975:(9 mg/m2 ) plus prednisone (60 mg/m2 ) daily for four days every 6 weeks or the same MP schedule with bortezomib, 1.3 mg/m2 iv on days 1, 8, 11, 22, 25, 29, and 32 of every 6 week cycle for 4 cycles then once weekly for 4 weeks for 5 cycles. Time- to- progression (TTP) was the primary efficacy endpoint. Overall survival (OS), progression-free survival (PFS), and response rate (RR) were secondary endpoints. Eligible people were age > 65 years. A total of 682 people were randomized: 338 to receive MP and 344 to the combination of bortezomib plus MP. Demographics and baseline disease characteristics were similar between the two groups.
40:
546:
523:
932:
are likely to be involved, proteasome inhibition may prevent degradation of pro-apoptotic factors, thereby triggering programmed cell death in neoplastic cells. Bortezomib causes a rapid and dramatic change in the levels of intracellular peptides that are produced by the proteasome. Some intracellular peptides have been shown to be biologically active, and so the effect of bortezomib on the levels of intracellular peptides may contribute to the biological and/or side effects of the drug.
31:
971:(FDA) for use in multiple myeloma, based on the results from the SUMMIT Phase II trial. In 2008, bortezomib was approved in the United States for initial treatment of people with multiple myeloma. Bortezomib was previously approved in 2005, for the treatment of people with multiple myeloma who had received at least one prior therapy and in 2003, for the treatment of more refractory multiple myeloma.
4004:
2068:
2005:
866:
978:
The trial was stopped following a pre-specified interim analysis showing a statistically significant improvement in TTP with the addition of bortezomib to MP (median 20.7 months) compared with MP (median 15 months) . OS, PFS, and RR also were significantly superior for the bortezomib-MP combination.
931:
proteins, and also rids the cell of abnormal or misfolded proteins. Clinical and preclinical data support a role for the proteasome in maintaining the immortal phenotype of myeloma cells, and cell-culture and xenograft data support a similar function in solid tumor cancers. While multiple mechanisms
940:
After subcutaneous administration, peak plasma levels are ~25-50 nM and this peak is sustained for 1-2 hrs. After intravenous injection, peak plasma levels are ~500 nM but only for ~5 minutes, after which the levels rapidly drop as the drug distributes to tissues (volume of distribution is ~500 L).
974:
The 2008 approval was based on an international, multicenter, open label, active-control trial in previously untreated people with symptomatic multiple myeloma. People were randomized to receive either nine cycles of oral melphalan (M) plus prednisone (P) or MP plus bortezomib. People received M
1011:
per person, and because studies reviewed by NICE reported that it could only extend the life expectancy by an average of six months over standard treatment. However, the company later proposed a performance-linked cost reduction for multiple myeloma, and this was accepted.
764:) on days 1,4,8, and 11 of a 21-day cycle for a maximum of eight cycles in heavily pretreated people with relapsed/refractory multiple myeloma. The phase III demonstrated the superiority of bortezomib over a high-dose dexamethasone regimen (e.g. median
1842:
Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Grishunina M, et al. (May 2011). "Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study".
982:
In August 2014, bortezomib was approved in the United States for the retreatment of adults with multiple myeloma who had previously responded to
Velcade therapy and relapsed at least six months following completion of prior treatment.
768:
6.2 vs 3.5 months, and 1-year survival 80% vs 66%). New studies show that bortezomib may potentially help recover from vincristine treatment in treating acute lymphoblastic leukemia, when replacing vincristine in the process.
1451:
Pour L, Adam Z, Buresova L, Krejci M, Krivanova A, Sandecka V, et al. (April 2009). "Varicella-zoster virus prophylaxis with low-dose acyclovir in patients with multiple myeloma treated with bortezomib".
873:
proteasome. The bortezomib molecule is in the center colored by atom type (boron = pink, carbon = cyan, nitrogen = blue, oxygen = red), surrounded by the local protein surface. The blue patch is catalytic
1174:
845:
Ocular side effects such as chalazion or hordeolum (stye) may be more common in women and have led to discontinuation of treatment. Acute interstitial nephritis has also been reported.
2085:
1250:
Joshi J, Tanner L, Gilchrist L, Bostrom B (August 2019). "Switching to
Bortezomib may Improve Recovery From Severe Vincristine Neuropathy in Pediatric Acute Lymphoblastic Leukemia".
221:
1200:
941:
Both routes provide equal drug exposures and generally comparable therapeutic efficacy. Elimination half life is 9–15 hours and the drug is primarily cleared by hepatic metabolism.
1295:"Multiple drug combinations of bortezomib, lenalidomide, and thalidomide for first-line treatment in adults with transplant-ineligible multiple myeloma: a network meta-analysis"
618:
706:. This includes multiple myeloma in those who have and have not previously received treatment. It is generally used together with other medications. It is given by injection.
2045:
660:
InChI=1S/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17-/m0/s1
2114:"Velcade (Bortezomib) Receives 2 New FDA Indications: For Retreatment of Patients with Multiple Myeloma and for First-Line Treatment of Patients with Mantle-Cell Lymphoma"
4064:
3870:
967:
In May 2003, seven years after the initial synthesis, bortezomib (marketed as
Velcade by Millennium Pharmaceuticals Inc.) was approved in the United States by the
2207:
4069:
176:
2175:
1990:
2282:
730:
1046:
857:(EGCG), which were expected to have a synergistic effect, instead were found to reduce the effectiveness of bortezomib in cell culture experiments.
4034:
1162:
948:
of bortezomib are determined by quantifying proteasome inhibition in peripheral blood mononuclear cells taken from people receiving the drug.
70:
2093:
3930:
2982:
772:
Bortezomib was also evaluated together with other drugs for the treatment of multiple myelomas in adults. It was seen that bortezomib plus
632:
1228:
2811:
2791:
2776:
1640:"Bortezomib-mediated 26S proteasome inhibition causes cell-cycle arrest and induces apoptosis in CD-30+ anaplastic large cell lymphoma"
1390:"PAD combination therapy (PS-341/bortezomib, doxorubicin and dexamethasone) for previously untreated patients with multiple myeloma"
2053:
956:
Bortezomib was originally made in 1995 at
Myogenics. The drug (PS-341) was tested in a small Phase I clinical trial on people with
927:
with high affinity and specificity. In normal cells, the proteasome regulates protein expression and function by degradation of
1878:
1801:
1738:
1624:
1487:
1437:
1342:
3198:
1356:
3958:
2275:
745:
1293:
Piechotta V, Jakob T, Langer P, Monsef I, Scheid C, Estcourt LJ, et al. (Cochrane
Haematology Group) (November 2019).
3565:
2717:
2673:
2551:
2260:
2211:
677:
121:
2476:
1585:"Green tea polyphenols block the anticancer effects of bortezomib and other boronic acid-based proteasome inhibitors"
744:
Bortezomib was approved for medical use in the United States in 2003 and in the
European Union in 2004. It is on the
652:
3975:
2268:
2992:
1754:"Pharmacokinetic and pharmacodynamic study of two doses of bortezomib in patients with relapsed multiple myeloma"
206:
102:
4054:
4039:
3611:
2613:
2503:
1027:
986:
In
October 2014, bortezomib was approved in the United States for the treatment of treatment-naïve people with
2237:
1815:
Voorhees PM, Dees EC, O'Neil B, Orlowski RZ (December 2003). "The proteasome as a target for cancer therapy".
