25:
185:
125:(i.e., a uniform substance that has the same composition throughout the material). The perfect pure chemical will pass all attempts to separate and purify it further. Thirdly, and here we focus on the common chemical definition, it should not contain any trace of any other kind of chemical species. In reality, there are no absolutely 100% pure chemical compounds, as there is always some small amount of
268:
286:, the elements added to the original crystal structure, contain a different number of electrons then the base formula. Semiconductors that are p-doped contains a small amount of elements that have less valence electrons then the other elements in the crystal. N-doping is the opposite and the dopant contains more valence electrons.
145:
to acquire certain properties of a material such as the color in gemstones or conductivity in semiconductors. Impurities may also affect crystallization as they can act as nucleation sites that start crystal growth. Impurities can also play a role in nucleation of other phase transitions in the form of defects.
144:
of a chemical or commercial product. During production, impurities may be purposely or accidentally added to the substance. The removal of unwanted impurities may require the use of separation or purification techniques such as distillation or zone refining. In other cases, impurities might be added
207:
No matter what method is used, it is usually impossible to separate an impurity completely from a material. The reason that it is impossible to remove impurities completely is of thermodynamic nature and is predicted by the second law of thermodynamics. Removing impurities completely means reducing
224:
Occasionally, we may want to include impurities in a material to change its properties. These impurities can be naturally occurring and left unaltered in a material or be intentionally added during synthesis. These types of impurities can show up in our day-to-day lives such as different colors in
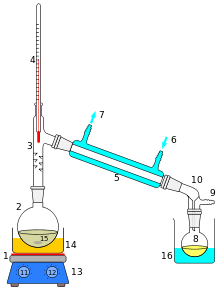
204:. This is done by heating the water so it boils and leaves behind the salt. The water is cooled and the gas turns back to a pure liquid. Impurities are usually physically removed from liquids and gases. Removal of sand particles from metal ore is one example with solids.
215:
Impurities in pharmaceuticals and therapeutics are of special concern and the last couple of decades have witnessed a fair number of scandals, from insecure ingredients and incorrect dosage forms to intentionally fortified medications and accidental contaminations.
136:
have been established by various organizations that attempt to define the permitted levels of various impurities in a manufactured product. Strictly speaking, a material's level of purity can only be stated as being more or less pure than some other material.
334:
Impurities play an important role in the nucleation of other phase transitions. For example, the presence of foreign elements may have important effects on the mechanical and magnetic properties of metal alloys. Iron atoms in copper cause the renowned
310:
below its melting point without becoming a solid. This occurs because the liquid has nothing to condense around so the solid cannot form a natural crystalline solid. The solid is eventually formed when
363:
of a new phase is lower at a point defect. In order for the nucleus of a new phase to be stable, it must reach a critical size. This threshold size is often lower at an impurity site.
714:
707:
468:
700:
843:
161:
and leaf pieces in blank white papers. The removal of impurities is usually done chemically. For example, in the manufacturing of
306:
around the impurities and becomes a crystalline solid. If there are no impurities then the liquid is said to be pure and can be
614:
486:
630:
Urwin, Stephanie J.; Levilain, Guillaume; Marziano, Ivan; Merritt, Jeremy M.; Houson, Ian; Ter Horst, Joop H. (2020-08-21).
252:. Pure beryl will appear colorless but this rarely occurs and the presence of trace elements change its color. The green of
877:
278:
is a process where impurities are purposefully added to semiconductors to increase electrical conductivity and improve a
212:. What technicians can do is to increase the purity of a material to as near 100% as possible or economically feasible.
765:
68:
46:
39:
904:
818:
339:
where the conduction electron spins form a magnetic bound state with the impurity atom. Magnetic impurities in
153:
Impurities can become unwanted when they prevent the working nature of the material. Examples include ash and
209:
181:, another purification method, is an economically important method for the purification of semiconductors.
256:
are from impurities such as chromium, vanadium, or iron. A manganese impurity will give a pink gem called
208:
their concentration to zero. This would require an infinite amount of work and energy as predicted by the
228:
An example of when impurities are wanted is shown in gems. These gems have slight impurities that act as
750:
782:
33:
838:
596:
360:
792:
275:
50:
909:
823:
632:"A Structured Approach To Cope with Impurities during Industrial Crystallization Development"
568:
192:
However, some kinds of impurities can be removed by physical means. A mixture of water and
110:
692:
8:
442:
201:
899:
664:
631:
541:
506:
141:
94:
417:
760:
735:
669:
651:
610:
546:
528:
482:
86:
113:
of the material or compound. Firstly, a pure chemical should appear in at least one
755:
745:
659:
643:
602:
536:
518:
474:
356:
328:
316:
299:
261:
873:
340:
320:
257:
170:
133:
184:
132:
The levels of impurities in a material are generally defined in relative terms.
