121:
90:
157:. Previously it was discovered that the hydroxylation of butter yellow or its demethylated derivatives exist within rats, and thus rats were deemed suitable for the comparison. Absorption spectra were examined to confirm the results of the experiment, which found that the product of the Milas hydroxylation was one of the obtained products.
132:
One variation of the Milas hydroxylation (shown in the mechanism above) requires stoichiometric amounts of osmium tetroxide, which is toxic (highly volatile) and expensive. Furthermore, in Milas's own experiments his yields ranged from 37.6% to 60.2% for the cis-vicinal diol. Note that a vicinal diol
148:
In 1949, the Milas hydroxylation was applied to a study of the demethylation of N-Dimethyl-p-Amino-azobenzene, otherwise known as butter yellow. Hydrogen peroxide in tertiary butyl alcohol with osmium tetroxide as a catalyst (Milas reagents) was examined to determine the parallels of the reaction
136:
The catalyst, osmium tetroxide, also known as Merck osmic acid, dissolves readily in tertiary butyl alcohol which implies that the solution in which the reaction occurs is stable, unless isobutylene is already present. In the presence of isobutylene most of the osmium tetroxide is reduced to an
137:
insoluble black colloidal oxide. This colloidal oxide is a very active catalyst in decomposition of hydrogen peroxide. Thus, in aqueous solutions osmium tetroxide decomposes hydrogen peroxide, whereas an anhydrous tertiary butyl alcohol decomposes at a much slower rate.
112:
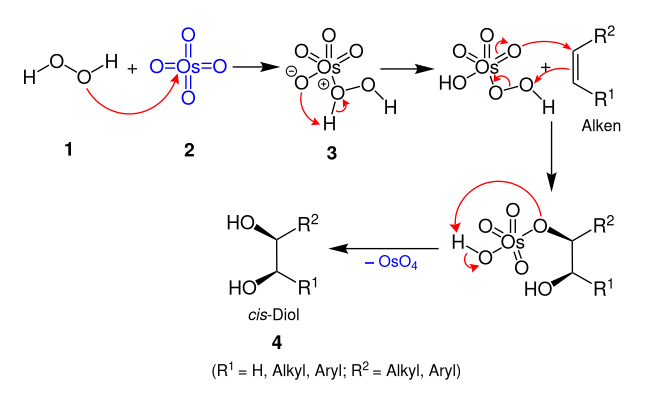
The proposed mechanism for the Milas hydroxylation involves the initial combination of hydrogen peroxide and the osmium tetroxide catalyst to form an intermediate, which then adds to the alkene, followed by a cleavage that forms the product and regenerates the
246:
Milas, Nicholas A.; Trepagnier, Joseph H.; Nolan, John T.; Iliopulos, Miltiadis I. (September 1959). "A Study of the
Hydroxylation of Olefins and the Reaction of Osmium Tetroxide with 1,2-Glycols".
302:"An Osmium(III)/Osmium(V) Redox Couple Generating OsV(O)(OH) Center for cis-1,2-Dihydroxylation of Alkenes with H2O2: Os Complex with a Nitrogen-Based Tetradentate Ligand"
273:
Milas, Nicholas A. (November 1937). "The
Hydroxylation of Unsaturated Substances. III. The Use of Vanadium Pentoxide and Chromium Trioxide as Catalysts of Hydroxylation".
133:
is a molecule in which two hydroxy atoms are located on adjacent carbon atoms. Vicinal diols can be oxidized to aldehydes and ketones, rendering their synthesis useful.
140:
The Upjohn dihydroxylation and the
Sharpless asymmetric dihydroxylation both result in cis-vicinal diols as well, and do not require the toxic, expensive catalyst.
67:
352:
478:
101:
230:
375:"An improved catalytic OsO4 oxidation of olefins to cis-1,2-glycols using tertiary amine oxides as the oxidant"
473:
340:
374:
483:
414:"Demethylation of N : N-Dimethyl-p-Amino-azobenzene (Butter Yellow) with Hydrogen Peroxide"
339:
Ouellette, Robert J.; Rawn, J. David (2018-01-01), Ouellette, Robert J.; Rawn, J. David (eds.),
97:
300:
Hideki
Sugimoto, Sugimoto; Kazuhiro Kitayama, Kitayama; Seiji, Mori; Shinobu, Itoh (2012).
8:
179:
Milas, Nicholas A.; Sussman, Sidney (July 1936). "The
Hydroxylation of the Double Bond".
60:
449:
79:
390:
441:
433:
394:
348:
321:
226:
83:
71:
35:
453:
425:
386:
313:
282:
255:
222:
218:
188:
75:
52:
467:
437:
398:
301:
299:
120:
445:
413:
325:
286:
259:
192:
317:
89:
429:
70:
in the 1930s. The cis-diol is formed by reaction of alkenes with
96:
The reaction has been superseded in synthetic chemistry by the
56:
245:
63:
373:
VanRheenen, V.; Kelly, R. C.; Cha, D. Y. (1976-06-01).