363:
3533:
3516:
3391:
3155:
2533:
2182:
1978:
3265:
2861:
2049:
1984:
1168:
968:
831:
and other treatment options for people with advanced disease. Bortezomib is associated with a high rate of
541:
414:
4074:
3467:
828:
502:
463:
165:
2857:
2827:
3994:
2647:
961:
2752:
3833:
3713:
3703:
1134:
1074:
3885:
3818:
3788:
3406:
2656:
854:
518:
3963:
3688:
3403:
2889:
2737:
2664:
2249:
1542:
Cheungpasitporn W, Leung N, Rajkumar SV, Cornell LD, Sethi S, Angioi A, et al. (July 2015).
283:
158:
52:
1752:
Reece DE, Sullivan D, Lonial S, Mohrbacher AF, Chatta G, Shustik C, et al. (January 2011).
39:
4049:
4024:
3768:
3758:
3491:
3333:
1681:"Alterations of the intracellular peptidome in response to the proteasome inhibitor bortezomib"
796:
695:
484:
264:
4044:
4029:
3040:
2687:
1872:
1795:
1732:
1618:
1481:
1431:
1336:
804:
722:
714:
354:
1935:
Adams J, Kauffman M (2004). "Development of the proteasome inhibitor
Velcade (Bortezomib)".
1069:
403:
318:
3438:
3315:
2836:
1692:
987:
734:
703:
423:
291:
2046:"Velcade (bortezomib) is Approved for Initial Treatment of Patients with Multiple Myeloma"
8:
3723:
3623:
3602:
3323:
3145:
2977:
2708:
2604:
765:
309:
169:
1696:
1583:
Golden EB, Lam PY, Kardosh A, Gaffney KJ, Cadenas E, Louie SG, et al. (June 2009).
545:
522:
4059:
2781:
2290:
2130:
2113:
1960:
1917:
1778:
1753:
1715:
1680:
1679:
Gelman JS, Sironi J, Berezniuk I, Dasgupta S, Castro LM, Gozzo FC, et al. (2013).
1419:
1319:
1294:
1275:
749:
718:
132:
1905:
1856:
3905:
3479:
2313:
2135:
1952:
1909:
1860:
1824:
1783:
1720:
1661:
1606:
1565:
1524:
1469:
1411:
1406:
1389:
1324:
1279:
1267:
1220:
1216:
474:
234:
94:
1964:
1921:
1423:
1388:
Oakervee HE, Popat R, Curry N, Smith P, Morris C, Drake M, et al. (June 2005).
3910:
3667:
3382:
3016:
3001:
2722:
2125:
1944:
1901:
1852:
1773:
1765:
1710:
1700:
1651:
1596:
1555:
1514:
1461:
1401:
1363:
1314:
1306:
1259:
1212:
1142:
957:
945:
820:
812:
807:
in 30% of people resulting in pain. This can be worse in people with pre-existing
699:
558:
343:
256:
189:
1109:
717:, shortness of breath, rash and abdominal pain. Other severe side effects include
80:
4008:
3349:
3311:
3102:
2524:
2439:
1705:
1601:
1584:
1263:
908:
892:
274:
2153:
1007:
initially recommended against
Velcade in October 2006, due to its cost of about
3969:
3503:
3094:
2588:
2452:
2339:
2309:
2296:
1501:
Dennis M, Maoz A, Hughes D, Sanchorawala V, Sloan JM, Sarosiek S (March 2019).
1769:
1310:
760:
Two open-label trials established the efficacy of bortezomib (with or without
4018:
3843:
3803:
3173:
3046:
2927:
2870:
2628:
2577:
2072:
2009:
1913:
896:
777:
761:
726:
710:
534:
3890:
3854:
3738:
3728:
3682:
3678:
3640:
3548:
3338:
3277:
3273:
3254:
3249:
3228:
3217:
3031:
3025:
3011:
3006:
2937:
2932:
2885:
2771:
2638:
2618:
2547:
2496:
2486:
2300:
2292:
2139:
1956:
1864:
1828:
1787:
1724:
1665:
1656:
1639:
1610:
1569:
1528:
1473:
1465:
1415:
1328:
1271:
1224:
1139:
World Health
Organization model list of essential medicines: 21st list 2019
904:
824:
773:
184:
22:
1948:
3935:
3915:
3900:
3895:
3880:
3838:
3808:
3748:
3718:
3662:
3635:
3508:
3449:
3421:
3411:
3364:
3328:
3244:
3206:
3188:
3183:
3163:
3140:
3111:
3089:
3069:
3059:
2910:
2895:
2875:
2761:
2692:
2678:
2633:
2566:
2561:
2538:
2513:
2481:
2424:
2387:
2364:
2349:
2344:
2329:
1560:
1543:
836:
816:
88:
1147:
383:
3925:
3828:
3823:
3798:
3793:
3783:
3778:
3773:
3743:
3733:
3698:
3693:
3673:
3628:
3616:
3591:
3586:
3576:
3558:
3553:
3543:
3538:
3496:
3484:
3472:
3431:
3426:
3396:
3359:
3354:
3292:
3287:
3233:
3178:
3130:
3116:
3064:
3051:
3020:
2953:
2942:
2915:
2905:
2900:
2846:
2801:
2796:
2697:
2661:
2593:
2583:
2572:
2556:
2508:
2491:
2467:
2419:
2407:
2359:
924:
808:
785:
738:
594:
394:
1519:
1502:
3940:
3920:
3875:
3865:
3859:
3763:
3708:
3657:
3581:
3571:
3526:
3513:
3454:
3416:
3344:
3282:
3223:
3212:
3168:
3125:
3084:
3078:
3074:
3036:
2963:
2958:
2948:
2920:
2880:
2841:
2816:
2806:
2766:
2412:
2402:
2397:
2392:
2354:
928:
888:
875:
839:
781:
329:
74:
3849:
3813:
3753:
3521:
3459:
3239:
3135:
3107:
2786:
2623:
1541:
1021:
832:
800:
443:
374:
116:
1841:
803:
are the most common adverse events. Bortezomib is associated with
617:
490:
2742:
2643:
2457:
2318:
2071:
This article incorporates text from this source, which is in the
2008:
This article incorporates text from this source, which is in the
1201:"Bortezomib: a review of its use in people with multiple myeloma"
923:
atom in bortezomib is proposed to bind the catalytic site of the
900:
788:
may result in a large increase in the progression-free survival.
878:
residue whose activity is blocked by the presence of bortezomib.
2382:
1892:
Larkin M (November 1999). "(In)famous trials brought to life".
454:
1814:
1751:
1678:
920:
870:
608:
279:
30:
1500:
507:
1249:
1004:
434:
1292:
1979:"Drug Approval Package: Velcade (Bortezomib) NDA #021602"
1133:
709:
Common side effects include nausea, diarrhea, tiredness,
1637:
1151:. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
935:
891:
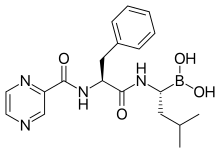
and can be written as Pyz-Phe-boroLeu, which stands for
1582:
1503:"Bortezomib ocular toxicities: Outcomes with ketotifen"
1387:
865:
746:
World Health Organization's List of Essential Medicines
1450:
3992:
1638:
Bonvini P, Zorzi E, Basso G, Rosolen A (April 2007).
