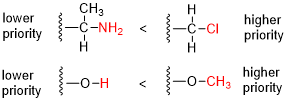512:
719:
246:. The IUPAC presentation of the rules constitute the official, formal standard for their use, and it notes that "the method has been developed to cover all compounds with ligancy up to 4... and… ligancy 6… for all configurations and conformations of such compounds." Nevertheless, though the IUPAC documentation presents a thorough introduction, it includes the caution that "it is essential to study the original papers, especially the 1966 paper, before using the sequence rule for other than fairly simple cases."
370:
1688:
1201:
451:
689:-symmetrical molecules where the rings lie approximately at right angles to each other and each molecule cannot be superposed on its mirror image. The spiro carbon, C, is a stereogenic centre, and priority can be assigned a>a′>b>b′, in which one ring (both give the same answer) contains atoms a and b adjacent to the spiro carbon, and the other contains a′ and b′. The configuration at C may then be assigned as for any other stereocentre.
785:
25:
862:
430:) by traversing bonds in all possible paths starting at the stereocenter. When the traversal encounters an atom through which the current path has already passed, a phantom atom is generated in order to keep the tree finite. A single atom of the original molecule may appear in many places (some as phantoms, some not) in the tree.
249:
A recent paper argues for changes to some of the rules (sequence rules 1b and 2) to address certain molecules for which the correct descriptors were unclear. However, a different problem remains: in rare cases, two different stereoisomers of the same molecule can have the same CIP descriptors, so the
377:
If an atom, A, is double-bonded to another atom, then atom A should be treated as though it is "connected to the same atom twice". An atom that is double-bonded has a higher priority than an atom that is single bonded. When dealing with double bonded priority groups, one is allowed to visit the same
381:
When B is replaced with a list of attached atoms, A itself, but not its "phantom", is excluded in accordance with the general principle of not doubling back along a bond that has just been followed. A triple bond is handled the same way except that A and B are each connected to two phantom atoms of
339:
If there is a tie, the atoms at distance 2 from the stereocenter have to be considered: a list is made for each group of further atoms bonded to the one directly attached to the stereocenter. Each list is arranged in order of decreasing atomic number Z. Then the lists are compared atom by atom; at
474:
have been assigned their priorities, the molecule is oriented in space so that the group with the lowest priority is pointed away from the observer. If the substituents are numbered from 1 (highest priority) to 4 (lowest priority), then the sense of rotation of a curve passing through 1, 2 and 3
343:
If there is still a tie, each atom in each of the two lists is replaced with a sublist of the other atoms bonded to it (at distance 3 from the stereocenter), the sublists are arranged in decreasing order of atomic number Z, and the entire structure is again compared atom by atom. This process is
483:, the lowest priority group (most times hydrogen) is positioned behind the plane or the hatched bond going away from the reader. The highest priority group will have an arc drawn connecting to the rest of the groups, finishing at the group of third priority. An arc drawn clockwise, has the
200:
so that the configuration of the entire molecule can be specified uniquely by including the descriptors in its systematic name. A molecule may contain any number of stereocenters and any number of double bonds, and each usually gives rise to two possible isomers. A molecule with an integer
1041:. A meso compound is superposable on its mirror image, therefore it reduces the number of stereoisomers predicted by the 2 rule. This occurs because the molecule obtains a plane of symmetry that causes the molecule to rotate around the central carbon–carbon bond. One example is meso-
877:
is connected to one hydrogen atom (not shown, priority 4) and three carbon atoms. The isopropenyl group has priority 1 (carbon atoms only), and for the two remaining carbon atoms, priority is decided with the carbon atoms two bonds removed from the stereocenter, one part of the
125:
An example of the prioritisation of structure within the CIP system. Priority is assigned according to the substitution of elements with higher atomic numbers, or other attached groups. In red is the substituent which determines the final priority (image
1315:)-stereocenter, as the priority of the chemical group has to be taken into account. That is, the absolute stereochemistry of the product is determined on its own and not by considering which face it was attacked from. In the above-mentioned example, if
503:
for 'right' and 'left', respectively. When naming an organic isomer, the abbreviation for either rectus or sinister assignment is placed in front of the name in parentheses. For example, 3-methyl-1-pentene with a rectus assignment is formatted as
665:. Some professionals have proposed a new rule to account for this. This rule states that "non-covalent interactions have a fictitious number between 0 and 1" when assigning priority. Compounds in which this occurs are referred to as
959:, or stereoisomers that are not enantiomers because they are not related as mirror-image copies. Pseudoephedrine and ephedrine are given different names because, as diastereomers, they have different chemical properties, even for
607:
For double bonded molecules, Cahn–Ingold–Prelog priority rules (CIP rules) are followed to determine the priority of substituents of the double bond. If both of the high priority groups are on the same side of the double bond
851:= 1). Lowest priority (i.e. number 4) is given to the hydrogen atom and as this atom points away from the viewer, the counterclockwise decrease in priority over the three remaining substituents completes the assignment as
1030:). This holds true also for compounds having more than two stereocenters: if two stereoisomers have at least one descriptor in common, they are diastereomers. If all the descriptors are opposite, they are enantiomers.
1728:
122:
454:
Two examples of stereocenters. The lowest substituent (number 4) is shown only by a wavy line, and is assumed to be behind the rest of the molecule. Both centers shown are
42:
373:
This example showcases the "divide and duplicate rule" for double bonds. The vinyl group (C=C) or alkene portion has a higher priority over the alkane (C−C) portion.
