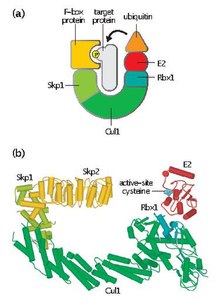
20:(a) SCF contains three core subunits—the RING protein Rbx1, the cullin Cul1, and Skp1. Rbx1 binds the E2–ubiquitin conjugate. The target protein binds to an F-box protein that is bound to the enzyme core via interactions with the Skp1 subunit. After binding of a target protein to the F-box protein, the ubiquitin is transferred from E2 and attached via a peptide bond to a lysine side chain in the target protein. (b) A composite model structure for human SCF derived from X-ray structures of human Rbx1–Cul1–Skp1–Skp2 complex and the E2 enzyme Ubc7. The target protein (not shown here) interacts with the F-box protein Skp2, which thereby positions the substrate for ubiquitination by the E2 enzyme. Ubiquitin is not shown in this model but at the start of the reaction it would be bound to the E2 enzyme at the active-site cysteine shown in blue. (Adapted from Zheng, N. et al.: Nature 2002, 416:703–709.) (PDB 1fbv, 1ldk, 1fqr)
66:(FBP) – FBP contributes to the substrate specificity of the SCF complex by first aggregating to target proteins independently of the complex. Each FBP (e.g. Skp2) may recognize several different substrates in a manner that is dependent on post-translational modifications such as phosphorylation or glycosylation. FBP then binds to Skp1 of the SCF complex using an F-box motif, bringing the target protein into proximity with the functional E2 ubiquitin-conjugating enzyme. FBP is also essential in regulating SCF activity during the course of the cell cycle. SCF levels are thought to remain constant throughout the cell-cycle. Instead, FBP affinity for protein substrates is regulated through cyclin-CDK-mediated phosphorylation of target proteins.
17:
1271:
1259:
159:
of nine possible sites are phosphorylated—and Swi5 for degradation. Since Sic1 normally prevents premature entry into S-phase by inhibiting Cyclin B-CDK1, targeting Sic1 for degradation promotes S-phase entry. Fbw7 is known to be a haplo-insufficient tumor suppressor gene implicated in several sporadic carcinomas, for which one mutant allele is enough to disturb the wild type phenotype.
101:, also known as budding yeast. Temperature-sensitive cell division cycle (Cdc) mutants—such as Cdc4, Cdc34, and Cdc53—arrested in G1 with unreplicated DNA and multiple elongated buds. The phenotype was attributed to a failure to degrade Sic1, an inhibitor of S cyclin-CDK complexes. These findings indicated that proteolysis is important in the G1/S transition.
165:
Cyclin F is an FBP that is associated with amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). Mutations that prevent phosphorylation of Cyclin F alter the activity of SCF-Cyclin F, which likely affects downstream processes pertinent to neuron degeneration in ALS and FTD. Normally,
104:
Next, biochemical studies revealed that Cdc34 is an E2 enzyme that physically interacts with an E3 ubiquitin ligase complex containing Skp1, Cdc4, and several other proteins. Skp1’s known binding partners—specifically Skp2, Cyclin F, and Cdc4—were found to share an approximately 40 residue motif that
194:
The plant hormone auxin binds Tir1 (Transport
Inhibitor Response 1). Tir1 is an Auxin Signaling F-box Protein (AFB) that acts as an auxin receptor. Auxin-bound Tir1 stimulates binding of SCF-Tir1 to the AUX/IAA repressor. Subsequent degradation of the repressor results in activation of AUX/IAA (i.e.
158:
Fbw7, which is the human homolog of cdc4 in yeast, is an FBP that targets Cyclin E, Myc, Notch and c-Jun for degradation. Fbw7 is stable throughout the cell cycle and is localized to the nucleus due to the presence of a nuclear localization sequence (NLS). SCF-Fbw7 targets Sic1—when at least six out
174:
Recently, SCF complexes have become an attractive anti-cancer target because of their upregulation in some human cancers and their biochemically distinct active sites. Though many of the aforementioned FBPs have been implicated in cancer, cytotoxicity has been a limiting factor of drug development.