853:
Polyphenols derived from green tea extract including
2210:. Euro Pharma Today. 21 January 2009. Archived from
823:
can also occur and be dose-limiting. However, these
362:
1544:"Bortezomib-induced acute interstitial nephritis"
731:reversible posterior leukoencephalopathy syndrome
4065:Drugs developed by Takeda Pharmaceutical Company
4016:
342:
2107:
2105:
2103:
2040:
2038:
1024:, a proteasome inhibitor that is given by mouth
960:. It was brought to further clinical trials by
741:, cellular complexes that break down proteins.
317:
2036:
2034:
2032:
2030:
2028:
2026:
2024:
2022:
2020:
2018:
4070:World Health Organization essential medicines
2276:
1934:
1198:
733:. It is in the class of medications known as
2208:"More Velcade-Style Risk-Sharing In The UK?"
2100:
1928:
1672:
1631:
1444:
1381:
1194:
1192:
640:O=C(N(C(=O)N(B(O)O)CC(C)C)Cc1ccccc1)c2nccnc2
120:
2015:
1299:The Cochrane Database of Systematic Reviews
1243:
869:Bortezomib bound to the core particle in a
248:In general: ℞ (Prescription only)
2283:
2269:
1104:
1102:
1100:
1098:
1096:
1094:
544:
521:
402:
38:
29:
2129:
2086:"Millennium: The Takeda Oncology Company"
1777:
1714:
1704:
1655:
1600:
1559:
1518:
1405:
1318:
1189:
1146:
1064:
1062:
1060:
1058:
1056:
1054:
422:
4035:Drugs developed by Johnson & Johnson
1252:Journal of Pediatric Hematology/Oncology
1110:"Bortezomib Monograph for Professionals"
864:
2111:
1357:"Highlights Of Prescribing Information"
1091:
517:
382:
93:
4017:
1891:
1127:
1051:
993:
535:
240:
2264:
1548:Nephrology, Dialysis, Transplantation
1141:. Geneva: World Health Organization.
936:Pharmacokinetics and pharmacodynamics
483:
462:
228:
111:
79:
2176:"Summary of Velcade Response Scheme"
1993:from the original on 5 December 2019
1758:Cancer Chemotherapy and Pharmacology
848:
489:
188:
57:Velcade, Chemobort, Bortecad, others
2552:ribonucleotide reductase inhibitors
2118:American Health & Drug Benefits
2052:(FDA). 23 June 2008. Archived from
1163:"2022 First Generic Drug Approvals"
442:
333:
13:
2718:Ribonucleotide reductase inhibitor
2674:Ribonucleotide reductase inhibitor
2156:. BBC News Online. 20 October 2006
2154:"NHS watchdog rejects cancer drug"
1877:: CS1 maint: overridden setting (
1800:: CS1 maint: overridden setting (
1737:: CS1 maint: overridden setting (
1623:: CS1 maint: overridden setting (
1486:: CS1 maint: overridden setting (
1436:: CS1 maint: overridden setting (
1341:: CS1 maint: overridden setting (
791:
14:
4086:
2477:Dihydrofolate reductase inhibitor
2230:
1177:from the original on 30 June 2023
215:
143:
4002:
2066:
2003:
1407:10.1111/j.1365-2141.2005.05519.x
1217:10.2165/00003495-200969070-00006
578:
569:
2200:
2168:
2146:
2092:. 8 August 2014. Archived from
2078:
1971:
1885:
1835:
1808:
1745:
1576:
1535:
1494:
1454:Clinical Lymphoma & Myeloma
860:
665:Key:GXJABQQUPOEUTA-RDJZCZTQSA-N
2614:Thymidylate synthase inhibitor
2504:Thymidylate synthase inhibitor
1507:American Journal of Hematology
1394:British Journal of Haematology
1349:
1286:
1155:
1040:
1028:Peter Elliott (pharmacologist)
755:
584:
563:
1:
2534:Adenosine deaminase inhibitor
2375:Block microtubule disassembly
1906:10.1016/s0140-6736(05)76886-0
1857:10.1016/s1470-2045(11)70081-x
1199:Curran MP, McKeage K (2009).
1033:
842:can reduce the risk of this.
827:are usually mild relative to
575:
2244:. National Cancer Institute.
2050:Food and Drug Administration
1985:Food and Drug Administration
1706:10.1371/journal.pone.0053263
1602:10.1182/blood-2008-07-171389
1264:10.1097/MPH.0000000000001529
1169:Food and Drug Administration
998:
969:Food and Drug Administration
914:
882:
690:, sold under the brand name
7:
1015:
887:The drug is an N-protected
829:bone marrow transplantation
780:as well as bortezomib plus
10:
4091:
962:Millennium Pharmaceuticals
951:
553:Chemical and physical data
3953:
3834:Omacetaxine mepesuccinate
3714:Ciltacabtagene autoleucel
3704:Brexucabtagene autoleucel
3650:
3601:
3381:
3374:
3310:
3264:
3197:
3154:
2991:
2976:
2856:
2826:
2751:
2736:
2707:
2603:
2523:
2466:
2449:
2438:
2374:
2327:
2308:
2254:National Cancer Institute
1770:10.1007/s00280-010-1283-3
1311:10.1002/14651858.CD013487
1135:World Health Organization
1075:European Medicines Agency
1047:Baxter Healthcare Pty Ltd
737:. It works by inhibiting
673:
648:
628:
606:
593:
557:
552:
533:
501:
473:
453:
433:
413:
393:
373:
353:
328:
308:
303:
290:
273:
263:
255:
205:
200:
175:
157:
131:
101:
87:
69:
61:
51:
46:
37:
28:
3886:Talimogene laherparepvec
3819:Nadofaragene firadenovec
3789:Lisocabtagene maraleucel
2738:Topoisomerase inhibitors
2657:DNA polymerase inhibitor
2124:(Spec Feature): 135–40.
2112:Raedler L (March 2015).
1817:Clinical Cancer Research
855:epigallocatechin gallate
3689:Axicabtagene ciloleucel
2293:chemotherapeutic agents
748:. It is available as a
3871:Sitimagene ceradenovec
3769:Idecabtagene vicleucel
3334:Methyl aminolevulinate
2048:(Press release). U.S.
1657:10.1038/sj.leu.2404528
1466:10.3816/CLM.2009.n.036
879:
696:anti-cancer medication
4055:Proteasome inhibitors
4040:Janssen Pharmaceutica
3345:Porphyrin derivatives
3041:Melphalan flufenamide
2688:Hypomethylating agent
2297:antineoplastic agents
1949:10.1081/CNV-120030218
868:
805:peripheral neuropathy
723:tumour lysis syndrome
715:low white blood cells
3674:Asparagine depleters
3603:Receptor antagonists
3517:+abiraterone acetate
1937:Cancer Investigation
1845:The Lancet. Oncology
988:mantle cell lymphoma
735:proteasome inhibitor
704:mantle cell lymphoma
694:among others, is an
286:extensively involved
3724:Denileukin diftitox
3624:Retinoid X receptor
3324:Aminolevulinic acid
3146:Triethylenemelamine
2978:Crosslinking of DNA
2709:Deoxyribonucleotide
2648:+gimeracil/oteracil
2242:NCI Drug Dictionary
2096:on 1 November 2018.
