217:
is highest. It is sent to another column rectifying the argon to the desired purity from which liquid is returned to the same location in the LP column. Use of modern structured packings which have very low pressure drops enable argon with less than 1 ppm impurities. Though argon is present in less to 1% of the incoming, the air argon column requires a significant amount of energy due to the high reflux ratio required (about 30) in the argon column. Cooling of the argon column can be supplied from cold expanded rich liquid or by liquid nitrogen.
243:
277:
106:
251:
126:
196:) and cooled against product (and waste) cryogenic streams. Part of the air liquefies to form a liquid that is enriched in oxygen. The remaining gas is richer in nitrogen and is distilled to almost pure nitrogen (typically < 1ppm) in a high pressure (HP) distillation column. The condenser of this column requires
287:
can provide alternate, lower-energy approaches to air separation. For example, a number of approaches are being explored for oxygen generation. Polymeric membranes operating at ambient or warm temperatures, for example, may be able to produce oxygen-enriched air (25-50% oxygen). Ceramic membranes can
216:
Because the boiling point of argon (87.3 K at standard conditions) lies between that of oxygen (90.2 K) and nitrogen (77.4 K), argon builds up in the lower section of the low pressure column. When argon is produced, a vapor side draw is taken from the low pressure column where the argon concentration
207:
Alternatively the condenser may be cooled by interchanging heat with a reboiler in a low pressure (LP) distillation column (operating at 1.2-1.3 bar abs.) when the ASU is producing pure oxygen. To minimize the compression cost the combined condenser/reboiler of the HP/LP columns must operate with a
291:
Membrane gas separation is used to provide oxygen-poor and nitrogen-rich gases instead of air to fill the fuel tanks of jet liners, thus greatly reducing the chances of accidental fires and explosions. Conversely, membrane gas separation is currently used to provide oxygen-enriched air to pilots
220:
Finally the products produced in gas form are warmed against the incoming air to ambient temperatures. This requires a carefully crafted heat integration that must allow for robustness against disturbances (due to switch over of the molecular sieve beds). It may also require additional external
288:
provide high-purity oxygen (90% or more) but require higher temperatures (800-900 deg C) to operate. These ceramic membranes include ion transport membranes (ITM) and oxygen transport membranes (OTM). Air
Products and Chemicals Inc and Praxair are developing flat ITM and tubular OTM systems.
184:
from the air, since these can be a problem in the subsequent air distillation that could lead to explosions. The molecular sieves bed must be regenerated. This is done by installing multiple units operating in alternating mode and using the dry co-produced waste gas to desorb the
208:
temperature difference of only 1-2 K, requiring plate fin brazed aluminium heat exchangers. Typical oxygen purities range in from 97.5% to 99.5% and influences the maximum recovery of oxygen. The refrigeration required for producing liquid products is obtained using the
596:
168:
state (gas or liquid) of the products. Typical pressures range between 5 and 10 bar gauge. The air stream may also be compressed to different pressures to enhance the efficiency of the ASU. During compression water is condensed out in inter-stage
133:
The cryogenic separation process requires a very tight integration of heat exchangers and separation columns to obtain a good efficiency and all the energy for refrigeration is provided by the compression of the air at the inlet of the unit.
121:
in the early 20th century and is still used today to produce high purity gases. He developed it in the year 1895; the process remained purely academic for seven years before it was used in industrial applications for the first time (1902).
295:
Oxygen-enriched air can be obtained exploiting the different solubility of oxygen and nitrogen. Oxygen is more soluble than nitrogen in water, so if air is degassed from water, a stream of 35% oxygen can be obtained.
688:
Galli, F; Comazzi, A; Previtali, D; Manenti, F; Bozzano, G; Bianchi, C. L.; Pirola, C (2017). "Production of oxygen-enriched air via desorption from water: Experimental data, simulations and economic assessment".
