469:
591:
InChI=1S/C59H89N19O13S/c60-37(14-5-19-67-57(61)62)48(82)72-38(15-6-20-68-58(63)64)52(86)75-22-8-18-43(75)54(88)77-30-35(80)26-44(77)50(84)70-28-47(81)71-40(27-36-13-9-23-92-36)49(83)74-41(31-79)53(87)76-29-34-12-2-1-10-32(34)24-46(76)55(89)78-42-17-4-3-11-33(42)25-45(78)51(85)73-39(56(90)91)16-7-21-69-59(65)66/h1-2,9-10,12-13,23,33,35,37-46,79-80H,3-8,11,14-22,24-31,60H2,(H,70,84)(H,71,81)(H,72,82)(H,73,85)(H,74,83)(H,90,91)(H4,61,62,67)(H4,63,64,68)(H4,65,66,69)/t33-,35+,37+,38-,39-,40-,41-,42-,43-,44-,45-,46+/m0/s1
549:
31:
1241:
725:
to market
Firazyr in the European Union's 27 member states, as well as Switzerland, Liechtenstein and Iceland, making it the first product to be approved in all EU countries for the treatment of hereditary angioedema. In the US, the drug was granted FDA approval in August 2011.
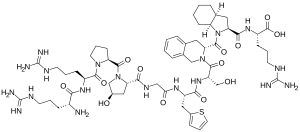
249:)-2-amino]-5-(diaminomethylideneamino)pentanoyl]pyrrolidine-2-carbonyl]-4-hydroxypyrrolidine-2-carbonyl]amino]acetyl]amino]-3-thiophen-2-ylpropanoyl]amino]-3-hydroxypropanoyl]-3,4-dihydro-1
194:
1081:
1276:
861:
149:
1033:
571:
C1CC2(C1)C(N2C(=O)3CC4=CC=CC=C4CN3C(=O)(CO)NC(=O)(CC5=CC=CS5)NC(=O)CNC(=O)6C(CN6C(=O)7CCCN7C(=O)(CCCN=C(N)N)NC(=O)(CCCN=C(N)N)N)O)C(=O)N(CCCN=C(N)N)C(=O)O
607:
673:
Bradykinin is a peptide-based hormone that is formed locally in tissues, very often in response to a trauma. It increases vessel permeability,
61:
785:
563:
1026:
800:
928:
1019:
253:-isoquinoline-3-carbonyl]-2,3,3a,4,5,6,7,7a-octahydroindole-2-carbonyl]amino]-5-(diaminomethylideneamino)pentanoic acid
1261:
614:
583:
98:
693:
receptors. Icatibant acts as a bradykinin inhibitor by blocking the binding of native bradykinin to the bradykinin B
237:
179:
79:
1266:
316:
889:"Randomized Trial of Icatibant for Angiotensin-Converting Enzyme Inhibitor-Induced Upper Airway Angioedema"
367:
437:
786:
https://www.tga.gov.au/resources/prescription-medicines-registrations/icatibant-wkt-wockhardt-bio-pty-ltd
448:
887:
Sinert R, Levy P, Bernstein JA, Body R, Sivilotti ML, Moellman J, et al. (September–October 2017).
1231:
1192:
718:
status in
Australia, the EU, Switzerland, and the US for the treatment of hereditary angioedema (HAE).