955:
rather than ephedrine. All four of these isomers are named 2-methylamino-1-phenyl-1-propanol in systematic nomenclature. However, ephedrine and pseudoephedrine are
234:
The key article setting out the CIP sequence rules was published in 1966, and was followed by further refinements, before it was incorporated into the rules of the
89:
1669:
61:
1500:, that make some of the stereoisomers "degenerate" (identical), so that this mathematical expression overestimates the number. See Clayden, op. cit., p. 317.
1554:
68:
2143:
2071:
1896:
1593:
336:) of the atoms directly attached to the stereocenter; the group having the atom of higher atomic number Z receives higher priority (i.e. number 1).
75:
2515:
882:
group (O, O, C, priority number 2) and one part of an alkene (C, C, H, priority number 3). The resulting counterclockwise rotation results in
466:
sp hybridized isomer contains four different substituents. All four substituents are assigned prorites based on its atomic numbers. After the
57:
1261:
are formed. When one face of a molecule is shielded by substituents or geometric constraints compared to the other face the faces are called
320:
descriptors are assigned by using a system for ranking priority of the groups attached to each stereocenter. This procedure, often known as
1963:"Is it possible to extend the Cahn-Ingold-Prelog priority rules to supramolecular structures and coordination compounds using lone pairs?"
1424:
1623:"Algorithmic Analysis of Cahn-Ingold-Prelog Rules of Stereochemistry: Proposals for Revised Rules and a Guide for Machine Implementation"
1683:
1925:
2191:
2534:
301:
171:
231:
of fewer than 4 (but including ligancy of 6 as well, this term referring to the "number of neighboring atoms" bonded to a center).
1621:
Hanson, Robert M.; Mayfield, John; Vainio, Mikko; Yerin, Andrey; Redkin, Dmitry
Vladimirovich; Musacchio, Sophia (30 July 2018).
1559:
1523:
1496:
The "usually" has its basis in the fact that molecules with chiral centers nevertheless may have mirror planes of symmetry, e.g.
1586:
IUPAC Chemical
Nomenclature and Structure Representation Division (2013). "P-9". In Favre, Henri A.; Powell, Warren H. (eds.).
82:
2048:
2021:
1605:
1437:
1396:
1862:
Cahn, R. S. (March 1964). "An introduction to the sequence rule: A system for the specification of absolute configuration".
406:) is classified as "Z." The stereoisomer with two higher priority groups on opposite sides of a carbon-carbon double bond (
1701:
1846:
1471:
1810:
Prelog, Vladlmir; Helmchen, Guenter (August 1982). "Basic
Principles of the CIP-System and Proposals for a Revision".
774:= 9). Allowing fluorine (lowest priority, number 4) to point away from the viewer the rotation is clockwise hence the
2598:
1937:
1794:
243:
108:
831:= 6) but priority is given to the latter because the carbon atom in the COOH group is connected to a second oxygen (
324:, is the heart of the CIP system. The overview in this section omits some rules that are needed only in rare cases.
2390:
1622:
2593:
1926:
International Union of Pure and
Applied Chemistry. Commission on the Nomenclature of Organic Chemistry (1993).
1769:
46:
2557:
2184:
1141:). To begin, the lowest-numbered (according to IUPAC systematic numbering) stereogenic center is given the
727:
562:
It is possible in rare cases that two substituents on an atom differ only in their absolute configuration (
344:
repeated recursively, each time with atoms one bond farther from the stereocenter, until the tie is broken.
2495:
340:
the earliest difference, the group containing the atom of higher atomic number Z receives higher priority.
2371:
2293:
2107:
1597:
1307:)-enantiomer. However, one should note that adding a chemical group to the prochiral center from the
402:-isomer. A stereoisomer that contains two higher priority groups on the same face of the double bond (
250:
CIP system may not be able to unambiguously name a stereoisomer, and other systems may be preferable.
2603:
2574:
2177:
658:
1932:. Robert Panico, Warren H. Powell, Jean-Claude Richer. Oxford: Blackwell Scientific Publications.
1426:
Rules for the
Nomenclature of Organic Chemistry: Section E: Stereochemistry (Recommendations 1974)
630:
word meaning "together"). If the high priority groups are on opposite sides of the double bond (
2240:
1585:
1514:
391:
155:
35:
1239:, both faces are identical and there is only one reaction product. When the nucleophile attacks
2466:
2231:
1443:
1345:
666:
273:
Assignment of priorities to the groups attached to each stereocenter or double-bonded atom; and
242:, in 1974. The rules have since been revised, most recently in 2013, as part of the IUPAC book
2011:
2413:
2353:
1340:
1254:
1228:
734:)-configuration would be a very simple chiral compound. The priorities are assigned based on
463:
163:
2009:
2417:
1871:
1587:
8:
1273:) also apply when assigning the face of a molecular group. The faces are then called the
631:
609:
511:
423:
228:
223:. The CIP sequence rules contribute to the precise naming of every stereoisomer of every
1875:
267:
2328:
2126:
1992:
1650:
1510:
1038:
685:)-7,7'-bis(diphenylphosphaneyl)-2,2',3,3'-tetrahydro-1,1'-spirobi), represent chiral, C
239:
151:
899:
If a compound has more than one chiral stereocenter, each center is denoted by either
2054:
2044:
2017:
2010:
Harold Hart; Christopher M. Hadad; Leslie E. Craine; David J. Hart (1 January 2011).
