218:
80:
261:
1816:
1838:
335:
439:
138:), which results in the emission of visible light as these substances release their excess energy (see spectrum below for an explanation of which specific radical species produce which specific colors). As the combustion temperature of a flame increases (if the flame contains small particles of unburnt carbon or other material), so does the average energy of the electromagnetic radiation given off by the flame (see
29:
1827:
785:
411:
In fires (particularly house fires), the cooler flames are often red and produce the most smoke. Here the red color compared to typical yellow color of the flames suggests that the temperature is lower. This is because there is a lack of oxygen in the room and therefore there is incomplete combustion
298:
The colder part of a diffusion (incomplete combustion) flame will be red, transitioning to orange, yellow, and white as the temperature increases as evidenced by changes in the black-body radiation spectrum. For a given flame's region, the closer to white on this scale, the hotter that section of the
362:
When looking at a flame's temperature there are many factors which can change or apply. An important one is that a flame's color does not necessarily determine a temperature comparison because black-body radiation is not the only thing that produces or determines the color seen; therefore it is only
808:
environment, such as in orbit, natural convection no longer occurs and the flame becomes spherical, with a tendency to become bluer and more efficient. There are several possible explanations for this difference, of which the most likely is the hypothesis that the temperature is sufficiently evenly
286:
is produced, and the flame tends to take oxygen from the surfaces it touches. When the air inlet is opened, less soot and carbon monoxide are produced. When enough air is supplied, no soot or carbon monoxide is produced and the flame becomes blue. (Most of this blue had previously been obscured by
813:
reveal that diffusion flames in microgravity allow more soot to be completely oxidized after they are produced than do diffusion flames on Earth, because of a series of mechanisms that behave differently in microgravity when compared to normal gravity conditions. These discoveries have potential
764:
in 1817. The process depends on a fine balance of temperature and concentration of the reacting mixture, and if conditions are right it can initiate without any external ignition source. Cyclical variations in the balance of chemicals, particularly of intermediate products in the reaction, give
125:
One may investigate all the different parts of the flame from a candle with a cold metal spoon: Higher parts are water vapor, the result of combustion; yellow parts in the middle are soot; down just next to the candle wick is unburned wax. Goldsmiths use higher parts of a flame with a metallic
299:
flame is. The transitions are often apparent in fires, in which the color emitted closest to the fuel is white, with an orange section above it, and reddish flames the highest of all. A blue-colored flame only emerges when the amount of soot decreases and the
268:
depend on oxygen supply. On the left a rich fuel with no premixed oxygen produces a yellow sooty diffusion flame; on the right a lean fully oxygen premixed flame produces no soot and the flame color is produced by molecular radicals, especially CH and C2
830:
Flames do not need to be driven only by chemical energy release. In stars, subsonic burning fronts driven by burning light nuclei (like carbon or helium) to heavy nuclei (up to iron group) propagate as flames. This is important in some models of
277:
In a laboratory under normal gravity conditions and with a closed air inlet, a Bunsen burner burns with yellow flame (also called a safety flame) with a peak temperature of about 2,000 K (3,100 °F). The yellow arises from
1059:
Gregory P. Smith; David M. Golden; Michael
Frenklach; Nigel W. Moriarty; Boris Eiteneer; Mikhail Goldenberg; C. Thomas Bowman; Ronald K. Hanson; Soonho Song; William C. Gardiner Jr.; Vitali V. Lissianski; Zhiwei Qin.
109:
in the air, which gives off enough heat in the subsequent exothermic reaction to vaporize yet more fuel, thus sustaining a consistent flame. The high temperature of the flame causes the vaporized fuel molecules to
796:
In the year 2000, experiments by NASA confirmed that gravity plays an indirect role in flame formation and composition. The common distribution of a flame under normal gravity conditions depends on
765:
oscillations in the flame, with a typical temperature variation of about 100 °C (212 °F), or between "cool" and full ignition. Sometimes the variation can lead to an explosion.