994:"FDA Approves Shire's Firazyr (icatibant injection) for Acute Attacks of Hereditary Angioedema (HAE)"
834:
464:
1187:
1177:
135:
43:
658:
142:
862:"Jerini Receives European Commission Approval for Firazyr (Icatibant) in the Treatment of HAE"
417:
356:
1271:
1108:
922:
632:
307:
829:
271:
376:
993:
8:
1159:
262:
71:
468:
1072:
970:
943:
768:
678:
109:
975:
910:
760:
756:
207:
772:
296:
1164:
1011:
965:
955:
944:"Management of acute attacks of hereditary angioedema: potential role of icatibant"
900:
752:
485:
162:
1245:
689:, overheating and pain. These symptoms are mediated by activation of bradykinin B
681:
to contract. Bradykinin plays an important role as the mediator of pain. Surplus
905:
888:
647:
1255:
1182:
1133:
1092:
1087:
640:
697:
receptor. Little is known about the effects of icatibant on the bradykinin B
639:
deficiency. It is not effective in angioedema caused by medication from the
1168:
1128:
1123:
1114:
1077:
1067:
1053:
1043:
979:
914:
764:
685:
is responsible for the typical symptoms of inflammation, such as swelling,
674:
636:
157:
22:
1148:
1138:
715:
336:
960:
682:
655:
651:
521:
347:
721:
In the EU, the approval by the
European Commission (July 2008) allows
1202:
1197:
1062:
722:
282:
65:
631:, is a medication for the symptomatic treatment of acute attacks of
686:
397:
327:
93:
548:
1097:
865:
428:
1218:
539:
408:
453:
30:
893:
The
Journal of Allergy and Clinical Immunology. In Practice
387:
886:
1229:
1041:
795:
793:
315:
654:, which is a selective and specific antagonist of
1277:Drugs developed by Takeda Pharmaceutical Company
1253:
801:"Firazyr- icatibant acetate injection, solution"
790:
295:
270:
824:
822:
1027:
856:
854:
97:
819:
221:In general: ℞ (Prescription only)
1034:
1020:
467:
355:
969:
959:
941:
904:
851:
375:
463:
335:
1254:
704:
668:
213:
1015:
743:"Icatibant: HOE 140, JE 049, JE049".
436:
416:
201:
88:
70:
161:
948:Vascular Health and Risk Management
396:
286:
13:
927:: CS1 maint: overridden setting (
14:
1288:
188:
120:
1239:
757:10.2165/00126839-200405060-00006
502:
496:
29:
942:Longhurst HJ (September 2010).
709:
596:Key:QURWXBZNHXJZBE-SKXRKSCCSA-N
986:
935:
880:
779:
736:
514:
508:
490:
1:
729:
1042:Other hematological agents (
627:, sold under the brand name
7:
10:
1293:
1193:Lovotibeglogene autotemcel
906:10.1016/j.jaip.2017.03.003
480:Chemical and physical data
1211:
1157:
1106:
1051:
835:European Medicines Agency
604:
579:
559:
537:
520:
484:
479:
447:
427:
407:
386:
366:
346:
326:
306:
281:
261:
233:
228:
178:
173:
148:
134:
108:
78:
60:
52:
42:
37:
28:
1262:Anti-inflammatory agents
1188:Exagamglogene autotemcel
1178:Betibeglogene autotemcel
996:(Press release). Shire
675:dilates blood vessels
637:C1-esterase-inhibitor
635:(HAE) in adults with
633:hereditary angioedema
1267:Peptide therapeutics
1160:sickle cell disease
1082:+desoxyribonuclease
899:(5): 1402–1409.e3.
839:. 17 September 2018
714:Icatibant received
705:Society and culture
679:smooth muscle cells
669:Mechanism of action
197:(Prescription only)
25:
16:Pharmaceutical drug
1073:Desoxyribonuclease
961:10.2147/vhrm.s4332
807:. 16 December 2019
751:(6): 343–8. 2004.
650:consisting of ten
21:
1227:
1226:
864:(Press release).
622:
621:
550:Interactive image
449:CompTox Dashboard
217:
205:
192:
124:
91:
1284:
1244:
1243:
1242:
1235:
1165:beta thalassemia
1036:
1029:
1022:
1013:
1012:
1006:
1005:
1003:
1001:
990:
984:
983:
973:
963:
939:
933:
932:
926:
918:
908:
884:
878:
877:
875:
873:
858:
849:
848:
846:
844:
826:
817:
816:
814:
812:
797:
788:
783:
777:
776:
745:Drugs in R&D
740:
618:
617:
610:
552:
532:
530:
516:
510:
504:
498:
492:
472:
471:
457:
455:
440:
420:
400:
379:
359:
339:
319:
299:
289:
288:
274:
215:
212:
203:
200:
190:
187:
165:
122:
119:
101:
90:
87:
74:
33:
26:
24:
20:
1292:
1291:
1287:
1286:
1285:
1283:
1282:
1281:
1252:
1251:
1250:
1240:
1238:
1230:
1228:
1223:
1207:
1162:
1153:
1110:
1102:
1047:
1040:
1010:
1009:
999:
997:
992:
991:
987:
940:
936:
920:
919:
885:
881:
871:
869:
860:
859:
852:
842:
840:
828:
827:
820:
810:
808:
799:
798:
791:
784:
780:
742:
741:
737:
732:
712:
707:
700:
696:
692:
671:
662:
613:
611:
608:(what is this?)