372:
211:Comprehensive Organic Name Reactions and Reagents
465:
204:
202:
338:
199:
178:
74:and either ultraviolet light or a catalytic
411:
341:"16 - Alcohols: Reactions and Synthesis"
275:Journal of the American Chemical Society
248:Journal of the American Chemical Society
181:Journal of the American Chemical Society
119:
466:
272:
347:, Academic Press, pp. 463–505,
174:
172:
170:
102:Sharpless asymmetric dihydroxylation
13:
345:Organic Chemistry (Second Edition)
88:
14:
495:
167:
143:
405:
366:
332:
293:
266:
239:
223:10.1002/9780470638859.conrr437
127:
1:
391:10.1016/S0040-4039(00)78093-2
160:
107:
7:
479:Organic oxidation reactions
412:Anderson, Wm (March 1949).
10:
500:
41:
18:
209:"Milas Hydroxylation".
66:, and was developed by
124:
98:Upjohn dihydroxylation
93:
123:
92:
19:Milas hydroxylation
379:Tetrahedron Letters
312:(46): 19270–19280.
287:10.1021/ja01290a072
260:10.1021/ja01526a070
217:: 1948–1951. 2010.
193:10.1021/ja01298a065
149:with butter yellow
49:Milas hydroxylation
474:Addition reactions
125:
94:
80:vanadium pentoxide
424:(4142): 444–445.
385:(23): 1973–1976.
354:978-0-12-812838-1
318:10.1021/ja309566c
281:(11): 2342–2344.
254:(17): 4730–4733.
100:and later by the
84:chromium trioxide
72:hydrogen peroxide
68:Nicholas A. Milas
45:
44:
36:Addition reaction
491:
458:
457:
430:10.1038/163444b0
409:
403:
402:
370:
364:
363:
362:
361:
336:
330:
329:
306:ACS Publications
297:
291:
290:
270:
264:
263:
243:
237:
236:
206:
197:
196:
187:(7): 1302–1304.
176:
76:osmium tetroxide
53:organic reaction
16:
15:
499:
498:
494:
493:
492:
490:
489:
488:
464:
463:
462:
461:
410:
406:
371:
367:
359:
357:
355:
337:
333:
298:
294:
271:
267:
244:
240:
233:
208:
207:
200:
177:
168:
163:
146:
130:
116:
110:
27:Nicholas Milas
12:
11:
5:
497:
487:
486:
484:Name reactions
481:
476:
460:
459:
404:
365:
353:
331:
292:
265:
238:
231:
198:
165:
164:
162:
159:
145:
142:
129:
126:
114:
109:
106:
55:converting an
43:
42:
39:
38:
33:
32:Reaction type
29:
28:
25:
21:
20:
9:
6:
4:
3:
2:
496:
485:
482:
480:
477:
475:
472:
471:
469:
455:
451:
447:
443:
439:
435:
431:
427:
423:
419:
415:
408:
400:
396:
392:
388:
384:
380:
376:
369:
356:
350:
346:
342:
335:
327:
323:
319:
315:
311:
307:
303:
296:
288:
284:
280:
276:
269:
261:
257:
253:
249:
242:
234:
232:9780470638859
228:
224:
220:
216:
212:
205:
203:
194:
190:
186:
182:
175:
173:
171:
166:
158:
156:
152:
141:
138:
134:
122:
118:
105:
103:
99:
91:
87:
85:
81:
77:
73:
69:
65:
62:
58:
54:
50:
40:
37:
34:
31:
30:
26:
23:
22:
17:
421:
417:
407:
382:
378:
368:
358:, retrieved
344:
334:
309:
305:
295:
278:
274:
268:
251:
247:
241:
214:
210:
184:
180:
154:
150:
147:
144:Applications
139:
135:
131:
111:
95:
48:
46:
24:Named after
128:Limitations
468:Categories
360:2022-12-05
161:References
438:1476-4687
399:0040-4039
108:Mechanism
446:18115097
326:23113538
155:in vitro
454:4090289
153:versus
151:in vivo
61:vicinal
452:
444:
436:
418:Nature
397:
351:
324:
229:
57:alkene
51:is an
450:S2CID
82:, or
59:to a
442:PMID
434:ISSN
395:ISSN
349:ISBN
322:PMID
227:ISBN
64:diol
47:The
426:doi
422:163
387:doi
314:doi
310:136
283:doi
256:doi
219:doi
215:437
189:doi
113:OsO
470::
448:.
440:.
432:.
420:.
416:.
393:.
383:17
381:.
377:.
343:,
320:.
308:.
304:.
279:59
277:.
252:81
250:.
225:.
213:.
201:^
185:58
183:.
169:^
117:.
104:.
86:.
78:,
456:.
428::
401:.
389::
328:.
316::
289:.
285::
262:.
258::
235:.
221::
195:.
191::
115:4
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.