199:, the oxygen and fuel are premixed beforehand, which results in a different type of flame. Candle flames (a diffusion flame) operate through evaporation of the fuel which rises in a
736:. This high flame temperature is partially due to the absence of hydrogen in the fuel (dicyanoacetylene is not a hydrocarbon) thus there is no water among the combustion products.
402:
the combustion process is (a 1:1 stoichiometricity) assuming no dissociation will have the highest flame temperature; excess air/oxygen will lower it as will lack of air/oxygen
424:
of spontaneous combustion are exposed to oxygen, carbon monoxide and superheated hydrocarbons combust, and temporary temperatures of up to 2,000 °C (3,630 °F) occur.
760:
At temperatures as low as 120 °C (248 °F), fuel-air mixtures can react chemically and produce very weak flames called cool flames. The phenomenon was discovered by
233:. Virtually all the light produced is in the blue to green region of the spectrum below about 565 nanometers, accounting for the bluish color of sootless hydrocarbon flames.
1041:
1348:
126:
blow-pipe for melting gold and silver. Sufficient energy in the flame will excite the electrons in some of the transient reaction intermediates such as the
303:
from excited molecular radicals become dominant, though the blue can often be seen near the base of candles where airborne soot is less concentrated.
145:
Other oxidizers besides oxygen can be used to produce a flame. Hydrogen burning in chlorine produces a flame and in the process emits gaseous
1423:
1325:
315:
180:
occurring in the flame are very complex and typically involve a large number of chemical reactions and intermediate species, most of them
1432:
184:. For instance, a well-known chemical kinetics scheme, GRI-Mech, uses 53 species and 325 elementary reactions to describe combustion of
89:
Color and temperature of a flame are dependent on the type of fuel involved in the combustion, for example, when a lighter is held to a
1441:
1363:
835:. In thermonuclear flames, thermal conduction dominates over species diffusion, so the flame speed and thickness is determined by the
1523:
1302:
1148:
746:, produces the second-hottest-known natural flame with a temperature of over 4,525 °C (8,177 °F) when it burns in oxygen.
495:
This is a rough guide to flame temperatures for various common substances (in 20 °C (68 °F) air at 1 atm. pressure):
295:
in the flame, which emit most of their light well below ≈565 nanometers in the blue and green regions of the visible spectrum.
253:
flames, the most important factor determining color is oxygen supply and the extent of fuel-oxygen pre-mixing, which determines the
1732:
861:
1045:
1284:
1128:
1101:
395:
Temperature of atmosphere links to adiabatic flame temperature (i.e., heat will transfer to a cooler atmosphere more quickly)
1009:
792:, convection does not carry the hot combustion products away from the fuel source, resulting in a spherical flame front.
952:"Measurement of the distribution of temperature and emissivity of a candle flame using hyperspectral imaging technique"
800:, as soot tends to rise to the top of a flame (such as in a candle in normal gravity conditions), making it yellow. In
909:
482:
464:
1345:
412:
and the flame temperature is low, often just 600 to 850 °C (1,112 to 1,562 °F). This means that a lot of
1061:
1513:
405:
The distance from the source of the flame (i.e., the further from the source of the flame the lower temperature)
1488:
449:
871:
1454:
844:
354:
particles (as the flame is clearly a blue premixed complete combustion flame) but instead comes from the
951:
385:
The kind of fuel used (i.e., depends on how quickly the process occurs; how violent the combustion is)
291:
flame on the right shows that the blue color arises specifically due to emission of excited molecular
1636:
1424:
A candle flame strongly influenced and moved about by an electric field due to the flame having ions.
1248:
J. B. Conway; R. H. Wilson Jr.; A. V. Grosse (1953). "The
Temperature of the Cyanogen-Oxygen Flame".
1182:
N, and a
Chemical Method for the Production of Continuous Temperatures in the Range of 5000–6000K".
