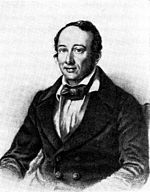31:
205:(Switzerland after 1815). His father was an artist and in 1805 moved the family to the Russian Empire to work as a tutor to a rich family. His mother was a tutor as well and Hess learned German and French at home. In 1817, his family moved to
288:
Like most of his colleagues, Hess was primarily an experimental chemist interested in the discovery and analysis of new substances. However, he also developed a strong interest for theoretical investigations. In particular, he wondered how
285:. It states that in a series of chemical reactions, the total energy gained or lost depends only on the initial and final states, regardless of the number or path of the steps. This is also known as the law of constant heat summation.
351:) went through seven editions and remained the standard Russian textbook for undergraduate chemistry until 1861. Hess was active as a teacher and mentor of young scientists, until his poor health forced him to retire, in 1848.
229:
was giving the lectures in German (an obvious advance for Hess). Under Osann's supervision, Hess made chemical analyses, but also had an interest in the lectures of
Professor of Physics
608:
603:
221:), where he went to a private school for two years, and then to Dorpat Gymnasium, which he finished in 1822. In autumn of the same year Hess studied medicine at the
421:
321:
317:
613:
593:
225:. During that time, the chemistry department was responsible for the Chemistry courses of the Medicine and Pharmacy departments and Professor
241:. He qualified as a physician in 1825. By application of Professors Osann and Engelhardt, Hess was sent to Sweden, to visit Swedish chemist
257:. According to the regulations of that time, new doctors had to practice at a Russian frontier town after having graduated. Hess went to
598:
245:. After this meeting Hess turned once and for all to chemistry. On his return to Russia, Hess joined an expedition to study the
400:
Köhler-Krützfeldt, Angela; Christmann, Klaus (2000). "Dem Begründer der
Thermochemie Hermann Heinrich Hess zum 150. Todestag".
269:
In 1830, Hess took up chemistry full-time, researching and teaching, and later became an adjunct professor of
Chemistry at the
237:. Hess graduated with honors from Dorpat University receiving a doctor of medicine degree with his dissertation entitled
301:
showed that the heat released when they formed was always the same, whether the reactions proceeded directly or through
428:
343:
After these two major discoveries, Hess was influential in the development of chemistry in Russia. His book
313:
stated a more general principle, in 1842. Hess was fully aware of the importance of his own contribution.
387:
333:
278:
270:
146:
171:
242:
181:; 7 August 1802 – 30 November 1850) was a Swiss-Russian chemist and doctor who formulated
519:
Leicester, Henry M. (1951-11-01). "Germain Henri Hess and the foundations of thermochemistry".
306:
230:
302:
234:
588:
583:
528:
8:
222:
105:
532:
494:
498:
486:
290:
555:
536:
478:
409:
360:
337:
329:
325:
310:
162:
556:"Recherches sur les quantités de chaleur dégagées dans les combinaisons chimiques"
274:
226:
186:
119:
469:"Academician German Ivanovich Hess (on the 200th Anniversary of His Birthday)".
383:
376:
250:
210:
80:
76:
482:
577:
490:
413:
298:
282:
182:
115:
95:
540:
372:
332:. A full explanation would only be given 45 years later, in terms of
136:
30:
277:, was published there in 1840. His principle, a progenitor for the
293:
relates to heat in chemical reactions. His experiments on various
368:
294:
258:
254:
246:
218:
206:
202:
198:
91:
58:
54:
214:
399:
239:
Something about
Curative Waters, Especially Those in Russia
359:
Hess' investigation of minerals included the analysis of
609:
Full members of the Saint
Petersburg Academy of Sciences
604:
Saint
Petersburg State Institute of Technology alumni
176:
382:
Hess died prematurely in 1850, at the age of 48, in
305:(1840). Hess thus formulated a special case of the
575:
354:
16:Swiss-Russian chemist and physician (1802–1850)
320:, which states that no heat is evolved in the
273:. His most famous paper, outlining his law on
264:
253:before he was appointed a medical doctor at
514:
512:
510:
508:
371:in his honour. He also discovered that the
192:
560:Comptes Rendus de l'Académie des Sciences
518:
464:
462:
460:
458:
456:
505:
614:Burials at Smolensky Lutheran Cemetery
576:
547:
453:
594:Swiss emigrants to the Russian Empire
553:
471:Russian Journal of Applied Chemistry
13:
554:Hess, Germain Henri (1840-01-01).
393:
316:In 1842, Hess proposed the law of
271:St. Petersburg Academy of Sciences
197:Hess was born on 7 August 1802 in
147:St. Petersburg Academy of Sciences
14:
625:
599:Chemists from the Russian Empire
29:
349:Fundamentals of Pure Chemistry
1:
521:Journal of Chemical Education
446:
355:Later research and final days
477:(7): 1200–1203. 2002-07-01.