1697:2013PLoSO...853263G
1369:on 19 February 2009
1079:. 17 September 2018
994:Society and culture
713:, fever, numbness,
224:(Prescription only)
25:
4075:Isobutyl compounds
3980:Never to phase III
2782:Etirinotecan pegol
2056:on 1 December 2011
1561:10.1093/ndt/gfv222
880:
750:generic medication
719:low blood pressure
21:
3990:
3989:
3949:
3948:
3906:Tigilanol tiglate
3383:Enzyme inhibitors
3306:
3305:
3302:
3301:
3002:Nitrogen mustards
2972:
2971:
2732:
2731:
2434:
2433:
2256:. 5 October 2006.
1520:10.1002/ajh.25382
1231:on 8 October 2011
964:in October 1999.
849:Drug interactions
797:Gastro-intestinal
685:
684:
619:Interactive image
503:CompTox Dashboard
244:
232:
219:
147:
114:
16:Chemical compound
4082:
4007:
4006:
4005:
3998:
3911:Tisagenlecleucel
3668:Arsenic trioxide
3379:
3378:
3312:Photosensitizers
3103:Alkyl sulfonates
3017:Cyclophosphamide
2989:
2988:
2928:Anthracenediones
2749:
2748:
2723:Hydroxycarbamide
2464:
2463:
2447:
2446:
2325:
2324:
2285:
2278:
2271:
2262:
2261:
2257:
2245:
2224:
2223:
2221:
2219:
2204:
2198:
2197:
2195:
2193:
2188:on 19 April 2009
2187:
2181:. Archived from
2180:
2172:
2166:
2165:
2163:
2161:
2150:
2144:
2143:
2133:
2109:
2098:
2097:
2082:
2076:
2070:
2069:
2065:
2063:
2061:
2042:
2013:
2007:
2006:
2002:
2000:
1998:
1975:
1969:
1968:
1932:
1926:
1925:
1889:
1883:
1882:
1876:
1868:
1839:
1833:
1832:
1812:
1806:
1805:
1799:
1791:
1781:
1749:
1743:
1742:
1736:
1728:
1718:
1708:
1676:
1670:
1669:
1659:
1635:
1629:
1628:
1622:
1614:
1604:
1580:
1574:
1573:
1563:
1539:
1533:
1532:
1522:
1498:
1492:
1491:
1485:
1477:
1448:
1442:
1441:
1435:
1427:
1409:
1385:
1379:
1378:
1376:
1374:
1368:
1362:. Archived from
1361:
1353:
1347:
1346:
1340:
1332:
1322:
1290:
1284:
1283:
1247:
1241:
1240:
1238:
1236:
1227:. Archived from
1196:
1187:
1186:
1184:
1182:
1173:. 3 March 2023.
1159:
1153:
1152:
1150:
1131:
1125:
1124:
1122:
1120:
1106:
1089:
1088:
1086:
1084:
1066:
1049:
1044:
1010:
958:multiple myeloma
946:pharmacodynamics
821:thrombocytopenia
813:myelosuppression
700:multiple myeloma
681:
680:
621:
601:
586:
580:
577:
571:
565:
548:
537:
526:
525:
511:
509:
493:
487:
466:
446:
426:
406:
386:
366:
346:
336:
335:
321:
295:
242:
239:
230:
227:
217:
214:
192:
145:
142:
124:
113:
110:
97:
83:
42:
33:
26:
24:
20:
4090:
4089:
4085:
4084:
4083:
4081:
4080:
4079:
4015:
4014:
4013:
4003:
4001:
3993:
3991:
3986:
3985:
3970:Clinical trials
3945:
3651:Other/ungrouped
3646:
3597:
3370:
3350:Porfimer sodium
3298:
3260:
3193:
3150:
2980:
2968:
2852:
2822:
2740:
2728:
2703:
2599:
2519:
2455:
2453:antimetabolites
2451:
2450:DNA precursors/
2442:
2440:DNA replication
2430:
2370:
2340:Vinca alkaloids
2316:
2304:
2289:
2248:
2236:
2233:
2228:
2227:
2217:
2215:
2214:on 10 July 2011
2206:
2205:
2201:
2191:
2189:
2185:
2178:
2174:
2173:
2169:
2159:
2157:
2152:
2151:
2147:
2110:
2101:
2090:.millennium.com
2084:
2083:
2079:
2067:
2059:
2057:
2044:
2043:
2016:
2004:
1996:
1994:
1989:. 13 May 2003.
1977:
1976:
1972:
1933:
1929:
1890:
1886:
1870:
1869:
1840:
1836:
1823:(17): 6316–25.
1813:
1809:
1793:
1792:
1750:
1746:
1730:
1729:
1677:
1673:
1636:
1632:
1616:
1615:
1595:(23): 5927–37.
1581:
1577:
1540:
1536:
1499:
1495:
1479:
1478:
1449:
1445:
1429:
1428:
1386:
1382:
1372:
1370:
1366:
1359:
1355:
1354:
1350:
1334:
1333:
1291:
1287:
1248:
1244:
1234:
1232:
1197:
1190:
1180:
1178:
1161:
1160:
1156:
1132:
1128:
1118:
1116:
1108:
1107:
1092:
1082:
1080:
1068:
1067:
1052:
1045:
1041:
1036:
1018:
1008:
1001:
996:
954:
938:
917:
909:carboxylic acid
893:pyrazinoic acid
885:
863:
851:
811:. In addition,
794:
792:Adverse effects
758:
676:
674:
669:
666:
661:
656:
655:
644:
641:
636:
635:
624:
599:
589:
583:
574:
568:
529:
505:
497:
469:
449:
429:
409:
389:
369:
349:
332:
324:
293:
265:Protein binding
257:Pharmacokinetic
251:
196:
160:
153:
134:
127:
17:
12:
11:
5:
4088:
4078:
4077:
4072:
4067:
4062:
4057:
4052:
4047:
4042:
4037:
4032:
4027:
4012:
4011:
3988:
3987:
3984:
3983:
3982:
3981:
3978:
3967:
3961:
3955:
3954:
3951:
3950:
3947:
3946:
3944:
3943:
3938:
3933:
3928:
3923:
3918:
3913:
3908:
3903:
3898:
3893:
3888:
3883:
3878:
3873:
3868:
3863:
3857:
3846:
3841:
3836:
3831:
3826:
3821:
3816:
3811:
3806:
3801:
3796:
3791:
3786:
3781:
3776:
3771:
3766:
3761:
3756:
3751:
3746:
3741:
3736:
3731:
3726:
3721:
3716:
3711:
3706:
3701:
3696:
3691:
3686:
3670:
3665:
3660:
3654:
3652:
3648:
3647:
3645:
3644:
3632:
3620:
3607:
3605:
3599:
3598:
3596:
3595:
3589:
3584:
3579:
3574:
3562:
3556:
3551:
3546:
3541:
3530:
3524:
3519:
3511:
3504:PARP inhibitor
3500:
3488:
3476:
3464:
3463:
3462:
3457:
3452:
3447:
3435:
3429:
3424:
3419:
3414:
3400:
3387:
3385:
3376:
3372:
3371:
3369:
3368:
3362:
3357:
3352:
3341:
3336:
3331:
3326:
3320:
3318:
3308:
3307:
3304:
3303:
3300:
3299:
3297:
3296:
3290:
3285:
3280:
3270:
3268:
3262:
3261:
3259:
3258:
3252:
3247:
3236:
3231:
3226:
3221:
3209:
3203:
3201:
3195:
3194:
3192:
3191:
3186:
3181:
3176:
3171:
3166:
3160:
3158:
3156:Platinum-based
3152:
3151:
3149:
3148:
3143:
3138:
3133:
3121:
3120:
3114:
3098:
3097:
3092:
3087:
3082:
3072:
3067:
3055:
3054:
3049:
3044:
3034:
3029:
3023:
3014:
3009:
2997:
2995:
2986:
2974:
2973:
2970:
2969:
2967:
2966:
2961:
2956:
2951:
2946:
2940:
2935:
2924:
2918:
2913:
2908:
2903:
2898:
2893:
2883:
2878:
2871:Anthracyclines
2866:
2864:
2854:
2853:
2851:
2850:
2844:
2832:
2830:
2824:
2823:
2821:
2820:
2814:
2809:
2804:
2799:
2794:
2789:
2784:
2779:
2774:
2769:
2757:
2755:
2746:
2734:
2733:
2730:
2729:
2727:
2726:
2713:
2711:
2705:
2704:
2702:
2701:
2695:
2683:
2682:
2669:
2668:
2652:
2651:
2641:
2636:
2631:
2626:
2621:
2609:
2607:
2601:
2600:
2598:
2597:
2591:
2589:Mercaptopurine
2580:
2575:
2570:
2564:
2559:
2543:
2542:
2529:
2527:
2521:
2520:
2518:
2517:
2511:
2500:
2494:
2489:
2484:
2472:
2470:
2461:
2444:
2436:
2435:
2432:
2431:
2429:
2428:
2416:
2410:
2405:
2400:
2395:
2390:
2378:
2376:
2372:
2371:
2369:
2368:
2362:
2357:
2352:
2347:
2335:
2333:
2322:
2306:
2305:
2291:Intracellular
2288:
2287:
2280:
2273:
2265:
2259:
2258:
2246:
2232:
2231:External links
2229:
2226:
2225:
2199:
2167:
2145:
2099:
2077:
2014:
1970:
1927:
1900:(9193): 1915.
