, Vitor C., et al. "Cu-Modified SrTiO3 Perovskites Toward
Enhanced Water–Gas Shift Catalysis: A Combined Experimental and Computational Study." ACS Applied Energy Materials (2021), 4, 1, 452–461
701:
482:
2655:
3134:
E. Proietti, F. Jaouen, M. Lefevre, N. Larouche, J. Tian, J. Herranz, and J.-P. Dodelet. 2011 Iron-based cathode catalyst with enhanced power density in polymer electrolyte membrane fuel cells"
384:
1320:
Very recently, a new class of ORR electrocatalysts have been introduced in the case of Pt-M (M-Fe and Co) systems with an ordered intermetallic core encapsulated within a Pt-rich shell. These
219:
3040:
C. Wang; H. Daimon; T. Onodera; T. Koda; S. Sun (2008). "A General
Approach to the Size- and Shape-Controlled Synthesis of Platinum Nanoparticles and Their Catalytic Reduction of Oxygen".
1035:
cheaper. However, acid leaching is a considerable issue and processing, mixing with catalyst to form ink, has proved tricky. Aromatic polymers, such as PEEK, are far cheaper than Teflon (
2224:
Schalenbach, Maximilian; Zillgitt, Marcel; Maier, Wiebke; Stolten, Detlef (2015-07-29). "Parasitic
Currents Caused by Different Ionic and Electronic Conductivities in Fuel Cell Anodes".
1258:
estimates that platinum-based catalysts will need to use roughly four times less platinum than is used in current PEM fuel cell designs in order to represent a realistic alternative to
1179:
stability, electrical conductivity, and corrosion resistance. However, defects in the TiN coating can easily lead to corrosion of the underlying material, most commonly steel.
1293:
Ni(111) surface has a higher oxygen reduction activity than pure Pt(111) by a factor of ten. The authors attribute this dramatic performance increase to modifications to the
2937:
Sagar Prabhudev; Matthieu Bugnet; Christina Bock; Gianluigi Botton (2013). "Strained Lattice with Persistent Atomic Order in Pt3Fe2 Intermetallic Core–Shell Nanocatalysts".
2692:
N. Tian; Z.-Y. Zhou; S.-G. Sun; Y. Ding; Z. L. Wang (2007). "Synthesis of tetrahexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity".
830:
626:
611:
345:
1782:
Schalenbach, Maximilian; Hoefner, Tobias; Paciok, Paul; Carmo, Marcelo; Lueke, Wiebke; Stolten, Detlef (2015-10-28). "Gas Permeation through Nafion. Part 1: Measurements".
1809:
Schalenbach, Maximilian; Hoeh, Michael A.; Gostick, Jeff T.; Lueke, Wiebke; Stolten, Detlef (2015-10-14). "Gas Permeation through Nafion. Part 2: Resistor Network Model".
1297:
of the surface, reducing its tendency to bond to oxygen-containing ionic species present in PEM fuel cells and hence increasing the number of available sites for oxygen
920:
patented SPX3 (POLYMERS COMPRISING SULFONATED 2,6-DIPHENYL-1,4-PHENYLENE OXIDE REPEATING UNITS -US 11434329 B2) are fluor free and therefore do not carry the PFAS risk.
2823:
1379:
Ni above: the ruthenium core of the particle alters the electronic structure of the platinum surface, rendering it better able to catalyze the oxidation of CO.
3225:
Serov, A.; Artyushkova, K.; Atanassov, P. (2014). "Fe-N-C Oxygen Reduction Fuel Cell Catalyst Derived from Carbendazim: Synthesis, Structure, and Reactivity".
3428:
3075:
S. Alayoglu; A. U. Nilekar; M. Mavrikakis; B. Eichhorn (2008). "Ru–Pt core–shell nanoparticles for preferential oxidation of carbon monoxide in hydrogen".
1354:(25%). Even tens of parts per million of CO can poison a pure platinum catalyst, so increasing platinum's resistance to CO is an active area of research.
1589:
1027:
2801:
2659:
1080:
17:
1266:
demonstrated additional ways to make further improvements to catalytic performance. For example, one study reports that high-index facets of platinum
153:
3184:
1317:
to deposit the catalyst over the membrane have also shown high catalyst utilization due to the reduced thickness of the deposited catalyst layers.
2831:
Proceedings of Asme 2011 5th International Conference on Energy Sustainability & 9th Fuel Cell Science, Engineering and Technology Conference
1183:
approaches, such as nature-inspired fractal models and computer simulations are being explored to optimize the function of these bipolar plates.
1654:
Millington, Ben; Du, Shangfeng; Pollet, Bruno G. (2011). "The Effect of Materials on Proton Exchange Membrane Fuel Cell Electrode Performance".
887:. Finally, the membrane must be resistant to the reducing environment at the cathode as well as the harsh oxidative environment at the anode.
2972:
Minna Cao, Dongshuang Wu & Rong Cao (2014). "Recent Advances in the Stabilization of Platinum Electrocatalysts for Fuel-Cell Reactions".
1274:
with large integers, such as Pt (730)) provide a greater density of reactive sites for oxygen reduction than typical platinum nanoparticles.
867:
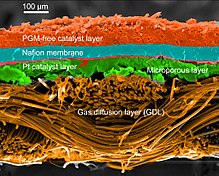
SEM micrograph of a PEMFC MEA cross-section with a non-precious metal catalyst cathode and Pt/C anode. False colors applied for clarity.
352:
The newly formed protons permeate through the polymer electrolyte membrane to the cathode side. The electrons travel along an external
2052:"Protic ionic liquid and ionic melts prepared from methanesulfonic acid and 1H-1,2,4-triazole as high temperature PEMFC electrolytes"
2050:
Jiangshui Luo; Jin Hu; Wolfgang Saak; Rüdiger Beckhaus; Gunther Wittstock; Ivo F. J. Vankelecom; Carsten Agert; Olaf Conrad (2011).
2311:
796:{\displaystyle E^{\circ }=1.2291\,\mathrm {V} \,\,\,{\frac {\mathrm {d} E^{\circ }}{\mathrm {d} T}}=-0.8456\,\mathrm {mV\,K^{-1}} }
577:{\displaystyle E^{\circ }=1.2291\,\mathrm {V} \,\,\,{\frac {\mathrm {d} E^{\circ }}{\mathrm {d} T}}=-0.8456\,\mathrm {mV\,K^{-1}} }
2084:
perfluorobutanesulfonate as an archetypal pure protic organic ionic plastic crystal electrolyte for all-solid-state fuel cells".
1735:
Yin, Xi; Lin, Ling; Chung, Hoon T; Komini Babu, Siddharth; Martinez, Ulises; Purdy, Geraldine M; Zelenay, Piotr (4 August 2017).
1232:
to obtain higher catalytic activity than the standard carbon-supported platinum particle catalysts used in current PEM fuel cells
3440:
Toyota's 2,000 or so Mirai sales in 2016 represented more than three times the megawattage of PEMFCs produced worldwide in 2014.
2869:
Shukla, S (2015). "Analysis of Low Platinum Loading Thin Polymer Electrolyte Fuel Cell Electrodes Prepared by Inkjet Printing".
1375:, a much less harmful fuel contaminant. The mechanism that produces this effect is conceptually similar to that described for Pt
996:. Furthermore, the start-up times of such a reformer reactor are of about half an hour. Alternatively, methanol, and some other
2675:
2523:
Ramaswamy, Padmini; Wong, Norman E.; Shimizu, George K. H. (2014). "MOFs as proton conductors – challenges and opportunities".