225:
The separated products are sometimes supplied by pipeline to large industrial users near the production plant. Long distance transportation of products is by shipping liquid product for large quantities or as
212:
in an expander which feeds compressed air directly to the low pressure column. Hence, a certain part of the air is not to be separated and must leave the low pressure column as a waste stream from its upper
265:(molecular sponge) is exposed to high pressure air, then the air is released and an adsorbed film of the desired gas is released. The size of compressor is much reduced over a liquefaction plant, and
717:
744:
149:
enclosure (commonly called a "cold box"). The cooling of the gases requires a large amount of energy to make this refrigeration cycle work and is delivered by an air
492:
117:
the components at their various boiling temperatures. The process can produce high purity gases but is energy-intensive. This process was pioneered by
157:
for cooling; the output of the expander helps drive the air compressor, for improved efficiency. The process consists of the following main steps:
50:. Cryogenic air separation units (ASUs) are built to provide nitrogen or oxygen and often co-produce argon. Other methods such as membrane,
451:
569:
Castle, W. F. (2002). "Air separation and liquefaction: Recent developments and prospects for the beginning of the new millennium".
261:
provides separation of oxygen or nitrogen from air without liquefaction. The process operates around ambient temperature; a
373:
Inerting with nitrogen storage tanks of ships and tanks for petroleum products, or for protecting edible oil products from
783:
754:
834:
746:
Innovations in
Industrial and Engineering Chemistry: A Century of Achievements and Prospects for the New Millennium
499:
71:
180:, which would freeze and plug the cryogenic equipment. Molecular sieves are often designed to remove any gaseous
844:
626:
Fainshtein, V. I. (2007). "Provision of explosion proof air separation units under contemporary conditions".
266:
542:
Agrawal, R. (1996). "Synthesis of
Distillation Column Configurations for a Multicomponent Separation".
854:
849:
258:
51:
334:
209:
193:
142:
200:
which is obtained from expanding the more oxygen rich stream further across a valve or through an
839:
406:
284:
270:
227:
55:
47:
479:
273:
is a similar process; the product gas is evolved from the zeolite at sub-atmospheric pressure.
113:
Pure gases can be separated from air by first cooling it until it liquefies, then selectively
416:
819:
386:
58:(VPSA) are commercially used to separate a single component from ordinary air. High purity
337:
process. Modern basic oxygen steelmaking uses almost two tons of oxygen per ton of steel.
8:
620:
Particulate matter from forest fires caused an explosion in the air separation unit of a
426:
391:
138:
90:
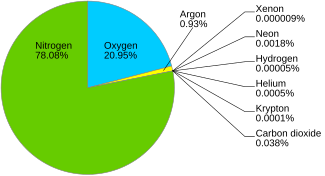
24:
164:
Air is compressed where the final delivery pressure is determined by recoveries and the
643:
146:
702:
674:
582:
779:
772:
750:
647:
362:
698:
670:
635:
578:
551:
411:
374:
173:
137:
To achieve the low distillation temperatures, an air separation unit requires a
401:
396:
365:
projects; cryogenic plants producing 3000 tons/day are found in some projects.
242:
189:
177:
150:
118:
639:
276:
828:
621:
431:
346:
269:
are made in this manner to provide oxygen-enriched air for medical purposes.
201:
197:
154:
231:
181:
114:
74:, require cryogenic distillation. Similarly, the only viable source of the
330:
421:
75:
555:
40:
743:
Flank, William H.; Abraham, Martin A.; Matthews, Michael A. (2009).
498:. Institution of Chemical Engineers. September 2010. Archived from
321:
Pure oxygen is delivered to large hospitals for use with patients.
63:
28:
292:
flying at great altitudes in aircraft without pressurized cabins.
350:
262:
82:
310:
94:
59:
32:
105:
165:
86:
67:
36:
801:
687:
250:
770:
Wingate, Philippa; Gifford, Clive; Treays, Rebecca (1992).
661:
Vinson, D. R. (2006). "Air separation control technology".
78:
790:
liquid
Nitrogen used in the Haber process to make ammonia.
176:
bed, which removes any remaining water vapour, as well as
125:
97:
is also recovered in advanced air separation processes.
129:
Distillation column in a cryogenic air separation plant
802:
Higman, Christopher; van der Burgt, Maarten (2008).
769:
742:
718:"Messer to build $ 50 million gas plant in McGregor"
589:
161:
Before compression the air is pre-filtered of dust.
