204:
190:
198:
perform intramolecular nucleophilic attack and cyclize. This reaction is a useful strategy for heterocycle formation. In the example below, Parham cyclization was used to in the cyclization of an isocyanate to form isoindolinone, which was then converted to a nitrone. The nitrone species further reacts with radicals and can be used as "spin traps" to study biological radical processes.
71:
Exchange rates usually follow the trend I > Br > Cl. Alkyl- and arylfluoride are generally unreactive toward organolithium reagents. Lithium–halogen exchange is kinetically controlled, and the rate of exchange is primarily influenced by the stabilities of the carbanion intermediates (sp > sp
24:
is a fundamental reaction that converts an organic halide into an organometallic product. The reaction commonly involves the use of electropositive metals (Li, Na, Mg) and organochlorides, bromides, and iodides. Particularly well-developed is the use of metal–halogen exchange for the preparation of
197:
Lithium–halogen exchange is a crucial part of Parham cyclization. In this reaction, an aryl halide (usually iodide or bromide) exchanges with organolithium to form a lithiated arene species. If the arene bears a side chain with an electrophillic moiety, the carbanion attached to the lithium will
67:
Lithium–halogen exchange is frequently used to prepare vinyl-, aryl- and primary alkyllithium reagents. Vinyl halides usually undergo lithium–halogen exchange with retention of the stereochemistry of the double bond. The presence of alkoxyl or related chelating groups accelerates lithium–halogen
93:
Li. A number of kinetic studies also support a nucleophilic pathway in which the carbanion on the lithium species attacks the halogen atom on the aryl halide. Another proposed mechanism involves single electron transfer with the generation of radicals. In reactions of secondary and tertiary
80:
Two mechanisms have been proposed for lithium–halogen exchange. One proposed pathway involves a nucleophilic mechanism that generates a reversible "ate-complex" intermediate. Farnham and
Calabrese crystallized an "ate-complex" lithium bis(pentafluorophenyl) iodinate complexed with
37:
Two kinds of lithium–halogen exchange can be considered: reactions involving organolithium compounds and reactions involving lithium metal. Commercial organolithium compounds are produced by the heterogeneous (slurry) reaction of lithium with organic bromides and chlorides:
181:
is used to perform lithium–halogen exchange with bromide. The nucleophilic carbanion center quickly undergoes carbolithiation to the double bond, generating an anion stabilized by the adjacent sulfone group. An intramolecular
516:
Knochel, P.; Dohle, W.; Gommermann, N.; Kneisel, F. F.; Kopp, F.; Korn, T.; Sapountzis, I.; Vu, V. A. (2003). "Highly
Functionalized Organomagnesium Reagents Prepared through Halogen–Metal Exchange".
114:
can be prepared by treating a preformed
Grignard reagent with an organic halide. This method offers the advantage that the Mg transfer tolerates many functional groups. A typical reaction involves
637:
Adam P. Smith; Scott A. Savage; J. Christopher Love; Cassandra L. Fraser (2002). "Synthesis of 4-, 5-, and 6-methyl-2,2'-bipyridine by a
Negishi Cross-coupling Strategy: 5-methyl-2,2'-bipyridine".
68:
exchange. Lithium halogen exchange is typically a fast reaction. It is usually faster than nucleophilic addition and can sometimes exceed the rate of proton transfer.
718:
Sotomayor, N.; Lete, E. (2003). "Aryl and
Heteroaryllithium Compounds by Metal–Halogen Exchange. Synthesis of Carbocyclic and Heterocyclic Systems".
320:
691:
Parham, W. P.; Bradsher, C. K. (1982). "Aromatic organolithium reagents bearing electrophilic groups. Preparation by halogen–lithium exchange".
226:
Gilman, Henry; Langham, Wright; Jacoby, Arthur L. (1939). "Metalation as a Side
Reaction in the Preparation of Organolithium Compounds".
189:
393:
Bailey, W. F.; Patricia, J. F. (1988). "The mechanism of the lithium–halogen
Interchange reaction: a review of the literature".