1228:
Much of the current research on catalysts for PEM fuel cells can be classified as having one of the following main objectives:
3024:
2846:
2628:
Lux, Lacey; Williams, Kia; Ma, Shengqian (2015). "Heat-treatment of metal–organic frameworks for green energy applications".
471:{\displaystyle {\frac {1}{2}}\mathrm {O} _{2}+{2\mathrm {H} }^{+}+\mathrm {2e} ^{-}\rightarrow \mathrm {H} _{2}\mathrm {O} }
3505:
2852:
3309:"Durability challenges and perspective in the development of PGM-free electrocatalysts for the oxygen reduction reaction"
2656:"Department of Energy Announces $ 39 million for Innovative Hydrogen and Fuel Cell Technologies Research and Development"
2189:
Gasteiger, H. A.; Panels, J. E.; Yan, S. G. (2004-03-10). "Dependence of PEM fuel cell performance on catalyst loading".
311:{\displaystyle E^{\circ }=0\,\mathrm {V} \,\,\,{\frac {\mathrm {d} E^{\circ }}{\mathrm {d} T}}=0\,\mathrm {mV\,K^{-1}} }
883:" the fuel cell. The membrane must also not allow either gas to pass to the other side of the cell, a problem known as
2673:
Hydrogen, Fuel Cells & Infrastructure Technologies Program Multi-Year Research, Development and Demonstration Plan
2086:
1609:
54:
2127:
992:
catalyst is necessary as some carbon monoxide will unavoidably reach the membrane. The level should not exceed 10
3686:
2307:"Compress effects on porosity, gas-phase tortuosity, and gas permeability in a simulated PEM gas diffusion layer"
3641:
2056:
1689:
Bratsch, Stephen G. (1989). "Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K".
1040:
have been shown as promising alternative electrolyte materials for high temperature (100–200 °C) PEMFCs.
3681:
3183:
Y. Wang; Daniela Fernanda Ruiz Diaz; Ken S. Chen; Zhe Wang; Xavier Cordobes Adroher (January–February 2020).
1604:
1506:
and Space Shuttle missions used fuel cells based on Bacon's design, developed by Pratt and Whitney Aircraft.
1450:. Initially, sulfonated polystyrene membranes were used for electrolytes, but they were replaced in 1966 by
3564:
851:
2436:"Investigation of fractal flow-fields in portable proton exchange membrane and direct methanol fuel cells"
879:
To function, the membrane must conduct hydrogen ions (protons) but not electrons as this would in effect "
3734:
3651:
3594:
1584:
1259:
839:
82:
1000:
can be fed to a PEM fuel cell directly without being reformed, thus making a direct methanol fuel cell (
3671:
3533:
3407:
1574:
1430:
690:{\displaystyle \mathrm {H} _{2}+{\frac {1}{2}}\mathrm {O} _{2}\rightarrow \mathrm {H} _{2}\mathrm {O} }
3463:
2482:
2435:
2388:
2349:
1876:
1837:
1737:"Effects of MEA Fabrication and Ionomer Composition on Fuel Cell Performance of PGM-Free ORR Catalyst"
3661:
3579:
3538:
1836:
Daud, W. R. W.; Rosli, R. E.; Majlan, E. H.; Hamid, S. A. A.; Mohamed, R.; Husaini, T. (2017-12-01).
1535:. The US Department of Energy estimates a 2016 price at $ 53/kW if 500,000 units per year were made.
1499:
1255:
1023:
370:
1486:
Aircraft, General Electric developed the first proton exchange membrane fuel cells (PEMFCs) for the
1155:
The practical efficiency of a PEMs is in the range of 50–60% . Main factors that create losses are:
807:
588:
322:
3666:
3574:
3498:
3016:
976:
46:
2743:
V. R. Stamenkovic; B. Fowler; B. S. Mun; G. Wang; P. N. Ross; C. A. Lucas; N. M. Marković (2007).
2348:
Li, Moucheng; Luo, Suzhen; Zeng, Chaoliu; Shen, Jianian; Lin, Haichao; Cao, Chu'nan (2004-06-01).
3656:
3589:
3569:
1955:
1594:
1579:
1036:
1019:
3676:
3615:
1442:
912:
1367:
was available for the reduction of oxygen, boosting the catalyst's oxygen reduction activity.
3584:
3548:
2483:"Numerical prediction of mass-exchange between cathode and anode channels in a PEM fuel cell"
2082:
1599:
1426:
361:
3008:
2936:
58:
3599:
3378:
3157:
3084:
2756:
2701:
2594:
2447:
2400:
2350:"Corrosion behavior of TiN coated type 316 stainless steel in simulated PEMFC environments"
2279:
2198:
2163:
2010:
1748:
1698:
1663:
1294:
3365:
2745:"Improved Oxygen Reduction Activity on Pt3Ni(111) via Increased Surface Site Availability"
8:
3755:
3620:
3491:
3307:
Martinez, Ulises; Babu, Siddharth Komini; Holby, Edward F.; Zelenay, Piotr (April 2018).
3009:
2558:
Li, Shun-Li; Xu, Qiang (2013). "Metal–organic frameworks as platforms for clean energy".
957:
863:
3161:
3088:
2760:
2705:
2598:
2451:
2404:
2283:
2202:
2167:
2014:
1752:
1702:
1667:
3528:
3289:
3242:
3207:
3108:
2989:
2919:
2782:
2725:
2330:
2103:
1714:
1552:
1468:
1457:, which proved to be superior in performance and durability to sulfonated polystyrene.
911:
chance of leakage . The proton-exchange membrane is commonly made of materials such as
353:
66:
2498:
2365:
1968:
3403:
3330:
3293:
3281:
3211:
3100:
3057:
3020:
2954:
2923:
2842:
2774:
2717:
2610:
2540:
2502:
2463:
2416:
2369:
2249:
2241:
2122:
2051:
2001:
Wainright, J. S. (1995). "Acid-Doped Polybenzimidazoles: A New Polymer Electrolyte".
1896:
1857:
1764:
1483:
1305:
1067:
993:
956:
A wide variety of solutions for managing the water exist including integration of an
108:
3246:
3182:
3112:
2993:
2882:
2786:
2672:
2334:
2107:
1718:
30:
3721:
3716:
3711:
3706:
3320:
3273:
3234:
3199:
3169:
3165:
3092:
3049:
2981:
2946:
2909:
2878:
2834:
2764:
2709:
2637:
2602:
2585:
Kitagawa, Hiroshi (2009). "Metal–organic frameworks: Transported into fuel cells".