120:
The eukaryotic cell cycle is regulated through the synthesis, degradation, binding interactions, post-translational modifications of regulatory proteins. Of these regulatory proteins, two ubiquitin ligases are crucial for progression through cell cycle checkpoints. The anaphase-promoting complex
150:
Skp2 is an FBP that binds CKIs such as p27 and p21. Skp2 binds p27 only when two conditions are met: p27 is phosphorylated by E/A/CKD2 and bound to Cks1. As a consequence of binding Skp2, p27 is ubiquitinated and targeted for degradation in late G1 and early S. SCF-Skp2 also targets p130 for
1215:
Devoto, Alessandra; Nieto-Rostro, Manuela; Xie, Daoxin; Ellis, Christine; Harmston, Rebecca; Patrick, Elaine; Davis, Jackie; Sherratt, Leigh; Coleman, Mark; Turner, John G. (November 2002). "COI1 links jasmonate signalling and fertility to the SCF ubiquitin-ligase complex inArabidopsis".
82:) – Cullin forms the major structural scaffold of the SCF complex and links the skp1 domain to the Rbx1 domain. Different combinations of Cullin and FBPs can generate on the order of a hundred types of E3 ubiquitin ligases that target different substrates.
154:
Beta-transducin repeat-containing protein (βTRCP) is an FBP that targets emi1—an APC/C-Cdh1 inhibitor—and wee1 for degradation during early mitosis. βTRCP recognizes these substrates after they are phosphorylated by Polo-like kinase 1 or Cyclin B-CDK1.
88:– Rbx1 contains a small, zinc-binding Really Interesting New Gene (RING) finger domain, to which the E2 ubiquitin-conjugating enzyme binds. This binding event allows the transferral of ubiquitin from E2 to a lysine residue on the target protein.
198:
The plant hormone
Jasmonate binds Coi1, an FBP. SCF-Coi1 then binds the JAZ transcription factor and targets it for degradation. Degradation of the JAZ transcription factor allows for the transcription of the jasmonate responsive genes.
105:
was coined the F-box motif. The F-box hypothesis that followed these discoveries proposed that F-box proteins recruit substrates targeted for degradation, and that Skp1 links the F-box protein to the core ubiquitination complex.
178:
Skp2-targeting anti-sense oligonucleotides and siRNAs are in the drug development pipeline. Preliminary studies have shown that Skp2 downregulation can inhibit the growth of melanomas, lung cancer cells, oral cancer cells, and
121:(APC) controls the metaphase-anaphase transition, while the SCF complex controls G1/S and G2/M transitions. Specifically, SCF has been shown to regulate centriole splitting from late telophase to the G1/S transition.
987:
Lee, Albert; Rayner, Stephanie L.; De Luca, Alana; Gwee, Serene S. L.; Morsch, Marco; Sundaramoorthy, Vinod; Shahheydari, Hamideh; Ragagnin, Audrey; Shi, Bingyang; Yang, Shu; Williams, Kelly L. (October 2017).
877:
Calhoun, Eric S.; Jones, Jessa B.; Ashfaq, Raheela; Adsay, Volkan; Baker, Suzanne J.; Valentine, Virginia; Hempen, Paula M.; Hilgers, Werner; Yeo, Charles J.; Hruban, Ralph H.; Kern, Scott E. (October 2003).
1270:
1258:
124:
SCF activity is largely regulated by post-translational modifications. For instance, ubiquitin-mediated autocatalytic degradation of FBPs is a mechanism of decreasing SCF activity.
162:
Fbxo4 is another tumor suppressor FBP that has been implicated in human carcinomas. SCF-fbxo4 plays a role in cell cycle control by targeting cyclin D1 for degradation.
990:"Casein kinase II phosphorylation of cyclin F at serine 621 regulates the Lys48-ubiquitylation E3 ligase activity of the SCF (cyclin F) complex"
935:
Yu, Yujiao; Nakagawa, Tadashi; Morohoshi, Akane; Nakagawa, Makiko; Ishida, Noriko; Suzuki, Naoki; Aoki, Masashi; Nakayama, Keiko (2019-09-30).
812:"A refined two-hybrid system reveals that SCFCdc4-dependent degradation of Swi5 contributes to the regulatory mechanism of S-phase entry"
147:
There are approximately seventy human FBPs, several of which are involved in cell cycle control as a component of SCF complexes.
706:"An Essential Domain within Cdc34p Is Required for Binding to a Complex Containing Cdc4p and Cdc53p inSaccharomyces cerevisiae"
186:βTRCP-targeting siRNAs have been shown to sensitize breast cancer cells and cervical cancer cells to existing chemotherapies.
