477:) on one or more of the carbons of the ring. Anomers are named "alpha" or "axial" and "beta" or "equatorial" when substituting a cyclic ring structure that has single bonds between the carbon atoms of the ring for example, a hydroxyl group, a methyl hydroxyl group, a methoxy group or another pyranose or furanose group which are typical single bond substitutions but not limited to these. Axial geometric isomerism will be perpendicular (90 degrees) to a reference plane and equatorial will be 120 degrees away from the axial bond or deviate 30 degrees from the reference plane.
185:
392:
345:
20:
283:, when looking at the source of light, the rotation of the plane of polarization may be either to the right (dextrorotary — d-rotary, represented by (+), clockwise), or to the left (levorotary — l-rotary, represented by (−), counter-clockwise) depending on which stereoisomer is dominant. For instance, sucrose and camphor are d-rotary whereas cholesterol is l-rotary.
301:
Stereoisomerism about double bonds arises because rotation about the double bond is restricted, keeping the substituents fixed relative to each other. If the two substituents on at least one end of a double bond are the same, then there is no stereoisomer and the double bond is not a stereocenter,
440:
Conformational isomerism is a form of isomerism that describes the phenomenon of molecules with the same structural formula but with different shapes due to rotations about one or more bonds. Different conformations can have different energies, can usually interconvert, and are very rarely
407:)-2-fluoro-3-methylpent-2-ene because the highest-priority groups on each side of the double bond are on the same side of the double bond. Fluoro is the highest-priority group on the left side of the double bond, and ethyl is the highest-priority group on the right side of the molecule.
472:
is an identity for single bonded ring structures where "cis" or "Z" and "trans" or "E" (geometric isomerism) needs to name the substitutions on a carbon atom that also displays the identity of chirality; so anomers have carbon atoms that have geometric isomerism and optical isomerism
791:
276:. For instance, by definition, in a Fischer projection the penultimate carbon of D-sugars are depicted with hydrogen on the left and hydroxyl on the right. L-sugars will be shown with the hydrogen on the right and the hydroxyl on the left.
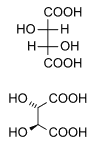
161:. Diastereomers seldom have the same physical properties. In the example shown below, the meso form of tartaric acid forms a diastereomeric pair with both levo- and dextro-tartaric acids, which form an enantiomeric pair.
96:
and how they interact with different enantiomers of other compounds. As a result, different enantiomers of a compound may have substantially different biological effects. Pure enantiomers also exhibit the phenomenon of
764:
460:
There are some molecules that can be isolated in several conformations, due to the large energy barriers between different conformations. 2,2',6,6'-Tetrasubstituted biphenyls can fit into this latter category.
457:
for this process, because there are lower-energy pathways. The conformational inversion of substituted cyclohexanes is a very rapid process at room temperature, with a half-life of 0.00001 seconds.
59:, which share the same molecular formula, but the bond connections or their order differs. By definition, molecules that are stereoisomers of each other represent the same structural isomer.
523:). This means that configurational isomers can be interconverted only by breaking covalent bonds to the stereocenter, for example, by inverting the configurations of some or all of the
92:
in one has the opposite configuration in the other. Two compounds that are enantiomers of each other have the same physical properties, except for the direction in which they rotate
360:-1,2-dichloroethene. Due to occasional ambiguity, IUPAC adopted a more rigorous system wherein the substituents at each end of the double bond are assigned priority based on their
178:
170:
372:, opposite). Since chlorine has a larger atomic number than hydrogen, it is the highest-priority group. Using this notation to name the above pictured molecules, molecule I is (
1341:
453:, the boat conformation represents the energy maximum on a conformational itinerary between the two equivalent chair forms; however, it does not represent the
403:-2-fluoro-3-methylpent-2-ene because the alkyl groups that form the backbone chain (i.e., methyl and ethyl) reside across the double bond from each other, or (
1346:
449:
where four of the carbon atoms form the "seat" of the chair, one carbon atom is the "back" of the chair, and one carbon atom is the "foot rest"; and a
610:
1082:
1207:
55:
and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in space. This contrasts with
1451:
817:
265:- labeling more commonly seen, explaining why these may appear reversed to those familiar with only the latter naming convention.