1884:
1834:
1807:
1744:
1671:
1630:
1575:
1534:
1513:(3): E80–E82.
1493:
1443:
1380:
1348:
1285:
1258:(6): 457–462.
1242:
1188:
1154:
1126:
1090:
1070:"Velcade EPAR"
1050:
1038:
1037:
1035:
1032:
1031:
1030:
1025:
1017:
1014:
1000:
997:
995:
992:
953:
950:
937:
934:
925:26S proteasome
916:
913:
884:
881:
862:
859:
850:
847:
793:
790:
757:
754:
698:used to treat
683:
682:
671:
670:
668:
667:
664:
662:
659:
651:
650:
649:
646:
645:
643:
642:
639:
631:
630:
629:
626:
625:
623:
622:
614:
612:
604:
603:
597:
591:
590:
587:
581:
572:
566:
561:
555:
554:
550:
549:
539:
531:
530:
528:
527:
514:
512:
499:
498:
496:
495:
479:
477:
471:
470:
468:
467:
459:
457:
451:
450:
448:
447:
439:
437:
431:
430:
428:
427:
419:
417:
411:
410:
408:
407:
399:
397:
391:
390:
388:
387:
379:
377:
371:
370:
368:
367:
359:
357:
351:
350:
348:
347:
339:
337:
326:
325:
323:
322:
314:
312:
306:
305:
301:
300:
297:
288:
287:
277:
271:
270:
267:
261:
260:
253:
252:
250:
249:
246:
237:
225:
211:
209:
203:
202:
198:
197:
195:
194:
181:
179:
173:
172:
163:
161:administration
155:
154:
152:
151:
149:
139:
137:
129:
128:
126:
125:
107:
105:
99:
98:
91:
85:
84:
77:
67:
66:
63:
59:
58:
55:
49:
48:
44:
43:
35:
34:
15:
9:
6:
4:
3:
2:
4087:
4076:
4073:
4071:
4068:
4066:
4063:
4061:
4058:
4056:
4053:
4051:
4050:Propionamides
4048:
4046:
4043:
4041:
4038:
4036:
4033:
4031:
4028:
4026:
4025:Boronic acids
4023:
4022:
4020:
4010:
4000:
3999:
3996:
3979:
3977:
3974:
3973:
3971:
3968:
3965:
3962:
3960:
3957:
3956:
3952:
3942:
3939:
3937:
3934:
3932:
3929:
3927:
3924:
3922:
3919:
3917:
3914:
3912:
3909:
3907:
3904:
3902:
3899:
3897:
3894:
3892:
3889:
3887:
3884:
3882:
3879:
3877:
3874:
3872:
3869:
3867:
3864:
3861:
3858:
3856:
3852:
3851:
3847:
3845:
3844:Tabelecleucel
3842:
3840:
3837:
3835:
3832:
3830:
3827:
3825:
3822:
3820:
3817:
3815:
3812:
3810:
3807:
3805:
3804:Lurbinectedin
3802:
3800:
3797:
3795:
3792:
3790:
3787:
3785:
3782:
3780:
3777:
3775:
3772:
3770:
3767:
3765:
3762:
3760:
3757:
3755:
3752:
3750:
3747:
3745:
3742:
3740:
3737:
3735:
3732:
3730:
3727:
3725:
3722:
3720:
3717:
3715:
3712:
3710:
3707:
3705:
3702:
3700:
3697:
3695:
3692:
3690:
3687:
3684:
3680:
3676:
3675:
3671:
3669:
3666:
3664:
3661:
3659:
3656:
3655:
3653:
3649:
3642:
3638:
3637:
3633:
3630:
3626:
3625:
3621:
3618:
3614:
3613:
3609:
3608:
3606:
3604:
3600:
3593:
3590:
3588:
3585:
3583:
3580:
3578:
3575:
3573:
3569:
3567:
3563:
3560:
3557:
3555:
3552:
3550:
3547:
3545:
3542:
3540:
3536:
3535:
3531:
3528:
3525:
3523:
3520:
3518:
3515:
3512:
3510:
3506:
3505:
3501:
3498:
3494:
3493:
3489:
3486:
3482:
3481:
3477:
3474:
3470:
3469:
3465:
3461:
3458:
3456:
3453:
3451:
3448:
3446:
3443:
3442:
3441:
3440:
3436:
3433:
3430:
3428:
3425:
3423:
3420:
3418:
3415:
3413:
3409:
3408:
3405:
3401:
3398:
3394:
3393:
3389:
3388:
3386:
3384:
3380:
3377:
3373:
3366:
3363:
3361:
3358:
3356:
3353:
3351:
3347:
3346:
3342:
3340:
3337:
3335:
3332:
3330:
3327:
3325:
3322:
3321:
3319:
3317:
3313:
3309:
3294:
3291:
3289:
3286:
3284:
3281:
3279:
3275:
3272:
3271:
3269:
3267:
3266:Intercalation
3263:
3256:
3253:
3251:
3248:
3246:
3242:
3241:
3237:
3235:
3232:
3230:
3227:
3225:
3222:
3219:
3215:
3214:
3210:
3208:
3205:
3204:
3202:
3200:
3196:
3190:
3187:
3185:
3182:
3180:
3177:
3175:
3174:Dicycloplatin
3172:
3170:
3167:
3165:
3162:
3161:
3159:
3157:
3153:
3147:
3144:
3142:
3139:
3137:
3134:
3132:
3129:
3127:
3123:
3122:
3118:
3115:
3113:
3109:
3106:
3104:
3100:
3099:
3096:
3093:
3091:
3088:
3086:
3083:
3080:
3076:
3073:
3071:
3068:
3066:
3063:
3061:
3057:
3056:
3053:
3050:
3048:
3047:Prednimustine
3045:
3042:
3038:
3035:
3033:
3030:
3027:
3024:
3022:
3018:
3015:
3013:
3010:
3008:
3005:
3003:
2999:
2998:
2996:
2994:
2990:
2987:
2984:
2979:
2975:
2965:
2962:
2960:
2957:
2955:
2952:
2950:
2947:
2944:
2941:
2939:
2936:
2934:
2930:
2929:
2925:
2922:
2919:
2917:
2914:
2912:
2909:
2907:
2904:
2902:
2899:
2897:
2894:
2891:
2887:
2884:
2882:
2879:
2877:
2873:
2872:
2868:
2867:
2865:
2863:
2862:Intercalation
2859:
2855:
2848:
2845:
2843:
2839:
2838:
2834:
2833:
2831:
2829:
2825:
2818:
2815:
2813:
2810:
2808:
2805:
2803:
2800:
2798:
2795:
2793:
2790:
2788:
2785:
2783:
2780:
2778:
2775:
2773:
2770:
2768:
2764:
2763:
2759:
2758:
2756:
2754:
2750:
2747:
2744:
2739:
2735:
2724:
2720:
2719:
2715:
2714:
2712:
2710:
2706:
2699:
2696:
2694:
2690:
2689:
2685:
2684:
2680:
2676:
2675:
2671:
2670:
2666:
2665:+daunorubicin
2663:
2659:
2658:
2654:
2653:
2649:
2645:
2642:
2640:
2637:
2635:
2632:
2630:
2629:Doxifluridine
2627:
2625:
2622:
2620:
2616:
2615:
2611:
2610:
2608:
2606:
2602:
2595:
2592:
2590:
2586:
2585:
2581:
2579:
2578:Rabacfosadine
2576:
2574:
2571:
2568:
2565:
2563:
2560:
2558:
2554:
2553:
2549:
2545:
2544:
2540:
2536:
2535:
2531:
2530:
2528:
2526:
2522:
2515:
2512:
2510:
2506:
2505:
2501:
2498:
2495:
2493:
2490:
2488:
2485:
2483:
2479:
2478:
2474:
2473:
2471:
2469:
2465:
2462:
2459:
2454:
2448:
2445:
2441:
2437:
2426:
2422:
2421:
2417:
2414:
2411:
2409:
2406:
2404:
2401:
2399:
2396:
2394:
2391:
2389:
2385:
2384:
2380:
2379:
2377:
2373:
2366:
2363:
2361:
2358:
2356:
2353:
2351:
2348:
2346:
2342:
2341:
2337:
2336:
2334:
2331:
2326:
2323:
2320:
2315:
2311:
2307:
2302:
2298:
2294:
2286:
2281:
2279:
2274:
2272:
2267:
2266:
2263:
2255:
2251:
2247:
2243:
2239:
2235:
2234:
2213:
2209:
2203:
2184:
2177:
2171:
2155:
2149:
2141:
2137:
2132:
2127:
2123:
2119:
2115:
2108:
2106:
2104:
2095:
2091:
2087:
2081:
2074:
2073:public domain
2055:
2051:
2047:
2041:
2039:
2037:
2035:
2033:
2031:
2029:
2027:
2025:
2023:
2021:
2019:
2011:
2010:public domain
1992:
1988:
1986:
1980:
1974:
1966:
1962:
1958:
1954:
1950:
1946:
1943:(2): 304–11.
1942:
1938:
1931:
1923:
1919:
1915:
1911:
1907:
1903:
1899:
1895:
1888:
1880:
1874:
1866:
1862:
1858:
1854:
1851:(5): 431–40.
1850:
1846:
1838:
1830:
1826:
1822:
1818:
1811:
1803:
1797:
1789:
1785:
1780:
1775:
1771:
1767:
1763:
1759:
1755:
1748:
1740:
1734:
1726:
1722:
1717:
1712:
1707:
1702:
1698:
1694:
1691:(1): e53263.
1690:
1686:
1682:
1675:
1667:
1663:
1658:
1653:
1650:(4): 838–42.
1649:
1645:
1641:
1634:
1626:
1620:
1612:
1608:
1603:
1598:
1594:
1590:
1586:
1579:
1571:
1567:
1562:
1557:
1554:(7): 1225–9.
1553:
1549:
1545:
1538:
1530:
1526:
1521:
1516:
1512:
1508:
1504:
1497:
1489:
1483:
1475:
1471:
1467:
1463:
1459:
1455:
1447:
1439:
1433:
1425:
1421:
1417:
1413:
1408:
1403:
1400:(6): 755–62.
1399:
1395:
1391:
1384:
1365:
1358:
1352:
1344:
1338:
1330:
1326:
1321:
1316:
1312:
1308:
1304:
1300:
1296:
1289:
1281:
1277:
1273:
1269:
1265:
1261:
1257:
1253:
1246:
1230:
1226:
1222:
1218:
1214:
1211:(7): 859–88.
1210:
1206:
1202:
1195:
1193:
1176:
1172:
1170:
1164:
1158:
1149:
1144:
1140:
1136:
1130:
1115:
1111:
1105:
1103:
1101:
1099:
1097:
1095:
1078:
1076:
1071:
1065:
1063:
1061:
1059:
1057:
1055:
1048:
1043:
1039:
1029:
1026:
1023:
1020:
1019:
1013:
1006:
991:
989:
984:
980:
976:
972:
970:
965:
963:
959:
949:
947:
942:
933:
930:
929:ubiquitylated
926:
922:
912:
910:
907:instead of a
906:
902:
898:
897:phenylalanine
894:
890:
877:
872:
867:
858:
856:
846:
843:
841:
838:
834:
830:
826:
822:
818:
814:
810:
806:
802:
798:
789:
787:
783:
779:
778:dexamethasone
775:
770:
767:
763:
762:dexamethasone
753:
751:
747:
742:
740:
736:
732:
728:
727:heart failure
724:
720:
716:
712:
711:low platelets
707:
705:
701:
697:
693:
689:
679:
672:
663:
658:
657:
654:
647:
638:
637:
634:
627:
620:
616:
615:
613:
610:
605:
598:
596:
592:
562:
560:
556:
551:
547:
543:
540:
538:
536:ECHA InfoCard
532:
524:
520:
519:DTXSID3040980
516:
515:
513:
504:
500:
492:
491:RCSB PDB
486:
481:
480:
478:
476:
472:
465:
461:
460:
458:
456:
452:
445:
441:
440:
438:
436:
432:
425:
421:
420:
418:
416:
412:
405:
401:
400:
398:
396:
392:
385:
381:
380:
378:
376:
372:
365:
361:
360:
358:
356:
352:
345:
341:
340:
338:
331:
327:
320:
316:
315:
313:
311:
307:
302:
299:9 to 15 hours
298:
296:
289:
285:
281:
278:
276:
272:
268:
266:
262:
258:
254:
247:
245: Rx-only
238:
236:
226:
223:
213:
212:
210:
208:
204:
199:
191:
186:
183:
182:
180:
178:
174:
171:
167:
164:
162:
156:
150:
141:
140:
138:
136:
130:
123:
118:
109:
108:
106:
104:
100:
96:
92:
90:
86:
82:
78:
76:
72:
68:
64:
60:
56:
54:
50:
47:Clinical data
45:
41:
36:
32:
27:
19:
4045:Orphan drugs
4030:Carboxamides
3891:Tazemetostat
3855:Alitretinoin
3848:
3759:Estramustine
3739:Elsamitrucin
3729:Eflornithine
3683:Pegaspargase
3679:Asparaginase
3672:
3641:Testolactone
3634:
3622:
3610:
3564:
3549:Panobinostat
3532:
3502:
3490:
3478:
3466:
3444:
3437:
3402:
3390:
3343:
3339:Padeliporfin
3278:Dactinomycin
3274:Streptomyces
3255:Temozolomide
3250:Mitozolomide
3238:
3229:Mitobronitol
3218:Procarbazine
3211:
3199:Nonclassical
3124:
3101:
3095:Streptozocin
3060:Nitrosoureas
3058:
3032:Chlorambucil
3026:Trofosfamide
3012:Chlormethine
3007:Bendamustine
3000:
2938:Mitoxantrone
2933:Losoxantrone
2926:
2886:Daunorubicin
2869:
2835:
2772:Camptothecin
2760:
2716:
2686:
2672:
2655:
2639:Fluorouracil
2619:Capecitabine
2612:
2582:
2546:
2532:
2502:
2497:Pralatrexate
2487:Methotrexate
2475:
2418:
2381:
2338:
2253:
2250:"Bortezomib"
2241:
2238:"Bortezomib"
2216:. Retrieved
2212:the original
2202:
2190:. Retrieved
2183:the original
2170:
2158:. Retrieved
2148:
2121:
2117:
2094:the original
2089:
2080:
2058:. Retrieved
2054:the original
1995:. Retrieved
1982:
1973:
1940:
1936:
1930:
1897:
1893:
1887:
1873:cite journal
1848:
1844:
1837:
1820:
1816:
1810:
1796:cite journal
1764:(1): 57–67.