2567:
2532:
2494:
2459:
2455:
2408:
2361:
2320:
2291:
2287:
2233:
2210:
2206:
2175:
2171:
2136:
2095:
2065:
2018:
1983:
1888:
1849:
1818:
1791:
1756:
1706:
1675:
1671:
1634:
1528:
1447:
62:
2729:
1914:
1145:{\displaystyle \eta ={\frac {\Delta G}{\Delta H}}=1-{\frac {T\Delta S}{\Delta H}}}
3760:
3325:
3308:
3203:
2679:
2412:
1853:
1339:
1314:
104:
100:
3185:"Materials, technological status, and fundamentals of PEM fuel cells – A review"
3074:
2049:
65:, which consumes electricity. They are a leading candidate to replace the aging
2838:
1892:
1495:
1487:
1472:
1464:
208:{\displaystyle \mathrm {H} _{2}\rightarrow \mathrm {2H} ^{+}+\mathrm {2e} ^{-}}
116:
3429:"Is Toyota's hydrogen fuel-cell fervor foolish, or foresighted? (with charts)"
1346:, a process which produces a mixture of gases that also contains CO (1–3%), CO
3749:
3636:
2506:
2467:
2420:
2373:
2245:
1900:
1861:
1822:
1795:
1566:
1476:
1357:
For example, one study reported that cube-shaped platinum nanoparticles with
1267:
1071:
1031:
880:
70:
2769:
2713:
2120:
1639:
3646:
3277:
3238:
3104:
3061:
3053:
2985:
2958:
2778:
2721:
2614:
2544:
2253:
2237:
1760:
1532:
1422:
1358:
1343:
1335:
1309:
1271:
1026:, can reach up to 220 °C without using any water management (see also
2898:"Analysis of Inkjet Printed PEFC Electrodes with Varying Platinum Loading"
2742:
1238:
to reduce the cost of the fuel cell due to use of platinum-based catalysts
871:
3262:"Kinetic Models for the Degradation Mechanisms of PGM-Free ORR Catalysts"
2914:
2897:
2824:"Maximizing the Use of Platinum Catalyst by Ultrasonic Spray Application"
1441:
Before the invention of PEM fuel cells, existing fuel cell types such as
1241:
to enhance the ORR activity of platinum group metal-free electrocatalysts
2641:
2571:
2536:
2140:
2099:
2069:
1520:
1298:
1285:
A second method of increasing the catalytic activity of platinum is to
112:
3404:"Fuel cell electric vehicles and hydrogen infrastructure: status 2012"
3334:
3285:
3261:
2950:
2606:
2022:
1987:
1768:
1736:
3514:
3096:
2442:. Selected papers presented at the Eighth Grove Fuel Cell Symposium.
2123:"Imidazolium methanesulfonate as a high temperature proton conductor"
1710:
989:
127:
94:
50:
3464:
The Application of Neural Networks to Fuel Processors for Fuel Cells
2744:
2325:
2306:
1289:
it with other metals. For example, it was recently shown that the Pt
1235:
to reduce the poisoning of PEM fuel cell catalysts by impurity gases
3698:
3402:
Eberle, Ulrich; Mueller, Bernd; von Helmolt, Rittmar (2012-07-15).
1546:
1515:
1491:
1015:
997:
984:
895:
891:
135:
2481:
Dutta, Sandip; Shimpalee, Sirivatch; Van Zee, J. W. (2001-06-01).
2154:
Litster, S.; McLean, G. (2004-05-03). "PEM fuel cell electrodes".
1627:
1325:
catalytic durability is attributable to a sustained atomic order.
1245:
Examples of these approaches are given in the following sections.
53:
being developed mainly for transport applications, as well as for
3039:
2691:
2434:
Tüber, K.; Oedegaard, A.; Hermann, M.; Hebling, C. (2004-05-14).
1560:
1524:
1454:
1363:
357:
3478:
2971:
856:
3379:"Collecting the History of Proton Exchange Membrane Fuel Cells"
2802:"Manufacturing of membrane electrode assemblies for fuel cells"
2223:
1503:
1451:
1011:
916:
131:
2121:
Jiangshui Luo; Olaf Conrad & Ivo F. J. Vankelecom (2013).
1308:
to apply the platinum catalyst to the electrolyte layer or to
934:
3. Light mass and high power density (transport applications)
3483:
3473:
3457:
1781:
1286:
366:
139:
123:
3148:
Litster, S.; McLean, G. (2004). "PEM fuel cell electrodes".
2433:
2270:
Litster, S.; Mclean, G. (2004). "PEM Fuel Cell Electrodes".
1808:
1461:
1001:
1338:. However, at the moment most hydrogen gas is produced by
3224:
2036:
2034:
2032:
1940:
PEM fuel cells: thermal and water management fundamentals
3401:
3306:
2395:. The World Energy Crisis: Some Vacuum-based Solutions.
1490:
in the early 1960s. The first mission to use PEMFCs was
142:
or hydrogen oxidation reaction (HOR) is represented by:
2029:
1915:"High Power Density Solid Oxide Fuel Cell | T2 Portal"
1734:
2480:
1083:
810:
704:
629:
591:
485:
387:
325:
222:
156:
3458:
Gas diffusion layer and catalyst layer, 3D animation
2522:
1835:
1542:
99:
A proton exchange membrane fuel cell transforms the
1937:
1590:
High Temperature Proton Exchange Membrane fuel cell
1074:of Hydrogen (ΔH = −285.84 kJ/mol) is 83% at 298 K.
1028:
High Temperature Proton Exchange Membrane fuel cell
3368:. Americanhistory.si.edu. Retrieved on 2013-04-19.
2833:. Art. FuelCell2011-54369. pp. 637–644.
2188:
1877:"Cold start of proton exchange membrane fuel cell"
1653:
1144:
824:
795:
689:
605:
576:
470:
339:
310:
207:
3747:
2265:
2263:
1467:series of spacecraft, but they were replaced by
1248:
1066:The maximal theoretical efficiency applying the
946:Fuel Cells based on PEM still have many issues:
2487:International Journal of Heat and Mass Transfer
1976:Journal of Industrial and Engineering Chemistry
1531:.) A large part of PEMFC production is for the
1004:). These devices operate with limited success.
845:
1969:"Polymer electrolyte membranes for fuel cells"
1304:Further efficiencies can be realized using an
3499:
3147:
3130:
3128:
3126:
3124:
3122:
2809:Singapore University of Technology and Design
2627:
2347:
2269:
2260:
2153:
1322:intermetallic core-shell (IMCS) nanocatalysts
3426:
3353:
1254:are not feasible for commercialization. The
1186:
1070:equation ΔG = −237.13 kJ/mol and using the
3506:
3492:
3259:
3119:
3006:
2682:, U.S. Department of Energy, October 2007.
2386:
1966:
3479:Open Source PEM Fuel Cell Simulation Tool
3468:IEEE Transactions on Vehicular Technology
3324:
2913:
2895:
2868:
2768:
2324:
2000:
1881:Progress in Energy and Combustion Science
1647:
1638:
778:
770:
729:
728:
727:
721:
559:
551:
510:
509:
508:
502:
293:
285:
247:
246:
245:
239:
126:side of the MEA. At the anode side it is
122:A stream of hydrogen is delivered to the
3260:Yin, Xi; Zelenay, Piotr (13 July 2018).
2799:
2584:
2312:International Journal of Energy Research
2304:
1838:"PEM fuel cell system control: A review"
870:
862:
855:
115:of hydrogen and oxygen gases to produce
29:
3427:Klippenstein, Matthew (24 April 2017).
1874:
1688:
1007:3. Limitation of Operating Temperature
14:
3748:
3015:. Boca Raton, FL: CRC Press. pp.
2902:Journal of the Electrochemical Society
2226:ACS Applied Materials & Interfaces
2193:. Eighth Ulmer Electrochemische Tage.
2003:Journal of the Electrochemical Society
1951:
1949:
1052:
3487:
2821:
2518:
2516:
2389:"Proton exchange membrane fuel cells"
1730:
1728:
1328:
3395:
2557:
1875:Luo, Yueqi; Jiao, Kui (2018-01-01).