925:
1737:
1459:
876:
191:
There are different methods of distributing the required components of combustion to a flame. In a
1505:
1449:
1381:"The conductive propagation of nuclear flames. I - Degenerate C + O and O + Ne + Mg white dwarfs"
1005:
460:
1322:
1641:
1631:
1579:
1209:
Thomas, N.; Gaydon, A. G.; Brewer, L. (1952). "Cyanogen Flames and the
Dissociation Energy of N
249:
emission and spectral line absorption playing smaller roles. In the most common type of flame,
217:
170:
111:
20:
1118:
897:
306:
Specific colors can be imparted to the flame by introduction of excitable species with bright
1830:
1429:
1058:
732:(4,990 °C; 9,010 °F), and at up to 6,000 K (5,730 °C; 10,340 °F) in
559:≈1,100 °C (≈2,012 °F) ; hot spots may be 1,300–1,400 °C (2,372–2,552 °F)
254:
1091:
1392:
1360:
1222:
963:
840:
836:
598:
374:
323:
238:
85:
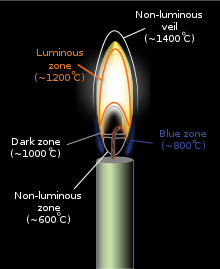
The interior of the luminous zone can be much hotter, beyond 1,500 °C (2,730 °F).
1837:
1299:
8:
1156:
801:
292:
226:
181:
127:
115:
1396:
1226:
1120:
Combustion
Phenomena: Selected Mechanisms of Flame Formation, Propagation and Extinction
967:
1481:
987:
832:
154:
1758:
1280:
1178:
Kirshenbaum, A. D.; A. V. Grosse (May 1956). "The
Combustion of Carbon Subnitride, NC
1124:
1097:
991:
979:
905:
307:
257:
and thus the temperature and reaction paths, thereby producing different color hues.
177:
146:
1683:
1662:
1400:
1257:
1230:
1191:
971:
809:
distributed that soot is not formed and complete combustion occurs. Experiments by
717:
149:(HCl) as the combustion product. Another of many possible chemical combinations is
68:
195:, oxygen and fuel diffuse into each other; the flame occurs where they meet. In a
1569:
1436:
1367:
1352:
1329:
1306:
819:
815:
413:
283:
192:
131:
119:
975:
363:
an estimation of temperature. Other factors that determine its temperature are:
1795:
1549:
1539:
856:
805:
789:
456:
399:
287:
the bright yellow emissions.) The spectrum of a premixed (complete combustion)
196:
44:
1014:
728:
burns in oxygen with a bright blue-white flame at a temperature of 5,260
50:
1862:
1856:
1819:
1518:
1474:
983:
866:
778:
545:
416:
is formed (which is a flammable gas) which is when there is greatest risk of
355:
279:
270:
265:
246:
242:
162:
260:
79:
1770:
1711:
1554:
761:
632:
319:
237:
Flame color depends on several factors, the most important typically being
200:
94:
63:
chemical reaction made in a thin zone. When flames are hot enough to have
1800:
1716:
1690:
1564:
1559:
421:
250:
1261:
1247:
1195:
221:
Spectrum of the blue (premixed, i.e., complete combustion) flame from a
1780:
1544:
1065:
797:
755:
515:
380:
371:; i.e., no loss of heat to the atmosphere (may differ in certain parts)
358:
emission of sodium atoms, specifically the very intense sodium D lines.
347:
339:
311:
230:
212:
158:
139:
60:
1234:
1785:
1775:
1765:
1706:
1678:
1574:
622:
618:
588:
577:
567:
417:
389:
368:
300:
150:
102:
1841:
1275:
Jones, John
Clifford (September 2003). "Low temperature oxidation".
1089:
467:. Statements consisting only of original research should be removed.
334:
1753:
1405:
1380:
1346:
Laminar Soot
Processes Experiment Shedding Light on Flame Radiation
739:
700:
690:
672:
662:
608:
523:
166:
98:
67:
gaseous components of sufficient density, they are then considered
33:
282:
of very fine soot particles that are produced in the flame. Also,
203:
of hot gas which then mixes with surrounding oxygen and combusts.
1277:
Hydrocarbon process safety: a text for students and professionals
1208:
1116:
1019:
564:
534:
97:
to vaporize (if this process happens in inert atmosphere without
950:
Zheng, Shu; Ni, Li; Liu, Huawei; Zhou, Huaichun (1 April 2019).
28:
1600:
904:. Cambridge, England: Cambridge University Press. p. 300.