1984:
1943:
1933:
1842:
1790:
1765:
1642:
1601:
1467:
1433:
1392:
131:
2153:
2130:
2081:
1996:
1906:
1654:
1152:
the relative stereodescriptors alpha (α) and beta (β) are used. In the α anomer the
718:
551:. If the thumb points in the direction of the fourth substituent, the enantiomer is
2157:
2148:
2116:
2085:
2076:
1974:
1910:
1901:
1879:
1819:
1634:
1568:
1532:
966:
More generally, for any pair of enantiomers, all of the descriptors are opposite: (
657:
centers are formed, the configuration must be specified. Without the presence of a
311:
224:
927:) stereoisomers, which are distinct mirror-image forms of each other, making them
2220:
2200:
1589:
Nomenclature of
Organic Chemistry: IUPAC Recommendations and Preferred Names 2013
1550:
1518:
1461:
1367:
1258:
1250:
1208:
960:
952:
824:
627:
544:
480:
419:
369:
159:
1295:-face. Hydride addition as in a reduction process from this side will form the (
2436:
1262:
578:. When this happens, the descriptor of the stereocenter is a lowercase letter (
2058:
1929:
A guide to IUPAC nomenclature of organic compounds : recommendations 1993
1389:
March's advanced organic chemistry : reactions, mechanisms, and structure
2587:
2152:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
2080:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
1988:
1905:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
1638:
1497:
1042:
1034:
956:
816:
735:
329:
2161:
2121:
2102:
2089:
1947:
1914:
1459:
1235:
group from two opposite sides or faces. When an achiral nucleophile attacks
570:). If the relative priorities of these substituents need to be established,
258:
The steps for naming molecules using the CIP system are often presented as:
2266:
1823:
1646:
1572:
1557:(1982). "Basic Principles of the CIP-System and Proposals for a Revision".
1536:
1350:
1288:
1245:
1204:
1078:
616:
602:
476:
471:
263:
216:
209:
186:
182:
167:
1979:
1962:
1927:
1224:
808:
654:
467:
358:
197:
1702:"Assigning Cahn-Ingold-Prelog (CIP) Priorities (2) - The Method of Dots"
1265:. The same rules that determine the stereochemistry of a stereocenter (
932:
928:
220:
121:
2038:
1883:
1200:
998:). Diastereomers have at least one descriptor in common; for example (
2215:
1674:. Abstracts of papers of the American Chemical Society. Vol. 254
908:
450:
1466:(2nd ed.). Oxford, UK: Oxford University Press. pp. 316f.
784:
24:
2448:
2103:"Basic terminology of stereochemistry (IUPAC Recommendations 1996)"
1316:
1240:
1232:
1090:
844:
800:
793:
767:
759:
2169:
1391:(6. ed.). Hoboken, NJ: Wiley-Interscience. pp. 155–162.
1236:
870:
751:
662:
354:
1509:
1362:
1220:
1149:
879:
840:
743:
697:
The following are examples of application of the nomenclature.
1460:
Clayden, Jonathan; Greeves, Nick & Warren, Stuart (2012).
861:
500:
235:
170:
of a molecule. The purpose of the CIP system is to assign an
547:: one wraps the molecule with the fingers in the direction
535:
A practical method of determining whether an enantiomer is
1620:
205:
describing the number of stereocenters will usually have
162:) are a standard process to completely and unequivocally
1671:
Comparing CIP implementations: The need for an open CIP
1101:*) means two centers having identical configurations, (
1069:
stereocenters occur in symmetrically positioned pairs.
637:), then the stereoisomer is assigned the configuration
2036:
1549:
1287:. In the example displayed on the right, the compound
1125:*) means two centers having opposite configurations, (
491:) assignment. An arc drawn counterclockwise, has the
677:
Some spiro compounds, for example the SDP ligands ((
1668:Mayfield, John; Lowe, Daniel; Sayle, Roger (2017).
1037:is an achiral molecule, despite having two or more
49:. Unsourced material may be challenged and removed.
1812:Angewandte Chemie International Edition in English
1687:
1323:= 17) were added to the prochiral center from the
1764:(2nd ed.). Garland Science. pp. 52–61.
1667:
799:highest priority (i.e. number 1) is given to the
586:) instead of the uppercase letter normally used.
236:International Union of Pure and Applied Chemistry
2585:
1521:(1966). "Specification of Molecular Chirality".
16:Naming convention for stereoisomers of molecules
1386:
1809:
1784:
1211:. The H atom has the lowest priority number 4.
1176:), whereas in the β anomer they are the same (
894:
2185:
2003:
701:
499:) assignment. The names are derived from the
1627:Journal of Chemical Information and Modeling
1215:Stereochemistry also plays a role assigning
868:
791:
725:
418:To handle a molecule containing one or more
2100:
1789:. Oxford University Press. pp. 38–39.
296:
2192:
2178:
1803:
1785:Okuyama, Tadashi; Maskill, Howard (2014).
1699:
1453:
1422:
1299:)-enantiomer and attack from the opposite
364:
2120:
1978:
1787:Organic Chemistry, A Mechanistic Approach
1492:
1490:
1072:
648:
109:Learn how and when to remove this message
1579:
1387:March, Jerry; Michael B., Smith (2007).