380:
343:
290:
241:
937:"Pathogenic mutations in the ALS gene CCNF cause cytoplasmic mislocalization of Cyclin F and elevated VCP ATPase activity"
449:
Schwob, E (1994-10-21). "The B-type cyclin kinase inhibitor p40SIC1 controls the G1 to S transition in S. cerevisiae".
406:
Patton, E (1998-06-01). "Combinatorial control in ubiquitin-dependent proteolysis: don't Skp the F-box hypothesis".
51:
of proteins involved in the cell cycle. The SCF complex also marks various other cellular proteins for destruction.
1276:
Hartmut C. Vodermaier (2004). "APC/C and SCF: Controlling Each Other and the Cell Cycle", Current
Biology, 14 (787)
1156:
Dharmasiri, Nihal; Dharmasiri, Sunethra; Estelle, Mark (May 2005). "The F-box protein TIR1 is an auxin receptor".
220:
Ou, Young; Rattner, J.B. (2004), "The
Centrosome in Higher Organisms: Structure, Composition, and Duplication",
44:
603:"Applying the auxin-inducible degradation (AID) system for rapid protein depletion in mammalian cells"
1264:
Morgan, David "Protein
Degradation in Cell-Cycle Control", The Cell Cycle; Principles of Control 2007
633:
360:
325:
755:"Nuclear-specific degradation of Far1 is controlled by the localization of the F-box protein Cdc4"
72:– Skp1 is an adaptor protein that is essential for the recognition and binding of F-box proteins.
649:"Deregulated proteolysis by the F-box proteins SKP2 and β-TrCP: tipping the scales of cancer"
620:
880:"BRAF and FBXW7 (CDC4, FBW7, AGO, SEL10) Mutations in Distinct Subsets of Pancreatic Cancer"
1165:
823:
8:
97:
The first hint that led to the discovery of the SCF complex came from genetic screens of
1169:
827:
1197:
1133:
1108:
1079:
1046:
1022:
989:
912:
879:
854:
811:
681:
648:
482:
335:
282:
895:
787:
754:
419:
273:
Fischer, Martin; Dang, Chi V.; DeCaprio, James A. (2018), "Control of Cell
Division",
233:
1241:
1233:
1229:
1189:
1181:
1138:
1084:
1066:
1027:
1009:
966:
958:
917:
899:
859:
841:
792:
774:
735:
727:
686:
668:
583:
575:
534:
526:
474:
466:
462:
431:
423:
376:
372:
339:
286:
247:
237:
486:
1290:
1225:
1201:
1173:
1128:
1120:
1074:
1058:
1017:
1001:
948:
907:
891:
849:
831:
782:
766:
717:
676:
660:
606:
565:
516:
458:
415:
368:
331:
278:
229:
32:
521:
504:
1124:
770:
48:
16:
570:
553:
1284:
1237:
1185:
1070:
1013:
962:
903:
845:
778:
731:
672:
579:
530:
470:
427:
63:
836:
1245:
1193:
1142:
1088:
1031:
970:
921:
863:
796:
722:
705:
690:
587:
538:
251:
180:
739:
478:
435:
953:
936:
1177:
1005:
601:
Lambrus, Bramwell G.; Moyer, Tyler C.; Holland, Andrew J. (2017-08-31).
505:"A hitchhiker's guide to the cullin ubiquitin ligases: SCF and its kin"
40:
810:
Kishi, T.; Ikeda, A.; Koyama, N.; Fukada, J.; Nagao, R. (2008-09-11).
112:
later contributed to the elucidation of other SCF complex components.
1045:
Skaar, Jeffrey R.; Pagan, Julia K.; Pagano, Michele (December 2014).
36:
1062:
664:
611:
602:
127:
Well-characterized cell cycle substrates of SCF complexes include:
503:
Willems, Andrew R.; Schwab, Michael; Tyers, Mike (November 2004).
75:
59:
SCF contains a variable F-box protein and three core subunits:
704:
Mathias, Neal; Steussy, C. Nic; Goebl, Mark G. (1998-02-13).
509:
Biochimica et
Biophysica Acta (BBA) - Molecular Cell Research
1155:
934:
304:
Morgan, David "Protein
Degradation in Cell-Cycle Control",
138:
85:
79:
69:
1214:
554:"APC/C and SCF: Controlling Each Other and the Cell Cycle"
876:
809:
137:
cyclin-dependent kinase inhibitors (CKIs): p27, p21,
986:
703:
600:
272:
151:degradation in a phosphorylation dependent manner.