1002:
321:(Latin, across), in reference to the relative position of substituents on either side of a double bond. A simple example of
973:
1200:
591:
138:
are stereoisomers not related through a reflection operation. They are not mirror images of each other. These include
1055:
1379:
801:
774:
1364:
1193:
745:
1374:
924:
1217:
949:
881:
515:
is a stereoisomer of a reference molecule that has the opposite configuration at a stereocenter (e.g.,
1027:
895:
1336:
857:
543:
648:
1441:
1262:
442:
435:
391:
987:
292:
143:
344:
586:
516:
685:
661:
1400:
1314:
1231:
1067:
909:
720:
364:. If the high-priority substituents are on the same side of the bond, it is assigned Z (Ger.
273:
102:
88:
of each other that are non-superposable. Human hands are a macroscopic analog of this. Every
68:
445:(which cyclohexane is an essential intermediate for the synthesis of nylon–6,6) including a
1410:
1395:
1246:
534:
is a diastereoisomer that has the opposite configuration at only one of the stereocenters.
8:
1308:
1185:
832:
1446:
1369:
1267:
1171:
1135:
1110:
269:
89:
1356:
1140:
1051:
797:
770:
550:
56:
52:
620:
1405:
1331:
1241:
1130:
1122:
624:
615:
454:
296:
280:
154:
105:
agent. In nature, only one enantiomer of most chiral biological compounds, such as
98:
16:
When molecules have the same atoms and bond structure but differ in 3D orientation
1415:
1303:
1257:
502:
barrier to rotation is high enough to allow for the isolation of the conformers.
158:
93:
84:, are two stereoisomers that are related to each other by a reflection: they are
36:
28:
1126:
699:
553:
atoms, there is a maximum of 2 different stereoisomers possible. As an example,
418:
are also used to describe the relative position of two substituents on a ring;
113:, which is achiral), is present. An optically active compound shows two forms:
184:
1435:
1298:
619:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
499:
495:
361:
139:
628:
1420:
1293:
1236:
1144:
524:
520:
130:
85:
388:, are always interchangeable. Consider the following fluoromethylpentene:
491:
486:
72:
1175:
1159:
1288:
558:
474:
106:
329:
isomerism is the 1,2-disubstituted ethenes, like the dichloroethene (C
48:
313:
Traditionally, double bond stereochemistry was described as either
700:"Geometric Isomers Definition And Examples | Chemistry Dictionary"
177:
169:
1003:"The Big Damn Post Of Carbohydrate-Related Chemistry Definitions"
882:"Conformational Isomer - an overview | ScienceDirect Topics"
110:
469:
24:
1050:
Morrison and Boyd
Organic Chemistry Sixth ed. pgs. 1170-1171
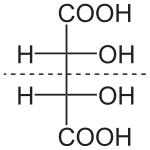
573:. Four of its six carbon atoms are stereogenic, which means
19:
988:"Anomeric Effect - an overview | ScienceDirect Topics"
1215:
368:, together). If they are on opposite sides, it is E (Ger.
494:
are stereoisomers resulting from hindered rotation about
272:
can be used to differentiate between L- and D- molecules
686:"Diastereomer - an overview | ScienceDirect Topics"
231:
1068:"Atropisomer - an overview | ScienceDirect Topics"
910:"Cyclohexane - an overview | ScienceDirect Topics"
818:"Cis–trans isomerism | NAL Agricultural Thesaurus"
257:- labeling of the isomers above is not the same as the
896:"Isomerism - Conformational isomers | Britannica"
796:(second ed.). W. A. Benjamin, Inc. p. 19.6.
769:(second ed.). W. A. Benjamin, Inc. p. 19.7.