729:
509:
343:
288:
222:
185:
106:
90:
64:
784:
1657:
1615:
733:
346:. The yellow color in this gas flame does not arise from the
118:, and these products then react with each other and with the
1605:
1595:
1497:
1356:
1310:
1152:
810:
720:, a compound of carbon and nitrogen with chemical formula C
351:
56:
1146:
1790:
122:
involved in the reaction of the following flame (fire).
1466:
1177:
310:
lines. In analytical chemistry, this effect is used in
1147:
Pearlman, Howard; Chapek, Richard M. (24 April 2000).
420:. When this occurs, combustible gases at or above the
114:, forming various incomplete combustion products and
93:. The applied heat causes the fuel molecules in the
1379:Timmes, F. X.; Woosley, S. E. (1 September 1992).
105:). In this state they can then readily react with
649:Max. flame temperature (in air, diffusion flame)
1854:
326:are used to produce brightly colored fireworks.
1142:
1140:
1090:Christopher W. Schmidt; Steve A. Symes (2008).
318:) to determine presence of some metal ions. In
1149:"Cool Flames and Autoignition in Microgravity"
949:
169:as an oxidizer of metallic fuels, e.g. in the
1482:
1378:
1311:National Aeronautics and Space Administration
1137:
1117:Jozef Jarosinski; Bernard Veyssiere (2009).
818:and private industry, especially concerning
428:
1489:
1475:
593:1,900–2,300 °C (3,452–4,172 °F)
583:1,700–1,950 °C (3,092–3,542 °F)
572:1,200–1,700 °C (2,192–3,092 °F)
1524:Native American use of fire in ecosystems
1404:
930:Science Questions with Surprising Answers
551:900–1,600 °C (1,652–2,912 °F)
483:Learn how and when to remove this message
1250:Journal of the American Chemical Society
1184:Journal of the American Chemical Society
783:
773:"Fire in space" redirects here. For the
540:900–1,500 °C (1,652–2,732 °F)
529:750–1,200 °C (1,382–2,192 °F)
333:
259:
216:
78:
27:
1733:International Flame Research Foundation
1279:. Tulsa, OK: PennWell. pp. 32–33.
862:International Flame Research Foundation
825:
1855:
1085:
1083:
712:
657:800–900 °C (1,472–1,652 °F)
627:Up to ≈2,300 °C (≈4,172 °F)
603:Up to ≈2,000 °C (≈3,632 °F)
1470:
1274:
161:and commonly used in rocket engines.
1826:
1430:Ultra-Low Emissions Low-Swirl Burner
1093:The analysis of burned human remains
1042:"Reaction of Chlorine with Hydrogen"
637:Up to 3,300 °C (5,972 °F)
432:
55:) is the visible, gaseous part of a
1447:
1080:
895:
13:
768:
14:
1874:
1417:
379:Percentage oxygen content of the
1836:
1825:
1815:
1814:
1096:. Academic Press. pp. 2–4.
685:1,027 °C (1,880.6 °F)
677:1,026 °C (1,878.8 °F)
437:
1514:Control of fire by early humans
1372:
1335:
1316:
1293:
1268:
1241:
1215:The Journal of Chemical Physics
1202:
1171:
514:~300 °C (~600 °F) (a
16:Visible, gaseous part of a fire
1110:
1052:
1034:
998:
943:
918:
889:
749:
706:1,390 °C (2,534 °F)
695:1,200 °C (2,192 °F)
613:2,020 °C (3,668 °F)
329:
1:
1300:Spiral flames in microgravity
882:
872:Oxidizing and reducing flames
1455:The Periodic Table of Videos
1426:(archived 30 September 2011)
1323:Candle Flame in Microgravity
667:990 °C (1,814 °F)
74:
7:
976:10.1016/j.ijleo.2019.02.077
926:"Do flames contain plasma?"
850:
463:the claims made and adding
316:flame emission spectroscopy
264:Different flame types of a
59:. It is caused by a highly
10:
1879:
1123:. CRC Press. p. 172.