1199:
510:
449:
433:
368:
238:(IUPAC), the official body that defines
120:
2496:Pseudoasymmetric (pseudochiral) centers
2476:CIP (Cahn–Ingold–Prelog) priority rules
1960:
1759:
1560:Angewandte Chemie International Edition
1524:Angewandte Chemie International Edition
1418:
1416:
1414:
1412:
1410:
1408:
931:. This compound also exists as the two
2586:
1487:
385:
2173:
1836:
1755:
1753:
1751:
1749:
1723:
1721:
438:
398:-isomer has higher priority than the
1861:
1841:(2nd ed.). Wiley. p. 203.
1405:
589:
47:adding citations to reliable sources
18:
2199:
2016:. Cengage Learning. pp. 177–.
1253:results. When the nucleophile is a
413:
390:If two substituents on an atom are
253:
58:"Cahn–Ingold–Prelog priority rules"
13:
2149:Compendium of Chemical Terminology
2077:Compendium of Chemical Terminology
1902:Compendium of Chemical Terminology
1746:
1718:
1311:-face will not always lead to an (
1081:may be denoted by the descriptors
1077:The relative configuration of two
860:
783:
717:
712:assignments for several compounds
672:
422:, one must first expand it into a
378:atom twice as one creates an arc.
219:each having an associated pair of
14:
2615:
2013:Organic Chemistry: A Short Course
1160:do have opposite configurations (
645:, German word meaning "opposed")
244:Nomenclature of Organic Chemistry
1700:Ashenhurst, James (2017-01-17).
1686:
1327:-face, this would result in an (
827:group (COOH) have carbon atoms (
23:
2137:
2094:
2065:
2037:Bruice, Paula Yurkanis (2007).
2030:
1954:
1919:
1890:
1855:
1830:
1778:
1762:Instant Notes Organic Chemistry
1693:
1243:, the faces are not identical (
859:
782:
716:
34:needs additional citations for
1837:Klein, David R. (2013-12-31).
1729:"3.6 Cahn-Ingold Prelog Rules"
1684:Abstract on publisher web site
1661:
1614:
1543:
1503:
1423:Cross, L.C; Klyne, W. (1974).
1380:
1219:to trigonal molecules such as
1061:) form. In meso compounds the
619:is assigned the configuration
1:
1907:pseudo-asymmetric carbon atom
1864:Journal of Chemical Education
1373:
1014:) are diastereomers, as are (
531:)-1,2,3-trichlorocyclopentane
361:is used to set the priority.
353:If two groups differ only in
1961:Elguero, José (2016-12-01).
728:bromochlorofluoroiodomethane
7:
1334:
982:) are enantiomers, as are (
895:Describing multiple centers
692:
661:interaction, a compound is
348:
227:molecule with all atoms of
10:
2620:
2558:Octahedral propeller twist
2294:Arene substitution pattern
2108:Pure and Applied Chemistry
726:The hypothetical molecule
600:
2572:
2556:
2533:
2514:
2494:
2474:
2465:
2447:
2435:
2412:
2389:
2370:
2352:
2327:
2292:
2265:
2239:
2232:Configuration descriptors
2230:
2207:
2043:. Pearson Prentice Hall.
704:
2599:Eponymous chemical rules
2575:Category:Stereochemistry
1760:Patrick, Graham (2004).
1706:Master Organic Chemistry
1639:10.1021/acs.jcim.8b00324
1195:
410:) is classified as "E."
297:Assignment of priorities
2467:Absolute configurations
2372:Three identical ligands
2162:10.1351/goldbook.R05308
2122:10.1351/pac199668122193
2090:10.1351/goldbook.R05260
1967:Chemistry International
1915:10.1351/goldbook.P04921
508:)-3-methyl-1-pentene.
365:Double and triple bonds
156:Christopher Kelk Ingold
148:CIP priority convention
2535:Relative configuration
2082:Relative Configuration
1824:10.1002/anie.198205671
1573:10.1002/anie.198205671
1537:10.1002/anie.196603851
1346:Descriptor (chemistry)
1212:
1073:Relative configuration
1053:) is the same as the (
865:
835:= 8) whereas in the CH
788:
722:
667:coordination compounds
649:Coordination compounds
532:
481:configurational isomer
459:
374:
127:
2594:Chemical nomenclature
2354:Syn and anti addition
1341:Chirality (chemistry)
1303:-face will give the (
1229:nucleophilic addition
1203:
864:
792:In the assignment of
787:
721:
514:
453:
434:Assigning descriptors
372:
124:
2384:(facies, meridonal)
2101:Moss, G. P. (1996).
1980:10.1515/ci-2016-0633
1733:Chemistry LibreTexts
1154:anomeric carbon atom
869:The stereocenter in
653:In some cases where
574:takes priority over
428:hierarchical digraph
240:organic nomenclature
43:improve this article
2391:In carbon skeletons
1876:1964JChEd..41..116C
1291:is viewed from the
1039:stereogenic centers
951:), which are named
555:; otherwise, it is
394:of each other, the
386:Geometrical isomers
1213:
866:
843:is connected to a
789:
723:
533:
475:distinguishes the
460:
375:
357:, then the larger
322:the sequence rules
262:Identification of
152:Robert Sidney Cahn
136:Cahn–Ingold–Prelog
128:
2581:
2580:
2568:
2567:
2461:
2460:
2115:(12): 2193–2222.