98:. The mechanistic studies of lithium–halogen exchange are complicated by the formation of aggregates of organolithium species.
458:
Rogers, H. R.; Houk, J. (1982). "Preliminary studies of the mechanism of metal-halogen exchange. The kinetics of reaction of
374:
304:
203:
261:
Seebach, D.; Neumann H. (1976). "Stereospecific preparation of terminal vinyllithium derivatives by Br/Li-exchange with
489:
Fischer, H. (1969). "Electron spin resonance of transient alkyl radicals during alkyllithium-alkyl halide reactions".
799:
664:
Toth, J. E.; Hamann, P. R.; Fuchs, P. L. (1988). "Studies culminating in the total synthesis of (dl)-morphine".
115:
49:
Most of this article is about the homogeneous (one-phase) reaction of preformed organolithium compounds:
60:
is commonly used. Gilman and Wittig independently discovered this method in the late 1930s. It is not a
82:
61:
17:
636:
85:. The "ate-complex" further reacts with electrophiles and provides pentafluorophenyl iodide and C
26:
8:
423:
Farnham, W. B.; Calabrese, J. C. (1986). "Novel hypervalent (10-I-2) iodine structures".
771:
746:
614:
589:
338:-butyllithium and 1-iodo-5-hexenes provides no evidence for single-electron transfer".
314:
747:"Synthesis of a mitochondria-targeted spin trap using a novel Parham-type cyclization"
351:
278:
776:
619:
533:
440:
406:
370:
300:
243:
766:
758:
727:
700:
673:
646:
609:
601:
568:
557:-Butyl-3-Bromo-5-Formylbenzoate Through Selective Metal-Halogen Exchange Reactions"
525:
498:
471:
432:
402:
347:
274:
235:
111:
95:
365:
Carey, Francis A. (2007). "Organometallic compounds of Group I and II metals".
175:
762:
793:
731:
650:
573:
552:
247:
174:
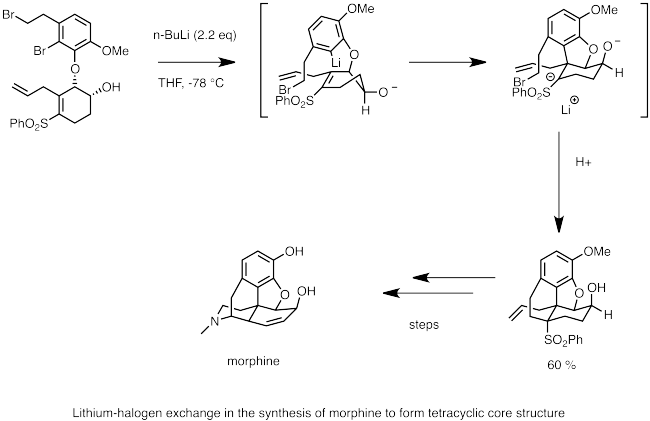
Below lithium–halogen exchange is a step in the synthesis of morphine. Here
780:
623:
605:
537:
529:
444:
294:
57:
134:
704:
677:
502:
475:
436:
239:
334:
Bailey, W. F.; et al. (1986). "Metal—halogen interchange between
663:
462:-butyllithium with substituted bromobenzenes in hexane solution".
94:
alkyllithium and alkyl halides, radical species were detected by
186:
2 reaction by the anion forms the cyclic backbone of morphine.
422:
515:
367:
Advanced
Organic Chemistry: Reaction and Synthesis Pt. B
690:
392:
297:
The
Preparation of Organolithium Reagents and Intermediates
551:
Arredondo, Juan D.; Li, Hongmei; Balsells, Jaume (2012).
46:
Often the lithium halide remains in the soluble product.
590:"Recent Advances of the Halogen–Zinc Exchange Reaction"
717:
457:
295:
Leroux F., Schlosser M., Zohar E., Marek I. (2004).
193:
Synthesis of morphine using lithium–halogen exchange
171:
Several examples can be found in organic syntheses.