1223:
3313:Current Opinion in Electrochemistry
1994:
1946:
1811:The Journal of Physical Chemistry C
1784:The Journal of Physical Chemistry C
1621:
1010:The most commonly used membrane is
360:side of the MEA, thus creating the
107:reaction of hydrogen and oxygen to
39:Proton-exchange membrane fuel cells
24:
3544:Proton-exchange membrane fuel cell
2560:Energy & Environmental Science
2513:
2087:Energy & Environmental Science
1725:
1133:
1125:
1101:
1093:
980:contaminants in the fuel/oxidant.
780:
775:
772:
751:
734:
723:
683:
672:
657:
632:
561:
556:
553:
532:
515:
504:
464:
453:
438:
419:
400:
295:
290:
287:
269:
252:
241:
195:
177:
159:
69:technology, which was used in the
25:
18:Proton exchange membrane fuel cell
3772:
3470:, Vol. 50 (1), pp. 125-143, 2001.
3451:
1610:Timeline of hydrogen technologies
1510:available (hydrogen and oxygen).
1169:
971:2. Vulnerability of the Catalyst
875:MEA fabrication methods for PEMFC
55:stationary fuel-cell applications
3474:Fuel Cell elements, 3D animation
2387:Vishnyakov, V. M. (2006-08-03).
2128:Journal of Materials Chemistry A
1967:Lee, J. S.; et al. (2006).
1559:
1545:
1460:PEM fuel cells were used in the
1382:
49:(PEM) fuel cells, are a type of
3687:Unitized regenerative fuel cell
3420:
3371:
3359:
3341:
3300:
3253:
3218:
3176:
3141:
3068:
3033:
3000:
2965:
2930:
2889:
2883:10.1016/j.electacta.2015.01.028
2862:
2815:
2793:
2736:
2685:
2666:
2648:
2621:
2578:
2551:
2474:
2427:
2380:
2341:
2298:
2217:
2182:
2147:
2114:
2076:
2043:
1960:
1931:
1415:
59:portable fuel-cell applications
3513:
3170:10.1016/j.jpowsour.2003.12.055
2460:10.1016/j.jpowsour.2003.11.078
2292:10.1016/j.jpowsour.2003.12.055
2211:10.1016/j.jpowsour.2003.09.013
2176:10.1016/j.jpowsour.2003.12.055
2057:Journal of Materials Chemistry
1938:Wang, Y.; Chen, K. S. (2013).
1907:
1868:
1829:
1802:
1775:
1682:
1676:10.1016/j.jpowsour.2010.12.043
983:PEM systems that use reformed
894:is relatively easy by using a
825:{\displaystyle \left(3\right)}
667:
606:{\displaystyle \left(2\right)}
448:
340:{\displaystyle \left(1\right)}
169:
13:
1:
3682:Solid oxide electrolyzer cell
3011:Fuel Cell Technology Handbook
2499:10.1016/S0017-9310(00)00257-X
2366:10.1016/S0010-938X(03)00187-2
1631:NASA Technical Reports Server
1615:
1605:Reversible hydrogen electrode
1249:Increasing catalytic activity
1061:
1043:
941:
923:2. Low Operating Temperature
83:membrane electrode assemblies
3565:Direct borohydride fuel cell
3462:Iwan, L., and Stengel, R., "
3326:10.1016/j.coelec.2018.04.010
3204:10.1016/j.mattod.2019.06.005
2413:10.1016/j.vacuum.2006.03.029
1854:10.1016/j.renene.2017.06.027
915:(PFSA, sold commercially as
902:
852:Polymer electrolyte membrane
846:Polymer electrolyte membrane
140:oxidation half-cell reaction
88:
47:polymer electrolyte membrane
7:
3652:Membrane electrode assembly
3595:Reformed methanol fuel cell
1585:Glossary of fuel cell terms
1538:
1260:internal combustion engines
840:standard hydrogen electrode
111:, as opposed to the direct
27:Power generation technology
10:
3777:
3672:Protonic ceramic fuel cell
3642:Electro-galvanic fuel cell
3534:Molten carbonate fuel cell
3408:Royal Society of Chemistry
2839:10.1115/FuelCell2011-54369
2822:Engle, Robb (2011-08-08).
1893:10.1016/j.pecs.2017.10.003
1575:Dynamic hydrogen electrode
1436:
890:Splitting of the hydrogen
849:
92:
76:
34:Diagram of a PEM fuel cell
3730:
3697:
3662:Photoelectrochemical cell
3629:
3608:
3580:Direct methanol fuel cell
3557:
3539:Phosphoric acid fuel cell
3521:
2305:Espinoza, Mayken (2015).
1256:U.S. Department of Energy
373:(ORR) is represented by:
371:oxygen reduction reaction
3667:Proton-exchange membrane
3575:Direct-ethanol fuel cell
3150:Journal of Power Sources
2800:Koraishy, Babar (2009).
2440:Journal of Power Sources
2272:Journal of Power Sources
2191:Journal of Power Sources
2156:Journal of Power Sources
1823:10.1021/acs.jpcc.5b04157
1796:10.1021/acs.jpcc.5b04155
1691:J. Phys. Chem. Ref. Data
1656:Journal of Power Sources
1187:Metal-organic frameworks
977:water gas shift reaction
81:PEMFCs are built out of
3657:Membraneless Fuel Cells
3590:Metal hydride fuel cell
3570:Direct carbon fuel cell
2770:10.1126/science.1135941
2714:10.1126/science.1140484
1595:Hydrogen sulfide sensor
1580:Gas diffusion electrode
1020:polybenzimidazole (PBI)
3677:Regenerative fuel cell
3616:Enzymatic biofuel cell
3383:americanhistory.si.edu
3278:10.1149/08513.1239ecst
3239:10.1002/aenm.201301735
3054:10.1002/anie.200800073
2986:10.1002/cctc.201300647
2238:10.1021/acsami.5b02182
1761:10.1149/07711.1273ecst
1443:solid-oxide fuel cells
1146:
913:perfluorosulfonic acid
876:
868:
860:
826:
797:
691:
607:
578:
472:
369:half-cell reaction or
341:
312:
209:
35:
3585:Formic acid fuel cell
3549:Solid oxide fuel cell
3136:Nature Communications
1600:Power-to-weight ratio
1514:such as low platinum
1496:Apollo space missions
1488:Gemini space missions
1165:Mass transport losses
1147:
874:
866:
859:
827:
798:
692:
608:
579:
473:
342:
313:
210:
103:liberated during the
93:Further information:
33:
2915:10.1149/2.1111607jes
1295:electronic structure
1081:
949:1. Water management
808:
702:
627:
589:
483:
385:
323:
220:
154:
3621:Microbial fuel cell
3162:2004JPS...130...61L
3089:2008NatMa...7..333A
3007:G. Hoogers (2003).
2871:Electrochimica Acta
2761:2007Sci...315..493S
2706:2007Sci...316..732T
2599:2009NatCh...1..689K
2452:2004JPS...131..175T
2405:2006Vacuu..80.1053V
2284:2004JPS...130...61L
2232:(29): 15746–15751.
2203:2004JPS...127..162G
2168:2004JPS...130...61L
2064:(28): 10426–10436.
2015:1995JElS..142L.121W
1919:technology.nasa.gov
1817:(45): 25156–25169.
1790:(45): 25145–25155.
1753:2017ECSTr..77k1273Y
1703:1989JPCRD..18....1B
1668:2011JPS...196.9013M
1475:program and in the
1469:Alkaline fuel cells
1053:Gas diffusion layer
958:electroosmotic pump
3529:Alkaline fuel cell
2896:Shukla, S (2016).