771:
145:, and the cold equipment has to be kept within an
100:
826:
544:Industrial & Engineering Chemistry Research
523:Latimer, R. E. (1967). "Distillation of Air".
172:The process air is generally passed through a
89:is the distillation of air using at least two
46:The most common method for air separation is
709:
188:Process air is passed through an integrated
625:
237:
361:Large amounts of oxygen are required for
275:
249:
241:
124:
104:
541:
522:
27:into its primary components, typically
827:
806:(2nd ed.). Elsevier. p. 324.
660:
571:International Journal of Refrigeration
568:
715:
691:Computers & Chemical Engineering
663:Computers & Chemical Engineering
309:Liquid oxygen for companies such as
820:Simulation of air separation plants
485:
13:
628:Chemical and Petroleum Engineering
109:Composition of dry atmospheric air
14:
866:
813:
703:10.1016/j.compchemeng.2016.07.031
675:10.1016/j.compchemeng.2006.05.038
449:
72:semiconductor device fabrication
56:vacuum pressure swing adsorption
795:
763:
736:
681:
299:
654:
614:
562:
535:
516:
473:
443:
221:refrigeration during start-up.
141:that operates by means of the
101:Cryogenic distillation process
1:
749:. American Chemical Society.
583:10.1016/S0140-7007(01)00003-2
525:Chemical Engineering Progress
437:
333:, oxygen is required for the
267:portable oxygen concentrators
254:Bottle of 4Å molecular sieves
368:
7:
380:
356:
304:
280:Membrane nitrogen generator
10:
871:
597:"How air separation works"
340:
316:
640:10.1007/s10556-007-0018-8
482:, (updated November 2007)
259:Pressure swing adsorption
52:pressure swing adsorption
335:basic oxygen steelmaking
324:
194:plate fin heat exchanger
835:Thermodynamic processes
271:Vacuum swing adsorption
238:Non-cryogenic processes
204:(a reverse compressor).
48:fractional distillation
595:
281:
255:
247:
234:for small quantities.
130:
110:
724:. Waco Tribune-Herald
480:NASA Earth Fact Sheet
417:Liquefaction of gases
345:Nitrogen used in the
285:Membrane technologies
279:
253:
245:
128:
108:
35:, and sometimes also
845:Industrial processes
669:(10–12): 1436–1446.
387:Louis Paul Cailletet
246:A nitrogen generator
210:Joule–Thomson effect
143:Joule–Thomson effect
91:distillation columns
722:Waco Tribune-Herald
427:Oxygen concentrator
407:Hampson–Linde cycle
392:Cryogenic gas plant
139:refrigeration cycle
282:
256:
248:
155:expansion turbines
153:. Modern ASUs use
131:
111:
774:Essential Science
556:10.1021/ie950323h
493:"Cool Inventions"
452:"Helium Recovery"
363:coal gasification
862:
855:Gas technologies
850:Industrial gases
808:
807:
799:
793:
792:
777:
767:
761:
760:
740:
734:
733:
731:
729:
716:Copeland, Mike.
713:
707:
706:
685:
679:
678:
658:
652:
651:
618:
612:
611:
609:
607:
593:
587:
586:
566:
560:
559:
550:(4): 1059–1071.
539:
533:
532:
520:
514:
513:
511:
510:
504:
497:
489:
483:
477:
471:
470:
468:
466:
456:
447:
412:Industrial gases
23:plant separates
16:Chemical process
870:
869:
865:
864:
863:
861:
860:
859:
825:
824:
816:
811:
800:
796:
786:
768:
764:
757:
741:
737:
727:
725:
714:
710:
686:
682:
659:
655:
634:(1–2): 96–101.
619:
615:
605:
603:
594:
590:
567:
563:
540:
536:
521:
517:
508:
506:
502:
495:
491:
490:
486:
478:
474:
464:
462:
454:
448:
444:
440:
383:
371:
359:
343:
327:
319:
307:
302:
240:
174:molecular sieve
103:
39:and other rare
25:atmospheric air
17:
12:
11:
5:
868:
858:
857:
852:
847:
842:
840:Gas separation
837:
823:
822:
815:
814:External links
812:
810:
809:
794:
784:
762:
755:
735:
708:
680:
653:
613:
588:
561:
534:
515:
484:
472:
450:Chrz, Vaclav.