860:
828:
312:
114:
893:
868:
655:
647:
532:
387:
295:
279:
178:
166:
126:
118:
802:
772:
673:
550:
523:
470:
Distillation
Control, Optimization, and Tuning: Fundamentals and Strategies
336:
197:
606:
507:"Chemical Impurities: An Epistemological Riddle with Serious Side Effects"
348:
307:
229:
122:
810:
352:
16:
Substance within a material that differs from its chemical composition
397:
382:
82:
850:
777:
478:
377:
355:. In general impurities are able to serve as initiation points for
225:
gemstones or by doping to tune the conductivity of semiconductors.
174:
505:
Abdin, Ahmad Yaman; Yeboah, Prince; Jacob, Claus (February 2020).
787:
511:
International
Journal of Environmental Research and Public Health
303:
253:
740:
344:
283:
158:
154:
98:
372:
324:
233:
106:
629:
722:
392:
232:
and give the stone its color. An example is the gem family
193:
162:
267:
140:
Impurities are either naturally occurring or added during
102:
347:
defects. Point defects can nucleate reversed domains in
271:
Three gems from the beryl family with different colors.
359:
because the energetic cost of creating a finite-size
200:, with water as the distillate and salt as the solid
891:
504:
121:. Secondly, a pure chemical should prove to be
708:
569:"Gem-Stones and Their Distinctive Characters"
289:
715:
701:
636:Organic Process Research & Development
663:
540:
522:
236:witch has the base chemical formula of Be
69:Learn how and when to remove this message
844:List of medicine contamination incidents
266:
183:
32:This article includes a list of general
723:Combined substance use and adulteration
466:
294:When an impure liquid is cooled to its
892:
148:
696:
500:
498:
117:and can also be characterized by its
878:List of reagent testing color charts
590:
588:
562:
560:
219:
165:, calcium carbonate is added to the
18:
594:
13:
766:List of polysubstance combinations
495:
343:can serve as generation sites for
38:it lacks sufficient corresponding
14:
921:
585:
567:Smith, George Frederick Herbert.
566:
557:
819:Counterfeit illegal drug selling
689:, Aristo-Wilson, Hong Kong, 2004
23:
687:Chemistry – A Modern View
623:
460:
435:
410:
351:and dramatically affect their
260:and iron creates the blue gem
1:
598:Doping in Conjugated Polymers
467:Robbins, Lanny (2011-05-27).
403:
319:occurs, but it forms into an
210:second law of thermodynamics
97:inside a confined amount of
7:
443:"Definition of HOMOGENEOUS"
366:
188:A basic distillation set up
10:
926:
751:Combined drug intoxication
595:Kar, Pradip (2013-08-23).
327:, instead, as there is no
859:
801:
728:
473:. Boca Raton: CRC Press.
298:the liquid, undergoing a
290:Impurities and nucleation
783:Polysubstance dependence
648:10.1021/acs.oprd.0c00166
418:"Definition of IMPURITY"
905:Environmental chemistry
839:Counterfeit medications
447:www.merriam-webster.com
422:www.merriam-webster.com
109:. They differ from the
53:more precise citations.
729:Combined substance use
524:10.3390/ijerph17031030
272:
189:
824:Clandestine chemistry
607:10.1002/9781118816639
601:(1 ed.). Wiley.
270:
187:
196:can be separated by
111:chemical composition
149:Unwanted impurities
95:chemical substances
685:Cheng, E. et al.,
331:in the structure.
273:
190:
887:
886:
761:Polysubstance use
736:Active metabolite
616:978-1-118-57380-8
488:978-0-429-10729-0
357:phase transitions
220:Wanted impurities
87:materials science
79:
78:
71:
917:
756:Drug interaction
746:Combination drug
717:
710:
703:
694:
693:
678:
677:
667:
642:(8): 1443–1456.