772:
753:
210:
18:
1809:
1746:
1725:
1699:
1671:
1650:
1624:
1588:
1532:
1504:
1444:(archived 31 August 2017)
1385:The Astrophysical Journal
898:"Laminar premixed flames"
429:Common flame temperatures
1738:The Combustion Institute
1496:
1460:University of Nottingham
877:The Combustion Institute
225:torch showing molecular
206:
1351:11 January 2014 at the
1328:26 October 2011 at the
742:, with the formula (CN)
83:Zones in a candle flame
1580:Spontaneous combustion
843:(often in the form of
793:
359:
274:
234:
171:magnesium/teflon/viton
165:can be used to supply
86:
49:
36:
21:Flame (disambiguation)
1305:19 March 2010 at the
787:
337:
324:pyrotechnic colorants
263:
220:
82:
31:
1435:13 June 2017 at the
1366:20 July 2012 at the
845:degenerate electrons
841:thermal conductivity
837:thermonuclear energy
826:Thermonuclear flames
775:Battlestar Galactica
375:Atmospheric pressure
245:emission, with both
239:black-body radiation
19:For other uses, see
1397:1992ApJ...396..649T
1262:10.1021/ja01098a517
1227:1952JChPh..20..369T
1196:10.1021/ja01590a075
968:2019Optik.183..222Z
896:Law, C. K. (2006).
713:Highest temperature
128:methylidyne radical
1068:on 29 October 2007
1048:on 20 August 2008.
902:Combustion physics
833:Type Ia supernovae
794:
504:Flame temperature
448:possibly contains
360:
275:
255:rate of combustion
235:
229:band emission and
155:nitrogen tetroxide
87:
37:
1850:
1849:
1759:List of wildfires
1450:"Coloured Flames"
1286:978-1-59370-004-1
1235:10.1063/1.1700426
1130:978-0-8493-8408-0
1103:978-0-12-372510-3
710:
709:
641:
640:
493:
492:
485:
450:original research
308:emission spectrum
178:chemical kinetics
147:hydrogen chloride
1870:
1840:
1829:
1828:
1818:
1817:
1663:Death by burning
1625:Individual fires
1491:
1484:
1477:
1468:
1467:
1463:
1448:Licence, Peter.
1442:7 Shades of Fire
1411:
1410:
1408:
1376:
1370:
1339:
1333:
1320:
1314:
1297:
1291:
1290:
1272:
1266:
1265:
1245:
1239:
1238:
1206:
1200:
1199:
1175:
1169:
1168:
1166:
1164:
1155:. Archived from
1144:
1135:
1134:
1114:
1108:
1107:
1087:
1078:
1077:
1075:
1073:
1064:. Archived from
1056:
1050:
1049:
1044:. Archived from
1038:
1032:
1031:
1029:
1027:
1002:
996:
995:
947:
941:
940:
938:
936:
922:
916:
915:
893:
814:applications in
718:Dicyanoacetylene
646:Material burned
643:
642:
518:in low gravity)
501:Material burned
498:
497:
488:
481:
477:
474:
468:
465:inline citations
441:
440:
433:
1878:
1877:
1873:
1872:
1871:
1869:
1868:
1867:
1853:
1852:
1851:
1846:
1805:
1742:
1721:
1695:
1667:
1646:
1620:
1584:
1570:Fire protection
1528:
1500:
1495:
1437:Wayback Machine
1420:
1415:
1414:
1377:
1373:
1368:Wayback Machine
1353:Wayback Machine
1340:
1336:
1330:Wayback Machine
1321:
1317:
1307:Wayback Machine
1298:
1294:
1287:
1273:
1269:
1246:
1242:
1212:
1207:
1203:
1181:
1176:
1172:
1162:
1160:
1145:
1138:
1131:
1115:
1111:
1104:
1088:
1081:
1071:
1069:
1057:
1053:
1040:
1039:
1035:
1025:
1023:
1015:"What Is Fire?"