441:
439:
436:
435:
434:
429:
424:
419:
414:
409:
404:
402:Gas to liquids
399:
397:Gas separation
394:
389:
382:
379:
370:
367:
358:
355:
342:
339:
326:
323:
318:
315:
306:
303:
301:
298:
239:
236:
223:
222:
218:
214:
205:
190:heat exchanger
186:
178:carbon dioxide
170:
162:
119:Carl von Linde
102:
99:
21:air separation
15:
9:
6:
4:
3:
2:
867:
856:
853:
851:
848:
846:
843:
841:
838:
836:
833:
832:
830:
821:
818:
817:
805:
798:
791:
787:
785:9780746010112
781:
776:
775:
766:
758:
756:9780841269637
752:
748:
747:
739:
723:
719:
712:
704:
700:
696:
692:
684:
676:
672:
668:
664:
657:
649:
645:
641:
637:
633:
629:
623:
622:Gas to Liquid
617:
602:
598:
592:
584:
580:
576:
572:
565:
557:
553:
549:
545:
538:
530:
526:
519:
505:on 2014-01-13
501:
494:
488:
481:
476:
460:
453:
446:
442:
433:
432:Siemens cycle
430:
428:
425:
423:
420:
418:
415:
413:
410:
408:
405:
403:
400:
398:
395:
393:
390:
388:
385:
384:
378:
376:
366:
364:
354:
352:
348:
347:Haber process
338:
336:
332:
322:
314:
312:
297:
293:
289:
286:
278:
274:
272:
268:
264:
260:
252:
244:
235:
233:
232:gas cylinders
229:
219:
215:
211:
206:
203:
199:
198:refrigeration
195:
191:
187:
183:
179:
175:
171:
167:
163:
160:
159:
158:
156:
152:
148:
144:
140:
135:
127:
123:
120:
116:
107:
98:
96:
92:
88:
84:
80:
77:
73:
69:
65:
61:
57:
53:
49:
44:
42:
38:
34:
30:
26:
22:
804:Gasification
803:
797:
789:
773:
765:
745:
738:
726:. Retrieved
721:
711:
694:
690:
683:
666:
662:
656:
631:
627:
616:
604:. Retrieved
600:
591:
574:
570:
564:
547:
543:
537:
528:
524:
518:
507:. Retrieved
500:the original
487:
475:
463:. Retrieved
458:
445:
372:
360:
344:
328:
320:
308:
300:Applications
294:
290:
283:
257:
228:dewar flasks
224:
182:hydrocarbons
136:
132:
112:
45:
20:
18:
778:. Usborne.
728:30 November
624:plant, see
577:: 158–172.
531:(2): 35–59.
465:30 November
331:steelmaking
192:(usually a
70:, used for
41:inert gases
829:Categories
606:9 November
509:2014-01-12
438:References
422:Liquid air
151:compressor
115:distilling
76:rare gases
54:(PSA) and
697:: 11–16.
648:110001679
375:oxidation
369:Inert gas
147:insulated
381:See also
357:Coal gas
349:to make
305:Rocketry
213:section.
202:expander
169:coolers.
64:nitrogen
29:nitrogen
351:ammonia
341:Ammonia
317:Medical
263:zeolite
83:krypton
782:
753:
646:
601:Messer
461:. CERN
311:SpaceX
185:water.
95:Helium
66:, and
60:oxygen
33:oxygen
644:S2CID
503:(PDF)
496:(PDF)
455:(PDF)
325:Steel
166:fluid
87:xenon
68:argon
37:argon
780:ISBN
751:ISBN
730:2022
608:2022
467:2022
459:CERN
79:neon
31:and
699:doi
695:102
671:doi
636:doi
579:doi
552:doi
329:In
230:or
85:,
19:An
831::
788:.
720:.
693:.
667:30
665:.
642:.
632:43
630:.
599:.
575:25
573:.
548:35
546:.
529:63
527:.
457:.
377:.
353:.
313:.
93:.
81:,
62:,
43:.
759:.
732:.
705:.
701::
677:.
673::
650:.
638::
610:.
585:.
581::
558:.
554::
512:.
469:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.