2050:978-0-13-199631-1
2040:Organic chemistry
2023:978-1-133-17283-3
1884:10.1021/ed041p116
1839:Organic Chemistry
1607:978-0-85404-182-4
1463:Organic Chemistry
1439:978-0-08-021019-3
1398:978-0-471-72091-1
1231:can approach the
1148:To designate two
890:
889:
796:
515:An example of a (
392:geometric isomers
132:organic chemistry
119:
118:
111:
93:
2611:
2550:
2549:
2544:
2543:
2516:Optical rotation
2472:
2471:
2237:
2236:
2194:
2187:
2180:
2171:
2170:
2164:
2141:
2135:
2134:
2124:
2098:
2092:
2069:
2063:
2062:
2034:
2028:
2027:
2007:
2001:
2000:
1982:
1958:
1952:
1951:
1923:
1917:
1894:
1888:
1887:
1859:
1853:
1852:
1834:
1828:
1827:
1807:
1801:
1800:
1782:
1776:
1775:
1757:
1744:
1743:
1741:
1740:
1725:
1716:
1715:
1713:
1712:
1697:
1691:
1690:
1689:
1682:
1680:
1679:
1665:
1659:
1658:
1633:(9): 1755–1765.
1618:
1612:
1611:
1583:
1577:
1576:
1547:
1541:
1540:
1507:
1501:
1494:
1485:
1484:
1482:
1480:
1457:
1451:
1450:
1448:
1442:. Archived from
1431:
1420:
1403:
1402:
1384:
1259:diastereoisomers
961:racemic mixtures
794:
702:
550:
543:is by using the
519:) descriptor: (1
414:Cyclic molecules
254:Steps for naming
215:
208:
204:
114:
107:
103:
100:
94:
92:
51:
27:
19:
2619:
2618:
2614:
2613:
2612:
2610:
2609:
2608:
2604:Stereochemistry
2584:
2583:
2582:
2577:
2564:
2552:
2547:
2546:
2541:
2540:
2529:
2510:
2490:
2457:
2443:
2431:
2408:
2385:
2366:
2348:
2323:
2288:
2261:
2226:
2225:
2221:Racemic mixture
2203:
2201:Stereochemistry
2198:
2168:
2167:
2142:
2138:
2099:
2095:
2070:
2066:
2051:
2035:
2031:
2024:
2008:
2004:
1959:
1955:
1940:
1924:
1920:
1895:
1891:
1860:
1856:
1849:
1835:
1831:
1808:
1804:
1797:
1783:
1779:
1772:
1758:
1747:
1738:
1736:
1727:
1726:
1719:
1710:
1708:
1698:
1694:
1677:
1675:
1666:
1662:
1619:
1615:
1608:
1584:
1580:
1548:
1544:
1508:
1504:
1495:
1488:
1478:
1476:
1474:
1458:
1454:
1446:
1440:
1429:
1421:
1406:
1399:
1385:
1381:
1376:
1368:Stereochemistry
1337:
1251:racemic product
1209:α-phenylethanol
1198:
1075:
953:pseudoephedrine
907:. For example,
897:
838:
825:carboxylic acid
822:
814:
695:
688:
675:
673:Spiro compounds
651:
605:
599:
548:
545:right-hand rule
448:
439:Stereocenters:
436:
416:
388:
367:
351:
299:
256:
213:
206:
202:
160:Vladimir Prelog
115:
104:
98:
95:
52:
50:
40:
28:
17:
12:
11:
5:
2617:
2607:
2606:
2601:
2596:
2579:
2578:
2573:
2570:
2569:
2566:
2565:
2562:
2560:
2554:
2553:
2539:
2537:
2531:
2530:
2521:(+)-, (−)- or
2520:
2518:
2512:
2511:
2500:
2498:
2492:
2491:
2480:
2478:
2469:
2463:
2462:
2459:
2458:
2453:
2451:
2445:
2444:
2441:
2439:
2437:Spiro compound
2433:
2432:
2422:
2420:
2410:
2409:
2395:
2393:
2387:
2386:
2376:
2374:
2368:
2367:
2358:
2356:
2350:
2349:
2340:
2338:
2325:
2324:
2298:
2296:
2290:
2289:
2278:
2276:
2263:
2262:
2252:
2250:
2234:
2228:
2227:
2224:
2223:
2218:
2212:
2211:
2209:
2205:
2204:
2197:
2196:
2189:
2182:
2174:
2166:
2165:
2136:
2093:
2064:
2049:
2029:
2022:
2002:
1953:
1938:
1918:
1889:
1854:
1848:978-1118454312
1847:
1829:
1818:(8): 567–583.
1802:
1795:
1777:
1770:
1745:
1717:
1692:
1660:
1613:
1606:
1578:
1542:
1531:(4): 385–415.
1502:
1498:meso compounds
1486:
1473:978-0199270293
1472:
1452:
1449:on 2016-04-07.
1438:
1404:
1397:
1378:
1377:
1375:
1372:
1371:
1370:
1365:
1360:
1348:
1343:
1336:
1333:
1331:)-enantiomer.
1263:diastereotopic
1197:
1194:
1158:reference atom
1145:* descriptor.