380:)-1,2-dichloroethene. It is not the case that Z and
441:isolatable. For example, there exists a variety of
31:
focuses on stereoisomers, red boxes in the picture.
974:"Anomer - an overview | ScienceDirect Topics"
310:where the two substituents at one end are both H.
1347:Ultraviolet–visible spectroscopy of stereoisomers
746:"Geometric Isomer Definition (Cis–Trans Isomers)"
1433:
577:-glucose is one of 2=16 possible stereoisomers.
163:
790:Roberts, John D.; Caserio, Marjorie C. (1977).
763:Roberts, John D.; Caserio, Marjorie C. (1977).
789:
762:
286:
1201:
399:The proper name for this molecule is either
224:
208:
191:
101:and can be separated only with the use of a
1208:
1194:
1115:Cold Spring Harbor Perspectives in Biology
1000:
639:Columbia Encyclopedia. "Stereoisomers" in
1134:
1108:
538:
376:)-1,2-dichloroethene and molecule II is (
1111:"The Origin of Biological Homochirality"
950:"What do the α- and β- forms look like?"
182:
175:
166:
18:
1080:
743:
356:-1,2-dichloroethene and molecule II is
1434:
1157:
922:
833:"E–Z notation for geometric isomerism"
1189:
830:
793:Basic Principles of Organic Chemistry
766:Basic Principles of Organic Chemistry
718:
659:
721:"geometric (cis / trans) isomerism"
505:
13:
1081:Metrano, Anthony J. (2018-06-09).
1025:
858:"What are Conformational Isomers?"
616:Compendium of Chemical Terminology
592:Backbone-dependent rotamer library
390:
343:
14:
1463:
1342:NMR spectroscopy of stereoisomers
546:states that for a structure with
51:in which molecules have the same
1380:Diastereomeric recrystallization
1001:Ashenhurst, James (2022-08-03).
947:
855:
183:
176:
168:
124:
1151:
1102:
1074:
1060:
1044:
1019:
994:
980:
966:
941:
916:
902:
888:
874:
849:
824:
810:
480:
422:if on the same side, otherwise
923:Reusch, William (2013-05-05).
783:
756:
737:
712:
692:
678:
653:
633:
604:
62:
1:
597:
429:
1452:Jacobus Henricus van 't Hoff
1375:Chiral column chromatography
1158:Brooks, Benjamin T. (1918).
831:Clark, Jim (November 2012).
719:Clark, Jim (February 2020).
660:Clark, Jim (November 2012).
513:configurational stereoisomer
7:
1127:10.1101/cshperspect.a032540
580:
287:Cis–trans and E–Z isomerism
10:
1468:
1337:Chiral derivatizing agents
1218:enantioselective synthesis
1160:"The German Chemical Myth"
1007:masterorganicchemistry.com
484:
464:
433:
290:
128:
66:
1388:
1355:
1324:
1276:
1224:
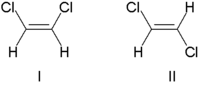
1164:The North American Review
1109:Blackmond, D. G. (2019).
744:Helmenstine, Anne Marie.
443:Cyclohexane conformations
317:(Latin, on this side) or
1263:Supramolecular chirality
1090:knowleslab.princeton.edu
436:Conformational isomerism
629:10.1351/goldbook.S05983
341:) isomers shown below.
193:(natural) tartaric acid
157:, and non-enantiomeric
23:The different types of
587:Descriptor (chemistry)
544:Le Bel-van't Hoff rule
539:Le Bel-van't Hoff rule
396:
349:
348:Dichloroethene isomers
32:
1401:Chiral pool synthesis
1315:Diastereomeric excess
561:and has the formula C
394:
347:
274:Chirality (chemistry)
69:Chirality (chemistry)
22:
1411:Asymmetric catalysis
1396:Asymmetric induction
279:The other refers to
220:dextro-tartaric acid
1309:Enantiomeric excess
662:"Optical isomerism"
395:Fluoromethylpentene
293:Cis–trans isomerism
226:meso-tartaric acid
205:levo-tartaric acid
1406:Chiral auxiliaries
1370:Kinetic resolution
1268:Inherent chirality
1253:-symmetric ligands
447:chair conformation
397:
350:
270:Fischer projection
218:-(-)-tartaric acid
203:-(+)-tartaric acid
90:stereogenic center
57:structural isomers
33:
1429:
1428:
1365:Recrystallization
1357:Chiral resolution
1032:chemistry.msu.edu
1026:Reusch, William.