1761:
1757:
1747:
1733:cite journal
1688:
1684:
1674:
1647:
1643:
1633:
1619:cite journal
1592:
1588:
1578:
1551:
1547:
1537:
1510:
1506:
1496:
1482:cite journal
1460:(2): 151–3.
1457:
1453:
1446:
1432:cite journal
1397:
1393:
1383:
1371:. Retrieved
1364:the original
1351:
1337:cite journal
1302:
1298:
1288:
1255:
1251:
1245:
1233:. Retrieved
1229:the original
1208:
1204:
1179:. Retrieved
1166:
1157:
1148:10665/325771
1138:
1129:
1117:. Retrieved
1113:
1081:. Retrieved
1073:
1042:
1002:
985:
981:
977:
973:
966:
955:
943:
939:
918:
905:boronic acid
886:
861:Pharmacology
852:
844:
837:prophylactic
825:side effects
799:effects and
795:
774:lenalidomide
771:
759:
743:
708:
691:
687:
686:
675:
464:ChEMBL325041
292:Elimination
207:Legal status
201:Legal status
166:Subcutaneous
103:License data
18:
3966:from market
3936:Vorasidenib
3916:Trabectedin
3901:Tiazofurine
3896:Tebentafusp
3881:Tagraxofusp
3839:Plitidepsin
3809:Mitoguazone
3749:Epacadostat
3719:Demecolcine
3663:Aflibercept
3636:Sex steroid
3509:Fuzuloparib
3450:Carfilzomib
3422:Palbociclib
3412:Abemaciclib
3365:Verteporfin
3329:Efaproxiral
3245:Dacarbazine
3207:Altretamine
3189:Satraplatin
3184:Oxaliplatin
3164:Carboplatin
3141:Triaziquone
3112:Mannosulfan
3090:Ranimustine
3070:Fotemustine
2911:Pirarubicin
2896:Doxorubicin
2890:+cytarabine
2876:Aclarubicin
2837:Podophyllum
2762:Camptotheca
2693:Azacitidine
2679:Gemcitabine
2634:Floxuridine
2567:Fludarabine
2562:Clofarabine
2548:Halogenated
2539:Pentostatin
2514:Raltitrexed
2482:Aminopterin
2425:Ixabepilone
2420:Epothilones
2388:Cabazitaxel
2365:Vinorelbine
2350:Vincristine
2345:Vinblastine
2330:microtubule
1373:19 December
1003:In the UK,
835:, although
817:neutropenia
756:Medical use
739:proteasomes
602: g·mol
542:100.125.601
319:179324-69-7
304:Identifiers
170:intravenous
89:MedlinePlus
62:Other names
53:Trade names
4019:Categories
3931:Verdinexor
3926:Venetoclax
3829:Oblimersen
3824:Navitoclax
3799:Lucanthone
3794:Lonidamine
3784:Lifileucel
3779:Ivosidenib
3774:Imetelstat
3744:Enasidenib
3734:Elesclomol
3699:Bexarotene
3694:Belzutifan
3629:Bexarotene
3617:Atrasentan
3592:Umbralisib
3587:Idelalisib
3577:Copanlisib
3559:Vorinostat
3554:Romidepsin
3544:Entinostat
3539:Belinostat
3497:Masoprocol
3485:Tiazofurin
3473:Anagrelide
3445:Bortezomib
3432:Seliciclib
3427:Ribociclib
3407:inhibitors
3397:Tipifarnib
3360:Temoporfin
3355:Talaporfin
3293:Plicamycin
3288:Mitomycins
3234:Pipobroman
3213:Hydrazines
3179:Nedaplatin
3131:Carboquone
3126:Aziridines
3117:Treosulfan
3065:Carmustine
3052:Uramustine
3021:Ifosfamide
2993:Alkylating
2954:Bisantrene
2943:Pixantrone
2916:Valrubicin
2906:Idarubicin
2901:Epirubicin
2847:Teniposide
2802:Lurtotecan
2797:Irinotecan
2698:Decitabine
2662:Cytarabine
2605:Pyrimidine
2594:Tioguanine
2584:Thiopurine
2573:Nelarabine
2557:Cladribine
2509:Pemetrexed
2492:Pemetrexed
2468:Folic acid
2408:Paclitaxel
2360:Vinflunine
2060:5 December
1997:5 December
1894:The Lancet
1119:13 October
1083:13 October
1034:References
809:neuropathy
786:prednisone
688:Bortezomib
607:3D model (
595:Molar mass
475:PDB ligand
424:69G8BD63PP
395:ChemSpider
355:IUPHAR/BPS
310:CAS Number
275:Metabolism
122:Bortezomib
23:Bortezomib
4060:Pyrazines
3976:Phase III
3964:Withdrawn
3941:Vosaroxin
3921:Veliparib
3876:Sotorasib
3866:Selinexor
3860:Tretinoin
3850:Retinoids
3764:Glasdegib
3709:Celecoxib
3658:Adagrasib
3582:Duvelisib
3572:Alpelisib
3527:Rucaparib
3514:Niraparib
3455:Oprozomib
3417:Alvocidib
3283:Bleomycin
3240:Triazenes
3224:Etoglucid
3169:Cisplatin
3085:Nimustine
3079:Semustine
3075:Lomustine
3037:Melphalan
2964:Menogaril
2959:Crisnatol
2949:Amsacrine
2921:Zorubicin
2881:Amrubicin
2842:Etoposide
2817:Topotecan
2812:Silatecan
2807:Rubitecan
2792:Gimatecan
2777:Cositecan
2767:Belotecan
2443:inhibitor
2413:Tesetaxel
2403:Ortataxel
2398:Larotaxel
2393:Docetaxel
2355:Vindesine
2218:14 August
2192:14 August
2160:14 August
1914:0140-6736
1280:195357104
1114:Drugs.com
999:Economics
915:Mechanism
889:dipeptide
883:Structure
876:threonine
840:acyclovir
782:melphalan
294:half-life
159:Routes of
133:Pregnancy
81:Monograph
75:Drugs.com
4009:Medicine
3814:Mitotane
3754:Eribulin
3522:Olaparib
3460:Ixazomib
3136:Thiotepa
3108:Busulfan
2787:Exatecan
2624:Carmofur
2332:assembly
2295: /
2140:26629279
1991:Archived
1965:23644211
1957:15199612
1922:53301933
1865:21507715
1829:14695130
1788:20306195
1725:23308178
1685:PLOS ONE
1666:17268529
1644:Leukemia
1611:19190249
1570:26109684
1529:30575098
1474:19406726
1424:34591121
1416:15953001
1329:31765002
1272:31233464
1235:26 March
1225:19441872
1175:Archived
1137:(2019).