550:
225:
711:
587:
260:
791:
219:
388:
386:
482:
418:
416:
319:: CS1 maint: multiple names: authors list (
657:
488:
383:
32:
588:Balkenhohl, Moritz; Knochel, Paul (2020).
770:
613:
572:
413:
744:
738:
290:
288:
228:Journal of the American Chemical Society
72:> sp) of the organolithium reagents.
581:
518:Angewandte Chemie International Edition
254:
792:
684:
333:
75:
451:
364:
327:
285:
358:
13:
202:
188:
14:
811:
630:
544:
509:
166:
101:
745:Quin, C.; et al. (2009).
594:Chemistry – A European Journal
207:Parham cyclization in MitoSpin
1:
369:(Kindle ed.). Springer.
352:10.1016/s0040-4039(00)84395-6
279:10.1016/s0040-4039(00)78926-x
213:
118:and aryl bromide or iodides:
407:10.1016/0022-328X(88)83017-1
7:
116:isopropylmagnesium chloride
10:
816:
107:Magnesium–halogen exchange
64:, as no salt is produced.
763:10.1016/j.tet.2009.07.081
53:R−Li + R′−X → R−X + R′−Li
800:Organometallic chemistry
732:10.2174/1385272033372987
651:10.15227/orgsyn.078.0051
574:10.15227/orgsyn.089.0460
62:salt metathesis reaction
33:Lithium–halogen exchange
18:organometallic chemistry
154:Zinc–halogen exchange:
137:metalate aryl halides:
42:2 Li + R−X → LiX + R−Li
27:organolithium compounds
606:10.1002/chem.201904794
530:10.1002/anie.200300579
208:
194:
141:ArBr + Li → ArMgBu
22:metal–halogen exchange
206:
192:
150:Zinc–halogen exchange
125:-PrMgCl + ArCl →
705:10.1021/ar00082a001
678:10.1021/jo00255a008
503:10.1021/j100845a044
476:10.1021/ja00366a024
437:10.1021/ja00269a055
299:. New York: Wiley.
240:10.1021/ja01870a036
162:Zn + R−I → Li + BuI
76:Mechanism and scope
395:J. Organomet. Chem
209:
195:
757:(39): 8154–8160.
672:(20): 4694–4708.
600:(17): 3688–3697.
561:Organic Syntheses
524:(36): 4302–4320.
497:(11): 3834–3838.
376:978-0-387-44899-2
346:(17): 1861–1864.
306:978-0-470-84339-0
273:(52): 4839–4842.
112:Grignard reagents
807:
785:
784:
774:
742:
736:
735:
715:
709:
708:
688:
682:
681:
661:
655:
654:
634:
628:
627:
617:
585:
579:
578:
576:
553:"Preparation of
548:
542:
541:
513:
507:
506:
486:
480:
479:
464:J. Am. Chem. Soc
455:
449:
448:
431:(9): 2449–2451.
425:J. Am. Chem. Soc
420:
411:
410:
390:
381:
380:
362:
356:
355:
340:Tetrahedron Lett
331:
325:
324:
318:
310:
292:
283:
282:
267:Tetrahedron Lett
265:-butyllithium".
258:
252:
251:
223:
129:-PrCl + ArMgCl
96:EPR spectroscopy
815:
814:
810:
809:
808:
806:
805:
804:
790:
789:
788:
743:
739:
720:Curr. Org. Chem
716:
712:
699:(10): 300–305.
689:
685:
662:
658:
635:
631:
586:
582:
549:
545:
514:
510:
487:
483:
456:
452:
421:
414:
391:
384:
377:
363:
359:
332:
328:
312:
311:
307:
293:
286:
259:
255:
224:
220:
216:
185:
169:
161:
144:
104:
92:
88:
78:
35:
12:
11:
5:
813:
803:
802:
787:
786:
737:
726:(3): 275–300.
710:
693:Acc. Chem. Res
683:
656:
629:
580:
543:
508:
481:
470:(2): 522–525.