1013:
1010:Wayback Machine
1003:
999:
948:
944:
934:
932:
924:
923:
919:
912:
894:
890:
885:
853:
828:
820:fuel efficiency
816:applied science
782:
771:
769:In microgravity
758:
752:
745:
727:
723:
715:
703:(forced draft)
489:
478:
472:
469:
454:
442:
438:
431:
414:carbon monoxide
408:
369:Adiabatic flame
332:
284:carbon monoxide
215:
209:
193:diffusion flame
137:
132:diatomic carbon
101:, it is called
84:
77:
24:
17:
12:
11:
5:
1876:
1866:
1865:
1848:
1847:
1845:
1844:
1833:
1822:
1810:
1807:
1806:
1804:
1803:
1798:
1796:Slash-and-burn
1793:
1788:
1783:
1778:
1773:
1768:
1763:
1762:
1761:
1750:
1748:
1744:
1743:
1741:
1740:
1735:
1729:
1727:
1723:
1722:
1720:
1719:
1714:
1709:
1703:
1701:
1697:
1696:
1694:
1693:
1688:
1687:
1686:
1675:
1673:
1669:
1668:
1666:
1665:
1660:
1654:
1652:
1648:
1647:
1645:
1644:
1639:
1634:
1628:
1626:
1622:
1621:
1619:
1618:
1613:
1608:
1603:
1598:
1592:
1590:
1586:
1585:
1583:
1582:
1577:
1572:
1567:
1562:
1557:
1552:
1550:Dust explosion
1547:
1542:
1540:Chain reaction
1536:
1534:
1530:
1529:
1527:
1526:
1521:
1519:Historic fires
1516:
1510:
1508:
1502:
1501:
1494:
1493:
1486:
1479:
1471:
1465:
1464:
1445:
1439:
1427:
1419:
1418:External links
1416:
1413:
1412:
1406:10.1086/171746
1371:
1334:
1315:
1292:
1285:
1267:
1240:
1221:(3): 369–374.
1210:
1201:
1179:
1170:
1136:
1129:
1109:
1102:
1079:
1062:"GRI-Mech 3.0"
1051:
1033:
997:
942:
917:
910:
887:
886:
884:
881:
880:
879:
874:
869:
864:
859:
857:Flame detector
852:
849:
827:
824:
770:
767:
754:Main article:
751:
748:
743:
725:
721:
714:
711:
708:
707:
704:
697:
696:
693:
687:
686:
683:
679:
678:
675:
669:
668:
665:
659:
658:
655:
651:
650:
647:
639:
638:
635:
629:
628:
625:
615:
614:
611:
605:
604:
601:
599:Hydrogen torch
595:
594:
591:
585:
584:
581:
574:
573:
570:
561:
560:
557:
553:
552:
549:
542:
541:
538:
537:(natural gas)
531:
530:
527:
520:
519:
512:
506:
505:
502:
491:
490:
445:
443:
436:
430:
427:
426:
425:
407:
406:
403:
400:stoichiometric
396:
393:
386:
383:
377:
372:
365:
331:
328:
301:blue emissions
208:
205:
197:premixed flame
163:Fluoropolymers
135:
76:
73:
15:
9:
6:
4:
3:
2:
1875:
1864:
1861:
1860:
1858:
1843:
1839:
1834:
1832:
1823:
1821:
1812:
1811:
1808:
1802:
1799:
1797:
1794:
1792:
1789:
1787:
1784:
1782:
1779:
1777:
1774:
1772:
1769:
1767:
1764:
1760:
1757:
1756:
1755:
1752:
1751:
1749:
1745:
1739:
1736:
1734:
1731:
1730:
1728:
1726:Organizations
1724:
1718:
1715:
1713:
1710:
1708:
1705:
1704:
1702:
1698:
1692:
1689:
1685:
1682:
1681:
1680:
1677:
1676:
1674:
1670:
1664:
1661:
1659:
1656:
1655:
1653:
1649:
1643:
1640:
1638:
1635:
1633:
1630:
1629:
1627:
1623:
1617:
1614:
1612:
1609:
1607:
1604:
1602:
1599:
1597:
1594:
1593:
1591:
1587:
1581:
1578:
1576:
1573:
1571:
1568:
1566:
1563:
1561:
1558:
1556:
1553:
1551:
1548:
1546:
1543:
1541:
1538:
1537:
1535:
1531:
1525:
1522:
1520:
1517:
1515:
1512:
1511:
1509:
1507:
1503:
1499:
1492:
1487:
1485:
1480:
1478:
1473:
1472:
1469:
1461:
1457:
1456:
1451:
1446:
1443:
1440:
1438:
1434:
1431:
1428:
1425:
1422:
1421:
1407:
1402:
1398:
1394:
1390:
1386:
1382:
1375:
1369:
1365:
1362:
1358:
1354:
1350:
1347:
1344:
1338:
1331:
1327:
1324:
1319:
1312:
1308:
1304:
1301:
1296:
1288:
1282:
1278:
1271:
1263:
1259:
1255:
1251:
1244:
1236:
1232:
1228:
1224:
1220:
1216:
1205:
1197:
1193:
1189:
1185:
1174:
1159:on 1 May 2010
1158:
1154:
1150:
1143:
1141:
1132:
1126:
1122:
1121:
1113:
1105:
1099:
1095:
1094:
1086:
1084:
1067:
1063:
1055:
1047:
1043:
1037:
1022:
1021:
1016:
1011:
1007:
1001:
993:
989:
985:
981:
977:
973:
969:
965:
961:
957:
953:
946:
931:
927:
921:
913:
911:0-521-87052-6
907:
903:
899:
892:
888:
878:
875:
873:
870:
868:
867:Olympic flame
865:
863:
860:
858:
855:
854:
848:
846:
842:
838:
834:
823:
821:
817:
812:
807:
803:
799:
791:
786:
780:
779:Fire in Space
777:episode, see
776:
766:
763:
757:
747:
741:
737:
735:
731:
719:
705:
702:
699:
698:
694:
692:
689:
688:
684:
681:
680:
676:
674:
671:
670:
666:
664:
661:
660:
656:
653:
652:
648:
645:
644:
636:
634:
631:
630:
626:
624:
620:
617:
616:
612:
610:
607:
606:
602:
600:
597:
596:
592:
590:
587:
586:
582:
579:
576:
575:
571:
569:
566:
563:
562:
558:
556:Candle flame
555:
554:
550:
547:
546:Bunsen burner
544:
543:
539:
536:
533:
532:
528:
525:
522:
521:
517:
513:
511:
508:
507:
503:
500:
499:
496:
487:
484:
476:
473:December 2019
466:
462:
458:
452:
451:
446:This section
444:
435:
434:
423:
419:
415:
410:
409:
404:
401:
397:
394:
391:
387:
384:
382:
378:
376:
373:
370:
367:
366:
364:
357:
356:spectral line
353:
349:
345:
341:
336:
327:
325:
321:
317:
313:
309:
304:
302:
296:
294:
290:
285:
281:
280:incandescence
272:
271:band emission
267:
266:Bunsen burner
262:
258:
256:
252:
248:
247:spectral line
244:
243:spectral band
240:
232:
228:
224:
219:
214:
204:
202:
198:
194:
189:
187:
183:
179:
174:
173:composition.
172:
168:
164:
160:
156:
152:
148:
143:
141:
133:
129:
123:
121:
117:
116:free radicals
113:
108:
104:
100:
96:
92:
81:
72:
70:
66:
62:
58:
54:
53:
52:
46:
42:
35:
30:
26:
22:
1771:Firefighting
1712:Fire worship
1610:
1555:Fire ecology
1453:
1388:
1384:
1374:
1342:
1337:
1318:
1295:
1276:
1270:
1253:
1249:
1243:
1218:
1214:
1204:
1187:
1183:
1173:
1161:. Retrieved
1157:the original
1119:
1112:
1092:
1070:. Retrieved
1066:the original
1054:
1046:the original
1036:
1024:. Retrieved
1018:
1006:Ghostarchive
1004:Archived at
1000:
959:
955:
945:
933:. Retrieved
929:
920:
901:
891:
839:release and
829:
806:zero gravity
802:microgravity
795:
774:
762:Humphry Davy
759:
738:
716:
633:Oxyacetylene
494:
479:
470:
447:
361:
350:emission of
320:pyrotechnics
305:
297:
276:
236:
201:laminar flow
190:
175:
144:
124:
88:
48:
40:
38:
25:
1801:Fire making
1717:Terra preta
1691:Firefighter
1565:Flash point
1560:Fire piston
1391:: 649–667.