1074:
1071:
896:
893:
892:
891:
888:
887:
867:
857:
856:
836:
820:
812:
790:
780:
779:
730:shown in its (
724:
714:
713:
694:
691:
686:
674:
671:
650:
647:
601:Main article:
598:
590:Double bonds:
588:
447:
437:
435:
432:
415:
412:
387:
384:
366:
363:
350:
347:
346:
345:
341:
337:
298:
295:
294:
293:
276:Assignment of
274:
271:
255:
252:
150:; named after
144:sequence rules
117:
116:
31:
29:
22:
15:
9:
6:
4:
3:
2:
2616:
2605:
2602:
2600:
2597:
2595:
2592:
2591:
2589:
2576:
2571:
2561:
2559:
2555:
2538:
2536:
2532:
2528:
2524:
2519:
2517:
2513:
2508:
2504:
2499:
2497:
2493:
2488:
2484:
2479:
2477:
2473:
2470:
2468:
2464:
2456:
2452:
2450:
2446:
2440:
2438:
2434:
2429:
2425:
2421:
2419:
2415:
2411:
2406:
2402:
2398:
2394:
2392:
2388:
2383:
2379:
2375:
2373:
2369:
2365:
2361:
2357:
2355:
2351:
2347:
2343:
2339:
2337:
2335:
2331:
2326:
2321:
2317:
2313:
2309:
2305:
2301:
2297:
2295:
2291:
2286:
2282:
2277:
2275:
2273:
2269:
2264:
2259:
2255:
2251:
2249:
2247:
2243:
2238:
2235:
2233:
2229:
2222:
2219:
2217:
2214:
2213:
2210:
2206:
2202:
2195:
2190:
2188:
2183:
2181:
2176:
2175:
2172:
2163:
2159:
2155:
2151:
2150:
2145:
2140:
2132:
2128:
2123:
2118:
2114:
2110:
2109:
2104:
2097:
2091:
2087:
2083:
2079:
2078:
2073:
2068:
2060:
2056:
2052:
2046:
2042:
2041:
2033:
2025:
2019:
2015:
2014:
2006:
1998:
1994:
1990:
1986:
1981:
1976:
1972:
1969:(in German).
1968:
1964:
1957:
1949:
1945:
1941:
1939:0-632-03702-4
1935:
1931:
1930:
1922:
1916:
1912:
1908:
1904:
1903:
1898:
1893:
1885:
1881:
1877:
1873:
1869:
1865:
1858:
1850:
1844:
1840:
1833:
1825:
1821:
1817:
1813:
1806:
1798:
1796:9780199693276
1792:
1788:
1781:
1773:
1767:
1763:
1756:
1754:
1752:
1750:
1734:
1730:
1724:
1722:
1707:
1703:
1696:
1685:
1673:
1672:
1664:
1656:
1652:
1648:
1644:
1640:
1636:
1632:
1628:
1624:
1617:
1609:
1603:
1599:
1595:
1591:
1590:
1582:
1574:
1570:
1567:(8): 567–58.
1566:
1562:
1561:
1556:
1552:
1546:
1538:
1534:
1530:
1526:
1525:
1520:
1516:
1512:
1506:
1499:
1493:
1491:
1475:
1469:
1465:
1464:
1456:
1445:
1441:
1435:
1428:
1427:
1419:
1417:
1415:
1413:
1411:
1409:
1400:
1394:
1390:
1383:
1379:
1369:
1366:
1364:
1361:
1359:
1357:
1353:
1349:
1347:
1344:
1342:
1339:
1338:
1332:
1330:
1326:
1322:
1318:
1314:
1310:
1306:
1302:
1298:
1294:
1290:
1286:
1284:
1279:
1277:
1272:
1268:
1264:
1260:
1256:
1252:
1248:
1247:
1242:
1238:
1234:
1230:
1226:
1222:
1218:
1210:
1206:
1202:
1193:
1191:
1187:
1183:
1179:
1175:
1171:
1167:
1163:
1159:
1155:
1151:
1146:
1144:
1140:
1136:
1132:
1128:
1124:
1120:
1116:
1112:
1108:
1104:
1100:
1096:
1092:
1088:
1084:
1080:
1079:stereoisomers
1070:
1068:
1064:
1060:
1056:
1052:
1048:
1044:
1043:tartaric acid
1040:
1036:
1035:meso compound
1031:
1029:
1025:
1021:
1017:
1013:
1009:
1005:
1001:
997:
993:
989:
985:
981:
977:
973:
969:
964:
962:
958:
957:diastereomers
954:
950:
946:
942:
938:
934:
930:
926:
922:
918:
914:
910:
906:
902:
885:
881:
876:
874:
863:
858:
854:
850:
846:
842:
834:
830:
826:
818:
817:hydroxymethyl
810:
806:
802:
798:
786:
781:
777:
773:
769:
765:
761:
757:
753:
749:
745:
741:
737:
736:atomic number
733:
729:
720:
715:
711:
707:
703:
700:
699:
698:
690:
684:
680:
670:
668:
664:
660:
656:
646:
644:
640:
636:
635:configuration
634:
629:
626:
622:
618:
614:
613:configuration
612:
604:
597:
593:
587:
585:
581:
577:
573:
569:
565:
560:
558:
554:
546:
542:
538:
530:
526:
522:
518:
513:
509:
507:
502:
498:
494:
490:
486:
482:
478:
477:stereoisomers
473:
469:
465:
457:
452:
446:
442:
431:
429:
425:
421:
411:
409:
405:
401:
397:
393:
383:
379:
371:
362:
360:
356:
342:
338:
335:
331:
330:atomic number
327:
326:
325:
323:
319:
318:
314:
309:
308:
304:
291:
287:
283:
279:
275:
272:
269:
265:
264:stereocenters
261:
260:
259:
251:
247:
245:
241:
237:
232:
230:
226:
222:
218:
217:diastereomers
211:
210:stereoisomers
199:
195:
193:
189:
184:
180:
178:
174:
169:
165:
161:
157:
153:
149:
145:
141:
137:
133:
123:
113:
110:
102:
99:February 2016
91:
88:
84:
81:
77:
74:
70:
67:
63:
60: –
59:
55:
54:Find sources:
48:
44:
38:
37:
32:This article
30:
26:
21:
20:
2526:
2522:
2506:
2502:
2486:
2482:
2475:
2454:
2427:
2423:
2404:
2400:
2396:
2381:
2377:
2363:
2359:
2345:
2341:
2333:
2329:
2319:
2315:
2311:
2307:
2303:
2299:
2284:
2280:
2271:
2267:
2257:
2253:
2245:
2241:
2147:
2139:
2112:
2106:
2096:
2075:
2067:
2039:
2032:
2012:
2005:
1973:(6): 30–31.