502:
1282:
1109:"SCF E3 Ubiquitin Ligases as Anticancer Targets"
1044:
816:Proceedings of the National Academy of Sciences
647:Frescas, David; Pagano, Michele (June 2008).
646:
134:transcriptional regulators: Myc, E2f1, p130
551:
189:
131:cyclin family proteins: Cyclin D, Cyclin E
1132:
1078:
1047:"SCF ubiquitin ligase-targeted therapies"
1021:
952:
911:
853:
835:
786:
721:
680:
610:
569:
552:Vodermaier, Hartmut C. (September 2004).
520:
361:"Ubiquitin-Dependent Protein Degradation"
219:
115:
15:
752:
166:Cyclin F targets E2f1 for degradation.
1283:
448:
405:
1102:
1100:
1098:
982:
980:
358:
306:The Cell Cycle; Principles of Control
25:Skp, Cullin, F-box containing complex
498:
496:
401:
399:
397:
320:
318:
316:
314:
268:
266:
264:
262:
260:
215:
213:
211:
1106:
13:
1095:
977:
336:10.1016/b978-1-4160-3703-3.x5001-7
283:10.1016/b978-0-323-35762-3.00017-2
143:centriole proteins: Cep250, Ninein
54:
14:
1302:
1107:Sun, L. Jia and Y. (2011-02-28).
884:The American Journal of Pathology
493:
394:
365:Comprehensive Natural Products II
311:
257:
208:
47:, SCF has important roles in the
1269:
1257:
1230:10.1046/j.1365-313x.2002.01432.x
373:10.1016/b978-008045382-8.00697-3
222:International Review of Cytology
1208:
1149:
1038:
928:
870:
803:
746:
710:Journal of Biological Chemistry
697:
640:
594:
545:
442:
367:, Elsevier, pp. 699–752,
352:
298:
277:, Elsevier, pp. 176–185,
108:Subsequent genetic studies in
1:
1051:Nature Reviews Drug Discovery
896:10.1016/s0002-9440(10)63485-2
420:10.1016/s0168-9525(98)01473-5
327:The Molecular Basis of Cancer
234:10.1016/s0074-7696(04)38003-4
202:
39:of proteins destined for 26S
522:10.1016/j.bbamcr.2004.09.027
463:10.1016/0092-8674(94)90193-7
92:
43:degradation. Along with the
7:
1113:Current Cancer Drug Targets
35:complex that catalyzes the
10:
1307:
1125:10.2174/156800911794519734
753:Blondel, M. (2000-11-15).
45:anaphase-promoting complex
571:10.1016/j.cub.2004.09.020
195:auxin-responsive) genes.
169:
31:) is a multi-protein E3
941:Human Molecular Genetics
771:10.1093/emboj/19.22.6085
359:Hegde, Ashok N. (2010),
99:Saccharomyces cerevisiae
837:10.1073/pnas.0806253105
190:Plant hormone signaling
723:10.1074/jbc.273.7.4040
628:Cite journal requires
110:Caenorhabditis elegans
21:
653:Nature Reviews Cancer
228:, Elsevier: 119–182,
116:Cell cycle regulation
19:
1178:10.1038/nature03543
1170:2005Natur.435..441D
1006:10.1098/rsob.170058
828:2008PNAS..10514497K
822:(38): 14497–14502.
954:10.1093/hmg/ddz119
408:Trends in Genetics
330:. Elsevier. 2008.
22:
1218:The Plant Journal
1164:(7041): 441–445.
947:(20): 3486–3497.
765:(22): 6085–6097.
564:(18): R787–R796.
382:978-0-08-045382-8
345:978-1-4160-3703-3
292:978-0-323-35762-3
243:978-0-12-364642-2
1298:
1273:
1261:
1250:
1249:
1212:
1206:
1205:
1153:
1147:
1146:
1136:
1104:
1093:
1092:
1082:
1042:
1036:
1035:
1025:
984:
975:
974:
956:
932:
926:
925:
915:
890:(4): 1255–1260.
874:
868:
867:
857:
839:
807:
801:
800:
790:
759:The EMBO Journal
750:
744:
743:
725:
716:(7): 4040–4045.