929:chemistry.msu.edu
576:
556:
551:asymmetric carbon
451:boat conformation
256:
252:
247:
246:
237:
217:
212:
202:
197:
120:
116:
53:molecular formula
45:spatial isomerism
1459:
1332:Optical rotation
1277:Chiral molecules
1242:Planar chirality
1210:
1203:
1196:
1187:
1186:
1180:
1179:
1170:(756): 729–735.
1155:
1149:
1148:
1138:
1106:
1100:
1099:
1097:
1096:
1087:
1078:
1072:
1071:
1064:
1058:
1048:
1042:
1041:
1039:
1038:
1023:
1017:
1016:
1014:
1013:
998:
992:
991:
984:
978:
977:
970:
964:
963:
961:
960:
954:chem.ucalgary.ca
945:
939:
938:
936:
935:
920:
914:
913:
906:
900:
899:
892:
886:
885:
878:
872:
871:
869:
868:
862:chem.ucalgary.ca
853:
847:
846:
844:
843:
828:
822:
821:
814:
808:
807:
787:
781:
780:
760:
754:
753:
741:
735:
734:
732:
731:
716:
710:
709:
707:
706:
696:
690:
689:
682:
676:
675:
673:
672:
657:
651:
641:Encyclopedia.com
637:
631:
608:
574:
554:
506:More definitions
455:transition state
302:e.g. propene, CH
281:Optical rotation
254:
250:
235:
215:
210:
200:
195:
187:
180:
172:
164:
118:
114:
99:optical activity
80:, also known as
1467:
1466:
1462:
1461:
1460:
1458:
1457:
1456:
1442:Stereochemistry
1432:
1431:
1430:
1425:
1416:Organocatalysis
1384:
1351:
1320:
1304:Racemic mixture
1272:
1258:Axial chirality
1252:
1225:Chirality types
1220:
1214:
1184:
1183:
1156:
1152:
1107:
1103:
1094:
1092:
1085:
1079:
1075:
1066:
1065:
1061:
1049:
1045:
1036:
1034:
1028:"Stereoisomers"
1024:
1020:
1011:
1009:
999:
995:
986:
985:
981:
972:
971:
967:
958:
956:
946:
942:
933:
931:
925:"Stereoisomers"
921:
917:
908:
907:
903:
894:
893:
889:
880:
879:
875:
866:
864:
854:
850:
841:
839:
837:chemguide.co.uk
829:
825:
816:
815:
811:
804:
788:
784:
777:
761:
757:
742:
738:
729:
727:
717:
713:
704:
702:
698:
697:
693:
684:
683:
679:
670:
668:
666:chemguide.co.uk
658:
654:
638:
634:
621:stereoisomerism
609:
605:
600:
583:
572:
568:
564:
557:-glucose is an
541:
508:
489:
483:
467:
438:
432:
340:
336:
332:
309:
305:
299:
291:Main articles:
289:
240:"racemic acid"
239:
234:
221:
219:
214:
204:
199:
194:
159:optical isomers
133:
127:
94:polarized light
82:optical isomers
75:
67:Main articles:
65:
47:, is a form of
41:stereoisomerism
37:stereochemistry
29:Stereochemistry
17:
12:
11:
5:
1465:
1455:
1454:
1449:
1444:
1427:
1426:
1424:
1423:
1418:
1413:
1408:
1403:
1398:
1392:
1390:
1386:
1385:
1383:
1382:
1377:
1372:
1367:
1361:
1359:
1353:
1352:
1350:
1349:
1344:
1339:
1334:
1328:
1326:
1322:
1321:
1319:
1318:
1312:
1306:
1301:
1296:
1291:
1286:
1280:
1278:
1274:
1273:
1271:
1270:
1265:
1260:
1255:
1250:
1244:
1239:
1234:
1228:
1226:
1222:
1221:
1213:
1212:
1205:
1198:
1190:
1182:
1181:
1150:
1121:(3): a032540.