1190:(9): 2020.
1026:27 November
962:: 222–231.
750:Cool flames
654:Animal fat
580:flame peak
422:flash point
392:of the fuel
330:Temperature
312:flame tests
251:hydrocarbon
1842:Wiktionary
1781:Fire whirl
1679:Pyromanias
1637:By country
1589:Components
1545:Combustion
1341:C. H. Kim
1256:(2): 499.
1072:8 November
883:References
798:convection
756:Cool flame
516:cool flame
457:improve it
381:atmosphere
348:black-body
340:flame test
231:Swan bands
213:Flame test
211:See also:
159:hypergolic
140:Black body
95:candle wax
61:exothermic
43:(from
32:Flames of
1786:Blue lava
1776:Firestorm
1766:Backdraft
1754:Wildfires
1707:Cremation
1575:Pyrolysis
992:126553613
984:0030-4026
623:blowtorch
621:blowlamp/
619:Acetylene
589:Magnesium
578:Backdraft
568:blowtorch
461:verifying
418:backdraft
390:oxidation
157:which is
151:hydrazine
130:(CH) and
112:decompose
103:pyrolysis
75:Mechanism
1857:Category
1820:Category
1433:Archived
1364:Archived
1349:Archived
1326:Archived
1303:Archived
1008:and the
851:See also
740:Cyanogen
701:Charcoal
691:Methanol
673:Gasoline
663:Kerosene
609:MAPP gas
524:Charcoal
293:radicals
182:radicals
167:fluorine
120:oxidizer
99:oxidizer
34:charcoal
1831:Commons
1700:Culture
1642:By year
1632:By type
1533:Science
1506:History
1393:Bibcode
1313:, 2000.
1223:Bibcode
1020:YouTube
964:Bibcode
935:26 June
565:Propane
535:Methane
455:Please
227:radical
65:ionized
1835:
1824:
1813:
1672:People
1601:Oxygen
1343:et al.
1332:. NASA
1283:
1163:13 May
1127:
1100:
990:
982:
908:
790:zero-G
548:flame
510:Butane
344:sodium
322:, the
289:butane
223:butane
186:biogas
107:oxygen
91:candle
69:plasma
51:flamma
1747:Other
1684:Child
1658:Arson
1651:Crime
1616:Smoke
1611:Flame
988:S2CID
956:Optik
734:ozone
682:Wood
526:fire
207:Color
47:
45:Latin
41:flame
1863:Fire
1606:Heat
1596:Fuel
1498:Fire
1361:HTML
1357:NASA
1281:ISBN
1165:2010
1153:NASA
1125:ISBN
1098:ISBN
1074:2007
1028:2019
980:ISSN
937:2022
906:ISBN
811:NASA
398:How
388:Any
352:soot
342:for
314:(or
241:and
176:The
153:and
57:fire
1791:Ash
1401:doi
1389:396
1258:doi
1231:doi
1213:".
1192:doi
972:doi
960:183
847:).
804:or
788:In
459:by
142:).
1859::
1458:.
1452:.
1399:.
1387:.
1383:.
1359:,
1355:.
1309:,
1254:75
1252:.
1229:.
1219:20
1217:.
1188:78
1186:.
1151:.
1139:^
1082:^
1017:.
1012::
986:.
978:.
970:.
958:.
954:.
928:.
900:.
822:.
338:A
188:.
134:(C
71:.
39:A
1490:e
1483:t
1476:v
1462:.
1409:.
1403::
1395::
1289:.
1264:.
1260::
1237:.
1233::
1225::
1211:2
1198:.
1194::
1180:4
1167:.
1133:.
1106:.
1076:.
1030:.
994:.
974::
966::
939:.
914:.
781:.
744:2
730:K
726:2
724:N
722:4
486:)
480:(
475:)
471:(
453:.
273:.
136:2
23:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.