1970:
1966:
1956:
1928:
1921:
1900:
1892:
1867:
1863:
1857:
1838:
1832:
1815:
1811:
1805:
1786:
1780:
1761:
1737:. Retrieved
1735:. 2014-08-05
1732:
1709:. Retrieved
1705:
1695:
1676:. Retrieved
1670:
1663:
1630:
1626:
1616:
1588:
1581:
1564:
1558:
1555:Helmchen, G.
1545:
1528:
1522:
1515:Ingold, C.K.
1505:
1477:. Retrieved
1462:
1455:
1444:the original
1425:
1388:
1382:
1355:
1351:
1328:
1324:
1320:
1312:
1308:
1304:
1300:
1296:
1292:
1289:acetophenone
1282:
1281:
1275:
1274:
1270:
1266:
1246:enantiotopic
1244:
1216:
1214:
1205:Acetophenone
1189:
1185:
1181:
1177:
1173:
1169:
1165:
1161:
1157:
1153:
1147:
1142:
1138:
1134:
1130:
1126:
1122:
1118:
1114:
1110:
1106:
1102:
1098:
1094:
1086:
1082:
1076:
1066:
1062:
1058:
1054:
1050:
1046:
1045:, in which (
1032:
1027:
1023:
1019:
1015:
1011:
1007:
1003:
999:
995:
991:
987:
983:
979:
975:
971:
967:
965:
948:
944:
940:
936:
924:
920:
916:
912:
911:exists in (1
904:
900:
898:
883:
872:
852:
848:
832:
828:
823:OH) and the
815:). Both the
807:= 7) in the
804:
778:assignment.
775:
771:
763:
755:
747:
739:
731:
709:
705:
696:
682:
678:
676:
659:non-covalent
652:
642:
638:
632:
624:
620:
617:stereoisomer
615:), then the
610:
606:
603:E-Z notation
595:
591:
583:
579:
575:
571:
567:
563:
561:
556:
552:
540:
536:
534:
528:
524:
520:
516:
505:
496:
492:
488:
484:
472:stereocenter
468:substituents
461:
455:
444:
440:
427:
417:
407:
403:
399:
395:
389:
380:
376:
352:
333:
328:Compare the
321:
316:
312:
306:
302:
300:
292:descriptors.
289:
285:
281:
277:
268:double bonds
257:
248:
233:
191:
187:
183:stereocenter
176:
172:
168:stereoisomer
147:
143:
139:
135:
129:
105:
96:
86:
79:
72:
65:
53:
41:Please help
36:verification
33:
1225:nucleophile
933:enantiomers
929:enantiomers
809:amino group
766:= 17) >
758:= 35) >
750:= 53) >
655:stereogenic
382:the other.
359:atomic mass
221:enantiomers
198:double bond
2588:Categories
2059:1046519135
1870:(3): 116.
1771:0203427610
1739:2022-11-18
1711:2022-11-18
1678:2020-07-22
1551:Prelog, V.
1519:Prelog, V.
1511:Cahn, R.S.
1479:2 February
1374:References
935:written (1
426:(called a
194:descriptor
179:descriptor
146:(also the
69:newspapers
2414:Secondary
2336:isomerism
2248:isomerism
2216:Chirality
1989:1365-2192
1257:molecule
963:of each.
909:ephedrine
875:)-carvone
839:OH group
819:group (CH
625:zusammen,
549:1 → 2 → 3
2449:Catenane
2418:tertiary
2274:notation
2131:98272391
1997:99300397
1948:27431284
1655:51876996
1647:30059222
1358:notation
1335:See also
1317:chloride
1249:) and a
1241:butanone
1233:carbonyl
1156:and the
1091:asterisk
1089:with an
943:) and (1
919:) and (1
845:hydrogen
801:nitrogen
768:fluorine
760:chlorine
693:Examples
681:)- and (
643:entgegen
493:sinister
458:isomers.
355:isotopes
349:Isotopes
196:to each
181:to each
2407:, cyclo
2270:–
1872:Bibcode
1237:acetone
1221:ketones
1150:anomers
1022:) and (
1006:) and (
990:) and (
974:) and (
797:-serine
752:bromine
663:achiral
479:. In a
229:ligancy
225:organic
185:and an
126:above).