450:
412:
382:
375:
357:
326:
305:
284:
253:
234:(1): 106–109.
217:
215:
212:
211:
210:
183:
168:
165:
164:
163:
159:
152:
151:
147:
146:
142:
131:
130:
109:
108:
103:
100:
90:
86:
77:
74:
55:
54:
44:
43:
34:
31:
9:
6:
4:
3:
2:
812:
801:
798:
797:
795:
782:
778:
773:
768:
764:
760:
756:
752:
748:
741:
733:
729:
725:
721:
714:
706:
702:
698:
694:
687:
679:
675:
671:
667:
660:
652:
648:
644:
640:
633:
625:
621:
616:
611:
607:
603:
599:
595:
591:
584:
575:
570:
566:
562:
558:
556:
547:
539:
535:
531:
527:
523:
519:
512:
504:
500:
496:
492:
491:J. Phys. Chem
485:
477:
473:
469:
465:
461:
454:
446:
442:
438:
434:
430:
426:
419:
417:
408:
404:
401:(1–2): 1–46.
400:
396:
389:
387:
378:
372:
368:
361:
353:
349:
345:
341:
337:
330:
322:
316:
308:
302:
298:
291:
289:
280:
276:
272:
268:
264:
257:
249:
245:
241:
237:
233:
229:
222:
218:
205:
201:
200:
199:
191:
187:
180:
179:-butyllithium
178:
172:
157:
156:
155:
149:
148:
140:
139:
138:
136:
135:ate complexes
128:
124:
121:
120:
119:
117:
113:
106:
105:
99:
97:
84:
73:
69:
65:
63:
59:
52:
51:
50:
47:
41:
40:
39:
30:
28:
23:
19:
754:
750:
740:
723:
719:
713:
696:
692:
686:
669:
666:J. Org. Chem
665:
659:
642:
638:
632:
597:
593:
583:
564:
560:
554:
546:
521:
517:
511:
494:
490:
484:
467:
463:
459:
453:
428:
424:
398:
394:
366:
360:
343:
339:
335:
329:
296:
270:
266:
262:
256:
231:
227:
221:
196:
176:
173:
170:
167:Applications
153:
132:
126:
122:
110:
102:Other metals
79:
70:
66:
58:Butyllithium
56:
48:
45:
36:
21:
15:
751:Tetrahedron
639:Org. Synth
214:References
133:Magnesium
315:cite book
248:0002-7863
794:Category
781:19888470
624:31742792
538:14502700
445:22175602
772:2767131
615:7155102
567:: 460.
145:+ BuBr
779:
769:
645:: 51.
622:
612:
536:
443:
373:
303:
246:
83:TMEDA
777:PMID
620:PMID
534:PMID
441:PMID
371:ISBN
321:link
301:ISBN
244:ISSN
158:LiBu
767:PMC
759:doi
728:doi
701:doi
674:doi
647:doi
610:PMC
602:doi
569:doi
526:doi
499:doi
472:doi
468:104
433:doi
429:108
403:doi
399:352
348:doi
275:doi
236:doi
16:In
796::
775:.
765:.
755:65
753:.
749:.
722:.
697:15
695:.
670:53
668:.
643:78
641:.
618:.
608:.
598:26
596:.
592:.
565:89
563:.
559:.
532:.
522:42
520:.
495:73
493:.
466:.
439:.
427:.
415:^
397:.
385:^
344:27
342:.
317:}}
313:{{
287:^
271:17
269:.
242:.
232:61
230:.
29:.
20:,
783:.
761::
734:.
730::
724:7
707:.
703::
680:.
676::
653:.
649::
626:.
604::
577:.
571::
555:t
540:.
528::
505:.
501::
478:.
474::
460:n
447:.
435::
409:.
405::
379:.
354:.
350::
336:t
323:)
309:.
281:.
277::
263:t
250:.
238::
184:N
182:S
177:n
160:3
143:2
127:i
123:i
91:5
89:H
87:6
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.