2678:2015-09-24 at the
2642:10.1039/c4ce01499e
2572:10.1039/c3ee40507a
2537:10.1039/c4cs00093e
2141:10.1039/C2TA00713D
2100:10.1039/C4EE02280G
2070:10.1039/C0JM04306K
1553:Electronics portal
1329:Reducing poisoning
1142:
877:
869:
861:
822:
793:
687:
618:Overall reaction:
603:
574:
468:
337:
308:
205:
67:alkaline fuel-cell
36:
3743:
3742:
3272:(13): 1239–1250.
3227:Adv. Energy Mater
3048:(19): 3588–3591.
3042:Angewandte Chemie
3026:978-0-8493-0877-2
2951:10.1021/nn4019009
2848:978-0-7918-5469-3
2755:(5811): 493–497.
2700:(5825): 732–735.
2607:10.1038/nchem.454
2531:(16): 5913–5932.
2493:(11): 2029–2042.
2399:(10): 1053–1065.
2354:Corrosion Science
2319:(11): 1528–1536.
2023:10.1149/1.2044337
1988:10.1021/ie050498j
1942:. Momentum Press.
1747:(11): 1273–1281.
1484:Pratt and Whitney
1306:Ultrasonic nozzle
1224:Catalyst research
1159:Activation losses
1140:
1108:
1068:Gibbs free energy
994:parts per million
907:1. Easy sealing
835:
834:
759:
653:
616:
615:
540:
396:
350:
349:
277:
109:electrical energy
45:), also known as
16:(Redirected from
3768:
3600:Zinc–air battery
3508:
3501:
3494:
3485:
3484:
3443:
3442:
3437:
3435:
3424:
3418:
3417:
3415:
3414:
3399:
3393:
3392:
3390:
3389:
3375:
3369:
3363:
3357:
3351:
3345:
3339:
3338:
3328:
3304:
3298:
3297:
3266:ECS Transactions
3257:
3251:
3250:
3222:
3216:
3215:
3189:
3180:
3174:
3173:
3145:
3139:
3132:
3117:
3116:
3097:10.1038/nmat2156
3077:Nature Materials
3072:
3066:
3065:
3037:
3031:
3030:
3014:
3004:
2998:
2997:
2969:
2963:
2962:
2945:(7): 6103–6110.
2934:
2928:
2927:
2917:
2908:(7): F677–F687.
2893:
2887:
2886:
2866:
2860:
2859:
2857:
2851:. Archived from
2828:
2819:
2813:
2812:
2806:
2797:
2791:
2790:
2772:
2740:
2734:
2733:
2689:
2683:
2670:
2664:
2663:
2658:. Archived from
2652:
2646:
2645:
2625:
2619:
2618:
2587:Nature Chemistry
2582:
2576:
2575:
2555:
2549:
2548:
2520:
2511:
2510:
2478:
2472:
2471:
2431:
2425:
2424:
2384:
2378:
2377:
2360:(6): 1369–1380.
2345:
2339:
2338:
2328:
2302:
2296:
2295:
2267:
2258:
2257:
2221:
2215:
2214:
2197:(1–2): 162–171.
2186:
2180:
2179:
2151:
2145:
2144:
2135:(6): 2238–2247.
2118:
2112:
2111:
2094:(4): 1276–1291.
2080:
2074:
2073:
2047:
2041:
2038:
2027:
2026:
1998:
1992:
1991:
1973:
1964:
1958:
1953:
1944:
1943:
1935:
1929:
1928:
1926:
1925:
1911:
1905:
1904:
1872:
1866:
1865:
1842:Renewable Energy
1833:
1827:
1826:
1806:
1800:
1799:
1779:
1773:
1772:
1741:ECS Transactions
1732:
1723:
1722:
1711:10.1063/1.555839
1686:
1680:
1679:
1662:(21): 9013–017.
1651:
1645:
1644:
1642:
1640:2060/20110014968
1625:
1569:
1564:
1563:
1555:
1550:
1549:
1529:hydrogen economy
1448:General Electric
1151:
1149:
1148:
1143:
1141:
1139:
1131:
1120:
1109:
1107:
1099:
1091:
831:
829:
828:
823:
821:
802:
800:
799:
794:
792:
791:
790:
760:
758:
754:
748:
747:
746:
737:
731:
726:
714:
713:
696:
694:
693:
688:
686:
681:
680:
675:
666:
665:
660:
654:
646:
641:
640:
635:
621:
620:
612:
610:
609:
604:
602:
583:
581:
580:
575:
573:
572:
571:
541:
539:
535:
529:
528:
527:
518:
512:
507:
495:
494:
477:
475:
474:
469:
467:
462:
461:
456:
447:
446:
441:
429:
428:
423:
422:
409:
408:
403:
397:
389:
379:
378:
376:At the cathode:
346:
344:
343:
338:
336:
317:
315:
314:
309:
307:
306:
305:
278:
276:
272:
266:
265:
264:
255:
249:
244:
232:
231:
214:
212:
211:
206:
204:
203:
198:
186:
185:
180:
168:
167:
162:
148:
147:
63:PEM electrolysis
21:
3776:
3775:
3771:
3770:
3769:
3767:
3766:
3765:
3746:
3745:
3744:
3739:
3726:
3693:
3625:
3604:
3553:
3517:
3512:
3454:
3448:
3446:
3433:
3431:
3425:
3421:
3412:
3410:
3400:
3396:
3387:
3385:
3377:
3376:
3372:
3364:
3360:
3354:
3346:
3342:
3305:
3301:
3258:
3254:
3233:(10): 1301735.
3223:
3219:
3192:Materials Today
3187:
3181:
3177:
3146:
3142:
3133:
3120:
3073:
3069:
3038:
3034:
3027:
3005:
3001:
2970:
2966:
2935:
2931:
2894:
2890:
2867:
2863:
2855:
2849:
2826:
2820:
2816:
2804:
2798:
2794:
2741:
2737:
2690:
2686:
2680:Wayback Machine
2671:
2667:
2654:
2653:
2649:
2626:
2622:
2583:
2579:
2556:
2552:
2521:
2514:
2479:
2475:
2432:
2428:
2385:
2381:
2346:
2342:
2326:10.1002/er.3348
2303:
2299:
2268:
2261:
2222:
2218:
2187:
2183:
2152:
2148:
2119:
2115:
2081:
2077:
2048:
2044:
2039:
2030:
1999:
1995:
1971:
1965:
1961:
1954:
1947:
1936:
1932:
1923:
1921:
1913:
1912:
1908:
1873:
1869:
1834:
1830:
1807:
1803:
1780:
1776:
1733:
1726:
1687:
1683:
1652:
1648:
1626:
1622:
1618:
1565:
1558:
1551:
1544:
1541:
1498:and subsequent
1494:. However, the
1439:
1418:
1411:
1399:
1395:
1385:
1378:
1374:
1353:
1350:(19–25%), and N
1349:
1340:steam reforming
1331:
1315:inkjet printing
1301:and reduction.