83:scholar
2563:Λ-, Δ-
2455:catena
2208:Topics
2154:Re, Si
2129:
2057:
2047:
2020:
1995:
1987:
1946:
1936:
1845:
1793:
1768:
1653:
1645:
1604:
1553:&
1470:
1436:
1395:
1363:Isomer
1255:chiral
1184:) or (
1168:) or (
1133:) or (
1109:) or (
1093:(*). (
847:atom (
841:carbon
803:atom (
744:iodine
628:German
485:rectus
464:chiral
420:cycles
212:, and
158:, and
134:, the
85:
78:
71:
64:
56:
2442:spiro
2312:ortho
2283:)-, (
2258:trans
2246:trans
2144:IUPAC
2127:S2CID
2072:IUPAC
1993:S2CID
1897:IUPAC
1651:S2CID
1594:IUPAC
1447:(PDF)
1430:(PDF)
1285:-face
1278:-face
1227:in a
1217:faces
1196:Faces
633:trans
501:Latin
470:of a
408:trans
90:JSTOR
76:books
2505:), (
2485:), (
2428:tert
2416:and
2364:anti
2342:endo
2330:Endo
2320:para
2316:meta
2055:OCLC
2045:ISBN
2018:ISBN
1985:ISSN
1944:OCLC
1934:ISBN
1843:ISBN
1791:ISBN
1766:ISBN
1643:PMID
1602:ISBN
1481:2016
1468:ISBN
1434:ISBN
1393:ISBN
1280:and
1223:. A
1207:and
1117:); (
1085:and
1065:and
880:keto
424:tree
310:and
284:and
266:and
164:name
62:news
2545:-,
2426:-,
2424:sec
2405:neo
2401:iso
2382:mer
2378:fac
2360:syn
2346:exo
2344:,
2334:exo
2310:- (
2306:-,
2302:-,
2256:-,
2254:cis
2242:cis
2158:doi
2156:".
2117:doi
2086:doi
2084:".
1975:doi
1911:doi
1909:".
1880:doi
1820:doi
1635:doi
1598:RSC
1569:doi
1533:doi
1269:or
1192:).
903:or
811:(NH
742:):
611:cis
582:or
566:or
539:or
404:cis
190:or
175:or
140:CIP
130:In
45:by
2590::
2527:l-
2525:,
2523:d-
2403:,
2399:,
2380:,
2362:,
2318:,
2314:,
2287:)-
2146:,
2125:.
2113:68
2111:.
2105:.
2074:,
2053:.
1991:.
1983:.
1971:38
1965:.
1942:.
1899:,
1878:.
1868:41
1866:.
1816:21
1814:.
1748:^
1731:.
1720:^
1704:.
1649:.
1641:.
1631:58
1629:.
1625:.
1600:.
1592:.
1565:21
1563:.
1527:.
1517:;
1513:;
1489:^
1432:.
1407:^
1325:Re
1309:Re
1301:Si
1293:Re
1283:Si
1276:Re
1121:*,
1097:*,
1033:A
947:,2
939:,2
923:,2
915:,2
886:.
855:.
669:.
559:.
527:,3
523:,2
462:A
166:a
154:,
142:)
2551:-
2548:L
2542:D
2509:)
2507:s
2503:r
2501:(
2489:)
2487:S
2483:R
2481:(
2430:-
2397:n
2332:-
2322:)
2308:p
2304:m
2300:o
2285:Z
2281:E
2279:(
2272:Z
2268:E
2260:-
2244:–
2193:e
2186:t
2179:v
2160::
2133:.
2119::
2088::
2061:.
2026:.
1999:.
1977::
1950:.
1913::
1886:.
1882::
1874::
1851:.
1826:.
1822::
1799:.
1774:.
1742:.
1714:.
1681:.
1657:.
1637::
1610:.
1596:–
1575:.
1571::
1539:.
1535::
1529:5
1483:.
1401:.
1356:Z
1354:–
1352:E
1329:R
1321:Z
1319:(
1313:S
1305:R
1297:S
1271:S
1267:R
1190:S
1188:,
1186:S
1182:R
1180:,
1178:R
1174:R
1172:,
1170:S
1166:S
1164:,
1162:R
1143:R
1139:R
1137:,
1135:S
1131:S
1129:,
1127:R
1123:S
1119:R
1115:S
1113:,
1111:S
1107:R
1105:,
1103:R
1099:R
1095:R
1087:S
1083:R
1067:S
1063:R
1059:R
1057:,
1055:S
1051:S
1049:,
1047:R
1028:S
1026:,
1024:S
1020:R
1018:,
1016:S
1012:R
1010:,
1008:R
1004:S
1002:,
1000:R
996:R
994:,
992:S
988:S
986:,
984:R
980:S
978:,
976:S
972:R
970:,
968:R
949:S
945:S
941:R
937:R
925:R
921:S
917:S
913:R
905:S
901:R
884:S
873:S
871:(
853:S
849:Z
837:2
833:Z
829:Z
821:2
813:2
805:Z
795:L
776:R
772:Z
770:(
764:Z
762:(
756:Z
754:(
748:Z
746:(
740:Z
738:(
732:R
710:S
708:/
706:R
687:2
683:S
679:R
641:(
639:E
623:(
621:Z
608:(
596:Z
594:/
592:E
584:s
580:r
576:S
572:R
568:S
564:R
557:S
553:R
541:S
537:R
529:S
525:s
521:R
517:s
506:R
504:(
497:S
495:(
489:R
487:(
456:S
445:S
443:/
441:R
400:E
396:Z
334:Z
332:(
317:Z
315:/
313:E
307:S
305:/
303:R
290:Z
288:/
286:E
282:S
280:/
278:R
270:;
214:2
207:2
203:n
192:Z
188:E
177:S
173:R
138:(
112:)
106:(
101:)
97:(
87:·
80:·
73:·
66:·
39:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.