122:
25:
1355:
1658:
962:
In this method the chemical equation is used to calculate the amount of one product which can be formed from each reactant in the amount present. The limiting reactant is the one which can form the smallest amount of the product considered. This method can be extended to any number of reactants more
945:
769:
1513:
1770:
569:
1152:
189:
of the other reactant (B) necessary to react with A. If the amount of B actually present exceeds the amount required, then B is in excess and A is the limiting reagent. If the amount of B present is less than required, then B is the limiting reagent.
1524:
1844:
This suggests a shortcut which works for any number of reagents. Just calculate this formula for each reagent, and the reagent that has the lowest value of this formula is the limiting reagent. We can apply this shortcut in the above example.
1840:
153:
of product formed is limited by this reagent, since the reaction cannot continue without it. If one or more other reagents are present in excess of the quantities required to react with the limiting reagent, they are described as
367:
1135:
Since the reactant amounts are given in grams, they must be first converted into moles for comparison with the chemical equation, in order to determine how many moles of Fe can be produced from either reactant.
1130:
125:
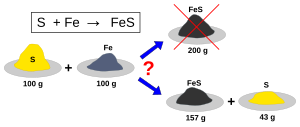
Equal masses of iron (Fe) and sulfur (S) react to form iron sulfide (FeS), but because of its higher atomic weight, iron is the limiting reagent and once all the iron is consumed some sulfur remains unreacted
776:
600:
1361:
1529:
1157:
1664:
417:
1350:{\displaystyle {\begin{aligned}{\ce {mol~Fe2O3}}&={\frac {\ce {grams~Fe2O3}}{\ce {g/mol~Fe2O3}}}\\&={\frac {20.0~{\ce {g}}}{159.7~{\ce {g/mol}}}}=0.125~{\ce {mol}}\end{aligned}}}
1653:{\displaystyle {\begin{aligned}{\ce {mol~Al}}&={\frac {\ce {grams~Al}}{\ce {g/mol~Al}}}\\&={\frac {8.00~{\ce {g}}}{26.98~{\ce {g/mol}}}}=0.297~{\ce {mol}}\end{aligned}}}
1814:
578:
are present, there will be an excess of (18 - 11.25) = 6.75 mol of unreacted oxygen when all the benzene is consumed. Benzene is then the limiting reagent.
177:, which describes the reaction, there are several equivalent ways to identify the limiting reagent and evaluate the excess quantities of other reagents.
173:
of a reaction since the theoretical yield is defined as the amount of product obtained when the limiting reagent reacts completely. Given the balanced
185:
This method is most useful when there are only two reactants. One reactant (A) is chosen, and the balanced chemical equation is used to determine the
216:
997:
940:{\displaystyle {\frac {\ce {mol\,O2}}{\ce {mol\,C6H6}}}={\frac {18\ {\ce {mol\,O2}}}{1.5\ {\ce {mol\,C6H6}}}}=12\ {\ce {mol\,O2}}}
764:{\displaystyle {\frac {\ce {mol\,O2}}{\ce {mol\,C6H6}}}={\frac {15\ {\ce {mol\,O2}}}{2\ {\ce {mol\,C6H6}}}}=7.5\ {\ce {mol\,O2}}}
89:
1508:{\displaystyle {\ce {mol~Fe}}=0.125\ {\ce {mol~Fe2O3}}\times {\frac {\ce {2~mol~Fe}}{\ce {1~mol~Fe2O3}}}=0.250~{\ce {mol~Fe}}}
1935:
61:
68:
1765:{\displaystyle {\ce {mol~Fe}}=0.297~{\ce {mol~Al}}\times {\frac {\ce {2~mol~Fe}}{\ce {2~mol~Al}}}=0.297~{\ce {mol~Fe}}}
564:{\displaystyle 1.5\ {\ce {mol\,C6H6}}\times {\frac {15\ {\ce {mol\,O2}}}{2\ {\ce {mol\,C6H6}}}}=11.25\ {\ce {mol\,O2}}}
1910:
108:
42:
75:
1885:
46:
57:
1957:
1785:
to produce 0.250 mol Fe. This means that the amount of Fe actually produced is limited by the Fe
1835:{\displaystyle {\frac {\mbox{Moles of Reagent X }}{\mbox{Stoichiometric Coefficient of Reagent X}}}}
1801:
It can be seen from the example above that the amount of product (Fe) formed from each reagent X (Fe
957:
35:
1962:
82:
396:
8:
186:
150:
395:
The amount of oxygen required for other quantities of benzene can be calculated using
1931:
1906:
1881:
988:
207:
174:
170:
142:
16:
Reactant introduced in deficit, totally consumed, and stopping the chemical reaction
972:
1875:
1854:
954:
is the reagent in excess, which confirms that benzene is the limiting reagent.