1022:Ixazomib
1016:See also
833:shingles
815:causing
801:asthenia
678:(verify)
375:DrugBank
177:ATC code
135:category
117:DailyMed
2743:S phase
2644:Tegafur
2458:S phase
2383:Taxanes
2319:M phase
2131:4665054
1779:3951913
1716:3538785
1693:Bibcode
1320:6876545
1181:30 June
1009:£18,000
990:(MCL).
952:History
903:with a
901:Leucine
692:Velcade
559:Formula
384:DB00188
330:PubChem
193:)
187: (
185:L01XG01
148: C
119::
95:a607007
3995:Portal
3959:WHO-EM
3568:(Pi3K)
2525:Purine
2328:Block
2138:
2128:
1963:
1955:
1920:
1912:
1863:
1827:
1786:
1776:
1723:
1713:
1664:
1609:
1568:
1527:
1472:
1422:
1414:
1327:
1317:
1305:(11).
1278:
1270:
1223:
729:, and
633:SMILES
600:384.24
455:ChEMBL
444:D03150
404:343402
344:387447
235:℞-only
233:
220:
115:
65:PS-341
3480:IMPDI
3375:Other
2186:(PDF)
2179:(PDF)
1987:(FDA)
1983:U.S.
1961:S2CID
1918:S2CID
1589:Blood
1420:S2CID
1367:(PDF)
1360:(PDF)
1276:S2CID
1205:Drugs
1171:(FDA)
1167:U.S.
1077:(EMA)
921:boron
871:yeast
776:plus
653:InChI
609:JSmol
482:BO2 (
280:Liver
3566:PIKI
3534:HDAC
2983:CCNS
2220:2009
2194:2009
2162:2009
2136:PMID
2062:2019
1999:2019
1953:PMID
1910:ISSN
1879:link
1861:PMID
1825:PMID
1802:link
1784:PMID
1739:link
1721:PMID
1662:PMID
1625:link
1607:PMID
1566:PMID
1525:PMID
1488:link
1470:PMID
1438:link
1412:PMID
1375:2022
1343:link
1325:PMID
1303:2019
1268:PMID
1237:2010
1221:PMID
1183:2023
1121:2019
1085:2019
1005:NICE
944:The
919:The
899:and
819:and
784:and
702:and
485:PDBe
435:KEGG
415:UNII
364:6391
259:data
71:AHFS
3612:ERA
3468:PhI
3439:PrI
3404:CDK
3316:PDT
2314:MIs
2310:SPs
2301:L01
2126:PMC
1945:doi
1902:doi
1898:354
1853:doi
1774:PMC
1766:doi
1711:PMC
1701:doi
1652:doi
1597:doi
1593:113
1556:doi
1515:doi
1462:doi
1402:doi
1398:129
1315:PMC
1307:doi
1260:doi
1213:doi
1143:hdl
766:TTP
508:EPA
334:CID
284:CYP
269:83%
190:WHO
168:,
4021::
3972::
3492:LI
3392:FI
2858:II
2828:II
2650:))
2252:.
2240:.
2134:.
2120:.
2116:.
2102:^
2088:.
2017:^
1981:.
1959:.
1951:.
1941:22
1939:.
1916:.
1908:.
1896:.
1875:}}
1871:{{
1859:.
1849:12
1847:.
1819:.
1798:}}
1794:{{
1782:.
1772:.
1762:67
1760:.
1756:.
1735:}}
1731:{{
1719:.
1709:.
1699:.
1687:.
1683:.
1660:.
1648:21
1646:.
1642:.
1621:}}
1617:{{
1605:.
1591:.
1587:.
1564:.
1552:30
1550:.
1546:.
1523:.
1511:94
1509:.
1505:.
1484:}}
1480:{{
1468:.
1456:.
1434:}}
1430:{{
1418:.
1410:.
1396:.
1392:.
1339:}}
1335:{{
1323:.
1313:.
1301:.
1297:.
1274:.
1266:.
1256:41
1254:.
1219:.
1209:69
1207:.
1203:.
1191:^
1165:.
1112:.
1093:^
1072:.
1053:^
911:.
895:,
752:.
725:,
721:,
573:25
567:19
488:,
282:,
241:EU
229:US
222:S4
216:AU
144:AU
112:US
3997::
3862:)
3853:(
3685:)
3681:/
3677:(
3643:)
3639:(
3631:)
3627:(
3619:)
3615:(
3594:)
3570:(
3561:)
3537:(
3529:)
3507:(
3499:)
3495:(
3487:)
3483:(
3475:)
3471:(
3434:)
3410:(
3399:)
3395:(
3367:)
3348:(
3314:/
3295:)
3276:(
3257:)
3243:(
3220:)
3216:(
3128::
3119:)
3110:(
3105::
3081:)
3077:(
3062::
3043:)
3039:(
3028:)
3019:(
3004::
2985:)
2981:(
2945:)
2931:(
2923:)
2892:)
2888:(
2874:(
2860:+
2849:)
2840:(
2819:)
2765:(
2753:I
2745:)
2741:(
2725:)
2721:(
2700:)
2691:(
2681:)
2677:(
2667:)
2660:(
2646:(
2617:(
2596:)
2587:(
2569:)
2555:(
2550:/
2541:)
2537:(
2516:)
2507:(
2499:)
2480:(
2460:)
2456:(
2427:)
2423:(
2415:)
2386:(
2367:)
2343:(
2321:)
2317:(
2312:/
2303:)
2299:(
2284:e
2277:t
2270:v
2222:.
2196:.
2164:.
2142:.
2122:8
2075:.
2064:.
2012:.
2001:.
1967:.
1947::
1924:.
1904::
1881:)
1867:.
1855::
1831:.
1821:9
1804:)
1790:.
1768::
1741:)
1727:.
1703::
1695::
1689:8
1668:.
1654::
1627:)
1613:.
1599::
1572:.
1558::
1531:.
1517::
1490:)
1476:.
1464::
1458:9
1440:)
1426:.
1404::
1377:.
1345:)
1331:.
1309::
1282:.
1262::
1239:.
1215::
1185:.
1145::
1123:.
1087:.
611:)
588:4
585:O
582:4
579:N
576:B
570:H
564:C
510:)
506:(
494:)
243::
231::
218::
146::
73:/
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.