605:
600:
597:
592:
587:
586:
575:
572:
567:
566:
555:
528:
526:
513:
507:
501:
495:
475:
451:
443:
423:
403:
382:
362:
342:
322:
302:
285:
277:
257:
254:
241:
240:
224:
169:
137:
130:
111:
104:
56:Hoe 140, JE 049
17:
12:
11:
5:
1290:
1280:
1279:
1274:
1269:
1264:
1249:
1248:
1225:
1224:
1222:
1221:
1215:
1213:
1209:
1208:
1206:
1205:
1200:
1195:
1190:
1185:
1180:
1174:
1172:
1158:Drugs used in
1155:
1154:
1152:
1151:
1146:
1141:
1136:
1131:
1126:
1120:
1118:
1107:Drugs used in
1104:
1103:
1101:
1100:
1095:
1090:
1085:
1075:
1070:
1065:
1059:
1057:
1049:
1048:
1039:
1038:
1031:
1024:
1016:
1008:
1007:
985:
934:
879:
868:. 15 July 2008
850:
830:"Firazyr EPAR"
818:
789:
778:
734:
733:
731:
728:
711:
708:
706:
703:
698:
694:
690:
670:
667:
660:
648:peptidomimetic
620:
619:
602:
601:
599:
598:
595:
593:
590:
582:
581:
580:
577:
576:
574:
573:
570:
562:
561:
560:
557:
556:
554:
553:
545:
543:
535:
534:
524:
518:
517:
511:
505:
499:
493:
488:
482:
481:
477:
476:
474:
473:
465:DTXSID20903963
460:
458:
445:
444:
442:
441:
433:
431:
425:
424:
422:
421:
413:
411:
405:
404:
402:
401:
395:as salt:
392:
390:
384:
383:
381:
380:
372:
370:
364:
363:
361:
360:
352:
350:
344:
343:
341:
340:
332:
330:
324:
323:
321:
320:
312:
310:
304:
303:
301:
300:
292:
290:
279:
278:
276:
275:
267:
265:
259:
258:
256:
255:
244:
236:
235:
234:
231:
230:
226:
225:
223:
222:
219:
210:
198:
184:
182:
176:
175:
171:
170:
168:
167:
154:
152:
146:
145:
140:
138:administration
132:
131:
129:
128:
126:
116:
114:
106:
105:
103:
102:
84:
82:
76:
75:
68:
58:
57:
54:
50:
49:
46:
40:
39:
35:
34:
15:
9:
6:
4:
3:
2:
1289:
1278:
1275:
1273:
1270:
1268:
1265:
1263:
1260:
1259:
1257:
1247:
1237:
1236:
1233:
1220:
1217:
1216:
1214:
1210:
1204:
1201:
1199:
1196:
1194:
1191:
1189:
1186:
1184:
1183:Crizanlizumab
1181:
1179:
1176:
1175:
1173:
1170:
1166:
1161:
1156:
1150:
1147:
1145:
1142:
1140:
1137:
1135:
1134:Conestat alfa
1132:
1130:
1127:
1125:
1122:
1121:
1119:
1116:
1112:
1105:
1099:
1096:
1094:
1093:Streptokinase
1091:
1089:
1088:Hyaluronidase
1086:
1083:
1079:
1076:
1074:
1071:
1069:
1066:
1064:
1061:
1060:
1058:
1055:
1050:
1045:
1037:
1032:
1030:
1025:
1023:
1018:
1017:
1014:
995:
989:
981:
977:
972:
967:
962:
957:
953:
949:
945:
938:
930:
924:
916:
912:
907:
902:
898:
894:
890:
883:
867:
863:
857:
855:
838:
836:
831:
825:
823:
806:
802:
796:
794:
787:
782:
774:
770:
766:
762:
758:
754:
750:
746:
739:
735:
727:
724:
719:
717:
702:
688:
684:
680:
676:
666:
664:
657:
653:
649:
644:
642:
641:ACE inhibitor
638:
634:
630:
626:
616:
609:
603:
594:
589:
588:
585:
578:
569:
568:
565:
558:
551:
547:
546:
544:
541:
536:
525:
523:
519:
489:
487:
483:
478:
470:
466:
462:
461:
459:
450:
446:
439:
438:ChEMBL1743581
435:
434:
432:
430:
426:
419:
415:
414:
412:
410:
406:
399:
394:
393:
391:
389:
385:
378:
374:
373:
371:
369:
365:
358:
354:
353:
351:
349:
345:
338:
334:
333:
331:
329:
325:
318:
314:
313:
311:
309:
305:
298:
294:
293:
291:
284:
280:
273:
269:
268:
266:
264:
260:
252:
248:
243:
242:
239:
232:
227:
220:
218: Rx-only
211:
209:
199:
196:
186:
185:
183:
181:
177:
172:
164:
159:
156:
155:
153:
151:
147:
144:
141:
139:
133:
127:
118:
117:
115:
113:
107:
100:
95:
86:
85:
83:
81:
77:
73:
69:
67:
63:
59:
55:
51:
47:
45:
41:
38:Clinical data
36:
32:
27:
19:
1272:Orphan drugs
1143:
1129:C1-inhibitor
1124:Berotralstat
1078:Fibrinolysin
1068:Chymotrypsin
998:. Retrieved
988:
951:
947:
937:
923:cite journal
896:
892:
882:
870:. Retrieved
841:. Retrieved
833:
809:. Retrieved
804:
781:
748:
744:
738:
720:
713:
710:Legal status
672:
645:
628:
624:
623:
612:
606:
250:
246:
180:Legal status
174:Legal status
143:Subcutaneous
80:License data
18:
1149:Lanadelumab
1139:Ecallantide
954:: 795–802.
716:orphan drug
677:and causes
652:amino acids
533: g·mol
418:CHEBI:68556
272:130308-48-4
229:Identifiers
53:Other names
44:Trade names
1256:Categories
1111:angioedema
1109:hereditary
730:References
701:receptor.
683:bradykinin
656:bradykinin
538:3D model (
522:Molar mass
377:7PG89G35Q7
348:ChemSpider
308:IUPHAR/BPS
263:CAS Number
238:IUPAC name
1203:Voxelotor
1198:Mitapivat
1144:Icatibant
1063:Bromelain
1052:Enzymes (
1000:28 August
866:Jerini AG
663:receptors
625:Icatibant
136:Routes of
110:Pregnancy
99:Icatibant
72:Monograph
66:Drugs.com
23:Icatibant
1246:Medicine
980:20859548
915:28552382
843:17 April
811:17 April
805:DailyMed
773:25491021
765:15563238
646:It is a
615:(verify)
357:16736634
328:DrugBank
150:ATC code
112:category
94:DailyMed
1098:Trypsin
971:2941790
872:22 July
687:redness
643:class.
629:Firazyr
486:Formula
337:DB06196
297:6918173
283:PubChem
166:)
160: (
158:B06AC02
125: C
96::
48:Firazyr
1232:Portal
1212:Others
978:
968:
913:
771:
763:
723:Jerini
564:SMILES
429:ChEMBL
398:D04492
208:℞-only
206:
193:
92:
1219:Hemin
1169:B06AX
1115:B06AC
1054:B06AA
837:(EMA)
769:S2CID
584:InChI
540:JSmol
409:ChEBI
1163:and
1002:2011
976:PMID
929:link
911:PMID
874:2008
845:2020
813:2020
761:PMID
388:KEGG
368:UNII
62:AHFS
1044:B06
966:PMC
956:doi
901:doi
753:doi
531:.54
529:304
454:EPA
317:667
287:CID
163:WHO
1258::
974:.
964:.
950:.
946:.
925:}}
921:{{
909:.
895:.
891:.
853:^
832:.
821:^
803:.
792:^
767:.
759:.
747:.
665:.
512:13
506:19
500:89
494:59
245:(2
214:EU
202:US
195:S4
189:AU
121:AU
89:US
1234::
1171:)
1167:(
1117:)
1113:(
1084:)
1080:(
1056:)
1046:)
1035:e
1028:t
1021:v
1004:.
982:.
958::
952:6
931:)
917:.
903::
897:5
876:.
847:.
815:.
775:.
755::
749:5
699:1
695:2
691:2
661:2
659:B
542:)
527:1
515:S
509:O
503:N
497:H
491:C
456:)
452:(
251:H
247:S
216::
204::
191::
123::
64:/
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.