958:
Method 2: Comparison of product amounts which can be formed from each reactant
1951:
373:
593:
required by the balanced equation with the mole ratio actually present:
362:{\displaystyle {\ce {2 C6H6(l) + 15 O2(g) -> 12 CO2(g) + 6 H2O(l)}}}
199:
149:
that is totally consumed when the chemical reaction is completed. The
984:
121:
24:
1777:
There is enough Al to produce 0.297 mol Fe, but only enough Fe
1125:{\displaystyle {\ce {Fe2O3(s) + 2 Al(s) -> 2 Fe(l) + Al2O3(s)}}}
146:
169:
The limiting reagent must be identified in order to calculate the
203:
581:
This conclusion can be verified by comparing the mole ratio of O
377:
180:
1479:
1466:
1415:
1402:
1275:
1262:
1233:
1220:
1190:
1177:
1107:
1094:
1025:
1012:
933:
902:
889:
862:
829:
816:
797:
757:
726:
713:
686:
653:
640:
621:
557:
526:
513:
486:
455:
442:
341:
310:
279:
248:
235:
1825:
1820:
1817:
1667:
1527:
1364:
1155:
1000:
779:
603:
420:
219:
1905:(4th ed.). New York: Houghton Mifflin Company.
1519:Moles of Fe which can be produced from reactant Al
399:(the rule of three). For example, if 1.5 mol C
49:. Unsourced material may be challenged and removed.
1900:
1834:
1793:present, which is therefore the limiting reagent.
1764:
1652:
1507:
1349:
1140:Moles of Fe which can be produced from reactant Fe
1124:
939:
763:
563:
361:
1926:Masterton, William L.; Hurley, Cecile N. (2008).
950:Since the actual ratio is larger than required, O
384:) is required to react with 2 moles of benzene (C
1949:
1925:
1873:
1880:. Jones & Bartlett Learning. p. 163.
162:(sometimes abbreviated as "xs"), or to be in
1874:Olmsted, John; Williams, Gregory M. (1997).
193:
1726:
1708:
1446:
1428:
1066:
1045:
922:
878:
851:
805:
786:
746:
702:
675:
629:
610:
546:
502:
475:
431:
330:
299:
268:
224:
181:Method 1: Comparison of reactant amounts
109:Learn how and when to remove this message
120:
1827:Stoichiometric Coefficient of Reagent X
1809:or Al) is proportional to the quantity
1950:
47:adding citations to reliable sources
18:
1928:Chemistry: Principles and Reactions
13:
14:
1974:
1930:(6 ed.). Cengage Learning.
1877:Chemistry: The Molecular Science
23:
206:, represented by the following
34:needs additional citations for
1919:
1894:
1867:
1117:
1111:
1077:
1071:
1060:
1056:
1050:
1035:
1029:
963:easily than the first method.
354:
348:
320:
314:
293:
289:
283:
258:
252:
1:
1860:
407:is present, 11.25 mol O
7:
1901:Zumdahl, Steven S. (2006).