388:Smolenskoe Lutheran cemetery
233:and Professor of Mineralogy
7:
427:(in German). Archived from
279:first law of thermodynamics
177:
10:
630:
265:Contributions to chemistry
336:, by the Swedish chemist
334:electrolytic dissociation
166:
152:
142:
132:
125:
111:
101:
87:
65:
40:
28:
21:
414:10.1002/ckon.20000070406
345:Osnovania Chistoy Khimii
193:Early life and education
185:, an early principle of
483:10.1023/A:1020761623694
422:"Hermann Heinrich Hess"
311:Julius Robert von Mayer
231:Georges-Frédéric Parrot
307:conservation of energy
367:Te), which was named
235:Moritz von Engelhardt
178:German Ivanovich Gess
281:, came to be called
243:Jöns Jakob Berzelius
223:University of Dorpat
167:Герман Иванович Гесс
106:University of Dorpat
533:1951JChEd..28..581L
386:. He was buried at
375:of sugars yielded
322:exchange reactions
227:Gottfried W. Osann
159:Germain Henri Hess
35:Germain Henri Hess
23:Germain Henri Hess
541:10.1021/ed028p581
309:two years before
291:chemical affinity
175:
156:
155:
127:Scientific career
621:
568:
567:
551:
545:
544:
516:
503:
502:
466:
442:
440:
439:
433:
426:
417:
361:silver telluride
338:Svante Arrhenius
330:aqueous solution
318:thermoneutrality
261:in August 1826.
180:
170:
168:
72:
69:13 December 1850
50:
48:
33:
19:
18:
629:
628:
624:
623:
622:
620:
619:
618:
574:
573:
572:
571:
552:
548:
517:
506:
468:
467:
454:
449:
437:
435:
431:
424:
420:
396:
394:Further reading
366:
357:
275:thermochemistry
267:
195:
187:thermochemistry
120:Thermochemistry
102:Alma mater
83:
74:
70:
61:
52:
46:
44:
36:
24:
17:
12:
11:
5:
627:
617:
616:
611:
606:
601:
596:
591:
586:
570:
569:
546:
504:
451:
450:
448:
445:
444:
443:
418:
395:
392:
384:St. Petersburg
377:saccharic acid
364:
356:
353:
266:
263:
211:Russian Empire
194:
191:
154:
153:
150:
149:
144:
140:
139:
134:
130:
129:
123:
122:
113:
112:Known for
109:
108:
103:
99:
98:
89:
85:
84:
77:St. Petersburg
75:
73:(aged 48)
67:
63:
62:
53:
42:
38:
37:
34:
26:
25:
22:
15:
9:
6:
4:
3:
2:
626:
615:
612:
610:
607:
605:
602:
600:
597:
595:
592:
590:
587:
585:
582:
581:
579:
565:
561:
557:
550:
542:
538:
534:
530:
526:
522:
515:
513:
511:
509:
500:
496:
492:
488:
484:
480:
476:
472:
465:
463:
461:
459:
457:
452:
434:on 2012-03-08
430:
423:
419:
415:
411:
407:
403:
398:
397:
391:
389:
385:
380:
378:
374:
370:
362:
352:
350:
346:
341:
339:
335:
331:
327:
323:
319:
314:
312:
308:
304:
303:intermediates
300:
299:sulfuric acid
296:
292:
286:
284:
280:
276:
272:
262:
260:
256:
252:
248:
244:
240:
236:
232:
228:
224:
220:
216:
212:
208:
204:
200:
190:
188:
184:
179:
173:
164:
160:
151:
148:
145:
141:
138:
135:
131:
128:
124:
121:
117:
114:
110:
107:
104:
100:
97:
93:
90:
86:
82:
78:
68:
64:
60:
56:
51:7 August 1802
43:
39:
32:
27:
20:
563:
559:
549:
524:
520:
474:
470:
436:. Retrieved
429:the original
405:
401:
381:
358:
348:
344:
342:
315:
287:
268:
238:
196:
158:
157:
143:Institutions
126:
71:(1850-12-13)
589:1850 deaths
584:1802 births
527:(11): 581.
324:of neutral
88:Nationality
578:Categories
566:: 759–763.
447:References
438:2010-06-24
408:(4): 193.
47:1802-08-07
499:195242226
491:1070-4272
373:oxidation
283:Hess' law
183:Hess' law
172:romanized
137:Chemistry
116:Hess' law
295:hydrates
529:Bibcode
402:Chemkon
369:hessite
259:Irkutsk
255:Irkutsk
249:of the
247:geology
219:Estonia
174::
163:Russian
92:Russian
497:
489:
207:Dorpat
203:France
199:Geneva
133:Fields
81:Russia
59:France
55:Geneva
495:S2CID
432:(PDF)
425:(PDF)
326:salts
251:Urals
215:Tartu
213:(now
96:Swiss
487:ISSN
66:Died
41:Born
537:doi
479:doi
410:doi
363:(Ag
328:in
297:of
580::
564:10
562:.
558:.
535:.
525:28
523:.
507:^
493:.
485:.
475:75
473:.
455:^
404:.
390:.
379:.
340:.
217:,
209:,
201:,
189:.
169:,
165::
118:,
79:,
57:,
543:.
539::
531::
501:.
481::
441:.
416:.
412::
406:7
365:2
347:(
161:(
94:-
49:)
45:(
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.