1292:
1251:
1226:
1211:
1189:
1172:
1132:
1121:
1119:
1100:
1092:
1090:
1082:
1079:
1078:
1064:
1055:
1046:
1024:phosphoric acid
944:
905:
854:
848:
811:
809:
806:
805:
783:
779:
771:
750:
749:
742:
738:
733:
732:
730:
722:
709:
705:
703:
700:
699:
682:
676:
671:
670:
661:
656:
655:
645:
636:
631:
630:
628:
625:
624:
592:
590:
587:
586:
564:
560:
552:
531:
530:
523:
519:
514:
513:
511:
503:
490:
486:
484:
481:
480:
463:
457:
452:
451:
442:
434:
433:
424:
418:
414:
413:
404:
399:
398:
388:
386:
383:
382:
326:
324:
321:
320:
298:
294:
286:
268:
267:
260:
256:
251:
250:
248:
240:
227:
223:
221:
218:
217:
199:
191:
190:
181:
173:
172:
163:
158:
157:
155:
152:
151:
105:electrochemical
101:chemical energy
97:
91:
79:
28:
23:
22:
15:
12:
11:
5:
3774:
3764:
3763:
3758:
3741:
3740:
3738:
3737:
3731:
3728:
3727:
3725:
3724:
3719:
3714:
3709:
3703:
3701:
3695:
3694:
3692:
3691:
3690:
3689:
3684:
3674:
3669:
3664:
3659:
3654:
3649:
3644:
3639:
3633:
3631:
3627:
3626:
3624:
3623:
3618:
3612:
3610:
3606:
3605:
3603:
3602:
3597:
3592:
3587:
3582:
3577:
3572:
3567:
3561:
3559:
3555:
3554:
3552:
3551:
3546:
3541:
3536:
3531:
3525:
3523:
3522:By electrolyte
3519:
3518:
3511:
3510:
3503:
3496:
3488:
3482:
3481:
3476:
3471:
3460:
3453:
3452:External links
3450:
3445:
3444:
3419:
3394:
3370:
3366:PEM Fuel Cells
3358:
3352:
3340:
3299:
3252:
3217:
3175:
3156:(1–2): 61–76.
3140:
3118:
3083:(4): 333–338.
3067:
3032:
3025:
2999:
2964:
2929:
2888:
2861:
2858:on 2014-03-28.
2847:
2814:
2792:
2735:
2684:
2665:
2662:on 2018-06-15.
2647:
2620:
2593:(9): 689–690.
2577:
2550:
2525:Chem. Soc. Rev
2512:
2473:
2446:(1): 175–181.
2426:
2379:
2340:
2297:
2278:(1–2): 61–76.
2259:
2216:
2181:
2162:(1–2): 61–76.
2146:
2113:
2075:
2042:
2028:
1993:
1959:
1945:
1930:
1906:
1867:
1828:
1801:
1774:
1724:
1681:
1646:
1619:
1617:
1614:
1613:
1612:
1607:
1602:
1597:
1592:
1587:
1582:
1577:
1571:
1570:
1556:
1540:
1537:
1482:Parallel with
1438:
1435:
1417:
1414:
1409:
1397:
1393:
1384:
1381:
1376:
1372:
1351:
1347:
1330:
1327:
1290:
1272:Miller indexes
1250:
1247:
1243:
1242:
1239:
1236:
1233:
1225:
1222:
1209:
1188:
1185:
1171:
1170:Bipolar plates
1168:
1167:
1166:
1163:
1160:
1153:
1152:
1138:
1135:
1130:
1127:
1124:
1118:
1115:
1112:
1106:
1103:
1098:
1095:
1089:
1086:
1063:
1060:
1054:
1051:
1045:
1042:
943:
940:
904:
901:
850:Main article:
847:
844:
833:
832:
820:
817:
814:
803:
789:
786:
782:
777:
774:
769:
766:
763:
757:
753:
745:
741:
736:
725:
720:
717:
712:
708:
697:
685:
679:
674:
669:
664:
659:
652:
649:
644:
639:
634:
614:
613:
601:
598:
595:
584:
570:
567:
563:
558:
555:
550:
547:
544:
538:
534:
526:
522:
517:
506:
501:
498:
493:
489:
478:
466:
460:
455:
450:
445:
440:
437:
432:
427:
421:
417:
412:
407:
402:
395:
392:
348:
347:
335:
332:
329:
318:
304:
301:
297:
292:
289:
284:
281:
275:
271:
263:
259:
254:
243:
238:
235:
230:
226:
215:
202:
197:
194:
189:
184:
179:
176:
171:
166:
161:
145:At the anode:
117:thermal energy
90:
87:
78:
75:
26:
9:
6:
4:
3:
2:
3773:
3762:
3759:
3757:
3754:
3753:
3751:
3736:
3733:
3732:
3729:
3723:
3720:
3718:
3715:
3713:
3710:
3708:
3705:
3704:
3702:
3700:
3696:
3688:
3685:
3683:
3680:
3679:
3678:
3675:
3673:
3670:
3668:
3665:
3663:
3660:
3658:
3655:
3653:
3650:
3648:
3645:
3643:
3640:
3638:
3635:
3634:
3632:
3628:
3622:
3619:
3617:
3614:
3613:
3611:
3609:Biofuel cells
3607:
3601:
3598:
3596:
3593:
3591:
3588:
3586:
3583:
3581:
3578:
3576:
3573:
3571:
3568:
3566:
3563:
3562:
3560:
3556:
3550:
3547:
3545:
3542:
3540:
3537:
3535:
3532:
3530:
3527:
3526:
3524:
3520:
3516:
3509:
3504:
3502:
3497:
3495:
3490:
3489:
3486:
3480:
3477:
3475:
3472:
3469:
3465:
3461:
3459:
3456:
3455:
3449:
3441:
3430:
3423:
3409:
3405:
3398:
3384:
3380:
3374:
3367:
3362:
3356:
3348:
3344:
3336:
3332:
3327:
3322:
3318:
3314:
3310:
3303:
3295:
3291:
3287:
3283:
3279:
3275:
3271:
3267:
3263:
3256:
3248:
3244:
3240:
3236:
3232:
3228:
3221:
3213:
3209:
3205:
3201:
3197:
3193:
3186:
3179:
3171:
3167:
3163:
3159:
3155:
3151:
3144:
3137:
3131:
3129:
3127:
3125:
3123:
3114:
3110:
3106:
3102:
3098:
3094:
3090:
3086:
3082:
3078:
3071:
3063:
3059:
3055:
3051:
3047:
3043:
3036:
3028:
3022:
3018:
3013:
3012:
3003:
2995:
2991:
2987:
2983:
2979:
2975:
2968:
2960:
2956:
2952:
2948:
2944:
2940:
2933:
2925:
2921:
2916:
2911:
2907:
2903:
2899:
2892:
2884:
2880:
2876:
2872:
2865:
2854:
2850:
2844:
2840:
2836:
2832:
2825:
2818:
2810:
2803:
2796:
2788:
2784:
2780:
2776:
2771:
2766:
2762:
2758:
2754:
2750:
2746:
2739:
2731:
2727:
2723:
2719:
2715:
2711:
2707:
2703:
2699:
2695:
2688:
2681:
2677:
2674:
2669:
2661:
2657:
2651:
2643:
2639:
2635:
2631:
2624:
2616:
2612:
2608:
2604:
2600:
2596:
2592:
2588:
2581:
2573:
2569:
2565:
2561:
2554:
2546:
2542:
2538:
2534:
2530:
2526:
2519:
2517:
2508:
2504:
2500:
2496:
2492:
2488:
2484:
2477:
2469:
2465:
2461:
2457:
2453:
2449:
2445:
2441:
2437:
2430:
2422:
2418:
2414:
2410:
2406:
2402:
2398:
2394:
2390:
2383:
2375:
2371:
2367:
2363:
2359:
2355:
2351:
2344:
2336:
2332:
2327:
2322:
2318:
2314:
2313:
2308:
2301:
2293:
2289:
2285:
2281:
2277:
2273:
2266:
2264:
2255:
2251:
2247:
2243:
2239:
2235:
2231:
2227:
2220:
2212:
2208:
2204:
2200:
2196:
2192:
2185:
2177:
2173:
2169:
2165:
2161:
2157:
2150:
2142:
2138:
2134:
2130:
2129:
2124:
2117:
2109:
2105:
2101:
2097:
2093:
2089:
2088:
2079:
2071:
2067:
2063:
2059:
2058:
2053:
2046:
2037:
2035:
2033:
2024:
2020:
2016:
2012:
2008:
2004:
1997:
1989:
1985:
1981:
1977:
1970:
1963:
1957:
1952:
1950:
1941:
1934:
1920:
1916:
1910:
1902:
1898:
1894:
1890:
1886:
1882:
1878:
1871:
1863:
1859:
1855:
1851:
1847:
1843:
1839:
1832:
1824:
1820:
1816:
1812:
1805:
1797:
1793:
1789:
1785:
1778:
1770:
1766:
1762:
1758:
1754:
1750:
1746:
1742:
1738:
1731:
1729:
1720:
1716:
1712:
1708:
1704:
1700:
1696:
1692:
1685:
1677:
1673:
1669:
1665:
1661:
1657:
1650:
1641:
1636:
1632:
1624:
1620:
1611:
1608:
1606:
1603:
1601:
1598:
1596:
1593:
1591:
1588:
1586:
1583:
1581:
1578:
1576:
1573:
1572:
1568:
1567:Energy portal
1562:
1557:
1554:
1548:
1543:
1536:
1534:
1530:
1526:
1522:
1517:
1511:
1507:
1505:
1501:
1497:
1493:
1489:
1485:
1480:
1478:
1477:Space Shuttle
1474:
1470:
1466:
1463:
1458:
1456:
1453:
1449:
1444:
1434:
1432:
1428:
1424:
1413:
1405:
1401:
1389:
1383:Lowering cost
1380:
1368:
1365:
1360:
1355:
1345:
1341:
1337:
1326:
1323:
1318:
1316:
1311:
1307:
1302:
1300:
1296:
1288:
1283:
1279:
1275:
1273:
1269:
1268:nanoparticles
1263:
1261:
1257:
1246:
1240:
1237:
1234:
1231:
1230:
1229:
1221:
1217:
1213:
1205:
1201:
1197:
1193:
1184:
1180:
1176:
1164:
1161:
1158:
1157:
1156:
1136:
1128:
1122:
1116:
1113:
1110:
1104:
1096:
1087:
1084:
1077:
1076:
1075:
1073:
1072:heating value
1069:
1059:
1050:
1041:
1038:
1033:
1032:sulfuric acid
1029:
1025:
1021:
1017:
1013:
1008:
1005:
1003:
999:
995:
991:
986:
981:
978:
972:
969:
965:
961:
959:
954:
950:
947:
939:
935:
932:
928:
924:
921:
918:
914:
908:
900:
897:
893:
888:
886:
885:gas crossover
882:
881:short circuit
873:
865:
858:
853:
843:
841:
818:
815:
812:
804:
787:
784:
767:
764:
761:
755:
743:
739:
718:
715:
710:
706:
698:
677:
662:
650:
647:
642:
637:
623:
622:
619:
599:
596:
593:
585:
568:
565:
548:
545:
542:
536:
524:
520:
499:
496:
491:
487:
479:
458:
443:
435:
430:
425:
415:
410:
405:
393:
390:
381:
380:
377:
374:
372:
368:
363:
359:
355:
333:
330:
327:
319:
302:
299:
282:
279:
273:
261:
257:
236:
233:
228:
224:
216:
200:
192:
187:
182:
174:
164:
150:
149:
146:
143:
141:
137:
133:
129:
128:catalytically
125:
120:
118:
114:
110:
106:
102:
96:
86:
84:
74:
72:
71:Space Shuttle
68:
64:
60:
56:
52:
48:
44:
40:
32:
19:
3647:Flow battery
3543:
3467:
3447:
3439:
3432:. Retrieved
3422:
3411:. Retrieved
3397:
3386:. Retrieved
3382:
3373:
3361:
3355:
3343:
3316:
3312:
3302:
3269:
3265:
3255:
3230:
3226:
3220:
3195:
3191:
3178:
3153:
3149:
3143:
3135:
3080:
3076:
3070:
3045:
3041:
3035:
3010:
3002:
2980:(1): 26–45.
2977:
2973:
2967:
2942:
2938:
2932:
2905:
2901:
2891:
2874:
2870:
2864:
2853:the original
2830:
2817:
2811:. p. 9.
2808:
2795:
2752:
2748:
2738:
2697:
2693:
2687:
2668:
2660:the original
2650:
2636:(1): 10–22.
2633:
2630:CrystEngComm
2629:
2623:
2590:
2586:
2580:
2563:
2559:
2553:
2528:
2524:
2490:
2486:
2476:
2443:
2439:
2429:
2396:
2392:
2382:
2357:
2353:
2343:
2316:
2310:
2300:
2275:
2271:
2229:
2225:
2219:
2194:
2190:
2184:
2159:
2155:
2149:
2132:
2126:
2116:
2091:
2085:
2078:
2061:
2055:
2045:
2006:
2002:
1996:
1979:
1975:
1962:
1939:
1933:
1922:. Retrieved
1918:
1909:
1884:
1880:
1870:
1845:
1841:
1831:
1814:
1810:
1804:
1787:
1783:
1777:
1744:
1740:
1694:
1690:
1684:
1659:
1655:
1649:
1630:
1623:
1533:Toyota Mirai
1512:
1508:
1500:Apollo-Soyuz
1481:
1459:
1446:Niedrach of
1440:
1423:fossil fuels
1419:
1416:Applications
1406:
1402:
1390:
1386:
1369:
1359:(100) facets
1356:
1344:hydrocarbons
1336:electrolysis
1332:
1321:
1319:
1310:carbon paper
1303:
1284:
1280:
1276:
1264:
1252:
1244:
1227:
1218:
1214:
1206:
1202:
1198:
1194:
1190:
1181:
1177:
1173:
1162:Ohmic losses
1154:
1065:
1056:
1047:
1009:
1006:
982:
973:
970:
966:
962:
955:
951:
948:
945:
936:
933:
929:
925:
922:
909:
906:
889:
884:
878:
836:
617:
375:
354:load circuit
351:
144:
121:
98:
80:
42:
38:
37:
3637:Blue energy
3319:: 224–232.
3198:: 178–203.
2974:ChemCatChem
2877:: 289–300.
2566:(6): 1656.
2009:(7): L121.
1982:: 175–183.
1848:: 620–638.
1697:(1): 1–21.
1521:automobiles
130:split into
3756:Fuel cells
3750:Categories
3515:Fuel cells
3413:2013-01-08
3388:2022-01-07
1924:2022-11-22
1633:(Report).
1616:References
1299:adsorption
1062:Efficiency
1044:Electrodes
942:Weaknesses
113:combustion
3350:981-1007.
3294:103125742
3212:201288395
2924:102257960
2507:0017-9310
2468:0378-7753
2421:0042-207X
2374:0010-938X
2246:1944-8244
1901:0360-1285
1887:: 29–61.