627:
621:
620:
592:
583:
582:
580:
579:
564:
555:
554:
544:
526:
502:
493:
492:
464:
458:
457:
455:
454:
439:
433:
432:
430:
429:
414:
329:long-range order
317:glass transition
300:phase transition
74:
67:
63:
60:
54:
49:this article by
40:inline citations
27:
26:
19:
925:
924:
920:
919:
918:
916:
915:
914:
890:
889:
888:
883:
874:Reagent testing
855:
797:
724:
721:
682:
681:
628:
624:
617:
593:
586:
577:
575:
565:
558:
503:
496:
489:
465:
461:
452:
450:
441:
440:
436:
427:
425:
416:
415:
411:
406:
369:
341:superconductors
321:amorphous solid
292:
251:
247:
243:
239:
222:
171:silicon dioxide
151:
75:
64:
58:
55:
45:Please help to
44:
28:
24:
17:
12:
11:
5:
923:
913:
912:
907:
902:
885:
884:
882:
881:
871:
865:
863:
861:Harm reduction
857:
856:
854:
853:
848:
847:
846:
841:
833:
832:
831:
826:
821:
815:Illegal drugs
813:
807:
805:
799:
798:
796:
795:
790:
785:
780:
775:
770:
769:
768:
758:
753:
748:
743:
738:
732:
730:
726:
725:
720:
719:
712:
705:
697:
691:
690:
680:
679:
622:
615:
584:
556:
494:
487:
479:10.1201/b10875
459:
434:
408:
407:
405:
402:
401:
400:
395:
390:
385:
380:
375:
368:
365:
313:dynamic arrest
291:
288:
282:function. The
280:semiconductors
249:
245:
241:
237:
221:
218:
167:blast furnaces
150:
147:
115:chemical phase
77:
76:
31:
29:
22:
15:
9:
6:
4:
3:
2:
922:
911:
908:
906:
903:
901:
898:
897:
895:
879:
875:
872:
870:
869:Drug checking
867:
866:
864:
862:
858:
852:
849:
845:
842:
840:
837:
836:
834:
830:
827:
825:
822:
820:
817:
816:
814:
812:
809:
808:
806:
804:
800:
794:
791:
789:
786:
784:
781:
779:
776:
774:
771:
767:
764:
763:
762:
759:
757:
754:
752:
749:
747:
744:
742:
739:
737:
734:
733:
731:
727:
718:
713:
711:
706:
704:
699:
698:
695:
688:
684:
683:
675:
671:
666:
661:
657:
653:
649:
645:
641:
637:
633:
626:
618:
612:
608:
604:
600:
599:
591:
589:
574:
573:gutenberg.org
570:
563:
561:
552:
548:
543:
538:
534:
530:
525:
520:
516:
512:
508:
501:
499:
490:
484:
480:
476:
472:
471:
463:
448:
444:
438:
423:
419:
413:
409:
399:
396:
394:
391:
389:
388:Semiconductor
386:
384:
381:
379:
376:
374:
371:
370:
364:
362:
358:
354:
350:
346:
342:
338:
332:
330:
326:
322:
318:
314:
309:
305:
301:
297:
296:melting point
287:
285:
281:
277:
269:
265:
263:
259:
255:
235:
231:
226:
217:
213:
211:
205:
203:
199:
195:
186:
182:
180:
179:Zone refining
176:
172:
168:
164:
160:
156:
146:
143:
138:
135:
130:
128:
127:contamination
124:
120:
119:phase diagram
116:
112:
108:
104:
100:
96:
92:
88:
84:
73:
70:
62:
52:
48:
42:
41:
35:
30:
21:
20:
910:Adulteration
803:Adulteration
773:Polypharmacy
686:
639:
635:
625:
597:
576:. Retrieved
572:
514:
510:
469:
462:
451:. Retrieved
449:. 2024-03-10
446:
437:
426:. Retrieved
424:. 2024-03-24
421:
412:
349:ferromagnets
337:Kondo effect
333:
304:crystallizes
293:
274:
230:chromophores
227:
223:
214:
206:
198:distillation
191:
152:
139:
131:
90:
80:
65:
59:January 2021
56:
37:
517:(3): 1030.
308:supercooled
123:homogeneous
51:introducing
894:Categories
835:Medicines
811:Drug fraud
578:2024-04-15
453:2024-03-27
428:2024-04-01
404:References
353:coercivity
262:aquamarine
169:to remove
91:impurities
34:references
900:Materials
656:1083-6160
533:1661-7827
398:Spin wave
383:Pollution
258:morganite
173:from the
142:synthesis
134:Standards
83:chemistry
851:Impurity
778:Polypill
674:32905065
551:32041209
378:Fineness
367:See also
254:emeralds
175:iron ore
793:Synergy
788:Prodrug
665:7461122
542:7038150
284:dopants
202:residue
47:improve
829:Lacing
741:Codrug
672:
662:
654:
613:
549:
539:
531:
485:
361:domain
345:vortex
276:Doping
159:metals
155:debris
99:liquid
36:, but
373:Dross
325:glass
234:beryl
107:solid
105:, or
670:PMID
652:ISSN
611:ISBN
547:PMID
529:ISSN
483:ISBN
393:Slag
323:– a
244:(SiO
194:salt
163:iron
93:are
85:and
660:PMC
644:doi
603:doi
537:PMC
519:doi
475:doi
315:or
177:.
157:in
103:gas
81:In
896::
668:.
658:.
650:.
640:24
638:.
634:.
609:.
587:^
571:.
559:^
545:.
535:.
527:.
515:17
513:.
509:.
497:^
481:.
445:.
420:.
302:,
264:.
240:Al
129:.
101:,
89:,
880:)
876:(
716:e
709:t
702:v
676:.
646::
619:.
605::
581:.
553:.
521::
491:.
477::
456:.
431:.
250:6
248:)
246:3
242:2
238:3
72:)
66:(
61:)
57:(
43:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.