1101:
1083:"Atropisomers"
1073:
1059:
1043:
1018:
993:
979:
965:
940:
915:
901:
887:
873:
848:
823:
809:
802:
782:
775:
755:
736:
711:
691:
677:
652:
632:
602:
601:
599:
596:
595:
594:
589:
582:
579:
570:
566:
562:
540:
537:
536:
535:
528:
527:in a compound.
507:
504:
485:Main article:
482:
479:
466:
463:
434:Main article:
431:
428:
352:Molecule I is
338:
334:
330:
307:
303:
288:
285:
245:
244:
242:
238:-tartaric acid
229:
228:
223:
213:-tartaric acid
207:
198:-tartaric acid
189:
188:
181:
174:
140:meso compounds
129:Main article:
126:
123:
117:-(+) form and
64:
61:
15:
9:
6:
4:
3:
2:
1464:
1453:
1450:
1448:
1445:
1443:
1440:
1439:
1437:
1422:
1419:
1417:
1414:
1412:
1409:
1407:
1404:
1402:
1399:
1397:
1394:
1393:
1391:
1387:
1381:
1378:
1376:
1373:
1371:
1368:
1366:
1363:
1362:
1360:
1358:
1354:
1348:
1345:
1343:
1340:
1338:
1335:
1333:
1330:
1329:
1327:
1323:
1316:
1313:
1310:
1307:
1305:
1302:
1300:
1299:Meso compound
1297:
1295:
1292:
1290:
1287:
1285:
1282:
1281:
1279:
1275:
1269:
1266:
1264:
1261:
1259:
1256:
1254:
1249:
1245:
1243:
1240:
1238:
1235:
1233:
1230:
1229:
1227:
1223:
1219:
1211:
1206:
1204:
1199:
1197:
1192:
1191:
1188:
1177:
1173:
1169:
1165:
1161:
1154:
1146:
1142:
1137:
1132:
1128:
1124:
1120:
1116:
1112:
1105:
1091:
1084:
1077:
1069:
1063:
1057:
1056:0-13-643669-2
1053:
1047:
1033:
1029:
1022:
1008:
1004:
997:
989:
983:
975:
969:
955:
951:
944:
930:
926:
919:
911:
905:
897:
891:
883:
877:
863:
859:
852:
838:
834:
827:
819:
813:
805:
799:
795:
794:
786:
778:
772:
768:
767:
759:
751:
747:
740:
726:
722:
715:
701:
695:
687:
681:
667:
663:
656:
650:
646:
642:
636:
630:
626:
622:
618:
617:
612:
607:
603:
593:
590:
588:
585:
584:
578:
560:
552:
549:
545:
533:
529:
526:
525:stereocenters
522:
518:
514:
510:
509:
503:
501:
500:steric strain
497:
493:
488:
478:
476:
475:enantiomerism
471:
462:
458:
456:
452:
448:
444:
437:
427:
425:
421:
417:
413:
408:
406:
402:
393:
389:
387:
383:
379:
375:
371:
367:
363:
362:atomic number
359:
355:
346:
342:
328:
324:
320:
316:
311:
298:
294:
284:
282:
277:
275:
271:
266:
264:
260:
243:
241:
230:
227:
222:
206:
190:
186:
179:
173:
171:
165:
162:
160:
156:
152:
150:
146:
141:
137:
136:Diastereomers
132:
125:Diastereomers
122:
112:
108:
104:
100:
95:
91:
87:
86:mirror images
83:
79:
74:
70:
60:
58:
54:
50:
46:
42:
38:
30:
26:
21:
1421:Biocatalysis
1294:Diastereomer
1284:Stereoisomer
1283:
1247:
1237:Stereocenter
1216:Concepts in
1167:
1163:
1153:
1118:
1114:
1104:
1093:. Retrieved
1089:
1076:
1062:
1046:
1035:. Retrieved
1031:
1021:
1010:. Retrieved
1006:
996:
982:
968:
957:. Retrieved
953:
943:
932:. Retrieved
928:
918:
904:
890:
876:
865:. Retrieved
861:
851:
840:. Retrieved
836:
826:
812:
792:
785:
765:
758:
749:
739:
728:. Retrieved
725:Chemguide.uk
724:
714:
703:. Retrieved
694:
680:
669:. Retrieved
665:
655:
644:
640:
635:
614:
606:
547:
542:
531:
512:
496:single bonds
492:Atropisomers
490:
481:Atropisomers
468:
459:
450:
446:
439:
423:
419:
415:
411:
409:
404:
400:
398:
385:
381:
377:
373:
369:
365:
357:
353:
351:
326:
322:
318:
314:
312:
300:
297:E–Z notation
278:
267:
262:
258:
248:
232:
225:
209:
192:
167:
148:
144:
135:
134:
131:Diastereomer
81:
77:
76:
44:
40:
34:
948:Hunt, Ian.
856:Hunt, Ian.
487:Atropisomer
384:, or E and
155:E-Z isomers
121:-(−) form.
107:amino acids
78:Enantiomers
73:Enantiomers
63:Enantiomers
1436:Categories
1289:Enantiomer
1095:2022-08-09
1037:2022-08-09
1012:2022-08-09
959:2022-08-09
934:2022-08-09
867:2022-08-09
842:2022-08-09
803:0805383298
776:0805383298
730:2022-08-09
705:2022-06-20
671:2022-08-09
598:References
559:aldohexose
498:where the
430:Conformers
410:The terms
1447:Isomerism
1389:Reactions
1232:Chirality
750:ThoughtCo
470:Anomerism
49:isomerism
1325:Analysis
1176:25151064
1145:30824575
643:, n.l.,
581:See also
521:E- vs Z-
517:R- vs S-
370:entgegen
366:zusammen
109:(except
1136:6396334
465:Anomers
151:isomers
111:glycine
25:isomers
1174:
1143:
1133:
1054:
800:
773:
532:epimer
261:- and
253:- and
103:chiral
1172:JSTOR
1086:(PDF)
611:IUPAC
424:trans
416:trans
401:trans
386:trans
358:trans
327:trans
319:trans
306:CH=CH
233:(1:1)
149:trans
43:, or
1317:(de)
1311:(ee)
1141:PMID
1052:ISBN
798:ISBN
771:ISBN
649:Link
645:2005
414:and
295:and
249:The
71:and
1168:208
1131:PMC
1123:doi
625:doi
623:".
530:An
519:or
420:cis
412:cis
382:cis
354:cis
323:cis
315:cis
145:cis
35:In
1438::
1166:.
1162:.
1139:.
1129:.
1119:11
1117:.
1113:.
1088:.
1030:.
1005:.
952:.
927:.
860:.
835:.
748:.
723:.
664:.
647:,
613:,
567:12
511:A
426:.
337:Cl
268:A
236:DL
153:,
142:,
39:,
27:.
1251:2
1248:C
1209:e
1202:t
1195:v
1178:.
1147:.
1125::
1098:.
1070:.
1040:.
1015:.
990:.
976:.
962:.
937:.
912:.
898:.
884:.
870:.
845:.
820:.
806:.
779:.
752:.
733:.
708:.
688:.
674:.
627::
575:D
571:6
569:O
565:H
563:6
555:D
548:n
473:(
405:Z
378:E
374:Z
339:2
335:2
333:H
331:2
325:–
308:2
304:3
263:l
259:d
255:L
251:D
216:D
211:D
201:L
196:L
147:–
119:L
115:D
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.