1862:0960-1481
1523:or other
1270:(that is
1134:Δ
1126:Δ
1117:−
1102:Δ
1094:Δ
1085:η
990:ruthenium
988:platinum-
903:Strengths
785:−
765:−
744:∘
711:∘
668:→
566:−
546:−
525:∘
492:∘
449:→
444:−
367:reduction
300:−
262:∘
229:∘
201:−
170:→
136:electrons
95:Fuel cell
89:Reactions
51:fuel cell
3735:Glossary
3699:Hydrogen
3247:97667736
3113:17600075
3105:18345004
3062:18399516
2994:97620646
2959:23773037
2939:ACS Nano
2787:39722200
2779:17218494
2722:17478717
2676:Archived
2615:21124353
2545:24733639
2335:93173199
2254:26154401
2108:84176511
1719:97185915
1539:See also
1525:vehicles
1516:catalyst
1492:Gemini V
1016:Chemours
998:biofuels
985:methanol
896:platinum
892:molecule
3722:Vehicle
3717:Storage
3712:Station
3707:Economy
3558:By fuel
3335:1459825
3286:1471365
3158:Bibcode
3085:Bibcode
2757:Bibcode
2749:Science
2702:Bibcode
2694:Science
2595:Bibcode
2448:Bibcode
2401:Bibcode
2280:Bibcode
2199:Bibcode
2164:Bibcode
2011:Bibcode
1769:1463547
1749:Bibcode
1699:Bibcode
1664:Bibcode
1527:. (See
1471:in the
1455:ionomer
1437:History
1371:form CO
1364:sulfate
362:current
358:cathode
356:to the
138:. This
132:protons
77:Science
3761:Proton
3630:Others
3434:13 May
3333:
3292:
3284:
3245:
3210:
3111:
3103:
3060:
3023:
2992:
2957:
2922:
2845:
2785:
2777:
2730:939992
2728:
2720:
2613:
2543:
2505:
2466:
2419:
2393:Vacuum
2372:
2333:
2252:
2244:
2106:
1899:
1860:
1767:
1717:
1504:Skylab
1473:Apollo
1465:Gemini
1452:Nafion
1342:light
1012:Nafion
917:Nafion
768:0.8456
719:1.2291
549:0.8456
500:1.2291
3466:" In
3290:S2CID
3243:S2CID
3208:S2CID
3188:(PDF)
3138:2(1),
3109:S2CID
2990:S2CID
2920:S2CID
2856:(PDF)
2827:(PDF)
2805:(PDF)
2783:S2CID
2726:S2CID
2331:S2CID
2104:S2CID
1972:(PDF)
1715:S2CID
1431:MCFCs
1427:SOFCs
1287:alloy
124:anode
43:PEMFC
3436:2017
3331:OSTI
3282:OSTI
3101:PMID
3058:PMID
3021:ISBN
3019:–3.
2955:PMID
2843:ISBN
2775:PMID
2718:PMID
2611:PMID
2541:PMID
2503:ISSN
2464:ISSN
2417:ISSN
2370:ISSN
2250:PMID
2242:ISSN
1897:ISSN
1858:ISSN
1765:OSTI
1462:NASA
1429:and
1037:PTFE
1002:DMFC
134:and
57:and
3321:doi
3274:doi
3235:doi
3200:doi
3166:doi
3154:130
3093:doi
3050:doi
2982:doi
2947:doi
2910:doi
2906:163
2879:doi
2875:156
2835:doi
2765:doi
2753:315
2710:doi
2698:316
2638:doi
2603:doi
2568:doi
2533:doi
2495:doi
2456:doi
2444:131
2409:doi
2362:doi
2321:doi
2288:doi
2276:130
2234:doi
2207:doi
2195:127
2172:doi
2160:130
2137:doi
2096:doi
2066:doi
2019:doi
2007:142
1984:doi
1889:doi
1850:doi
1846:113
1819:doi
1815:119
1792:doi
1788:119
1757:doi
1707:doi
1672:doi
1660:196
1635:hdl
1396:, O
1022:or
1014:by
3752::
3438:.
3406:.
3381:.
3329:.
3315:.
3311:.
3288:.
3280:.
3270:85
3268:.
3264:.
3241:.
3229:.
3206:.
3196:32
3194:.
3190:.
3164:.
3152:.
3121:^
3107:.
3099:.
3091:.
3079:.
3056:.
3046:47
3044:.
2988:.
2976:.
2953:.
2941:.
2918:.
2904:.
2900:.
2873:.
2841:.
2829:.
2807:.
2781:.
2773:.
2763:.
2751:.
2747:.
2724:.
2716:.
2708:.
2696:.
2634:17
2632:.
2609:.
2601:.
2589:.
2562:.
2539:.
2529:43
2527:.
2515:^
2501:.
2491:44
2489:.
2485:.
2462:.
2454:.
2438:.
2415:.
2407:.
2397:80
2391:.
2368:.
2358:46
2356:.
2352:.
2329:.
2317:39
2315:.
2309:.
2286:.
2274:.
2262:^
2248:.
2240:.
2228:.
2205:.
2170:.
2158:.
2131:.
2125:.
2102:.
2090:.
2062:21
2060:.
2054:.
2031:^
2017:.
2005:.
1980:12
1978:.
1974:.
1948:^
1917:.
1895:.
1885:64
1883:.
1879:.
1856:.
1844:.
1840:.
1813:.
1786:.
1763:.
1755:.
1745:77
1743:.
1739:.
1727:^
1713:.
1705:.
1695:18
1693:.
1670:.
1658:.
1502:,
960:.
842:.
119:.
73:.
3507:e
3500:t
3493:v
3416:.
3391:.
3337:.
3323::
3317:9
3296:.
3276::
3249:.
3237::
3231:4
3214:.
3202::
3172:.
3168::
3160::
3115:.
3095::
3087::
3081:7
3064:.
3052::
3029:.
3017:6
2996:.
2984::
2978:6
2961:.
2949::
2943:7
2926:.
2912::
2885:.
2881::
2837::
2789:.
2767::
2759::
2732:.
2712::
2704::
2644:.
2640::
2617:.
2605::
2597::
2591:1
2574:.
2570::
2564:6
2547:.
2535::
2509:.
2497::
2470:.
2458::
2450::
2423:.
2411::
2403::
2376:.
2364::
2337:.
2323::
2294:.
2290::
2282::
2256:.
2236::
2230:7
2213:.
2209::
2201::
2178:.
2174::
2166::
2143:.
2139::
2133:1
2110:.
2098::
2092:8
2072:.
2068::
2040:.
2025:.
2021::
2013::
1990:.
1986::
1927:.
1903:.
1891::
1864:.
1852::
1825:.
1821::
1798:.
1794::
1771:.
1759::
1751::
1721:.
1709::
1701::
1678:.
1674::
1666::
1643:.
1637::
1410:2
1408:H
1398:2
1394:2
1377:3
1373:2
1352:2
1348:2
1291:3
1210:2
1137:H
1129:S
1123:T
1114:1
1111:=
1105:H
1097:G
1088:=
819:)
816:3
813:(
788:1
781:K
776:V
773:m
762:=
756:T
752:d
740:E
735:d
724:V
716:=
707:E
684:O
678:2
673:H
663:2
658:O
651:2
648:1
643:+
638:2
633:H
600:)
597:2
594:(
569:1
562:K
557:V
554:m
543:=
537:T
533:d
521:E
516:d
505:V
497:=
488:E
465:O
459:2
454:H
439:e
436:2
431:+
426:+
420:H
416:2
411:+
406:2
401:O
394:2
391:1
334:)
331:1
328:(
303:1
296:K
291:V
288:m
283:0
280:=
274:T
270:d
258:E
253:d
242:V
237:0
234:=
225:E
196:e
193:2
188:+
183:+
178:H
175:2
165:2
160:H
41:(
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.