1848:
1796:
10:
1979:
983:) are reacted with 8.00 g
966:
194:Example for two reactants
1822:Moles of Reagent X
574:If in fact 18 mol O
1836:
1766:
1654:
1509:
1351:
1126:
987:(Al) in the following
941:
765:
565:
363:
126:
1837:
1767:
1655:
1510:
1352:
1127:
942:
766:
566:
364:
124:
1815:
1665:
1525:
1362:
1153:
998:
777:
601:
418:
397:cross-multiplication
217:
43:improve this article
1903:Chemical Principles
1481:
1468:
1417:
1404:
1277:
1264:
1235:
1222:
1192:
1179:
1109:
1096:
1027:
1014:
935:
904:
891:
864:
831:
818:
799:
759:
728:
715:
688:
655:
642:
623:
559:
528:
515:
488:
457:
444:
372:This means that 15
343:
312:
281:
250:
237:
1958:Chemical reactions
1832:
1829:
1824:
1762:
1650:
1648:
1505:
1469:
1456:
1405:
1392:
1347:
1345:
1265:
1252:
1223:
1210:
1180:
1167:
1122:
1097:
1084:
1015:
1002:
937:
923:
892:
879:
852:
819:
806:
787:
761:
747:
716:
703:
676:
643:
630:
611:
561:
547:
516:
503:
476:
445:
432:
359:
331:
300:
269:
238:
225:
127:
58:"Limiting reagent"
1937:978-0-495-12671-3
1830:
1828:
1823:
1760:
1757:
1754:
1750:
1740:
1738:
1735:
1732:
1729:
1720:
1717:
1714:
1711:
1697:
1694:
1691:
1687:
1677:
1674:
1671:
1644:
1640:
1630:
1627:
1619:
1615:
1606:
1602:
1582:
1580:
1577:
1574:
1566:
1561:
1558:
1555:
1541:
1538:
1535:
1503:
1500:
1497:
1493:
1483:
1472:
1459:
1455:
1452:
1449:
1440:
1437:
1434:
1431:
1408:
1395:
1391:
1388:
1384:
1374:
1371:
1368:
1341:
1337:
1327:
1324:
1316:
1312:
1303:
1299:
1279:
1268:
1255:
1251:
1248:
1240:
1226:
1213:
1209:
1206:
1183:
1170:
1166:
1163:
1116:
1100:
1087:
1076:
1069:
1055:
1048:
1034:
1018:
1005:
989:thermite reaction
926:
921:
917:
907:
895:
882:
877:
873:
855:
850:
846:
833:
822:
809:
804:
790:
785:
750:
745:
741:
731:
719:
706:
701:
697:
679:
674:
670:
657:
646:
633:
628:
614:
609:
550:
545:
541:
531:
519:
506:
501:
497:
479:
474:
470:
448:
435:
430:
426:
353:
346:
334:
319:
303:
288:
272:
257:
241:
228:
208:chemical equation
175:chemical equation
143:chemical reaction
135:limiting reactant
119:
118:
111:
93:
1970:
1942:
1941:
1923:
1917:
1916:
1898:
1892:
1891:
1871:
1841:
1839:
1838:
1833:
1831:
1826:
1821:
1819:
1771:
1769:
1768:
1763:
1761:
1758:
1755:
1752:
1748:
1741:
1739:
1736:
1733:
1730:
1727:
1721:
1718:
1715:
1712:
1709:
1703:
1698:
1695:
1692:
1689:
1685:
1678:
1675:
1672:
1669:
1659:
1657:
1656:
1651:
1649:
1645:
1642:
1638:
1631:
1629:
1628:
1625:
1624:
1617:
1613:
1608:
1607:
1604:
1600:
1595:
1587:
1583:
1581:
1578:
1575:
1572:
1571:
1564:
1562:
1559:
1556:
1553:
1551:
1542:
1539:
1536:
1533:
1514:
1512:
1511:
1506:
1504:
1501:
1498:
1495:
1491:
1484:
1482:
1480:
1477:
1470:
1467:
1464:
1457:
1453:
1450:
1447:
1441:
1438:
1435:
1432:
1429:
1423:
1418:
1416:
1413:
1406:
1403:
1400:
1393:
1389:
1386:
1382:
1375:
1372:
1369:
1366:
1356:
1354:
1353:
1348:
1346:
1342:
1339:
1335:
1328:
1326:
1325:
1322:
1321:
1314:
1310:
1305:
1304:
1301:
1297:
1292:
1284:
1280:
1278:
1276:
1273:
1266:
1263:
1260:
1253:
1249:
1246:
1245:
1238:
1236:
1234:
1231:
1224:
1221:
1218:
1211:
1207:
1204:
1202:
1193:
1191:
1188:
1181:
1178:
1175:
1168:
1164:
1161:
1131:
1129:
1128:
1123:
1121:
1120:
1114:
1108:
1105:
1098:
1095:
1092:
1085:
1080:
1074:
1067:
1059:
1053:
1046:
1038:
1032:
1026:
1023:
1016:
1013:
1010:
1003:
973:iron (III) oxide
946:
944:
943:
938:
936:
934:
931:
924:
919:
915:
908:
906:
905:
903:
900:
893:
890:
887:
880:
875:
871:
866:
865:
863:
860:
853:
848:
844:
839:
834:
832:
830:
827:
820:
817:
814:
807:
802:
800:
798:
795:
788:
783:
781:
770:
768:
767:
762:
760:
758:
755:
748:
743:
739:
732:
730:
729:
727:
724:
717:
714:
711:
704:
699:
695:
690:
689:
687:
684:
677:
672:
668:
663:
658:
656:
654:
651:
644:
641:
638:
631:
626:
624:
622:
619:
612:
607:
605:
570:
568:
567:
562:
560:
558:
555:
548:
543:
539:
532:
530:
529:
527:
524:
517:
514:
511:
504:
499:
495:
490:
489:
487:
484:
477:
472:
468:
463:
458:
456:
453:
446:
443:
440:
433:
428:
424:
368:
366:
365:
360:
358:
357:
351:
344:
342:
339:
332:
323:
317:
311:
308:
301:
292:
286:
280:
277:
270:
261:
255:
249:
246:
239:
236:
233:
226:
171:percentage yield
160:excess reactants
131:limiting reagent
114:
107:
103:
100:
94:
92:
51:
27:
19:
1978:
1977:
1973:
1972:
1971:
1969:
1968:
1967:
1948:
1947:
1946:
1945:
1938:
1924:
1920:
1913:
1899:
1895:
1888:
1872:
1868:
1863:
1855:Limiting factor
1851:
1818:
1816:
1813:
1812:
1808:
1804:
1799:
1792:
1788:
1784:
1780:
1751:
1722:
1704:
1702:
1688:
1668:
1666:
1663:
1662:
1647:
1646:
1641:
1620:
1616:
1609:
1603:
1596:
1594:
1585:
1584:
1567:
1563:
1552:
1550:
1543:
1532:
1528:
1526:
1523:
1522:
1494:
1478:
1473:
1465:
1460:
1442:
1424:
1422:
1414:
1409:
1401:
1396:
1385:
1365:
1363:
1360:
1359:
1344:
1343:
1338:
1317:
1313:
1306:
1300:
1293:
1291:
1282:
1281:
1274:
1269:
1261:
1256:
1241:
1237:
1232:
1227:
1219:
1214:
1203:
1201:
1194:
1189:
1184:
1176:
1171:
1160:
1156:
1154:
1151:
1150:
1147:
1143:
1110:
1106:
1101:
1093:
1088:
1070:
1049:
1028:
1024:
1019:
1011:
1006:
1001:
999:
996:
995:
982:
978:
969:
960:
953:
932:
927:
918:
901:
896:
888:
883:
874:
867:
861:
856:
847:
840:
838:
828:
823:
815:
810:
801:
796:
791:
782:
780:
778:
775:
774:
756:
751:
742:
725:
720:
712:
707:
698:
691:
685:
680:
671:
664:
662:
652:
647:
639:
634:
625:
620:
615:
606:
604:
602:
599:
598:
592:
588:
584:
577:
556:
551:
542:
525:
520:
512:
507:
498:
491:
485:
480:
471:
464:
462:
454:
449:
441:
436:
427:
419:
416:
415:
410:
406:
402:
391:
387:
383:
347:
340:
335:
313:
309:
304:
282:
278:
273:
251:
247:
242:
234:
229:
220:
218:
215:
214:
196:
183:
156:excess reagents
115:
104:
98:
95:
52:
50:
40:
28:
17:
12:
11:
5:
1976:
1966:
1965:
1960:
1944:
1943:
1936:
1918:
1911:
1893:
1886:
1865:
1864:
1862:
1859:
1858:
1857:
1850:
1847:
1806:
1802:
1798:
1795:
1790:
1786:
1782:
1778:
1775:
1774:
1773:
1772:
1747:
1744:
1725:
1707:
1701:
1684:
1681:
1660:
1637:
1634:
1623:
1612:
1599:
1593:
1590:
1588:
1586:
1570:
1549:
1546:
1544:
1531:
1530:
1517:
1516:
1515:
1490:
1487:
1476:
1463:
1445:
1427:
1421:
1412:
1399:
1381:
1378:
1357:
1334:
1331:
1320:
1309:
1296:
1290:
1287:
1285:
1283:
1272:
1259:
1244:
1230:
1217:
1200:
1197:
1195:
1187:
1174:
1159:
1158:
1145:
1141:
1133:
1132:
1119:
1113:
1104:
1091:
1083:
1079:
1073:
1065:
1062:
1058:
1052:
1044:
1041:
1037:
1031:
1022:
1009:
980:
976:
968:
965:
959:
956:
951:
948:
947:
930:
914:
911:
899:
886:
870:
859:
843:
837:
826:
813:
794:
771:
754:
738:
735:
723:
710:
694:
683:
667:
661:
650:
637:
618:
590:
586:
582:
575:
572:
571:
554:
538:
535:
523:
510:
494:
483:
467:
461:
452:
439:
423:
408:
404:
400:
389:
385:
381:
370:
369:
356:
350:
338:
329:
326:
322:
316:
307:
298:
295:
291:
285:
276:
267:
264:
260:
254:
245:
232:
223:
195:
192:
182:
179:
139:limiting agent
117:
116:
31:
29:
22:
15:
9:
6:
4:
3:
2:
1975:
1964:
1963:Stoichiometry
1961:
1959:
1956:
1955:
1953:
1939:
1933:
1929:
1922:
1914:
1912:0-618-37206-7
1908:
1904:
1897:
1889:
1883:
1879:
1878:
1870:
1866:
1856:
1853:
1852:
1846:
1842:
1810:
1794:
1745:
1742:
1723:
1705:
1699:
1682:
1679:
1661:
1635:
1632:
1621:
1610:
1597:
1591:
1589:
1568:
1547:
1545:
1521:
1520:
1518:
1488:
1485:
1474:
1461:
1443:
1425:
1419:
1410:
1397:
1379:
1376:
1358:
1332:
1329:
1318:
1307:
1294:
1288:
1286:
1270:
1257:
1242:
1228:
1215:
1198:
1196:
1185:
1172:
1149:
1148:
1139:
1138:
1137:
1102:
1089:
1081:
1063:
1042:
1039:
1020:
1007:
994:
993:
992:
990:
986:
974:
964:
955:
928:
912:
909:
897:
884:
868:
857:
841:
835:
824:
811:
792:
772:
752:
736:
733:
721:
708:
692:
681:
665:
659:
648:
635:
616:
596:
595:
594:
579:
552:
536:
533:
521:
508:
492:
481:
465:
459:
450:
437:
421:
414:
413:
412:
411:is required:
398:
393:
379:
376:of molecular
375:
336:
327:
324:
305:
296:
274:
265:
262:
243:
230:
221:
213:
212:
211:
209:
205:
201:
198:Consider the
191:
188:
178:
176:
172:
167:
165:
161:
157:
152:
148:
144:
140:
136:
132:
123:
113:
110:
102:
91:
88:
84:
81:
77:
74:
70:
67:
63:
60: –
59:
55:
54:Find sources:
48:
44:
38:
37:
32:This article
30:
26:
21:
20:
1927:
1921:
1902:
1896:
1876:
1869:
1843:
1811:
1800:
1776:
1134:
970:
961:
949:
580:
573:
394:
371:
197:
184:
168:
163:
159:
155:
138:
134:
130:
128:
105:
96:
86:
79:
72:
65:
53:
41:Please help
36:verification
33:
1952:Categories
1887:0815184506
1861:References
971:20.0 g of
597:required:
200:combustion
69:newspapers
1700:×
1420:×
1061:⟶
985:aluminium
460:×
294:⟶
164:abundance
99:June 2015
1849:See also
1797:Shortcut
773:actual:
147:reactant
967:Example
204:benzene
141:) in a
83:scholar
1934:
1909:
1884:
1756:
1749:
1734:
1728:
1716:
1710:
1693:
1686:
1673:
1639:
1614:
1601:
1576:
1557:
1537:
1499:
1492:
1454:
1448:
1436:
1430:
1390:
1383:
1370:
1336:
1311:
1298:
1250:
1208:
1165:
916:
872:
845:
740:
696:
669:
540:
496:
469:
425:
378:oxygen
187:amount
151:amount
85:
78:
71:
64:
56:
1746:0.297
1683:0.297
1636:0.297
1611:26.98
1554:grams
1489:0.250
1380:0.125
1333:0.125
1308:159.7
1205:grams
585:and C
537:11.25
374:moles
145:is a
90:JSTOR
76:books
1932:ISBN
1907:ISBN
1882:ISBN
1598:8.00
1295:20.0
133:(or
129:The
62:news
1753:mol
1731:mol
1713:mol
1690:mol
1670:mol
1643:mol
1626:mol
1573:mol
1534:mol
1496:mol
1451:mol
1433:mol
1387:mol
1367:mol
1340:mol
1323:mol
1247:mol
1162:mol
975:(Fe
920:mol
876:mol
869:1.5
849:mol
803:mol
784:mol
744:mol
737:7.5
700:mol
673:mol
627:mol
608:mol
544:mol
500:mol
473:mol
429:mol
422:1.5
202:of
158:or
137:or
45:by
1954::
1759:Fe
1737:Al
1719:Fe
1696:Al
1676:Fe
1579:Al
1560:Al
1540:Al
1502:Fe
1458:Fe
1439:Fe
1394:Fe
1373:Fe
1254:Fe
1212:Fe
1169:Fe
1086:Al
1068:Fe
1047:Al
1004:Fe
991::
913:12
842:18
666:15
466:15
392:)
380:(O
302:CO
297:12
266:15
210::
166:.
1940:.
1915:.
1890:.
1807:3
1805:O
1803:2
1791:3
1789:O
1787:2
1783:3
1781:O
1779:2
1743:=
1724:2
1706:2
1680:=
1633:=
1622:/
1618:g
1605:g
1592:=
1569:/
1565:g
1548:=
1486:=
1475:3
1471:O
1462:2
1444:1
1426:2
1411:3
1407:O
1398:2
1377:=
1330:=
1319:/
1315:g
1302:g
1289:=
1271:3
1267:O
1258:2
1243:/
1239:g
1229:3
1225:O
1216:2
1199:=
1186:3
1182:O
1173:2
1146:3
1144:O
1142:2
1118:)
1115:s
1112:(
1103:3
1099:O
1090:2
1082:+
1078:)
1075:l
1072:(
1064:2
1057:)
1054:s
1051:(
1043:2
1040:+
1036:)
1033:s
1030:(
1021:3
1017:O
1008:2
981:3
979:O
977:2
952:2
929:2
925:O
910:=
898:6
894:H
885:6
881:C
858:2
854:O
836:=
825:6
821:H
812:6
808:C
793:2
789:O
753:2
749:O
734:=
722:6
718:H
709:6
705:C
693:2
682:2
678:O
660:=
649:6
645:H
636:6
632:C
617:2
613:O
591:6
589:H
587:6
583:2
576:2
553:2
549:O
534:=
522:6
518:H
509:6
505:C
493:2
482:2
478:O
451:6
447:H
438:6
434:C
409:2
405:6
403:H
401:6
390:6
388:H
386:6
382:2
355:)
352:l
349:(
345:O
337:2
333:H
328:6
325:+
321:)
318:g
315:(
306:2
290:)
287:g
284:(
275:2
271:O
263:+
259:)
256:l
253:(
244:6
240:H
231:6
227:C
222:2
112:)
106:(
101:)
97:(
87:·
80:·
73:·
66:·
39:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.