701:
695:
694:
684:
644:
638:
637:
631:
626:
624:
616:
614:
598:
592:
591:
573:
549:
543:
542:
524:
515:(1–3): 133–170.
500:
491:
490:
446:
440:
439:
403:
392:
391:
390:
389:
356:
350:
349:
322:
309:
302:
296:
295:
270:
255:
254:
217:
33:ubiquitin ligase
1306:
1305:
1301:
1300:
1299:
1297:
1296:
1295:
1281:
1280:
1277:
1274:
1265:
1262:
1253:
1213:
1209:
1154:
1150:
1105:
1096:
1063:10.1038/nrd4432
1057:(12): 889–903.
1043:
1039:
985:
978:
933:
929:
875:
871:
808:
804:
751:
747:
702:
698:
665:10.1038/nrc2396
645:
641:
629:
627:
618:
617:
599:
595:
558:Current Biology
550:
546:
501:
494:
447:
443:
404:
395:
387:
385:
383:
357:
353:
346:
324:
323:
312:
303:
299:
293:
271:
258:
244:
218:
209:
205:
192:
172:
118:
95:
57:
55:Core components
12:
11:
5:
1304:
1294:
1293:
1279:
1278:
1275:
1268:
1266:
1263:
1256:
1252:
1251:
1224:(4): 457–466.
1207:
1148:
1119:(3): 347–356.
1094:
1037:
1000:(10): 170058.
976:
927:
869:
802:
745:
696:
659:(6): 438–449.
639:
630:|journal=
612:10.1101/182840
593:
544:
492:
457:(2): 233–244.
441:
414:(6): 236–243.
393:
381:
351:
344:
310:
297:
291:
256:
242:
206:
204:
201:
191:
188:
171:
168:
145:
144:
141:
135:
132:
117:
114:
94:
91:
90:
89:
83:
73:
67:
56:
53:
49:ubiquitination
37:ubiquitination
9:
6:
4:
3:
2:
1303:
1292:
1289:
1288:
1286:
1272:
1267:
1260:
1255:
1254:
1247:
1243:
1239:
1235:
1231:
1227:
1223:
1219:
1211:
1203:
1199:
1195:
1191:
1187:
1183:
1179:
1175:
1171:
1167:
1163:
1159:
1152:
1144:
1140:
1135:
1130:
1126:
1122:
1118:
1114:
1110:
1103:
1101:
1099:
1090:
1086:
1081:
1076:
1072:
1068:
1064:
1060:
1056:
1052:
1048:
1041:
1033:
1029:
1024:
1019:
1015:
1011:
1007:
1003:
999:
995:
991:
983:
981:
972:
968:
964:
960:
955:
950:
946:
942:
938:
931:
923:
919:
914:
909:
905:
901:
897:
893:
889:
885:
881:
873:
865:
861:
856:
851:
847:
843:
838:
833:
829:
825:
821:
817:
813:
806:
798:
794:
789:
784:
780:
776:
772:
768:
764:
760:
756:
749:
741:
737:
733:
729:
724:
719:
715:
711:
707:
700:
692:
688:
683:
678:
674:
670:
666:
662:
658:
654:
650:
643:
635:
622:
613:
608:
604:
597:
589:
585:
581:
577:
572:
567:
563:
559:
555:
548:
540:
536:
532:
528:
523:
518:
514:
510:
506:
499:
497:
488:
484:
480:
476:
472:
468:
464:
460:
456:
452:
445:
437:
433:
429:
425:
421:
417:
413:
409:
402:
400:
398:
384:
378:
374:
370:
366:
362:
355:
347:
341:
337:
333:
329:
328:
321:
319:
317:
315:
307:
301:
294:
288:
284:
280:
276:
269:
267:
265:
263:
261:
253:
249:
245:
239:
235:
231:
227:
223:
216:
214:
212:
207:
200:
196:
187:
184:
182:
176:
167:
163:
160:
156:
152:
148:
142:
140:
136:
133:
130:
129:
128:
125:
122:
113:
111:
106:
102:
100:
87:
84:
81:
77:
74:
71:
68:
65:
64:F-box protein
62:
61:
60:
52:
50:
46:
42:
38:
34:
30:
26:
18:
1221:
1217:
1210:
1161:
1157:
1151:
1116:
1112:
1054:
1050:
1040:
997:
994:Open Biology
993:
944:
940:
930:
887:
883:
872:
819:
815:
805:
762:
758:
748:
713:
709:
699:
656:
652:
642:
621:cite journal
596:
561:
557:
547:
512:
508:
454:
450:
444:
411:
407:
386:, retrieved
364:
354:
326:
305:
300:
274:
225:
221:
197:
193:
185:
181:glioblastoma
177:
173:
164:
161:
157:
153:
149:
146:
126:
123:
119:
109:
107:
103:
98:
96:
58:
28:
24:
23:
41:proteasomal
29:SCF complex
388:2019-12-01
275:Hematology
203:References
1238:0960-7412
1186:0028-0836
1071:1474-1776
1014:2046-2441
963:0964-6906
904:0002-9440
846:0027-8424
779:1460-2075
732:0021-9258
673:1474-175X
580:0960-9822
531:0167-4889
471:0092-8674
428:0168-9525
93:Discovery
1285:Category
1246:12445118
1194:15917797
1143:21247385
1089:25394868
1032:29021214
971:31577344
922:14507635
864:18787112
797:11080155
691:18500245
588:15380093
539:15571813
487:34939988
252:15364198
1291:Enzymes
1202:4428049
1166:Bibcode
1134:3323109
1080:4410837
1023:5666078
913:1868306
855:2567208
824:Bibcode
740:9461595
682:2711846
479:7954792
436:9635407
183:cells.
1244:
1236:
1200:
1192:
1184:
1158:Nature
1141:
1131:
1087:
1077:
1069:
1030:
1020:
1012:
969:
961:
920:
910:
902:
862:
852:
844:
795:
788:305831
785:
777:
738:
730:
689:
679:
671:
586:
578:
537:
529:
485:
477:
469:
434:
426:
379:
342:
289:
250:
240:
170:Cancer
76:Cullin
1198:S2CID
483:S2CID
1242:PMID
1234:ISSN
1190:PMID
1182:ISSN
1139:PMID
1085:PMID
1067:ISSN
1028:PMID
1010:ISSN
967:PMID
959:ISSN
918:PMID
900:ISSN
860:PMID
842:ISSN
793:PMID
775:ISSN
736:PMID
728:ISSN
687:PMID
669:ISSN
634:help
584:PMID
576:ISSN
535:PMID
527:ISSN
513:1695
475:PMID
467:ISSN
451:Cell
432:PMID
424:ISSN
377:ISBN
340:ISBN
308:2007
287:ISBN
248:PMID
238:ISBN
139:Wee1
86:RBX1
80:CUL1
70:Skp1
27:(or
1226:doi
1174:doi
1162:435
1129:PMC
1121:doi
1075:PMC
1059:doi
1018:PMC
1002:doi
949:doi
908:PMC
892:doi
888:163
850:PMC
832:doi
820:105
783:PMC
767:doi
718:doi
714:273
677:PMC
661:doi
607:doi
566:doi
517:doi
459:doi
416:doi
369:doi
332:doi
279:doi
230:doi
226:238
1287::
1240:.
1232:.
1222:32
1220:.
1196:.
1188:.
1180:.
1172:.
1160:.
1137:.
1127:.
1117:11
1115:.
1111:.
1097:^
1083:.
1073:.
1065:.
1055:13
1053:.
1049:.
1026:.
1016:.
1008:.
996:.
992:.
979:^
965:.
957:.
945:28
943:.
939:.
916:.
906:.
898:.
886:.
882:.
858:.
848:.
840:.
830:.
818:.
814:.
791:.
781:.
773:.
763:19
761:.
757:.
734:.
726:.
712:.
708:.
685:.
675:.
667:.
655:.
651:.
625::
623:}}
619:{{
605:.
582:.
574:.
562:14
560:.
556:.
533:.
525:.
511:.
507:.
495:^
481:.
473:.
465:.
455:79
453:.
430:.
422:.
412:14
410:.
396:^
375:,
363:,
338:.
313:^
285:,
259:^
246:,
236:,
224:,
210:^
1248:.
1228::
1204:.
1176::
1168::
1145:.
1123::
1091:.
1061::
1034:.
1004::
998:7
973:.
951::
924:.
894::
866:.
834::
826::
799:.
769::
742:.
720::
693:.
663::
657:8
636:)
632:(
615:.
609::
590:.
568::
541:.
519::
489:.
461::
438:.
418::
371::
348:.
334::
281::
232::
78:(
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.